Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells
Abstract
:1. Introduction
1.1. Motivation for HT-PEMFC
1.2. Low Range HT-PEMFC: 100–150 °C
1.3. High Range HT-PEMFC: 150–250 °C
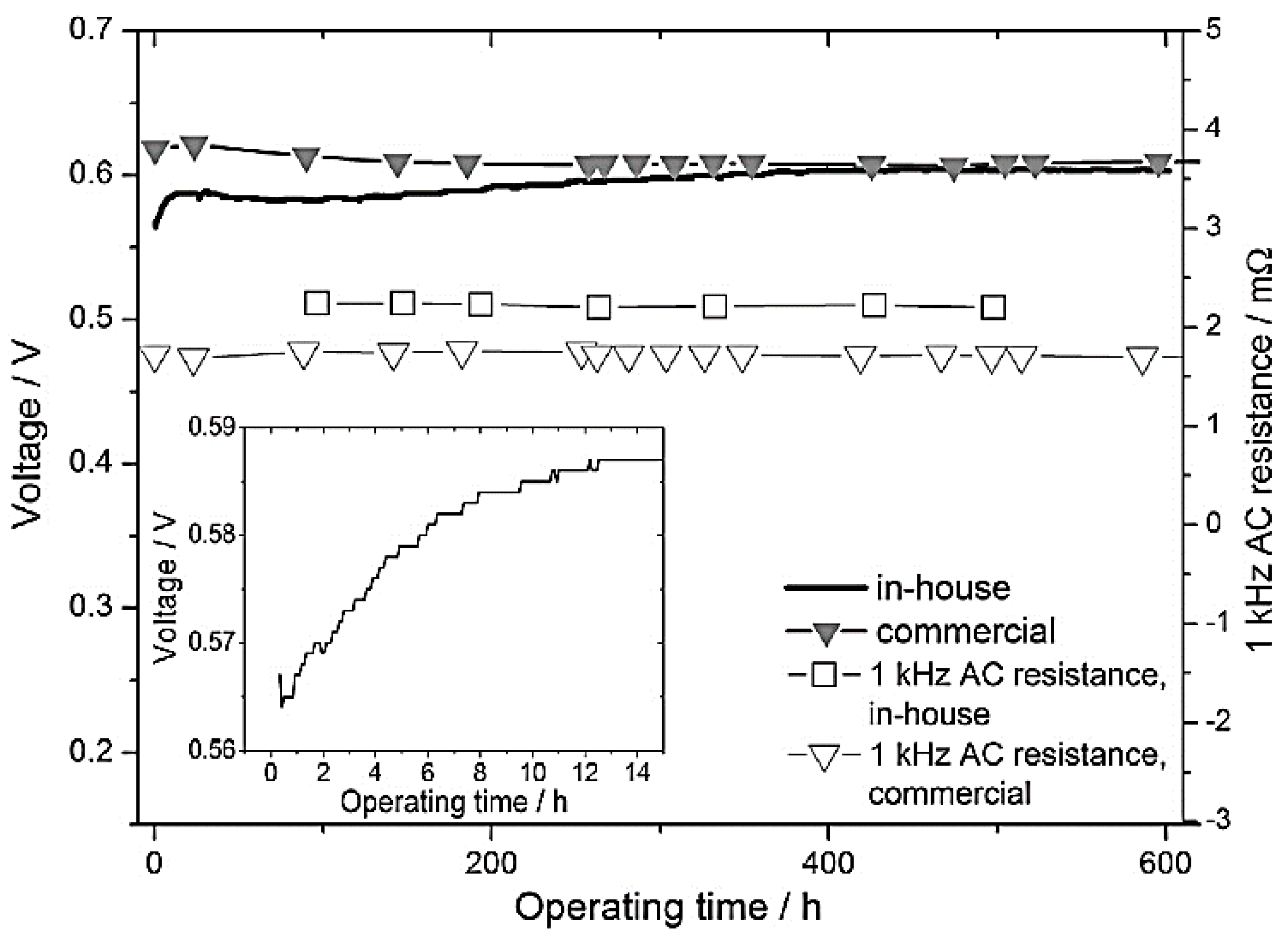
1.4. Challenges in Implementations
2. High Temperature Polymer Electrolyte Membranes
3. Acid Management
4. ORR Catalyst Activity in Free Electrolyte: Alternative Catalyst
5. Oxygen Permeability
5.1. Additives
5.2. Alternative Electrolytes
5.3. Effect of Doped Ionomer
6. Phosphate Adsorption
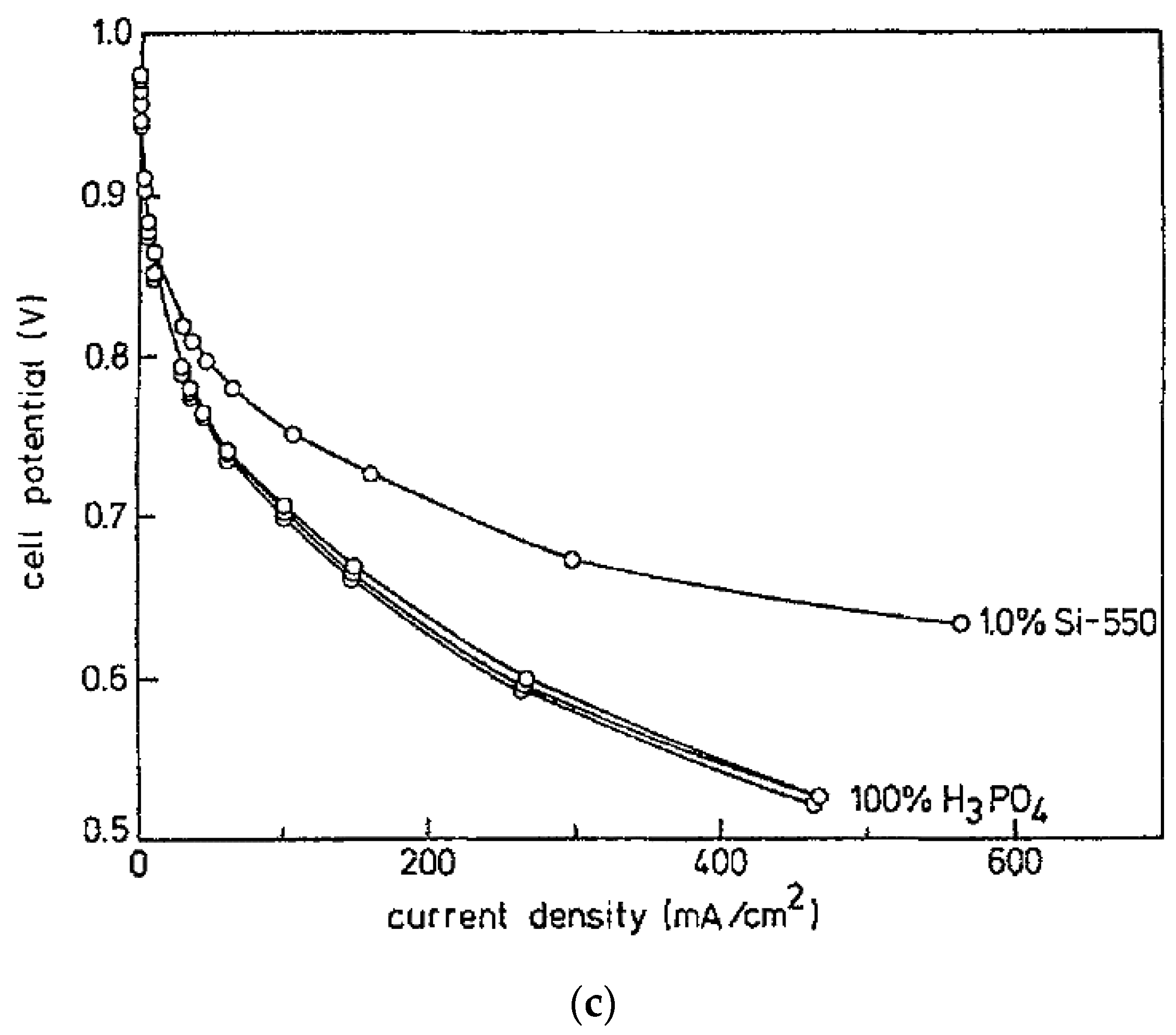
6.1. Additives
6.2. Alternative Acids
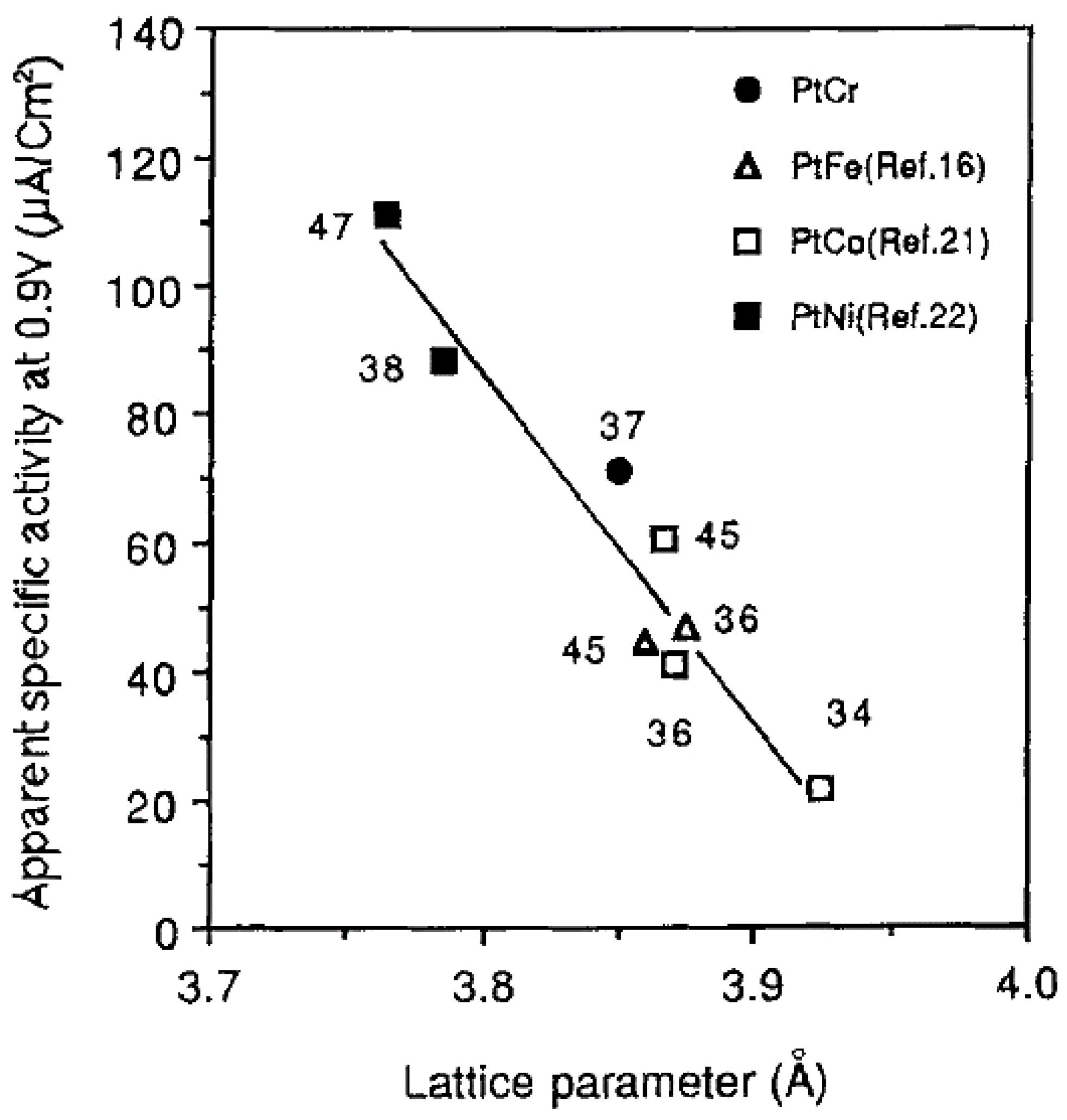
6.3. Impact of Catalyst
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Yang, C.; Costamagna, P.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Approaches and technical challenges to high temperature operation of PEMFC. J. Power Sources 2001, 103, 1–9. [Google Scholar] [CrossRef]
- Paddison, S.J. Proton conducting mechanism at low degree of hydration in sulfonic acid-based polymer electrolyte membranes. Ann. Rev. Mater. Res. 2003, 33, 289–319. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Wang, Z.; Gao, Y. PEMFC from low temperature to high temperature: Material challenges. J. Power Sources 2007, 167, 235–242. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Mathias, M.F. Fundamental research and development challenges in Polymer Electrolyte Fuel Cell Technology. Proton Conduct. Fuel Cells III Proc. Electr. Soc. Ser. 2002, 2005, 1–24. [Google Scholar]
- Dai, W.; Wang, H.; Yuan, X.-Z.; Martin, J.-J.; Yang, D.; Qiao, J.; Ma, J. A review on water balance in the membrane electrode assembly of PEMFC. Int. J. Hydrog. Energy 2009, 34, 9461–9478. [Google Scholar] [CrossRef]
- Subianto, S. Recent advances in polybenzimidazole/phosphoric acid membranes for high-temperature fuel cells. Polym. Int. 2014, 63, 1134–1144. [Google Scholar] [CrossRef]
- Morfopoulou, C.; Andreopoulou, A.K.; Kallitsis, J.K. The effect of structural variations on aromatic polyethers for high-temperature PEM fuel cells. J. Polym. Sci. A 2011, 49, 4325–4334. [Google Scholar] [CrossRef]
- Daletou, M.K.; Geormezi, M.; Vogli, E.; Voyiatzis, G.A.; Neophytides, S.G. The interaction of H3PO4 and steam with PBI and TPS polymeric membranes. A TGA and Raman study. J. Mater. Chem. A 2014, 2, 1117–1127. [Google Scholar] [CrossRef]
- Gourdoupi, N.; Triantafyllopoulos, N.; Deimede, V.; Pefkianakis, L.; Daletou, M.; Neophytides, S.; Kallitsis, J.K. Aromatic Polyether Copolymers and Polymer Blends and Fuel Cells Comprising the Same. U.S. Patent WO/2008/038162, 4 March 2008. [Google Scholar]
- Daletou, M.K.; Geormezi, M.; Pefkianakis, E.K.; Morfopoulou, C.; Kallitsis, J.K. Fully aromatic copolyethers for high temperature polymer electrolyte membrane fuel cells. Fuel Cells 2010, 10, 35–44. [Google Scholar] [CrossRef]
- Geormezi, M.; Deimede, V.; Gourdoupi, N.; Triantafyllopoulos, N.; Neophytides, S.; Kallitsis, J.K. Novel pyridine-based poly(ether sulfones) and their study in high temperature PEM fuel cells. Macromolecules 2008, 41, 9051–9056. [Google Scholar] [CrossRef]
