Enzymatic Degradation of Poly(ethylene 2,5-furanoate) Powders and Amorphous Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzymatic Hydrolysis of PEF Powders
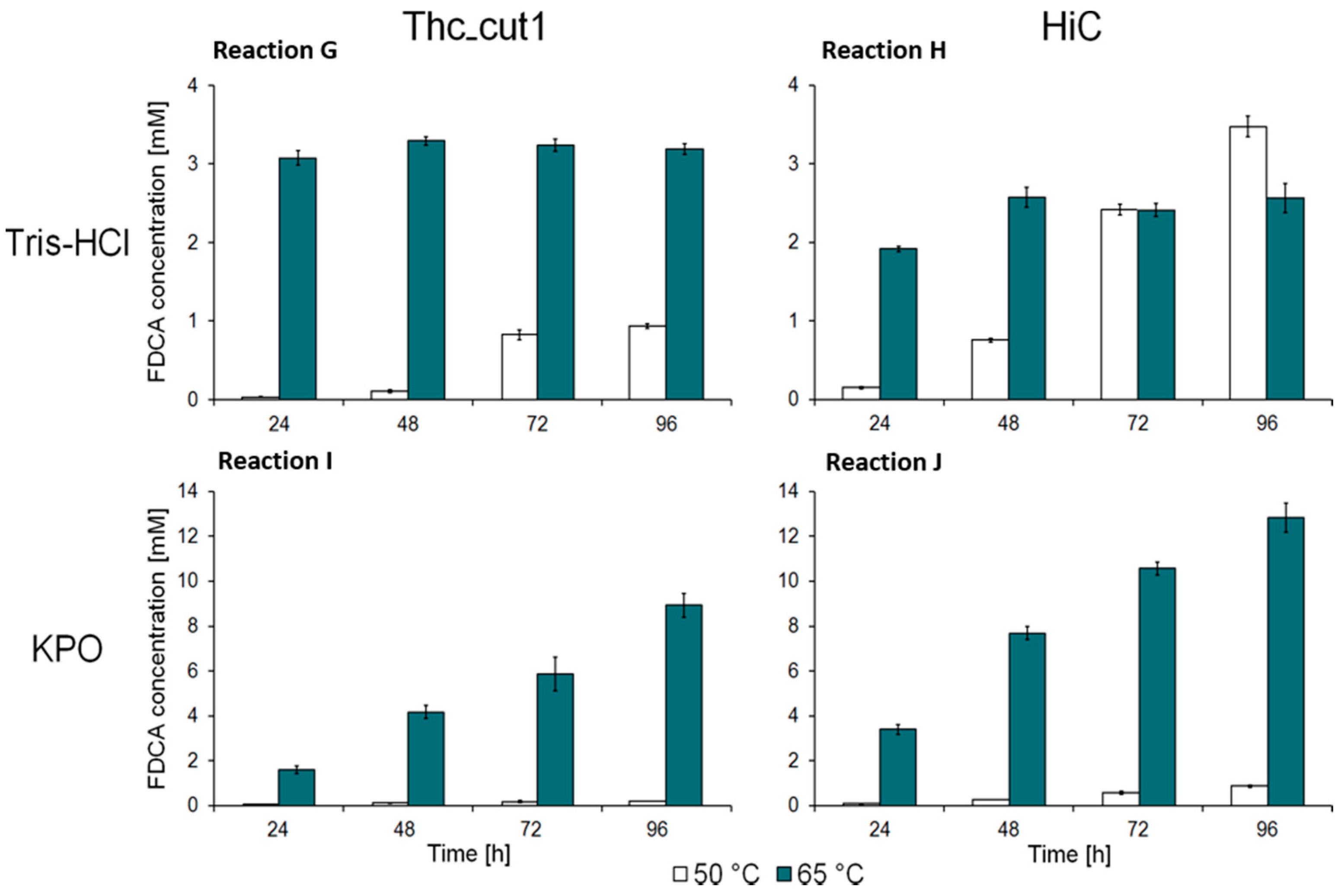
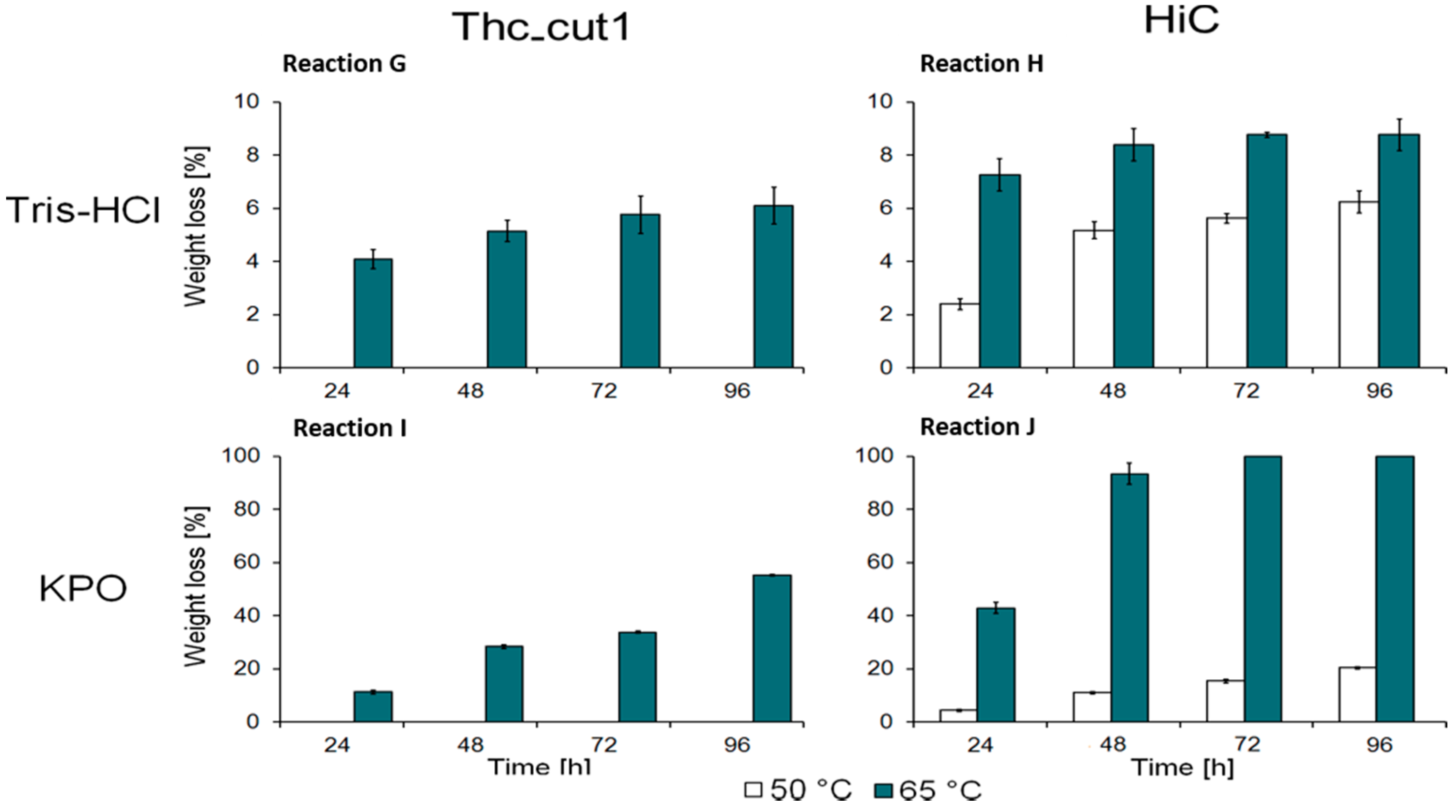
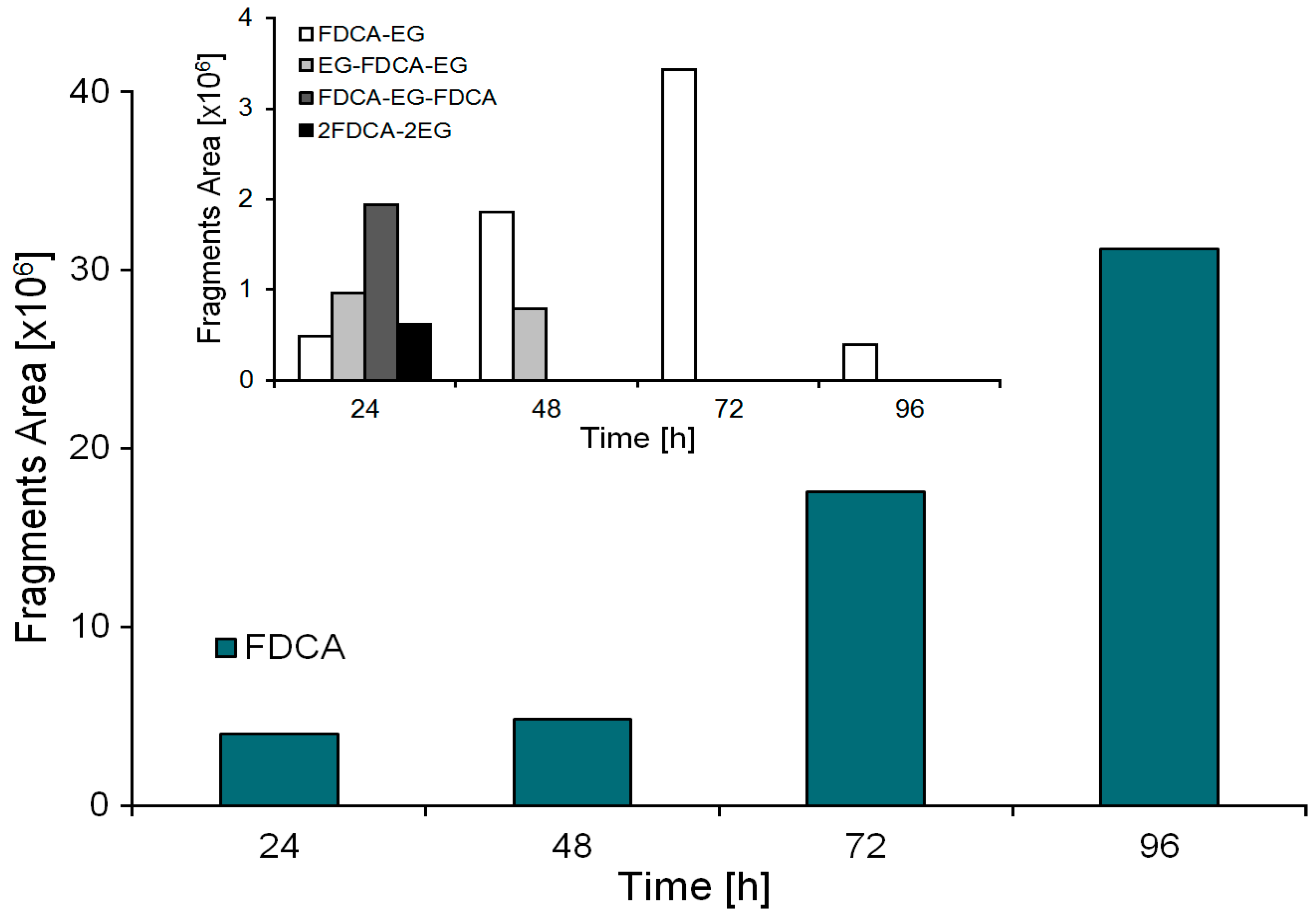
