Response Surface Methodology and Aspen Plus Integration for the Simulation of the Catalytic Steam Reforming of Ethanol
Abstract
:1. Introduction
2. Results and Discussion
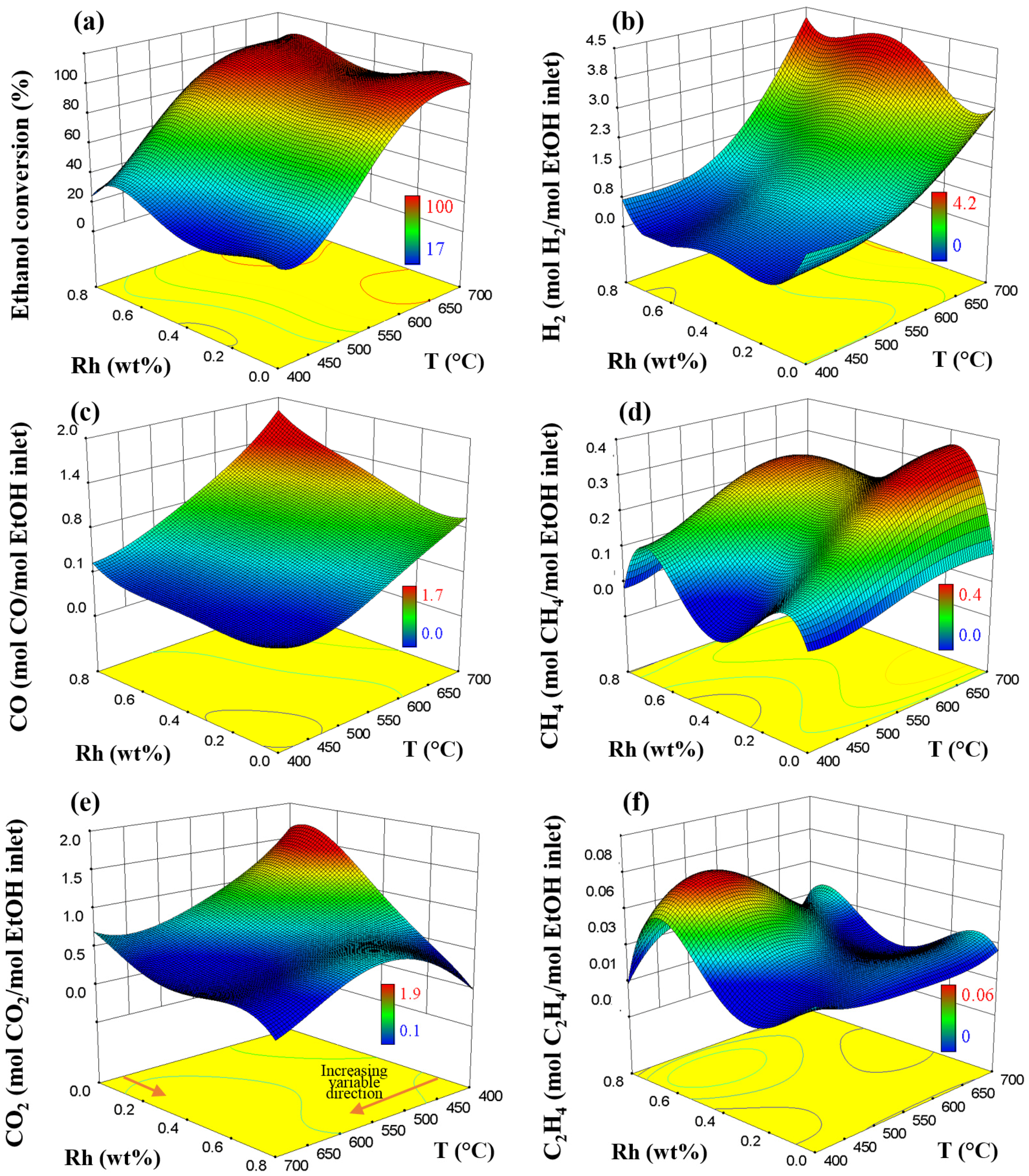
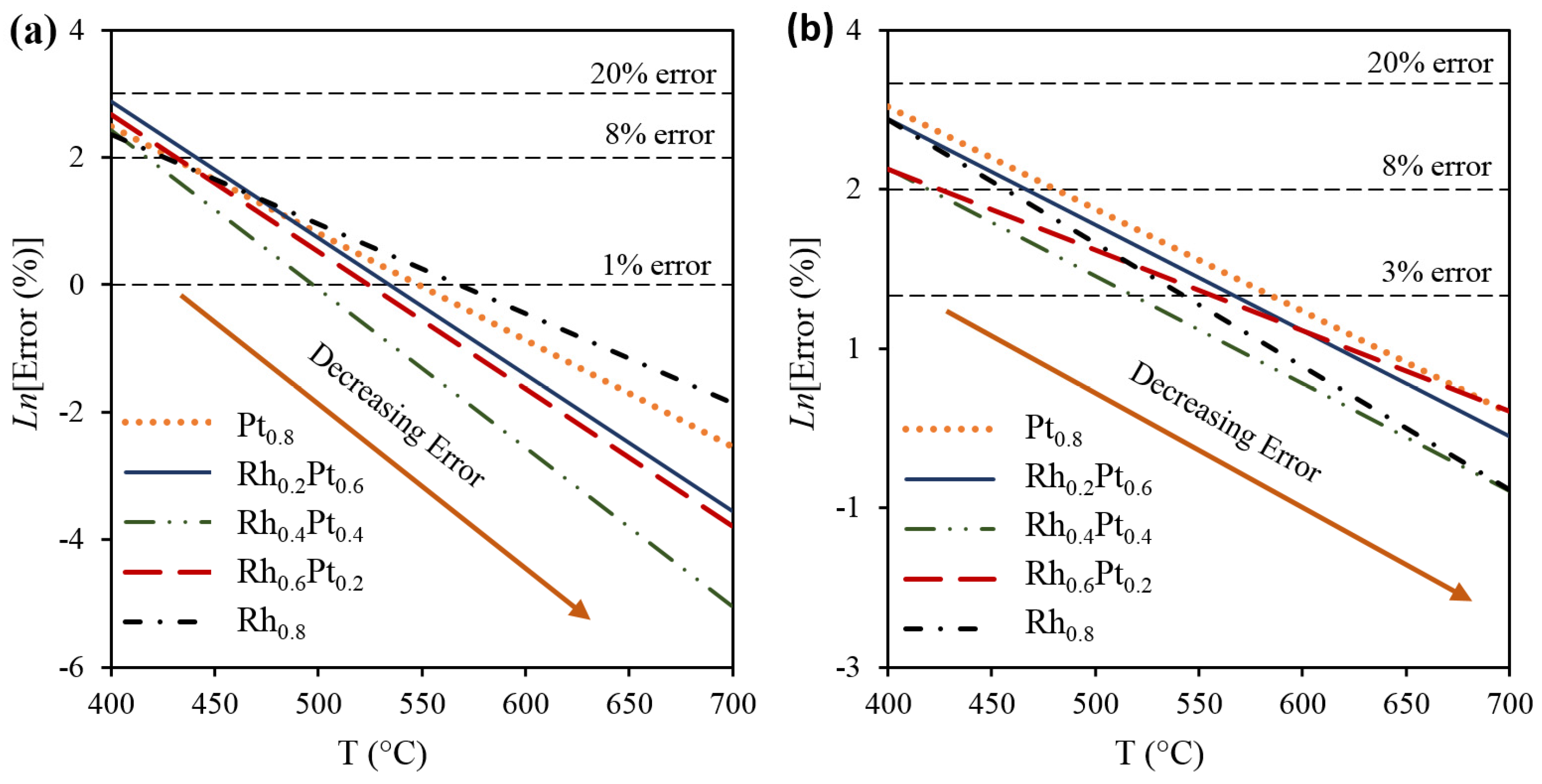
2.1. Effect of the Rh–Pt Ratio on the Catalytic Performance
2.2. Models of the SRE from the RSM
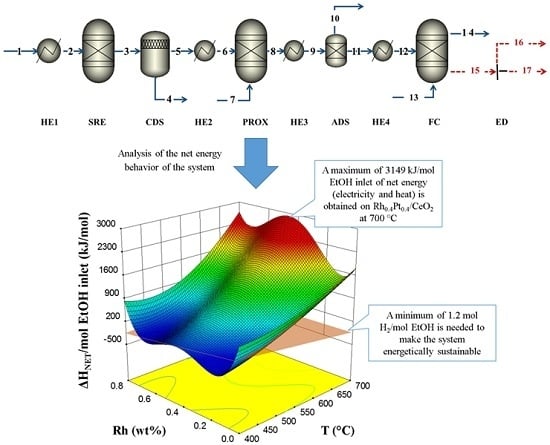
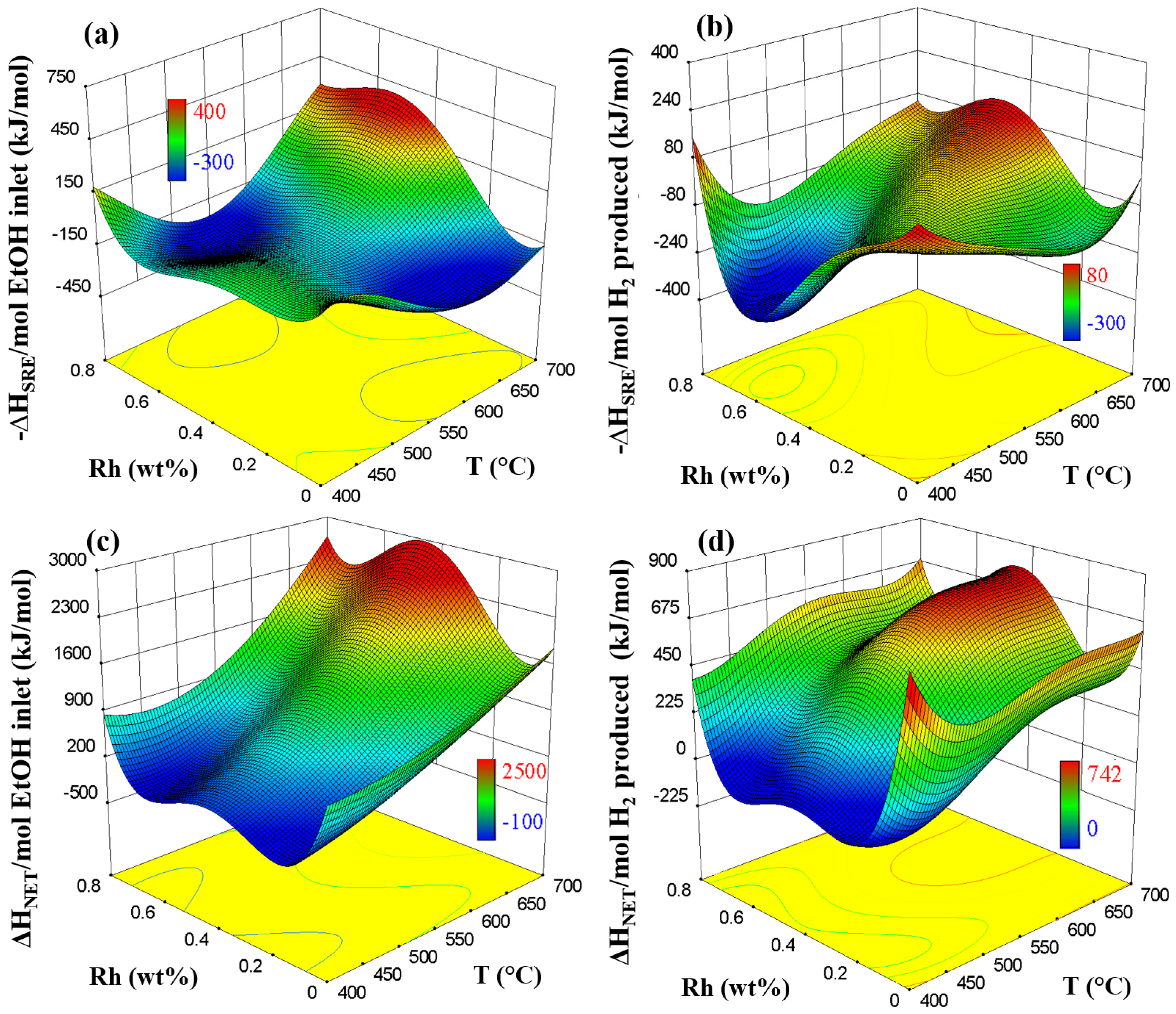
2.3. Energy Analysis from RSM and Aspen Plus Integration
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalytic Tests
3.3. Statistical Analysis
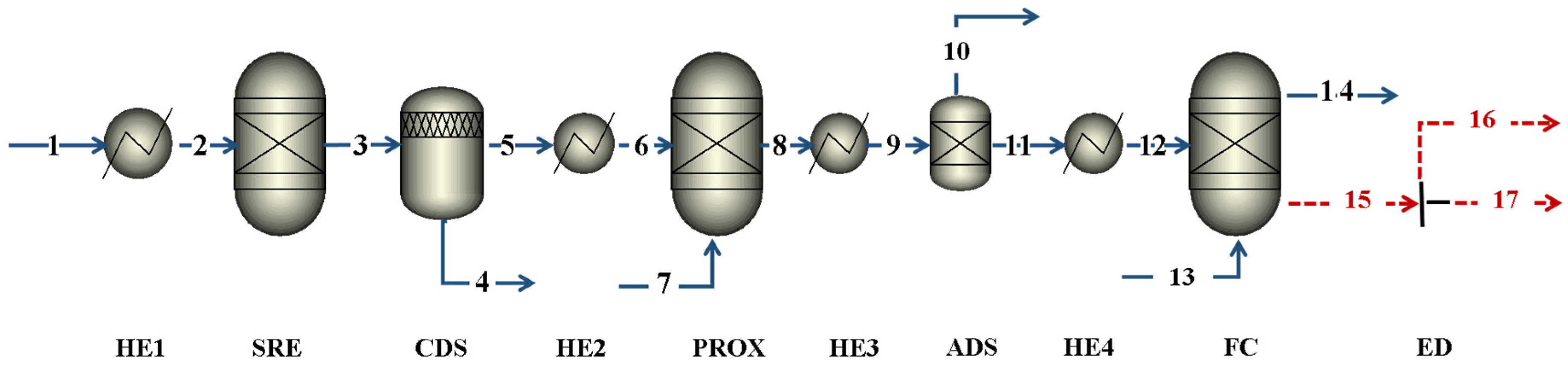
3.4. Simulation in Aspen Plus Software
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Rh Content (wt %) | T (°C) | Carbon Balance (%) | Yield (mol/mol EtOH Inlet) | Ethanol Conversion (%) | ||||
|---|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | Ethylene | ||||
| 0.0 | 700 | 93 | 3.11 | 1.09 | 0.13 | 0.64 | 3.0 × 10−6 | 100 |
| 0.0 | 680 | 96 | 2.84 | 0.92 | 0.16 | 0.85 | 2.9 × 105 | 100 |
| 0.0 | 660 | 91 | 2.52 | 0.98 | 0.19 | 0.65 | 5.0 × 105 | 100 |
| 0.0 | 640 | 94 | 2.19 | 0.72 | 0.32 | 0.84 | 2.4 × 10−4 | 100 |
| 0.0 | 620 | 92 | 1.96 | 0.82 | 0.18 | 0.83 | 1.1 × 105 | 99.3 |
| 0.0 | 600 | 92 | 1.66 | 0.78 | 0.01 | 0.85 | 2.4 × 10−4 | 90.5 |
| 0.0 | 580 | 97 | 1.60 | 0.65 | 0.03 | 0.91 | 1.6 × 10−4 | 82.8 |
| 0.0 | 560 | 99 | 1.28 | 0.36 | 0.08 | 1.08 | 4.5 × 10−4 | 76.9 |
| 0.0 | 540 | 95 | 1.26 | 0.30 | 0.06 | 0.96 | 3.3 × 105 | 71.1 |
| 0.0 | 520 | 96 | 1.19 | 0.10 | 0.09 | 1.09 | 5.0 × 105 | 68.1 |
| 0.0 | 500 | 107 | 1.11 | 0.09 | 0.02 | 1.02 | 9.7 × 105 | 49.7 |
| 0.0 | 480 | 109 | 1.13 | 0.07 | 0.04 | 0.82 | 3.5 × 10−4 | 36.8 |
| 0.0 | 460 | 109 | 1.13 | 0.12 | 0.00 | 0.82 | 5.3 × 10−4 | 37.7 |
| 0.0 | 440 | 106 | 1.15 | 0.01 | 0.00 | 0.81 | 3.7 × 105 | 35.5 |
| 0.0 | 420 | 107 | 1.15 | 0.00 | 0.00 | 0.73 | 4.0 × 10−4 | 29.8 |
| 0.0 | 400 | 108 | 0.96 | 0.00 | 0.00 | 0.81 | 6.6 × 10−3 | 33.7 |
| 0.2 | 700 | 97 | 3.15 | 0.97 | 0.31 | 0.65 | 2.1 × 10−4 | 100 |
| 0.2 | 680 | 93 | 2.87 | 0.78 | 0.44 | 0.63 | 1.1 × 10−4 | 100 |
| 0.2 | 660 | 92 | 2.54 | 0.84 | 0.46 | 0.55 | 2.9 × 10−4 | 100 |
| 0.2 | 640 | 92 | 2.28 | 0.84 | 0.38 | 0.63 | 2.1 × 10−4 | 100 |
| 0.2 | 620 | 95 | 1.80 | 0.86 | 0.45 | 0.60 | 2.2 × 10−4 | 100 |
| 0.2 | 600 | 89 | 1.49 | 0.80 | 0.35 | 0.61 | 0 | 99.1 |
| 0.2 | 580 | 94 | 1.29 | 0.74 | 0.27 | 0.82 | 7.7 × 105 | 97.4 |
| 0.2 | 560 | 89 | 1.18 | 0.39 | 0.43 | 0.59 | 0 | 81.6 |
| 0.2 | 540 | 93 | 1.02 | 0.32 | 0.25 | 0.60 | 1.0 × 10−4 | 65.4 |
| 0.2 | 520 | 91 | 0.53 | 0.13 | 0.24 | 0.59 | 1.3 × 10−4 | 57.0 |
| 0.2 | 500 | 96 | 0.46 | 0.10 | 0.15 | 0.71 | 1.5 × 10−4 | 51.8 |
| 0.2 | 480 | 104 | 0.35 | 0.06 | 0.06 | 0.89 | 7.0 × 10−4 | 46.6 |
| 0.2 | 460 | 110 | 0.31 | 0.02 | 0.04 | 1.02 | 1.3 × 10−3 | 44.0 |
| 0.2 | 440 | 102 | 0.15 | 0.01 | 0.05 | 0.51 | 2.3 × 10−3 | 26.6 |
| 0.2 | 420 | 105 | 0.18 | 0.01 | 0.04 | 0.80 | 1.9 × 10−3 | 37.7 |
| 0.2 | 400 | 108 | 0.10 | 0.01 | 0.02 | 0.90 | 7.2 × 105 | 39.0 |
| 0.4 | 700 | 96 | 4.10 | 1.34 | 0.26 | 0.31 | 7.7 × 105 | 100 |
| 0.4 | 680 | 100 | 3.81 | 1.25 | 0.25 | 0.50 | 6.0 × 105 | 100 |
| 0.4 | 660 | 96 | 3.32 | 1.24 | 0.26 | 0.42 | 8.2 × 105 | 100 |
| 0.4 | 640 | 90 | 2.90 | 0.89 | 0.41 | 0.50 | 1.0 × 10−4 | 100 |
| 0.4 | 620 | 91 | 2.68 | 0.98 | 0.50 | 0.34 | 0 | 100 |
| 0.4 | 600 | 79 | 2.38 | 0.95 | 0.18 | 0.43 | 4.5 × 10−3 | 100 |
| 0.4 | 580 | 93 | 2.08 | 0.94 | 0.17 | 0.75 | 4.5 × 10−3 | 100 |
