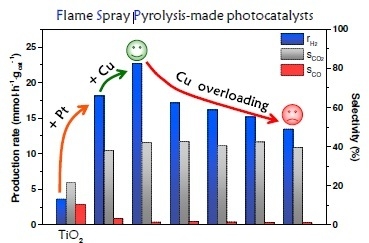
Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalyst Characterization
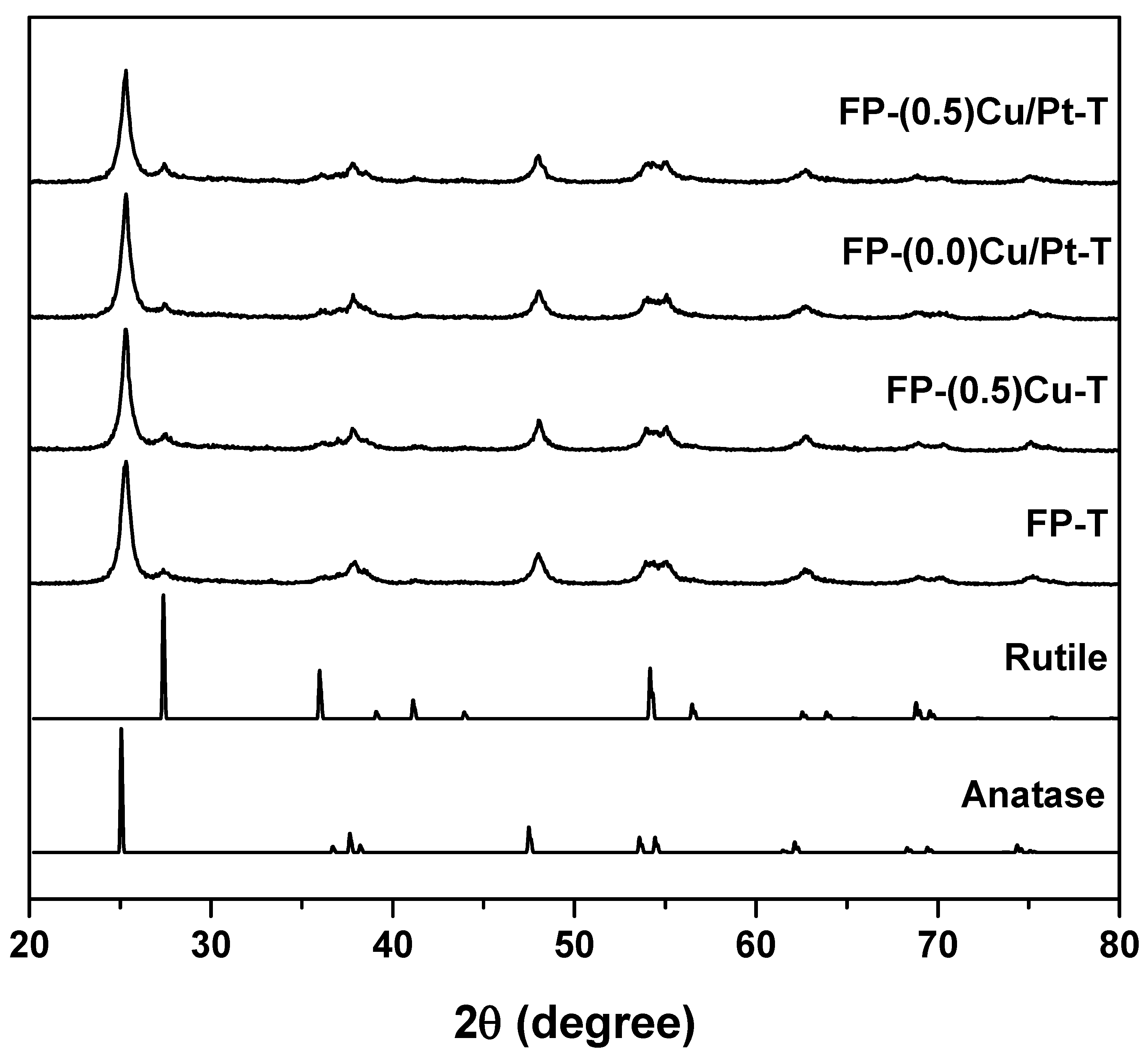
2.1.1. XRPD and BET Analyses
2.1.2. UV-VIS Absorption Properties
2.1.3. XPS Analysis
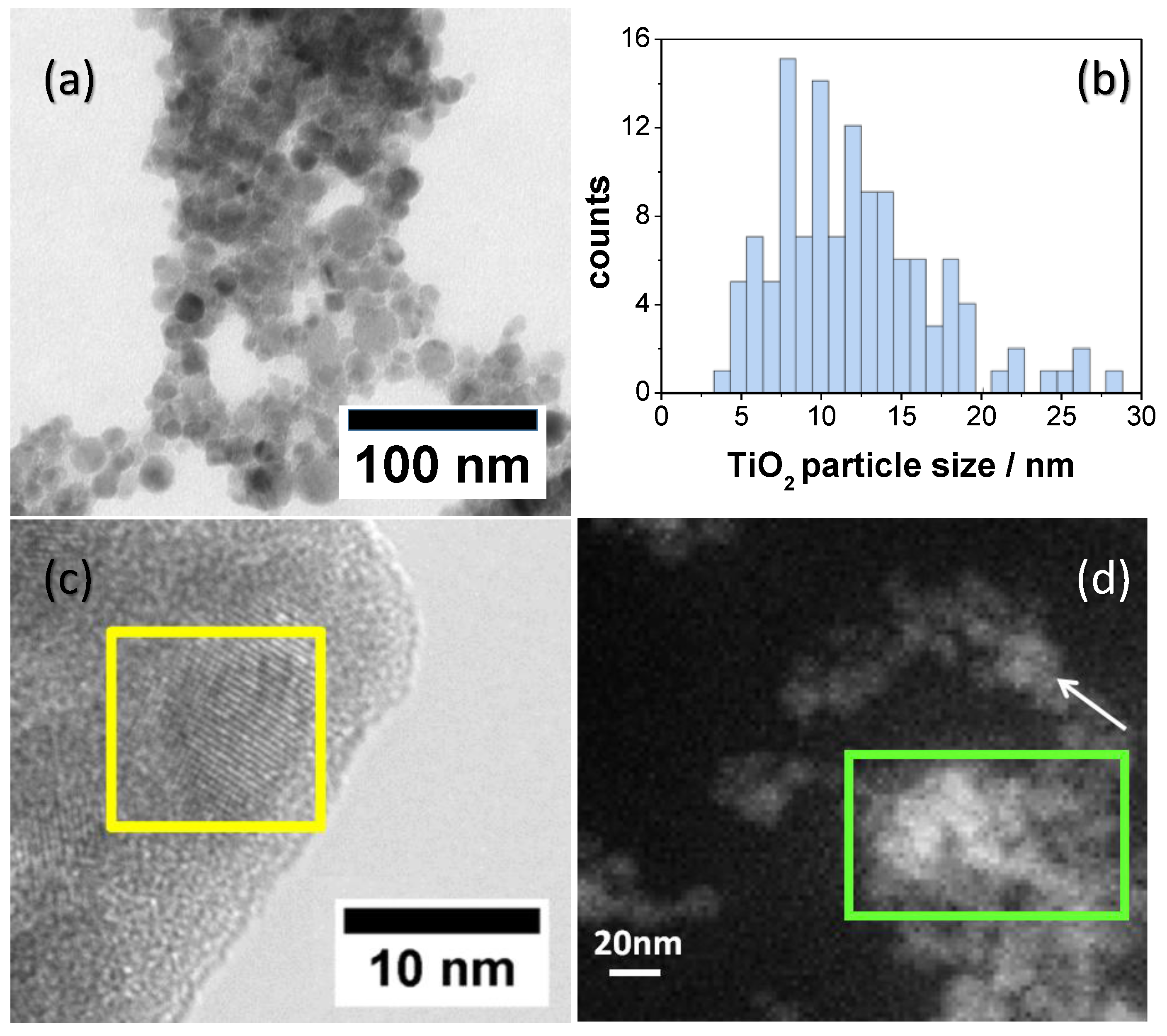
2.1.4. HRTEM Analysis
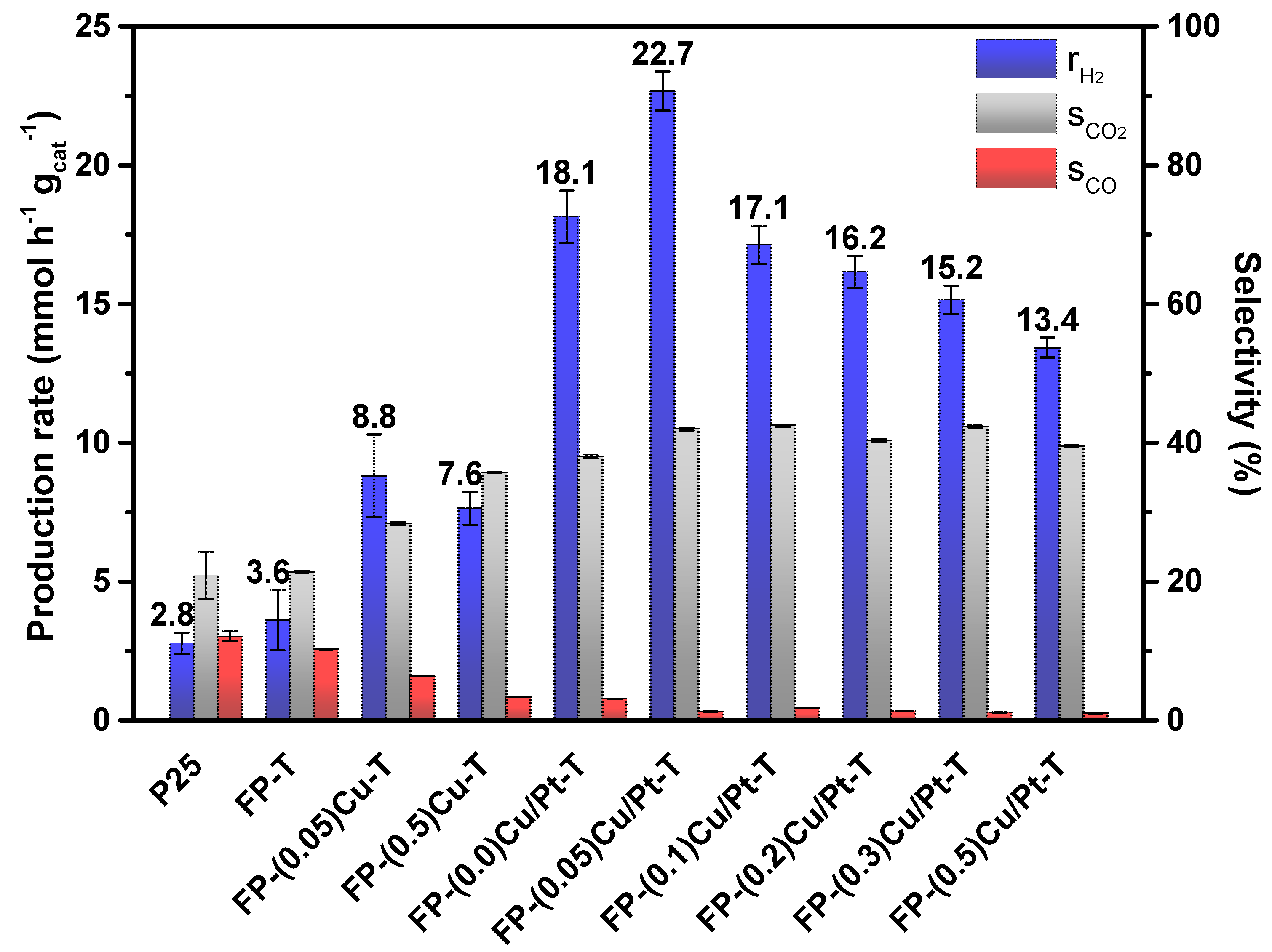
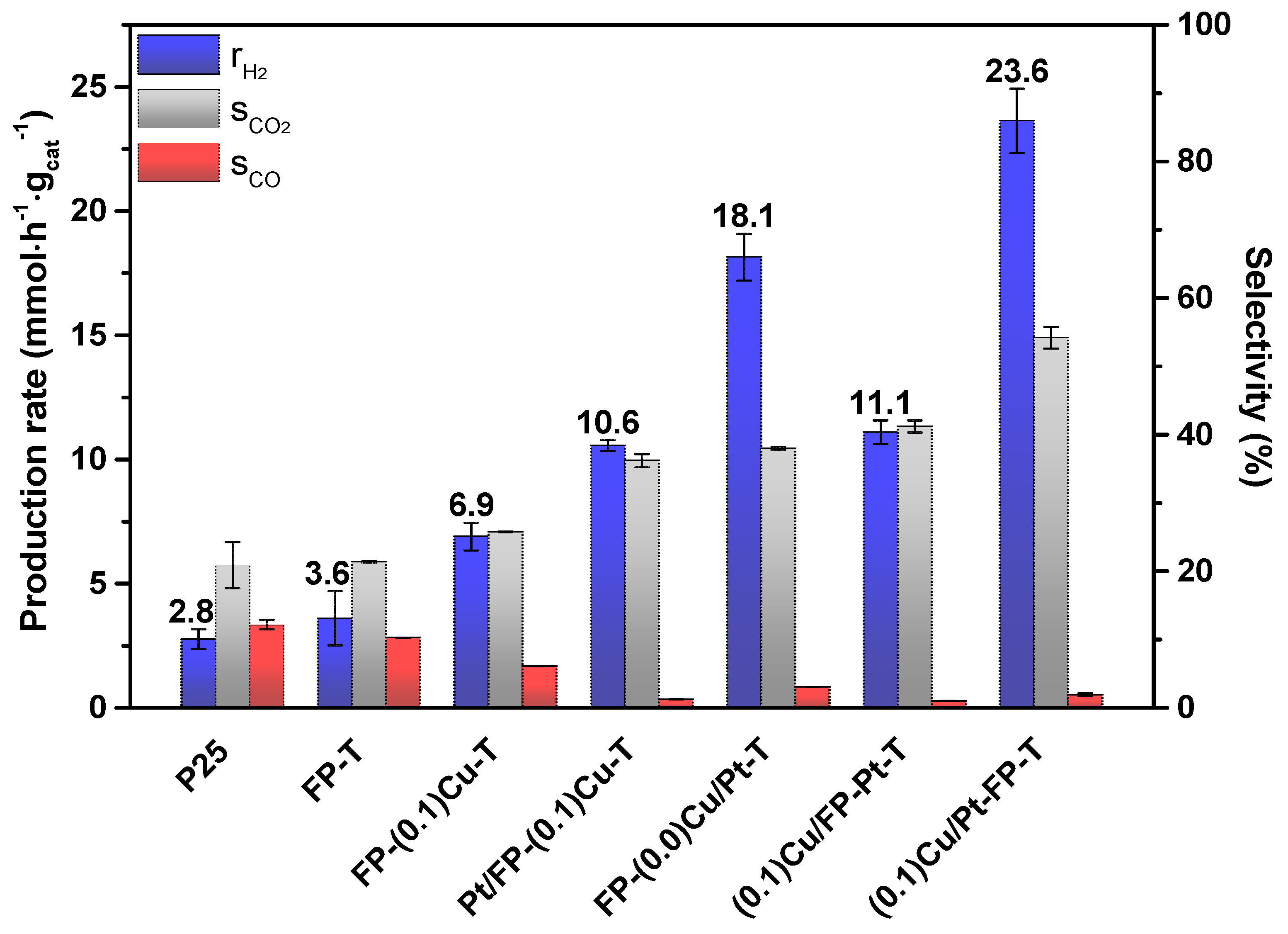
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Synthesis of the Photocatalysts
3.2. Characterization of the Photocatalysts
3.3. Photocatalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cherry, R.S. A hydrogen utopia? Int. J. Hydrogen Energy 2004, 29, 125–129. [Google Scholar] [CrossRef]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M.A. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.L.; Jia, Y.S.; Chen, X.B.; Han, H.X.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Forni, L.; Selli, E. Photocatalytic hydrogen production by liquid- and gas-phase reforming of CH3OH over flame-made TiO2 and Au/TiO2. Catal. Today 2009, 144, 69–74. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Noda, T.; Katsumata, H.; Suzuki, T.; Kaneco, S. Enhanced hydrogen production from aqueous methanol solution using TiO2/Cu as photocatalysts. Front. Chem. Sci. Eng. 2014, 8, 197–202. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Hernández-Gordillo, A.; Morales-Mendoza, G.; Lartundo-Rojas, L.; Mantilla, A.; Gomez, R. Enhancing the H2 evolution from water-methanol solution using Mn2+-Mn+3-Mn4+ redox species of Mn-doped TiO2 sol-gel photocatalysts. Catal. Today 2016, 266, 9–16. [Google Scholar] [CrossRef]
