TiO2 Solar Photocatalytic Reactor Systems: Selection of Reactor Design for Scale-up and Commercialization—Analytical Review
Abstract
:1. Introduction
2. Reactor Configurations
2.1. Parabolic Trough Reactor
2.2. Concentrating Falling Film Reactor
2.3. Compound Parabolic Concentrator
2.4. Tubular Reactor
2.5. Shallow Pond Reactor
2.6. Double-Skin Sheet Reactor
2.7. Flat Plate Reactor
2.8. Falling Film Photoreactor
2.9. Thin Film Cascade Photoreactor
2.10. Step Photoreactor
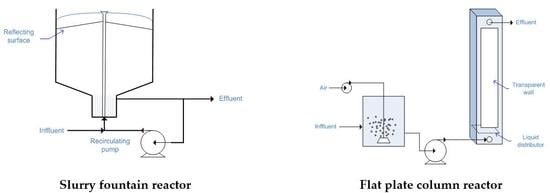
2.11. Fountain Photocatalytic Reactor
2.12. Slurry Bubble Column Reactor
2.13. Flat Plate Column Reactor
2.14. Pebble Bed Photoreactor
2.15. Flat Packed Bed Reactor
3. Comparison of Different Reactors Throughput
4. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.; Poulios, I.; Mantzavinos, D. Perspective Advanced oxidation processes for water treatment: Advances and trends for R & D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Blake, D. Bibliography of Work on the Heterogeneous Photocatalytic Removal of Hazardous Compounds from Water and Air, Update Number 4 to October 2001, NREL/TP-510–31319; National Renewable Energy Laboratory: Golden, CO, USA, 2001. [Google Scholar]
- Arana, J.; Melian, J.; Rodriguez, J.; Diaz, O.; Viera, A.; Pena, J.; Sosa, P.; Jimenez, V. TiO2-photocatalysis as a tertiary treatment of naturally treated wastewater. Catal. Today 2002, 76, 279–289. [Google Scholar] [CrossRef]
- Al-Bastaki, N. Performance of advanced methods for treatment of wastewater: UV/TiO2, RO and UF. Chem. Eng. Process 2004, 43, 935–940. [Google Scholar] [CrossRef]
- Likodimos, V.; Dionysiou, D.; Falaras, P. Clean water: Water detoxification using innovative photocatalysts. Res. Environ. Sci. Biotechnol. 2010, 9, 87–94. [Google Scholar] [CrossRef]
- Dimitroula, H.; Daskalaki, V.; Frontistis, Z.; Kondaides, D.; Panagiotopoulou, P.; Xekoukoulotakis, N.; Mantzavinos, D. Solar photocatlysis for the abatement of emerging micro-contaminants in wastewater: Synthesis, characterization and testing of various TiO2 samples. Appl. Catal. B: Environ. 2012, 117, 283–291. [Google Scholar] [CrossRef]
- Miranda-Garcia, N.; Maldonado, M.; Coronado, J.; Malato, S. Degradation study of 15 emerging contaminants at low concentrationby immobilized TiO2 in a pilot plant. Catal. Today 2010, 151, 107–113. [Google Scholar] [CrossRef]
- Agullo-Barcelo, M.; Polo-Lopez, M.; Lucena, F.; Jofre, J.; Fernandez-Ibanez, P. Solar advanced oxidation processes as disinfection tertiary treatments for real wastewater: Implicatons for water reclamation. Appl. Catal. B: Environ. 2013, 136, 341–350. [Google Scholar] [CrossRef]
- Dominguez-Espindola, R.; Silva-Martinez, S.; Ortiz-Hernandez, M.; Roman-Zubillaga, J.; Guardian-Tapia, R. Photocatlytic disinfection of municipal wastewater using TiO2 film and Ag/TiO2 powder under UV and solar light irradiation. Mex. J. Sci. Res. 2013, 2, 60–68. [Google Scholar]
- Navalon, S.; Alvaro, M.; Garcia, H.; Escrig, D.; Costa, V. Photocatalytic water disinfection of cryptosporidium parvum and Giardia lamlia using a fibrous ceramic TiO2 photocatalyst. Water Sci. Technol. 2009, 59, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Gamage, J.; Zhang, Z. Applications of photocatalytic disinfection. Int. J. Photoenergy 2010, 2010. [Google Scholar] [CrossRef]
- Maness, P.; Smolinski, S.; Blake, D.; Huang, Z.; Wolfrum, E.; Jacoby, W. Bacericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Sokmen, M.; Degerli, S.; Aslan, A. Photocatalytic disinfection of Giardia intestinals and acanthamoeba castellani cysts in water. Exp. Parasitol. 2008, 119, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, M.; Choung, S.; Ogino, K.; Miyata, S.; Kim, M.; Park, J.; Kim, J. The preparation of TiO2 nanometer photocatalyst film by a hydrothermal method and its sterilization performance for Giardia lamblia. Water Res. 2004, 38, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ede, S.; Hafner, L.; Dunlop, P.; Byrne, J.; Will, G. photocatalytic disinfection of bacterial pollutants using suspended and immobilized TiO2 powders. Photochem. Photobiol. 2012, 88, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Mccullagh, C.; Robertson, J.; Bahnemann, D.; Robertson, P. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B. Photocatalytic treatment of high concentration carbamazepine in synthetic hospital wastewater. J. Hazard. Mater. 2012, 199, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Ferro, G.; Manaia, C. Wastewater disinfection by solar heterogeeous photocatalysis: Effect on tetracycline resistant/sensetive enterococcus strains. Glob. Nest J. 2014, 16, 457–464. [Google Scholar]
- Ghaly, M.Y.; Jamil, T.S.; El-Seesy, I.E.; Souaya, E.R.; Nasr, R.A. Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2. Chem. Eng. J. 2011, 168, 446–454. [Google Scholar] [CrossRef]