| 0.4 | 560 | 92 | 1.54 | 0.65 | 0.30 | 0.59 | 1.2 × 10−2 | 85,8 |
| 0.4 | 540 | 96 | 0.91 | 0.50 | 0.21 | 0.68 | 1.2 × 10−2 | 75.3 |
| 0.4 | 520 | 93 | 0.90 | 0.47 | 0.09 | 0.65 | 1.2 × 10−2 | 68.5 |
| 0.4 | 500 | 103 | 0.81 | 0.22 | 0.07 | 0.69 | 1.2 × 10−2 | 47.4 |
| 0.4 | 480 | 108 | 0.65 | 0.10 | 0.11 | 0.75 | 1.1 × 10−2 | 41.6 |
| 0.4 | 460 | 104 | 0.48 | 0.01 | 0.00 | 0.69 | 2.1 × 10−3 | 31.0 |
| 0.4 | 440 | 107 | 0.37 | 0.01 | 0.00 | 0.75 | 2.2 × 10−4 | 31.3 |
| 0.4 | 420 | 110 | 0.34 | 0.02 | 0.00 | 0.66 | 1.1 × 10−4 | 23.5 |
| 0.4 | 400 | 109 | 0.32 | 0.02 | 0.00 | 0.66 | 2.2 × 10−7 | 24.4 |
| 0.6 | 700 | 90 | 4.12 | 1.48 | 0.19 | 0.13 | 5.6 × 10−6 | 100 |
| 0.6 | 680 | 100 | 3.40 | 1.28 | 0.22 | 0.50 | 2.4 × 10−4 | 100 |
| 0.6 | 660 | 93 | 3.02 | 1.28 | 0.32 | 0.27 | 4.9 × 10−4 | 100 |
| 0.6 | 640 | 93 | 2.41 | 0.96 | 0.28 | 0.61 | 4.8 × 10−4 | 99.7 |
| 0.6 | 620 | 93 | 1.96 | 0.85 | 0.35 | 0.66 | 1.0 × 10−3 | 100 |
| 0.6 | 600 | 92 | 1.67 | 0.94 | 0.29 | 0.61 | 5.2 × 10−4 | 99.5 |
| 0.6 | 580 | 91 | 1.49 | 0.84 | 0.26 | 0.71 | 4.3 × 10−4 | 99.4 |
| 0.6 | 560 | 94 | 1.24 | 0.83 | 0.43 | 0.62 | 8.6 × 105 | 100 |
| 0.6 | 540 | 100 | 0.95 | 0.74 | 0.27 | 0.73 | 0.13 | 100 |
| 0.6 | 520 | 93 | 0.72 | 0.69 | 0.17 | 0.75 | 7.7 × 10−2 | 94.9 |
| 0.6 | 500 | 94 | 0.43 | 0.51 | 0.20 | 0.90 | 7.8 × 10−3 | 87.4 |
| 0.6 | 480 | 107 | 0.20 | 0.19 | 0.19 | 0.89 | 0.13 | 70.1 |
| 0.6 | 460 | 101 | 0.18 | 0.18 | 0.12 | 0.59 | 7.7 × 10−2 | 52.0 |
| 0.6 | 440 | 92 | 0.13 | 0.12 | 0.13 | 0.44 | 7.8 × 10−3 | 44.0 |
| 0.6 | 420 | 93 | 0.09 | 0.09 | 0.00 | 0.37 | 3.8 × 10−2 | 33.7 |
| 0.6 | 400 | 90 | 0.03 | 0.03 | 0.04 | 0.29 | 1.2 × 10−2 | 29.1 |
| 0.8 | 700 | 98 | 4.07 | 1.71 | 0.04 | 0.19 | 4.5 × 10−3 | 100 |
| 0.8 | 680 | 95 | 3.93 | 1.65 | 0.05 | 0.19 | 2.4 × 105 | 100 |
| 0.8 | 660 | 95 | 3.34 | 1.57 | 0.07 | 0.25 | 3.7 × 10−4 | 99.8 |
| 0.8 | 640 | 91 | 2.65 | 1.42 | 0.08 | 0.33 | 4.8 × 10−4 | 99.6 |
| 0.8 | 620 | 89 | 2.23 | 1.25 | 0.09 | 0.43 | 3.1 × 10−4 | 99.2 |
| 0.8 | 600 | 97 | 1.89 | 1.31 | 0.09 | 0.41 | 1.1 × 105 | 93.7 |
| 0.8 | 580 | 89 | 1.39 | 0.85 | 0.11 | 0.61 | 1.8 × 10−3 | 89.3 |
| 0.8 | 560 | 100 | 1.08 | 0.80 | 0.16 | 0.62 | 3.5 × 10−3 | 79.2 |
| 0.8 | 540 | 104 | 1.10 | 0.72 | 0.08 | 0.76 | 4.2 × 10−3 | 74.5 |
| 0.8 | 520 | 97 | 0.96 | 0.61 | 0.09 | 0.62 | 3.9 × 10−3 | 69.5 |
| 0.8 | 500 | 91 | 0.95 | 0.58 | 0.01 | 0.46 | 2.1 × 10−3 | 62.0 |
| 0.8 | 480 | 92 | 0.83 | 0.51 | 0.01 | 0.36 | 1.2 × 10−3 | 51.8 |
| 0.8 | 460 | 89 | 0.67 | 0.37 | 0.00 | 0.30 | 2.6 × 10−3 | 45.4 |
| 0.8 | 440 | 95 | 0.65 | 0.34 | 0.00 | 0.26 | 7.5 × 10−4 | 35.1 |
| 0.8 | 420 | 99 | 0.67 | 0.31 | 0.00 | 0.29 | 1.2 × 10−3 | 31.5 |
| 0.8 | 400 | 96 | 0.62 | 0.30 | 0.00 | 0.18 | 4.7 × 10−4 | 28.3 |
| Mean | 97 | - | - | - | - | - | - | |
| Standard deviation | 6.5 | - | - | - | - | - | - | |
References
- Rhodes, C.J. The 2015 Paris climate change conference: Cop21. Sci. Prog. 2016, 99, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Vajpai, S.; Patel, S.; Pant, K.K. Kinetic modeling of steam reforming of ethanol for the production of hydrogen over Co/Al2O3 catalyst. Chem. Eng. J. 2007, 125, 139–147. [Google Scholar] [CrossRef]
- Quintero, J.A.; Montoya, M.I.; Sánchez, O.J.; Giraldo, O.H.; Cardona, C.A. Fuel ethanol production from sugarcane and corn: Comparative analysis for a Colombian case. Energy 2008, 33, 385–399. [Google Scholar] [CrossRef]
