Morpholine-Modified Pd/γ-Al2O3@ASMA Pellet Catalyst with Excellent Catalytic Selectivity in the Hydrogenation of p-Chloronitrobenzene to p-Chloroaniline
Abstract
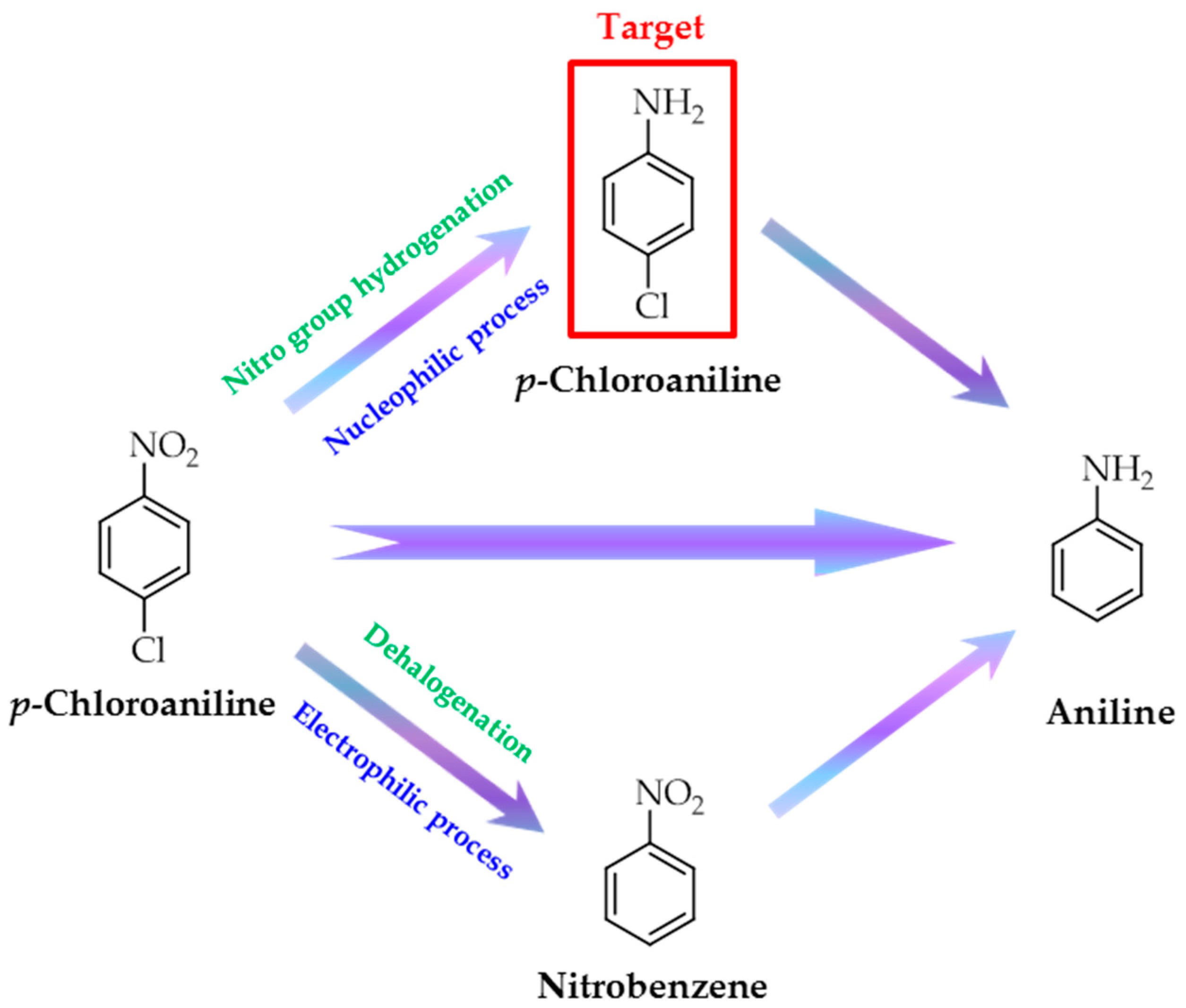
:1. Introduction
2. Results and Discussion
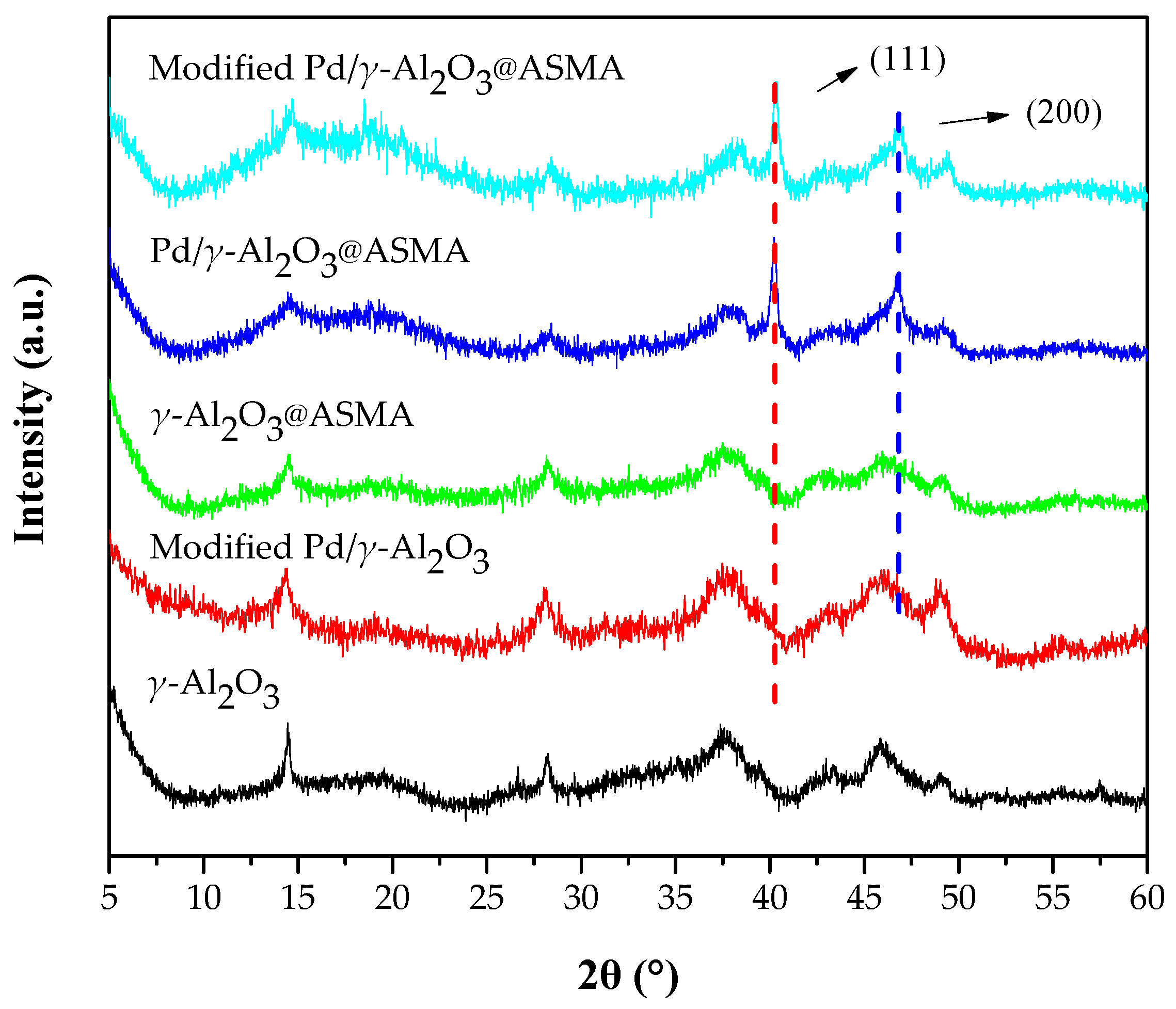
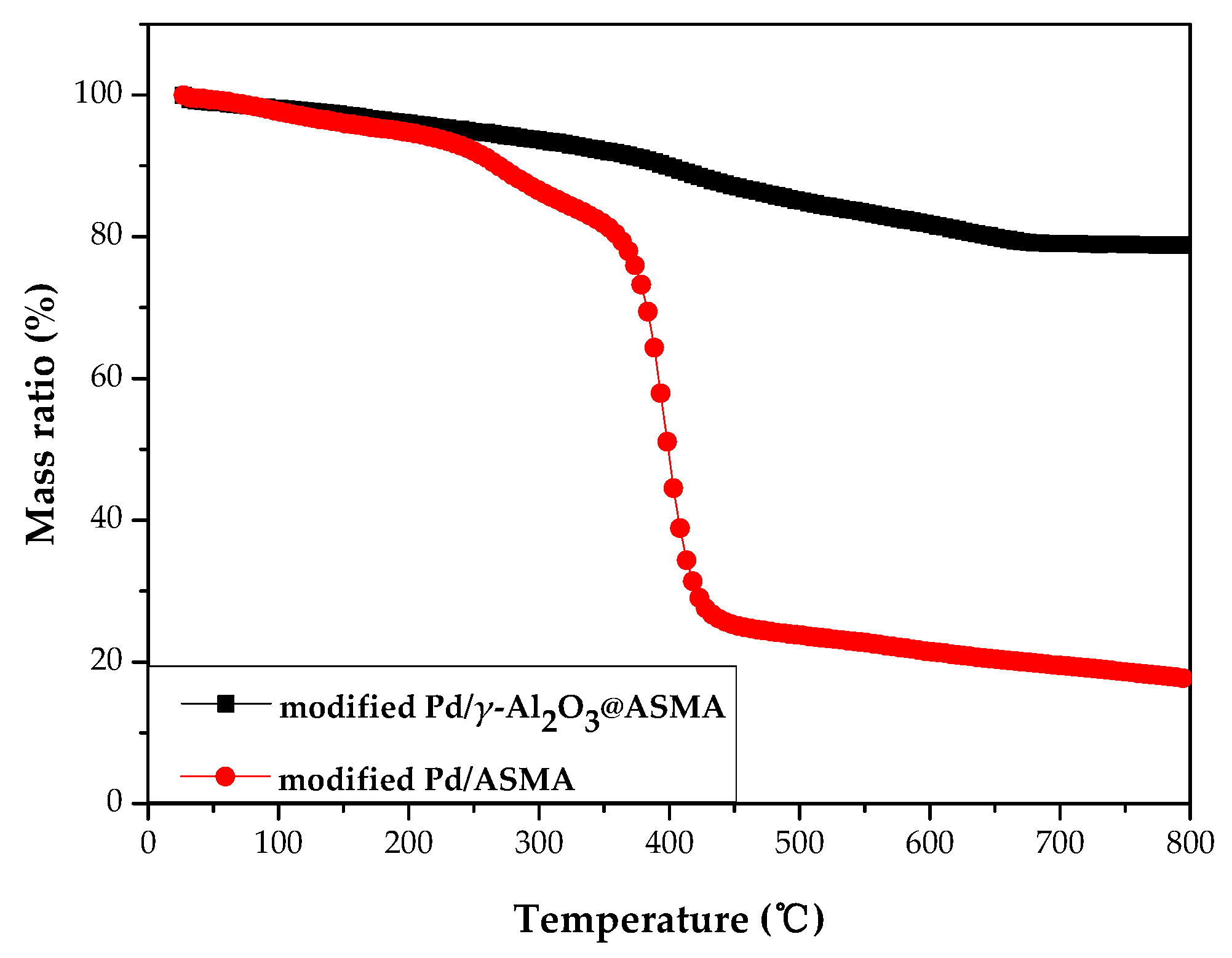
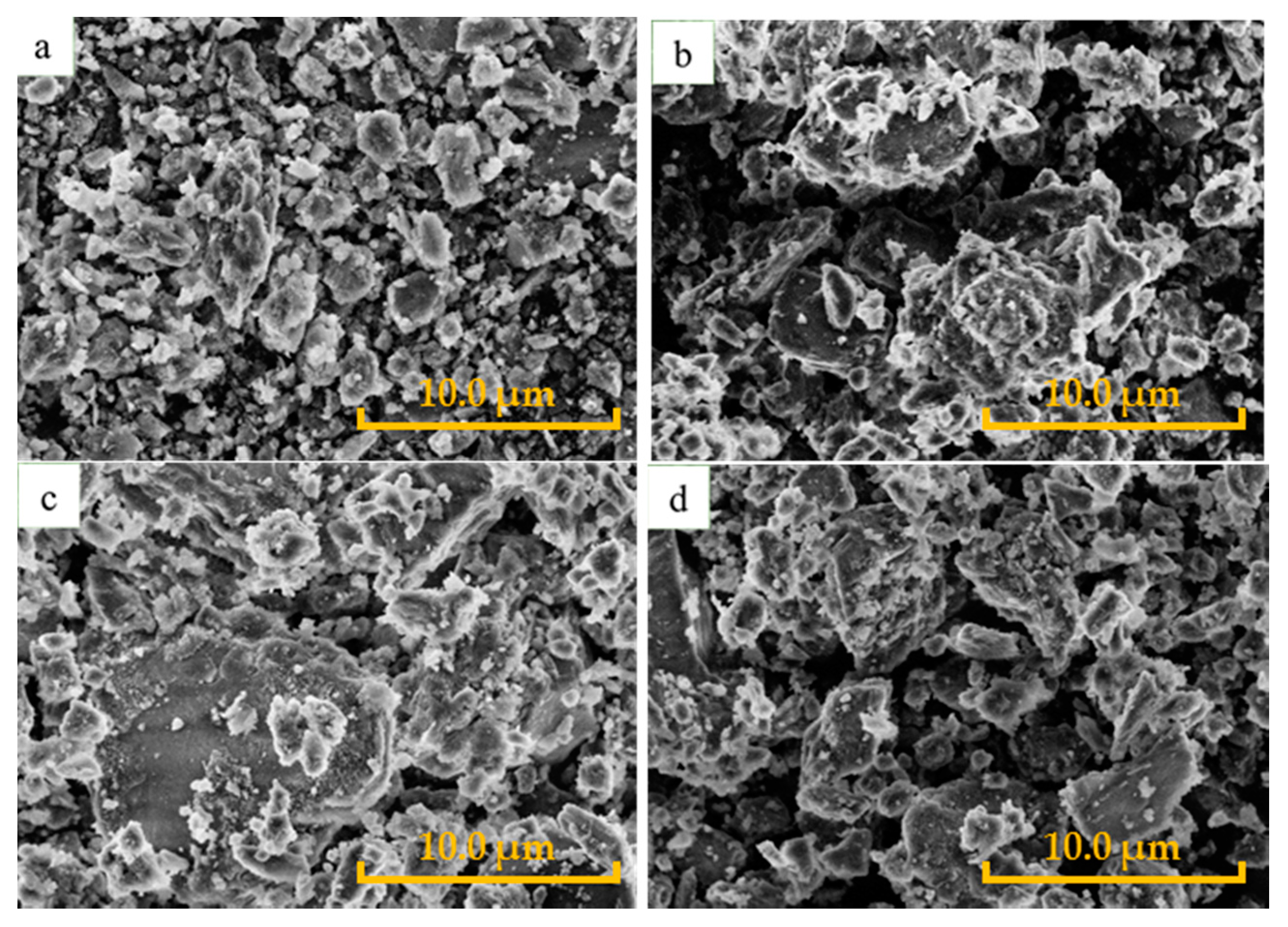
2.1. Catalyst Characterization
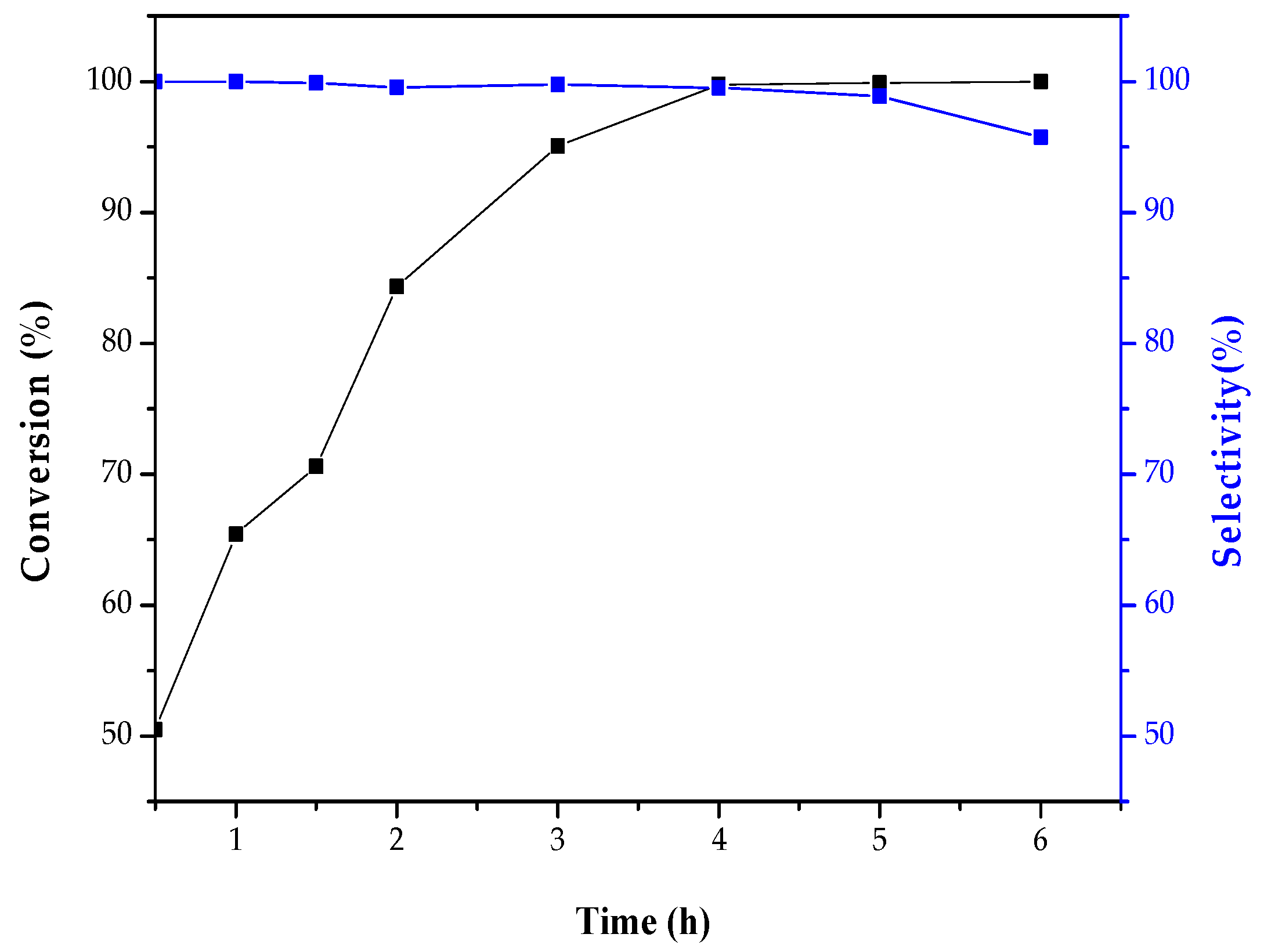
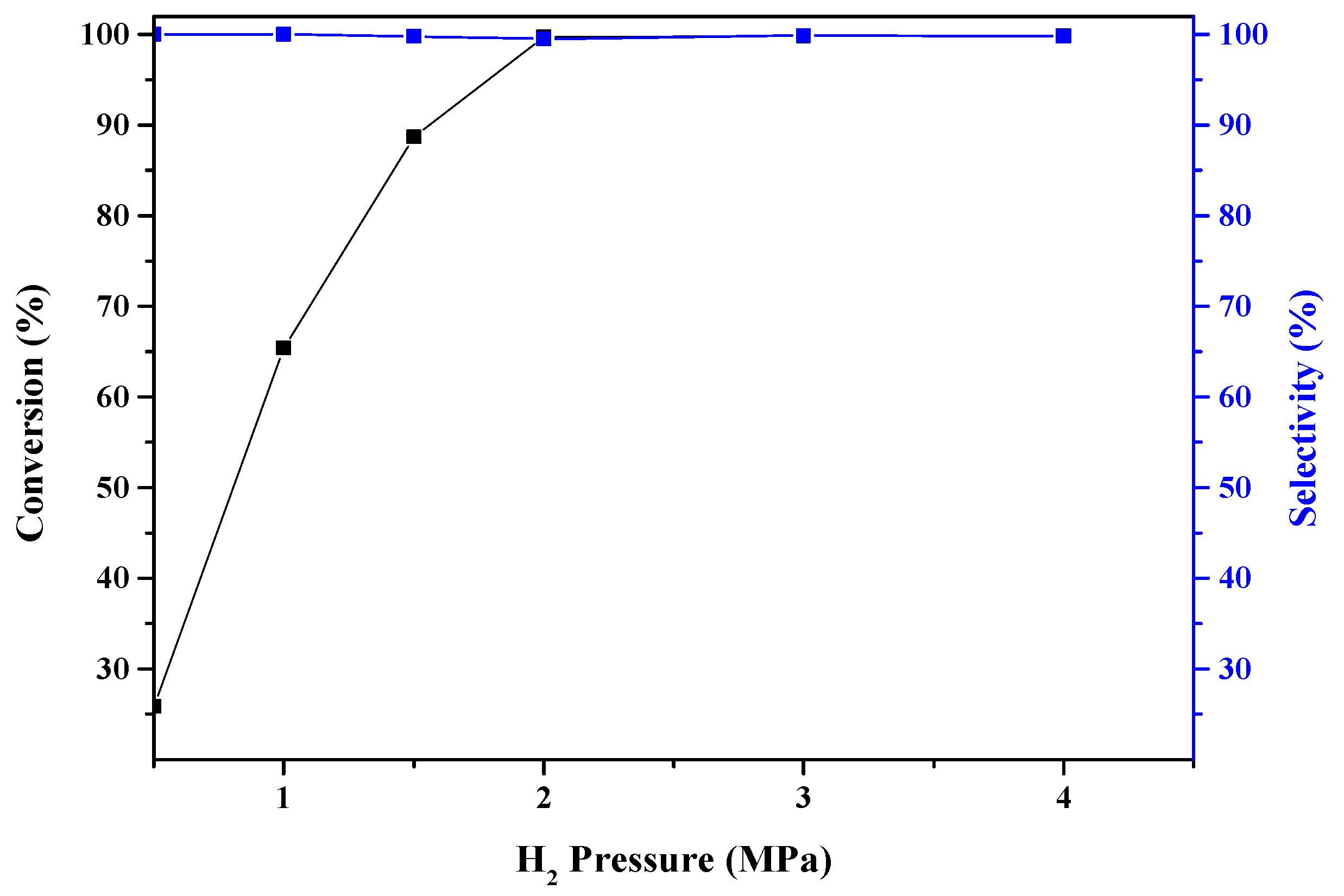
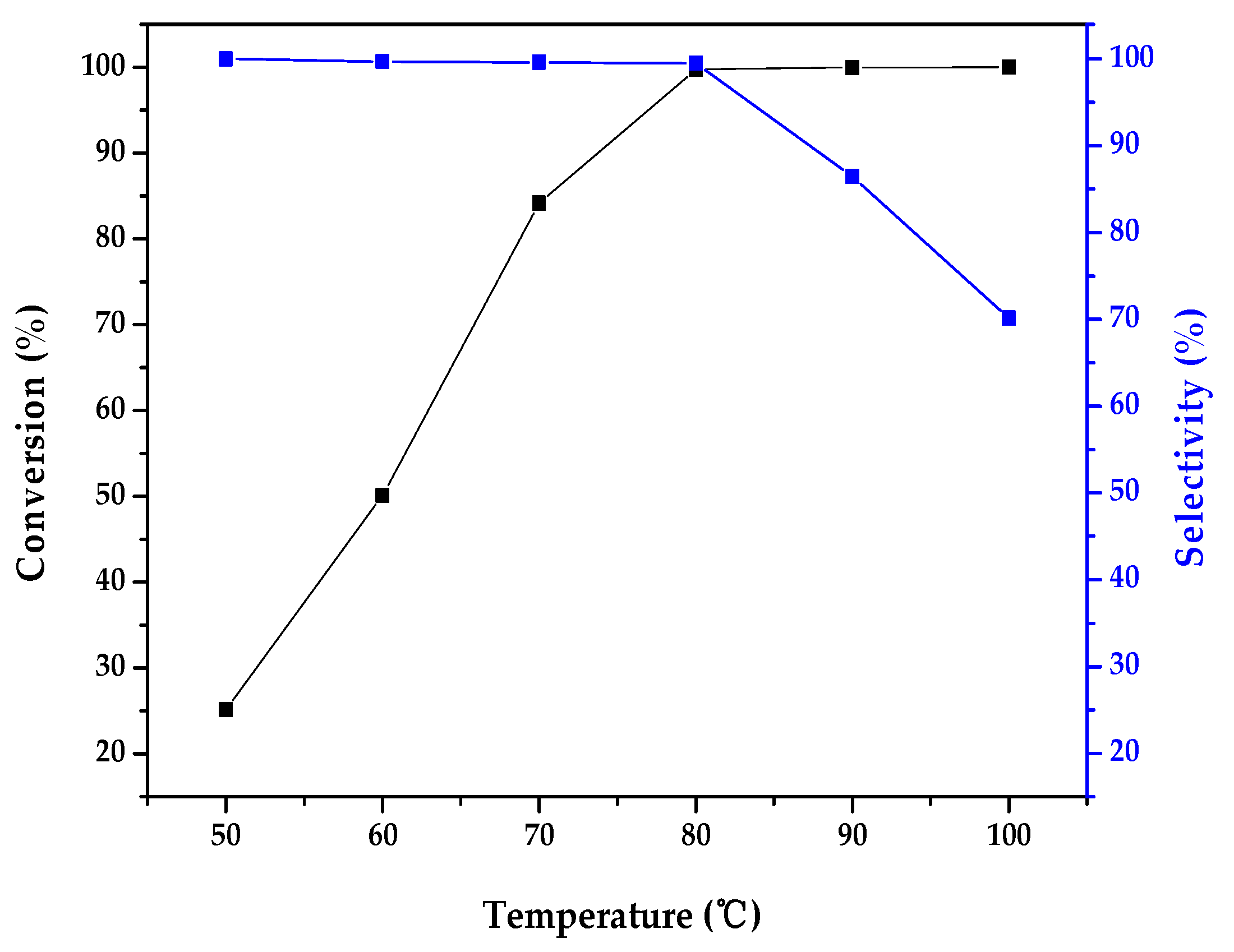
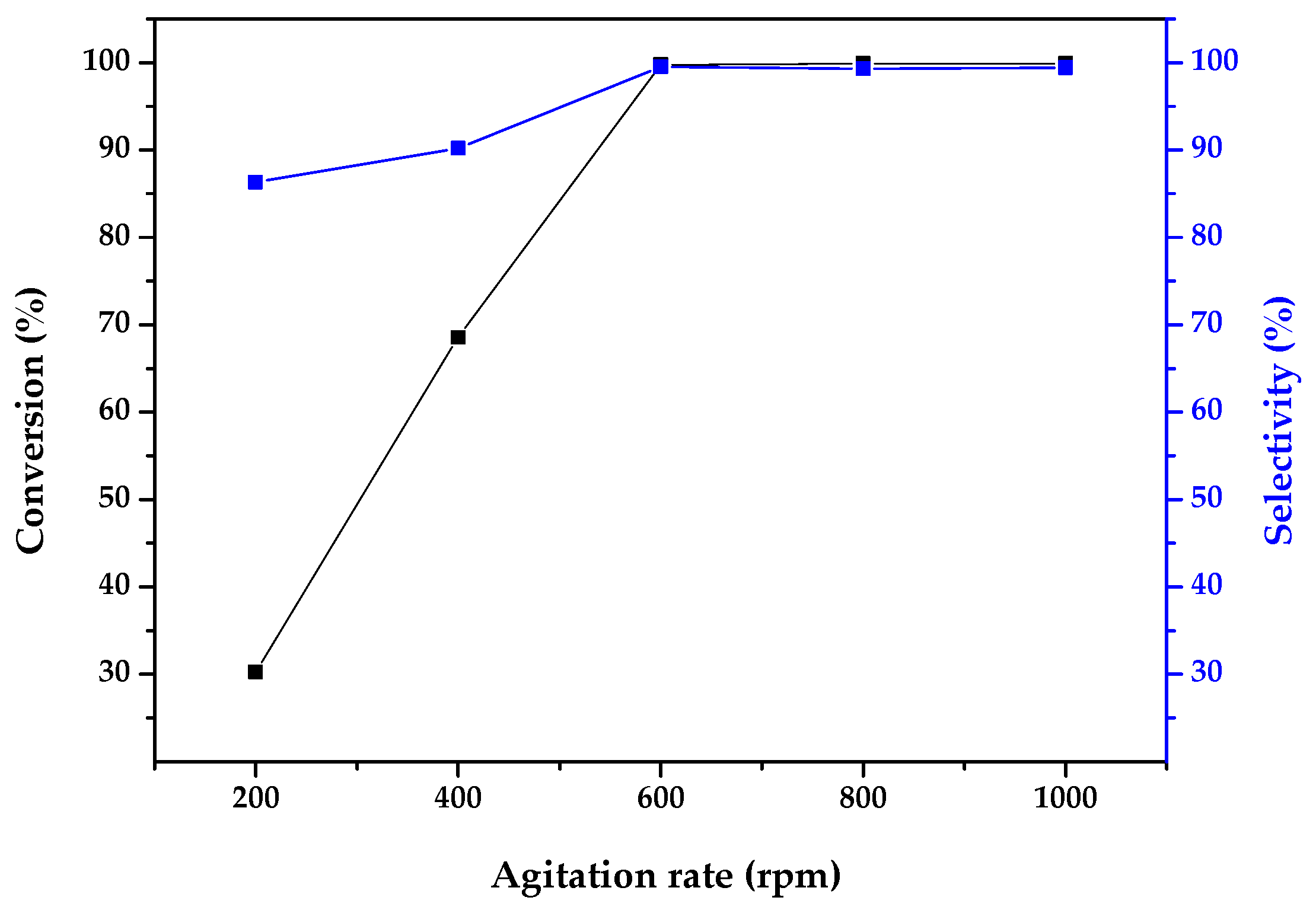
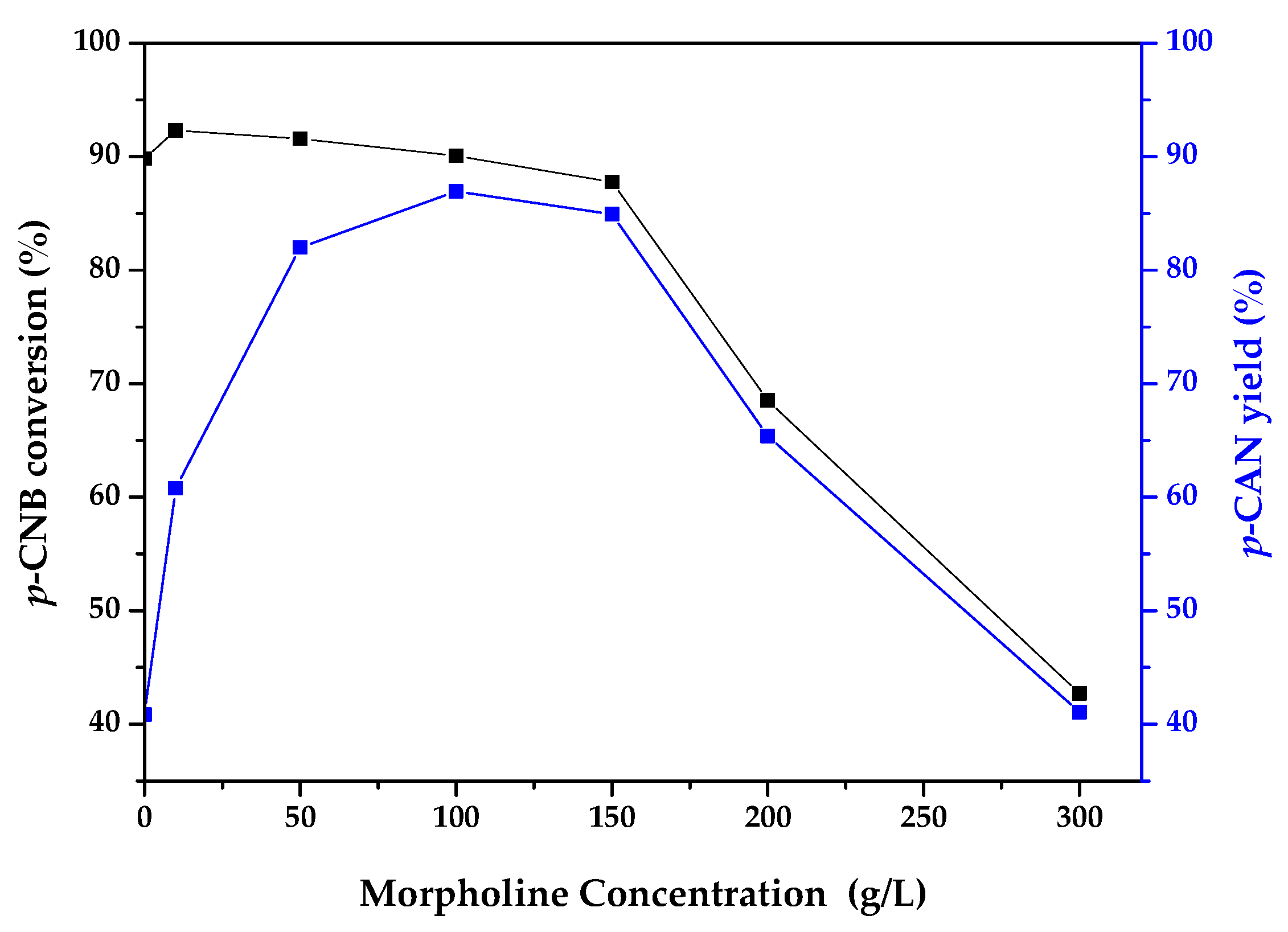
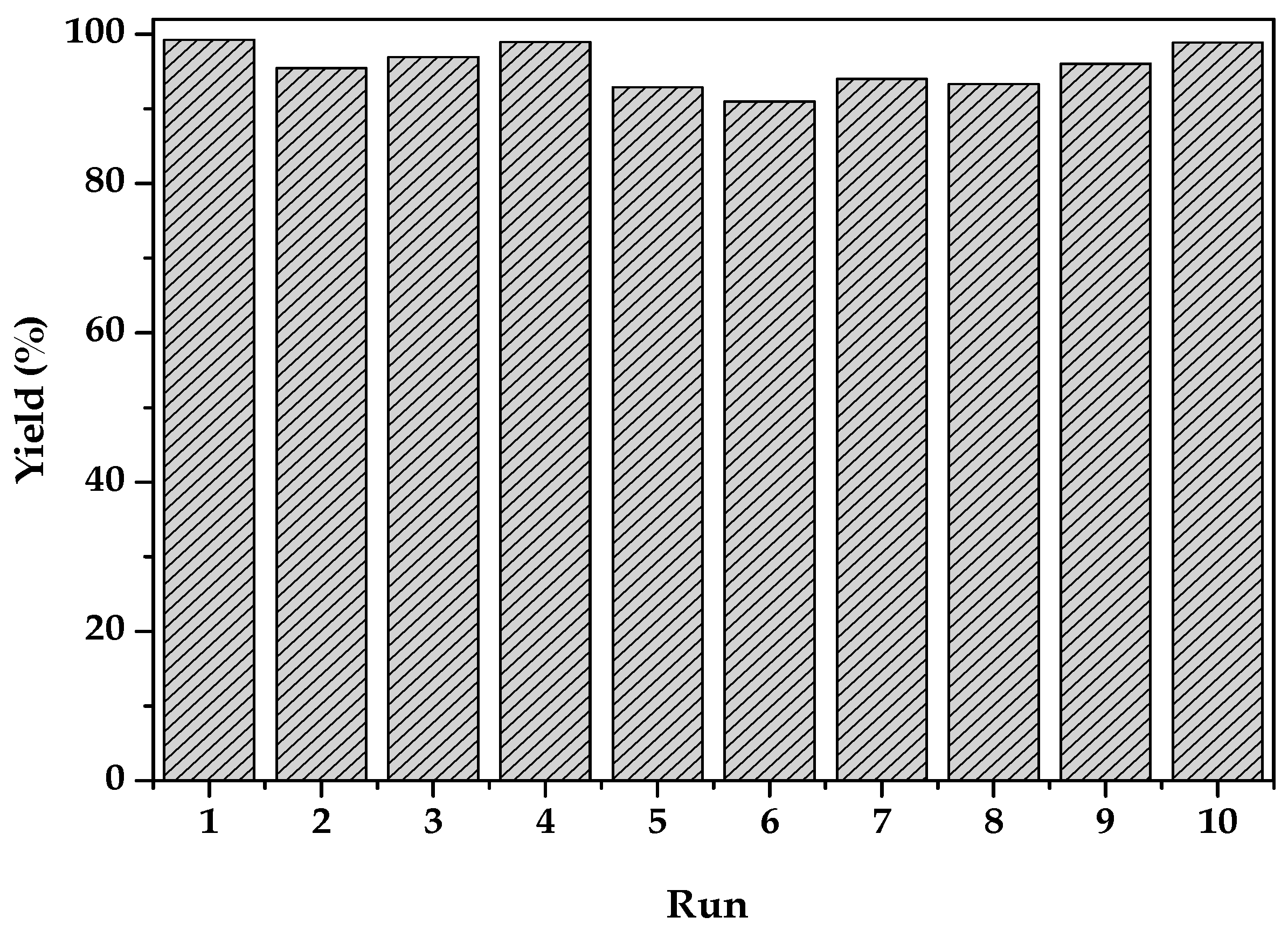
2.2. Catalytic Activity
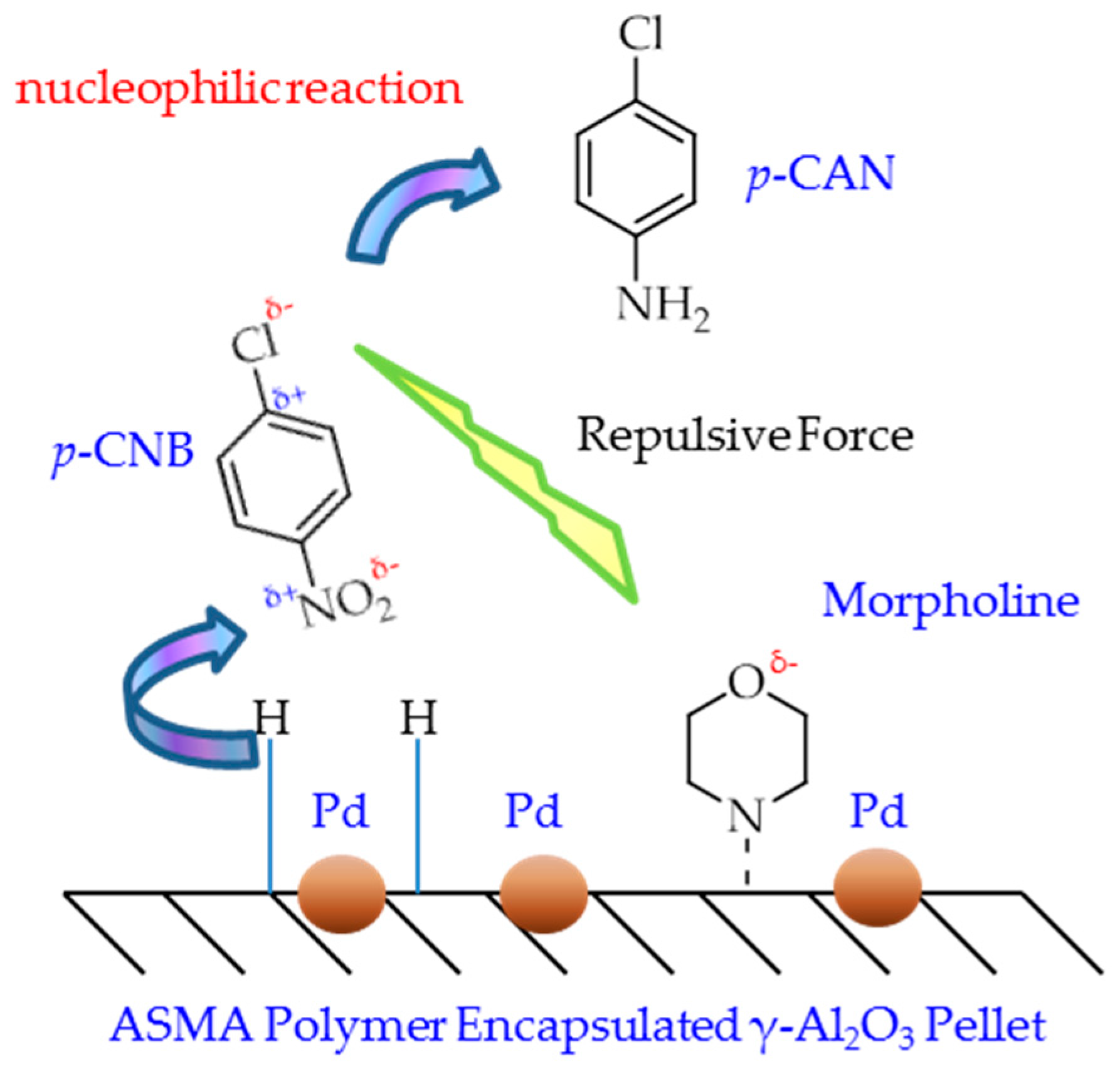
2.3. Catalytic Mechanism Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Hydrogenation of p-CNB Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, B.; Chen, Y.-W. Hydrogenation of p-chloronitrobenzene on Mo, La, Fe, and W-modified NiCoB nanoalloy catalysts. J. Non Cryst. Solids 2010, 356, 839–847. [Google Scholar] [CrossRef]
- Lin, M.H.; Zhao, B.; Chen, Y.W. Hydrogenation of p-Chloronitrobenzene over Mo-Modified NiCoB Nanoalloy Catalysts: Effect of Mo Content. Ind. Eng. Chem. Res. 2009, 48, 7037–7043. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Molga, E.J.; Gelder, K.B.V. Catalytic hydrogenation reactors for the fine chemicals industries. Their design and operation. Chem. Eng. Process. 1997, 36, 17–27. [Google Scholar] [CrossRef]