- Kansal, S.; Singh, M.; Sud, D. Effluent quality at kraft/soda agro-based paper mills and its treatment using a heterogeneous photocatalytic system. Desalination 2008, 228, 183–190. [Google Scholar] [CrossRef]
- Amat, A.; Arques, A.; Lopez, F.; Miranda, M. Solar photo-catalysis to remove paper mill wastewater pollutants. Sol. Energy 2005, 79, 393–401. [Google Scholar] [CrossRef]
- Rodrigues, A.; Boroski, M.; Shimada, N.; Garcia, J.; Nozaki, J.; Hioka, N. Treatment of paper pulp and paper mill wastewater by coagulation-flocculation followed by heterogeneous photocatalysis. J. Photochem. Photobiol. A: Chem. 2008, 194, 1–10. [Google Scholar] [CrossRef]
- Banu, J.; Anandan, S.; Kaliappan, S.; Yeom, I. Treatment of dairy wastewater using anaerobic and solar photocatalytic methods. Sol. Energy 2008, 82, 812–819. [Google Scholar] [CrossRef]
- Pekakis, P.; Xekoukoulotakis, N.; Mantzavinos, D. Treatment of textile dyehouse wastewater by TiO2 photocatalysis. Water Res. 2006, 40, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Saquib, M.; Tariq, M.A.; Faisal, M.; Muneer, M. Photocatalytic degradation of two selected dye derivatives in aqueous suspensions of titanium dioxide. Desalination 2008, 219, 301–311. [Google Scholar] [CrossRef]
- Luo, M.; Bowden, D.; Brimblecombe, P. Removal of dyes from water using a TiO2 photocatalyst supported on black sand. Water Air Soil Pollut. 2009, 198, 233–241. [Google Scholar] [CrossRef]
- Oliveira, D.F.M.; Batista, P.S.; Muller, P.S., Jr.; Velani, V.; Franca, M.; Souza, D.; Machado, A. Evaluating the effectiveness of photocatalysts based on Titanium dioxide in the degradation of the dye Ponceau 4R. Dyes Pigment. 2011, 92, 563–572. [Google Scholar] [CrossRef]
- Zayani, G.; Bousseimi, L.; Pichat, P.; Mhenni, F.; Ghrabi, A. Photocatalytic Degradation of four Textile Azo Dyes in Aqueous TiO2 Suspensions: Practical Outcomes and Revisited Pathways. J. Adv. Oxid. Technol. 2006, 9, 65–78. [Google Scholar]
- Kasanen, J.; Salstela, J.; Suvanto, M.; Pakkanen, T. Photocatalytic degradation of methylene blue in water solution by multilayer TiO2 coating on HDPE. Appl. Surf. Sci. 2011, 258, 1738–1743. [Google Scholar] [CrossRef]
- Sokmen, M.; Tatlidil, I.; Breen, C.; Clegg, F.; Buruk, C.; Sivlim, T.; Akkan, S. A new nano-TiO2 immobilized biodegradable polymer with self-cleaning properties. J. Hazard. Mater. 2011, 187, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Toor, A.; Verma, A.; Jotshi, C.; Bajpai, P.; Singh, V. Photocatalytic degradation of direct yellow 12 dye using UV/TiO2 in a shallow pond slurry reactor. Dyes Pigment. 2006, 68, 53–60. [Google Scholar] [CrossRef]
- Gernjak, W.; Maldonado, M.I.; Malato, S.; Caceres, J.; Krutzler, T.; Glaser, A.; Bauer, R. Pilot-plant treatment of olive mill wastewater (OMW) by solar TiO2 photocatalysis and solar photo-Fenton. Sol. Energy 2004, 77, 567–572. [Google Scholar] [CrossRef]
- Nogueira, V.; Lopes, I.; Rocha-Santos, T.; Goncalves, F.; Duarte, A.; Pereira, R. Photocatalytic treatment of olive oil mill wastewater using TiO2 and Fe2O3 nanomaterials. Water Air Soil Pollut. 2016, 227. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharamceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.; Shaik, B.; Nolan, K.; Urell, K.; Oelgemoller, M.; Tobin, J.; Morrissey, A. Treatment options for wastewater effluents from pharmaceutical companies. Int. J. Environ. Sci. Technol. 2011, 8, 649–666. [Google Scholar] [CrossRef]
- Anheden, M.; Goswami, D.; Svedberg, G. photocatalytic treatment of wastewater from 5-Flurouracil manufacturing. Sol. Energy Eng. 1996, 118, 2–8. [Google Scholar] [CrossRef]
- Augugliaro, V.; Gracia-Lopez, E.; Loddo, V.; Malato, S.; Maldonado, I.; Marci, G.; Molinari, R.; Palmisano, L. Degradation of lincomycin in aqueous medium: Coupling of solar photocatalysis and membrane separation. Sol. Energy 2005, 79, 402–408. [Google Scholar] [CrossRef]
- Torbina, V.; Vodyankin, A.; Ivanchikova, I.; Kholdeeva, O.; Vodyankina, O. Support pretreatment effect on the catalytic properties and reusability of silica-supported Titania catalysts in 2,3,6-Trimethylphenol oxidation with Hydrogen peroxide. Kinet. Catal. 2015, 56, 369–374. [Google Scholar] [CrossRef]
- Tasbihi, M.; Ngah, C.; Aziz, N.; Mansor, A.; Abdullah, A.; Teong, L.; Mohamed, A. Lifetime and regeneration studies of various supported TiO2 photocatalysts for degradation of phenol under UV-C light in a batch reactor. Ind. Eng. Chem. Res. 2007, 46, 9006–9014. [Google Scholar] [CrossRef]
- Zainudin, N.; Abdullah, A.; Mohamed, A. Development of supported TiO2 photocatalyst based on adsorbent for photocatalytic degradation of phenol. In Proceedings of the International Conference on Environment 2008, Penang, Malaysia, 15–17 December 2008.