- Daletou, M.K.; Gourdoupi, N.; Kallitsis, J.K. Proton conducting membranes based on blends of PBI with aromatic polyethers containing pyridine units. J. Membr. Sci. 2005, 252, 115–122. [Google Scholar] [CrossRef]
- Pefkianakis, E.K.; Deimede, V.; Daletou, M.K.; Gourdoupi, N.; Kallitsis, J.K. Novel polymer electrolyte membrane, based on pyridine containing poly(ether sulfone), for application in high-temperature fuel cells. Macromol. Rapid Commun. 2005, 26, 1724–1728. [Google Scholar] [CrossRef]
- Appleby, A.J.; Baker, B.S. Oxygen reduction on platinum in trifluoromethane sulfonic acid. J. Electrochem. Soc. 1978, 125, 404–406. [Google Scholar] [CrossRef]
- Zelenay, P.; Habib, M.A.; Bockris, J.O.M. Adsorption from solution on platinum: An in situ FTIR and radiotracer study. Langmuir 1986, 2, 393–405. [Google Scholar] [CrossRef]
- Hsueh, K.-L.; Chang, H.H.; Chin, D.-T. Electrode kinetics of oxygen reduction on platinum in trifluoromethanesulphonic acid. Electrochim. Acta 1985, 30, 1137–1142. [Google Scholar] [CrossRef]
- Kunz, H.R. Electrolyte migration in fuel cell modeling. Electrochem. Soc. Proc. 1999, 99–14, 191–207. [Google Scholar]
- Mukerjee, S. Innovative non-PGM catalysts for high-temperature PEMFCs. In 2016 DOE Hydrogen and Fuel Cell Program Review; DOE Hydrogen and Fuel Cell Program: Washington, DC, USA, June 2016. [Google Scholar]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High temperature proton exchange membranes based on polybenzimidazoles for fuel cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.-T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sánchez, E.M.; Gómez-Romero, P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef] [PubMed]
- Herring, A.M. Inorganic–polymer composite membranes for proton exchange membrane fuel cells. J. Macromol. Sci. C 2006, 46, 245–296. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M. Composite membranes for medium-temperature PEM fuel cells. Annu. Rev. Mater. Res. 2003, 33, 129–154. [Google Scholar] [CrossRef]
- Costamagna, P.; Yang, C.; Bocarsly, A.B.; Srinivasan, S. Nafion® 115/zirconium phosphate composite membranes for operation of PEMFCs above 100 °C. Electrochim. Acta 2002, 47, 1023–1033. [Google Scholar] [CrossRef]
- Atkins, J.R.; Sides, C.R.; Creager, S.E.; Harris, J.L.; Pennington, W.T.; Thomas, B.H.; DesMarteau, D.D. Effect of equivalent weight on water sorption, PTFE-like crystallinity, and ionic conductivity in bis[(perfluoroalkyl)sulfonyl] imide perfluorinated ionomers. J. New Mater. Electr. Syst. 2003, 6, 9–15. [Google Scholar]
- Damay, F.; Klein, L.C. Transport properties of Nafion™ composite membranes for proton-exchange membranes fuel cells. Solid State Ion. 2003, 162–163, 261–267. [Google Scholar] [CrossRef]
- Ruffmann, B.; Silva, H.; Schulte, B.; Nunes, S.P. Organic/inorganic composite membranes for application in DMFC. Solid State Ion. 2003, 162–163, 269–275. [Google Scholar] [CrossRef]