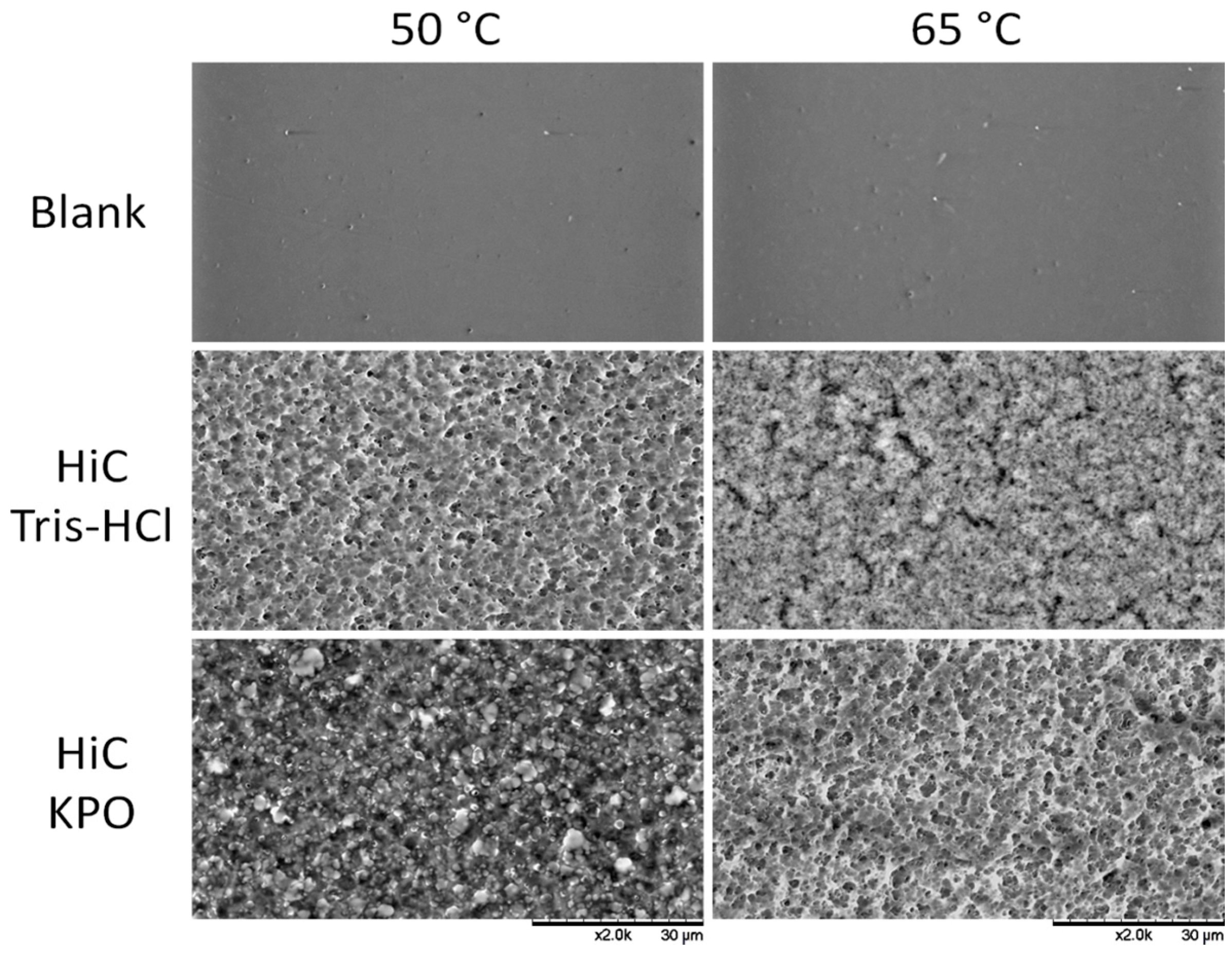
2.2. Enzymatic Hydrolysis of PEF Films
3. Materials and Methods
3.1. Chemicals, Reagents and Enzymes
3.2. Esterase Activity and Protein Concentration Assays
3.3. Poly(ethylene 2,5-furanoate) Powders & Films Hydrolysis
3.4. High-Performance Liquid Chromatography (HPLC-DAD)
3.5. Scanning Electron Microscopy (SEM)
3.6. LC-MS/TOF Analysis
3.7. Size Exclusion Chromatography (SEC)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The danube so colourful: A potpourri of plastic litter outnumbers fish larvae in europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A. Emerging issues in our global environment. In UNEP Year Book; UNEP: Nairobi, Kenya, 2014. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- E. Bioplastics. Available online: http://www.european-bioplastics.org/market/ (accessed on 22 August 2017).
