Metal-Catalyzed Intra- and Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: A Review
Abstract
:1. Introduction
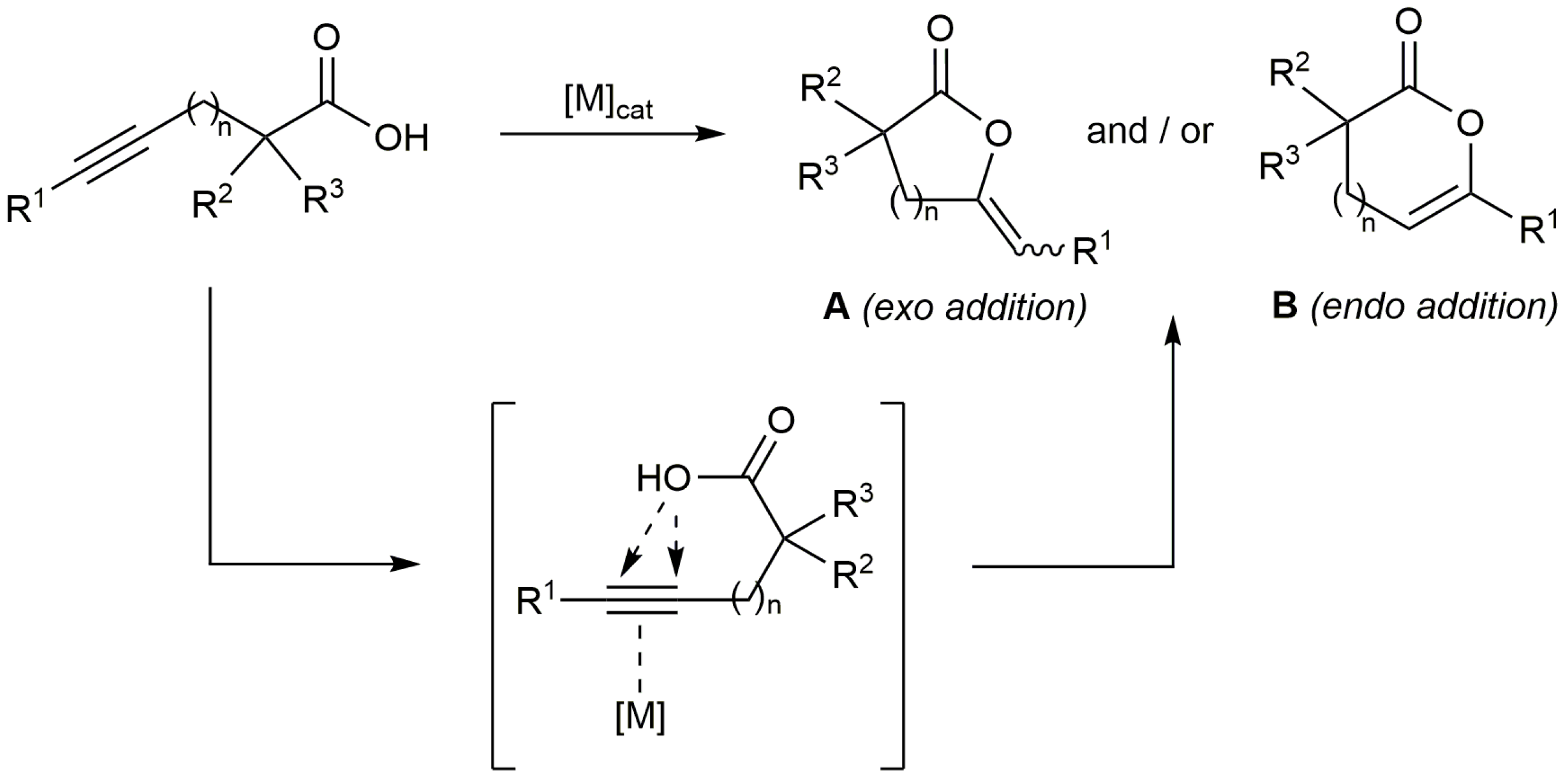
2. Intramolecular Processes
2.1. Cycloisomerization of Preformed or In Situ Generated Alkynoic Acids
2.2. Tandem and Cascade Processes Involving the Cycloisomerization of an Alkynoic Acid
3. Intermolecular Processes
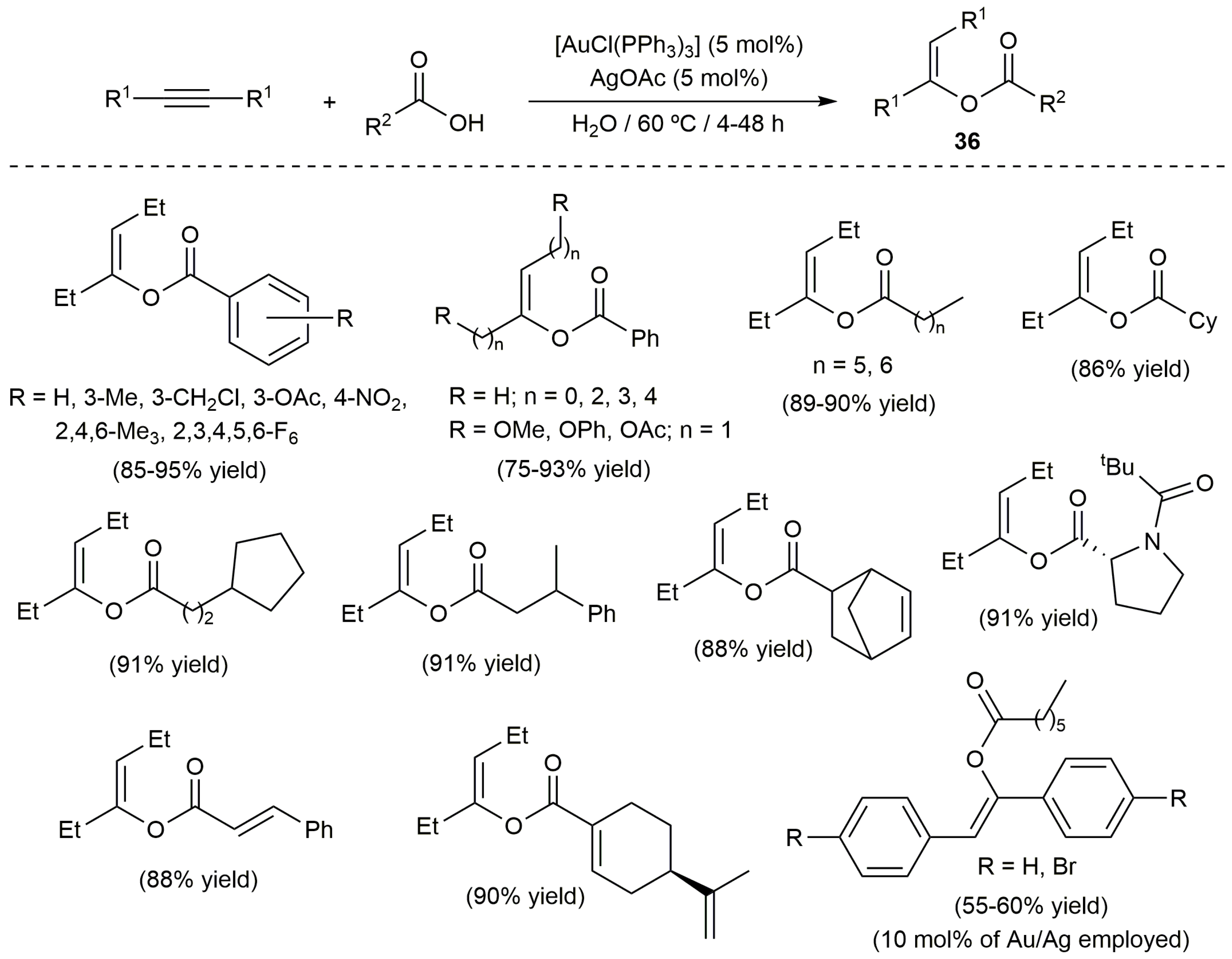
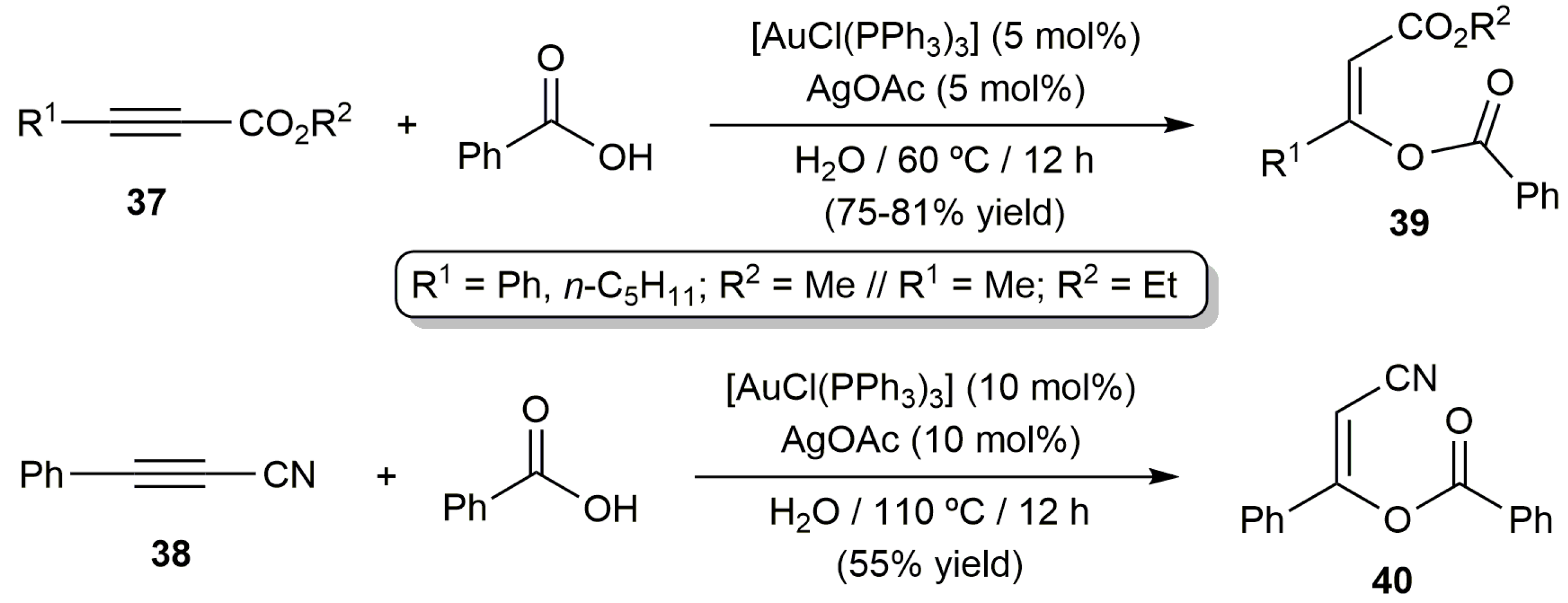
3.1. Catalytic Addition of Carboxylic Acids to Terminal and Internal Alkynes
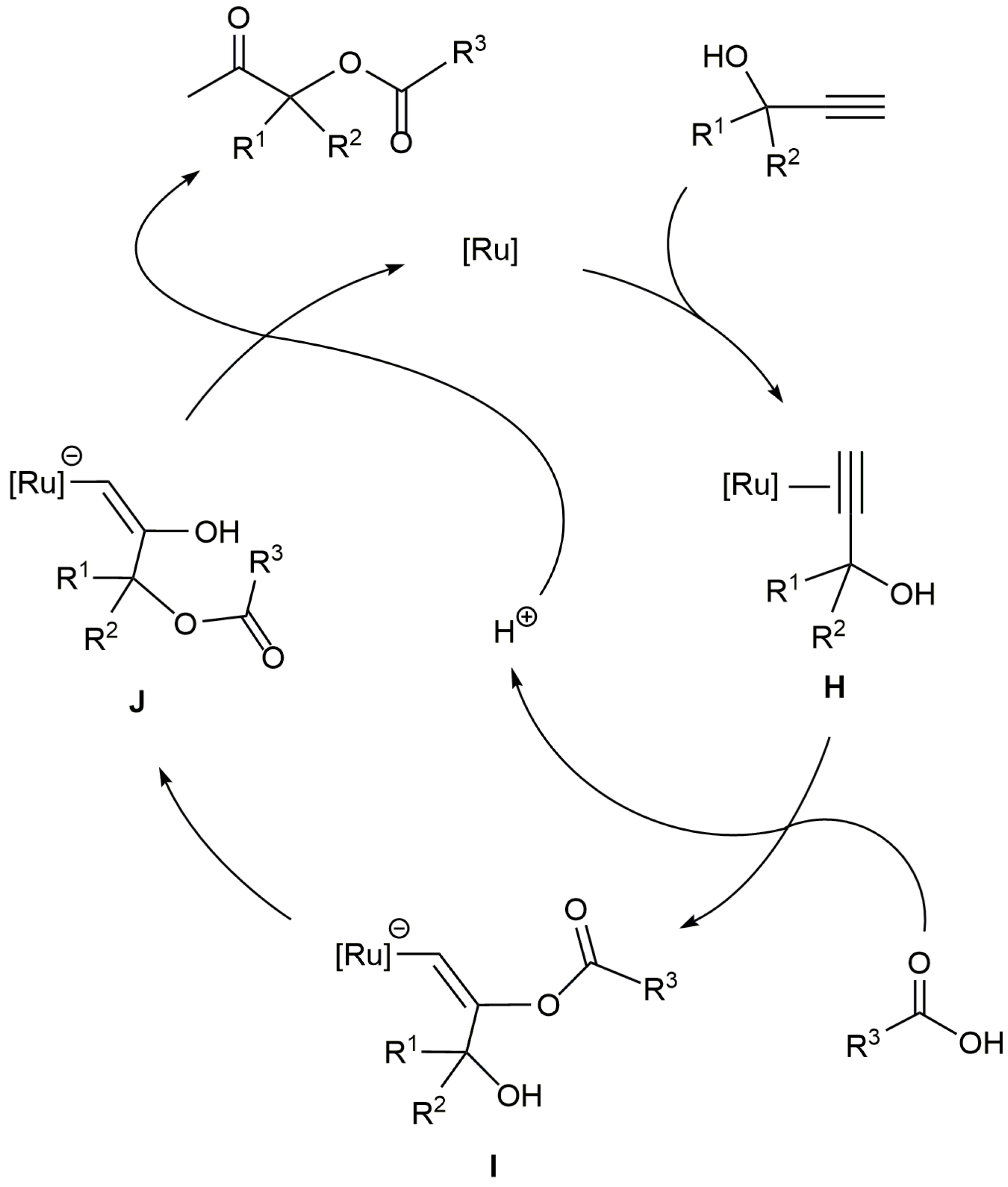
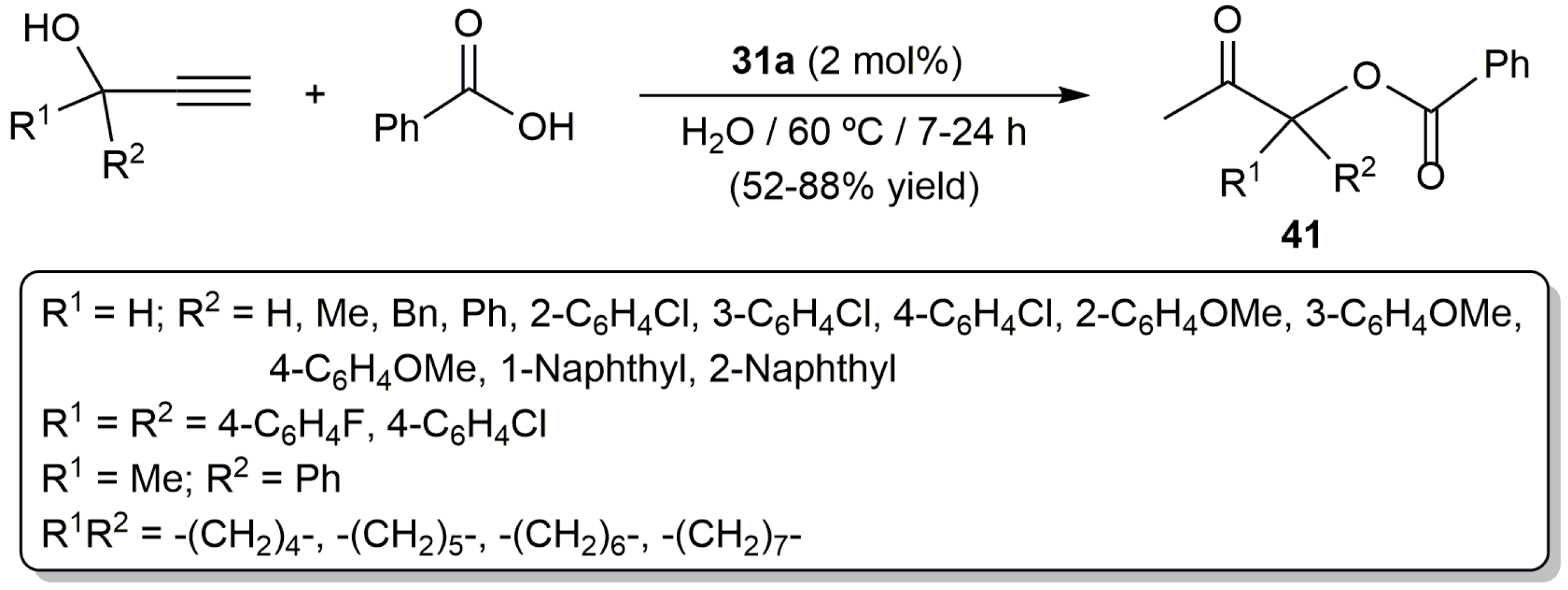
3.2. Catalytic Addition of Carboxylic Acids to Terminal Propargylic Alcohols
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-metal-catalyzed addition of heteroatom-hydrogen bonds to alkynes. Chem. Rev. 2004, 104, 3079–3159. [Google Scholar] [CrossRef] [PubMed]
- Beller, M.; Seayad, J.; Tillack, A.; Jiao, H. Catalytic Markovnikov and anti-Markovnikov functionalization of alkenes and alkynes: Recent developments and trends. Angew. Chem. Int. Ed. 2004, 43, 3368–3398. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Kavthe, R.D.; Shinde, V.S. Transition metal-catalyzed addition of C-, N- and O-nucleophiles to unactivated C–C multiple bonds. Tetrahedron 2012, 68, 8079–8146. [Google Scholar] [CrossRef]
- Hintermann, L. Recent developments in metal-catalyzed additions of oxygen nucleophiles to alkenes and alkynes. Top. Organomet. Chem. 2010, 31, 123–155. [Google Scholar]
- Bruneau, C. Group 8 metals-catalyzed O–H bond addition to unsaturated molecules. Top. Organomet. Chem. 2013, 43, 203–230. [Google Scholar]
- Abbiati, G.; Beccalli, E.M.; Rossi, E. Groups 9 and 10 metals-catalyzed O-H bond addition to unsaturated molecules. Top. Organomet. Chem. 2013, 43, 231–290. [Google Scholar]
- Rao, Y.S. Recent advances in the chemistry of unsaturated lactones. Chem. Rev. 1976, 76, 625–694. [Google Scholar] [CrossRef]
- Laduwahetty, T. Saturated and unsaturated lactones. Contemp. Org. Synth. 1995, 2, 133–149. [Google Scholar] [CrossRef]
- Libiszewska, K. Lactones as biologically active compounds. Biotechnol. Food Sci. 2011, 75, 45–53. [Google Scholar]
- Janecki, T. (Ed.) Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527334148. [Google Scholar]
- Neaţu, F.; Toullec, P.Y.; Michelet, V.; Pârvulescu, V.I. Heterogeneous Au and Rh catalysts for the cycloisomerization reactions of γ-acetylenic carboxylic acids. Pure Appl. Chem. 2009, 81, 2387–2396. [Google Scholar] [CrossRef]
