Analysis of Anodes of Microbial Fuel Cells When Carbon Brushes Are Preheated at Different Temperatures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preheating Temperature Selection
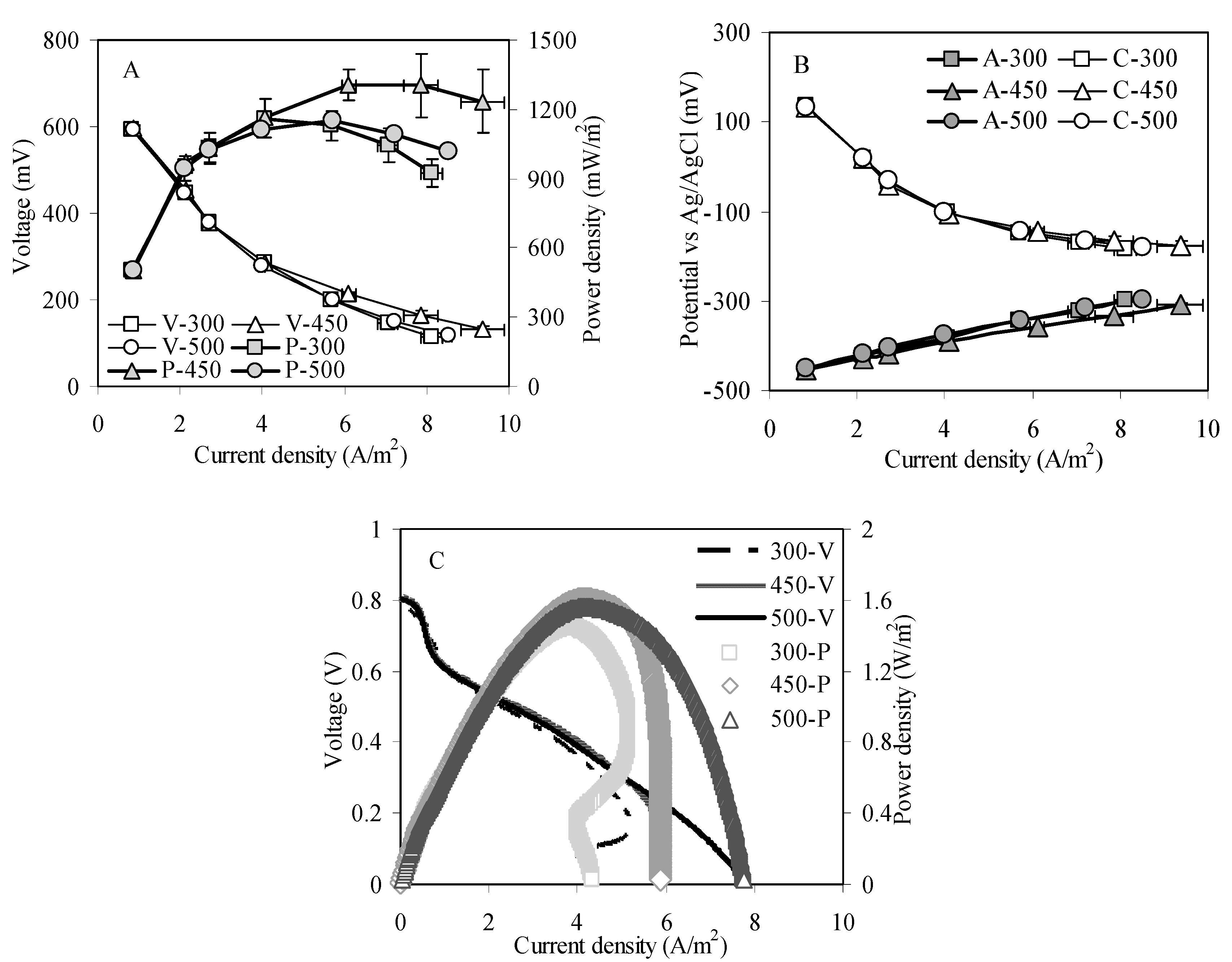
2.2. MFC Reactors Performance
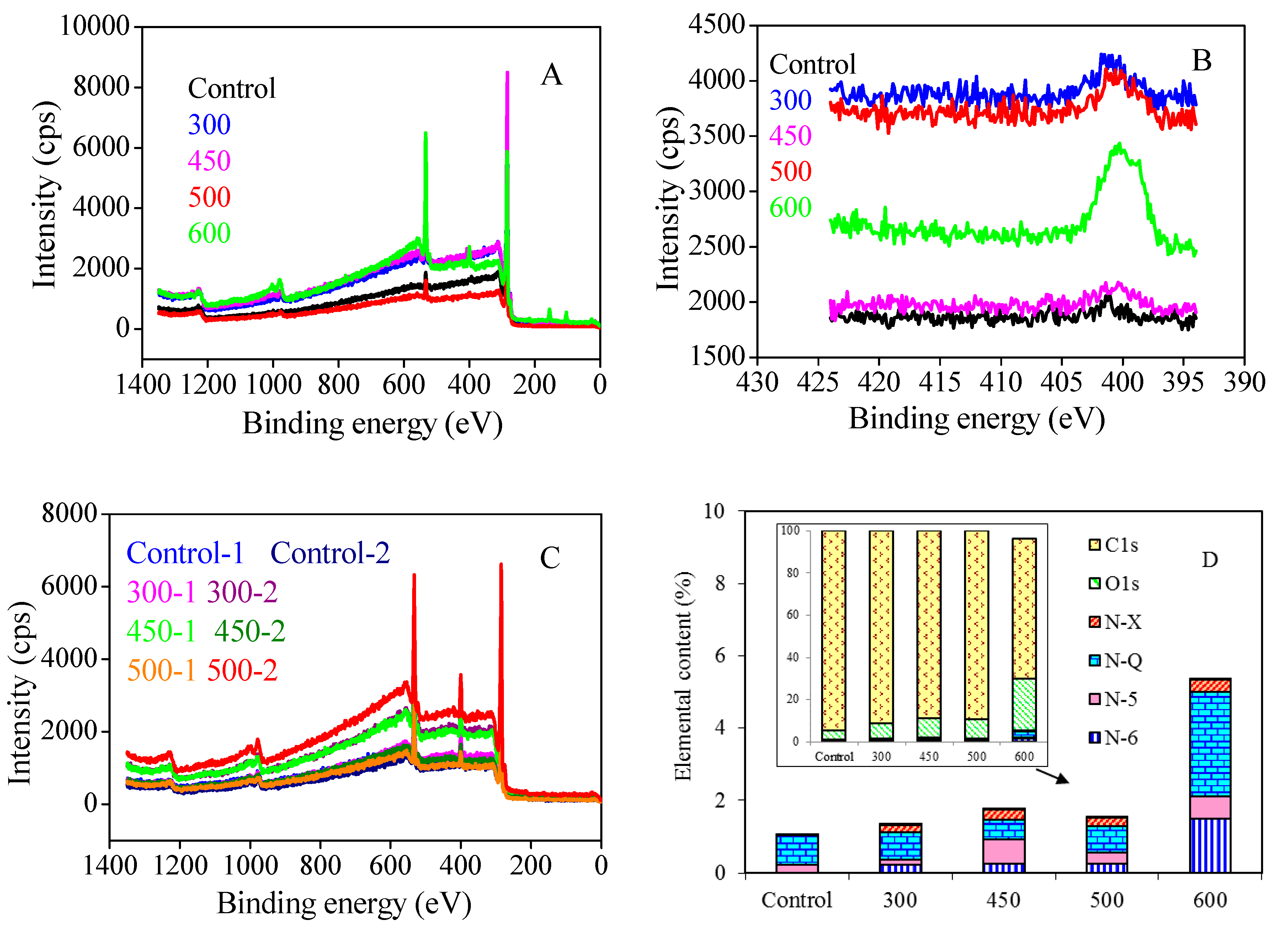
2.3. XPS Analysis on Carbon Fiber Surface
2.4. The Anode Microbial Community
3. Materials and Methods
3.1. MFC Construction with Heat-Treated Anodes
3.2. MFC Operation
3.3. Analysis Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allen, R.M.; Bennetto, H.P. Microbial fuel-cells: Electricity production from carbohydrates. Appl. Biochem. Biotechnol. 1993, 39, 27–40. [Google Scholar] [CrossRef]
- Wingard, L.B.; Shaw, C.H.; Castner, J.F. Bioelectrochemical fuel cells. Enzyme Microb. Technol. 1982, 4, 137–142. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciense. Enzyme Microb. Technol. 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Wallack, M.J.; Kim, K.-Y.; He, W.; Feng, Y.; Saikaly, P.E. Assessment of Microbial Fuel Cell Configurations and Power Densities. Environ. Sci. Technol. Lett. 2015, 2, 206–214. [Google Scholar] [CrossRef]
- Cusick, R.D.; Kim, Y.; Logan, B.E. Energy Capture from Thermolytic Solutions in Microbial Reverse-Electrodialysis Cells. Science 2012, 335, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.S.; Jang, J.K.; Gil, G.C.; Kim, M.; Kim, H.J.; Cho, B.W.; Kim, B.H. Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens. Bioelectron. 2004, 19, 607–613. [Google Scholar] [CrossRef]
- Kim, B.H.; Chang, I.S.; Gil, G.C.; Park, H.S.; Kim, H.J. Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnol. Lett. 2003, 25, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, H.; Fallgren, P.H.; Jin, S.; Ren, Z.J. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015, 33, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.J.; Zhang, X.Y.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, X.; Liang, P.; Cao, X.X.; Sun, H.T.; Huang, X. Stacked Microbial Desalination Cells to Enhance Water Desalination Efficiency. Environ. Sci. Technol. 