- Zulfakar, M.; Hairul, N.; Akmal, H.; Rahman, M.A. Photocatalytic degradation of phenol in a fluidized bed reactor utilizing immobilized TiO2 photocatalyst: Characterization and process studies. J. Appl. Sci. 2011, 11, 2320–2326. [Google Scholar] [CrossRef]
- Haarstrick, A.; Kut, O.; Heinzle, E. TiO2-assissted degradation of environmentally relevant organic compounds in wastewater using a novel fluidized bed photreactor. Environ. Sci. Technol. 1996, 30, 817–824. [Google Scholar] [CrossRef]
- Sivlim, T.; Akkan, S.; Altin, I.; Koc, M.; Sokmen, M. TiO2 immobilized biodegradable polymer for photocatalytic removal of chlorophenol. Water Air Soil Pollut. 2012, 223, 3955–3964. [Google Scholar] [CrossRef]
- Seck, E.; Dona-Rodriguez, J.; Fernandez-Rodriguez, C.; Portillo-Carrizo, D.; Hernandez-Rodriguez, M.; Gonzalez-Diaz, O.; Perez-Pena, J. Solar photocatalytic removal of herbicides from real wastewater by using sol-gel synthesized nanocrystalline TiO2: Operational parameters optimization and toxicity studies. Sol. Energy 2013, 87, 150–157. [Google Scholar] [CrossRef]
- Jimenez, M.; Maldonado, M.; Rodriguez, E.; Hernandez-Ramirez, A.; Saggioro, E.; Carra, I.; Perez, J. Supported TiO2 solar photocatalysis at semi-pilot scale: Degradation of pesticides found in citrus processing industry wastewater, reactivity and influence of photogenerated species. J. Chem. Technol. Biotechnol. 2015, 90, 149–157. [Google Scholar] [CrossRef]
- Echavia, G.; Matzusawa, F.; Negishi, N. Photocatalytic degradation of organophosphate and phosphonoglycine pesticides using TiO2 immobilized on silica gel. Chemosphere 2009, 76, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Shavisi, Y.; Sharifnia, S.; Hosseini, S.; Khadivi, M. Application of TiO2/perlite photocatalysis for degradation of ammonia in wastewater. J. Ind. Eng. Chem. 2014, 20, 278–283. [Google Scholar] [CrossRef]
- Shavisi, Y.; Sharifnia, S.; Zendehzaban, M.; Mirghavami, M.; Kakehazar, S. Application of solar light for degradation of ammonia in petrochemical wastewater by a floating TiO2/LECA photocatalyst. J. Ind. Eng. Chem. 2014, 20, 2806–2813. [Google Scholar] [CrossRef]
- Singla, P.; Pandey, O.; Singh, K. Study of photocatalytic degradation of environmentally harmful phthalate esters using Ni-doped TiO2 nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 849–856. [Google Scholar] [CrossRef]
- Czech, B. Advanced photooxidation of surfactants in wastewater. Chemik 2012, 66, 1314–1325. [Google Scholar]
- Alrousan, D.; Polo-Lopez, M.; Dunlop, P.; Fernandez-Ibanez, P.; Byrne, J. Solar photocatalytic disinfection of water with immobilised titanium dioxide in re-circulating flow CPC reactors. Appl. Catal. B: Environ. 2012, 128, 126–134. [Google Scholar] [CrossRef]
- Nakano, K.; Obuchi, E.; Takagi, S.; Yamamoto, R.; Tanizaki, T.; Taketomi, M.; Eguchi, M.; Ichida, K.; Suzuki, M.; Hashimoto, A. Photocatlytic treatment of water containing dinitrophenol and city water over TiO2/SiO2. Sep. Purif. Technol. 2004, 34, 67–72. [Google Scholar] [CrossRef]
- Motegh, M.; Ommen, J.; Appel, P.; Kreutzer, M. Scale-up study of a multiphase photocatalytic reactor—Degradation of Cyanide in water over TiO2. Environ. Sci. Technol. 2014, 48, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Blanco, J.; Vidal, A.; Fernandez, P.; Caceres, J.; Trincado, P.; Oliveira, J.; Vincent, M. New large solar photocatalytic plant: Set-up and preliminary results. Chemosphere 2002, 47, 235–240. [Google Scholar] [CrossRef]
- Fostier, A.; Pereira, M.; Rath, S.; Guimaraes, J. Arsenic removal from water employing heterogeneous photocatalysis with TiO2 immobilized in PET bottles. Chemosphere 2008, 72, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Deedar, N.A.; Aslam, I. Evaluation of the adsorption potential of titanium dioxide nanoparticles for arsenic removal. J. Environ. Sci. 2009, 21, 402–408. [Google Scholar]