- Bruneau, C.; Neveux, M.; Kabouche, Z.; Ruppin, C.; Dixneuf, P.H. Ruthenium-catalyzed additions to alkynes: Synthesis of activated esters and their use in acylation reactions. Synlett 1991, 11, 755–763. [Google Scholar] [CrossRef]
- Kumar, M.; Bagchi, S.; Sharma, A. The first vinyl acetate mediated organocatalytic transesterification of phenols: A step towards sustainability. New J. Chem. 2015, 39, 8329–8336. [Google Scholar] [CrossRef]
- Liu, X.; Coutelier, O.; Harrison, S.; Tassaing, T.; Marty, J.-D.; Destarac, M. Enhanced solubility of polyvinyl esters in scCO2 by means of vinyl trifluorobutyrate monomer. ACS Macro Lett. 2015, 4, 89–93. [Google Scholar] [CrossRef]
- Foarta, F.; Landis, C.R. Condensation oligomers with sequence control but without coupling reagents and protecting groups via asymmetric hydroformylation and hydroacyloxylation. J. Org. Chem. 2016, 81, 11250–11255. [Google Scholar] [CrossRef] [PubMed]
- Jena, R.K.; Das, U.K.; Ghorai, A.; Bhattacharjee, M. Ruthenium-catalyzed addition of carboxylic acids to progargylic alcohols: An easy route to O-dienyl esters and their tandem atom-transfer radical polymerization. Eur. J. Org. Chem. 2016, 6015–6021. [Google Scholar] [CrossRef]
- Konrad, T.M.; Schmitz, P.; Leitner, W.; Franciò, G. Highly enantioselective Rh-catalysed hydrogenation of 1-alkyl vinyl esters using phosphine-phosphoramidite ligands. Chem. Eur. J. 2013, 19, 13299–13303. [Google Scholar] [CrossRef] [PubMed]
- González-Liste, P.J.; León, F.; Arribas, I.; Rubio, M.; García-Garrido, S.E.; Cadierno, V.; Pizzano, A. Highly stereoselective synthesis and hydrogenation of (Z)-1-alkyl-2-arylvinyl acetates: A wide scope procedure for the preparation of chiral homobenzylic esters. ACS Catal. 2016, 6, 3056–3060. [Google Scholar] [CrossRef]
- León, F.; González-Liste, P.J.; García-Garrido, S.E.; Arribas, I.; Rubio, M.; Cadierno, V.; Pizzano, A. Broad scope synthesis of ester precursors of nonfunctionalized chiral alcohols based on the asymmetric hydrogenation of α,β-dialkyl-, α,β-diaryl-, and α-alkyl-β-aryl-vinyl esters. J. Org. Chem. 2017, 82, 5852–5867. [Google Scholar] [CrossRef] [PubMed]
- Takeno, M.; Kikuchi, S.; Morita, S.-I.; Nishiyama, Y.; Ishii, Y. A new coupling reaction of vinyl esters with aldehydes catalyzed by organosamarium compounds. J. Org. Chem. 1995, 60, 4974–4975. [Google Scholar] [CrossRef]
- Goossen, L.J.; Paetzold, J. Decarbonylative Heck olefination of enol esters: Salt-free and environmentally friendly access to vinyl arenes. Angew. Chem. Int. Ed. 2004, 43, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Geibel, I.; Dierks, A.; Schmidtmann, M.; Christoffers, J. Formation of δ-lactones by cerium-catalyzed, Baeyer-Villiger-type coupling of β-oxoesters, enol acetates, and dioxygen. J. Org. Chem. 2016, 81, 7790–7798. [Google Scholar] [CrossRef] [PubMed]
