Carbon-Based Air-Breathing Cathodes for Microbial Fuel Cells
Abstract
:1. Introduction
| O2 + 2H2O + 4e− → 4OH− | 0.401 V vs. SHE | (1) |
| O2 + H2O + 2e− → HO2− + OH− | −0.065 V vs. SHE | (2) |
| HO2− + H2O + 2e− → 3OH− | 0.867 V vs. SHE | (3) |
| 2HO2− → 2OH− + O2 | (4) |
2. Results and Discussion
2.1. RRDE Results
2.2. Electrode Characterization
2.3. Electrode Resistivity
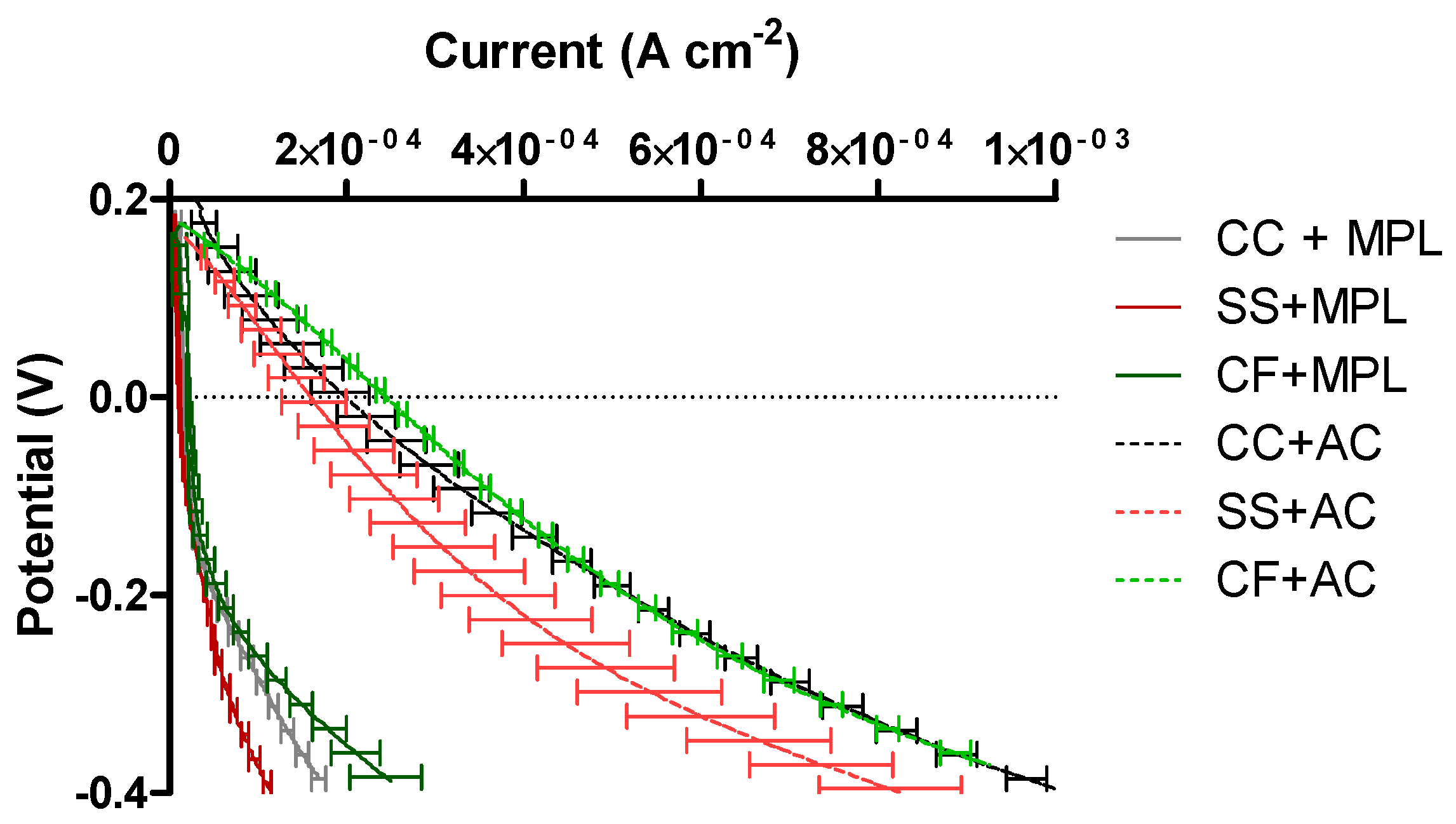
2.4. Cathode Polarization Curve in “Pristine” Conditions
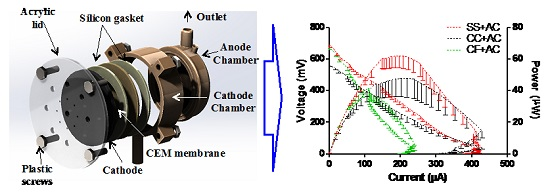
2.5. MFCs’ Power Performance
3. Materials and Methods
3.1. Electrode Preparation and Characterization
3.2. Electrical Resistivity
3.3. Rotating Ring Disk Electrode (RRDE) Analysis on Carbon Black and Activated Carbon Catalysts
3.4. Linear Sweep Voltammetry on Cathode Electrode
3.5. MFC Design and Operation
3.6. Testing of Different Cathode Electrodes
3.7. MFC Monitoring
3.8. Inoculation and Polarization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Winfield, J.; Gajda, I.; Walter, A.; Papaharalabos, G.; Merino-Jimenez, I.; Pasternak, G.; You, J.; Tremouli, A.; Stinchcombe, A.; et al. Chapter 12: The practical implementation of microbial fuel cell technology. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing/Elsevier: Cambridge, UK, 2015; pp. 357–380. [Google Scholar]
- Ieropoulos, I.; Ledezma, P.; Stinchcombe, A.; Papaharalabos, G.; Melhuish, C.; Greenman, J. Waste to real energy: The first MFC powered mobile phone. Phys. Chem. Chem. Phys. 2013, 15, 15312–15316. [Google Scholar] [CrossRef] [PubMed]
- Melhuish, C.; Ieropoulos, I.; Greenman, J.; Horsfield, I. Energetically autonomous robots: Food for thought. Auton. Robot. 2006, 21, 187–198. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C.; Horsfield, I. EcoBot-III: A robot with guts. In Proceedings of the Alife XII Conference, Odense, Denmark, 19–23 August 2010.
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal—Microbial fuel cell technology field trials in the context of sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Kim, J.R.; Oh, S.E.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Hamelers, H.V.M.; TerHeijne, A.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Strik, D.P.B.T.B.; Buisman, C.J.N. New applications and performance of bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Cristiani, P.; Carvalho, M.L.; Guerrini, E.; Daghio, M.; Santoro, C.; Li, B. Cathodic and anodic biofilms in single chamber microbial fuel cells. Bioelectrochemistry 2013, 92, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Cremins, M.; Pasaogullari, U.; Guilizzoni, M.; Casalegno, A.; Mackay, A.; Li, B. Evaluation of water transport and oxygen presence in single chamber microbial fuel cells with carbon-based cathodes. J. Electrochem. Soc. 2013, 160, 128–134. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Jang, J.K.; Moon, H.S.; Chang, I.S.; Kim, B.H. Improved performance of microbial fuel cell using membrane-electrode assembly. J. Microbiol. Biotechnol. 2005, 15, 438–441. [Google Scholar]