- Goswami, D. Engineering of Solar Photocatalytic Detoxification and Disinfection Processes. Adv. Sol. Energy 1995, 10, 165–210. [Google Scholar]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibanez, P. Solar photocatalysis: Materials, reactors, some commercial and pre-industeralized applications. A comprehensive approach. Appl. Catal. B: Environ. 2015, 170, 90–123. [Google Scholar] [CrossRef]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic water treatment by Titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Braham, R.; Harris, A. Review of Major Design and Scale-up Considerations for Solar Photocatalytic Reactors. Ind. Eng. Chem. Res. 2009, 48, 8890–8905. [Google Scholar] [CrossRef]
- Lee, S.; Mills, A. Detoxification of water by semiconductor photocatalysis. J. Ind. Eng. Chem. 2004, 10, 173–187. [Google Scholar]
- Malato, S.; Fernandez-Ibanez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Alfano, O.; Bahnemann, D.; Cassano, A.; Dillert, R.; Goslich, R. Photocatalysis in water environments using artificial and solar light. Catal. Today 2000, 58, 199–230. [Google Scholar] [CrossRef]
- Bahnemann, D. Photocatalytic water treatment: Solar energy applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Adesina, A. Industrial exploitation of photocatalysis: Progress, perspectives and prospects. Catal. Surv. Asia 2004, 8, 265–273. [Google Scholar] [CrossRef]
- Blanco-Galvez, J.; Fernandez-Ibanez, P.; Malato-Rodriguez, S. Solar photocatalytic detoxification and disinfection of water: Recent overview. Sol. Energy Eng. 2007, 129, 4–15. [Google Scholar] [CrossRef]
- Pacheco, J.; Tyner, C. Enhancement of processes for solar photocatalytic detoxification of water. In Proceedings of the ASME Solar Energy Conference on Solar Engineering 1990, Miami, FL, USA, 1–4 April 1990; pp. 163–166.
- Mehos, M.; Turchi, C. Field testing solar photocatalytic detoxification on TCE-contaminated groundwater. Environ. Prog. 1993, 12, 194–199. [Google Scholar] [CrossRef]
- Minero, C.; pelizzeti, E.; Malato, S.; Blanco, J. Large solar plant photocatalytic water decontamination: Degradation of pentachlorophenol. Chemosphere 1993, 26, 2013–2119. [Google Scholar] [CrossRef]
- Goswami, D.; Klausner, J.; Mathur, G.; Martin, A.; Schanze, K.; Wyness, P.; Turchi, C.; Marchand, E. Solar photocatalytic treatment of groundwater at Tyndall AFB: Field test results. In Proceedings of the 1993 American solar energy society annual conference, Washigton, DC, USA, 1993.
- Bousseimi, L.; Geissen, S.; Schroeder, H. Textile wastewater treatment and reuse by solar catalysis: Results from a pilot plant in Tunisia. Water Sci. Technol. 2004, 49, 331–337. [Google Scholar]
- Dillert, R.; Vollmer, S.; Gross, E.; Schober, M.; Bahnemann, D. Solar-catalytic treatment of an industrial wastewater. Z. Phys. Chem. 1999, 213, 141–147. [Google Scholar] [CrossRef]
- Blanco, J.; Malato, S.; Fernandez, P.; Vidal, A.; Morales, A.; Trincado, P.; Oliveira, J.; Minero, C.; Musci, M.; Casalle, C.; et al. Compound parabolic concentrator technology development to commercial solar detoxification applications. Sol. Energy 1999, 67, 317–330. [Google Scholar] [CrossRef]
- Mozia, S.; Brozek, P.; Przepiorski, J.; Tryba, B.; Morawski, A. Immobilized TiO2 for phenol degradation in a pilot-scale photocatalytic reactor. J. Nanomater. 2012, 2012, 16. [Google Scholar] [CrossRef]
- Hendarsa, A.; Hermansyah, H.; Slamet. A novel photobiodegradation technology for hydrocarbon wastewater treatment. Avaliable online: http://www.ijens.org/Vol_13_I_01/130301-2525-IJET-IJENS.pdf (accessed on 9 September 2016).