- González-Liste, P.J.; Francos, J.; García-Garrido, S.E.; Cadierno, V. The intermolecular hydro-oxycarbonylation of internal alkynes: Current state of the art. Arkivoc 2018, Part II. 17–39. [Google Scholar]
- Cornils, B.; Herrmann, W.A. (Eds.) Aqueous-Phase Organometallic Catalysis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004; ISBN 3527307125. [Google Scholar]
- Li, C.-J.; Chan, T.-H. Comprehensive Organic Reactions in Aqueous Media, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9780471761297. [Google Scholar]
- Dixneuf, P.H.; Cadierno, V. (Eds.) Metal-Catalyzed Organic Reactions in Water; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527331888. [Google Scholar]
- Benaglia, M. (Ed.) Recoverable and Recyclable Catalysts; John Wiley & Sons: Chichester, UK, 2009; ISBN 9780470681954. [Google Scholar]
- Chen, L.; Li, C.-J. Catalytic reactions of alkynes in water. Adv. Synth. Catal. 2006, 348, 1459–1484. [Google Scholar] [CrossRef]
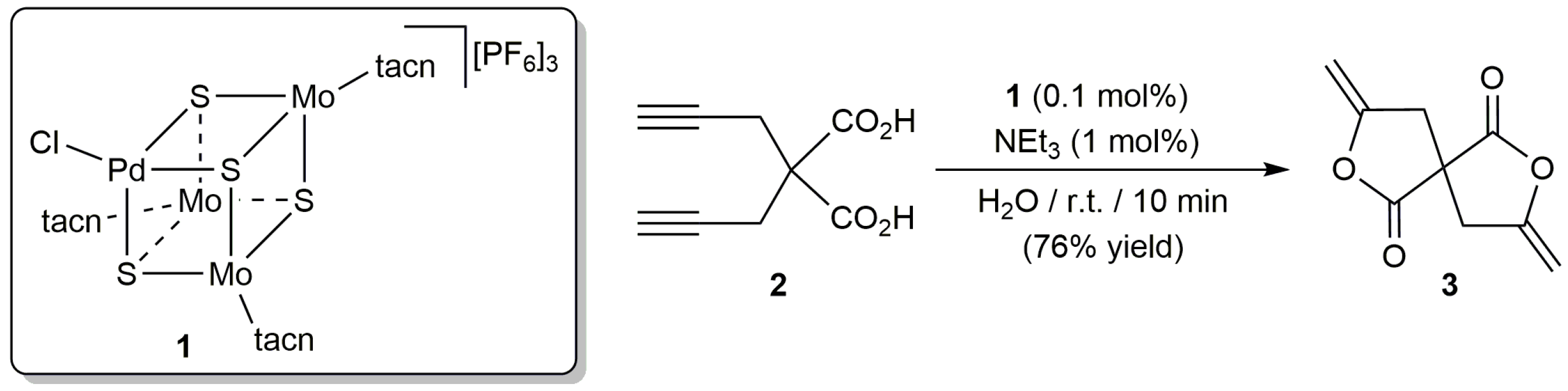
- Wakabayashi, T.; Ishii, Y.; Ishikawa, K.; Hidai, M. A novel catalyst with a cuboidal PdMo3S4 core for the cyclization of alkynoic acids to enol lactones. Angew. Chem. Int. Ed. 1996, 35, 2123–2124. [Google Scholar] [CrossRef]
- García-Álvarez, J.; Díez, J.; Vidal, C. Pd(II)-catalyzed cycloisomerisation of γ-alkynoic acids and one-pot tandem cycloisomerisation/CuAAC reactions in water. Green Chem. 2012, 14, 3190–3196. [Google Scholar] [CrossRef]
- Phillips, A.D.; Gonsalvi, L.; Romerosa, A.; Vizza, F.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA): Transition metal complexes and related catalytic, medicinal and photoluminescent applications. Coord. Chem. Rev. 2004, 248, 955–993. [Google Scholar] [CrossRef]
- Bravo, J.; Bolaño, S.; Gonsalvi, L.; Peruzzini, M. Coordination chemistry of 1,3,5-triaza-7-phosphaadamantane (PTA) and derivatives. Part II. The quest for tailored ligands, complexes and related applications. Coord. Chem. Rev. 2010, 254, 555–607. [Google Scholar] [CrossRef]
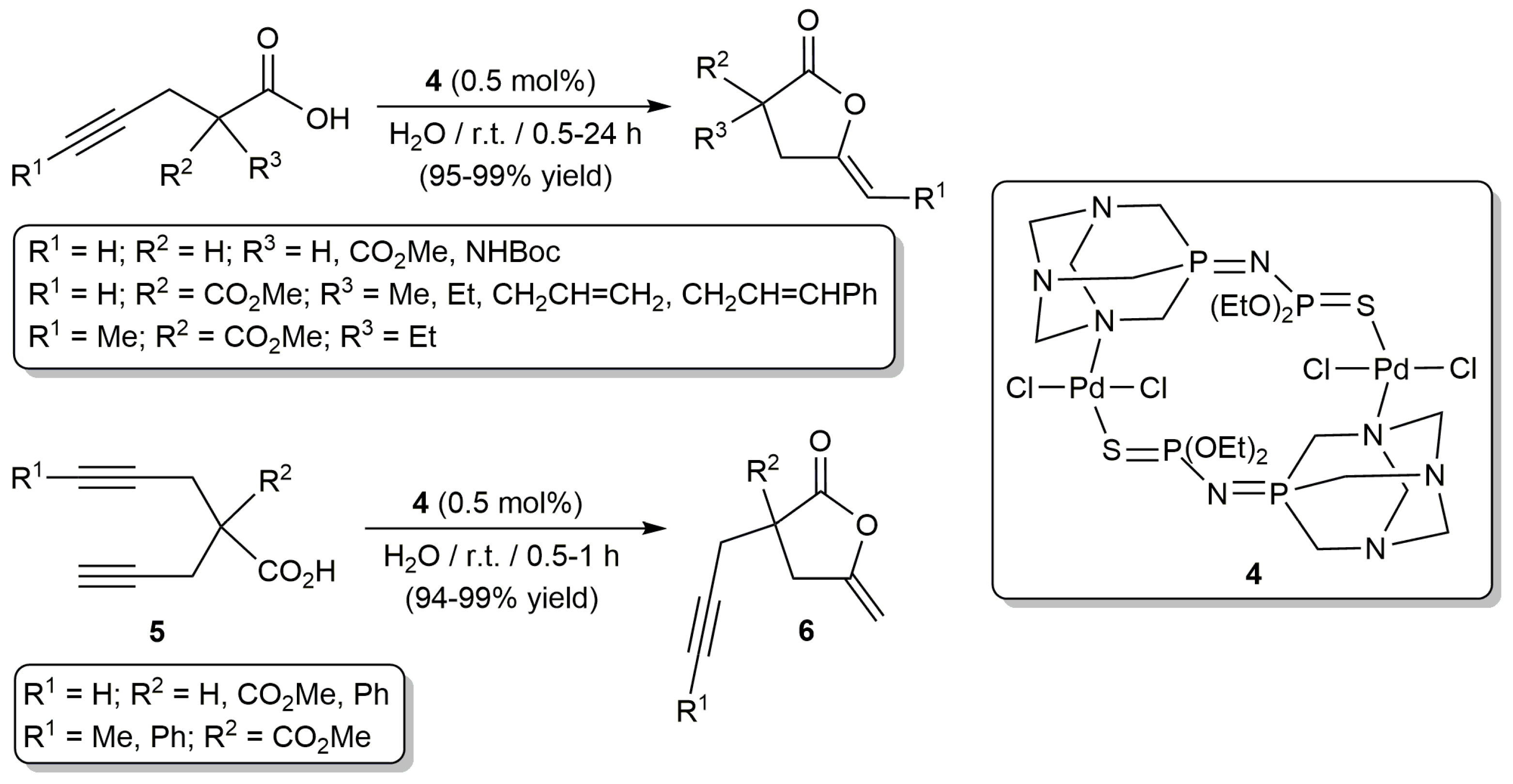
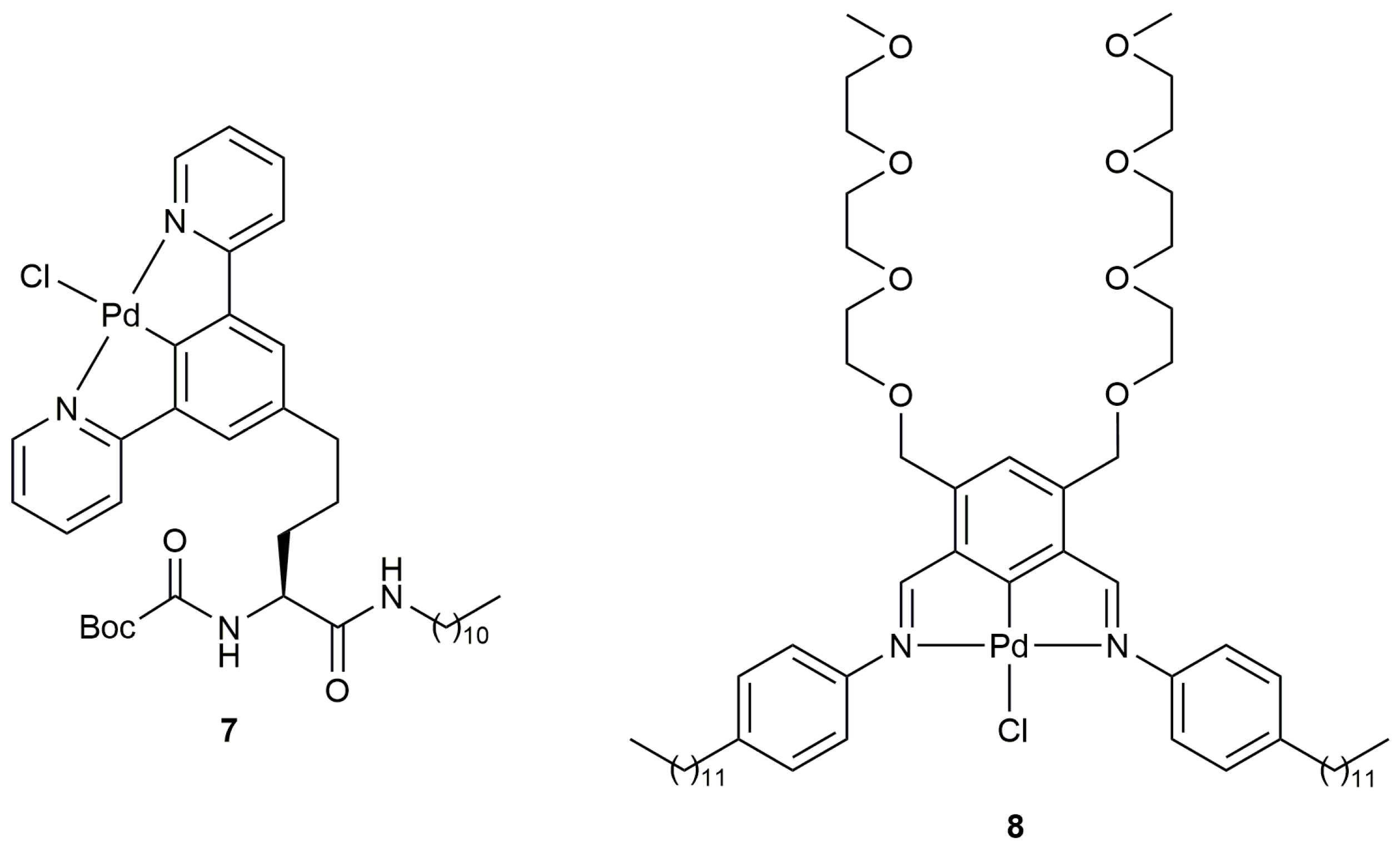
- Ogata, K.; Sasano, D.; Yokoi, T.; Isozaki, K.; Seike, H.; Takaya, H.; Nakamura, M. Pd-complex bound amino acid-based supramolecular gel catalyst for intramolecuar addition-cyclization of alkynoic acids in water. Chem. Lett. 2012, 41, 498–500. [Google Scholar] [CrossRef]
