The Applications of Morphology Controlled ZnO in Catalysis
Abstract
:1. Introduction
2. Applications to Thermo-Catalysis
2.1. Methanol Synthesis
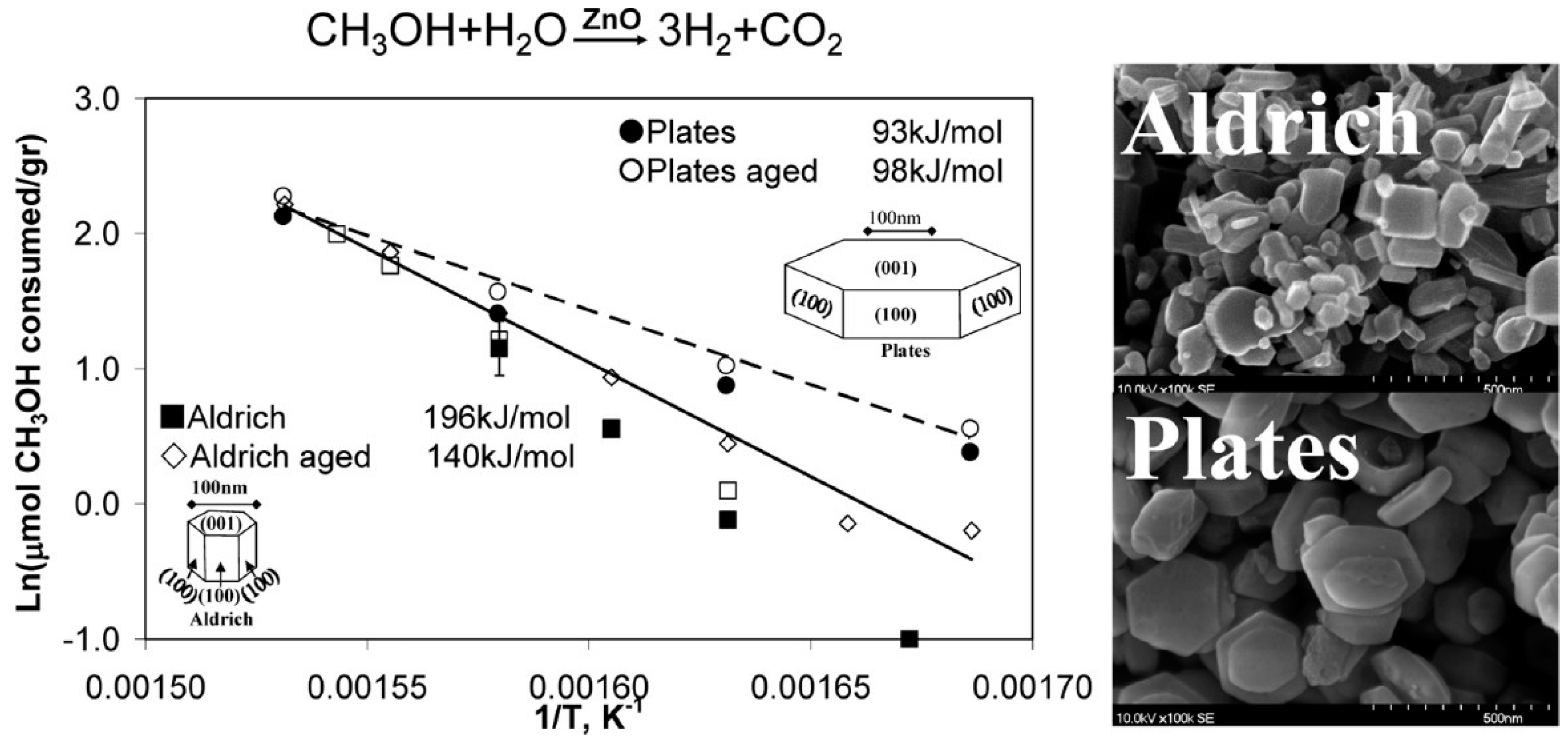
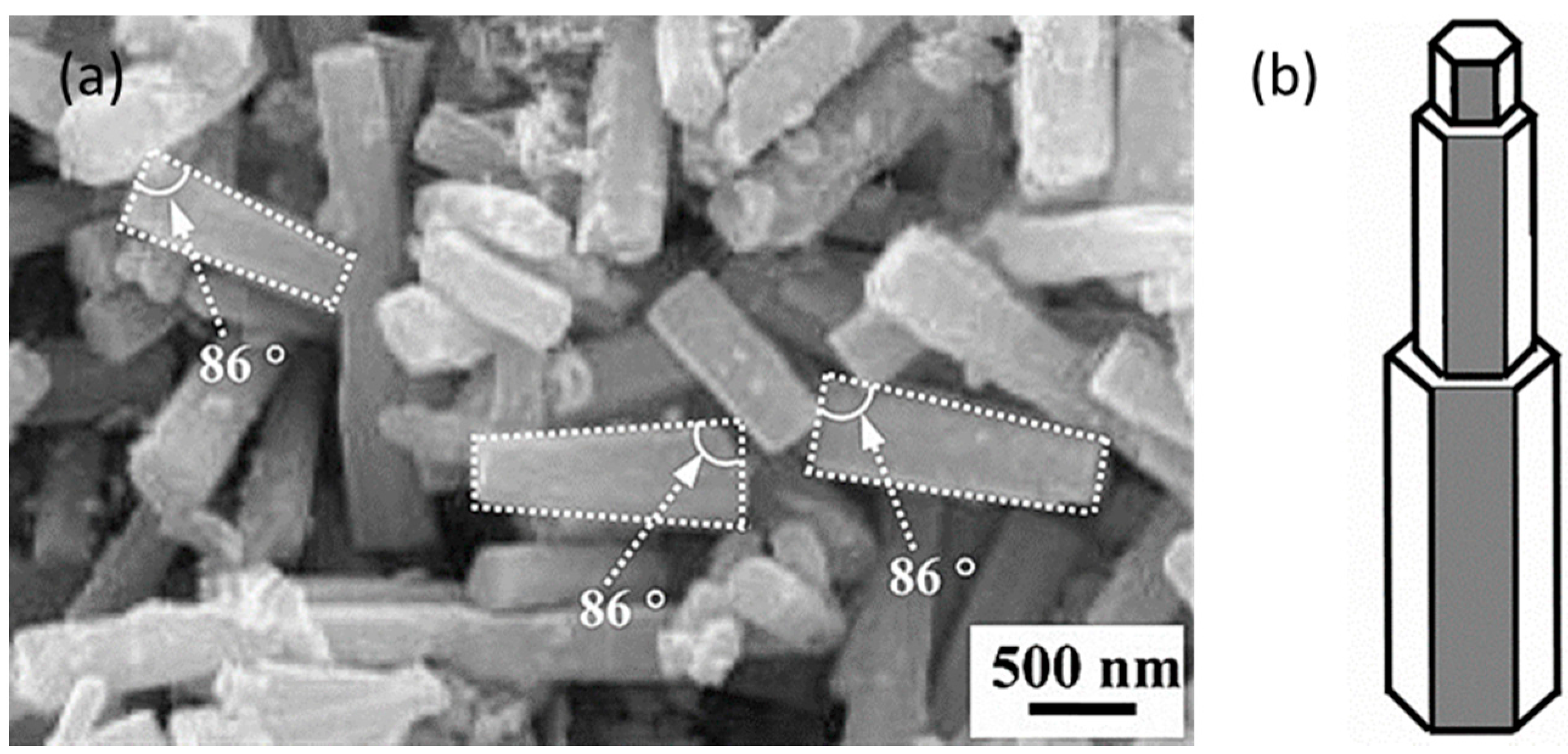
2.2. Methanol Steam Reforming (MSR)
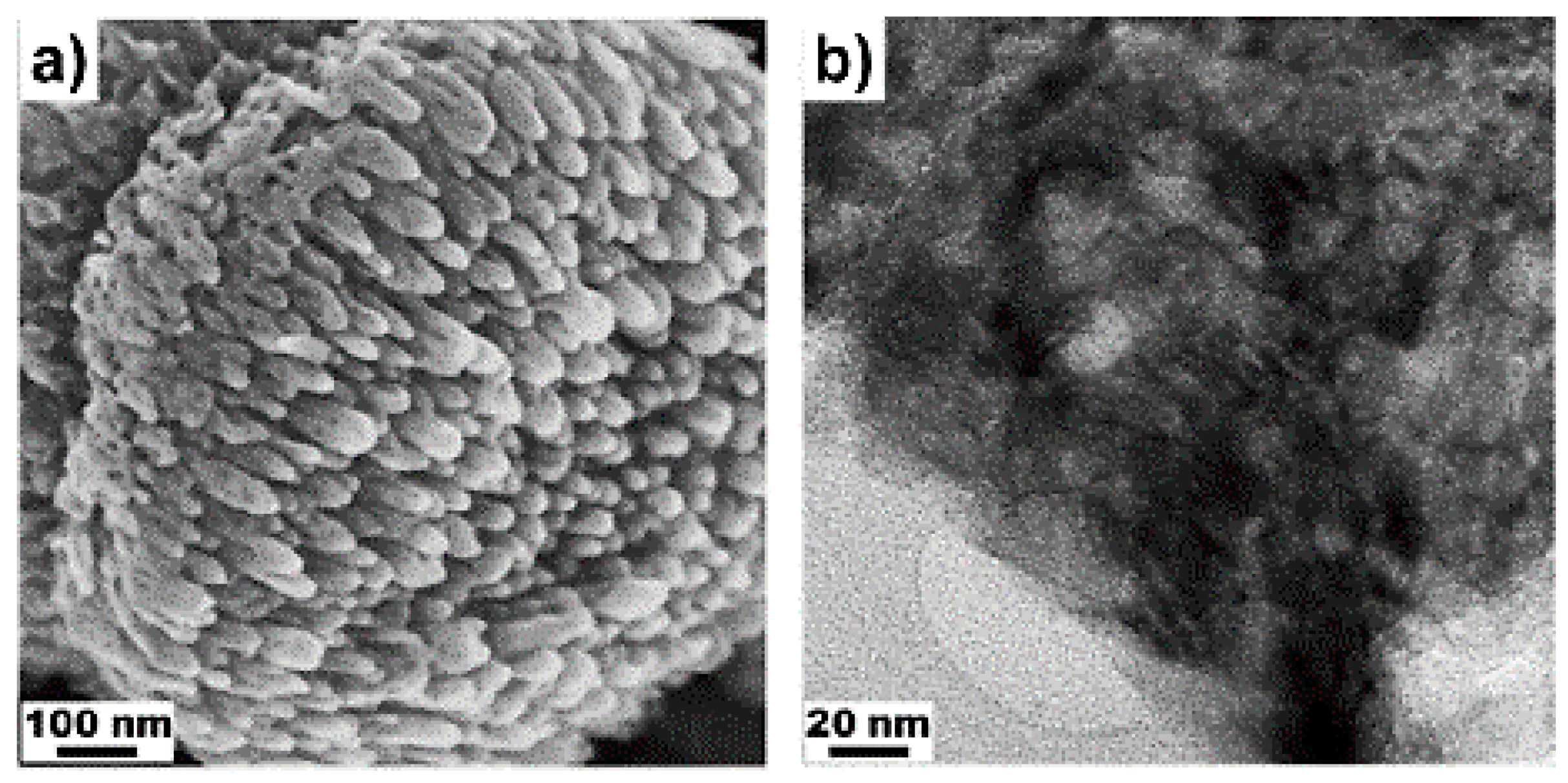
2.3. Biomass Related Catalytic Reactions
2.4. CO Oxidation
2.5. Other Thermo-Catalytic Reactions
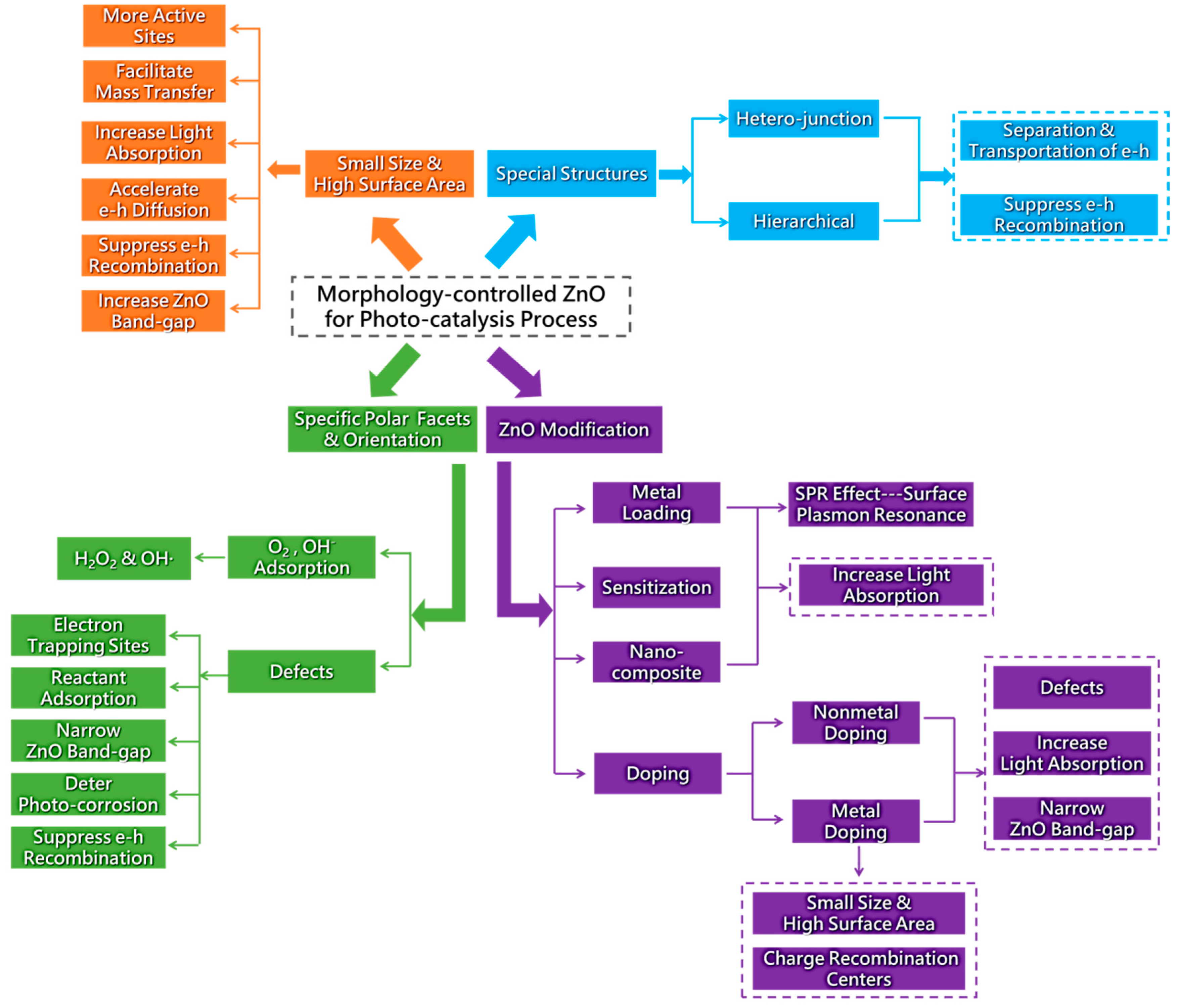
3. Applications to Photo-Catalysis
3.1. Degradation of Organic Dyes
3.1.1. Degradation of Methylene Blue (MB)
3.1.2. Photo-Degradation of Methyl Orange (MO)
3.1.3. Photo-Degradation of Rhodamine B (RhB)
3.1.4. Photo-Degradation of Other Organic Dyes
3.2. Photo-Degradation of Phenol and other Pollutants
4. Application to Photo-Assisted Electro-Catalysis
5. Conclusions and Outlook
- High specific surface area is beneficial for catalytic performance and some ZnO morphologies with meporous structures have been reported in the applications of catalysis [142,207,240,249]. However, this is a challenge because the morphology controlled synthesis may be destroyed by pore-forming materials and more research should be carried out.