- Silva, C.; Wang, W.; Faria, J. Photocatalytic and photochemical degradation of mono-, di- and tri-azo dyes in aqueous solution under UV irradiation. J. Photochem. Photobiol. 2006, 181, 314–324. [Google Scholar] [CrossRef]
- Serrano, B.; de Lasa, H. Photocatalytic Degradation of Water Organic Pollutants. Kinetic Modeling and Energy Efficiency. Ind. Eng. Chem. Res. 1997, 36, 4705–4711. [Google Scholar] [CrossRef]
- Gaya, U.; Abdullah, A. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C: Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bolton, J.; Bircher, K.; Tumas, W.; Tolman, C. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Bandala, E.; Estrada, C. Comparison of Solar Collection Geometries for Application to Photocatalytic Degradation of Organic Contaminants. J. Sol. Energy Eng. 2005, 129, 22–26. [Google Scholar] [CrossRef]
- Rao, N.; Chaturvedi, V.; Puma, G. Novel pebble bed photocatalytic reactor for solar treatment of textile wastewater. Chem. Eng. J. 2012, 184, 90–97. [Google Scholar] [CrossRef]
- Shan, A.; Ghazi, T.; Rashid, S. Immobilization of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A: Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Chong, M.; Jin, B.; Chow, C.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Tokarsky, J.; Matejka, V.; Neuwirthova, L.; Vontorova, J.; Kutlakova, K.; Kukutschova, J.; Capkova, P. A low-cost photoactive composite quartz sand/TiO2. Chem. Eng. J. 2013, 222, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Prairie, M.; Pacheco, J.; Evans, L. Solar Detoxification of water Containing Chlorinated Solvents and Heavy Metals via TiO2 Photocatalysis. In Proceedings of the 1992 ASME International Solar Energy Conference on Solar Engineering, Maui, HI, USA, 4–8 April 1992; Volume 1, pp. 1–8.
- Malato, S.; Blanco, J.; Vidal, A.; Richter, C. Photocatalysis with solar energy at a pilot-plant scale: An overview. Appl. Catal. B: Environ. 2002, 37, 1–15. [Google Scholar] [CrossRef]
- De Lasa, H.; Serrano, B.; Salaices, M. Photocatalytic Reaction Engineering; Springer: New York, NY, USA, 2005. [Google Scholar]
- Bahnemann, D.; Dillert, R.; Dzengel, J.; Goslich, R.; Sagawe, G.; Schumacher, H.; Benz, V. Field Studies of Solar Water Detoxification Using Non Light Concentrating Reactors. J. Adv. Oxid. Technol. 1999, 4, 11–19. [Google Scholar]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Morales-Mejia, J.; Hernandez, P.; Santiago, L.; Almanza, R. Solar photocatalytic oxidation of Triclosan with TiO2 immobilized on volcanic porous stones on a CPC pilot scale reactor. Energy Procedia 2014, 57, 3014–3020. [Google Scholar] [CrossRef]
- Navntoft, C.; Araujo, P.; Litter, M.; Apella, M.; Fernandez, D.; Puchulu, M.; Hidalgo, M.; Blesa, M. Field tests of the solar water detoxification SOLWATER reactor in Los Pereyra, Tucuman, Argentina. J. Sol. Energy Eng. 2007, 129, 127–134. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suarez, S.; sanchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal B: Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Crittenden, J.; Zhang, Y.; Hand, D.; Perram, D.; Marchand, E. Solar detoxification of fuel-contaminated groundwater using fixed-bed photocatalysts. Water Environ. Res. 1996, 68, 270–278. [Google Scholar] [CrossRef]
- Oberg, V.; Goswami, D.; Svedberg, G. On Photocatalytic Detoxification of Water Containing Volatile Organic Compounds. In Proceedings of the 1994 ASME International Solar Engineering Conference, San Francico, CA, USA, 27–30 March 1994; pp. 147–153.
- Vargas, R.; Nunez, O. Photocatalytic degradation of oil industry hydrocarbon models at alboratory and at pilot-plant scale. Sol. Energy 2010, 84, 345–351. [Google Scholar] [CrossRef]
- Wyness, P.; Klausner, J.; Goswami, D.; Schanze, K. Performance of nonconcentrating solar photocatalytic oxidation reactors, Part II: Shallow pond configuration. J. Sol. Energy Eng. 1994, 116, 8–13. [Google Scholar] [CrossRef]
- Kanki, T.; Hamasaki, S.; Sano, N.; Toyoda, A.; Hirano, K. Water purification in a fluidized bed photocatalytic reactor using TiO2-coated ceramic particles. Chem. Eng. J. 2005, 108, 155–160. [Google Scholar] [CrossRef]