- Alisch, M.; Feuerhack, A.; Müller, H.; Mensak, B.; Andreaus, J.; Zimmermann, W. Biocatalytic modification of polyethylene terephthalate fibres by esterases from actinomycete isolates. Biocatal. Biotransf. 2004, 22, 347–351. [Google Scholar] [CrossRef]
- Heumann, S.; Eberl, A.; Pobeheim, H.; Liebminger, S.; Fischer-Colbrie, G.; Almansa, E.; Cavaco-Paulo, A.; Gubitz, G.M. New model substrates for enzymes hydrolysing polyethyleneterephthalate and polyamide fibres. J. Biochem. Biophys. Methods 2006, 69, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vertommen, M.; Nierstrasz, V.A.; van der Veer, M.; Warmoeskerken, M.M.C.G. Enzymatic surface modification of poly(ethylene terephthalate). J. Biotechnol. 2005, 120, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.D. Enzymatic degradation of poly (ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Ferrario, V.; Pellis, A.; Cespugli, M.; Guebitz, G.M.; Gardossi, L. Nature Inspired Solutions for Polymers: Will Cutinase Enzymes Make Polyesters and Polyamides Greener? Catalysts 2017, 6, 205. [Google Scholar] [CrossRef]
- Pellis, A.; Cantone, S.; Ebert, C.; Gardossi, L. Evolving biocatalysis to meet bioeconomy challenges and opportunities. New Biotechnol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Then, J.; Wei, R.; Oeser, T.; Barth, M.; Belisário-Ferrari, M.R.; Schmidt, J.; Zimmermann, W. Ca2+ and Mg2+ binding site engineering increases the degradation of polyethylene terephthalate films by polyester hydrolases from Thermobifida fusca. Biotechnol. J. 2015, 10, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Alisch-Mark, M.; Herrmann, A.; Zimmermann, W. Increase of the hydrophilicity of polyethylene terephthalate fibres by hydrolases from Thermomonospora fusca and Fusarium solani f. sp. pisi. Biotechnol. Lett. 2006, 28, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, S.; Haernvall, K.; Scaini, D.; Ghazaryan, G.; Zumstein, M.Z.; Sander, M.; Pellis, A.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene furanoate) thin films of various crystallinities. Green Chem. 2017. [Google Scholar] [CrossRef]
- Vecchiato, S.; Ahrens, J.; Pellis, A.; Scaini, D.; Mueller, B.; Herrero Acero, E.; Guebitz, G.M. Enzymatic Functionalization of HMLS-Polyethylene Terephthalate Fabrics Improves the Adhesion to Rubber. ACS Sustain. Chem. Eng. 2017, 5, 6456–6465. [Google Scholar] [CrossRef]
- Silva, C.M.; Carneiro, F.; O’Neill, A.; Fonseca, L.P.; Cabral, J.S.; Guebitz, G.M.; Cavaco-Paulo, A. Cutinase-A new tool for biomodification of synthetic fibers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2448–2450. [Google Scholar] [CrossRef] [Green Version]