- Elfasakhany, A. Investigations on the effects of ethanol–methanol–gasoline blends in a spark-ignition engine: Performance and emissions analysis. Eng. Sci. Technol. Int. J. 2015, 18, 713–719. [Google Scholar] [CrossRef]
- Chiu, W.-C.; Horng, R.-F.; Chou, H.-M. Hydrogen production from an ethanol reformer with energy saving approaches over various catalysts. Int. J. Hydrogen Energy 2013, 38, 2760–2769. [Google Scholar] [CrossRef]
- De Lima, S.; Da Cruz, I.; Jacobs, G.; Davis, B.; Mattos, L.; Noronha, F.B. Steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Pt/CeZrO2 catalyst. J. Catal. 2008, 257, 356–368. [Google Scholar] [CrossRef]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Rabenstein, G.; Hacker, V. Hydrogen for fuel cells from ethanol by steam-reforming, partial-oxidation and combined auto-thermal reforming: A thermodynamic analysis. J. Power Sources 2008, 185, 1293–1304. [Google Scholar] [CrossRef]
- Liberatori, J.W.C.; Ribeiro, R.U.; Zanchet, D.; Noronha, F.B.; Bueno, J.M.C. Steam reforming of ethanol on supported nickel catalysts. Appl. Catal. A 2007, 327, 197–204. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Remiro, A.; Bilbao, J.; Gayubo, A.G. Effect of calcination/reduction conditions of Ni/La2O3–αAl2O3 catalyst on its activity and stability for hydrogen production by steam reforming of raw bio-oil/ethanol. Appl. Catal. B 2014, 147, 402–410. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Yuan, M.; Xu, T.; Liu, Y. Steam reforming of ethanol over Ni/Ce0.7Pr0.3O2 catalyst. J. Rare Earths 2012, 30, 670–675. [Google Scholar] [CrossRef]
- Bilala, M.; Jackson, S.D. Ethanol steam reforming over Rh and Pt catalysts: Effect of temperature and catalyst deactivation. Catal. Sci. Technol 2013, 3, 754–766. [Google Scholar] [CrossRef]
- Guerrero, L.; Castilla, S.; Cobo, M. Advances in ethanol reforming for the production of hydrogen. Quim. Nova 2014, 37, 850–856. [Google Scholar] [CrossRef]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G. Hydrogen production by ethanol steam reforming (ESR) over CeO2 supported transition metal (Fe, Co, Ni, Cu) catalysts: Insight into the structure-activity relationship. Catalysts 2016, 6, 39. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Steam reforming of bio-ethanol to produce hydrogen over Co/CeO2 catalysts derived from Ce1−xCoxO2−y precursors. Catalysts 2016, 6, 26. [Google Scholar] [CrossRef]
- Domínguez, M.; Taboada, E.; Molins, E.; Llorca, J. Co-Fe-Si aerogel catalytic honeycombs for low temperature ethanol steam reforming. Catalysts 2012, 2, 386–399. [Google Scholar] [CrossRef]
- Sheng, P.Y.; Chiu, W.W.; Yee, A.; Morrison, S.J.; Idriss, H. Hydrogen production from ethanol over bimetallic Rh-M/CeO2 (M = Pd or Pt). Catal. Today 2007, 129, 313–321. [Google Scholar] [CrossRef]
- Sheng, P.-Y.; Yee, A.; Bowmaker, G.A.; Idriss, H. H2 production from ethanol over Rh–Pt/CeO2 catalysts: The role of Rh for the efficient dissociation of the carbon–carbon bond. J. Catal. 2002, 208, 393–403. [Google Scholar] [CrossRef]
- Jacobs, G.; Keogh, R.A.; Davis, B.H. Steam reforming of ethanol over Pt/ceria with co-fed hydrogen. J. Catal. 2007, 245, 326–337. [Google Scholar] [CrossRef]
- Zou, J.; Yu, B.; Zhang, S.; Zhang, J.; Chen, Y.; Cui, L.; Xu, T.; Cai, W. Hydrogen production from ethanol over Ir/CeO2 catalyst: Effect of the calcination temperature. Fuel 2015, 159, 741–750. [Google Scholar] [CrossRef]