- Keshmiri, M.; Mohseni, M.; Troczynski, T. Development of novel TiO2 sol-gel-derived composite and its photocatalytic activities for trichloroethylene oxidation. Appl. Catal. B: Envieon. 2004, 53, 209–219. [Google Scholar] [CrossRef]
- Van Well, M.; Dillert, R.; Bahnemann, D.; Benz, V.; Mueller, M. A novel nonconcentrating reactor for solar water detoxification. J. Sol. Energy Eng. 1997, 119, 114–119. [Google Scholar] [CrossRef]
- Dillert, R.; Cassano, A.; Goslich, R.; Bahnemann, D. Large scale studies in solar catalytic wastewater treatment. Catal. Today 1999, 54, 267–282. [Google Scholar] [CrossRef]
- Plantard, G.; Janin, T.; Goetz, V.; Brosillon, S. Solar photocatalysis treatment of phytosanitry refuses: Efficiency of industrial photocatalysts. Appl. Catal B: Environ. 2012, 115, 38–44. [Google Scholar] [CrossRef]
- Wyness, P.; Klausner, J.; Goswami, D.; Schanze, K. Performance of nonconcentrating solar photocatalytic oxidation reactors, Part I: Flat-plate configuration. J. Sol. Energy Eng. 1994, 116, 2–7. [Google Scholar] [CrossRef]
- Zayani, G.; Bousselmi, L.; Mhenni, F.; Ghrabi, A. Solar photocatalytic degradation of commercial textile azo dye: Performance of pilot scale thin film fixed-bed reactor. Desalination 2009, 246, 344–352. [Google Scholar] [CrossRef]
- Bekbolet, M.; Linder, M.; Weichgrebe, D.; Bahnemann, D. Photocatalytic detoxification with the thin-film fixed-bed reactor (TFFBR): Clean-up of highly polluted landfill effluents using a novel TiO2 photocatalyst. Sol. Energy 1996, 56, 455–469. [Google Scholar] [CrossRef]
- Bockelmann, D.; Weichgrebe, D.; Goslisch, R.; Bahnemann, D. Concentrating versus non-concentrating reactors for solar water detoxification. Sol. Energy Mater. Sol. Cells 1995, 38, 441–451. [Google Scholar] [CrossRef]
- Chan, A.H.C.; Chan, C.K.; Barford, J.P.; Porter, J.F. Solar photocatalytic thin film cascade reactor for treatment of benzoic acid cotaining wastewater. Water Res. 2003, 37, 1125–1135. [Google Scholar] [CrossRef]
- Chan, A.; Porter, J.; Barford, J.; Chan, C. Photocatalytic thin film cascade reactor for treatment of organic compounds in wastewater. Water Sci. Technol. 2001, 44, 187–195. [Google Scholar] [PubMed]
- Thu, H.B.; Karkmaz, M.; Puzenat, E.; Guillard, C.; Herrmann, J.M. From the fundamentals of photocatalysis to its applications in environment protection and in solar purification of water in arid countries. Res. Chem. Intermed. 2005, 31, 449–461. [Google Scholar] [CrossRef]
- Shama, G.; Peppiatt, C.; Biguzzi, M. A Novel thin film photoreactor. J. Chem. Technol. Biotechnol. 1996, 65, 56–64. [Google Scholar] [CrossRef]
- Puma, G. Dimensionless analysis of photocatalytic reactors using suspended solid photocatalysts. Chem. Eng. Res. Des. 2005, 83, 820–826. [Google Scholar] [CrossRef]
- Puma, G.L.; Yue, P.L. A novel fountain photocatalytic reactor: Model development and experimental validation. Chem. Eng. Sci. 2001, 56, 2733–2744. [Google Scholar] [CrossRef]
- Puma, G.L.; Yue, P.L. A novel fountain photocatalytic reactor for water treatment and purification; modeling and design. Ind. Eng. Chem. Res. 2001, 40, 5162–5169. [Google Scholar] [CrossRef]
- Kamble, S.; Sawant, S.; Pangarkar, V. Novel Solar-based Photocatalytic Reactor for Degradation of Refractory Pollutants. AIChE J. 2004, 50, 1647–1650. [Google Scholar] [CrossRef]
- Otalvaro-Martin, H.; Mueses, M.; Machuca-Martinez, F. Boundary layer of photon absorption applied to heterogeneous photocatalytic solar flat plate reactor design. Int. J. Photoenergy 2014, 2014, 930439. [Google Scholar]
- Vaiano, V.; Sacco, O.; Pisano, D.; Sannino, D.; Ciambelli, P. From the design to the development of a continuous fixed bed photoreactor for photocatalytic degradation of organic pollutants in wastewater. Chem. Eng. Sci. 2015, 137, 152–160. [Google Scholar] [CrossRef]
- Rao, N.; Chaturvedi, V. Photoactivity of TiO2-coated pebbles. Ind. Eng. Chem. Res. 2007, 46, 4406–4414. [Google Scholar] [CrossRef]
- Matthews, R. Photooxidative degradation of coloured organics in water using supported catalysts TiO2 on Sand. Water Res. 1991, 25, 1169–1176. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghasemi, M.; Rafsanjani, H. Study of differrent parameters in TiO2 nanoparticles formation. J. mater. Sci. Eng. 2011, 5, 87–93. [Google Scholar]