- Kim, M.; Hyun, M.S.; Gadd, G.M.; Kim, G.T.; Lee, S.J.; Kim, H.J. Membrane-electrode assembly enhances performance of a microbial fuel cell type biological oxygen demand sensor. Environ. Technol. 2009, 30, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Min, B.; Logan, B.E. Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl. Microbiol. Biotechnol. 2005, 68, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Guilizzoni, M.; Correa Baena, J.P.; Pasaogullari, U.; Casalegno, A.; Li, B.; Babanova, S.; Artyushkova, K.; Atanassov, P. The effect of carbon surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon 2014, 67, 128–139. [Google Scholar] [CrossRef]
- Rabaey, K.; Rodrıguez, J.; Blackall, L.L.; Keller, J.; Gross, P.; Batstone, D.; Verstrate, W.; Nealson, K.H. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007, 1, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Phan, T.; Wanger, G.; Nealson, K.H.; Sekiguchi, Y.; Gorby, Y.A.; Bretschger, O. Microbial population and functional dynamics associated with surface potential and carbon metabolism. ISME J. 2014, 8, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Wu, A.; Yamanaka, Y.; Nealson, K.H.; Bretschger, O. Identifying the microbial communities and operational conditions for optimized wastewater treatment in microbial fuel cells. Water Res. 2013, 47, 7120–7130. [Google Scholar] [CrossRef] [PubMed]
- Xafenias, N.; Mapelli, V. Performance and bacterial enrichment of bioelectrochemical systems during methane and acetate production. Int. J. Hydrog. Energy 2014, 39, 21864–21875. [Google Scholar] [CrossRef]
- Erable, B.; Feron, D.; Bergel, A. Microbial catalysis of the oxygen reduction reaction for microbial fuel cells: A review. ChemSusChem 2012, 5, 975–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshminarayanaiah, N. Transport Phenomena in Membranes; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Fischer, P.; Heitbaum, J. Mechanistic aspects of cathodic oxygen reduction. J. Electroanal. Chem. 1980, 112, 231–238. [Google Scholar] [CrossRef]
- Pivovar, B.S.; Smyrl, W.; Cussler, E.L. Electro-osmosis in Nafion 117, Polystyrene Sulfonic Acid, and Polybenzimidazole. J. Electrochem. Soc. 2005, 152, A53–A60. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Hill, R.F. Ion and solvent transport in ion-exchange membranes. J. Electrochem. Soc. 1990, 137, 886–893. [Google Scholar] [CrossRef]