- Aili, D.; Hansen, M.K.; Pan, C.; Li, Q.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Phosphoric acid doped membranes based on Nafion®, PBI and their blends—Membrane preparation, characterization and steam electrolysis testing. Int. J. Hydrog. Energy 2011, 36, 6985–6993. [Google Scholar] [CrossRef]
- Si, Y.; Kunz, H.R.; Fenton, J.M. Nafion-teflon-Zr (HPO4)2 composite membranes for high-temperature PEMFCs. J. Electrochem. Soc. 2004, 151, A623–A631. [Google Scholar] [CrossRef]
- Kwak, S.H.; Yang, T.H.; Kim, C.S.; Yoon, K.H. Polymer composite membrane incorporated with a hygroscopic material for high-temperature PEMFC. Electrochim. Acta 2004, 50, 653–657. [Google Scholar] [CrossRef]
- Kwak, S.H.; Yang, T.H.; Kim, C.S.; Yoon, K.H. Nafion/mordenite hybrid membrane for high-temperature operation of polymer electrolyte membrane fuel cell. Solid State Ion. 2003, 160, 309–315. [Google Scholar] [CrossRef]
- Ramani, V.; Kunz, H.R.; Fenton, J.M. Investigation of Nafion®/HPA composite membranes for high temperature/low relative humidity PEMFC operation. J. Membr. Sci. 2004, 232, 31–44. [Google Scholar] [CrossRef]
- Wasmus, S.; Valeriu, A.; Mateescu, G.D.; Tryk, D.A.; Savinell, R.F. Characterization of H3PO4-Equili-brated Nafion(r) 117 Membranes Using 1H and 31P NMR Spectroscopy. Solid State Ion. 1995, 80, 87–92. [Google Scholar] [CrossRef]
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Teranishi, K.; Kawata, K.; Tsushima, S.; Hirai, S. Degradation mechanism of PEMFC under open circuit operation. Electrochem. Solid State 2006, 9, A475–A477. [Google Scholar] [CrossRef]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved PEMFC performance and life. J. Power Sources 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Madden, T.; Weiss, D.; Cipollini, N.; Condit, D.; Gummalla, M.; Burlatsky, S.; Atrazhev, V. Degradation of polymer-electrolyte membranes in fuel cells: I. experimental. J. Electrochem. Soc. 2009, 156, B657–B662. [Google Scholar] [CrossRef]
- Tang, H.; Peikang, H.S.; Jiang, S.P.; Wang, F.; Pan, M. A degradation study of Nafion proton exchange membrane of PEM fuel cells. J. Power Sources 2007, 170, 85–92. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Bonville, L.J.; Kunz, H.R.; Slattery, D.K.; Fenton, J.M. Fuel cell perfluorinated sulfonic acid membrane degradation correlating accelerated stress testing and lifetime. Chem. Rev. 2012, 112, 6075–6103. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, W.H.J.; Diniz da Costa, J.C.; Lu, G.Q. Solid acid membranes for high temperature (>140 °C) proton exchange membrane fuel cells. J. Power Sources 2005, 142, 223–237. [Google Scholar] [CrossRef]
- Zaidi, S.M. J.; Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Proton conducting composite membranes from polyether ether ketone and heteropolyacids for fuel cell applications. J. Membr. Sci. 2000, 173, 17–34. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, L.; Mukerjee, S.; Ofer, D.; Nair, B. An investigation of proton conduction in select PEM’s and reaction layer interfaces-designed for elevated temperature operation. J. Membr. Sci. 2003, 219, 123–136. [Google Scholar] [CrossRef]