- Hamasaka, G.; Uozumi, Y. Cyclization of alkynoic acids in water in the presence of a vesicular self-assembled amphiphilic pincer palladium complex catalyst. Green Chem. 2014, 50, 14516–14518. [Google Scholar] [CrossRef] [PubMed]
- Michelet, V.; Toullec, P.Y.; Genêt, J.-P. Cycloisomerization of 1,n-enynes: Challenging metal-catalyzed rearrangements and mechanistic insights. Angew. Chem. Int. Ed. 2008, 47, 4268–4315. [Google Scholar] [CrossRef] [PubMed]
- Soriano, E.; Marco-Contelles, J. Mechanistic insights on the cycloisomerization of polyunsaturated precursors catalyzed by platinum and gold complexes. Acc. Chem. Res. 2009, 42, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Espinosa, N.A.; Mallet-Ladeira, S.; Monot, J.; Martin-Vaca, B.; Bourissou, D. Efficient synthesis of unsaturated δ- and ε-lactones/lactams by catalytic cycloisomerization: When Pt outperforms Pd. Adv. Synth. Catal. 2016, 358, 2324–2331, and references therein. [Google Scholar] [CrossRef]
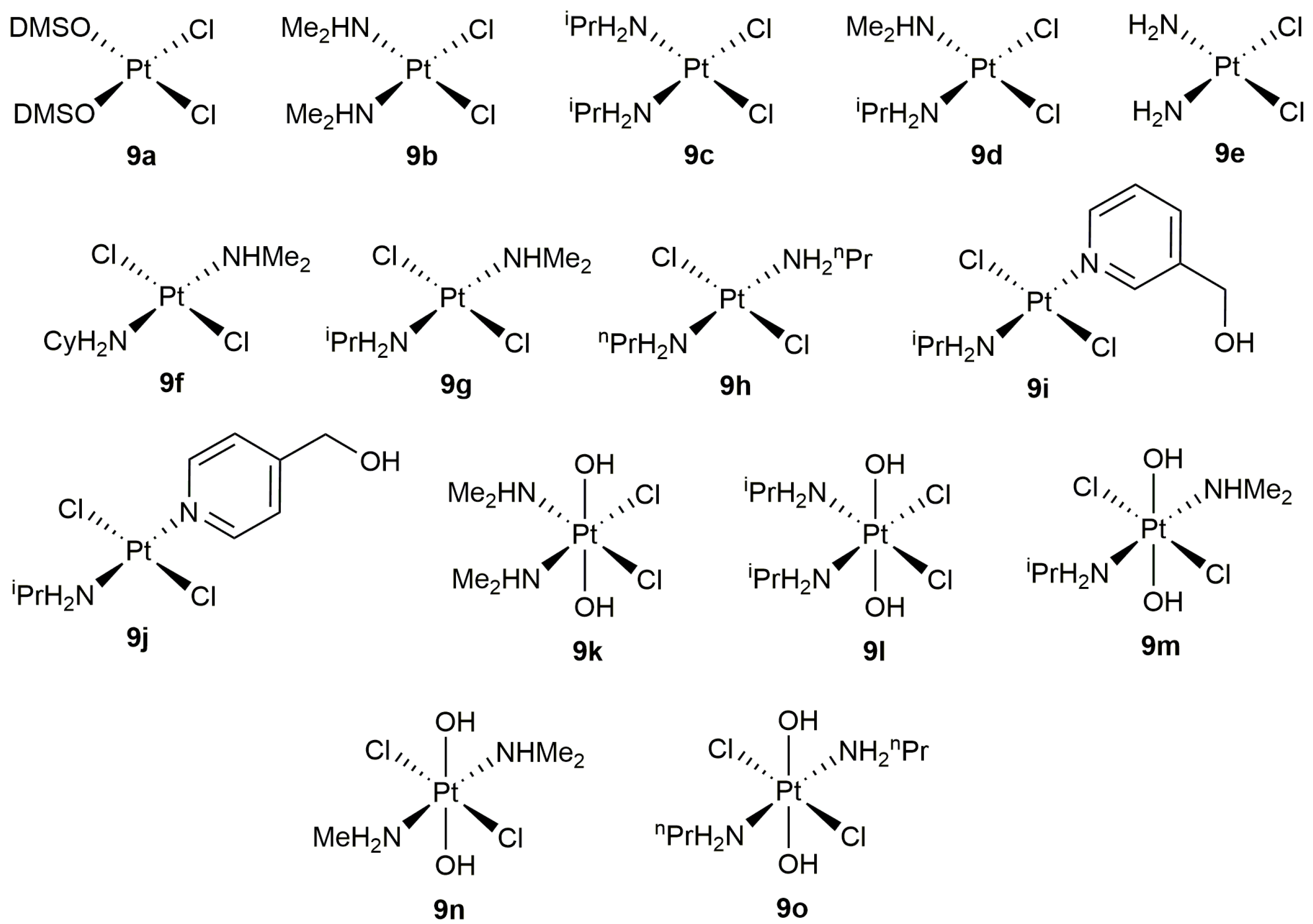
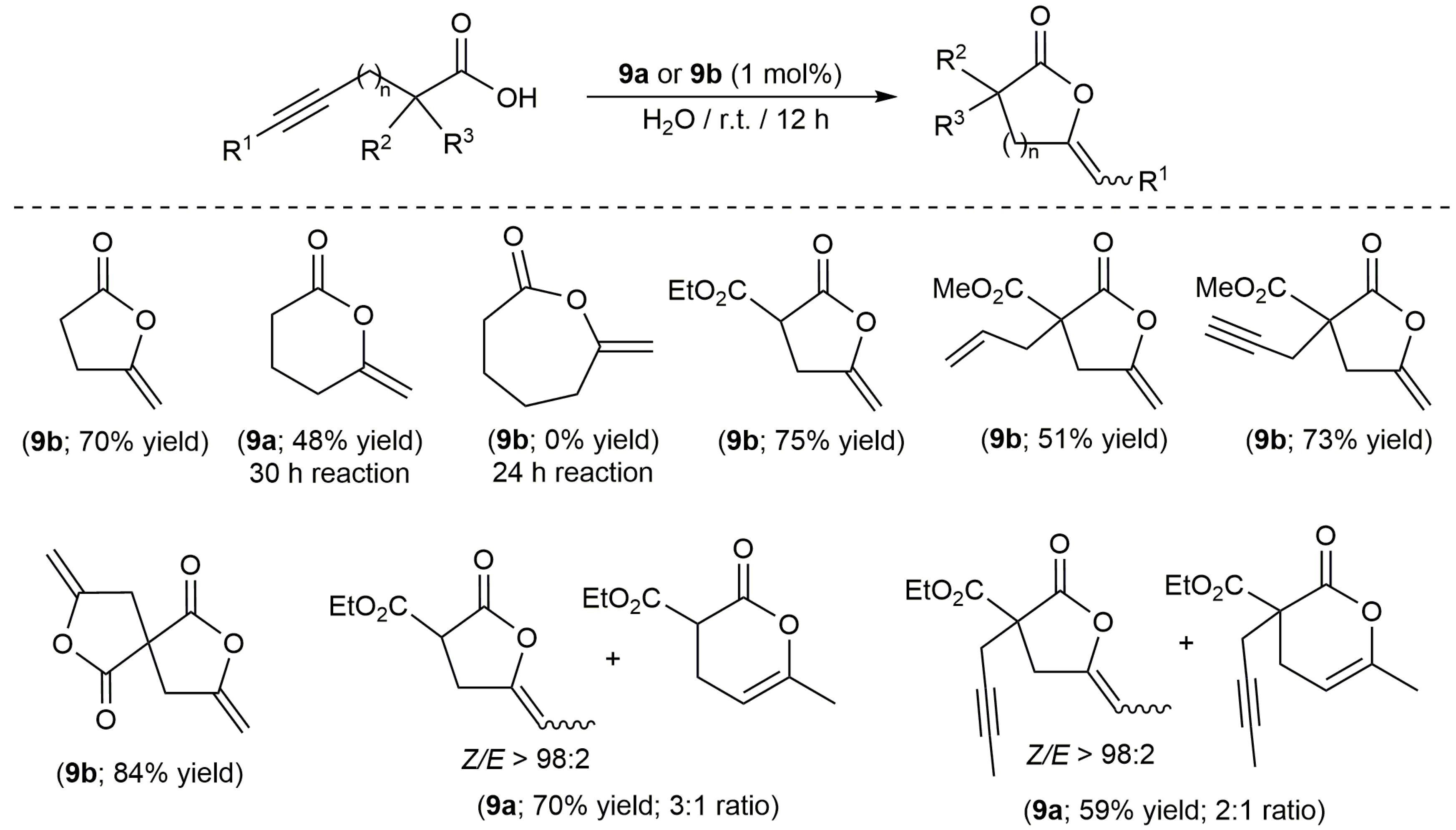
- Alemán, J.; del Solar, V.; Navarro-Ranninger, C. Anticancer platinum complexes as non-innocent compounds for catalysis in aqueous media. Chem. Commun. 2010, 46, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.; del Solar, V.; Cubo, L.; Quiroga, A.G.; Navarro-Ranninger, C. New reactions of anticancer-platinum complexes and their intriguing behavior under various experimental conditions. Dalton Trans. 2010, 39, 10601–10607. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K.; Toste, F.D. (Eds.) Modern Gold Catalyzed Synthesis; Wiley-VCH: Weinheim, Germany, 2012; ISBN 9783527319527. [Google Scholar]
- Toste, F.D.; Michelet, V. (Eds.) Gold Catalysis: An Homogeneous Approach; Imperial College Press: London, UK, 2014; ISBN 9781848168527. [Google Scholar]
- Genin, E.; Toullec, P.Y.; Antoniotti, S.; Brancour, C.; Genêt, J.-P.; Michelet, V. Room temperature Au(I)-catalyzed exo-selective cycloisomerization of acetylenic acids: An entry to functionalized γ-lactones. J. Am. Chem. Soc. 2006, 128, 3112–3113. [Google Scholar] [CrossRef] [PubMed]
- Toullec, P.Y.; Genin, E.; Antoniotti, S.; Genêt, J.-P.; Michelet, V. Au2O3 as a stable and efficient catalyst for the selective cycloisomerization of γ-acetylenic carboxylic acids to γ-alkylidene-γ-butyrolactones. Synlett 2008, 707–711. [Google Scholar]
- Tomás-Mendivil, E.; Toullec, P.Y.; Díez, J.; Conejero, S.; Michelet, V.; Cadierno, V. Cycloisomerization versus hydration reactions in aqueous media: A Au(III)-NHC catalyst that makes the difference. Org. Lett. 2012, 14, 2520–2523. [Google Scholar] [CrossRef] [PubMed]