- One-dimensional ZnO nanostructures have been intensively investigated in photoelectrochemical (PEC) cells to split water and large enhancements have been achieved [74,77,79,82,83,84]. Furthermore, ZnO nanorods have been reported in the applications of photoelectron-oxidation of ethanol and methanol [83,84]; ZnO has also been applied as an important photoelectrocatalyst component in the methanol production from CO2 by photoelectrocatalytic reduction. Therefore, applications of morphology controlled ZnO in the photoelectron oxidation of organic substances to generate hydrogen or electricity and photoelectron reduction of CO2 to produce fuels should be further investigated.
- ZnO based proper hierarchical architectures and hetero-junctions have been proven to be favorable for enhancing thermocatalytic properties [264]. Morphology controlled ZnO with special hierarchical architectures and hetero-junctions should be well developed and utilized in all kinds of thermocatalytic reactions. Similarly, metal and nonmetal doping can modify the electronic structures of ZnO; however, there is a lack of research on the thermocatalytic reactions of morphology controlled ZnO with doping. The development of these types of ZnO nanostructures on one hand will contribute to the improvement of catalytic performance; on the other hand, can be served as model catalyst to investigate complicated catalytic theories.
Acknowledgments
Conflicts of Interest
References
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Segets, D.; Gradl, J.; Taylor, R.K.; Vassilev, V.; Peukert, W. Analysis of optical absorbance spectra for the determination of ZnO nanoparticle size distribution, solubility, and surface energy. ACS Nano 2009, 3, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Bacaksiz, E.; Parlak, M.; Tomakin, M.; Özçelik, A.; Karakız, M.; Altunbaş, M. The effects of zinc nitrate, zinc acetate and zinc chloride precursors on investigation of structural and optical properties of ZnO thin films. J. Alloys Compd. 2008, 466, 447–450. [Google Scholar] [CrossRef]
- Wang, J.; Cao, J.; Fang, B.; Lu, P.; Deng, S.; Wang, H. Synthesis and characterization of multipod, flower-like, and shuttle-like ZnO frameworks in ionic liquids. Mater. Lett. 2005, 59, 1405–1408. [Google Scholar] [CrossRef]
- Wang, Z.L. Splendid one-dimensional nanostructures of zinc oxide: A new nanomaterial family for nanotechnology. ACS Nano 2008, 2, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Matoussi, A. Electrical conduction and dielectric studies of ZnO pellets. Phys. B Condens. Matter 2012, 407, 3441–3447. [Google Scholar] [CrossRef]
- Cheng, S.; Yan, D.; Chen, J.; Zhuo, R.; Feng, J.; Li, H.; Feng, H.; Yan, P. Soft-template synthesis and characterization of ZnO and ZnO hollow spheres. J. Phys. Chem. C 2009, 113, 13630–13635. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Liu, B.; Yang, H. Charge separation between wurtzite ZnO polar {001} surfaces and their enhanced photocatalytic activity. Appl. Catal. B 2015, 163, 189–197. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Huang, Y.; Ge, J.; Zheng, W.; Tedsree, K.; Collier, P.; Hong, X.; Tsang, S.C. Morphology-dependent interactions of ZnO with Cu nanoparticles at the materials’ interface in selective hydrogenation of CO2 to CH3OH. Angew. Chem. Int. Ed. 2011, 50, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fan, Y.; Pei, Y.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. Shape effect of ZnO crystals as cocatalyst in combined reforming–hydrogenolysis of glycerol. ACS Catal. 2013, 3, 2280–2287. [Google Scholar] [CrossRef]
- Schumann, J.; Eichelbaum, M.; Lunkenbein, T.; Thomas, N.; Alvarez Galvan, M.C.; Schlogl, R.; Behrens, M. Promoting strong metal support interaction: Doping ZnO for enhanced activity of Cu/ZnO:M (M = Al, Ga, Mg) catalysts. ACS Catal. 2015, 5, 3260–3270. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Dagle, V.L.; Halevi, B.; Datye, A.K.; Wang, Y. Influence of ZnO facets on Pd/ZnO catalysts for methanol steam reforming. ACS Catal. 2014, 4, 2379–2386. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.-L.; Pelligra, C.; Chen, C.-H.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joesten, R.; Suib, S.L. ZnO with different morphologies synthesized by solvothermal methods for enhanced photocatalytic activity. Chem. Mater. 2009, 21, 2875–2885. [Google Scholar] [CrossRef]
- Kuld, S.; Thorhauge, M.; Falsig, H.; Elkjær, C.F.; Helveg, S.; Chorkendorff, I.; Sehested, J. Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 2016, 352, 969–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, K.; Ludviksson, A.; Zhang, R.; Yoshihara, J.; Campbell, C. Growth model for metal films on oxide surfaces: Cu on ZnO(000–1). Phys. Rev. B 1993, 47, 13782. [Google Scholar] [CrossRef]
- Jacek, G.; Fabio, F.; Claudine, N. Polarity of oxide surfaces and nanostructures. Rep. Prog. Phys. 2008, 71, 016501. [Google Scholar]
- Schimpf, S.; Rittermeier, A.; Zhang, X.; Li, Z.A.; Spasova, M.; van den Berg, M.W.; Farle, M.; Wang, Y.; Fischer, R.A.; Muhler, M. Stearate-based Cu colloids in methanol synthesis: Structural changes driven by strong metal–support interactions. ChemCatChem 2010, 2, 214–222. [Google Scholar] [CrossRef]
- Sirbuly, D.J.; Law, M.; Yan, H.; Yang, P. Semiconductor nanowires for subwavelength photonics integration. J. Phys. Chem. B 2005, 109, 15190–15213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, F.; Lu, Y.; Xu, M.; Li, Q. Induction of zinc particles on the morphology and photoluminescent property of globular Zn/ZnO core/shell nanorod heterojunction array architectures. J. Exp. Nanosci. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Heo, Y.; Tien, L.; Norton, D.; Pearton, S.; Kang, B.; Ren, F.; LaRoche, J. Pt∕ZnO nanowire schottky diodes. Appl. Phys. Lett. 2004, 85, 3107–3109. [Google Scholar] [CrossRef]
- Barth, S.; Hernandez-Ramirez, F.; Holmes, J.D.; Romano-Rodriguez, A. Synthesis and applications of one-dimensional semiconductors. Prog. Mater. Sci. 2010, 55, 563–627. [Google Scholar] [CrossRef]
- Devan, R.S.; Patil, R.A.; Lin, J.H.; Ma, Y.R. One-dimensional metal-oxide nanostructures: Recent developments in synthesis, characterization, and applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Wu, J.-J.; Liu, S.-C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Kahn, M.L.; Monge, M.; Snoeck, E.; Maisonnat, A.; Chaudret, B. Spontaneous formation of ordered 2D and 3D superlattices of ZnO nanocrystals. Small 2005, 1, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, M.R.; Henley, S.J.; Emerson, N.G.; Silva, S.R.P. From 1d and 2D ZnO nanostructures to 3D hierarchical structures with enhanced gas sensing properties. Nanoscale 2014, 6, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, W.; Liu, J.; Wang, F.; Kong, J.; Qiu, S.; He, C.; Luan, L. Synthesis of nestlike ZnO hierarchically porous structures and analysis of their gas sensing properties. ACS Appl. Mater. Int. 2012, 4, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Schneider, J.J. Assembly of one dimensional inorganic nanostructures into functional 2D and 3D architectures. Synthesis, arrangement and functionality. Chem. Soc. Rev. 2012, 41, 5285–5312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, W.; Duan, G.; Cao, B.; Sun, F.; Lu, F. Superhydrophobicity of 2D ZnO ordered pore arrays formed by solution-dipping template method. J. Colloid Interface Sci. 2005, 287, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gao, S.; Wang, J. One-pot synthesis of Ag/ZnO self-assembled 3D hollow microspheres with enhanced photocatalytic performance. J. Phys. Chem. C 2008, 112, 16792–16800. [Google Scholar] [CrossRef]
- Vayssieres, L.; Keis, K.; Lindquist, S.-E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350–3352. [Google Scholar] [CrossRef]
- Banerjee, D.; Lao, J.; Wang, D.; Huang, J.; Ren, Z.; Steeves, D.; Kimball, B.; Sennett, M. Large-quantity free-standing ZnO nanowires. Appl. Phys. Lett. 2003, 83, 2061–2063. [Google Scholar] [CrossRef]
- Hahn, Y.-B. Zinc oxide nanostructures and their applications. Korean J. Chem. Eng. 2011, 28, 1797–1813. [Google Scholar] [CrossRef]
- Frade, T.; Jorge, M.M.; Gomes, A. One-dimensional ZnO nanostructured films: Effect of oxide nanoparticles. Mater. Lett. 2012, 82, 13–15. [Google Scholar] [CrossRef]
- Wu, J.-J.; Liu, S.-C.; Wu, C.-T.; Chen, K.-H.; Chen, L.-C. Heterostructures of ZnO-Zn coaxial nanocables and ZnO nanotubes. Appl. Phys. Lett. 2002, 81, 1312–1314. [Google Scholar] [CrossRef]
- Chen, W.; Liu, W.; Hsieh, S.; Tsai, T. Preparation of nanosized ZnO using α brass. Appl. Surf. Sci. 2007, 253, 6749–6753. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Duan, J.; Ai, H.; Tu, P. A low-temperature synthesis of multi whisker-based zinc oxide micron crystals. Mater. Lett. 2005, 59, 3710–3714. [Google Scholar] [CrossRef]
- Huang, Y.; He, J.; Zhang, Y.; Dai, Y.; Gu, Y.; Wang, S.; Zhou, C. Morphology, structures and properties of ZnO nanobelts fabricated by Zn-powder evaporation without catalyst at lower temperature. J. Mater. Sci. 2006, 41, 3057–3062. [Google Scholar] [CrossRef]
- Nikoobakht, B.; Wang, X.; Herzing, A.; Shi, J. Scalable synthesis and device integration of self-registered one-dimensional zinc oxide nanostructures and related materials. Chem. Soc. Rev. 2013, 42, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Tien, L.; Pearton, S.; Norton, D.; Ren, F. Synthesis and microstructure of vertically aligned ZnO nanowires grown by high-pressure-assisted pulsed-laser deposition. J. Mater. Sci. 2008, 43, 6925–6932. [Google Scholar] [CrossRef]
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.; Kim, Y.-S.; Seo, H.-K.; Shin, H.-S. Room temperature synthesis of needle-shaped ZnO nanorods via sonochemical method. Appl. Surf. Sci. 2007, 253, 7622–7626. [Google Scholar] [CrossRef]
- Hughes, W.L.; Wang, Z.L. Formation of piezoelectric single-crystal nanorings and nanobows. J. Am. Chem. Soc. 2004, 126, 6703–6709. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ji, P.; He, M.; Li, J. Growth and structure of pure ZnO micro/nanocombs. J. Nanomater. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wang, Z.L. Spontaneous polarization-induced nanohelixes, nanosprings, and nanorings of piezoelectric nanobelts. Nano Lett. 2003, 3, 1625–1631. [Google Scholar] [CrossRef]
- Snure, M.; Tiwari, A. Synthesis, characterization, and green luminescence in ZnO nanocages. J. Nanosci. Nanotechnol. 2007, 7, 481–485. [Google Scholar] [CrossRef]
- Chiu, W.; Khiew, P.; Cloke, M.; Isa, D.; Tan, T.; Radiman, S.; Abd-Shukor, R.; Hamid, M.A.; Huang, N.; Lim, H. Photocatalytic study of two-dimensional ZnO nanopellets in the decomposition of methylene blue. Chem. Eng. J. 2010, 158, 345–352. [Google Scholar] [CrossRef]