- Pietrowski, M.; Wojciechowska, M. The origin of increased chemoselectivity of platinum supported on magnesium fluoride in the hydrogenation of chloronitrobenzene. Catal. Today 2011, 169, 217–222. [Google Scholar] [CrossRef]
- Menini, C.; Park, C.; Brydson, R.; Keane, M.A. Low-Temperature (553 K) Catalytic Growth of Highly Ordered Carbon Filaments during Hydrodechlorination Reactions. J. Phys. Chem. B 2000, 104, 4281–4284. [Google Scholar] [CrossRef]
- Liang, M.; Wang, X.; Liu, H.; Liu, H.; Wang, Y. Excellent catalytic properties over nanocomposite catalysts for selective hydrogenation of halonitrobenzenes. J. Catal. 2008, 255, 335–342. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Berguerand, C.; Yuranov, I.; Kiwi-Minsker, L. Chemoselective hydrogenation of nitroarenes: Boosting nanoparticle efficiency by confinement within highly porous polymeric framework. J. Catal. 2013, 301, 103–111. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, C.; Cen, J.; Feng, F.; Ma, L.; Lu, C.; Li, X. The Modification of Diphenyl Sulfide to Pd/C Catalyst and Its Application in Selective Hydrogenation of p-Chloronitrobenzene. Chin. J. Chem. Eng. 2014, 22, 1111–1116. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Amorim, C.; Keane, M.A. Gas phase hydrogenation of p-chloronitrobenzene over Pd–Ni/Al2O3. Appl. Catal. A 2014, 473, 41–50. [Google Scholar] [CrossRef]