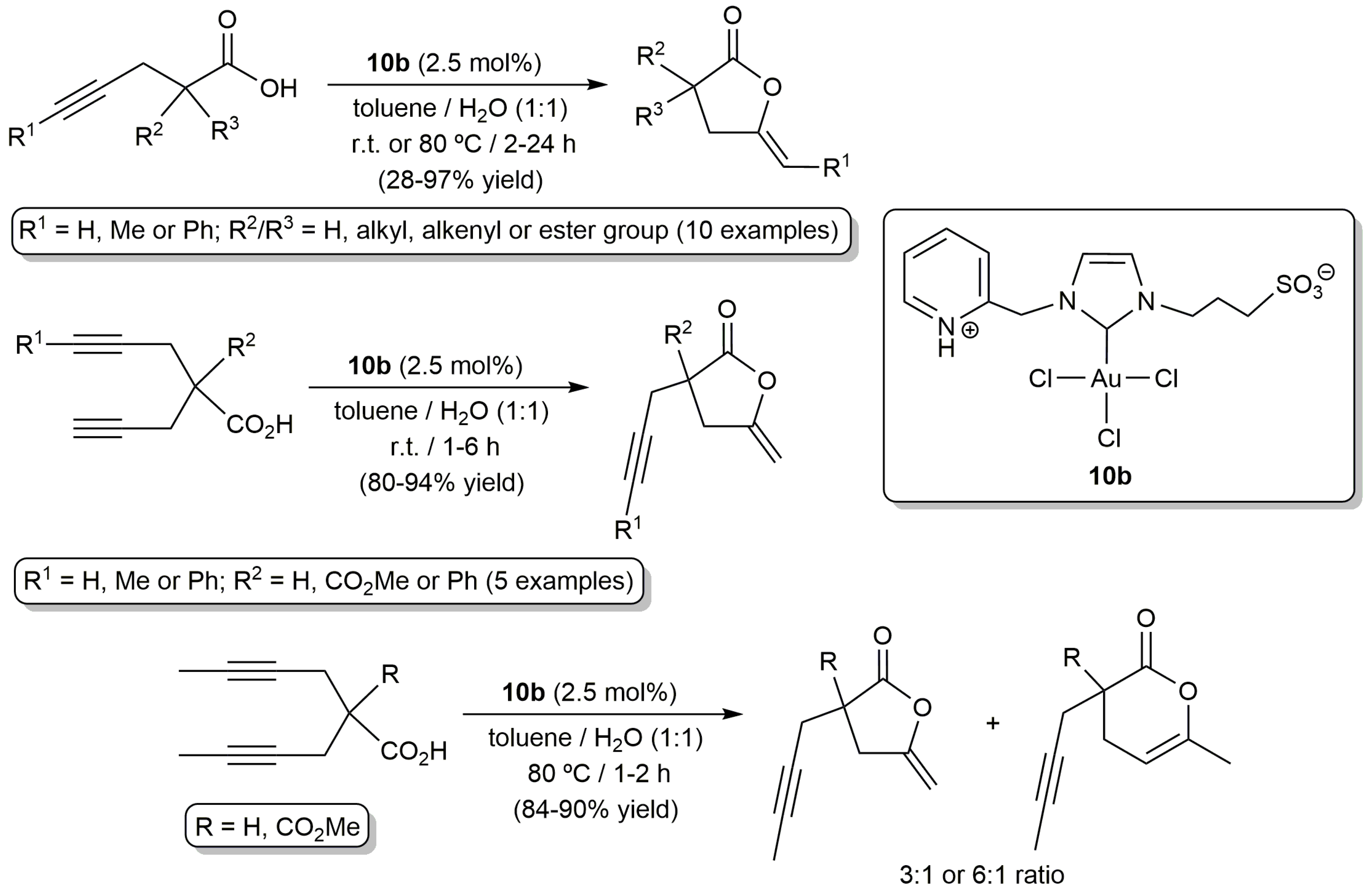
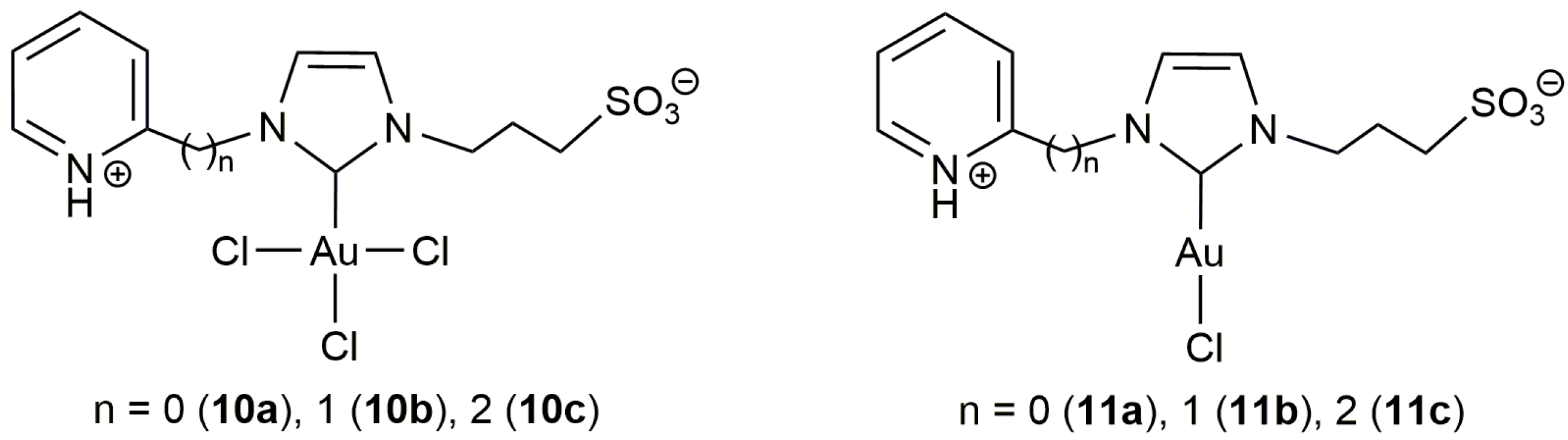
- Tomás-Mendivil, E.; Toullec, P.Y.; Borge, J.; Conejero, S.; Michelet, V.; Cadierno, V. Water-soluble gold(I) and gold(III) complexes with sulfonated N-heterocyclic carbene ligands: Synthesis, characterization, and application in the catalytic cycloisomerization of γ-alkynoic acids into enol-lactones. ACS Catal. 2013, 3, 3086–3098. [Google Scholar] [CrossRef]
- Ferré, M.; Cattoën, X.; Wong Chi Man, M.; Pleixats, R. Sol-gel immobilized N-heterocyclic carbene gold complex as a recyclable catalyst for the rearrangement of allylic esters and the cycloisomerization of γ-alkynoic acids. ChemCatChem 2016, 8, 2824–2831. [Google Scholar] [CrossRef]
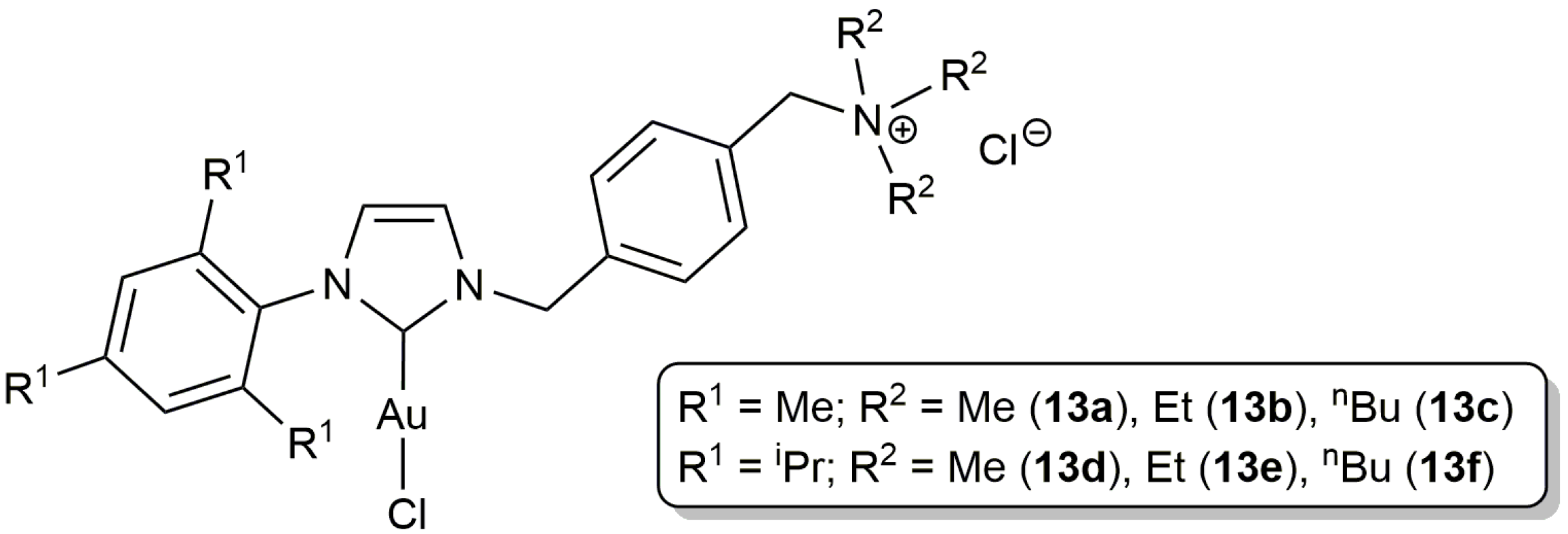
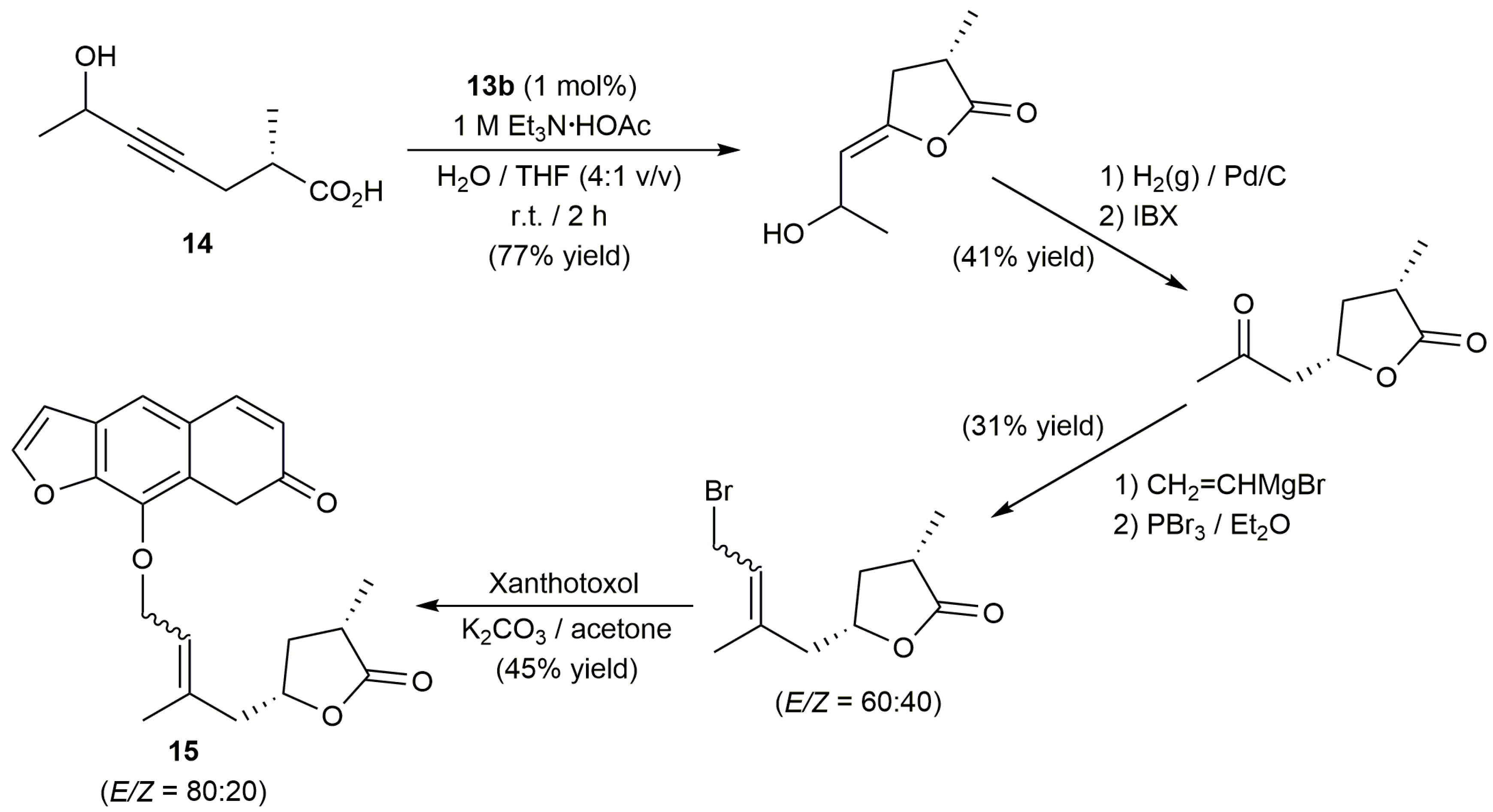
- Belger, K.; Krause, N. Smaller, faster, better: Modular synthesis of unsymmetrical ammonium salt-tagged NHC-gold(I) complexes and their application as recyclable catalysts in water. Org. Biomol. Chem. 2015, 13, 8556–8560. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Álvarez, M.J.; Vidal, C.; Díez, J.; García-Álvarez, J. Introducing deep eutectic solvents as biorenewable media for Au(I)-catalysed cycloisomerisation of γ-alkynoic acids: An unprecedented catalytic system. Chem. Commun. 2014, 50, 12927–12929. [Google Scholar] [CrossRef] [PubMed]
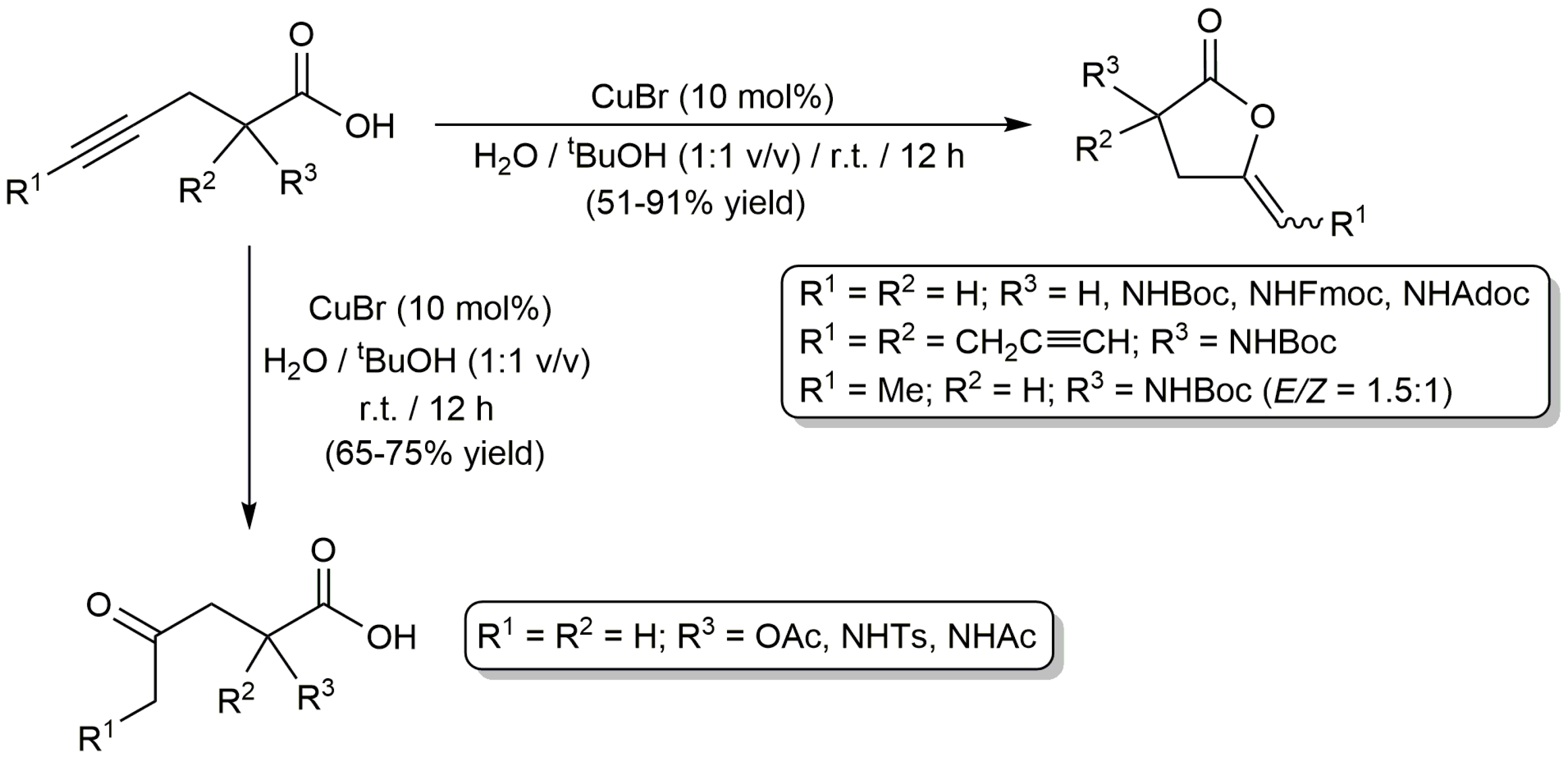
- Mindt, T.L.; Schibli, R. Cu(I)-catalyzed intramolecular cyclization of alkynoic acids in aqueous media: A “click side reaction”. J. Org. Chem. 2007, 72, 10247–10250. [Google Scholar] [CrossRef] [PubMed]
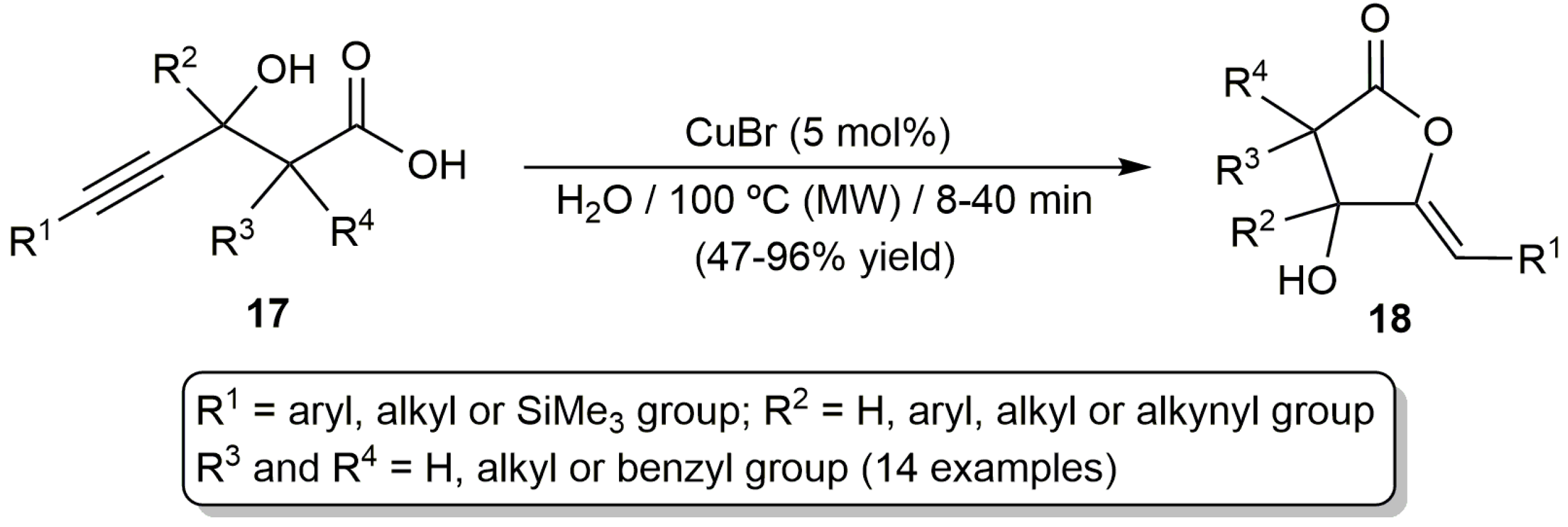
- López-Reyes, M.E.; Toscano, R.A.; López-Cortés, J.G.; Alvarez-Toledano, C. Fast and efficient synthesis of Z-enol-γ-lactones through a cycloisomerization reaction of β-hydroxy-γ-alkynoic acids catalyzed by copper(I) under microwave heating in water. Asian J. Org. Chem. 2015, 4, 545–551. [Google Scholar] [CrossRef]
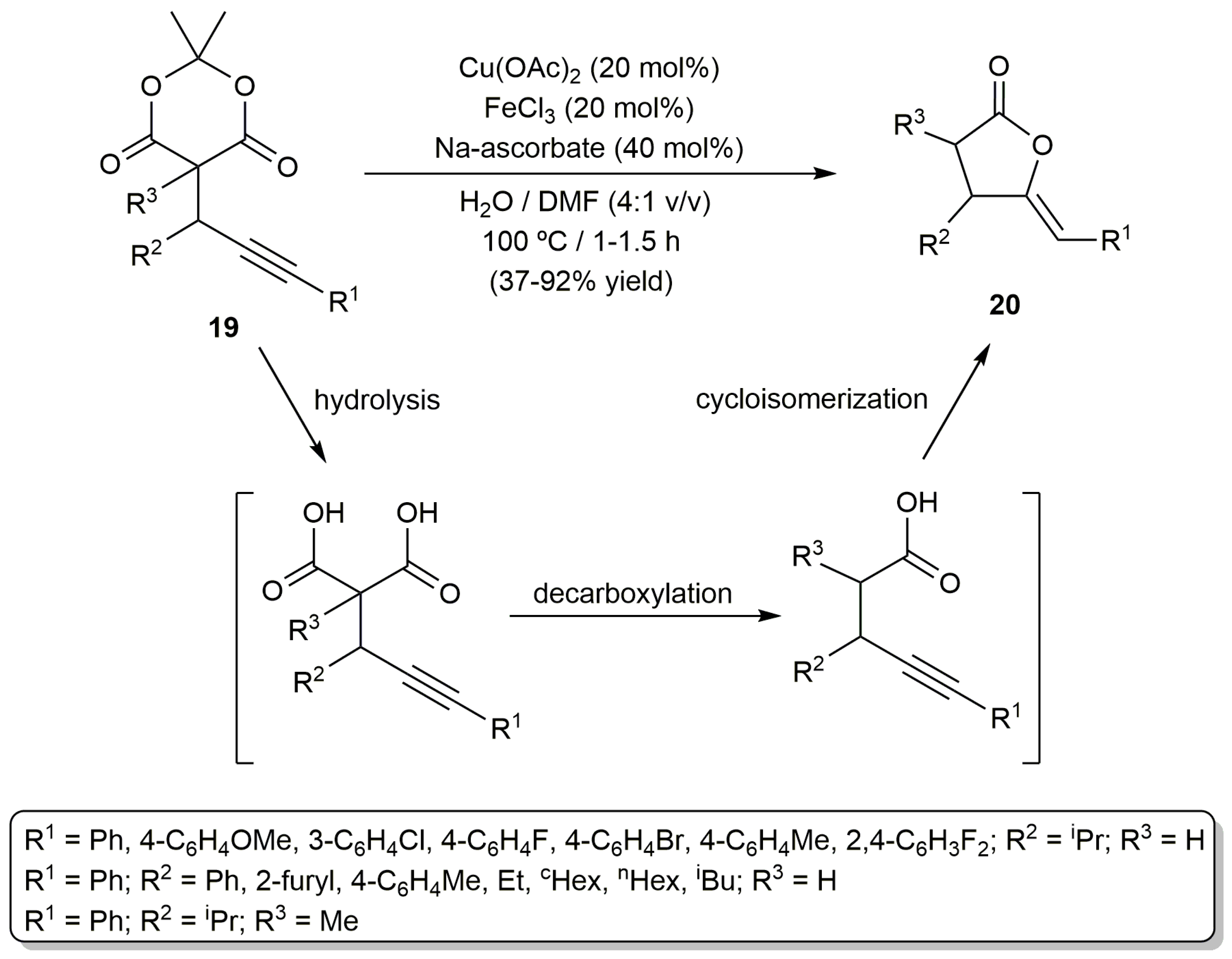
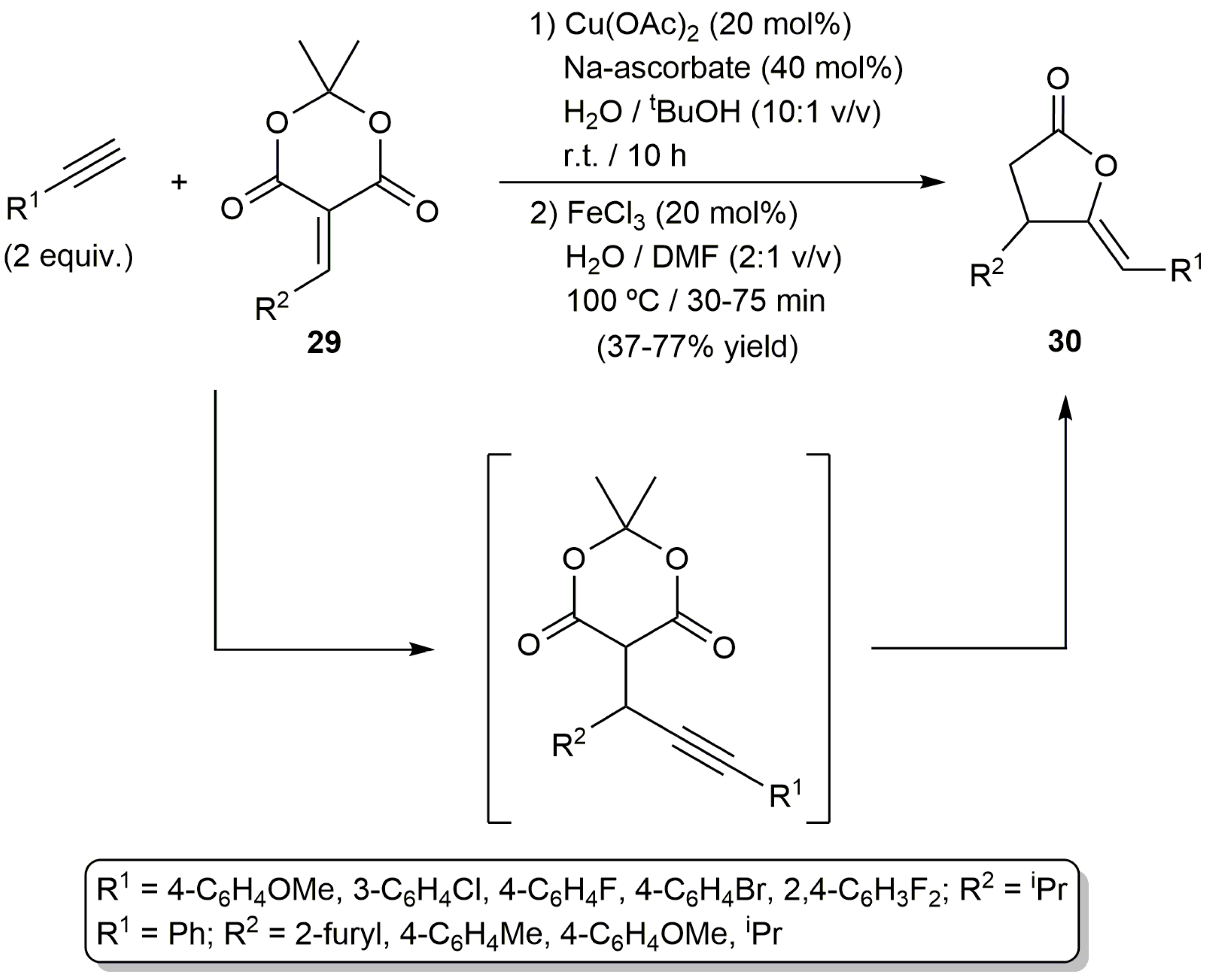
- Li, S.; Jia, W.; Jiao, N. Copper/iron-cocatalyzed highly selective tandem reactions: Efficient approaches to Z-γ-alkylidene lactones. Adv. Synth. Catal. 2009, 351, 569–575. [Google Scholar] [CrossRef]
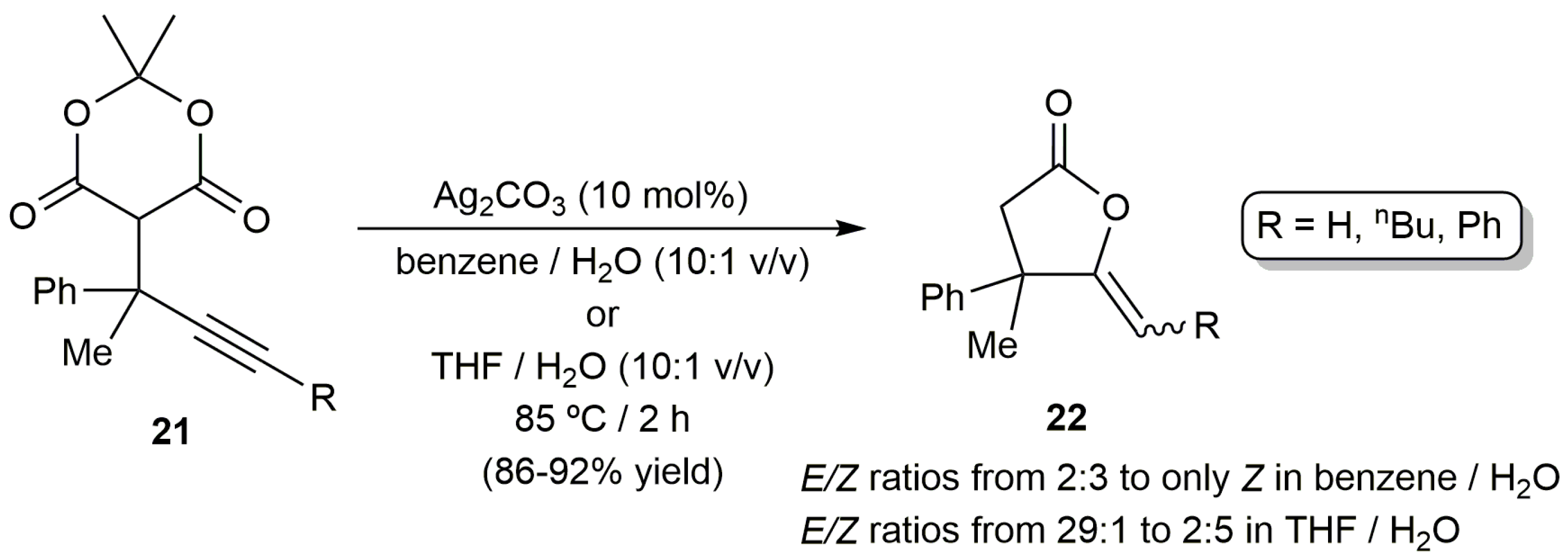
- Jia, W.; Li, S.; Yu, M.; Chen, W.; Jiao, N. AgNO3 catalyzed cyclization of propargyl-Meldrum´s acids in aqueous solvent: Highly selective synthesis of Z-γ-alkylidene lactones. Tetrahedron Lett. 2009, 50, 5406–5408. [Google Scholar] [CrossRef]