- Wang, Q.; An, N.; Bai, Y.; Hang, H.; Li, J.; Lu, X.; Liu, Y.; Wang, F.; Li, Z.; Lei, Z. High photocatalytic hydrogen production from methanol aqueous solution using the photocatalysts CuS/TiO2. Int. J. Hydrogen Energy 2013, 38, 10739–10745. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Choi, H.J.; Kang, M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2. Int. J. Hydrogen Energy 2007, 32, 3841–3848. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Selli, E. TiO2-based materials for photocatalytic hydrogen production. J. Energy Chem. 2017, 26, 250–258. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Huang, W. Influences of TiO2 phase structures on the structures and photocatalytic hydrogen production of CuOx/TiO2 photocatalysts. Appl. Surf. Sci. 2016, 389, 760–767. [Google Scholar] [CrossRef]
- Luna, A.L.; Novoseltceva, E.; Louarn, E.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Synergetic effect of Ni and Au nanoparticles synthesized on titania particles for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 191, 18–28. [Google Scholar] [CrossRef]
- Barrios, C.E.; Albiter, E.; Gracia y Jimenez, J.M.; Tiznado, H.; Romo-Herrera, J.; Zanella, R. Photocatalytic hydrogen production over titania modified by gold—Metal (palladium, nickel and cobalt) catalysts. Int. J. Hydrogen Energy 2016, 41, 1–14. [Google Scholar] [CrossRef]