- Calles, J.; Carrero, A.; Vizcaíno, A.; Lindo, M. Effect of Ce and Zr addition to Ni/SiO2 catalysts for hydrogen production through ethanol steam reforming. Catalysts 2015, 5, 58–76. [Google Scholar] [CrossRef]
- Gutierrez, A.; Karinen, R.; Airaksinen, S.; Kaila, R.; Krause, A.O.I. Autothermal reforming of ethanol on noble metal catalysts. Int. J. Hydrogen Energy 2011, 36, 8967–8977. [Google Scholar] [CrossRef]
- Contreras, J.L.; Salmones, J.; Colín-Luna, J.A.; Nuño, L.; Quintana, B.; Córdova, I.; Zeifert, B.; Tapia, C.; Fuentes, G.A. Catalysts for H2 production using the ethanol steam reforming (a review). Int. J. Hydrogen Energy 2014, 39, 18835–18853. [Google Scholar] [CrossRef]
- Moura, J.S.; Souza, M.O.G.; Bellido, J.D.A.; Assaf, E.M.; Opportus, M.; Reyes, P.; do Carmo Rangel, M. Ethanol steam reforming over rhodium and cobalt-based catalysts: Effect of the support. Int. J. Hydrogen Energy 2012, 37, 3213–3224. [Google Scholar] [CrossRef] [Green Version]
- Cobo, M.; Pieruccini, D.; Abello, R.; Ariza, L.; Córdoba, L.F.; Conesa, J.A. Steam reforming of ethanol over bimetallic RhPt/La2O3: Long-term stability under favorable reaction conditions. Int. J. Hydrogen Energy 2013, 38, 5580–5593. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J.P. Water gas shift catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Mei, Z.; Li, Y.; Fan, M.; Zhao, L.; Zhao, J. Effect of the interactions between Pt species and ceria on Pt/ceria catalysts for water gas shift: The XPS studies. Chem. Eng. J. 2015, 259, 293–302. [Google Scholar] [CrossRef]
- Holgado, J.P.; Munuera, G. XPS/TPR study of the reducibility of M/CeO2 catalysts (M = Pt, Rh): Does junction effect theory apply? Stud. Surf. Sci. Catal. 1995, 96, 109–122. [Google Scholar]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Cifuentes, B.; Valero, M.F.; Conesa, J.A.; Cobo, M. Hydrogen production by steam reforming of ethanol on Rh-Pt catalysts: Influence of CeO2, ZrO2, and La2O3 as supports. Catalysts 2015, 5, 1872–1896. [Google Scholar] [CrossRef]
- Idriss, H.; Scott, M.; Llorca, J.; Chan, S.C.; Chiu, W.; Sheng, P.-Y.; Yee, A.; Blackford, M.A.; Pas, S.J.; Hill, A.J.; et al. A phenomenological study of the metal-oxide interface: The role of catalysis in hydrogen production from renewable resources. ChemSusChem 2008, 1, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Kaila, R.K.; Gutiérrez, A.; Slioor, R.; Kemell, M.; Leskelä, M.; Krause, A.O.I. Zirconia-supported bimetallic RhPt catalysts: Characterization and testing in autothermal reforming of simulated gasoline. Appl. Catal. B 2008, 84, 223–232. [Google Scholar] [CrossRef]
- Song, H.; Ozkan, U.S. Economic analysis of hydrogen production through a bio-ethanol steam reforming process: Sensitivity analyses and cost estimations. Int. J. Hydrogen Energy 2010, 35, 127–134. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Cifuentes, B.; Hernández, M.; Monsalve, S.; Cobo, M. Hydrogen production by steam reforming of ethanol on a RhPt/CeO2/SiO2 catalyst: Synergistic effect of the Si:Ce ratio on the catalyst performance. Appl. Catal. A 2016, 523, 283–293. [Google Scholar] [CrossRef]
- Geravand, E.; Shariatinia, Z.; Yaripour, F.; Sahebdelfar, S. Synthesis of copper–silica nanosized catalysts for 2-butanol dehydrogenation and optimization of preparation parameters by response surface method. Chem. Eng. Res. Des. 2015, 96, 63–77. [Google Scholar] [CrossRef]
- Rossetti, I.; Compagnoni, M.; Torli, M. Process simulation and optimization of H2 production from ethanol steam reforming and its use in fuel cells. 2. Process analysis and optimization. Chem. Eng. J. 2015, 281, 1036–1044. [Google Scholar] [CrossRef]