- Scott, K.; Taama, W.M.; Argyropoulos, P. Performance of the direct methanol fuel cell with radiation-grafted polymer membranes. J. Membr. Sci. 2000, 171, 119–130. [Google Scholar] [CrossRef]
- Pohl, H.A.; Chartoff, R.P. Carriers and unpaired spins in some organic semiconductors. J. Polym. Sci. A 1964, 2, 2787–2806. [Google Scholar] [CrossRef]
- Aharoni, S.M.; Signorelli, A.J. Electrical resistivity and ESCA studies on neutral poly(alkylbenzimidazoles), their salts, and complexes. J. Appl. Polym. Sci. 1979, 23, 2653–2660. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Wainright, J.S.; Litt, M.H.; Savinell, R.F. Conductivity of PBI membranes for high-temperature polymer electrolyte fuel cells. J. Electrochem. Soc. 2004, 151, A8–A16. [Google Scholar] [CrossRef]
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Membr. Sci. 2003, 226, 169–184. [Google Scholar] [CrossRef]
- Gulledge, A.L.; Chen, X.; Benicewicz, B.C. Investigation of sequence isomer effects in AB-polybenzimidazole polymers. J. Polym. Sci. A Polym. Chem. 2014, 52, 619–628. [Google Scholar] [CrossRef]
- Savinell, R.; Yeager, E.; Tryk, D.; Landau, U.; Wainright, J.; Weng, D.; Lux, K.; Litt, M.; Rogers, C. A polymer electrolyte for operation at temperatures up to 200 °C. J. Electrochem. Soc. 1994, 141, L46–L48. [Google Scholar] [CrossRef]
- Hazarika, M.; Jana, T. Proton exchange membrane developed from novel blends of polybenzimidazole and poly(vinyl-1,2,4-triazole). ACS Appl. Mater. Interfaces 2012, 4, 5256–5265. [Google Scholar] [CrossRef] [PubMed]
- Samms, S.R.; Wasmus, S.; Savinell, R.F. Thermal stability of proton conducting acid doped polybenzimidazole in simulated fuel cell environments. J. Electrochem. Soc. 1996, 143, 1225–1232. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Hu, J.; Zhai, Y.; Xu, D.; Shao, Z.-G. Studies of performance degradation of a high temperature PEMFC based on H3PO4-doped PBI. J. Power Sources 2006, 162, 547–552. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Chayer, M. Jerkiewicz Energetics of the underpotential deposition of hydrogen on platinum electrodes I. Absence of coadsorbed species. J. Electrochem. Soc. 1997, 144, 3034–3041. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Sheng, L.; Xu, H.; Guo, X.; Fang, J.; Fang, L.; Yin, J. Synthesis and properties of novel sulfonated polybenzimidazoles from disodium 4,6-bis(4-carboxyphenoxy)benzene-1,3-disulfonate. J Power Sources 2011, 196, 3039–3047. [Google Scholar] [CrossRef]
- Mader, J.A.; Benicewicz, B.C. Synthesis and Properties of Segmented Block Copolymers of Functionalised Polybenzimidazoles for High-Temperature PEM Fuel Cells. Fuel Cells 2011, 11, 222–237. [Google Scholar] [CrossRef]
- Mader, J.A.; Benicewicz, B.C. Sulfonated polybenzimidazoles for high temperature PEM fuel cells. Macromolecules 2010, 43, 6706–6715. [Google Scholar] [CrossRef]
- Jensen, J.O.; Li, Q.; Pan, C.; Bjerrum, N.J.; Rudbeck, H.C.; Steenberg, T. Ongoing efforts addressing degradation of high temperature PEMFC. In Proceedings of the 18th World Hydrogen Energy Conference, Essen, Germany, 6–20 May 2010.