- Guebitz, G.M.; Cavaco-Paulo, A. Enzymes go big: Surface hydrolysis and functionalisation of synthetic polymers. Trends Biotechnol. 2008, 26, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, R.; Silva, C.; O’Neill, A.; Micaelo, N.; Guebitz, G.M.; Soares, C.M.; Casal, M.; Cavaco-Paulo, A. Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6,6 fibers. J. Biotechnol. 2007, 128, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero Acero, E.; Ribitsch, D.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Steinkellner, G.; Gruber, K.; Schwab, H.; Guebitz, G.M. Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol. Bioeng. 2013, 110, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Eberl, A.; Heumann, S.; Brückner, T.; Araujo, R.; Cavaco-Paulo, A.; Kaufmann, F.; Kroutil, W.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene terephthalate) and bis(benzoyloxyethyl) terephthalate by lipase and cutinase in the presence of surface active molecules. J. Biotechnol. 2009, 143, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Herrero Acero, E.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast Crystallization and Melting Behavior of a Long-Spaced Aliphatic Furandicarboxylate Biobased Polyester, Poly (dodecylene 2, 5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Knoop, J.R.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon Dioxide Sorption and Transport in Amorphous Poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly (ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly(ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Pellis, A.; Guarneri, A.; Brandauer, M.; Herrero Acero, E.; Peerlings, H.; Gardossi, L.; Guebitz, G.M. Exploring mild enzymatic sustainable routes for the synthesis of bio-degradable aromatic-aliphatic oligoesters. Biotechnol. J. 2016, 11, 642–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Woortman, A.J.J.; van Ekenstein, G.; Loos, K. A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Pellis, A.; Haernvall, K.; Pichler, C.M.; Ghazaryan, G.; Breinbauer, R.; Guebitz, G.M. Enzymatic hydrolysis of poly(ethylene furanoate). J. Biotechnol. 2016, 235, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, D.; Zitzenbacher, S.; Zartl, B.; Gamerith, C.; Pellis, A.; Jungbauer, A.; Łyskowski, A.; Steinkellner, G.; Gruber, K.; Tscheliessnig, R.; et al. Small cause, large effect: Structural characterization of cutinases from Thermobifida cellulosilytica. Biotechnol. Bioeng. 2017, 114, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Gamerith, C.; Ghazaryan, G.; Ortner, A.; Herrero Acero, E.; Guebitz, G.M. Ultrasound-enhanced enzymatic hydrolysis of poly(ethylene terephthalate). Bioresource Technol. 2016, 218, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Gamerith, C.; Zartl, B.; Pellis, A.; Guillamot, F.; Marty, A.; Herrero Acero, E.; Guebitz, G.M. Enzymatic recovery of polyester building blocks from polymer blends. Process Biochem. 2017, 59, 58–64. [Google Scholar] [CrossRef]