- Ye, G.; Xie, D.; Qiao, W.; Grace, J.R.; Lim, C.J. Modeling of fluidized bed membrane reactors for hydrogen production from steam methane reforming with Aspen Plus. Int. J. Hydrogen Energy 2009, 34, 4755–4762. [Google Scholar] [CrossRef]
- Chang, H.; Liau, J.-S.; Ho, C.-D.; Wang, W.-H. Simulation of membrane distillation modules for desalination by developing user’s model on Aspen Plus platform. Desalination 2009, 249, 380–387. [Google Scholar] [CrossRef]
- Sangal, V.; Kumar, V.; Mishra, M. Optimization of a divided wall column for the separation of C4-C6 normal paraffin mixture using Box-Behnken design. Chem. Ind. Chem. Eng. Q. 2013, 19, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Yu, B.; Zhang, S.; Xu, T.; Wang, D.; Cai, W. Hydrogen production from ethanol steam reforming over Rh/CeO2 catalyst. Catal. Commun. 2015, 58, 137–140. [Google Scholar] [CrossRef]
- Auprêtre, F.; Descorme, C.; Duprez, D. Bio-ethanol catalytic steam reforming over supported metal catalysts. Catal. Commun. 2002, 3, 263–267. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Petallidou, K.C.; Efstathiou, A.M. The effect of La3+-doping of CeO2 support on the water-gas shift reaction mechanism and kinetics over Pt/Ce1−xLaxO2−δ. Appl. Catal. B 2013, 136–137, 225–238. [Google Scholar] [CrossRef]
- Scarabello, A.; Dalle Nogare, D.; Canu, P.; Lanza, R. Partial oxidation of methane on Rh/ZrO2 and Rh/Ce–ZrO2 on monoliths: Catalyst restructuring at reaction conditions. Appl. Catal. B 2015, 174–175, 308–322. [Google Scholar] [CrossRef]
- Jakdetchai, O.; Nakajima, T. Mechanism of the water–gas shift reaction over Cu(110), Cu(111) and Cu(100) surfaces: An AM1-d study. J. Mol. Struct. THEOCHEM 2002, 619, 51–58. [Google Scholar] [CrossRef]
- Diagne, C.; Idriss, H.; Kiennemann, A. Hydrogen production by ethanol reforming over Rh/CeO2–ZrO2 catalysts. Catal. Commun. 2002, 3, 565–571. [Google Scholar] [CrossRef]
- Silva, A.M.; Costa, L.O.O.; Barandas, A.P.M.G.; Borges, L.E.P.; Mattos, L.V.; Noronha, F.B. Effect of the metal nature on the reaction mechanism of the partial oxidation of ethanol over CeO2-supported Pt and Rh catalysts. Catal. Today 2008, 133–135, 755–761. [Google Scholar] [CrossRef]
- Liu, H.-H.; Wang, Y.; Jia, A.-P.; Wang, S.-Y.; Luo, M.-F.; Lu, J.-Q. Oxygen vacancy promoted CO oxidation over Pt/CeO2 catalysts: A reaction at Pt–CeO2 interface. Appl. Surf. Sci. 2014, 314, 725–734. [Google Scholar] [CrossRef]
- Diagne, C.; Idriss, H.; Pearson, K.; Gómez-García, M.A.; Kiennemann, A. Efficient hydrogen production by ethanol reforming over Rh catalysts. Effect of addition of Zr on CeO2 for the oxidation of CO to CO2. Comptes Rendus Chim. 2004, 7, 617–622. [Google Scholar] [CrossRef]
- Cavallaro, S. Ethanol steam reforming on Rh/Al2O3 catalysts. Energy Fuels 2000, 14, 1195–1199. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Hungría, A.B.; Munuera, G.; Gamarra, D. Preferential oxidation of CO in rich H2 over CuO/CeO2: Details of selectivity and deactivation under the reactant stream. Appl. Catal. B 2006, 65, 207–216. [Google Scholar] [CrossRef]
- Llera, I.; Mas, V.; Bergamini, M.L.; Laborde, M.; Amadeo, N. Bio-ethanol steam reforming on Ni based catalyst. Kinetic study. Chem. Eng. Sci. 2012, 71, 356–366. [Google Scholar] [CrossRef]