- Hanaor, D.; Sorrell, C. Sand supported mixed-phase TiO2 photocatalysts for water decontamination applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar] [CrossRef]
- Remoundaki, E.; Vidali, R.; Kousi, P.; Hatzikioseyian, A.; Tsezos, M. Photolytic and photocatalytic alterations of humic substances in UV (254 nm) and solar concentric parabolic concentrator (CPC) reactors. Desalination 2009, 248, 843–851. [Google Scholar] [CrossRef]










| Reactor Type | Treated Water | Pollutant | Treated Volume | Erection Year | Location, Country | Ref. |
|---|---|---|---|---|---|---|
| PTR a | Deionized water | Salycilic acid | 1100 L | 1989 | Albuquerque, NM, USA | [67] |
| PTR | Contaminated groundwater | Trichloroethylene | 15 L/min | 1990 | Lawrence Livermore National Laboratories (LLNL), Livermore, CA, USA | [68] |
| PTR | Industrial wastewater | Pentachlorophenol | 837 L | 1991 | Platforma Solar de Almeria (PSA), Spain | [69] |
| Tubular | Pretreated groundwater | BTEX b | 530 L | 1993 | Tyndall Air Force Base, FL, USA | [70] |
| CPC c | Deionized water | Cyanide | 247 L | 1996 | Platforma Solar de Almeria (PSA), Spain | [54] |
| Fixed-bed thin-film | Textile wastewater | TOC d | 1000 L | 1998 | Menzel Temimi, Tunsia | [71] |
| DSSR e | Biologically pretreated industrial wastewater | TOC | 500 L | 1998 | Volkswagen factory, Wolfsburg, Germany | [72] |
| CPC | Raw water | Cyanide | 1000 L | 1999 | Hidrocen, Madrid, Spain | [73] |
| Packed Tubular | City water | Aromatic organics | 1000 L/day | 2004 | Kitakyushu City, Japan | [52] |
| Shallow tank | Tap water | Phenol | 1350 L | 2011 | Szczecin, Poland | [74] |
| Open tank | Oil and gas wastewater | Oil, phenol and ammonia | 4000 L | 2011 | Grati, East Java,, Indonesia | [75] |
| Reactor Type | Pollutant and Concentration | Treated Volume/Flow Rate | Water Matrix | TiO2 conc a | UV-A (W/m2) | Destruction Percent (%) | Reactor Footprint (m2) | Treatment Duration | Throughput (L/h/m2) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PTR | Salicylic Acid | 1100 L | Deionized water | 1 g/L | NA | 96 | 465 | 40 min | 3.54 | [67] |
| 30 mg/L | ||||||||||
| PTR | Trichloroethylene | 15 L/min | Filtered groundwater | 1 g/L | NA | 90 | 154 | Single-pass mode | 5.82 | [68] |
| 0.1 mg/L | ||||||||||
| PTR | Pentachlorophenol | 838 L | Deionized water | 1 g/L | 24–30 | 100 | 384 | 20 min | 6.55 | [69] |
| 10 mg/L | ||||||||||
| PTR | COD | 3.5 L | Diluted paper mill effluent | 0.75 g/L | 35–45 | 70.5 | 3.7 | 3 h | 0.32 | [19] |
| 2075 mg/L | ||||||||||
| CPC | Cyanide, 50 mg/L | 1000 L | Tap water | 0.2 g/L | 30 | 76 | 100 | 2 h | 5.16 | [54] |
| DCA b, 50 mg/L | 100 | 1 h | 10.58 | |||||||
| CPC | Cyanide | 247 L | Deionized water | 0.2 g/L | 30 | 100 | 8.9 | 1.5 h | 18.72 | [54] |
| 50 mg/L | ||||||||||
| CPC | Dichlorophenoxyacetic acid, 58 mg/L | 5 L | water containing herbicides | 1 g/L | 30 | 80 | 0.248 | 1.3 h c | 15.05 | [44] |
| Bentazon, 32 mg/L | 100 | |||||||||
| CPC | DCA | 39 L | Deionized water | 0.2 g/L | 30 | 100 d | 3 | 0.5 h | 21.97 | [54] |
| 50 mg/L | ||||||||||
| CPC | DOC | 35 L | Synthetic wastewater | 0.2 g/L | 30 | 18 e | 3.08 | 5.5 h | 2.06 | [89] |
| 200 mg/L | ||||||||||
| CPC | COD | 24 L | Real cardboard industry effluent | 2 g/L | NA | 38 | 2.15 | 7 h | 1.59 | [21] |
| 11,000 mg/L | ||||||||||
| CPC | COD | 30 L | Olive oil mill effluent | 1 g/L | NA | 12 f | 3.08 | 32 h | 0.3 | [32] |
| 89,000 mg/L | ||||||||||
| Tubular | BTEX | 530 L | Filtered groundwater | 1 g/L g | 45.3 | 75 | 37.2 | 3 h | 4.74 | [70] |
| 2 mg/L | ||||||||||
| Tubular | Naphthalene (15 mg/L) & Dibenzo-thiophene (1.2 mg/L) mixture | 15 L | Deionized water | 1.5 g/L | 30 | 92 | NA | 21 min | Could not be calculated | [95] |
| Shallow pond | Chlorophenol | 28.8 L | Tap water | 3 g/L | 31.4 | 88 | 0.57 | 2 h | 25.3 | [96] |
| 9 mg/L | ||||||||||
| DSSR | Dichloroacetic acid | 25 L | Deionized water | 7.5 g/L | NA | 100 | 1.37 | 3.5 h | 5.21 | [99] |
| 50 mg/L | ||||||||||
| DSSR | TOC | 500 L | Biologically treated wastewater | 5 g/L | 13.3 | 40 | 27.6 | 5.5 h | 3.36 | [100] |