- Jose-Yacaman, M.; Gutierrez-Wing, C.; Miki, M.; Yang, D.-Q.; Piyakis, K.; Sacher, E. Surface diffusion and coalescence of mobile metal nanoparticles. J. Phys. Chem. B 2005, 109, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-assembly of metal oxides into three-dimensional nanostructures: Synthesis and application in catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Dai, Z.; Liang, J.; Xu, L.; Yu, W.; Qian, Y. Synthesis of ZnO three-dimensional architectures and their optical properties. Solid State Commun. 2005, 136, 304–307. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.; Sun, F.; He, X. Selective growth and properties of zinc oxide nanostructures. Scr. Mater. 2006, 55, 795–798. [Google Scholar] [CrossRef]
- Bitenc, M.; Orel, Z.C. Synthesis and characterization of crystalline hexagonal bipods of zinc oxide. Mater. Res. Bull. 2009, 44, 381–387. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Zinc oxide nanostructures: Synthesis and properties. J. Nanosci. Nanotechnol. 2005, 5, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.-C.; Wang, C.; Park, W.I. ZnO nanorods: Synthesis, characterization and applications. Semicond. Sci. Technol. 2005, 20, S22–S34. [Google Scholar] [CrossRef]
- Heo, Y.W.; Norton, D.; Tien, L.; Kwon, Y.; Kang, B.; Ren, F.; Pearton, S.; LaRoche, J. ZnO nanowire growth and devices. Mater. Sci. Eng. A-Struct. R: Rep. 2004, 47, 1–47. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoc, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: From basics towards applications. Phys. Status Solidi B 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, physics and applications. Chem. Phys. Chem. 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 1, 013001. [Google Scholar] [CrossRef] [PubMed]
- Ellmer, K.; Klein, A.; Rech, B. Transparent Conductive Zinc Oxide: Basics and Applications in Thin Film Solar Cells; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Wöll, C. The chemistry and physics of zinc oxide surfaces. Prog. Surf. Sci. 2007, 82, 55–120. [Google Scholar] [CrossRef]
- McCluskey, M.; Jokela, S. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Djurišić, A.; Ng, A.; Chen, X. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quant. Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ng, A.M.C. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Liu, X.; Leung, Y.H. Zinc oxide films and nanomaterials for photovoltaic applications. Phys. Status Solidi-R 2014, 8, 123–132. [Google Scholar] [CrossRef]
- Feng, K.; Li, W.; Xie, S.; Lu, X. Nickel hydroxide decorated hydrogenated zinc oxide nanorod arrays with enhanced photoelectrochemical performance. Electrochim. Acta 2014, 137, 108–113. [Google Scholar] [CrossRef]
- Kozuka, Y.; Tsukazaki, A.; Kawasaki, M. Challenges and opportunities of ZnO-related single crystalline heterostructures. Appl. Phys. Rev. 2014, 1, 011303. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Al-Dossary, O.; Umar, A. ZnO nanostructured thin films: Depositions, properties and applications-a review. Mater. Express 2015, 5, 3–23. [Google Scholar] [CrossRef]
- Sengupta, D.; Das, P.; Mondal, B.; Mukherjee, K. Effects of doping, morphology and film-thickness of photo-anode materials for dye sensitized solar cell application-a review. Renew. Sustain. Energy Rev. 2016, 60, 356–376. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Johar, M.A.; Afzal, R.A.; Alazba, A.A.; Manzoor, U. Photocatalysis and Band gap Engineering Using ZnO Nanocomposites. Adv. Mater. Sci. Eng. 2015, 2015, 934587. [Google Scholar]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water. Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- KSR, K.K.R. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar]
- Kulmas, M.; Paterson, L.; Höflich, K.; Bashouti, M.Y.; Wu, Y.; Göbelt, M.; Ristein, J.; Bachmann, J.; Meyer, B.; Christiansen, S. Composite nanostructures of TiO2 and ZnO for water splitting application: Atomic layer deposition growth and density functional theory investigation. Adv. Funct. Mater. 2016, 26, 4882–4889. [Google Scholar] [CrossRef]
- Sudha, D.; Sivakumar, P. Review on the photocatalytic activity of various composite catalysts. Chem. Eng. Process. 2015, 97, 112–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, characterization, and applications of ZnO nanowires. J. Nanomater. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Djerdj, I.; Jagličić, Z.; Arčon, D.; Niederberger, M. Co-doped ZnO nanoparticles: Minireview. Nanoscale 2010, 2, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Antoniadou, M.; Lianos, P. Production of electricity by photoelectrochemical oxidation. Appl. Catal. B 2010, 99, 307–313. [Google Scholar] [CrossRef]
- Leelavathi, A.; Madras, G.; Ravishankar, N. New insights into electronic and geometric effects in the enhanced photoelectrooxidation of ethanol using ZnO nanorod/ultrathin au nanowire hybrids. J. Am. Chem. Soc. 2014, 136, 14445. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Hsueh, Y.-C.; Kei, C.-C.; Lin, C.-T.; Perng, T.-P. Fabrication of high-activity hybrid Pt@ZnO catalyst on carbon cloth by atomic layer deposition for photoassisted electro-oxidation of methanol. J. Phys. Chem. C 2013, 117, 11610–11618. [Google Scholar] [CrossRef]
- Hewlett, R.M.; McLachlan, M.A. Surface structure modification of ZnO and the impact on electronic properties. Adv. Mater. 2016, 28, 3893–3921. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, S.; Lim, H.; Talib, Z.; Pandikumar, A.; Huang, N. Aerosol-assisted chemical vapor deposition of metal oxide thin films for photoelectrochemical water splitting. Int. J. Hydrog. Energy 2015, 40, 2115–2131. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.-S.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Nano-architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Miao, J.; Tao, H.B.; Hung, S.F.; Wang, H.Y.; Yang, H.B.; Chen, J.; Chen, R.; Liu, B. One-dimensional hybrid nanostructures for heterogeneous photocatalysis and photoelectrocatalysis. Small 2015, 11, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Rajaambal, S.; Sivaranjani, K.; Gopinath, C.S. Recent developments in solar H2 generation from water splitting. J. Chem. Sci. 2015, 127, 33–47. [Google Scholar] [CrossRef]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Solid solution of gan and ZnO as a stable photocatalyst for overall water splitting under visible light. Chem. Mater. 2010, 22, 612–623. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Lingampalli, S.R.; Dey, S.; Roy, A. Solar photochemical and thermochemical splitting of water. Philos. Trans. R. Soc. A 2016, 374, 20150088. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Chi, L.; Nair, A.K.; Shangguan, W.; Jiang, Z. Recent advances in visible-light-driven photoelectrochemical water splitting: Catalyst nanostructures and reaction systems. Nano-Micro Lett. 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Mao, S.S.; Shen, S.; Guo, L. Nanomaterials for renewable hydrogen production, storage and utilization. Prog. Nat. Sci. Mater. 2012, 22, 522–534. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tan, C.-S. A review: CO2 utilization. Aerosol. Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Schüth, F. Chemical compounds for energy storage. Chem. Ing. Tech. 2011, 83, 1984–1993. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Centi, G.; Duplan, J.L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef] [PubMed]
- Studt, F.; Behrens, M.; Kunkes, E.L.; Thomas, N.; Zander, S.; Tarasov, A.; Schumann, J.; Frei, E.; Varley, J.B.; Abild-Pedersen, F. The mechanism of CO and CO2 hydrogenation to methanol over Cu-based catalysts. ChemCatChem 2015, 7, 1105–1111. [Google Scholar] [CrossRef]
- Vesborg, P.C.; Chorkendorff, I.; Knudsen, I.; Balmes, O.; Nerlov, J.; Molenbroek, A.M.; Clausen, B.S.; Helveg, S. Transient behavior of Cu/ZnO-based methanol synthesis catalysts. J. Catal. 2009, 262, 65–72. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; John Wiley & Sons: Virginia, VA, USA, 2008. [Google Scholar]
- Wang, H.; Lu, Z.; Lu, D.; Li, C.; Fang, P.; Wang, X. The synthesis of Cu/plate-like ZnO nanostructures and their self-assembly mechanism. Solid State Sci. 2016, 55, 69–76. [Google Scholar] [CrossRef]
- Lei, H.; Nie, R.; Wu, G.; Hou, Z. Hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts with different ZnO morphology. Fuel 2015, 154, 161–166. [Google Scholar] [CrossRef]
- Güder, F.; Frei, E.; Kücükbayrak, U.M.; Menzel, A.; Thomann, R.; Luptak, R.; Hollaender, B.; Krossing, I.; Zacharias, M. Engineered high aspect ratio vertical nanotubes as a model system for the investigation of catalytic methanol synthesis over Cu/ZnO. ACS Appl. Mater. Int. 2014, 6, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Sun, J.; Li, Y.; Datye, A.K.; Wang, Y. Bimetallic catalysts for hydrogen generation. Chem. Soc. Rev. 2012, 41, 7994–8008. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.D.; Zhang, H.; Sun, J.; Wang, Y. Supported metal catalysts for alcohol/sugar alcohol steam reforming. Dalton Trans. 2014, 43, 11782–11802. [Google Scholar] [CrossRef] [PubMed]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Holladay, J.D.; Wang, Y. A review of recent advances in numerical simulations of microscale fuel processor for hydrogen production. J. Power Sources 2015, 282, 602–621. [Google Scholar] [CrossRef]
- Xia, G.; Holladay, J.D.; Dagle, R.A.; Jones, E.O.; Wang, Y. Development of highly active Pd-ZnO/Al2O3 catalysts for microscale fuel processor applications. Chem. Eng. Technol. 2005, 28, 515–519. [Google Scholar] [CrossRef]
- Danwittayakul, S.; Dutta, J. Two step copper impregnated zinc oxide microball synthesis for the reduction of activation energy of methanol steam reformation. Chem. Eng. J. 2013, 223, 304–308. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ishibe, H. High temperature steam reforming of methanol over Cu/ZnO/ZrO2 catalysts. Appl. Catal. B 2009, 91, 524–532. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ishibe, H. Suppression of CO by-production in steam reforming of methanol by addition of zinc oxide to silica-supported copper catalyst. J. Catal. 2009, 268, 282–289. [Google Scholar] [CrossRef]
- Liu, S.; Takahashi, K.; Fuchigami, K.; Uematsu, K. Hydrogen production by oxidative methanol reforming on Pd/ZnO: Catalyst deactivation. Appl. Catal. A 2006, 299, 58–65. [Google Scholar] [CrossRef]
- Lin, Y.-G.; Hsu, Y.-K.; Chen, S.-Y.; Lin, Y.-K.; Chen, L.-C.; Chen, K.-H. Nanostructured zinc oxide nanorods with copper nanoparticles as a microreformation catalyst. Angew. Chem. Int. Ed. 2009, 48, 7586–7590. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.M.; Conant, T.; Datye, A.K. Controlling ZnO morphology for improved methanol steam reforming reactivity. Phys. Chem. Chem. Phys. 2008, 10, 5584–5590. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Conant, T.; Datye, A. The role of PdZn alloy formation and particle size on the selectivity for steam reforming of methanol. J. Catal. 2006, 243, 420–427. [Google Scholar] [CrossRef]