2011, 45, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Wang, Z.; Chen, X.; Zhang, X.; Zuo, J.; Liang, P.; Huang, X. Self-Driven Desalination and Advanced Treatment of Wastewater in a Modularized Filtration Air Cathode Microbial Desalination Cell. Environ. Sci. Technol. 2016, 50, 7254–7262. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular microbial fuel cells for efficient electricity generation. Environ. Sci. Technol. 2005, 39, 8077–8082. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, Z.; Liu, L.; Zhang, F.; Liang, S. Treatment of Oil Wastewater and Electricity Generation by Integrating Constructed Wetland with Microbial Fuel Cell. Materials 2016, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yi, S.; Cao, X.; Fang, Z.; Li, X. Reductive dechlorination of hexachlorobenzene subjected to several conditions in a bioelectrochemical system. Ecotoxicol. Environ. Saf. 2017, 139, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Habibul, N.; Hu, Y.; Sheng, G.-P. Microbial fuel cell driving electrokinetic remediation of toxic metal contaminated soils. J. Hazard. Mater. 2016, 318, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, L.; Deng, W.; Tan, Y.; Xie, Q. Macroporous graphitic carbon foam decorated with polydopamine as a high-performance anode for microbial fuel cell. J. Power Sources 2017, 363, 27–33. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.Q.; Li, B.K. Novel electrode materials to enhance the bacterial adhesion and increase the power generation in microbial fuel cells (MFCs). Water Sci. Technol. 2009, 59, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.J.; Xiang, C.L.; Yang, L.N.; Sun, L.X.; Xu, F.; Cao, Z. A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int. J. Hydrogen Energy 2008, 33, 4856–4862. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, C.M.; Bao, S.J.; Bao, Q.L. Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J. Power Sources 2007, 170, 79–84. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High power density microbial fuel cell with flexible 3D graphene–nickel foam as anode. Nanoscale 2013, 5, 10283–10290. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.A.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.A.; Feng, Y.J.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of Carbon Mesh Anodes and the Effect of Different Pretreatment Methods on Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6870–6874. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.J.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air-cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Watson, V.J.; Logan, B.E. Analysis of polarization methods for elimination of power overshoot in microbial fuel cells. Electrochem. Commun. 2011, 13, 54–56. [Google Scholar] [CrossRef]
- Saito, T.; Mehanna, M.; Wang, X.; Cusick, R.D.; Feng, Y.J.; Hickner, M.A.; Logan, B.E. Effect of nitrogen addition on the performance of microbial fuel cell anodes. Bioresour. Technol. 2011, 102, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Pels, J.R.; Kapteijn, F.; Moulijn, J.A.; Zhu, Q.; Thomas, K.M. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Lin, Z.-Q.; Yuan, S.-J.; Li, W.-W.; Chen, J.-J.; Sheng, G.-P.; Yu, H.-Q. Denitrification in an integrated bioelectro-photocatalytic system. Water Res. 2017, 109, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Y.; Zhou, A.; Wang, A.; Wu, Z.; Lv, L.; Li, X.; Zhang, K.; Zhu, T. Removal of azo dye in an up-flow membrane-less bioelectrochemical system integrated with bio-contact oxidation reactor. Chem. Eng. J. 2017, 326, 454–461. [Google Scholar] [CrossRef]
- Chen, H.; Gao, X.; Wang, C.; Shao, J.; Xu, X.; Zhu, L. Efficient 2,4-dichloronitrobenzene removal in the coupled BES-UASB reactor: Effect of external voltage mode. Bioresour. Technol. 2017, 241, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wang, F.; Zhai, W.; Scott, K.; Wang, X.; Diao, G. Efficient removal of nitrobenzene and concomitant electricity production by single-chamber microbial fuel cells with activated carbon air-cathode. Bioresour. Technol. 