- Ahmar, S.; Fillion, E. Expedient synthesis of complex γ-butyrolactones from 5-(1-arylalkylidene) Meldrum´s acids via sequential conjugate alkynylation/Ag(I)-catalyzed lactonization. Org. Lett. 2014, 16, 5748–5751. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, D.D.; Repič, O.; Blacklock, T.J. A mild method for ring-opening aminolysis of lactones. Tetrahedron Lett. 2001, 42, 2439–2441. [Google Scholar] [CrossRef]
- Guo, W.; Gómez, J.E.; Martínez-Rodríguez, L.; Bandeira, N.A.G.; Bo, C.; Kleij, A.W. Metal-free synthesis of N-aryl amides using organocatalytic ring-opening aminolysis of lactones. ChemSusChem 2017, 10, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Campbell, L.; Dixon, D.J. A Au(I)-catalyzed N-acyl iminium ion cyclization cascade. J. Am. Chem. Soc. 2007, 129, 12070–12071. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Zhuo, Y.; Zhang, D.; Zhang, L.; Sun, H.; Jiang, H.; Liu, H. Gold(I)-catalyzed tandem transformation: A simple approach for the synthesis of pyrrolo/pyrido[2,1-a][1,3]benzoxazinones and pyrrolo/pyrido[2,1-a]quinazolinones. J. Org. Chem. 2010, 75, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Lakshmi, P.G.V.V.; Sridhar, B.; Patra, S.; Bhadra, M.P.; Patra, C.R. New linearly and angularly fused quinazolinones: Synthesis through gold(I)-catalyzed cascade reactions and anticancer activities. Eur. J. Org. Chem. 2012, 1790–1799. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Yang, N.; Chen, Y.; Zhou, Y.; Ji, X.; Zhang, L.; Wang, J.; Xie, X.; Liu, H. Gold(I)-catalyzed cascade approach for the synthesis of tryptamine-based polycyclic privileged scaffolds as α1-adrenergenic receptor antagonists. J. Org. Chem. 2013, 76, 10802–10811. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Zhou, Y.; Zhao, F.; Chen, X.; Zheng, L.; Jiang, H.; Liu, H. Gold-catalyzed tandem reaction in water: An efficient and convenient synthesis of fused polycyclic indoles. Green Chem. 2012, 14, 1888–1895. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhai, Y.; Ji, X.; Liu, G.; Feng, E.; Ye, D.; Zhao, L.; Jiang, H.; Liu, H. Gold(I)-catalyzed one-pot tandem coupling/cyclization: An efficient synthesis of pyrrolo-/pyrido[2,1-b]benzo[d][1,3]oxazin-1-ones. Adv. Synth. Catal. 2010, 352, 373–378. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, H.-F. Synthesis of phthalides via Pd/CNTs-catalyzed reaction of terminal alkynes and o-iodobenzoic acid under copper- and ligand-free conditions. Tetrahedron Lett. 2007, 48, 8449–8452. [Google Scholar] [CrossRef]