- Kotesh Kumar, M.; Bhavani, K.; Naresh, G.; Srinivas, B.; Venugopal, A. Plasmonic resonance nature of Ag-Cu/TiO2 photocatalyst under solar and artificial light: Synthesis, characterization and evaluation of H2O splitting activity. Appl. Catal. B Environ. 2016, 199, 282–291. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Wei, Z.; Siuzdak, K.; Kouame, N.A.; Kowalska, E.; Remita, H.; Zaleska-Medynska, A. Enhanced photocatalytic, electrochemical and photoelectrochemical properties of TiO2 nanotubes arrays modified with Cu, AgCu and Bi nanoparticles obtained via radiolytic reduction. Appl. Surf. Sci. 2016, 387, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver-and copper-modified decahedral anatase titania particles as visible light-responsive plasmonic photocatalyst. J. Photonics Energy 2017, 7, 12008. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sakamoto, H.; Sugano, Y.; Ichikawa, S.; Hirai, T. Pt-Cu bimetallic alloy nanoparticles supported on anatase TiO2: Highly active catalysts for aerobic oxidation driven by visible light. ACS Nano 2013, 7, 9287–9297. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Chen, M.; Li, N.; Hua, X.; Wang, K.; Xu, T. Effect of TiO2 Surface Structure on the Hydrogen Production Activity of the Pt@CuO/TiO2 Photocatalysts for Water Splitting. ChemCatChem 2014, 6, 842–847. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Boensch, D.; Scott, J.; Ng, Y.H.; Amal, R. Hydrogen evolution via glycerol photoreforming over Cu-Pt nanoalloys on TiO2. Appl. Catal. A Gen. 2016, 518, 221–230. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Chiarello, G.L.; Pedroni, M.; Livraghi, S.; Giamello, E.; Selli, E. High Photocatalytic Hydrogen Production on Cu(II) Pre-grafted Pt/TiO2. Appl. Catal. B Environ. 2017, 209, 417–428. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Mädler, L.; Beydoun, D.; Pratsinis, S.E.; Amal, R. Direct (one-step) synthesis of TiO2 and Pt/TiO2 nanoparticles for photocatalytic mineralisation of sucrose. Chem. Eng. Sci. 2005, 60, 5852–5861. [Google Scholar] [CrossRef]
- Strobel, R.; Baiker, A.; Pratsinis, S.E. Aerosol flame synthesis of catalysts. Adv. Powder Technol. 2006, 17, 457–480. [Google Scholar] [CrossRef]
- Pratsinis, S.E.; Vemury, S. Particle formation in gases: A review. POWDER Technol. 1996, 88, 267–273. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Selli, E.; Forni, L. Photocatalytic hydrogen production over flame spray pyrolysis-synthesised TiO2 and Au/TiO2. Appl. Catal. B Environ. 2008, 84, 332–339. [Google Scholar] [CrossRef]
- Teleki, A.; Bjelobrk, N.; Pratsinis, S.E. Flame-made Nb- and Cu-doped TiO2 sensors for CO and ethanol. Sens. Actuators B Chem. 2008, 130, 449–457. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Rossetti, I.; Lopinto, P.; Migliavacca, G.; Forni, L. Preparation by flame spray pyrolysis of ABO3±δ catalysts for the flameless combustion of methane. Catal. Today 2006, 117, 549–553. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Rossetti, I.; Forni, L.; Lopinto, P.; Migliavacca, G. Solvent nature effect in preparation of perovskites by flame pyrolysis. 2. Alcohols and alcohols + propionic acid mixtures. Appl. Catal. B Environ. 2007, 72, 227–232. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Scavini, M.; Grunwaldt, J.-D.; Selli, E. One step flame-made fluorinated Pt/TiO2 photocatalysts for hydrogen production. Appl. Catal. B Environ. 