| 16.7 mg/L | ||||||||||
| Flat plate reactor | Chlorophenol | 56.8 L | Deionized water | 1 g/L | 37.5 | 60 | 2.73 | 2.5 h | 11.1 | [102] |
| 12.85 mg/L | ||||||||||
| reverse flow flat plate | Pyrimethanil | 15 L | Deionized water | 1.5 g/L | 30 | 100 | 0.3 | 10.37 h | 4.82 | [101] |
| 23 mg/L | ||||||||||
| Fountain reactor | Salicylic acid | 12 L/h | Deionized water | 1 g/L | 68.5 | 20 | 0.64 | Continuous flow | 18.75 | [111] |
| 20 mg/L | ||||||||||
| Slurry bubble column reactor | nitrobenzene | 19.5 L | Deionized water | 3 g/L | NA | 70 | NA | 4 h | Could not be calculated h | [113] |
| chlorobenzene | 0.15 g/L | 96 | 4 h | |||||||
| phenol | 2 g/L | 25 | 6 h | |||||||
| 100 mg/L each |
| Reactor Type | Pollutant and Concentration | Volume/ Flowrate | Water Matrix | Support | UV-A W/m2 | Destruction Percent (%) | Reactor Footprint m2 | Treatment Duration | Throughput L/h/m2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| One packed tube | BTEX | 2.4 L/h | pretreated groundwater | Silica gel | 3.2 | 100 | 0.375 a | Single-pass mode | 6.4 | [93] |
| 2 mg/L | ||||||||||
| Packed CPC | Triclosan | 21 L | Water-ethanol solution | porous stones | 30 | 50.5 | 1.71 | 2 h | 6.14 | [92] |
| 12 mg/L | ||||||||||
| Packed CPC | Humic acids | 50 L | Deionized water | Ahlstrom paper | NA | 100 | 1 | 12 h | 4.16 | [120] |
| 5 mg/L | ||||||||||
| Packed CPC | Amazil | 8 L | Secondary treated municipal WW | Glass spheres | 30 | 70 | 0.25 | 13.3 h b | 2.41 | [45] |
| Acetamiprid | 10 | |||||||||
| thiabendazole | 10 | |||||||||
| Packed CPC | 15 emerging contaminant | 10 L | Secondary treated municipal WW | Glass spheres | 30 | 90 c | 0.3 | 2.3 h | 14.7 | [92] |
| 0.1 mg/L each | ||||||||||
| Packed CPC (Solwater) | F.coliforms | 20 L | Ground water | Ahlstrom paper | 30 | 100 | 1 d | 6 h | 4.98 | [91] |
| Enterococcus faccalis | ||||||||||
| Packed Tubular | TOC | 100 L /h e | City water | Silica beads | NA | Variable through 3 month | NA | Single-pass mode | Could not be calculated | [52] |
| TFFBR | TOC | 1.5 L/h | pretreated landfill leachate | reactor surface | 100 | 52 | 0.7 | Single-pass mode | 2.16 | [104] |
| 66 mg/L | ||||||||||
| TFFBR pilot plant | dyes | 730 L | Well water | reactor surface | NA | 47 | 25 | 8 h | 3.65 | [103] |
| 44.8 mg/L | ||||||||||
| Fluidized bed shallow tank | Phenol | 1 L | Deionized water | ceramic carriers | 30 | 100 | 0.0375 | 13 h | 2.05 | [97] |
| Bisphenol A | ||||||||||
| 10 mg/L each | 100 | 12 h | 2.22 | |||||||
| Floating bed shallow tank | Ammonia | 5 L | Petrochemical plant effluent | Light expanded clay aggregate | 35–45 | 59 | 0.071 | 21 h f | 3.35 | [47] |
| 975 mg/L | ||||||||||
| Shallow tank | Phenol | 1350 L | Tap water | Photospheres | 330 | 50 | 1.8 | 15 h | 50 | [74] |
| Steel mesh | 47 | |||||||||
| 25 mg/L | ||||||||||
| Filtercloth | 80 | |||||||||
| Thin film Cascade | Benzoic acid | 7 L | Deionized water | reactor plates | 17.4 | 30 | 1.68 | 3 h | 1.39 | [106] |
| 100 mg/L | ||||||||||
| Step reactor | Pesticides mixture | 25 L | Tap water | Ahlstrom paper | 30 | 80 | 1 | 4.5 h | 5.56 | [108] |
| 8 mg/L | ||||||||||
| Pebble bed | dyes | 10 L | Simulated dyehouse effluent | pebbles | 18.9 | 15 | 0.234 | 5 h | 8.52 | [81] |
| 83 mg/L | ||||||||||
| Flat packed bed | E-coli | 1 L | Deionized water | quartz sand | 3.7 | No complete sterilization | 0.07 | 1.6 h | 8.57 | [119] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 Solar Photocatalytic Reactor Systems: Selection of Reactor Design for Scale-up and Commercialization—Analytical Review. Catalysts 2016, 6, 138. https://doi.org/10.3390/catal6090138
Abdel-Maksoud Y, Imam E, Ramadan A. TiO2 Solar Photocatalytic Reactor Systems: Selection of Reactor Design for Scale-up and Commercialization—Analytical Review. Catalysts. 2016; 6(9):138. https://doi.org/10.3390/catal6090138
Chicago/Turabian StyleAbdel-Maksoud, Yasmine, Emad Imam, and Adham Ramadan. 2016. "TiO2 Solar Photocatalytic Reactor Systems: Selection of Reactor Design for Scale-up and Commercialization—Analytical Review" Catalysts 6, no. 9: 138. https://doi.org/10.3390/catal6090138