- Munera, J.; Irusta, S.; Cornaglia, L.; Lombardo, E.; Vargascesar, D.; Schmal, M. Kinetics and reaction pathway of the CO2 reforming of methane on Rh supported on lanthanum-based solid. J. Catal. 2007, 245, 25–34. [Google Scholar] [CrossRef]
- da Silva, A.M.; de Souza, K.R.; Jacobs, G.; Graham, U.M.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Steam and CO2 reforming of ethanol over Rh/CeO2 catalyst. Appl. Catal. B 2011, 102, 94–109. [Google Scholar] [CrossRef]
- Yee, A.; Morrison, S.; Idriss, H. The reactions of ethanol over M/CeO2 catalysts. Catal. Today 2000, 63, 327–335. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Özkara-Aydınoğlu, Ş.; Özensoy, E.; Aksoylu, A.E. The effect of impregnation strategy on methane dry reforming activity of Ce promoted Pt/ZrO2. Int. J. Hydrogen Energy 2009, 34, 9711–9722. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.Y.; Lee, D.W.; Park, S.J.; Lee, K.Y.; Lee, K.H. Study on a catalytic membrane reactor for hydrogen production from ethanol steam reforming. Int. J. Hydrogen Energy 2009, 34, 2947–2954. [Google Scholar] [CrossRef]
- Ciambelli, P.; Palma, V.; Ruggiero, A. Low temperature catalytic steam reforming of ethanol. 2. Preliminary kinetic investigation of Pt/CeO2 catalysts. Appl. Catal. B 2010, 96, 190–197. [Google Scholar] [CrossRef]
- Zanchet, D.; Santos, J.B.O.; Damyanova, S.; Gallo, J.M.R.; Bueno, J.M.C. Toward understanding metal-catalyzed ethanol reforming. ACS Catal. 2015, 5, 3841–3863. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J.; Shari, L.K.; Adams, W. Handbook for Experimenters; Stat-Ease, Inc.: Minneapoliscity, MN, USA, 2009. [Google Scholar]
- Lopes, D.G.; da Silva, E.P.; Pinto, C.S.; Neves, N.P.; Camargo, J.C.; Ferreira, P.F.P.; Furlan, A.L.; Lopes, D.G. Technical and economic analysis of a power supply system based on ethanol reforming and PEMFC. Renew. Energy 2012, 45, 205–212. [Google Scholar] [CrossRef]
- Liao, X.; Chu, W.; Dai, X.; Pitchon, V. Bimetallic Au-Cu supported on ceria for PROX reaction: Effects of Cu/Au atomic ratios and thermal pretreatments. Appl. Catal. B 2013, 142–143, 25–37. [Google Scholar] [CrossRef]
- Grande, C.A.; Lopes, F.V.S.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Adsorption of off-gases from steam methane reforming (H2, CO2, CH4, CO and N2) on activated carbon. Sep. Sci. Technol. 2008, 43, 1338–1364. [Google Scholar] [CrossRef]
- Hernández, L.; Kafarov, V. Use of bioethanol for sustainable electrical energy production. Int. J. Hydrogen Energy 2009, 34, 7041–7050. [Google Scholar] [CrossRef]
- Elmer, T.; Worall, M.; Wu, S.; Riffat, S.B. Fuel cell technology for domestic built environment applications: State of-the-art review. Renew. Sustain. Energy Rev. 2015, 42, 913–931. [Google Scholar] [CrossRef]
- Martavaltzi, C.S.; Pampaka, E.P.; Korkakaki, E.S.; Lemonidou, A.A. Hydrogen production via steam reforming of methane with simultaneous CO2 capture over CaO−Ca12Al14O33. Energy Fuels 2010, 24, 2589–2595. [Google Scholar] [CrossRef]
- Rydén, M.; Ramos, P. H2 production with CO2 capture by sorption enhanced chemical-looping reforming using NiO as oxygen carrier and CaO as CO2 sorbent. Fuel Process. Technol. 2012, 96, 27–36. [Google Scholar] [CrossRef]
- Ogo, S.; Shimizu, T.; Nakazawa, Y.; Mukawa, K.; Mukai, D.; Sekine, Y. Steam reforming of ethanol over K promoted Co catalyst. Appl. Catal. A 2015, 495, 30–38. [Google Scholar] [CrossRef]
