CuO Nanorods-Decorated Reduced Graphene Oxide Nanocatalysts for Catalytic Oxidation of CO
Abstract
:1. Introduction
2. Results and Discussion
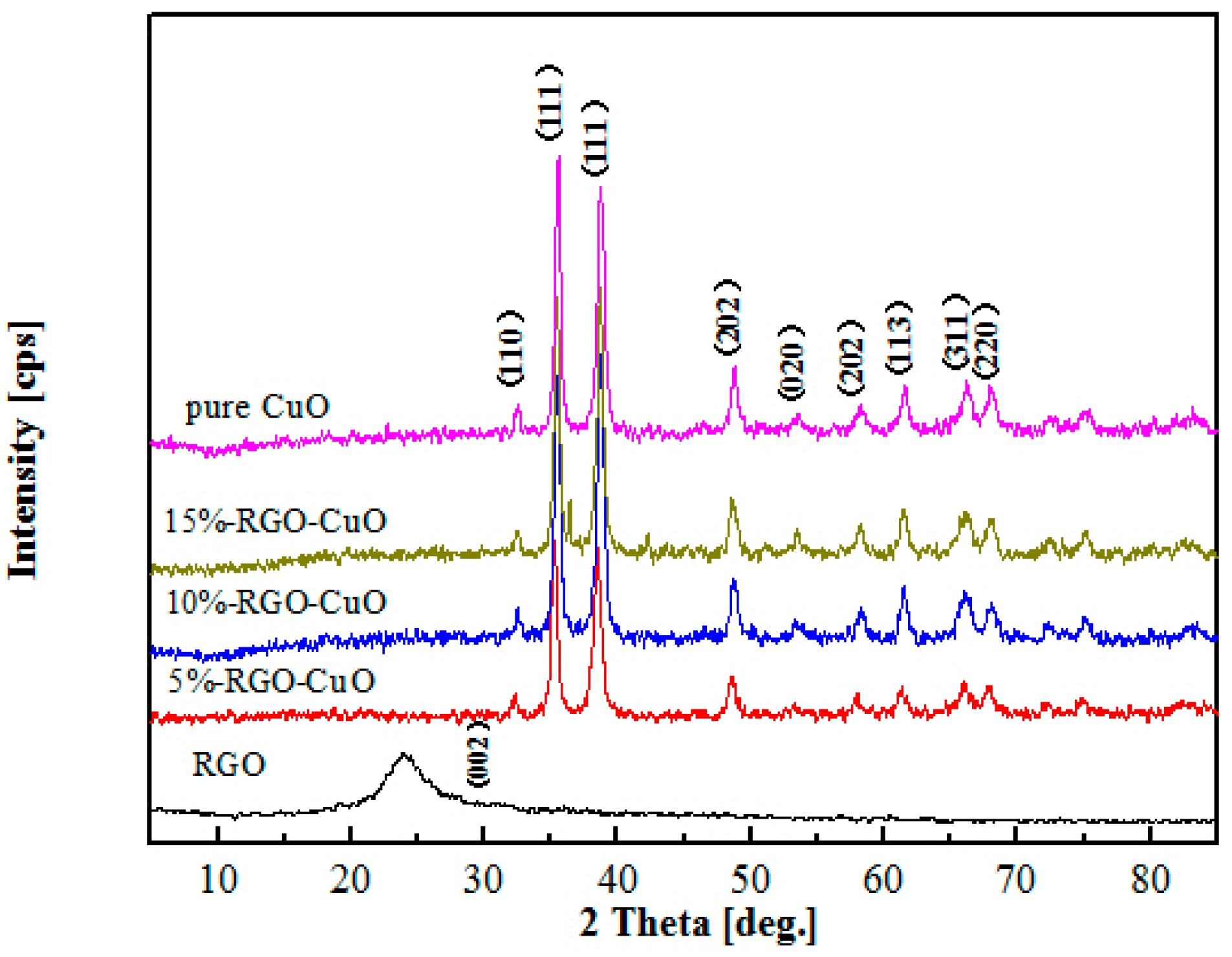
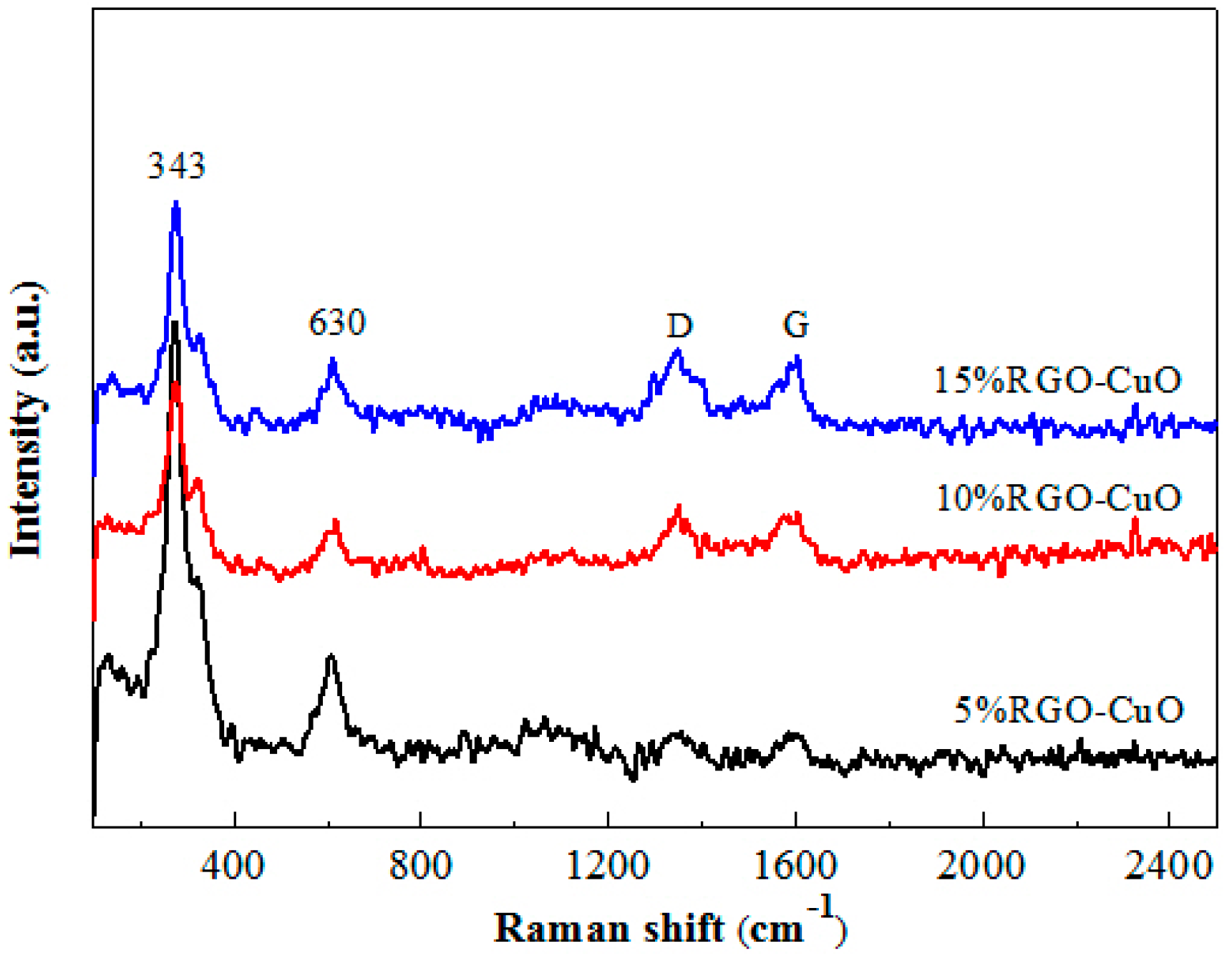
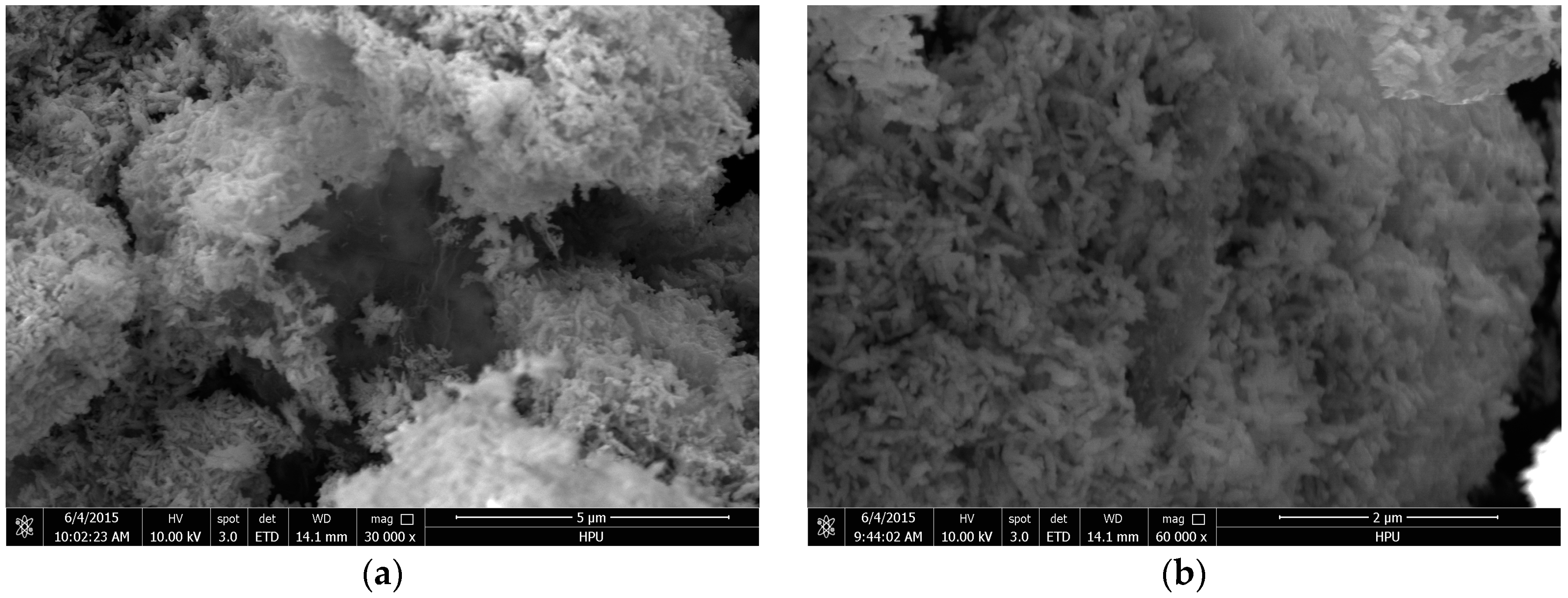
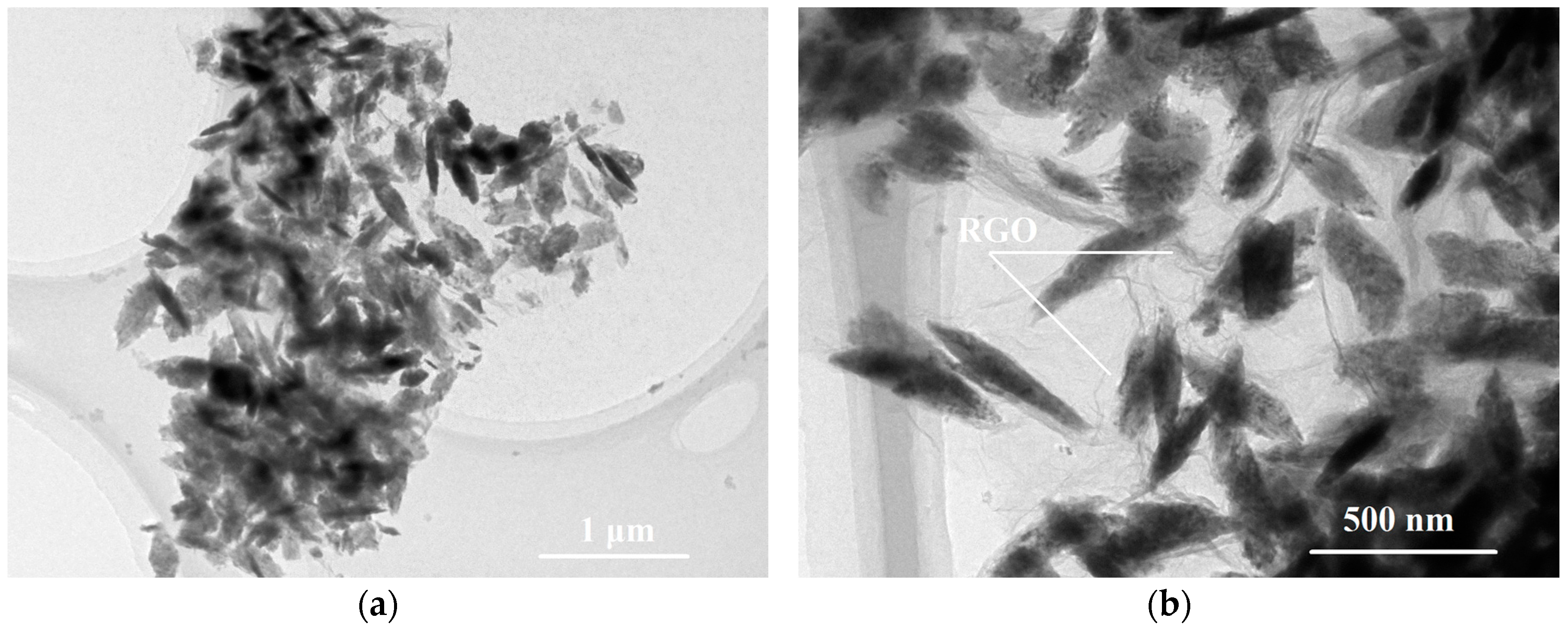
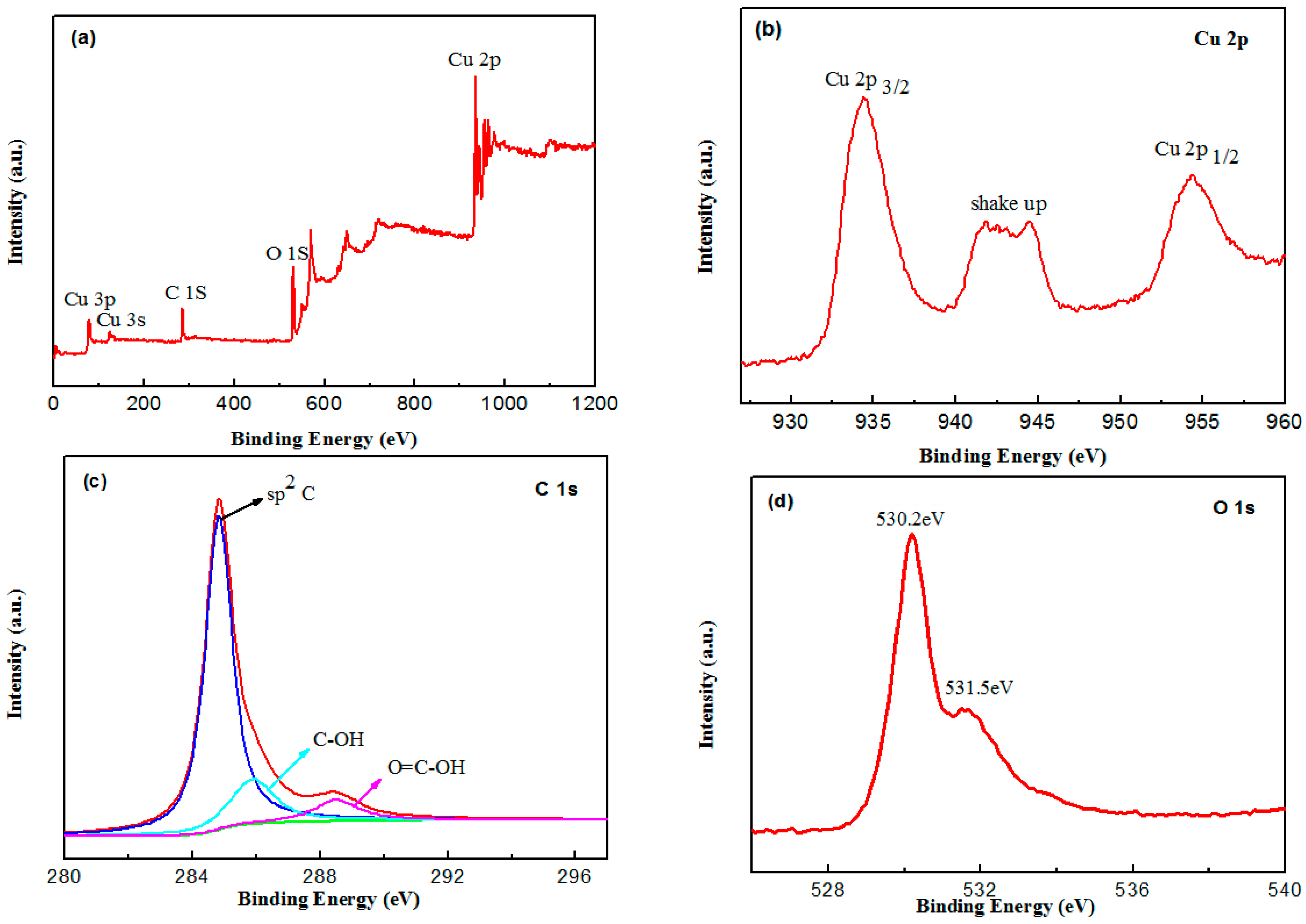
2.1. Catalyst Characterization
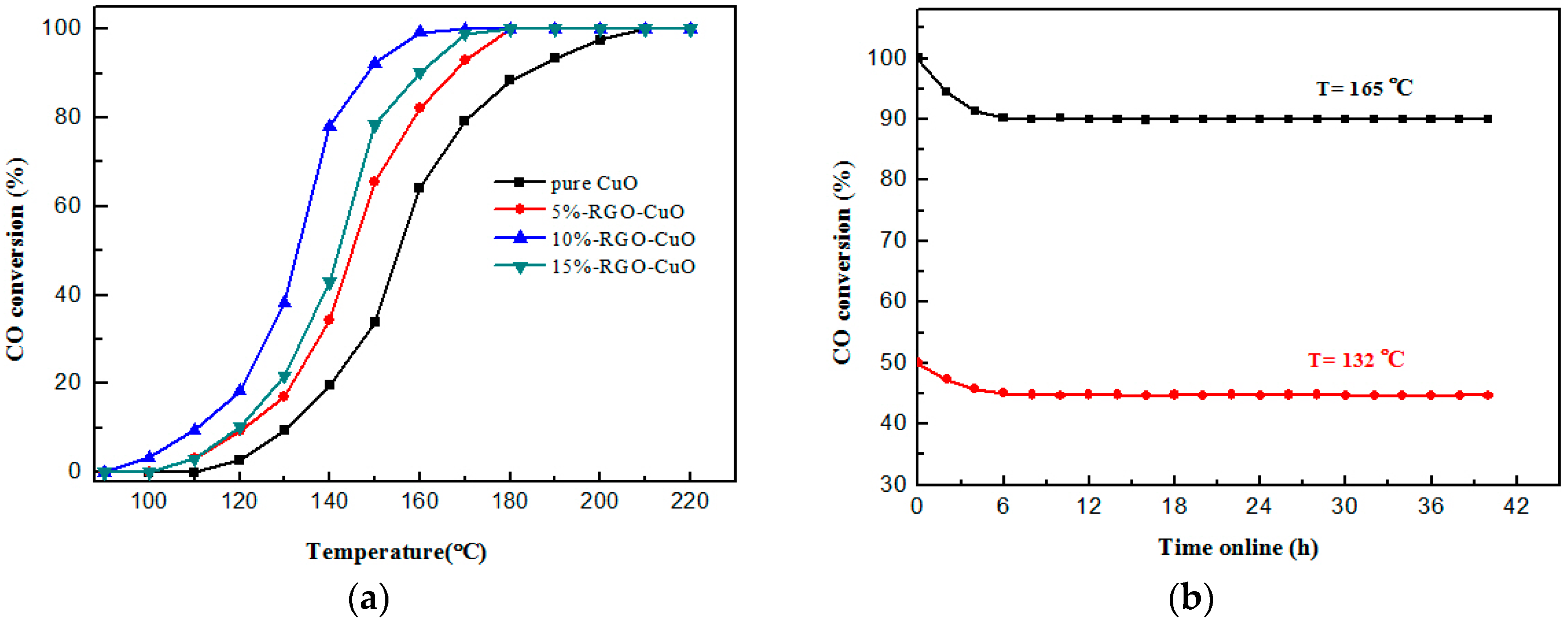
2.2. Catalytic Activity
3. Materials and Methods
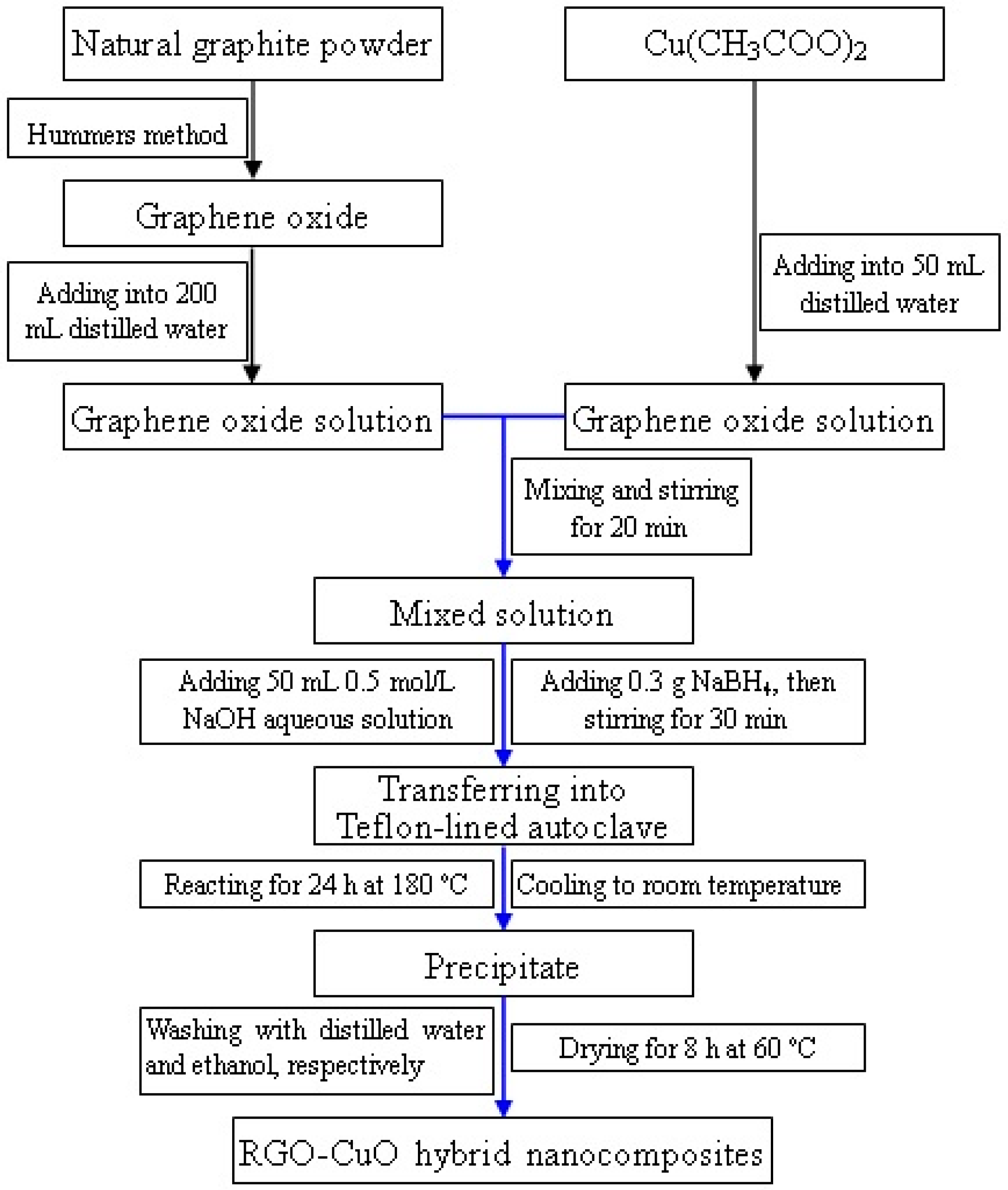
3.1. Synthesis
3.2. Characterizations
3.3. Catalytic Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aguila, G.; Guerrero, S.; Araya, P. Effect of the preparation method and calcination temperature on the oxidation activity of CO at low temperature on CuO-CeO2/SiO2 catalysts. Appl. Catal. A Gen. 2013, 462–463, 56–63. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Zhang, Y.; Wu, S.; Yuan, Z. Preparation, characterization and catalytic behavior of nanostructured mesoporous CuO/Ce0.8Zr0.2O2 catalysts for low-temperature CO oxidation. Appl. Catal. B Environ. 2008, 78, 120–128. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wang, J.; Song, J.; Li, Q.; Dong, M.; Liu, C. CO oxidation over graphene supported palladium catalyst. Appl. Catal. B: Environ. 2012, 125, 189–196. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Cenet, M.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. Elementary steps in the catalytic oxidation of carbon monoxide on platinum metals. Adv. Catal. 1979, 28, 1–78. [Google Scholar]
- Zhu, D.; Duan, D.; Han, Y.; He, Y.; Chen, Y.; Zhang, W.; Yan, Z.; Wang, J.; Yuan, F. Noble Metal-Free Ceria-Zirconia Solid Solutions Templated by Tabacco Materials for Catalytic Oxidation of CO. Catalysis 2016, 6, 135. [Google Scholar]
- Cao, J.; Wang, Y.; Yu, X.; Wang, S.; Wu, S.; Yuan, Z. Mesoporous CuO–Fe2O3 composite catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. B Environ. 2008, 79, 26–34. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, R.; Chen, B.; Bion, N.; Duprez, D.; Royer, S. Activity of perovskite-type mixed oxides for the low-temperature CO oxidation: Evidence of oxygen species participation from the solid. J. Catal. 2012, 295, 45–48. [Google Scholar] [CrossRef]
- Luo, M.; Ma, J.; Lu, J.; Song, Y.; Wang, Y. High-surface area CuO–CeO2 catalysts prepared by a surfactant-templated method for low-temperature CO oxidation. J. Catal. 2007, 246, 52–59. [Google Scholar] [CrossRef]
- Lin, H.; Chiu, H.; Tsai, H.; Chien, S.; Wang, C. Synthesis, characterization and catalytic oxidation of carbon monoxide over cobalt oxide. Catal. Lett. 2003, 88, 169–174. [Google Scholar] [CrossRef]