- García-Álvarez, J.; Díez, J.; Gimeno, J. A highly efficient copper(I) catalyst for the 1,3-dipolar addition of azides with terminal and 1-iodoalkynes in water: Regioselective synthesis of 1,4-disubstituted and 1,4,5-trisubstituted 1,2,3-triazoles. Green Chem. 2010, 12, 2127–2130. [Google Scholar] [CrossRef]
- Denard, C.A.; Hartwig, J.F.; Zhao, H. Multistep one-pot reactions combining biocatalysts and chemical catalysts for asymmetric synthesis. ACS Catal. 2013, 3, 2856–2864. [Google Scholar] [CrossRef]
- Gröger, H.; Hummel, W. Combining the ‘two worlds’ of chemocatalysis and biocatalysis towards multi-step one-pot processes in aqueous media. Curr. Opin. Chem. Biol. 2014, 19, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.; Balskus, E.P. Opportunities for merging chemical and biological synthesis. Curr. Opin. Biotechnol. 2014, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Álvarez, M.J.; Ríos-Lombardía, N.; Schumacher, S.; Pérez-Iglesias, D.; Morís, F.; Cadierno, V.; García-Álvarez, J.; González-Sabín, J. Combination of metal-catalyzed cycloisomerizations and biocatalysis in aqueous media: Asymmetric construction of chiral alcohols, lactones and γ-hydroxy-carbonyl compounds. ACS Catal. 2017, 7, 7753–7759. [Google Scholar] [CrossRef]
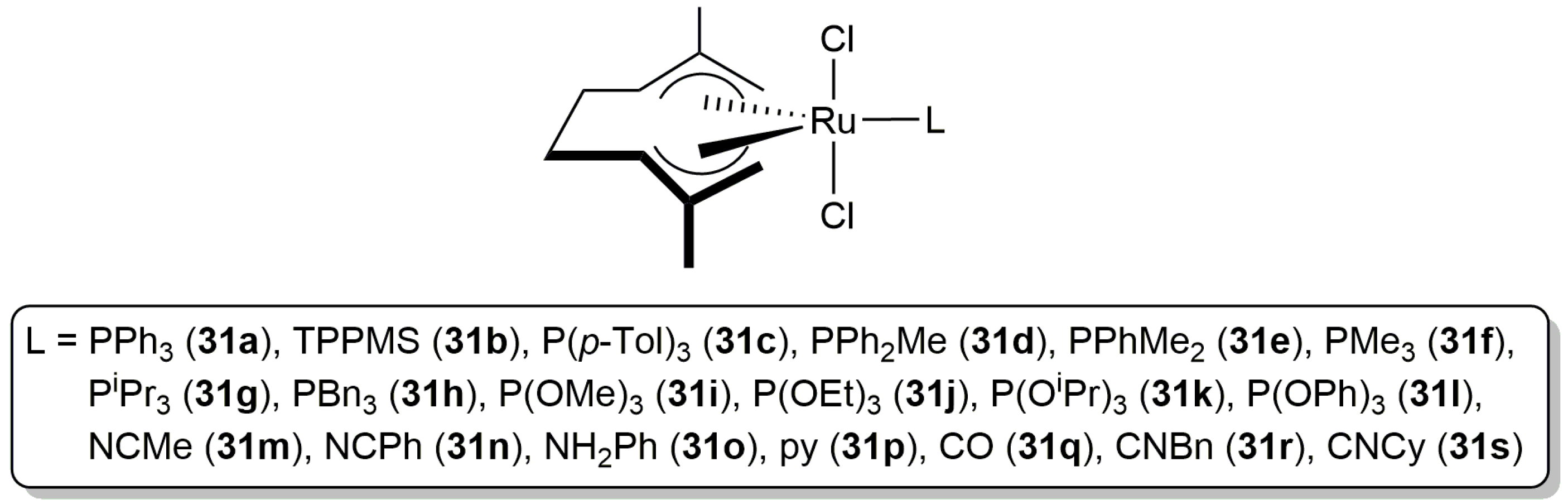
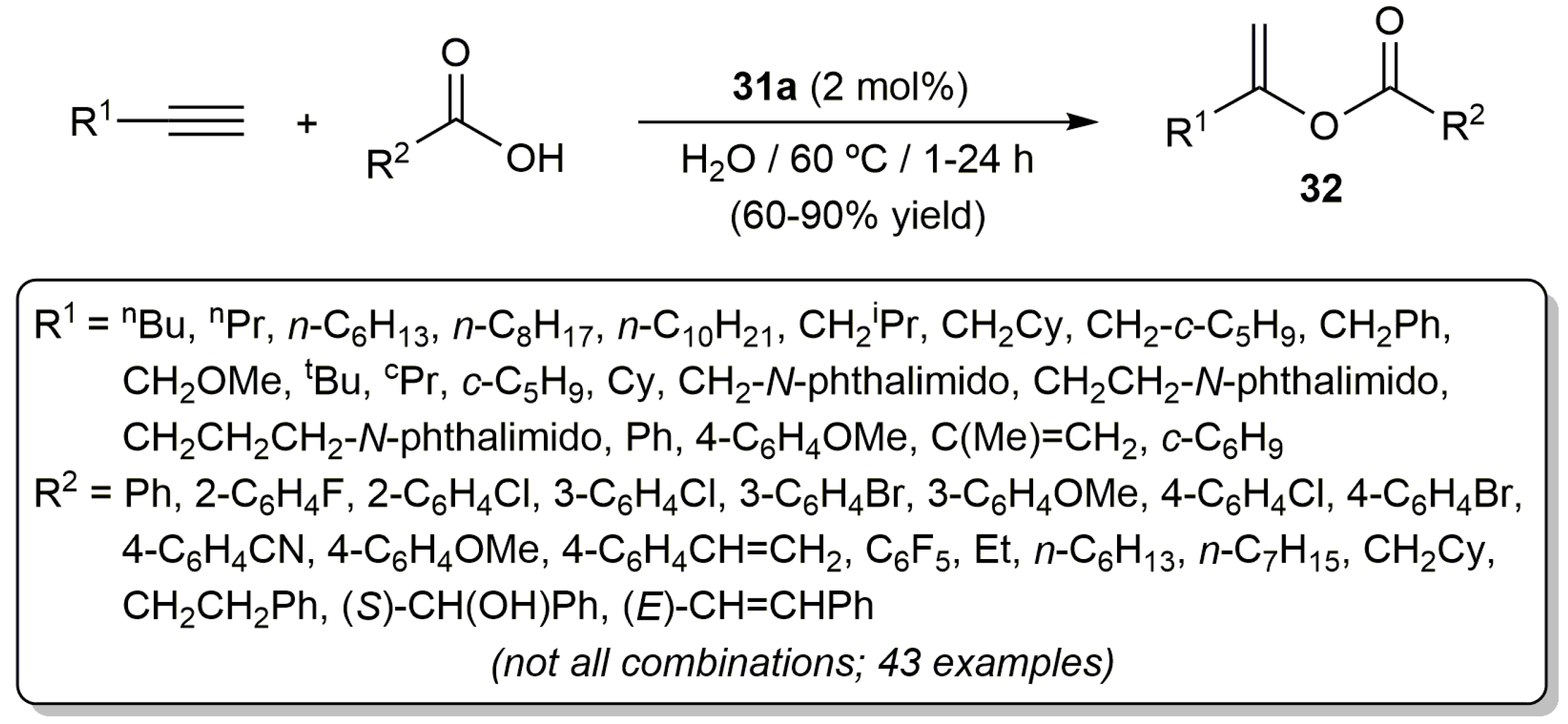
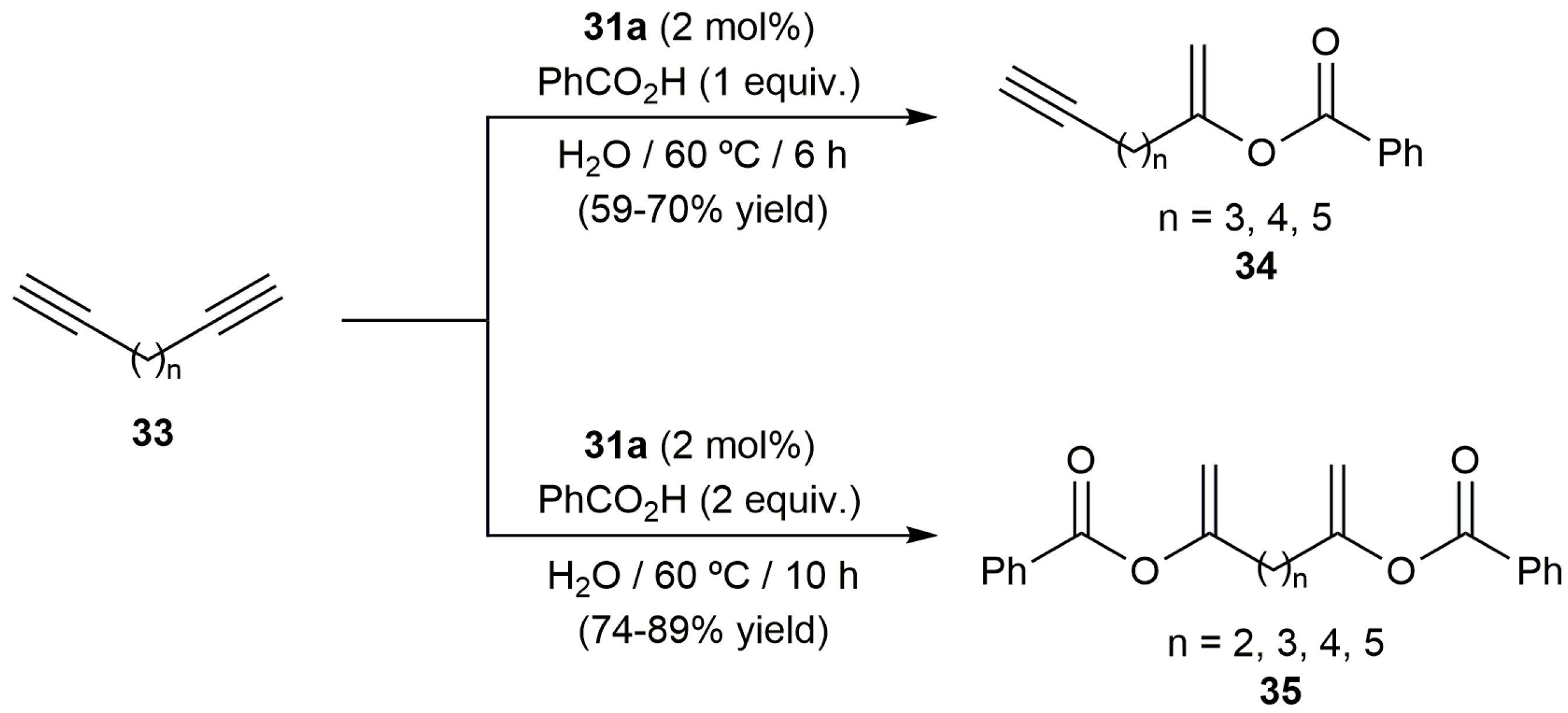
- Cadierno, V.; Francos, J.; Gimeno, J. Ruthenium(IV)-catalyzed Markovnikov addition of carboxylic acids to terminal alkynes in aqueous medium. Organometallics 2011, 30, 852–862. [Google Scholar] [CrossRef]
- Chanda, A.; Fokin, V.V. Organic synthesis ”on water”. Chem. Rev. 2009, 109, 725–748. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.N.; Coyne, A.G. Water: Nature’s reaction enforcer—Comparative effects for organic synthesis “in-water” and “on-water”. Chem. Rev. 2010, 110, 6302–6337. [Google Scholar] [CrossRef] [PubMed]
- Nicks, F.; Aznar, R.; Sainz, D.; Muller, G.; Demonceau, A. Novel, highly efficient and selective ruthenium catalysts for the synthesis of vinyl esters from carboxylic acids and alkynes. Eur. J. Org. Chem. 2009, 5020–5027. [Google Scholar] [CrossRef]
- Kleman, P.; González-Liste, P.J.; García-Garrido, S.E.; Cadierno, V.; Pizzano, A. Highly enantioselective hydrogenation of 1-alkylvinyl benzoates: A simple, nonenzymatic access to chiral 2-alkanols. Chem. Eur. J. 2013, 19, 16209–16212. [Google Scholar] [CrossRef] [PubMed]