- Dagle, R.A.; Chin, Y.-H.; Wang, Y. The effects of PdZn crystallite size on methanol steam reforming. Top. Catal. 2007, 46, 358–362. [Google Scholar] [CrossRef]
- Halevi, B.; Lin, S.; Roy, A.; Zhang, H.; Jeroro, E.; Vohs, J.; Wang, Y.; Guo, H.; Datye, A.K. High CO2 selectivity of ZnO powder catalysts for methanol steam reforming. J. Phys. Chem. C 2013, 117, 6493–6503. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Concepción, P.H.; Silva, H.; Mendes, A. Ultraselective low temperature steam reforming of methanol over PdZn/ZnO catalysts—Influence of induced support defects on catalytic performance. Appl. Catal. B 2014, 154–155, 316–328. [Google Scholar] [CrossRef]
- Maceiras, R.; Vega, M.; Costa, C.; Ramos, P.; Márquez, M. Effect of methanol content on enzymatic production of biodiesel from waste frying oil. Fuel 2009, 88, 2130–2134. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K. Prospects of 2nd generation biodiesel as a sustainable fuel—Part 1: Selection of feedstocks, oil extraction techniques and conversion technologies. Renew. Sustain. Energy Rev. 2016, 55, 1109–1128. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K.; Hazrat, M.A. Prospects of 2nd generation biodiesel as a sustainable fuel—Part 2: Properties, performance and emission characteristics. Renew. Sustain. Energy Rev. 2016, 55, 1129–1146. [Google Scholar] [CrossRef]
- Takisawa, K.; Kanemoto, K.; Kartikawati, M.; Kitamura, Y. Simultaneous hydrolysis-esterification of wet microalgal lipid using acid. Bioresour. Technol. 2013, 149, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, S.; Kitamura, Y. Evaluation of hydrolysis–esterification biodiesel production from wet microalgae. Bioresour. Technol. 2016, 214, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y. Controllable growth of “multi-level tower” ZnO for biodiesel production. Ceram. Int. 2011, 37, 3193–3202. [Google Scholar] [CrossRef]
- Di Cosimo, J.; Dıez, V.; Xu, M.; Iglesia, E.; Apesteguıa, C. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Van Santen, R.A.; Neurock, M. Molecular Heterogeneous Catalysis: A Conceptual and Computational Approach; John Wiley & Sons: Eindhoved, The Netherlands, 2009. [Google Scholar]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics; John Wiley & Sons: Lyngby, Denmark, 2006. [Google Scholar]
- Yan, S.; Salley, S.O.; Ng, K.S. Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La2O3 catalysts. Appl. Catal. A 2009, 353, 203–212. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Liu, C.; Wang, Y. Distinct water activation on polar/non-polar facets of ZnO nanoparticles. J. Catal. 2015, 331, 57–62. [Google Scholar] [CrossRef]
- Morales, M.V.; Asedegbega-Nieto, E.; Iglesias-Juez, A.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Role of exposed surfaces on zinc oxide nanostructures in the catalytic ethanol transformation. ChemSusChem 2015, 8, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lawrence, N.J.; Wu, T.S.; Liu, J.; Kent, P.; Soo, Y.L.; Cheung, C.L. Pd/CeO2-x nanorod catalysts for CO oxidation: Insights into the origin of their regenerative ability at room temperature. ChemCatChem 2014, 6, 2937–2946. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Li, T.; Zhao, Z.; Gong, X.-Q.; Chen, Y.; Duan, A.; Jiang, G.; Wei, Y. Interfacial effects of CeO2-supported Pd nanorod in catalytic CO oxidation: A theoretical study. J. Phys. Chem. C 2015, 119, 12923–12934. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Q.; Yang, Y.; Li, C.; Huang, T.; Sun, G.; Zhang, S.; Ma, D.; Li, X. Facile and mild strategy to construct mesoporous CeO2-CuO nanorods with enhanced catalytic activity toward co oxidation. ACS Appl. Mater. Interfaces 2015, 7, 23538–23544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, H.; Li, J.; Zhao, H.; Zhu, B.; Huang, W.; Zhang, S. Au/BiPO4 nanorod catalysts: Synthesis, characterization and their catalytic performance for CO oxidation. RSC Adv. 2016, 6, 15304–15312. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Z.; Henkelman, G.; Song, W. Computational design of a CeO2-supported Pd-based bimetallic nanorod for CO oxidation. J. Phys. Chem. C 2016, 120, 5557–5564. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Song, W.; Lin, H.-J.; Wang, S.; Biswas, S.; Mollahosseini, M.; Kuo, C.-H.; Gao, P.-X.; Suib, S.L. Manganese oxide nano-array based monolithic catalysts: Tunable morphology and high efficiency for CO oxidation. ACS Appl. Mater. Interfaces 2016, 8, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, B.; Song, Y.; Huang, Y.; Liu, J.J. Hetero-epitaxially anchoring Au nanoparticles onto ZnO nanowires for CO oxidation. Chem. Commun. 2015, 51, 15332–15335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, B.; Song, Y.; Tang, H.; Huang, Y.; Liu, J. Highly active and sintering-resistant heteroepitaxy of Au nanoparticles on ZnO nanowires for CO oxidation. J. Energy Chem. 2016, 25, 361–370. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.-H.; Luo, Y.-C.; Mou, C.-Y.; Lin, S.D.; Cheng, H.; Chen, J.-M.; Lee, J.-F.; Lin, T.-S. Strong metal-support interactions between gold nanoparticles and ZnO nanorods in CO oxidation. J. Am. Chem. Soc. 2012, 134, 10251–10258. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.; Machado, B.; Bacsa, R.; Serp, P.; Dražić, G.; Faria, J.; Figueiredo, J. Catalytic performance of Au/ZnO nanocatalysts for CO oxidation. J. Catal. 2010, 273, 191–198. [Google Scholar] [CrossRef]
- Liu, M.-H.; Chen, Y.-W.; Liu, X.; Kuo, J.-L.; Chu, M.-W.; Mou, C.-Y. Defect-mediated gold substitution doping in ZnO mesocrystals and catalysis in CO oxidation. ACS Catal. 2015, 6, 115–122. [Google Scholar] [CrossRef]
- Peng, S.-Y.; Xu, Z.-N.; Chen, Q.-S.; Wang, Z.-Q.; Lv, D.-M.; Sun, J.; Chen, Y.; Guo, G.-C. Enhanced stability of Pd/ZnO catalyst for CO oxidative coupling to dimethyl oxalate: Effect of Mg2+ doping. ACS Catal. 2015, 5, 4410–4417. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmed, S.; Ahmed, K.W.; Al-Saleh, M.A. Simultaneous hydrodesulfurization of dibenzothiophene and substituted dibenzothiophenes over phosphorus modified CoMo/Al2O3 catalysts. Fuel. Process. Technol. 2012, 98, 39–44. [Google Scholar] [CrossRef]
- Gao, Q.; Ofosu, T.N.; Ma, S.-G.; Komvokis, V.G.; Williams, C.T.; Segawa, K. Catalyst development for ultra-deep hydrodesulfurization (HDS) of dibenzothiophenes. I: Effects of Ni promotion in molybdenum-based catalysts. Catal. Today 2011, 164, 538–543. [Google Scholar] [CrossRef]
- Fujikawa, T.; Kimura, H.; Kiriyama, K.; Hagiwara, K. Development of ultra-deep HDS catalyst for production of clean diesel fuels. Catal. Today 2006, 111, 188–193. [Google Scholar] [CrossRef]
- Farag, H.; Mochida, I. A comparative kinetic study on ultra-deep hydrodesulfurization of pre-treated gas oil over nanosized MoS2, CoMo-sulfide, and commercial CoMo/Al2O3 catalysts. J. Colloid Interface Sci. 2012, 372, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Han, H.; Yang, M.; Wang, L.; Zhang, Y.; Jiang, Z.; Li, C. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: The effect of ZnO particle size on the adsorption performance. Appl. Catal. B 2012, 119, 13–19. [Google Scholar] [CrossRef]
- Petzold, F.G.; Jasinski, J.; Clark, E.L.; Kim, J.H.; Absher, J.; Toufar, H.; Sunkara, M.K. Nickel supported on zinc oxide nanowires as advanced hydrodesulfurization catalysts. Catal. Today 2012, 198, 219–227. [Google Scholar] [CrossRef]
- Tang, G.; Tian, S.; Zhou, Z.; Wen, Y.; Pang, A.; Zhang, Y.; Zeng, D.; Li, H.; Shan, B.; Xie, C. ZnO micro/nanocrystals with tunable exposed (0001) facets for enhanced catalytic activity on the thermal decomposition of ammonium perchlorate. J. Phys. Chem. C 2014, 118, 11833–11841. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water. Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, L.; Yu, H.; Huang, Q.; Zhang, Y.C. Synthesis of Sn-doped ZnO nanorods and their photocatalytic properties. Mater. Res. Bull. 2011, 46, 1107–1112. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M.; Kim, H.-Y.; Lee, S.-Y.; Park, S.-J. Electrospun ZnO hybrid nanofibers for photodegradation of wastewater containing organic dyes: A review. J. Ind. Eng. Chem. 2015, 21, 26–35. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-M.; Sin, J.-C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalin. Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Souza, R.P.; Freitas, T.K.F.S.; Domingues, F.S.; Pezoti, O.; Ambrosio, E.; Ferrari-Lima, A.M.; Garcia, J.C. Photocatalytic activity of TiO2, ZnO and Nb2O5 applied to degradation of textile wastewater. J. Photochem. Photobiol. A 2016, 329, 9–17. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.; Patil, A. Effect of morphology and crystallite size on solar photocatalytic activity of zinc oxide synthesized by solution free mechanochemical method. J. Mol. Catal. A Chem. 2009, 308, 32–40. [Google Scholar] [CrossRef]
- Ali, A.M.; Emanuelsson, E.A.; Patterson, D.A. Photocatalysis with nanostructured zinc oxide thin films: The relationship between morphology and photocatalytic activity under oxygen limited and oxygen rich conditions and evidence for a mars van krevelen mechanism. Appl. Catal. B 2010, 97, 168–181. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-G.; Hsu, Y.-K.; Chen, Y.-C.; Chen, L.-C.; Chen, S.-Y.; Chen, K.-H. Visible-light-driven photocatalytic carbon-doped porous ZnO nanoarchitectures for solar water-splitting. Nanoscale 2012, 4, 6515–6519. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baruah, A.; Tonda, S.; Kumar, B.; Shanker, V.; Sreedhar, B. Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core-shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale 2014, 6, 4830–4842. [Google Scholar] [CrossRef] [PubMed]
- Kochuveedu, S.T.; Jang, Y.H.; Jang, Y.J.; Kim, D.H. Visible light active photocatalysis on block copolymer induced strings of ZnO nanoparticles doped with carbon. J. Mater. Chem. A 2013, 1, 898–905. [Google Scholar] [CrossRef]
- Kadam, S.R.; Mate, V.R.; Panmand, R.P.; Nikam, L.K.; Kulkarni, M.V.; Sonawane, R.S.; Kale, B.B. A green process for efficient lignin (biomass) degradation and hydrogen production via water splitting using nanostructured C, N, S-doped ZnO under solar light. RSC Adv. 2014, 4, 60626–60635. [Google Scholar] [CrossRef]
- Geng, B.Y.; Wang, G.Z.; Jiang, Z.; Xie, T.; Sun, S.H.; Meng, G.W.; Zhang, L.D. Synthesis and optical properties of S-doped zno nanowires. Appl. Phys. Lett. 2003, 82, 4791–4793. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Lee, J.S.; Lee, K.-H. Carbon-doped ZnO nanostructures synthesized using vitamin C for visible light photocatalysis. CrystEngComm 2010, 12, 3929–3935. [Google Scholar] [CrossRef]
