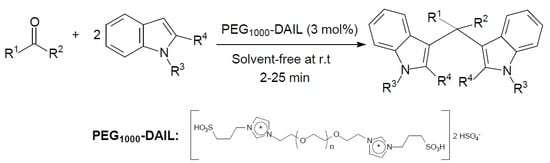
PEG1000-Based Dicationic Acidic Ionic Liquid/Solvent-Free Conditions: An Efficient Catalytic System for the Synthesis of Bis(Indolyl)methanes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Different Reaction Conditions
2.2. Effects of Different Substrates
2.3. Effects of Different Catalysts
3. Experimental Section
3.1. Materials and Methods
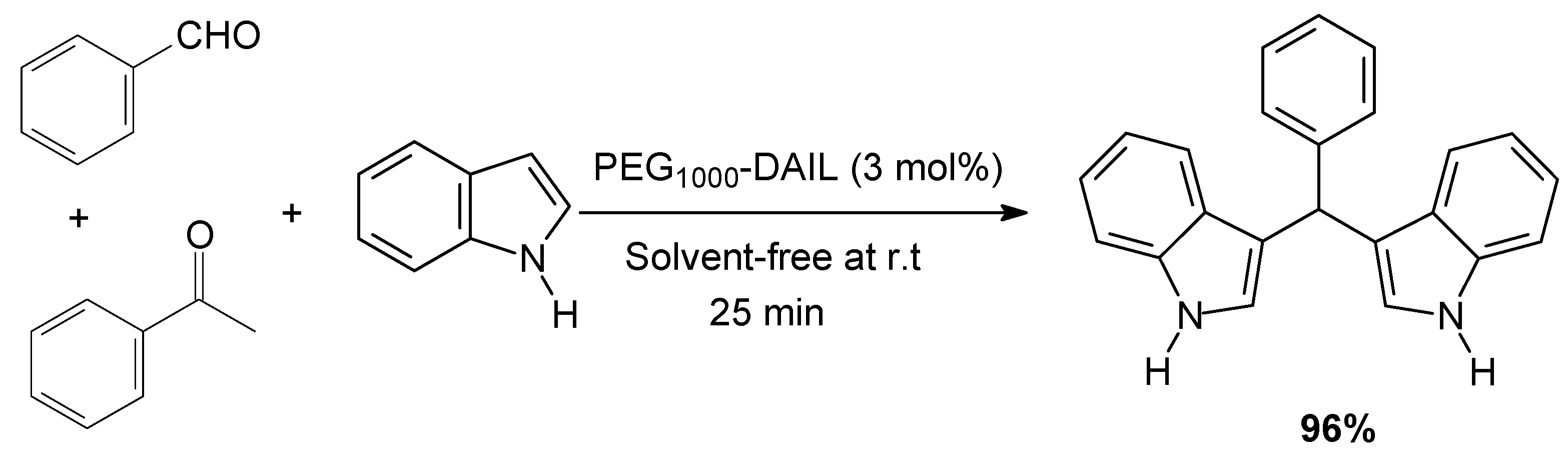
3.2. Typical Procedure for the Synthesis of Bis(indolyl)methanes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hibino, S.; Chozi, T. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2001, 18, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Shiri, M.; Zolfigol, M.A.; Kruger, H.G.; Tanbakouchian, Z. Bis- and trisindolylmethanes (BIMs and TIMs). Chem. Rev. 2010, 110, 2250–2293. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Potts, B.C.M.; Faulkner, D.J.; Smith, K. 6-Bromotryptamine derivatives from the gulf of California tunicate didemnum candidum. J. Nat. Prod. 1991, 54, 564–569. [Google Scholar] [CrossRef]
- Garbe, T.R.; Kobayashi, M.; Shimizu, N.; Takesue, N.; Ozawa, M.; Yukawa, H. Indolyl carboxylic acids by condensation of indoles with α-keto acids. J. Nat. Prod. 2000, 63, 596–598. [Google Scholar] [CrossRef]
- Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Lett. 2008, 269, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Li, B.; Zhang, G. Synthesis and cytotoxic activity of N-acetylated triindolylmethanes. Heterocycle 2003, 60, 1307–1315. [Google Scholar]
- Karthikeyan, K.; Sivaprasad, G. Synthesis of some bis(indolyl)methanes catalyzed by ascorbic acid under mild conditions. Org. Prep. Proced. Int. 2015, 47, 449–453. [Google Scholar] [CrossRef]
- Zare, A.; Bahrami, F.; Merajoddin, M.; Bandari, M.; Moosavi-Zare, A.R.; Zolfigol, M.A.; Hasaninejad, A.; Shekouhy, M.; Beyzavi, M.H.; Khakyzadeh, V.; et al. Efficient preparation of sulfonylimines, imidazoles and bis(indolyl)methanes catalyzed by [Et3NSO3H]Cl. Org. Prep. Proced. Int. 2013, 45, 211–219. [Google Scholar] [CrossRef]
- Handy, S.; Westbrook, N.M. A mild synthesis of bis(indolyl)methanes using a deep eutectic solvent. Tetrahedron Lett. 2014, 55, 4969–4971. [Google Scholar] [CrossRef]
- Selvakumar, K.; Shanmugaprabha, T.; Annapoorani, R.; Sami, P. One-pot three-component synthesis of bis(indolyl)methanes under solvent-free condition using heteropoly-11-tungsto-1-vanadophosphoric acid supported on natural clay as catalyst. Synth. Commun. 2017, 47, 913–927. [Google Scholar] [CrossRef]