- Mader, J.; Xiao, L.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole/acid complexes as high-temperature membranes. Adv. Polym. Sci. 2008, 216, 63–124. [Google Scholar]
- Daletou, M.K.; Kallitsis, J.K.; Voyiatzis, G.; Neophytides, S.G. The interaction of water vapors with H3PO4 imbibed electrolyte based on PBI/polysulfone copolymer blends. J. Membr. Sci. 2009, 326, 76–83. [Google Scholar] [CrossRef]
- Kalamaras, I.; Daletou, M.K.; Neophytides, S.G.; Kallitsis, J.K. Thermal crosslinking of aromatic polyethers bearing pyridine groups for use as high temperature polymer electrolytes. J. Membr. Sci. 2012, 415–416, 42–50. [Google Scholar] [CrossRef]
- Papadimitriou, K.D.; Geormezi, M.; Neophytides, S.G.; Kallitsis, J. K Covalent cross-linking in phosphoric acid of pyridine based aromatic polyethers bearing side double bonds for use in high temperature polymer electrolyte membrane fuel cells. J. Membr. Sci. 2013, 433, 1–9. [Google Scholar] [CrossRef]
- Cappadonia, M.; Niemzig, O.; Stimming, U. Preliminary study on the ionic conductivity of a polyphosphate composite. Solid State Ion. 1999, 125, 333–337. [Google Scholar] [CrossRef]
- Heo, P.; Kajiyama, N.; Kobayashi, K.; Nagao, M.; Sano, M.; Hibino, T. Proton conduction in Sn0.95Al0.05P2O7PBI–PTFE composite membrane. Electrochem. Solid-State Lett. 2008, 11, B91–B95. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yoshida, T.; Kawamura, G.; Muto, H.; Sakai, M.; Matsuda, A. Inorganic–organic composite electrolytes consisting of polybenzimidazole and Cs-substituted heteropoly acids and their application for medium temperature fuel cells. J. Mater. Chem. 2010, 20, 6359–6366. [Google Scholar] [CrossRef]
- Liu, X.; Yu, E.H.; Scott, K. Preparation and evaluation of a highly stable palladium yttrium platinum core–shell–shell structure catalyst for oxygen reduction reactions. Appl. Catal. B Environ. 2015, 162, 593–601. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, X.; Cheng, J.; Scott, K. A polybenzimidazole/ionic-liquid-graphite-oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2015, 274, 922–927. [Google Scholar] [CrossRef]
- Staiti, P.; Minutoli, M.; Hocevar, S. Membranes based on phosphotungstic acid and polybenzimidazole for fuel cell application. J. Power Sources 2000, 90, 231–235. [Google Scholar] [CrossRef]
- Staiti, P.; Minutoli, M. Influence of composition and acid treatment on proton conduction of composite polybenzimidazole membranes. J. Power Sources 2001, 94, 9–13. [Google Scholar] [CrossRef]
- Staiti, P. Proton conductive membranes based on silicotungstic acid/silica and polybenzimidazole. Mater. Lett. 2001, 47, 241–246. [Google Scholar] [CrossRef]
- Lobato, J.; Canizares, P.; Rodrigo, M.A.; Úbeda, D.; Pinar, F.J. Promising TiOSO4 composite polybenzimidazole-based membranes for high temperature PEMFCs. ChemSusChem 2011, 4, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Yan, L.; Han, S.; Yue, B.; Feng, Q.; Xie, L.; Chen, J.; Zhang, D.; Sun, C. Enhancing the high-temperature proton conductivity of phosphoric acid doped poly(2,5-benzimidazole) by preblending boron phosphate nanoparticles to the raw materials. J. Power Sources 2012, 211, 161–168. [Google Scholar] [CrossRef]
- Suryani; Liu, Y.L. Preparation and properties of nanocomposite membranes of polybenzimidazole/sulfonated silica nanoparticles for proton exchange membranes. J. Membr. Sci. 2009, 332, 121–128. [Google Scholar] [CrossRef]
- Aili, D.; Allward, T.; Alfaro, S.M.; Hartmann-Thompson, C.; Steenberg, T.; Hjuler, H.A.; Li, Q.; Jensen, J.O.; Stark, E.J. Polybenzimidazole and sulfonated polyhedral oligosilsesquioxanecomposite membranes for high temperature polymer electrolytemembrane fuel cells. Electrochim. Acta 2014, 140, 182–190. [Google Scholar] [CrossRef]
- Lobato, J.; Canizares, P.; Rodrigo, M.A.; Úbeda, D.; Pinar, F.J. A novel titanium PBI-based composite membrane for high temperature PEMFCs. J. Membr. Sci. 2011, 369, 105–111. [Google Scholar] [CrossRef]
- Plackett, D.; Siu, A.; Li, Q.; Pan, C.; Jensen, J.O.; Nielsen, S.F.; Permyakova, A.A.; Bjerrum, N.J. High-temperature proton exchange membranes based on polybenzimidazole and clay composites for fuel cells. J. Membr. Sci. 2011, 383, 78–87. [Google Scholar] [CrossRef]
- Kurdakova, V.; Quartarone, E.; Mustarelli, P.; Magistris, A.; Caponetti, E.; Saladino, M.L. PBI-based composite membranes for polymer fuel cells. J. Power Sources 2010, 195, 7765–7769. [Google Scholar] [CrossRef]
- Ghosh, S.; Maity, S.; Jana, T. Polybenzimidazole/silica nanocomposites: Organic-inorganic hybrid membranes for PEM fuel cell. J. Mater. Chem. 2011, 21, 14897–14906. [Google Scholar] [CrossRef]