2017, 229, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Zhang, B.; Li, J.; Lv, Q.; Wang, S.; Gu, Q. Enhanced vanadium (V) reduction and bioelectricity generation in microbial fuel cells with biocathode. J. Power Sources 2017, 359, 379–383. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, D. Cometabolic degradation of chloramphenicol via a meta-cleavage pathway in a microbial fuel cell and its microbial community. Bioresour. Technol. 2017, 229, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, W.; Cao, L.; Yang, J. A spontaneous catalytic membrane reactor to dechlorinate 2,4,6-TCP as an organic pollutant in wastewater and to reclaim electricity simultaneously. Chem. Eng. J. 2016, 285, 573–580. [Google Scholar] [CrossRef]
- Gao, C.; Liu, L.; Yang, F. Development of a novel proton exchange membrane-free integrated MFC system with electric membrane bioreactor and air contact oxidation bed for efficient and energy-saving wastewater treatment. Bioresour. Technol. 2017, 238, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.A.; Schrorder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, X.; Feng, Y.; Lee, H.; Liu, J.; Shi, X.; Qu, Y.; Ren, N. Electricity generation using eight amino acids by air–cathode microbial fuel cells. Fuel 2012, 102, 478–482. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Liang, S.; Liu, J.; Lv, J.; Feng, Y. Analysis of Anodes of Microbial Fuel Cells When Carbon Brushes Are Preheated at Different Temperatures. Catalysts 2017, 7, 312. https://doi.org/10.3390/catal7110312
Yang Q, Liang S, Liu J, Lv J, Feng Y. Analysis of Anodes of Microbial Fuel Cells When Carbon Brushes Are Preheated at Different Temperatures. Catalysts. 2017; 7(11):312. https://doi.org/10.3390/catal7110312
Chicago/Turabian StyleYang, Qiao, Shengna Liang, Jia Liu, Jiangwei Lv, and Yujie Feng. 2017. "Analysis of Anodes of Microbial Fuel Cells When Carbon Brushes Are Preheated at Different Temperatures" Catalysts 7, no. 11: 312. https://doi.org/10.3390/catal7110312