- Fan, G.-Y.; Huang, W.-J. Synthesis of ruthenium/reduced graphene oxide composites and application for the selective hydrogenation of halonitroaromatics. Chin. Chem. Lett. 2014, 25, 359–363. [Google Scholar] [CrossRef]
- Beswick, O.; Lamey, D.; Muriset, F.; Lagrange, T.; Oberson, L.; Yoon, S.; Sulman, E.; Dyson, P.J.; Kiwi-Minsker, L. Ni-based structured catalyst for selective 3-phase hydrogenation of nitroaromatics. Catal. Today 2016, 273, 244–251. [Google Scholar] [CrossRef]
- Fu, T.; Hu, P.; Wang, T.; Dong, Z.; Xue, N.; Peng, L.; Guo, X.; Ding, W. High selectivity to p-chloroaniline in the hydrogenation of p-chloronitrobenzene on Ni modified carbon nitride catalyst. Chin. J. Catal. 2015, 36, 2030–2035. [Google Scholar] [CrossRef]
- Chen, T.; Li, D.; Jiang, H.; Xiong, C. High-performance Pd nanoalloy on functionalized activated carbon for the hydrogenation of nitroaromatic compounds. Chem. Eng. J. 2015, 259, 161–169. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Preparation and characterization of PdO nanoparticles on trivalent metal (B, Al and Ga) substituted MCM-41: Excellent catalytic activity in supercritical carbon dioxide. J. Colloid Interface Sci. 2014, 420, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kratky, V.; Kralik, M.; Mecarova, M.; Stolcova, M.; Zalibera, L.; Hronec, M. Effect of catalyst and substituents on the hydrogenation of chloronitrobenzenes. Appl. Catal. A 2002, 235, 225–231. [Google Scholar] [CrossRef]
- Tu, W.; Cao, S.; Yang, L.; Wang, W. Modification effects of magnetic supports and bimetallic structures on palladium nanocluster catalysts. Chem. Eng. J. 2008, 143, 244–248. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Hugon, A.; Delannoy, L.; Louis, C.; Keane, M.A. Pd-promoted selective gas phase hydrogenation of p-chloronitrobenzene over alumina supported Au. J. Catal. 2009, 262, 235–243. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Ilchenko, I.A.; Sheinker, V.N.; Bulatov, A.V. Heterogenization of palladium(II) chelates on a sibunite. Transit. Met. Chem. 1991, 16, 293–295. [Google Scholar] [CrossRef]
- Yu, Z.; Liao, S.; Xu, Y.; Yang, B.; Yu, D. Hydrogenation of nitroaromatics by polymer-anchored bimetallic palladium-ruthenium and palladium-platinum catalysts under mild conditions. J. Mol. Catal. A Chem. 1997, 120, 247–255. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Malpartida, I.; Alemany, L.J. Transient study of the dry reforming of methane over Pt supported on different γ-Al2O3. Catal. Today 2010, 149, 380–387. [Google Scholar] [CrossRef]