- Kim, S.; Myles, T.D.; Kunz, H.R.; Kwak, D.; Wang, Y.; Maric, R. The effect of binder content on the performance of a high temperature polymer electrolyte membrane fuel cell produced with reactive spray deposition technology. Electrochim. Acta 2015, 177, 190–200. [Google Scholar] [CrossRef]
- Myles, T.D.; Kim, S.; Maric, R.; Mustain, W.E. Application of a coated film catalyst layer model to a high temperature polymer electrolyte membrane fuel cell with low catalyst loading produced by reactive spray deposition technology. Catalysts 2015, 5, 1673–1691. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Papavasiliou, J.; Daletou, M.K.; Kallitsis, J.K.; Ionnides, T.; Neophytides, S. Reforming methanol to electricity in a high temperature PEM fuel cell. Appl. Catal. B-Environ. 2009, 90, 628–632. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; Wiley: West Sussex, UK, 2003; pp. 177–187. [Google Scholar]
- Mench, M. Fuel Cell Engines; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Breault, R.D. Stack materials and stack design. In Handbook of Fuel Cells-Fundamentals, Technology and Applications; Vielstich, W., Lamm, A., Gasteiger, H.A., Eds.; Wiley: New York, NY, USA, 2003; Volume 4, pp. 797–810. [Google Scholar]
- Breault, R.D. Method for Reducing Electrolyte Loss from an Electrochemical Cell. U.S. Patent 4,414,291, 8 November 1983. [Google Scholar]
- Yu, S.; Xiao, L.; Benicewicz, B.C. Durability studies of PBI-based high temperature PEMFCs. Fuel Cells 2008, 8, 165–174. [Google Scholar] [CrossRef]
- Wannek, C.; Kohnen, B.; Oetjen, H.-F.; Lippert, H.; Mergel, J. Durability of ABPBI-based MEAs for high temperature PEMFCs at different operating conditions. Fuel Cells 2008, 8, 87–95. [Google Scholar] [CrossRef]
- Lobato, J.; Canizares, P.; Rodrigo, M.A.; Ubeda, D.; Pinar, F.J. Enhancement of the fuel cell performance of a high temperature proton exchange membrane fuel cell running with titanium composite polybenzimidazole-based membranes. J. Power Sources 2011, 196, 8265–8271. [Google Scholar] [CrossRef]
- Pinar, F.J.; Canizares, P.; Rodrigo, M.A.; Ubeda, D.; Lobato, J. Titanium composite PBI-based membranes for high temperature polymer electrolyte membrane fuel cells. Effect on titanium dioxide amount. RSC Adv. 2012, 2, 1547–1556. [Google Scholar] [CrossRef]
- Pinar, F.J.; Canizares, P.; Rodrigo, M.A.; Ubeda, D.; Lobato, J. Long-term testing of a high-temperature proton exchange membrane fuel cell short stack operated with improved polybenzimidazole-based composite membranes. J. Power Sources 2015, 274, 177–185. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Y.; Duan, W.; Wang, W.; Lin, Y.; Wang, Y.; Liu, J. Nanostructured electrocatalysts for PEM fuel cells and redox flow batteries: A selected review. ACS Catal. 2015, 5, 7288–7298. [Google Scholar] [CrossRef]
- Zhang, J. Recent advances in cathode electrocatalysts for PEM fuel cells. Front. Energy 2011, 5, 137–148. [Google Scholar] [CrossRef]
- Luczak, F.J.; Landsman, D.A. Ternary Fuel Cell Catalysts Containing Platinum, Cobalt and Chromium. U.S. Patent 4,447,506, 8 May 1984. [Google Scholar]
- Luczak, F.J.; Douglas, A. Ordered Ternary Fuel Cell Catalysts Containing Platinum and Cobalt. U.S. Patent 4,711,829, 8 December 1987. [Google Scholar]
- Luczak, F.J.; Landsman, D.A. Ordered Ternary Fuel Cell Catalysts Containing Platinum and Cobalt and Method for Making the Catalysts. U.S. Patent 4,677,092, 30 June 1987. [Google Scholar]
- Landsman, D.A.; Luczak, F.J. Noble Metal-Chromium Alloy Catalysts and Electrochemical Cell. U.S. Patent 4,316,944, 23 February 1982. [Google Scholar]
- Beard, B.C.; Ross, P.N. The structure and activity of Pt-Co alloys as oxygen reduction electrocatalysts. J. Electrochem. Soc. 1990, 137, 3368–3374. [Google Scholar] [CrossRef]
- Glass, J.T.; Cahen, G.L.; Stoner, G.E.; Taylor, E.J. The effect of metallurgical variables on the electrocatalytic properties of PtCr alloys. J. Electrochem. Soc. 1987, 134, 58–65. [Google Scholar] [CrossRef]
- Paulus, U.A.; Wokaun, A.; Scherer, G.G.; Schmidt, T.J.; Stamenkovic, V.; Radmilovic, V.; Markovic, N.M.; Ross, P.N. Oxygen reduction on carbon-supported Pt-Ni and Pt-Co alloy catalysts. J. Phys. Chem. B 2002, 106, 4181–4191. [Google Scholar] [CrossRef]
- Min, M.-K.; Cho, J.; Cho, K.; Kim, H. Particle size and alloying effects of Pt-based alloy catalysts for fuel cell applications. Electrochim. Acta 2000, 45, 4211–4217. [Google Scholar] [CrossRef]
- Aricò, A.S.; Stassi, A.; Modica, E.; Ornelas, R.; Gatto, I.; Passalacqua, E.; Antonucci, V. Performance and degradation of high temperature polymer electrolyte fuel cell catalysts. J. Power Sources 2008, 178, 525–536. [Google Scholar] [CrossRef]
- Parrondo, J.; Mijangos, F.; Rambabu, B. Platinum/tin oxide/carbon cathode catalyst for high temperature PEM fuel cell. J. Power Sources 2010, 195, 3977–3983. [Google Scholar] [CrossRef]