- Bhirud, A.P.; Sathaye, S.D.; Waichal, R.P.; Nikam, L.K.; Kale, B.B. An eco-friendly, highly stable and efficient nanostructured p-type N-doped ZnO photocatalyst for environmentally benign solar hydrogen production. Green Chem. 2012, 14, 2790–2798. [Google Scholar] [CrossRef]
- Bae, S.Y.; Seo, H.W.; Park, J. Vertically aligned sulfur-doped ZnO nanowires synthesized via chemical vapor deposition. J. Phys. Chem. B 2004, 108, 5206–5210. [Google Scholar] [CrossRef]
- Jang, Y.J.; Simer, C.; Ohm, T. Comparison of zinc oxide nanoparticles and its nano-crystalline particles on the photocatalytic degradation of methylene blue. Mater. Res. Bull. 2006, 41, 67–77. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, L.; Wang, C.; Lun, N.; Qi, Y. Sol-gel growth of hexagonal faceted ZnO prism quantum dots with polar surfaces for enhanced photocatalytic activity. ACS Appl. Mater. Int. 2010, 2, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liu, X.; Han, Q.; Wang, X. Controlled morphologies and optical properties of ZnO films and their photocatalytic activities. J. Alloys Compd. 2011, 509, 9255–9263. [Google Scholar] [CrossRef]
- Boppella, R.; Anjaneyulu, K.; Basak, P.; Manorama, S.V. Facile synthesis of face oriented ZnO crystals: Tunable polar facets and shape induced enhanced photocatalytic performance. J. Phys. Chem. C 2013, 117, 4597–4605. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile solid state synthesis of zno hexagonal nanogranules with excellent photocatalytic activity. Appl. Surface Sci. 2014, 292, 520–530. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, W.; Ma, X.; Pan, C.; Zong, R.; Zhu, Y. The surface oxygen vacancy induced visible activity and enhanced UV activity of a ZnO1−x photocatalyst. Catal. Sci. Technol. 2013, 3, 3136–3146. [Google Scholar] [CrossRef]
- Shidpour, R.; Simchi, A.; Ghanbari, F.; Vossoughi, M. Photo-degradation of organic dye by zinc oxide nanosystems with special defect structure: Effect of the morphology and annealing temperature. Appl. Catal. A 2014, 472, 198–204. [Google Scholar] [CrossRef]
- Liu, T.-J.; Wang, Q.; Jiang, P. Morphology-dependent photo-catalysis of bare zinc oxide nanocrystals. RSC Adv. 2013, 3, 12662–12670. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Rajendra Kumar, R.T. Surfactant free, simple, morphological and defect engineered ZnO nanocatalyst: Effective study on sunlight driven and reusable photocatalytic properties. J. Photochem. Photobiol. B 2016, 329, 35–45. [Google Scholar] [CrossRef]
- Jeong, H.W.; Choi, S.-Y.; Hong, S.H.; Lim, S.K.; Han, D.S.; Abdel-Wahab, A.; Park, H. Shape-dependent charge transfers in crystalline ZnO photocatalysts: Rods versus plates. J. Phys. Chem. C 2014, 118, 21331–21338. [Google Scholar] [CrossRef]
- Khokhra, R.; Singh, R.K.; Kumar, R. Effect of synthesis medium on aggregation tendencies of ZnO nanosheets and their superior photocatalytic performance. J. Mater. Sci. 2015, 50, 819–832. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Wan, Q.; Dai, G.; Zhou, C.; Zou, B. Controllable ZnO architectures by ethanol amine-assisted hydrothermal reaction for enhanced photocatalytic activity. J. Phys. Chem. C 2011, 115, 2769–2775. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2014, 16, 12741–12749. [Google Scholar] [CrossRef] [PubMed]
- Rajbongshi, B.M.; Samdarshi, S.K. ZnO and CO-ZnO nanorods—Complementary role of oxygen vacancy in photocatalytic activity of under UV and visible radiation flux. Mater. Sci. Eng. B 2014, 182, 21–28. [Google Scholar] [CrossRef]
- Pawar, R.C.; Choi, D.-H.; Lee, J.-S.; Lee, C.S. Formation of polar surfaces in microstructured ZnO by doping with Cu and applications in photocatalysis using visible light. Mater. Chem. Phys. 2015, 151, 167–180. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Chu, J.; Chen, Y. Electrospun pd-doped ZnO nanofibers for enhanced photocatalytic degradation of methylene blue. J. Sol-Gel Sci. Technol. 2013, 66, 139–144. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Ibrahim, A.A.; Bouzid, H.; Al-Sayari, S.A. Highly efficient photocatalyst based on Ce doped ZnO nanorods: Controllable synthesis and enhanced photocatalytic activity. Chem. Eng. J. 2013, 229, 225–233. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, D.; Song, Z.; Shan, C.; Zhang, Z.; Li, B.; Shen, D. Gold nanoparticles modified ZnO nanorods with improved photocatalytic activity. J. Colloid. Interface Sci. 2011, 363, 175–181. [Google Scholar] [CrossRef]
- Egelhaaf, H.-J.; Oelkrug, D. Luminescence and nonradiative deactivation of excited states involving oxygen defect centers in polycrystalline ZnO. J. Cryst. Growth 1996, 161, 190–194. [Google Scholar] [CrossRef]
- Goto, H.; Hanada, Y.; Ohno, T.; Matsumura, M. Quantitative analysis of superoxide Ion and hydrogen peroxide produced from molecular oxygen on photoirradiated TiO2 particles. J. Catal. 2004, 225, 223–229. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Green emission to probe photoinduced charging events in ZnO-Au nanoparticles. Charge distribution and fermi-level equilibration. J. Phys. Chem. B 2003, 107, 7479–7485. [Google Scholar] [CrossRef]
- Kayaci, F.; Vempati, S.; Ozgit-Akgun, C.; Biyikli, N.; Uyar, T. Enhanced photocatalytic activity of homoassembled ZnO nanostructures on electrospun polymeric nanofibers: A combination of atomic layer deposition and hydrothermal growth. Appl. Catal. B 2014, 156, 173–183. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Yang, G.; Chen, L.; Guo, P.; Zhang, L. A facile synthesis of ZnO/CNT hierarchical microsphere composites with enhanced photocatalytic degradation of methylene blue. RSC Adv. 2015, 5, 72476–72481. [Google Scholar] [CrossRef]
- Chang, Y.-C. Complex ZnO/ZnS nanocable and nanotube arrays with high performance photocatalytic activity. J. Alloys Compd. 2016, 664, 538–546. [Google Scholar] [CrossRef]
- Avci, A.; Eskizeybek, V.; Gülce, H.; Haspulat, B.; Şahin, Ö.S. ZnO-TiO2 nanocomposites formed under submerged dc arc discharge: Preparation, characterization and photocatalytic properties. Appl. Phys. A 2014, 116, 1119–1125. [Google Scholar] [CrossRef]
- Wang, M.H.; Cui, L.Y.; Li, S.Y.; Li, Z.X.; Ma, T.L.; Luan, G.Y.; Liu, W.; Zhang, F.L. Facile fabrication hybrids of TiO2@ZnO tubes with enhanced photocatalytic properties. RSC Adv. 2016, 6, 58452–58457. [Google Scholar] [CrossRef]
- Liu, X.; Huang, W.; Cheng, H.; Huang, B.; Bai, D.; Fu, F.; Wu, H.; Li, L. In-situ observation of hydrothermal growth of ZnO nanowires on patterned Zn substrate and their photocatalytic performance. Appl. Surface Sci. 2015, 356, 240–248. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Peng, D.; Chen, T.; Dong, S.; Chang, Y. Synthesis and properties of Au/ZnO nanorods as a plasmonic photocatalyst. Phys. E 2016, 78, 41–48. [Google Scholar] [CrossRef]
- Li, C.; Ahmed, T.; Ma, M.; Edvinsson, T.; Zhu, J. A facile approach to ZnO/CdS nanoarrays and their photocatalytic and photoelectrochemical properties. Appl. Catal. B 2013, 138, 175–183. [Google Scholar] [CrossRef]
- Li, G.; Yi, Z.; Wang, H.; Jia, C.; Zhang, W. Factors impacted on anisotropic photocatalytic oxidization activity of ZnO: Surface band bending, surface free energy and surface conductance. Appl. Catal. B 2014, 158–159, 280–285. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Li, X.; Lang, J.; Liu, F.; Yang, L.; Zhai, H.; Gao, M.; Zhao, X. Effect of polar and non-polar surfaces of ZnO nanostructures on photocatalytic properties. J. Alloys Compd. 2012, 528, 28–33. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Yang, J.; Lang, J.; Lü, S.; Wei, M.; Meng, X.; Kou, C.; Li, X. Comparison of photocatalytic activity of ZnO rod arrays with various diameter sizes and orientation. J. Alloys Compd. 2013, 580, 205–210. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Yang, J.; Lang, J.; Cao, J.; Liu, F.; Fan, H.; Gao, M.; Jiang, Y. Size-controlled fabrication of ZnO micro/nanorod arrays and their photocatalytic performance. Mater. Chem. Phys. 2013, 141, 929–935. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Enhanced photocatalytic performance of WO3 loaded Ag–ZnO for acid black 1 degradation by UV-a light. J. Mol. Catal. A Chem. 2013, 366, 54–63. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H. Enhancement of photocatalytic activity of sprayed nitrogen-containing ZnO powders by coupling with metal oxides during the acetaldehyde decomposition. Chemosphere 2004, 54, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
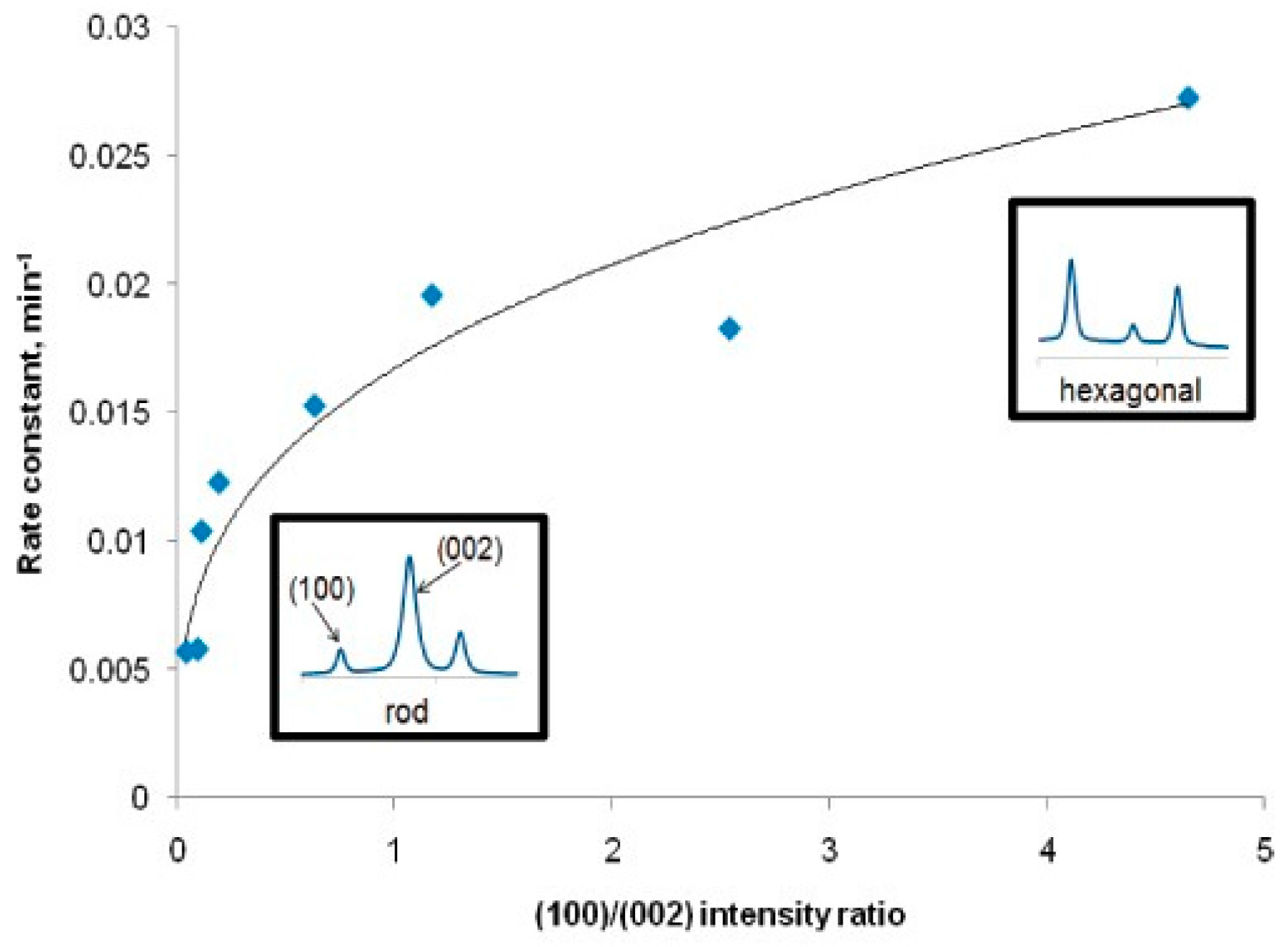
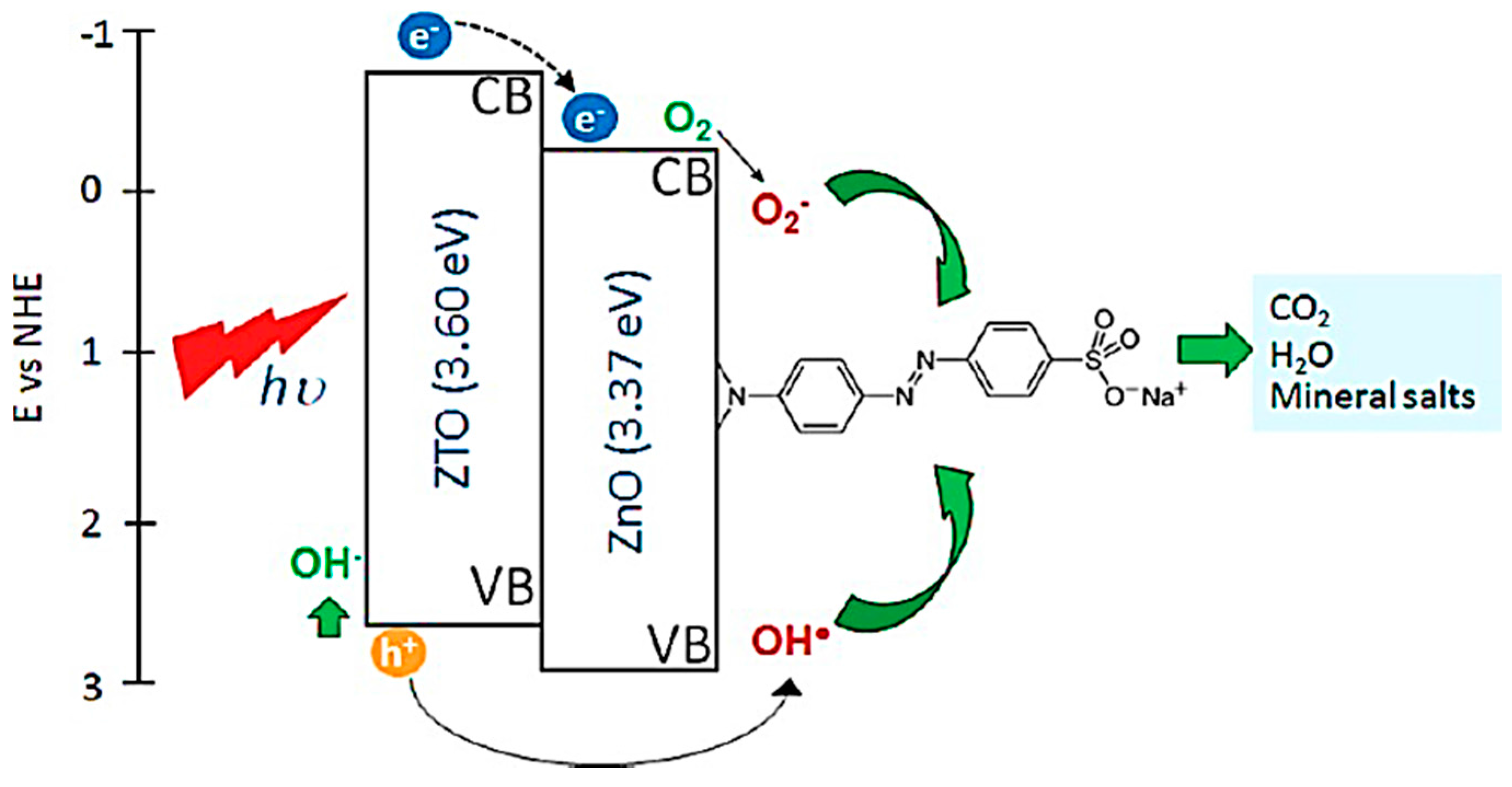
- Danwittayakul, S.; Jaisai, M.; Koottatep, T.; Dutta, J. Enhancement of photocatalytic degradation of methyl orange by supported zinc oxide nanorods/zinc stannate (ZnO/ZTO) on porous substrates. Ind. Eng. Chem. Res. 2013, 52, 13629–13636. [Google Scholar] [CrossRef]
- Gong, W.; Pan, G.; Shang, F.; Wang, F.; Zhou, Z.; Liu, C.; Zhao, M.; Zi, Z.; Wei, Y.; Lv, J.; et al. Effect of ethylene glycol monomethyl ether ratio in mixed solvent on surface morphology, wet ability and photocatalytic properties of ZnO thin films. J. Mater. Sci.-Mater. El. 2014, 25, 2948–2956. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Ning, L.; Zhang, C.; Zhao, H.; Liu, B.; Yang, H. Superior photocatalytic activity of porous wurtzite ZnO nanosheets with exposed {001} facets and a charge separation model between polar (001) and (00) surfaces. Chem. Eng. J. 2015, 264, 557–564. [Google Scholar] [CrossRef]
- Hong, Y.; Tian, C.; Jiang, B.; Wu, A.; Zhang, Q.; Tian, G.; Fu, H. Facile synthesis of sheet-like ZnO assembly composed of small ZnO particles for highly efficient photocatalysis. J. Mater. Chem. 2013, 1, 5700–5708. [Google Scholar] [CrossRef]
- Xiao, S.; Li, H.; Liu, L.; Lian, J. Glucose-assisted generation of assembled mesoporous ZnO sheets with highly efficient photocatalytic performance. Mater. Sci. Semicond. Process. 2015, 39, 680–685. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lo, S.-L. Effects of operational conditions of microwave-assisted synthesis on morphology and photocatalytic capability of zinc oxide. Chem. Eng. J. 2011, 170, 411–418. [Google Scholar] [CrossRef]
- Gomez-Solís, C.; Ballesteros, J.; Torres-Martínez, L.; Juárez-Ramírez, I.; Torres, L.D.; Zarazua-Morin, M.E.; Lee, S.W. Rapid synthesis of ZnO nano-corncobs from nital solution and its application in the photodegradation of methyl orange. J. Photochem. Photobiol. B 2015, 298, 49–54. [Google Scholar] [CrossRef]
- Srikhaow, A.; Smith, S.M. Photocatalytic activity of flower-like ZnO derived by a d-glucose-assisted sonochemical method. Res. Chem. Intermed. 2013, 39, 1545–1553. [Google Scholar] [CrossRef]