- Skotak, M.; Karpinski, Z.; Juszczyk, W.; Pielaszek, J.; Kepinski, L.; Kazachkin, D.; Kovalchuk, V.; Ditri, J. Characterization and catalytic activity of differently pretreated Pd/Al2O3 catalysts: The role of acid sites and of palladium–alumina interactions. J. Catal. 2004, 227, 11–25. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El-Din, M.R.N.; Morsi, R.E.; Elsabee, M.Z. Demulsification efficiency of some novel styrene/maleic anhydride ester copolymers. J. Appl. Polym. Sci. 2008, 108, 2301–2311. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dabbawala, A.A.; Hwang, J.-S. Poly (styrene-co-divinylbenzene) amine functionalized polymer supported ruthenium nanoparticles catalyst active in hydrogenation of xylose. Catal. Commun. 2013, 41, 52–55. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, Y.; Yu, B.; Zhang, H.; Xu, H.; Xu, J.; Liu, Z. Polyurea-supported metal nanocatalysts: Synthesis, characterization and application in selective hydrogenation of o-chloronitrobenzene. J. Colloid Interface Sci. 2014, 424, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Qiu, T.; Guo, L.; Ye, J.; Yuan, Y.; Li, X. The aminolysis of styrene–maleic anhydride copolymers for a new modifier used in urea-formaldehyde resins. Int. J. Adhes. Adhes. 2016, 66, 138–146. [Google Scholar] [CrossRef]