- Hao, X.D.; Wang, C.C.; Lan, L.; Von Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Luo, N.; Miley, G.H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 2007, 173, 77–85. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, X.; Wang, X.; Lee, H.; Liu, J.; Qu, Y.; He, W.; Senthil Kumar, S.M.; Kim, B.H.; Ren, N. Effects of sulfide on microbial fuel cells with platinum and nitrogen- doped carbon powder cathodes. Biosens. Bioelectron. 2012, 35, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Serov, A.; Stariha, L.; Kodali, M.; Gordon, J.; Babanova, S.; Bretschger, O.; Artyushkova, K.; Atanassov, P. Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ. Sci. 2016, 9, 2346–2353. [Google Scholar] [CrossRef]
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering materials and biology to boost performance of microbial fuel cells: A critical review. Energy Environ. Sci. 2009, 1, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Liew, K.B.; Wan Daud, W.R.; Ghasemi, M.; Leonga, J.X.; Lima, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrog. Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Cheng, S.A.; Liu, H.; Logan, B.E. Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ. Sci. Technol. 2006, 40, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Harnisch, F.; Schroder, U.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Application of pyrolysed iron (II) phthalocyanine and CoTMPP based oxygen reduction catalysts as cathode materials in microbial fuel cells. Electrochem. Commun. 2005, 7, 1405–1410. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Lozano-Blanco, L.J.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process. Technol. 2015, 138, 284–297. [Google Scholar] [CrossRef]
- Antolini, E. Composite materials for polymer electrolyte membrane microbial fuel cells. Biosens. Bioelectron. 2015, 69, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Hou, Y.; Reesh, I.M.A.; Chen, J.; He, Z. Oxygen reduction reaction catalysts in microbial fuel cells for energy-efficient wastewater treatment: A review. Mater. Horiz. 2016. [Google Scholar] [CrossRef]
- Mustakeem, M. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, C.; Zheng, Y.; Chen, S.; Zhao, F. Abiotic oxygen reduction reaction catalysts used in microbial fuel cells. ChemElectroChem 2014, 1, 1813–1821. [Google Scholar] [CrossRef]
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Agrios, A.; Pasaogullari, U.; Li, B. Effect of gas diffusion layer (GDL) and microporous layer (MPL) on cathode performance in microbial fuel cells. Int. J. Hydrog. Energy 2011, 36, 13096–13104. [Google Scholar] [CrossRef]
- Papaharalabos, G.; Greenman, J.; Melhuish, C.; Santoro, C.; Cristiani, P.; Li, B.; Ieropoulos, I. Increased power output from micro porous layer (MPL) cathode microbial fuel cells (MFC). Int. J. Hydrog. Energy 2013, 38, 11552–11558. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of Multitudinous Active Sites for Oxygen Reduction Reaction in Transition Metal–Nitrogen–Carbon Electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Park, S.S.; Jung, Y. Influence of the Electrode Materials on the Electrochemical Performance of Room Temperature Li-SO2 Rechargeable Battery. Int. J. Electrochem. Sci. 2015, 10, 7574–7581. [Google Scholar]