- Chen, S.; Si, R.; Taylor, E. Synthesis of Pd/Fe3O4 hybrid nanocatalysts with controllable interface and enhanced catalytic activities for CO oxidation. J. Phys. Chem. C 2012, 116, 12969–12976. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Manzoli, M.; Boccuzzi, F.; Tabakova, T.; Papavasiliou, J.; Idakiev, V. Catalytic performance and characterization of Au/doped-ceria catalysts for the preferential CO oxidation reaction. J. Catal. 2008, 256, 237–247. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E. The effect of the crystalline phase of alumina on the selective CO oxidation in a hydrogen-rich stream over Ru/Al2O3. Appl. Catal. B Environ. 2010, 96, 41–50. [Google Scholar] [CrossRef]
- Chen, H. First-principles study of CO adsorption and oxidation on Ru-doped CeO2 (111) surface. J. Phys. Chem. C 2012, 116, 6239–6246. [Google Scholar] [CrossRef]
- Chua, Y.P.G.; Gunasooriya, G.T.K.K.; Saeys, M.; Seebauer, E.G. Controlling the CO oxidation rate over Pt/TiO2 catalysts by defect engineering of the TiO2 support. J. Catal. 2014, 311, 306–313. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Kardash, T.Y.; Stonkus, O.A. In situ XRD, XPS, TEM, and TPR study of highly active in CO oxidation CuO nanopowders. J. Phys. Chem. C 2013, 117, 14588–14599. [Google Scholar] [CrossRef]
- Xiao, J.; Wan, L.; Wang, X.; Kuang, Q.; Dong, S.; Xiao, F.; Wang, S. Mesoporous Mn3O4–CoO core–shell spheres wrapped by carbon nanotubes: A high performance catalyst for the oxygen reduction reaction and CO oxidation. J. Mater. Chem. A 2014, 2, 3794–3800. [Google Scholar] [CrossRef]
- Hu, L.; Sun, K.; Peng, Q.; Xu, B.; Li, Y. Surface active sites on Co3O4 nanobelt and nanocube model catalysts for CO oxidation. Nano Res. 2010, 3, 363–368. [Google Scholar] [CrossRef]
- Radwan, N.R.E.; El-Shall, M.S.; Hassan, H.M.A. Synthesis and characterization of nanoparticle Co3O4, CuO and NiO catalysts prepared by physical and chemical methods to minimize air pollution. Appl. Catal. A Gen. 2007, 331, 8–18. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, W.; Tao, Y.; Wang, G.; Cui, X.; Zhang, C.; Wu, T.; Dong, G. Preparation, characterization and CO oxidation of nanometer Co3O4. Chem. Res. Chin. Univ. 1999, 4, 637–639. [Google Scholar]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Yoo, E.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.; Liu, J.; Chen, T.; Yang, H.; Li, C. CeO2 nanoparticles/graphene nanocomposite-based high performance supercapacitor. Dalton. Trans. 2011, 40, 6388–6391. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, J.; Chen, Z. Fe-anchored graphene oxide: A low-cost and easily accessible catalyst for low-temperature CO oxidation. J. Phys. Chem. C 2012, 116, 2507–2514. [Google Scholar] [CrossRef]
- Song, E.; Wen, Z.; Jiang, Q. CO catalytic oxidation on copper-embedded graphene. J. Phys. Chem. C 2011, 115, 3678–3683. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Xu, S.; Gu, Z. Graphene-CuO Nanocomposite. J. Nanosci. Nanotechnol. 2010, 10, 6690–6693. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Hsu, T.; Sun, C.; Nien, Y.; Pu, N.; Ger, M. Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 2012, 82, 152–157. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: Synthesis and host-guest inclusion for enhanced electrochemical performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; He, M.; Lu, J.; Luo, M.; Fang, P.; Xie, Y. Comparative Study of CuO Species on CuO/Al2O3, CuO/CeO2-Al2O3 and CuO/La2O-Al2O3 Catalysts for CO Oxidation. Chin. J. Chem. Phys. 2007, 20, 582–586. [Google Scholar] [CrossRef]
- Morales, J.; Sánchez, L.; Martín, F.; Ramos-Barrado, J.R.; Sánchez, M. Use of low-temperature nanostructured CuO thin films deposited by spray-pyrolysis in lithium cells. Thin. Solid Films 2005, 474, 133–140. [Google Scholar] [CrossRef]
- Gao, L.; Yue, W.; Tao, S.; Fan, L. Novel strategy for preparation of graphene-Pd, Pt composite, and its enhanced electrocatalytic activity for alcohol oxidation. Langmuir 2012, 29, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wen, Z.; Zhang, H.; Cao, G.; Sun, Q.; Cao, J. CuO Nanorods-Decorated Reduced Graphene Oxide Nanocatalysts for Catalytic Oxidation of CO. Catalysts 2016, 6, 214. https://doi.org/10.3390/catal6120214
Wang Y, Wen Z, Zhang H, Cao G, Sun Q, Cao J. CuO Nanorods-Decorated Reduced Graphene Oxide Nanocatalysts for Catalytic Oxidation of CO. Catalysts. 2016; 6(12):214. https://doi.org/10.3390/catal6120214
Chicago/Turabian StyleWang, Yan, Zhihui Wen, Huoli Zhang, Guohua Cao, Qi Sun, and Jianliang Cao. 2016. "CuO Nanorods-Decorated Reduced Graphene Oxide Nanocatalysts for Catalytic Oxidation of CO" Catalysts 6, no. 12: 214. https://doi.org/10.3390/catal6120214