- Plessers, E.; Fu, G.; Tan, C.; De Vos, D.; Roeffaers, M. Zr-Based MOF-808 as Meerwein–Ponndorf–Verley Reduction Catalyst for Challenging Carbonyl Compounds. Catalysts 2016, 6, 104. [Google Scholar] [CrossRef]
- Kozell, V.; Giannoni, T.; Nocchetti, M.; Vivani, R.; Piermatti, O.; Vaccaro, L. Immobilized Palladium Nanoparticles on Zirconium Carboxy-Aminophosphonates Nanosheets as an Efficient Recoverable Heterogeneous Catalyst for Suzuki–Miyaura and Heck Coupling. Catalysts 2017, 7, 186. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Y.; Zhang, F.; Zhong, Y.; Zhu, W. Catalytic hydrogenation of 2,3,5-trimethylbenzoquinone over Pd nanoparticles confined in the cages of MIL-101(Cr). Chem. Eng. J. 2014, 239, 33–41. [Google Scholar] [CrossRef]
- Han, D.; Li, X.; Zhang, L.; Wang, Y.; Yan, Z.; Liu, S. Hierarchically ordered meso/macroporous γ-alumina for enhanced hydrodesulfurization performance. Microporous Mesoporous Mater. 2012, 158, 1–6. [Google Scholar] [CrossRef]
- Vermeesch, I.; Groeninckx, G. Chemical Modification of Poly(styrene-co-maleic anhydride) with Primary N-alkylamines by Reactive Extrusion. J. Appl. Polym. Sci. 1994, 53, 1365–1373. [Google Scholar] [CrossRef]