- Chandam, D.; Mulik, A.; Patil, P.; Jagdale, S.; Patil, D.; Sankpal, S.; Deshmukh, M. Oxalic acid dihydrate: Proline (LTTM) as a new generation solvent for synthesis of 3,3-diaryloxindole and chromone based bis(indolyl)alkanes: Green, chromatography free protocol. J. Mol. Liq. 2014, 207, 14–20. [Google Scholar] [CrossRef]
- Maleki, B.; Kahoo, G.E.; Tayebee, R. One-pot synthesis of polysubstituted imidazoles catalyzed by an ionic liquid. Org. Prep. Proced. Int. 2015, 48, 461–472. [Google Scholar] [CrossRef]
- Hojati, S.F.; Raouf, H. Ionic Liquid for One-pot Synthesis of Spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles]. Org. Prep. Proced. Int. 2016, 48, 474–480. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Du, C.; Zhang, L. Salicylaldoxime-functionalized poly(ethylene glycol)-grafted dicationic ionic liquid as a water-soluble and recyclable ligand for palladium-catalyzed Mizoroki-Heck reactions in aqueous phase. Synth. Commun. 2017, 47, 886–891. [Google Scholar] [CrossRef]
- Budania, S.; Shelke, G.M.; Kumar, A. Synthesis of 3-alkenylated indole and bis(indol-3-yl) derivatives catalyzed by sulfonic acid-functionalized ionic liquid under ultrasound irradiation. Synth. Commun. 2017, 47, 646–654. [Google Scholar] [CrossRef]
- Jain, P.; Kumar, A. Probing the solute-solvent interactions in the binary mixtures of ionic liquids with water and alcohols by conductance, viscosity and IR spectroscopy. J. Mol. Liq. 2017, 238, 270–280. [Google Scholar] [CrossRef]
- Sarma, P.; Saikia, S.; Borah, R. Studies on -SO3H functionalized Brønsted acidic imidazolium ionic liquids (ILs) for one-pot, two-step synthesis of 2-styrylquinolines. Synth. Commun. 2016, 46, 1187–1196. [Google Scholar] [CrossRef]
- Artemenko, S.; Haddad, S.; Mazur, V. Azeotrope breaking potential of ionic liquids in separation processes. J. Mol. Liq. 2017, 235, 49–52. [Google Scholar] [CrossRef]
- Zhi, H.; Lü, C.; Zhang, Q.; Luo, J. A new PEG-1000-based dicationic ionic liquid exhibiting temperature-dependent phase behavior with toluene and its application in one-pot synthesis of benzopyrans. Chem. Commun. 2009, 2878–2880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhi, H.; Luo, J. A facile and efficient protocol for esterification and acetalization in a PEG1000-D(A)IL/toluene thermoregulated catalyst-media combinedsystems. J. Mol. Catal. Chem. 2013, 379, 46–52. [Google Scholar] [CrossRef]
- Fang, D.; Yang, J.; Jiao, C. Thermal-regulated PEG1000-based ionic liquid/PM for one-pot three-component synthesis of 2,4,5-trisubstituted imidazoles. Catal. Sci. Technol. 2011, 1, 243–245. [Google Scholar] [CrossRef]