- Rao, C.V.; Parrondo, J.; Ghatty, S.L.; Rambabu, B. High temperature polymer electrolyte membrane fuel cell performance of PtxCoy/C cathodes. J. Power Sources 2010, 195, 3425–3430. [Google Scholar] [CrossRef]
- Jalan, V.M.; Taylor, E.J. Importance of interatomic spacing in catalytic reduction of oxygen in phosphoric acid. J. Electrochem. Soc. 1983, 130, 2299–2302. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, Y.G.; Chung, J.S. Effect of surface roughening on the catalytic activity of Pt-Cr electrocatalysts for the oxygen reduction in phosphoric acid fuel cell. J. Electrochem. Soc. 1995, 142, 1531–1538. [Google Scholar] [CrossRef]
- Daube, K.A.; Paffett, M.T.; Gottesfeld, S.; Campbell, C.T. Combined electrochemical/surface science investigations of Pt/Cr alloy electrodes. J. Vac. Sci. Technol. A 1986, 4, 1617–1620. [Google Scholar] [CrossRef]
- Paffett, M.T.; Beery, J.G.; Gottesfeld, S. Oxygen reduction at Pt0.65Cr0.35, Pt0.2Cr0.8 and roughened platinum. J. Electrochem Soc. 1988, 135, 1431–1436. [Google Scholar] [CrossRef]
- Zagudaeva, N.M.; Tarasevich, M.R. Electrochemical characteristics of platinum-based binary catalysts for middle-temperature hydrogen-air fuel cells with phosphoric acid electrolyte. Russ. J. Electrochem. 2010, 46, 530–536. [Google Scholar] [CrossRef]
- Schenk, A.; Grimmer, C.; Perchthaler, M.; Weinberger, S.; Pichler, B.; Heinzl, C.; Scheu, C.; Mautner, F.-A.; Bitschnau, B.; Hacker, V. Platinum-cobalt catalysts for the oxygen reduction reaction in high temperature proton exchange membrane fuel cells—Long term behavior under ex-situ and in-situ conditions. J. Power Sources 2014, 266, 313–322. [Google Scholar] [CrossRef]
- Zamora, H.; Canizares, P.; Rodrigo, M.A.; Lobato, J. Improving of micro porous layer based on advanced carbon materials for high temperature proton exchange membrane fuel cell electrodes. Fuel Cells 2015, 15, 375–383. [Google Scholar] [CrossRef]
- Zamora, H.; Plaza, J.; Canizares, P.; Lobato, J.; Rodrigo, M.A. Improved electrodes for high temperature proton exchange membrane fuel cells using carbon nanospheres. ChemSusChem 2016, 9, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Lobato, J.; Zamora, H.; Canizares, P.; Plaza, J.; Rodrigo, M.A. Microporous layer based on SiC for high temperature proton exchange membrane fuel cells. J. Power Sources 2015, 288, 288–295. [Google Scholar] [CrossRef]
- Lobato, J.; Zamora, H.; Plaza, J.; Canizares, P.; Rodrigo, M.A. Enhancement of high temperature PEMFC stability using catalysts based on Pt supported on SiC based materials. Appl. Catal. B Environ. 2016, 198, 516–524. [Google Scholar] [CrossRef]
- Lobato, J.; Zamora, H.; Plaza, J.; Rodrigo, M.A. Composite titanium silicon carbide as a promising catalyst support for high-temperature proton-exchange membrane fuel cell electrodes. ChemCatChem 2016, 8, 848–854. [Google Scholar] [CrossRef]
- Razaq, M.; Yeager, E.; DesMarteau, D.D.; Singh, S. Perfluorosulfonimide as an additive in phosphoric acid fuel cell. J. Electrochem. Soc. 1989, 136, 385–390. [Google Scholar] [CrossRef]
- Gang, X.; Hjuler, H.A.; Olsen, C.; Berg, R.W.; Bjerrum, N.J. Electrolyte additives for phosphoric acid fuel cells. J. Electrochem. Soc. 1993, 140, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Qingfeng, L.; Gang, X.; Hjuler, H.A.; Berg, R.W.; Bjerrum, N.J. Limiting current of oxygen reduction on gas-diffusion electrodes for phosphoric acid fuel cells. J. Electrochem. Soc. 1994, 141, 3114–3119. [Google Scholar] [CrossRef] [Green Version]
- Qingfeng, L.; Gang, X.; Hjuler, H.A.; Berg, R.W.; Bjerrum, N.J. Oxygen reduction on gas diffusion electrodes for phosphoric acid fuel cells by a potential decay method. J. Electrochem Soc. 1995, 142, 3250–3256. [Google Scholar] [CrossRef] [Green Version]
- Qingfeng, L.; Hjuler, H.A.; Bjerrum, N.J. Oxygen reduction on carbon supported platinum catalysts in high temperature polymer electrolytes. Electrochim. Acta 2000, 45, 4219–4226. [Google Scholar] [CrossRef]
- Hong, S.-G.; Kwon, K.; Lee, M.-J.; Yoo, D.Y. Performance enhancement of phosphoric acid-based proton exchange membrane fuel cells by using ammonium trifluoromethanesulfonate. Electrochem. Commun. 2009, 11, 1124–1126. [Google Scholar] [CrossRef]
- Mamlouk, M.; Scott, K. The effect of electrode parameters on performance of a phosphoric acid-doped PBI membrane fuel cell. Int. J. Hydrog. Energy 2010, 35, 784–793. [Google Scholar] [CrossRef]
- Mamlouk, M.; Scott, K. Phosphoric acid-doped electrodes for a PBI polymer membrane fuel cell. Int. J. Energy Res. 2011, 35, 507–519. [Google Scholar] [CrossRef]