- Johnson, J.B.; Funk, G. Determination of carboxylic acid anhydrides by reaction with morpholine. Anal. Chem. 1955, 27, 1464–1465. [Google Scholar] [CrossRef]
- Antonetti, C.; Oubenali, M.; Raspolli Galletti, A.M.; Serp, P.; Vannucci, G. Novel microwave synthesis of ruthenium nanoparticles supported on carbon nanotubes active in the selective hydrogenation of p-chloronitrobenzene to p-chloroaniline. Appl. Catal. A 2012, 421, 99–107. [Google Scholar] [CrossRef]
- Baumeister, P.; Blaser, H.U.; Scherrer, W. Chemoselective Hydrogenation of Aromatic Chloronitro Compounds with Amidine Modified Nickel Catalysts. Stud. Surf. Sci. Catal. 1991, 59, 321–328. [Google Scholar]
- Shen, J.-H.; Chen, Y.-W. Catalytic properties of bimetallic NiCoB nanoalloy catalysts for hydrogenation of p-chloronitrobenzene. J. Mol. Catal. A Chem. 2007, 273, 265–276. [Google Scholar] [CrossRef]
- Coq, B.; Tijani, A.; Dutartre, R.; Figuéras, F. Influence of support and metallic precursor on the hydrogenation of p-chloronitrobenzene over supported platinum catalysts. J. Mol. Catal. 1993, 79, 253–264. [Google Scholar] [CrossRef]
- Menini, C.; Park, C.; Shin, E.-J.; Tavoularis, G.; Keane, M.A. Catalytic hydrodehalogenation as a detoxification methodology. Catal. Today 2000, 62, 355–366. [Google Scholar] [CrossRef]


| Material | SABET a (m2/g) | PDavg b (nm) | PVtotal c (cm3/g) |
|---|---|---|---|