- Jhi, S.-H.; Kwon, Y.-K.; Bradley, K.; Gabriel, J.-C.P. Hydrogen storage by physisorption: Beyond carbon. Solid State Commun. 2004, 129, 769–773. [Google Scholar] [CrossRef]
- Fang, B.; Kim, J.H.; Kim, M.-S.; Bonakdarpour, A.; Lam, A.; Wilkinson, D.P.; Yu, S.-J. Fabrication of hollow core carbon spheres with hierarchical nanoarchitecture for ultrahigh electrical charge storage. J. Mater. Chem. 2012, 22, 19031–19038. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.L.; Simon, P.; Fauvarque, J.F.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Tylus, U.; Jia, Q.; Strickland, K.; Ramaswamy, N.; Serov, A.; Atanassov, P.; Mukerjee, S. Elucidating oxygen reduction active sites in pyrolyzed metal–nitrogen coordinated non-precious-metal electrocatalyst systems. J. Phys. Chem. C 2014, 118, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.H.; Serov, A.; Artyushkova, K.; Atanassov, P. A mechanistic study of 4-aminoantipyrine and iron derived non-platinum group metal catalyst on the oxygen reduction reaction. Electrochim. Acta 2013, 90, 656–665. [Google Scholar] [CrossRef]
- Santoro, C.; Lei, Y.; Li, B.; Cristiani, P. Power generation from wastewater using single chamber microbial fuel cells (MFCs) with platinum-free cathodes and pre-colonized anodes. Biochem. Eng. J. 2012, 62, 8–16. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Simultaneous electricity generation and microbially-assisted electrosynthesis in ceramic MFCs. Bioelectrochemistry 2015, 104, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Artyushkova, K.; Babanova, S.; Atanassov, P.; Ieropoulos, I.; Grattieri, M.; Cristiani, P.; Trasatti, S.; Li, B.; Schuler, A.J. Parameters Characterization and Optmization of Activated Carbon (AC) Cathodes for Microbial Fuel Cell Applications. Bioresour. Technol. 2014, 163, 54–63. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Electricity generation and struvite recovery from human urine using microbial fuel cells. J. Chem. Technol. Biotechnol. 2015, 91, 647–654. [Google Scholar] [CrossRef]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino-Jimenez, I.; Santoro, C.; Rojas-Carbonell, S.; Greenman, J.; Ieropoulos, I.; Atanassov, P. Carbon-Based Air-Breathing Cathodes for Microbial Fuel Cells. Catalysts 2016, 6, 127. https://doi.org/10.3390/catal6090127
Merino-Jimenez I, Santoro C, Rojas-Carbonell S, Greenman J, Ieropoulos I, Atanassov P. Carbon-Based Air-Breathing Cathodes for Microbial Fuel Cells. Catalysts. 2016; 6(9):127. https://doi.org/10.3390/catal6090127
Chicago/Turabian StyleMerino-Jimenez, Irene, Carlo Santoro, Santiago Rojas-Carbonell, John Greenman, Ioannis Ieropoulos, and Plamen Atanassov. 2016. "Carbon-Based Air-Breathing Cathodes for Microbial Fuel Cells" Catalysts 6, no. 9: 127. https://doi.org/10.3390/catal6090127