- Hu, Y.L.; Jiang, H.; Zhu, J.; Lu, M. Facile and efficient hydrolysis of organic halides, epoxides, and esters with water catalyzed by ferric sulfate in a PEG1000-DAIL[BF4]/toluene temperature-dependent biphasic system. New J. Chem. 2011, 35, 292–298. [Google Scholar] [CrossRef]
- Ren, Y.M.; Yang, R.C. Efficient method for the synthesis of 3-arylbenzoquinoline,pyranoquinoline, and thiopyranoquinoline derivatives using PEG1000-based dicationic acidic ionic liquid as recyclable catalyst via a one-pot multicomponent reaction. Synth. Commun. 2016, 46, 1318–1325. [Google Scholar] [CrossRef]
- Ren, Y.M.; Zhang, Z.; Jin, S. Convenient and efficient method for synthesis of 2,4,6-triarylpyridines using catalytic amount of PEG1000-based dicationic acidic ionic liquid under solvent-free conditions. Synth. Commun. 2016, 46, 528–535. [Google Scholar] [CrossRef]
- Ren, Y.M.; Jin, S.; Yan, H.J.; Zhang, Z. PEG1000-based dicationic acidic ionic liquid catalyzed one-pot synthesis of 4-aryl-3-methyl-1-phenyl-1H-benzo[h]pyrazolo[3,4-b]quinoline-5,10-diones via multicomponent reactions. Catalysts 2015, 5, 1649–1656. [Google Scholar] [CrossRef]
- Ren, Y.M.; Shao, J.J.; Wu, Z.C.; Yang, R.C.; Zhang, Z.; Tao, T.X. PEG1000-based dicationic acidic ionic liquid as an efficient catalyst for mannich-type reaction in water. Synth. Commun. 2014, 44, 2529–2534. [Google Scholar] [CrossRef]
- Ren, Y.M.; Shao, J.J.; Wu, Z.C.; Xu, M.D. PEG1000-based dicationic acidic ionic liquid catalyzed one-pot synthesis of 1,4-dihydropyridines via the hantzsch reaction. Org. Prep. Proced. Int. 2014, 46, 545–550. [Google Scholar] [CrossRef]
- Ren, Y.M.; Shao, J.J.; Wu, Z.C.; Zhang, S.; Tao, T.X. Facile protection of carbonyl compounds as oxathiolanes and thioacetals promoted by PEG1000-based dicationic acidic ionic liquid as chemoselective and recyclable catalyst. J. Mol. Liq. 2014, 196, 392–394. [Google Scholar] [CrossRef]
- Chen, G.; Guo, C.Y.; Qiao, H.; Ye, M.; Qiu, X.; Yue, C. Well-dispersed sulfated zirconia nanoparticles as high-efficiency catalysts for the synthesis of bis(indolyl)methanes and biodiesel. Catal. Commun. 2013, 41, 70–74. [Google Scholar] [CrossRef]
- Xu, X.F.; Xiong, Y.; Ling, X.G.; Xie, X.M.; Yuan, J.; Zhang, S.T.; Song, Z.R. A practical synthesis of bis(indolyl)methanes catalyzed by BF3·Et2O. Chin. Chem. Lett. 2014, 25, 406–410. [Google Scholar] [CrossRef]
- Kundu, S.K.; Islam, S.; Hajra, A.; Majee, A. Tetrabutylammonium tribromide as efficient catalyst in the synthesis of bis(indolyl)methanes. Russ. J. Org. Chem. 2010, 46, 126–128. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Du, G.; Dai, B.; Jian, T. Synthesis of bis(indolyl)methanes catalyzed by cyclic phosphoric acid. Chin. J. Org. Chem. 2013, 33, 988–994. [Google Scholar] [CrossRef]