| γ-Al2O3 | 231.31 | 4.83 | 0.318 |
| γ-Al2O3@ASMA | 232.98 | 5.82 | 0.429 |
| Pd/γ-Al2O3@ASMA | 145.13 | 2.41 | 0.132 |
| Modified Pd/γ-Al2O3@ASMA | 62.34 | 2.41 | 0.066 |
| Pd Loading (wt %) | Conversion (%) | Selectivity (%) |
|---|---|---|
| 0.14 | 84.62 | 100 |
| 0.27 | 99.74 | 99.51 |
| 0.53 | 92.38 | 91.30 |
| 1.06 | 94.77 | 80.22 |
| 2.12 | 98.82 | 71.52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Xu, W.; Thapa, K.B.; Yang, X.; Liang, J.; Zhu, L.; Zhu, J. Morpholine-Modified Pd/γ-Al2O3@ASMA Pellet Catalyst with Excellent Catalytic Selectivity in the Hydrogenation of p-Chloronitrobenzene to p-Chloroaniline. Catalysts 2017, 7, 292. https://doi.org/10.3390/catal7100292
Wang W, Xu W, Thapa KB, Yang X, Liang J, Zhu L, Zhu J. Morpholine-Modified Pd/γ-Al2O3@ASMA Pellet Catalyst with Excellent Catalytic Selectivity in the Hydrogenation of p-Chloronitrobenzene to p-Chloroaniline. Catalysts. 2017; 7(10):292. https://doi.org/10.3390/catal7100292
Chicago/Turabian StyleWang, Wei, Wenlong Xu, Kedar Bahadur Thapa, Xiaorui Yang, Jinhua Liang, Liyan Zhu, and Jianliang Zhu. 2017. "Morpholine-Modified Pd/γ-Al2O3@ASMA Pellet Catalyst with Excellent Catalytic Selectivity in the Hydrogenation of p-Chloronitrobenzene to p-Chloroaniline" Catalysts 7, no. 10: 292. https://doi.org/10.3390/catal7100292