- Schmidt, J.; Oeser, T.; Belisário-Ferrari, M.R.; Barth, M.; Then, J.; Zimmermann, W. Effect of Tris, MOPS, and phosphate buffers on the hydrolysis of polyethylene terephthalate films by polyester hydrolases. FEBS Open Bio 2016, 6, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Gamerith, C.; Vastano, M.; Ghorbanpour, S.M.; Zitzenbacher, S.; Ribitsch, D.; Zumstein, M.T.; Sander, M.; Herrero Acero, E.; Pellis, A.; Guebitz, G.M. Enzymatic Degradation of Aromatic and Aliphatic Polyesters by P. pastoris Expressed Cutinase 1 from Thermobifida cellulosilytica. Front. Microbiol. 2017, 8, 938. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The closure of the cycle: Enzymatic synthesis and functionalization of bio-based polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Stoclet, G.; Gobius du Sart, G.; Yeniad, B.; de Vos, S.; Lefebvre, J.M. Isothermal crystallization and structural characterization of poly(ethylene-2,5-furanoate). Polymer 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Weber, H.; Obersriebnig, M.; Srebotnik, E.; Guebitz, G.M. Biocatalyzed approach for the surface functionalization of poly(l-lactic acid) films using hydrolytic enzyme. Biotechnol. J. 2015, 10, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
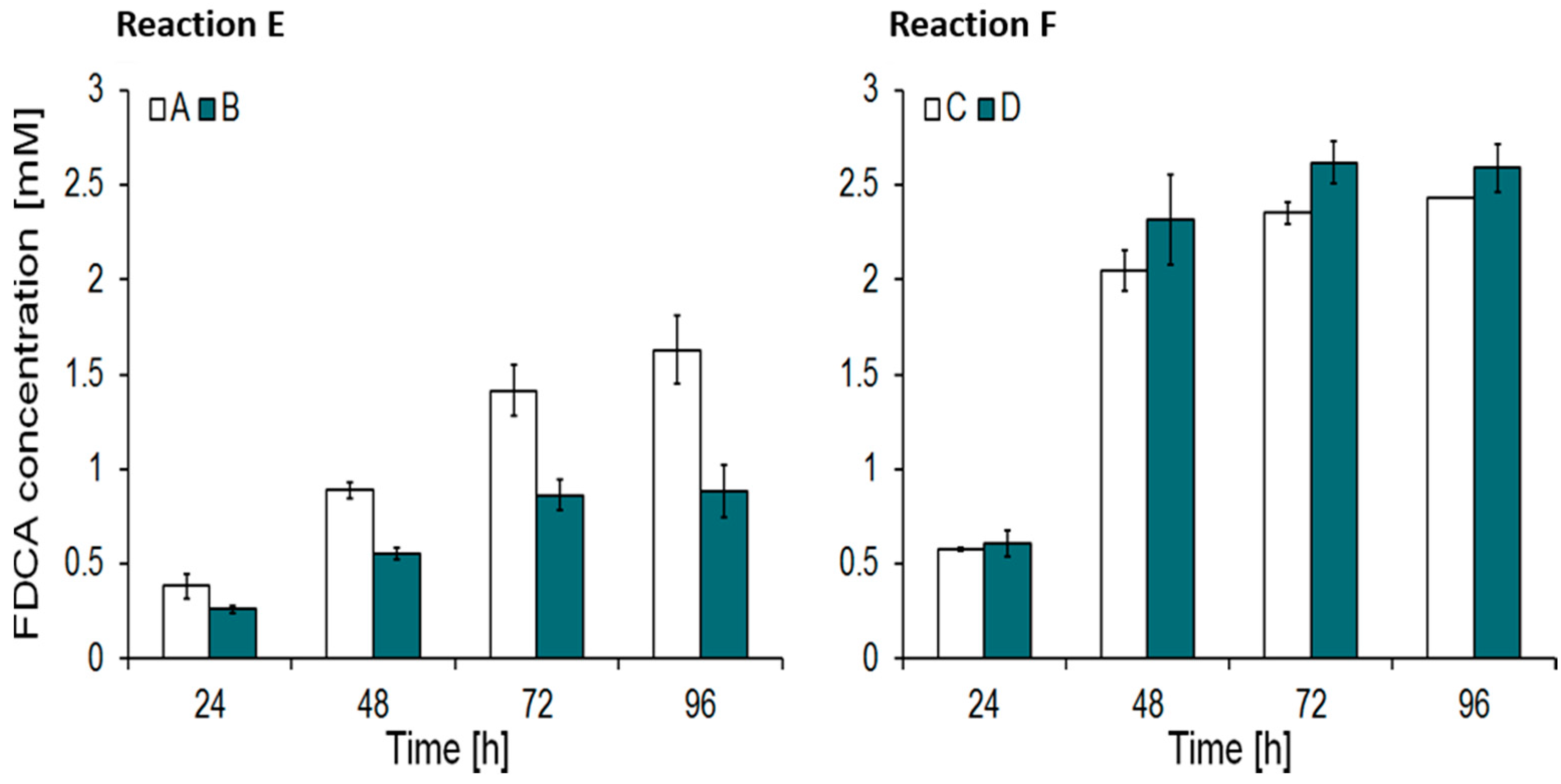
| Sample Name | Mn (kDa) * | Mw (kDa) * | PDI * | Diameter (µm) | Crystallinity (%) |
|---|---|---|---|---|---|
| A | 55 | 104 | 1.9 | d < 80 | 46 |
| B | 55 | 104 | 1.9 | 180 < d < 425 | 46 |
| C | 18 | 29 | 1.8 | d < 180 | amorphous |
| D | 18 | 29 | 1.8 | 180 < d < 425 | amorphous |
| Sample Name | Enzyme | Buffer |
|---|---|---|
| G | Thc_Cut1 | Tris-HCl (0.1 M, pH 7) |
| H | HiC | |
| I | Thc_Cut1 | KPO (1 M, pH 8) |
| J | HiC |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G.M. Enzymatic Degradation of Poly(ethylene 2,5-furanoate) Powders and Amorphous Films. Catalysts 2017, 7, 318. https://doi.org/10.3390/catal7110318
Weinberger S, Canadell J, Quartinello F, Yeniad B, Arias A, Pellis A, Guebitz GM. Enzymatic Degradation of Poly(ethylene 2,5-furanoate) Powders and Amorphous Films. Catalysts. 2017; 7(11):318. https://doi.org/10.3390/catal7110318
Chicago/Turabian StyleWeinberger, Simone, Judit Canadell, Felice Quartinello, Bahar Yeniad, Andrea Arias, Alessandro Pellis, and Georg M. Guebitz. 2017. "Enzymatic Degradation of Poly(ethylene 2,5-furanoate) Powders and Amorphous Films" Catalysts 7, no. 11: 318. https://doi.org/10.3390/catal7110318