- Freni, S.; Maggio, G.; Cavallaro, S. Ethanol steam reforming in a molten carbonate fuel cell: A thermodynamic approach. J. Power Sources 1996, 62, 67–73. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.Y.; Cifuentes, B.; Cobo, M. Hydrogen from glucose: A combined study of glucose fermentation, bioethanol purification, and catalytic steam reforming. Int. J. Hydrogen Energy 2016, 41, 5640–5651. [Google Scholar] [CrossRef]
- Ghelamallah, M.; Granger, P. Supported-induced effect on the catalytic properties of Rh and Pt-Rh particles deposited on La2O3 and mixed α-Al2O3-La2O3 in the dry reforming of methane. Appl. Catal. A 2014, 485, 172–180. [Google Scholar] [CrossRef]
- Mondal, T.; Pant, K.K.; Dalai, A.K. Catalytic oxidative steam reforming of bio-ethanol for hydrogen production over Rh promoted Ni/CeO2–ZrO2 catalyst. Int. J. Hydrogen Energy 2015, 40, 2529–2544. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; da Silva, E.G.P.; Dos Santos, W.N.L.; Quintella, C.M.; David, J.M.; de Andrade, J.B.; Breitkreitz, M.C.; Jardim, I.C.S.F.; Neto, B.B. Statistical designs and response surface techniques for the optimization of chromatographic systems. J. Chromatogr. A 2007, 1158, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.A.; Barton, P.I. Combining coal gasification and natural gas reforming for efficient polygeneration. Fuel Process. Technol. 2011, 92, 639–655. [Google Scholar] [CrossRef]
| Reaction | Description | |
|---|---|---|
| (Steam reforming–ethanol) | Equation (1) | |
| (Water gas shift reaction) | Equation (2) | |
| (Dehydrogenation–ethanol) | Equation (3) | |
| (Dehydration–ethanol) | Equation (4) | |
| (Decomposition–ethanol) | Equation (5) | |
| (Decomposition–acetaldehyde) | Equation (6) | |
| (Steam reforming–acetaldehyde) | Equation (7) | |
| (Steam reforming–methane) | Equation (8) | |
| (Dehydrogenation–methane) | Equation (9) | |
| (Steam reforming–ethylene) | Equation (10) | |
| Catalyst 1 | Rh (wt %) | Pt (wt %) | Rh–Pt (wt ratio) | Weight Loss of Used Catalyst Samples (mg carbon/(gcat·h)) 2 |
|---|---|---|---|---|
| CeO2 | 0 | 0 | 0:0 | 4.06 |
| Rh0.8/CeO2 | 0.8 | 0 | 1:0 | 1.00 |
| Rh0.6Pt0.2/CeO2 | 0.6 | 0.2 | 3:1 | 0.36 |
| Rh0.4Pt0.4/CeO2 | 0.4 | 0.4 | 1:1 | 0.42 |
| Rh0.2Pt0.6/CeO2 | 0.2 | 0.6 | 1:3 | 0.52 |
| Pt0.8/CeO2 | 0 | 0.8 | 0:1 | 3.31 |
| Variable*/Parameter | Ethanol Conversion (%) | H2 Yield (mol H2/mol EtOH Inlet) | CO Yield (mol CO/mol EtOH Inlet) | |||
| Coded Factors | Actual Factors | Coded Factors | Actual Factors | Coded Factors | Actual Factors | |
| - | NS* | 4.9 × 103 | NS* | −34 | NS* | 32 |
| T | NS* | −35 | NS* | 0.29 | NS* | −0.22 |
| C | −3.3 × 1010 | −2.2 × 103 | 2.1 × 109 | −41 | 2.4 × 108 | −21 |
| T*C | 3.9 × 107 | 11 | NS* | 0.1 | NS* | 0.1 |
| T2 | 1.5 × 105 | 9.1 × 10−2 | 3.4 × 103 | −8.8 × 10−4 | NS* | 5.6 × 10−4 |
| C2 | −5.0 × 1010 | 3.9 × 102 | 3.1 × 109 | 1.0 × 102 | 3.8 × 108 | 13 |
| T2*C | 3.1 × 105 | −1.8 × 10−2 | 6.9 × 103 | −1.2 × 10−4 | NS* | −1.7 × 10−4 |
| T*C2 | NS* | −3.8 | NS* | −9.2 × 10−2 | NS* | −2.0 × 10−2 |
| T3 | 1.5 × 103 | −1.0 × 10−4 | NS* | 1.1 × 10−6 | NS* | −6.0 × 10−7 |
| C3 | −3.6 × 109 | 1.6 × 103 | 2.1 × 109 | −1.4 × 102 | 2.5 × 108 | −14 |
| T2*C2 | 1.5 × 105 | 2.8 × 10−3 | 3.5 × 103 | 6.2 × 105 | NS* | 2.3 × 105 |
| T3*C | 1.6 × 103 | 9.4 × 10−6 | NS* | −1.1 × 10−3 | NS* | 9.1 × 10−8 |
| T*C3 | NS* | 0.71 | NS* | −5.2 × 10−10 | NS* | −4.7 × 10−3 |
| T4 | 21 | 4.2 × 10−8 | NS* | 85 | NS* | 2.4 × 10−10 |
| C4 | −8.5 × 109 | −1.4 × 103 | 5.3 × 108 | 85 | 6.2 × 107 | 10 |
| Model | Quartic | Quartic | Quartic | |||
| F-value | 164.08 | 480.03 | 200.86 | |||