2014, 160–161, 144–151. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Zuliani, A.; Grigioni, I.; Chiarello, G.L.; Meda, L.; Selli, E. Photocatalytic activity of one step flame-made fluorine doped TiO2. Appl. Catal. A Gen. 2016, 521, 220–226. [Google Scholar] [CrossRef]
- Reyes-Garcia, E.A.; Sun, Y.; Reyes-Gil, K.R.; Raftery, D. Solid-state NMR and EPR analysis of carbon-doped titanium dioxide photocatalysts (TiO2-xCx). Solid State Nucl. Magn. Reson. 2009, 35, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, H.; Tan, X.; Lian, J. IR and XPS investigation of visible-light photocatalysis-Nitrogen-carbon-doped TiO2 film. Appl. Surf. Sci. 2006, 253, 1988–1994. [Google Scholar] [CrossRef]
- Caruso, T.; Lenardi, C.; Agostino, R.G.; Amati, M.; Bongiorno, G.; Mazza, T.; Policicchio, A.; Formoso, V.; Maccallini, E.; Colavita, E.; et al. Electronic structure of cluster assembled nanostructured TiO2 by resonant photoemission at the Ti L2,3 edge. J. Chem. Phys. 2008, 128, 94704. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- DeMeo, D.; MacNaughton, S.; Sonkusale, S.; Vandervelde, T. Electrodeposited Copper Oxide and Zinc Oxide Core-Shell Nanowire Photovoltaic Cells. In Nanowires—Implementations and Applications; Hashim, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 141–156. ISBN 978-953-307-318-7. [Google Scholar]