- Misra, M.; Kapur, P.; Singla, M.L. Surface plasmon quenched of near band edge emission and enhanced visible photocatalytic activity of Au@ZnO core-shell nanostructure. Appl. Catal. B 2014, 150, 605–611. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, L.; Lian, J. Enhanced photocatalytic performance of supported Fe doped ZnO nanorod arrays prepared by wet chemical method. Catal. Lett. 2014, 144, 347–354. [Google Scholar] [CrossRef]
- Huang, J.; Liu, S.; Kuang, L.; Zhao, Y.; Jiang, T.; Liu, S.; Xu, X. Enhanced photocatalytic activity of quantum-dot-sensitized one-dimensionally-ordered ZnO nanorod photocatalyst. J. Environ. Sci. 2013, 25, 2487–2491. [Google Scholar] [CrossRef]
- Lu, F.; Cai, W.; Zhang, Y. Zno hierarchical micro/nanoarchitectures: Solvothermal synthesis and structurally enhanced photocatalytic performance. Adv. Funct. Mater. 2008, 18, 1047–1056. [Google Scholar] [CrossRef]
- Lu, H.; Wang, S.; Zhao, L.; Li, J.; Dong, B.; Xu, Z. Hierarchical ZnO microarchitectures assembled by ultrathin nanosheets: Hydrothermal synthesis and enhanced photocatalytic activity. J. Mater. Chem. 2011, 21, 4228–4234. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.C.; Men, L.; Wang, X.; He, D.N.; Chai, Y.C.; Zhao, B.; Ghoshroy, S.; Tang, Q.W. A highly efficient TiO2@ZnO n-p-n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zhao, F.; Yue, X.; Wang, D.; Lin, Y. Enhanced solar light-driven photocatalytic activity of BiOBr–ZnO heterojunctions with effective separation and transfer properties of photo-generated chargers. New J. Chem. 2015, 39, 6659–6666. [Google Scholar] [CrossRef]
- Huang, M.; Yan, Y.; Feng, W.; Weng, S.; Zheng, Z.; Fu, X.; Liu, P. Controllable tuning various ratios of ZnO polar facets by crystal seed-assisted growth and their photocatalytic activity. Cryst. Growth Des. 2014, 14, 2179–2186. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.; Xiang, L.; Komarneni, S. Defect-rich ZnO nanosheets of high surface area as an efficient visible-light photocatalyst. Appl. Catal. B 2016, 192, 8–16. [Google Scholar] [CrossRef]
- Fan, J.; Li, T.; Heng, H. Hydrothermal growth of ZnO nanoflowers and their photocatalyst application. Bull. Mater. Sci. 2016, 39, 19–26. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Yang, J.; Lang, J.; Wei, M.; Meng, X.; Lü, S.; Sui, Y. Enhanced photocatalytic activity of ZnO microflower arrays synthesized by one-step etching approach. J. Mol. Catal. A Chem. 2013, 378, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Wang, D.; Xie, T.; Chen, L.; Lin, Y. A comparative study on plate-like and flower-like ZnO nanocrystals surface photovoltage property and photocatalytic activity. Mater. Chem. Phys. 2011, 129, 281–287. [Google Scholar] [CrossRef]
- Li, B.X.; Wang, Y.F. Facile Synthesis and Enhanced Photocatalytic Performance of Flower-like ZnO Hierarchical Microstructures. J. Phys. Chem. C 2010, 114, 890–896. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, S.; Li, X.; Liu, Y. Amino acids assisted hydrothermal synthesis of hierarchically structured ZnO with enhanced photocatalytic activities. Appl. Surface Sci. 2016, 384, 83–91. [Google Scholar] [CrossRef]
- Wang, S.W.; Yu, Y.; Zuo, Y.H.; Li, C.Z.; Yang, J.H.; Lu, C.H. Synthesis and photocatalysis of hierarchical heteroassemblies of ZnO branched nanorod arrays on Ag core nanowires. Nanoscale 2012, 4, 5895–5901. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Guo, N.; Li, L.; Li, R.; Ji, G.; Gan, S. Fabrication of porous 3D flower-like Ag/ZnO heterostructure composites with enhanced photocatalytic performance. Appl. Surface Sci. 2015, 332, 32–39. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Xue, Y.; Li, B.; Geng, B. ZnO nanorods/ZnSe heteronanostructure arrays with a tunable microstructure of ZnSe shell for visible light photocatalysis. J. Mater. Chem. 2014, 2, 17502–17510. [Google Scholar] [CrossRef]
- Chennakesavulu, K.; Reddy, M.M.; Reddy, G.R.; Rabel, A.; Brijitta, J.; Vinita, V.; Sasipraba, T.; Sreeramulu, J. Synthesis, characterization and photo catalytic studies of the composites by tantalum oxide and zinc oxide nanorods. J. Mol. Struct. 2015, 1091, 49–56. [Google Scholar] [CrossRef]
- Li, X.; Hu, Z.; Liu, J.; Li, D.; Zhang, X.; Chen, J.; Fang, J. Ga doped ZnO photonic crystals with enhanced photocatalytic activity and its reaction mechanism. Appl. Catal. B 2016, 195, 29–38. [Google Scholar] [CrossRef]
- Yin, Q.; Qiao, R.; Li, Z.; Zhang, X.L.; Zhu, L. Hierarchical nanostructures of nickel-doped zinc oxide: Morphology controlled synthesis and enhanced visible-light photocatalytic activity. J. Alloys Compd. 2015, 618, 318–325. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, W.; Qiao, R.; Ke, X.; Hu, Y.; Li, Z. Glucose-assisted transformation of Ni-doped-ZnO@carbon to a Ni-doped-ZnO@void@SiO2 core–shell nanocomposite photocatalyst. RSC Adv. 2016, 6, 38653–38661. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, N.; Li, L.; Li, R.; Ji, G.; Gan, S. Preparation of porous 3D Ce-doped ZnO microflowers with enhanced photocatalytic performance. RSC Adv. 2015, 5, 59887–59894. [Google Scholar] [CrossRef]
- Chandrasekhar, M.; Nagabhushana, H.; Vidya, Y.; Anantharaju, K.; Sharma, S.; Premkumar, H.; Prashantha, S.; Prasad, B.D.; Shivakumara, C.; Saraf, R. Synthesis of Eu3+-activated ZnO superstructures: Photoluminescence, judd-ofelt analysis and sunlight photocatalytic properties. J. Mol. Catal. A Chem. 2015, 409, 26–41. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, J.; Yu, S.; Alcocer, E.J.; Shahid, M.; Wang, Z.; Pan, W. Synergistic effect of N-decorated and Mn2+ doped ZnO nanofibers with enhanced photocatalytic activity. Sci. Rep. 2016, 6, 32711. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, S.; Jang, J.-W.; Jung, S.-H.; Oh, E.; Lee, B.R.; Lee, K.-H. Large-scale fabrication of sub-20-nm-diameter ZnO nanorod arrays at room temperature and their photocatalytic activity. J. Phys. Chem. C 2009, 113, 10452–10458. [Google Scholar] [CrossRef]
- Hafez, H.S. Highly active ZnO rod-like nanomaterials: Synthesis, characterization and photocatalytic activity for dye removal. Phys. E 2012, 44, 1522–1527. [Google Scholar] [CrossRef]
- Leelavathi, A.; Madras, G.; Ravishankar, N. Origin of enhanced photocatalytic activity and photoconduction in high aspect ratio ZnO nanorods. Phys. Chem. Chem. Phys. 2013, 15, 10795–10802. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Subramanian, B.; Djaoued, Y.; Robichaud, J.; Sharma, T.; Bruning, R. Facile synthesis of mesoporous nanocrystalline ZnO bipyramids and spheres: Characterization, and photocatalytic activity. Mater. Chem. Phys. 2015, 155, 162–170. [Google Scholar] [CrossRef]
- Lan, S.; Liu, L.; Li, R.; Leng, Z.; Gan, S. Hierarchical hollow structure ZnO: Synthesis, characterization, and highly efficient adsorption/photocatalysis toward congo red. Ind. Eng. Chem. Res. 2014, 53, 3131–3139. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Mahjoub, A.R.; Abazari, R. Green synthesis of ZnO hollow sphere nanostructures by a facile route at room temperature with efficient photocatalytic dye degradation properties. RSC Adv. 2015, 5, 107378–107388. [Google Scholar] [CrossRef]
- Yu, C.; Yang, K.; Xie, Y.; Fan, Q.; Jimmy, C.Y.; Shu, Q.; Wang, C. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 2013, 5, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Lamba, R.; Umar, A.; Mehta, S.K.; Anderson, W.A.; Kansal, S.K. Visible-light-driven photocatalytic properties of self assembled cauliflower-like AgCl/ZnO hierarchical nanostructures. J. Mol. Catal. A Chem. 2015, 408, 189–201. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. CeO2-ZnO hexagonal nanodisks: Efficient material for the degradation of direct blue 15 dye and its simulated dye bath effluent under solar light. J. Alloys. Compd. 2015, 620, 67–73. [Google Scholar] [CrossRef]
- Mohan, R.; Krishnamoorthy, K.; Kim, S.-J. Enhanced photocatalytic activity of Cu-doped ZnO nanorods. Solid State Commun. 2012, 152, 375–380. [Google Scholar] [CrossRef]
- Wan, X.; Liang, X.; Zhang, C.; Li, X.; Liang, W.; Xu, H.; Lan, S.; Tie, S. Morphology controlled syntheses of Cu-doped ZnO, tubular Zn(Cu)O and Ag decorated tubular Zn(Cu)O microcrystals for photocatalysis. Chem. Eng. J. 2015, 272, 58–68. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, Z.-Y.; Du, G.-H.; Halasa, M.; Su, B.-L. High-yield synthesis of single-crystalline ZnO hexagonal nanoplates and accounts of their optical and photocatalytic properties. Appl. Phys. A 2007, 86, 181–185. [Google Scholar] [CrossRef]
- Liu, D.; Lv, Y.; Zhang, M.; Liu, Y.; Zhu, Y.; Zong, R.; Zhu, Y. Defect-related photoluminescence and photocatalytic properties of porous ZnO nanosheets. J. Mater. Chem. 2014, 2, 15377–15388. [Google Scholar] [CrossRef]
- Clament Sagaya Selvam, N.; Vijaya, J.J.; Kennedy, L.J. Effects of morphology and Zr doping on structural, optical, and photocatalytic properties of ZnO nanostructures. Ind. Eng. Chem. Res. 2012, 51, 16333–16345. [Google Scholar] [CrossRef]
- Rezapour, M.; Talebian, N. Comparison of structural, optical properties and photocatalytic activity of ZnO with different morphologies: Effect of synthesis methods and reaction media. Mater. Chem. Phys. 2011, 129, 249–255. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Satoshi, I.; Abdullah, A.Z.; Mohamed, A.R. Enhanced sunlight photocatalytic performance over Nb2O5/ZnO nanorod composites and the mechanism study. Appl. Catal. A 2014, 471, 126–135. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. Fabrication of ZnO nanorods via a green hydrothermal method and their light driven catalytic activity towards the erasure of phenol compounds. Mater. Lett. 2016, 167, 141–144. [Google Scholar] [CrossRef]
- Wu, S.-Z.; Li, N.; Zhang, W.-D. Attachment of ZnO nanoparticles onto layered double hydroxides microspheres for high performance photocatalysis. J. Porous Mater. 2014, 21, 157–164. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Lee, K.-T.; Mohamed, A.R. Preparation and photocatalytic properties of visible light-driven samarium-doped ZnO nanorods. Ceram. Int. 2013, 39, 5833–5843. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Lee, K.-T.; Mohamed, A.R. Preparation of rare earth-doped ZnO hierarchical micro/nanospheres and their enhanced photocatalytic activity under visible light irradiation. Ceram. Int. 2014, 40, 5431–5440. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Satoshi, I.; Lee, K.-T.; Mohamed, A.R. Sunlight photocatalytic activity enhancement and mechanism of novel europium-doped ZnO hierarchical micro/nanospheres for degradation of phenol. Appl. Catal. B 2014, 148, 258–268. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Lee, K.-T.; Mohamed, A.R. Preparation of cerium-doped ZnO hierarchical micro/nanospheres with enhanced photocatalytic performance for phenol degradation under visible light. J. Mol. Catal. A Chem. 2015, 409, 1–10. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Lee, K.-T.; Mohamed, A.R. Photocatalytic performance of novel samarium-doped spherical-like ZnO hierarchical nanostructures under visible light irradiation for 2,4-dichlorophenol degradation. J. Colloid Interface Sci. 2013, 401, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Visible light photocatalytic activity of Fe3+-doped ZnO nanoparticle prepared via Sol-Gel technique. Chemosphere 2013, 91, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-R.; Yin, X.; Zhang, Y.; Xu, Y.-J. One-pot, high-yield synthesis of one-dimensional ZnO nanorods with well-defined morphology as a highly selective photocatalyst. RSC Adv. 2013, 3, 5956–5965. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Xu, Y.-J. Morphology control, defect engineering and photoactivity tuning of ZnO crystals by graphene oxide–a unique 2D macromolecular surfactant. Phys. Chem. Chem. Phys. 2014, 16, 5589–5599. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, J.; Peng, T. New insight into the enhanced photocatalytic activity of N-, C- and S-doped ZnO photocatalysts. Appl. Catal. B 2016, 181, 220–227. [Google Scholar] [CrossRef]