| Probability F | <1 × 10−4 | <1 × 10−4 | <1 × 10−4 | |||
| Standard deviation | 5.4 | 0.12 | 0.07 | |||
| Mean | 72.0 | 1.53 | 0.55 | |||
| R2 | 0.97 | 0.990 | 0.977 | |||
| Adjusted R2 | 0.97 | 0.998 | 0.973 | |||
| Predicted R2 | 0.96 | 0.984 | 0.964 | |||
| Adequate precision | 37.09 | 81.95 | 57.84 | |||
| Lack of Fit | 74 | 73 | 79 | |||
| Variable*/Parameter | CO2 Yield (mol CO2/mol EtOH Inlet) | CH4 Yield (mol CH4/mol EtOH Inlet) | Ethylene Yield (mol Ethylene/mol EtOH Inlet) | |||
| Coded Factors | Actual Factors | Coded Factors | Actual Factors | Coded Factors | Actual Factors | |
| - | NS* | 34 | NS* | 4.9 | NS* | 2.1 |
| T | −6.0 × 105 | −0.23 | 2.1 × 105 | −3.5 × 10−2 | NS* | −1.5 × 10−2 |
| C | −3.4 × 108 | 2.1 | −5.1 × 108 | −0.52 | NS* | −3.2 × 10−2 |
| T*C | −1.8 × 106 | 1.7 × 10−2 | 6.4 × 105 | 2.9 × 10−4 | −5.9 × 10−2 | 1.8 × 10−4 |
| T2 | −4.0 × 103 | 6.1 × 10−4 | NS* | 8.9 × 105 | NS* | 3.9 × 105 |
| C2 | −5.1 × 108 | −57 | −7.7 × 108 | −6.0 | 0.14 | −7.4 × 105 |
| T2*C | −8.1 × 103 | −5.7 × 105 | NS* | 2.2 × 105 | NS* | −2.8 × 10−7 |
| T*C2 | −1.8 × 106 | 0.13 | 6.4 × 105 | −2.3 × 10−2 | NS* | −4.5 × 10−7 |
| T3 | NS* | −7.5 × 10−7 | NS* | −9.7 × 10−8 | NS* | −4.5 × 10−8 |
| C3 | −3.4 × 108 | 40 | −5.2 × 108 | 27 | NS* | 3.3 × 10−6 |
| T2*C2 | −4.0 × 103 | −7.2 × 105 | NS* | 4.1 × 10−6 | NS* | 1.2 × 10−10 |
| T3*C | NS* | 3.6 × 10−8 | NS* | −1.8 × 10−8 | NS* | 1.6 × 10−10 |
| T*C3 | −6.1 × 105 | −3.3 × 10−2 | 2.2 × 105 | 1.2 × 10−2 | NS* | 2.1 × 10−9 |
| T4 | NS* | 3.5 × 10−10 | NS* | 3.9 × 10−11 | NS* | 1.9 × 10−11 |
| C4 | −8.6 × 107 | −14 | −1.3 × 108 | −21 | −0.14 | −2.2 × 10−8 |
| Model | Quartic | Quartic | - | Quartic | - | |
| F-value | 42.55 | 47.39 | - | 3.2 | - | |
| Probability F | <1 × 10−4 | <1 × 10−4 | - | 7 × 10−4 | - | |
| Standard deviation | 0.12 | 0.04 | - | 0.02 | - | |
| Mean | 0.63 | 0.13 | - | 0.01 | - | |
| R2 | 0.902 | 0.911 | - | 0.851 | - | |
| Adjusted R2 | 0.880 | 0.892 | - | 0.828 | - | |
| Predicted R2 | 0.812 | 0.864 | - | 0.820 | - | |
| Adequate precision | 30.77 | 26.01 | - | 7.65 | - | |
| Lack of Fit | 72 | 74 | - | 73 | - | |
| Rh Content in Catalyst (wt %) 1 | T (°C) | Yield (mol/mol EtOH Inlet) 2 | Ethanol Conversion (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CH4 | CO2 | ||||||||
| E | S | E | S | E | S | E | S | E | S | ||
| 0 | 430 | 1.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.8 | 36 | 33 |
| 0.2 | 630 | 2.1 | 2.0 | 0.7 | 0.9 | 0.6 | 0.4 | 0.1 | 0.6 | 96 | 100 |
| 0.4 | 530 | 1.0 | 0.9 | 0.5 | 0.5 | 0.2 | 0.2 | 0.7 | 0.7 | 71 | 72 |
| 0.6 | 470 | 0.2 | 0.3 | 0.4 | 0.2 | 0.2 | 0.2 | 0.3 | 0.7 | 66 | 61 |
| 0.8 | 610 | 2.1 | 2.1 | 1.4 | 1.3 | 0.1 | 0.1 | 0.4 | 0.4 | 96 | 97 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes, B.; Figueredo, M.; Cobo, M. Response Surface Methodology and Aspen Plus Integration for the Simulation of the Catalytic Steam Reforming of Ethanol. Catalysts 2017, 7, 15. https://doi.org/10.3390/catal7010015
Cifuentes B, Figueredo M, Cobo M. Response Surface Methodology and Aspen Plus Integration for the Simulation of the Catalytic Steam Reforming of Ethanol. Catalysts. 2017; 7(1):15. https://doi.org/10.3390/catal7010015
Chicago/Turabian StyleCifuentes, Bernay, Manuel Figueredo, and Martha Cobo. 2017. "Response Surface Methodology and Aspen Plus Integration for the Simulation of the Catalytic Steam Reforming of Ethanol" Catalysts 7, no. 1: 15. https://doi.org/10.3390/catal7010015