- Lide, D.R.R.; Haynes, W.M.M.; Baysinger, G.; Berger, L.I.; Roth, D.L.; Zwillinger, D.; Frenkel, M.; Goldberg, R.N. CRC Handbook of Chemistry and Physics; Internet, V., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Vorontsov, A.V.; Dubovitskaya, V.P. Selectivity of photocatalytic oxidation of gaseous ethanol over pure and modified TiO2. J. Catal. 2004, 221, 102–109. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Paola, D.; Selli, E. Effect of titanium dioxide crystalline structure on the photocatalytic production of hydrogen. Photochem. Photobiol. Sci. 2011, 10, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.; Scott, J.; Ng, Y.H.; Jiang, Y.; Amal, R. CuOx dispersion and reducibility on TiO2 and its impact on photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 12499–12506. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Rossetti, I.; Forni, L. Flame-spray pyrolysis preparation of perovskites for methane catalytic combustion. J. Catal. 2005, 236, 251–261. [Google Scholar] [CrossRef]
- Morikawa, T.; Irokawa, Y.; Ohwaki, T. Enhanced photocatalytic activity of TiO2−xNx loaded with copper ions under visible light irradiation. Appl. Catal. A Gen. 2006, 314, 123–127. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Prati, L.; Canton, P.; Selli, E. Effects of gold nanoparticles deposition on the photocatalytic activity of titanium dioxide under visible light. Phys. Chem. Chem. Phys. 2009, 11, 7171–7180. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Rizzi, R. Quanto: A Rietveld program for quantitative phase analysis of polycrystalline mixtures. J. Appl. Crystallogr. 2001, 34, 392–397. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the size and internal structure of colloidal particles by means of Röntgen rays. Göttinger Nachrichten Math. Phys. 1918, 2, 98–100. [Google Scholar]