| Entry | PEG1000-DAIL (mol %) | t (min) | Yield (%) a |
|---|---|---|---|
| 1 | 0 | 20 | trace |
| 2 | 1 | 20 | 75 |
| 3 | 2 | 12 | 85 |
| 4 b | 3 | 3 | 98, 98, 97, 97, 95, 94, 90 |
| 5 | 4 | 3 | 98 |
| 6 | 5 | 3 | 98 |
| Entry | R1 | R2 | R3 | R4 | t (min) | Product | Yield (%) a |
|---|---|---|---|---|---|---|---|
| 1 | C6H5 | H | H | H | 3 | 3a | 98 |
| 2 | 4-ClC6H4 | H | H | H | 2 | 3b | 98 |
| 3 | 2-ClC6H4 | H | H | H | 2 | 3c | 98 |
| 4 | 4-CH3C6H4 | H | H | H | 10 | 3d | 96 |
| 5 | 4-CH3OC6H4 | H | H | H | 10 | 3e | 95 |
| 6 | 2-CH3OC6H4 | H | H | H | 8 | 3f | 93 |
| 7 | 4-NO2C6H4 | H | H | H | 2 | 3g | 98 |
| 8 | 3-NO2C6H4 | H | H | H | 3 | 3h | 94 |
| 9 | 4-BrC6H4 | H | H | H | 2 | 3i | 95 |
| 10 | 4-HOC6H4 | H | H | H | 10 | 3j | 96 |
| 11 | C6H5 | H | CH3 | H | 3 | 3k | 94 |
| 12 | 4-ClC6H4 | H | CH3 | H | 2 | 3l | 96 |
| 13 | 4-CH3C6H4 | H | CH3 | H | 10 | 3m | 95 |
| 14 | 3-NO2C6H4 | H | CH3 | H | 3 | 3n | 95 |
| 15 | 4-NO2C6H4 | H | CH3 | H | 2 | 3o | 97 |
| 16 | C6H5 | H | H | CH3 | 4 | 3p | 96 |
| 17 | 4-NO2C6H4 | H | H | CH3 | 3 | 3q | 98 |
| 18 | 4-CH3C6H4 | H | H | CH3 | 10 | 3r | 96 |
| 19 | CH3(CH2)3 | H | H | H | 15 | 3s | 92 |
| 20 | C6H5CH=CH | H | H | H | 5 | 3t | 95 |
| 21 | C6H5 | CH3 | H | H | 25 | 3u | 95 |
| 22 | 4-NO2C6H4 | CH3 | H | H | 18 | 3v | 97 |
| 23 | CH3 | CH3 | H | H | 50 | 3w | 73 |
| 24 | (CH2)5 | H | H | 40 | 3x | 81 |
| Entry | Catalysts | Amount (mmol) | Solvents | T (°C) | t (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | PEG1000-DAIL | 0.03 | No | r.t. | 0.05 | 98 |
| 2 | Ascorbic Acid | 0.14 | EtOH | r.t. | 0.5 | 89 [7] |
| 3 | Sulfated Zirconia | 0.30 | No | r.t. | 24 | 97 [29] |
| 4 | BF3∙Et2O | 0.15 | Et2O | r.t. | 2 | 90 [30] |
| 5 | Bu4NBr3 | 0.08 | EtOH | r.t. | 2.5 | 72 [31] |
| 6 | Cyclic phosphoric acid | 0.01 | CH2Cl2 | r.t. | 2.5 | 97 [32] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.-M.; Xu, M.-D.; Wang, X. PEG1000-Based Dicationic Acidic Ionic Liquid/Solvent-Free Conditions: An Efficient Catalytic System for the Synthesis of Bis(Indolyl)methanes. Catalysts 2017, 7, 300. https://doi.org/10.3390/catal7100300
Ren Y-M, Xu M-D, Wang X. PEG1000-Based Dicationic Acidic Ionic Liquid/Solvent-Free Conditions: An Efficient Catalytic System for the Synthesis of Bis(Indolyl)methanes. Catalysts. 2017; 7(10):300. https://doi.org/10.3390/catal7100300
Chicago/Turabian StyleRen, Yi-Ming, Mao-Dong Xu, and Xiong Wang. 2017. "PEG1000-Based Dicationic Acidic Ionic Liquid/Solvent-Free Conditions: An Efficient Catalytic System for the Synthesis of Bis(Indolyl)methanes" Catalysts 7, no. 10: 300. https://doi.org/10.3390/catal7100300