- Liao, F.; Zeng, Z.; Eley, C.; Lu, Q.; Hong, X.; Tsang, S.C. Electronic modulation of a copper/zinc oxide catalyst by a heterojunction for selective hydrogenation of carbon dioxide to methanol. Angew. Chem. Int. Ed. 2012, 51, 5832–5836. [Google Scholar] [CrossRef] [PubMed]





| Type of Catalytic Reactions | Catalyst | ZnO Morphology | Morphology-Dependent Promotion Mechanisms | Reference |
|---|---|---|---|---|
| Methanol synthesis | Cu-ZnO | Triangular | The formation of Cu-Zn colloids act as reservoir for ZnOx species | [31] |
| Cu/ZnO/Al2O3 | Nanoplates, Nanotubes | The exposure of ZnO (002) polar facets which enhances the SMSI, promotes electron transportation and leads to the formation of oxygen vacancies | [10] | |
| Cu-ZnO | Nanoplates | The exposed polar (002) facets in plate-like ZnO showed a much stronger interaction with Cu and a higher number of surface oxygen vacancies existed at the materials’ interface. Thus CO2 was likely to be activated by the generated vacancies and the Cu phase at the interface assisted molecule rearrangement (formate) and hydrogenation to methanol | [101] | |
| Cu-ZnO | Nanorods filament-like | The stronger Cu-ZnO interaction and more oxygen vacancies caused by the more exposure of (002) polar face | [102] | |
| Cu-ZnO | Nanotubes | High surface area leads to an excellent CO2 conversion; the high surface mobility of Cu results in low methanol selectivity | [103] | |
| Methanol steam reforming | Cu-ZnO | Nanorods | The large surface area enhances Cu dispersion, offers an effective surface contact between reactants and catalysts and enhances SMSI | [113] |
| Pd-ZnO | Nanorods | The morphology controlled ZnO not only acts as the support and Zn source for Pd-Zn alloy, but also facilitates one of the intermediate formation steps in MSR | [114] | |
| Pd-ZnO | Needle-like | Exposure of ZnO non-polar facet will influence Pd-Zn structure and particle size to influence product selectivity | [13] | |
| Pd-ZnO | Nanoplate | Plate-like ZnO possesses the lowest activation energy that leads to the highest specific activity | [117] | |
| Pd-ZnO | Nanoballs assembled by nanosheets | Higher oxygen vacancy concentration results in the high H2 selectivity in MSR reaction | [118] | |
| Combined reforming hydrogenolysis of glycerol | NiMo-ZnO | Nanodisk, Nanorods | CO2 adsorbed on the (100) non-polar plane can make the un-occupied Zn cations more electron-deficient, enhancing the Lewis acidity of the non-polar plane | [11] |
| Bio-diesel production | ZnO | Multi-level tower | Multi-level tower ZnO predominantly exposed active O2− on (0002), (100), (100) and (010) facets shows strong basic sites that results in obvious enhancement of catalytic activity and high yield of bio-diesel | [124] |
| Bio-ethanol conversion | ZnO | Needle-like, Flake-like | H2O was strongly adsorbed on ZnO (001)/(00) polar facets which prohibits the formation of the oxygen vacancy and thus adsorption/dissociation of H2O, leading to high ethanol conversion rate but low acetone selectivity. Acetaldehyde reaction to form acetone via the ketonization pathway mainly occurs on the ZnO (100) non-polar facet on which water dissociation is highly favored | [129] |
| Ethanol conversion | ZnO | Brick, Disk, Needle prism | The presence of acidic hydroxyl groups on polar surface seems to be responsible for the formation of ethylene and condensation products. | [130] |
| CO oxidation | Au-ZnO | Nanowires | Compared with Au/ZnO-P exposing various highly active ZnO facets, although Au/ZnO-NW catalyst predominantly exposing stable low energy ZnO (100) facets, exhibited relatively weaker interactions between Au nanoparticles and ZnO. It is mainly because of the higher stability of ZnO-NW that prohibits the sintering of Au particles and then displays higher stability at high pretreatment temperature | [137,138] |
| Au-ZnO | Nanorods | The formation of Au-O-Zn linkage or Au-Zn alloy was caused by different SMSI due to different pretreatment conditions. | [139] | |
| Au-ZnO | Nanorods | The SMSI leading to a unique structure of a number of gold nanocrystals epitaxial oriented on the ZnO nanorod | [140] | |
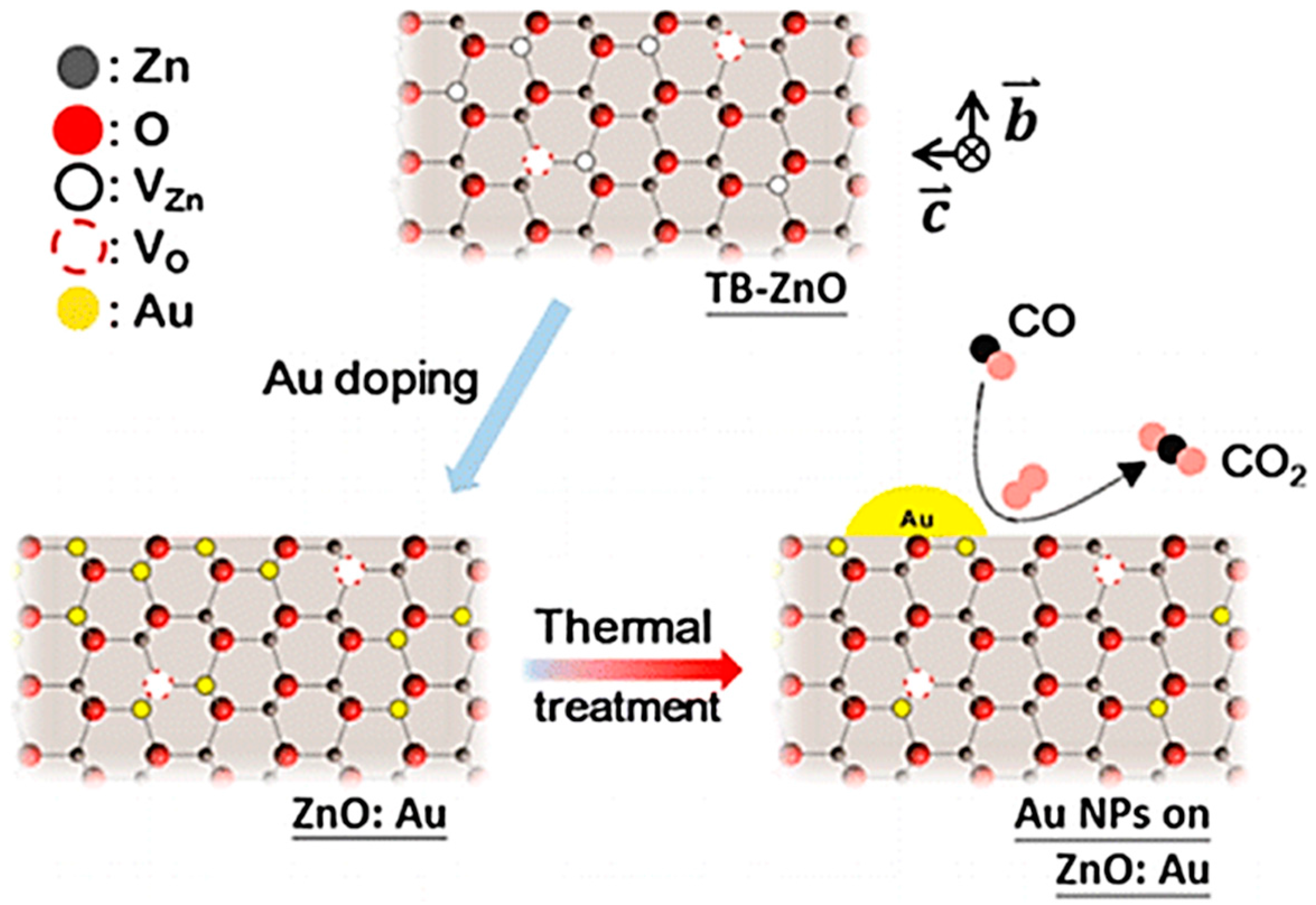
| Au-ZnO | Twin-brush | The SMSI lead to a large amount of Zn- and O-vacancy defects in TB-ZnO which provides a strong driving force for introducing gold particles into the lattice of ZnO which then eventually move out of ZnO lattice to form highly dispersed Au nanoparticles | [141] | |
| Mg-ZnO (doping) | Flower-like ZnO microspheres | Mg2+ doped ZnO support promotes a strong metal-support interaction caused by the formation of Zn-Mg oxide solid solution, effectively restrain the sintering of the active Pd NPs; retard the growth of Pd NPs, and enhance the catalytic stability | [142] | |
| Hydro-desulfurization | Ni-ZnO | Nanowires | ZnO supports can ensure the generation of highly active nickel species during deep desulfurization through the reported regenerative step. The enhanced metal-support interactions and short diffusion paths between Ni and ZnO NWs lead to improved activity and sulfur uptake capacity | [148] |
| Decomposition of ammonium perchlorate | ZnO | Nanorods Nanoplates | Highly exposed (0001) facets will promote perchloric acid adsorption and diffusion and facilitate the formation of active oxygen species, leading to the more complete oxidation reaction of ammonia in the catalytic decomposition of AP | [149] |
| Type of Catalytic Reactions | Catalyst | ZnO Morphology | Morphology-Dependent Promotion Mechanisms | Reference |
|---|---|---|---|---|
| Degradation of Methyl blue | ZnO | Nanorods, hexagonal plate | The hexagonal ZnO QDs have more polar O-terminated (00) and Zn-terminated (001) facets which are facile to the adsorption of oxygen molecules and OH− ions, leading to higher generation rate of H2O2 and ·OH radicals, more active for photo-catalysis than the non-polar surfaces on hexagonal rods. | [170,171] |
| ZnO | Hexagonal prisms, plates and rose-like twinned crystal films | High percentage of polar facets and concentration of oxygen vacancies. | [172] | |
| ZnO | Nanorods, nanoplates | ZnO rods were more active for the decomposition of MB and phenol, the production of ·OH radicals and the generation of photocurrents, all of which were associated with single-electron transfer reactions. ZnO nanoplates were more effective for the production of molecular hydrogen and hydrogen peroxide, both of which are initiated by two-electron transfer reactions. | [179] | |
| ZnO | Flower-like interwoven grown nanosheets, un-aggregated nanosheets | The oxygen vacancies not only act as the active centers or trap centers for photo-induced charges but also narrow the bandgap of ZnO by the broadening of the valance band. | [180] | |
| ZnO | Flower-like, Spindle-like, sword-like an dumbella-like | The flower-like structures possessed more irregular Zn sites than that of the other ZnO structures, so more naked Zn atoms on the defects prolong the binding of S and N atoms in the MB molecules, promoting photodegration reactions. Therefore, a relatively lower crystallinity and more defects were responsible for the enhanced photo-catalytic activity. | [181] | |
| Co-ZnO (doping) | Nanorods | The high visible light activity of Co-ZnO was ascribed to its reduced band gap and associated red-shifted absorption. | [182,183] | |
| Cu-ZnO (doping) | Rod-like, disk sphere-like | The existence of (001) polar surfaces, oxygen vacancies, and increased optical absorbance at visible wavelengths | [184] | |
| Pd-ZnO (doping) | Nanofiber | The PdO/ZnO heterostructures and the increase of the surface area | [185] | |
| ZnO/Au (doping) | Nanorods | The high photo-degradation efficiency was probably due to a broader adsorption band that was caused by discrepancies, such as O vacancies, Zn vacancies, Zn interstitials, O interstitials, donor acceptor pairs, and surface state. | [187] | |
| ZnO-CNT | Microsphere | The high surface area, enhanced light absorption, and suppression of charge carrier recombination originated from the interaction between ZnO and CNT | [192] | |
| ZnO- ZnS | Nanocable nanotube | The increased separation and transfer rate of photo-induced electrone-hole pairs on the surface of ZnO/ZnS nanocable and the high surface-to-volume ratio | [193] | |
| TiO2-ZnO | Nano-spheres, nanorods | The suppression of electron-hole pair recombination and the synergetic effects between hexagonal wurtzite ZnO and rutile TiO2 phases that related to ZnO morphology | [194] | |
| Degradation of Methyl orange | ZnO | Nanowires | The formation of oxygen vacancies; the decreased ZnO nanowire diameter size could increase the effective band gap then the photo-generated electrons and holes have a higher reducing/oxidizing power. | [196] |
| ZnO | Nanorods | The higher aspect ratio and larger specific surface area will enhance light photon capture efficiency, promote the generation of photo-induced charges and accelerate their transferrate. | [197] | |
| ZnO | Nanorod arrays | The smaller particle size could lead to a large surface area, providing more active sites; a high orientation of ZnO arrays could increase the polar facet exposing, leading to an enhanced photo-catalytic activity. | [201] | |
| ZnO | Nanorod arrays | The high orientation led to the increased quantity of exposed (0001) facet per unit area which decreased the recombination of photo-generated electron-hole pairs. | [202] | |
| ZnO/ZTO coating polyester fiber membranes orporous ceramic substrates | Nanorods | The coupling of metal oxides to match the electrochemical potential of semiconductors to direct the electron flow following photo-generated electron-hole pair creation enhances the photo-catalytic activity. | [205] | |