- Adams, A.A.; Foley, R.T.; Barger, H.J., Jr. The electroreduction of air in trifluoromethanesulfonic acid monohydrate. J. Electrochem. Soc. 1977, 124, 1228–1230. [Google Scholar] [CrossRef]
- Enayetullah, M.A.; Devilbiss, T.D.; Bockris, J.O.M. Activation parameters for oxygen reduction kinetics in trifluoromethane sulfonic acid systems. J. Electrochem. Soc. 1989, 136, 3369–3376. [Google Scholar] [CrossRef]
- George, M. Aqueous Trifluoromethanesulfonic Acid Fuel Cells; Technical Report, U.S. Army Mobility Equipment Research and Development Command, Contract No. DAAK70–78-C-0103; Ft. Belvoir Defense Technical Information Center: Fort Belvoir, VA, USA, 1979. [Google Scholar]
- Baker, B. Electrolyte for Hydrocarbon Air Fuel Cells; Final Technical Report, U.S. Army Mobility Equipment Research and Development Command, Contract No. DAAK02–73-C-0084; Ft. Belvoir Defense Technical Information Center: Fort Belvoir, VA, USA, 1975. [Google Scholar]
- Liu, Z.; Wainright, J.S.; Litt, M.H.; Savinell, R.F. Study of the oxygen reduction reaction (ORR) at Pt interfaced with phosphoric acid doped polybenzimidazole at elevated temperature and low relative humidity. Electrochim. Acta 2006, 51, 3914–3923. [Google Scholar] [CrossRef]
- Kwon, K.; Lee, M.-J. Effect of calixpyrrole on electrochemical properties of Pt electrocatalyst in phosphoric acid electrolyte. Electrochim. Acta 2008, 54, 513–517. [Google Scholar] [CrossRef]
- Striebel, K.A.; Andricacos, P.C.; Cairns, E.J.; Ross, P.N.; McLarnon, F.R. Oxygen reduction in tetrafluoroethane-l,2-disulfonic acid. J. Electrochem. Soc. 1985, 132, 2381–2384. [Google Scholar] [CrossRef]
- Saffarian, H.; Ross, P.; Behr, F.; Gard, G. Electrochemical properties of perfluoroalkane disulfonic [HSO3(CF2)nSO3H] acids relevant to fuel cell technology. J. Electrochem. Soc. 1992, 139, 2391–2397. [Google Scholar] [CrossRef]
- Kanamura, K.; Tanaka, A.; Gervasio, D.; Kennedy, V.; Adzic, R.; Yeager, E.B.; Burton, D.; Guneratne, R. Perfluoro-ethylene-1,2-bis-phosphonic acid fuel cell electrolyte. J. Electrochem. Soc. 1996, 143, 2765–2770. [Google Scholar] [CrossRef]
- Heider, E.; Ignatiev, N.; Jörissen, L.; Wenda, A.; Zeis, R. Fluoroalkyl phosphoric acid derivatives—Model compounds to study the adsorption of electrolyte species on polycrystalline platinum. Electrochem. Commun. 2014, 48, 24–27. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Bejan, D.; Willner, H. Perfluoroalkylphosphorus acids synthesis, properties and applications in catalysis. Oggi/Chem. Today 2011, 29, 32–34. [Google Scholar]
- Herath, M.B.; Creager, S.E.; Kitaygorodskiy, A.; DesMarteau, D.D. Perfluoroalkyl phosphonic and phosphinic acids as proton conductors for anhydrous proton-exchange membranes. ChemPhysChem 2010, 11, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Herath, M.B.; Creager, S.E.; Kitaygorodskiy, A.; DesMarteau, D.D. Effect of perfluoroalkyl chain length on proton conduction in fluoroalkylated phosphonic, phosphinic, and sulfonic acids. J. Phys. Chem. B 2010, 114, 14972–14976. [Google Scholar] [CrossRef] [PubMed]
- Heider, E.; Jusys, Z.; Behm, R.J.; Jörissen, L.; Zeis, R. Perfluoroalkyl-phosphonic acid adsorption on polycrystalline platinum and its influence on the oxygen reduction reaction. J. Phys. Chem. C 2015, 119, 18859–18869. [Google Scholar] [CrossRef]
- Mack, F.; Galbiati, S.; Gogel, V.; Jörissen, L.; Zeis, R. Evaluation of electrolyte additives for high-temperature polymer electrolyte fuel cells. ChemElectroChem 2016, 3, 770–773. [Google Scholar] [CrossRef]
- Lee, K.-S.; Yoo, S.J.; Ahn, D.; Kim, S.-K.; Hwang, S.J.; Sung, Y.-E.; Kim, H.-J.; Cho, E.; Henkensmeier, D.; Lim, T.-H.; et al. Phosphate adsorption and its effect on oxygen reduction reaction for PtxCoy alloy and Aucore-Ptshell electrocatalysts. Electrochim. Acta 2011, 56, 8802–8810. [Google Scholar] [CrossRef]
- He, Q.; Shyam, B.; Nishijima, M.; Ramaker, D.; Mukerjee, S. Mitigating phosphate anion poisoning of cathodic Pt/C catalysts in phosphoric acid fuel cells. J. Phys. Chem. C 2013, 117, 4877–4887. [Google Scholar] [CrossRef]
- Tanaka, A.; Adzic, R.; Nikolic, B. Oxygen reduction on single crystal platinum electrodes in phosphoric acid solutions. J. Serb. Chem. Soc. 1999, 64, 695–705. [Google Scholar]



© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myles, T.; Bonville, L.; Maric, R. Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells. Catalysts 2017, 7, 16. https://doi.org/10.3390/catal7010016
Myles T, Bonville L, Maric R. Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells. Catalysts. 2017; 7(1):16. https://doi.org/10.3390/catal7010016
Chicago/Turabian StyleMyles, Timothy, Leonard Bonville, and Radenka Maric. 2017. "Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells" Catalysts 7, no. 1: 16. https://doi.org/10.3390/catal7010016