| Photocatalyst | Anatase (%) | Rutile (%) | dA (nm) | SSA (m2 g−1) |
|---|---|---|---|---|
| FP-T | 88.6 | 11.4 | 13.9 | 110 |
| FP-(0.05)Cu-T | 83.2 | 16.8 | 13.8 | 116 |
| FP-(0.1)Cu-T | 91.8 | 8.2 | 14.3 | 119 |
| FP-(0.5)Cu-T | 78.9 | 21.1 | 17.1 | 115 |
| FP-(0.0)Cu/Pt-T | 87.3 | 12.7 | 14.0 | 131 |
| FP-(0.05)Cu/Pt-T | 91.9 | 8.1 | 12.8 | 153 |
| FP-(0.1)Cu/Pt-T | 87.7 | 12.3 | 13.4 | 130 |
| FP-(0.2)Cu/Pt-T | 82.4 | 17.6 | 14.9 | 127 |
| FP-(0.3)Cu/Pt-T | 83.5 | 16.5 | 14.9 | 129 |
| FP-(0.5)Cu/Pt-T | 82.0 | 18.0 | 15.2 | 121 |
| Photocatalyst | Concentration (at%) | O/Ti Ratio | ||
|---|---|---|---|---|
| O 1s | Ti 2p | C 1s | ||
| FP-(0.0)Cu/Pt-T | 55.4 | 23.5 | 19.7 | 2.36 |
| FP-(0.05)Cu/Pt-T | 54.6 | 23.1 | 21.0 | 2.36 |
| FP-(0.5)Cu/Pt-T | 53.5 | 22.2 | 23.4 | 2.41 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernareggi, M.; Dozzi, M.V.; Bettini, L.G.; Ferretti, A.M.; Chiarello, G.L.; Selli, E. Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production. Catalysts 2017, 7, 301. https://doi.org/10.3390/catal7100301
Bernareggi M, Dozzi MV, Bettini LG, Ferretti AM, Chiarello GL, Selli E. Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production. Catalysts. 2017; 7(10):301. https://doi.org/10.3390/catal7100301
Chicago/Turabian StyleBernareggi, Massimo, Maria Vittoria Dozzi, Luca Giacomo Bettini, Anna Maria Ferretti, Gian Luca Chiarello, and Elena Selli. 2017. "Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production" Catalysts 7, no. 10: 301. https://doi.org/10.3390/catal7100301