| ZnO | Nanofilms | The influence of morphologies on water contact angles before and after UV irradiation which will alter the ZnO hydrophilicity; when the film is irradiated by UV, it will produce electron-hole pairs. Surface oxygen vacancies are formed when some holes react with the lattice oxygen. | [206] | |
| ZnO | Nanosheets | The superior photocatalytic activity was attributed to the exposed (0001) facets and a high specific surface area. | [207] | |
| ZnO | Nano-corncobs | ZnO nano-corncobs hold a lot of polar planes and the polar planes are favorable for oxygen vacancy production which could deter photo corrosion, decrease the recombination of electron-hole pairs and then improve the catalyst chemical stability. | [211] | |
| ZnO | Flower-like | The high specific surface area and the number of oxygen vacancies in materials. | [212] | |
| ZnO, ZnO/Au | Nanorods | Both noble metal nanoparticles’ SPR effect and one-dimensional ZnO nanostructures’ morphology-dependent effects contribute to the high photo-catalytic properties of MO degradation. | [197] | |
| CdSe and core-shell CdSe/ZnS modified ZnO | Nanorods | CdSe QD-sensitized ZnO nanorods with stronger light-harvesting capability exhibited much improved photo-catalytic performance due to its relatively small band gap. ZnO assembled with core-shell CdSe/ZnS shows lower photo-catalytic ability due to the higher carrier transport barrier originated from ZnS shell layers. | [215] | |
| ZnO | Nano-sheet built networks on a hexagonal-pyramid-like microcrystal | The external surfaces that consistof a basal plane (0001) and lateral planes (011) present a strong enhancement of photocatalytic performance. | [216] | |
| ZnO | Nanosheets | The large specific surface areas and high proportion of active polar (0001) facets. | [217] | |
| TiO2@ZnO | Shell@core-nanostructured TiO2@ZnO n-p-n heterojunction | The driving force of inner electric field, efficiently promoting the separation and transfer of photo generated electron-hole pairs. | [218] | |
| BiOBr–ZnO | Flower-like | The effective separation and transfer of photo-generated charges are responsible for an improved photocatalytic performance due to the existence of the interfacial electric field located between BiOBr and ZnO. | [219] | |
| Degradation of Rhodamine B | ZnO | Nanorods, nanosheets | The high ratio of ZnO polar facets will lead to a longer lifetime of the photo-generated charge carriers or a more efficient separation of the photo-excited electron-hole pairs, enhancing the high photo-degradation efficiency | [220] |
| ZnO | Nanosheets | A higher surface area that increases surface oxygen vacancies to improve the visible-light absorption and act as active sites for photocatalytic reactions. A synergistic effect of surface oxygen vacancies and Ag3PO4 coupling; efficient charge transfer between ZnO and Ag3PO4 through energy level matching that leads to hybridization property. | [221] | |
| ZnO | Micro-flower arrays | The high surface area can promote organic dye adsorption and enhance oxygen vacancies density which serve as the electron capturing center; More efficient separation and transfer properties of photogenerated charges | [223,224] | |
| ZnO | Flower-like hierarchical | The open and porous nanostructured surface layer which significantly facilitates the diffusion and mass transportation of RhB molecules and oxygen species. | [225] | |
| Ag-ZnO | ZnO branched nanorods | The decisive roles of the synergistic effect of the hierarchical morphologies and the unique metal-semiconductor heterojunction as well as the presence of the dominant (001) surface of ZnO BNRs. | [227] | |
| Ga-ZnO (doping) | Porous | The slow photon effect from the photonic crystal structure and increased specific surface area after Ga doping | [231] | |
| Ni-ZnO (doping) | Nanosphere | Higher fraction of exposed polar facets, smaller particle size, and larger surface area. | [232] | |
| Ce-ZnO (doping) | Nanoflowers | The hierarchical ZnO microflowers ensured a large specific surface area which is favorable for enhancing the light absorption and light propagation. Ce-doped ZnO microflowers illustrated a noticeable red-shift in the absorption band in the visible light region allowing visible light absorption. | [234] | |
| Eu3+-ZnO | Lotus leaf, cabbage leaf, mushroom-like | The excellent photocatalytic activity and stability for the degradation of RhB (94%) under sunlight was attributed to the crystallite size, band gap, oxygen vacancies, and morphology. | [235] | |
| N/Mn2+-ZnO (codoping) | Nanofibers | The huge band gap decrease, originated from the synergetic effects of both Mn2+ doping and N decoration, which significantly enhanced the absorption of ZnO nanofibers in the range of visible-light. | ||
| Degradation of Yellow GR | ZnO | Nanorods | ZnO nanorods have higher visible light harvesting efficiency and oxygen vacancies which are favorable for photo-catalytic reactions. | [238] |
| Degradation of Orange G | ZnO | Nanorods | The easy separation of photogenerated charge carriers in the higher aspect-ratio structures and the presence of favourable sites on the prism planes which contribute to enhanced oxygen chemisorption, and then promote production of reactive radicals. | [239] |
| Degaradation of Crystal violet | ZnO | Square-bipyramid, bicones and spheres | Both high crystallinity and accessible meso-porous structure (high surface area) are essential for high photo-catalytic efficiency. | [240] |
| Degradation of Congo Red | ZnO | Hierarchical hollow structure | The large surface area and porous structures, which significantly facilitates diffusion and mass transportation of CR molecules and delays the recombination of photo-generated electron-hole pairs in the photo-catalysis process. | [241] |
| Degradation of Orange Ⅱ | Pt-ZnO | Microspheres | The unique hierarchical nanostructures will lead to high light collection efficiency and a fast motion of charge carriers. At the same time, these specific structures can allow more effective transport for the reactant molecules to reach the active sites on the framework walls, hence enhancing the efficiency of photocatalysis. | [243] |
| Degradation of Malachite Green | AgCl-ZnO | Cauliflower-like | The excellent catalytic performance was ascribed to the effectively extended absorption edge into the visible region, efficient electron–hole separation and strong adsorption capability, originating from the special AgCl/ZnO hierarchical nanostructures. | [244] |
| Degradation of Direct Blue DB-15 | CeO2-ZnO composite | Hexagonal nano-disk | Under solar light irradiation, the photo-generated electrons from CB of CeO2 transferred to the CB of ZnO, the photo-generated holes transferred from the VB of ZnO to the VB of CeO2. Thus, the recombination of the electron-hole pairs is prevented and H2O2 and ·OH radicals are produced, leading to enhanced photo-catalytic efficiency. | [245] |
| Degradation of Resazurin | Cu-ZnO (doping) | Nanorods | The surface defects caused by Cu doping could serve as favorable trap sites of the electrons or holes, suppressing their recombination and consequently increasing their photocatalytic activities. The bigger surface to volume ratio in nanorods, results in more surface oxygen vacancies and thus also increased surface activity. | [246] |
| Acid Orange 7 | Cu-ZnO (doping) | Prism-like | More defects and weaker PL emission introduced by Cu doping which also lead to the changing of ZnO morphology. The amount of high active (0001) planes and surface roughness were also thought to increase the catalysts property. | [247] |
| Degradation of Phenol | ZnO | Hexagonal nano-plates | The unique surface nanofeatures with high ratio of polar facets, high concentration of defects and the well-built 2-dimentional nanostructures containing boundaries on plates are desirable for the enhanced photo-catalytic properties. | [248] |
| ZnO | Nanosheets | A large specific surface area can provide more active sites for the reaction and then facilitates the diffusion and mass transportation of the pollutants and hydroxyl radicals during the photochemical reactions. | [249] | |
| ZnO | Cauliflower, truncated hexagonal conical, tubular, rod, hourglass and spherical particles | The interior cavities were found in conical ZnO and multiple reflection of UV light within the interior cavities could occur, which allowed for the more efficient use of the incident light, leading to an improved catalytic activity. | [14] | |
| ZnO | Nano-flakes, nano-rods and spherical nanoparticles | The narrow crystal size distribution, high surface area, and greater amount of oxygen vacancies lead to an enhanced catalytic activity. | [250] | |
| ZnO | Well-faceted hexagonal, spherical, particles and nearly hexagonal rods | The high photo-catalytic activity arose from the narrow particle size distribution, high surface area, and high ratio of polar planes and increased the concentration of surface defects. | [251] | |
| Nb2O5/ZnO, Cr2O3/ZnO composites | Nanorods | The extended light absorption region and fast charge transfer rate, caused by the incorporation of Nb2O5 into ZnO. | [252,253] | |
| ZnO/ZnMgAl-CO3-LDHs | Microspheres | The formation of a hetero-junction can lower the recombination rate of the photo-excited electron-hole pairs and the small size of ZnO could improve light absorption properties. | [254] | |
| Aerobic oxidation of alcohols | ZnO | Nanorods | The efficient separation of photo-generated charge carriers and its favorable, selective absorption toward the reactant of alcohols rather than the product of aldehydes, induced by the special 1D morphology of ZnO nanorods. | [261] |
| Reduction of Cr (VI) | GO-ZnO (doping) | Prismatic rod, Hexagonal tube-like | The oxygenated functional groups and the unique structures of 2D GO played pivotal roles in tuning the morphology of ZnO and increasing oxygen vacancies concentration. The introduction of oxygen vacancies and the intimate interfacial interaction between ZnO and reduced grapheneoxide (RGO) extended the light absorption edge of RGO-ZnO to the visible light region. | [262] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts 2016, 6, 188. https://doi.org/10.3390/catal6120188
Sun Y, Chen L, Bao Y, Zhang Y, Wang J, Fu M, Wu J, Ye D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts. 2016; 6(12):188. https://doi.org/10.3390/catal6120188
Chicago/Turabian StyleSun, Yuhai, Limin Chen, Yunfeng Bao, Yujun Zhang, Jing Wang, Mingli Fu, Junliang Wu, and Daiqi Ye. 2016. "The Applications of Morphology Controlled ZnO in Catalysis" Catalysts 6, no. 12: 188. https://doi.org/10.3390/catal6120188