- Kleman, P.; González-Liste, P.J.; García-Garrido, S.E.; Cadierno, V.; Pizzano, A. Asymmetric hydrogenation of 1-alkyl and 1-aryl vinyl benzoates: A broad scope procedure for the highly enantioselective synthesis of 1-substituted ethyl benzoates. ACS Catal. 2014, 4, 4398–4408. [Google Scholar] [CrossRef]
- González-Liste, P.J.; García-Garrido, S.E.; Cadierno, V. Gold(I)-catalyzed addition of carboxylic acids to internal alkynes in aqueous medium. Org. Biomol. Chem. 2017, 15, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Scheid, G.; Kuit, W.; Ruijter, E.; Orru, R.V.A.; Henke, E.; Bornscheuer, U.; Wessjohann, L.A. A new route to protected acyloins and their enzymatic resolution with lipases. Eur. J. Org. Chem. 2004, 1063–1074. [Google Scholar] [CrossRef]
- Carpino, P.A.; Griffith, D.A.; Sakya, S.; Dow, R.L.; Black, S.C.; Hadcock, J.R.; Iredale, P.A.; Scott, D.O.; Fichtner, M.W.; Rose, C.R.; et al. New bicyclic cannabinoid receptor-1 (CB1-R) antagonists. Bioorg. Med. Chem. Lett. 2006, 16, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-L.; Wu, Z.-W.; Song, S.-Y.; Liao, X.-D.; Zhu, Y.; Huang, Y.-S. An improved synthesis of nomegestrol acetate. Org. Process Res. Dev. 2014, 18, 431–436. [Google Scholar] [CrossRef]
- Kaila, N.; Janz, K.; DeBernardo, S.; Bedard, P.W.; Camphausen, R.T.; Tam, S.; Tsao, D.H.H.; Keith, J.C.; Nickerson-Nutter, C.; Shilling, A.; Young-Sciame, R.; Wang, Q. Synthesis and biological evaluation of quinoline salicylic acids as P-Selectin antagonists. J. Med. Chem. 2007, 50, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.F.M.; Radics, G.; Windle, H.; Serra, H.O.; Simplício, A.L.; Kedziora, K.; Fallon, P.G.; Kelleher, D.P.; Gilmer, J.F. Design, synthesis, and pharmacological effects of a cyclization-activated steroid prodrug for colon targeting in inflammatory bowel disease. J. Med. Chem. 2009, 52, 3205–3211. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, J.; Korb, M.; Rüffer, T.; Gäbler, C.; Lang, H. Atom economic ruthenium-catalyzed synthesis of bulky β-oxo esters. Adv. Synth. Catal. 2015, 357, 4069–4081, and references therein. [Google Scholar] [CrossRef]
- Mitsudo, T.; Hori, Y.; Yamakawa, Y.; Watanabe, Y. Ruthenium-catalyzed selective addition of carboxylic acids to alkynes. A new synthesis of enol esters. J. Org. Chem. 1987, 52, 2230–2239. [Google Scholar] [CrossRef]
- Bauer, E. Transition-metal-catalyzed functionalization of propargylic alcohols and their derivatives. Synthesis 2012, 44, 1131–1151. [Google Scholar] [CrossRef]
- Cadierno, V.; Francos, J.; Gimeno, J. Ruthenium-catalyzed synthesis of β-oxo esters in aqueous medium: Scope and limitations. Green Chem. 2010, 12, 135–143. [Google Scholar] [CrossRef]


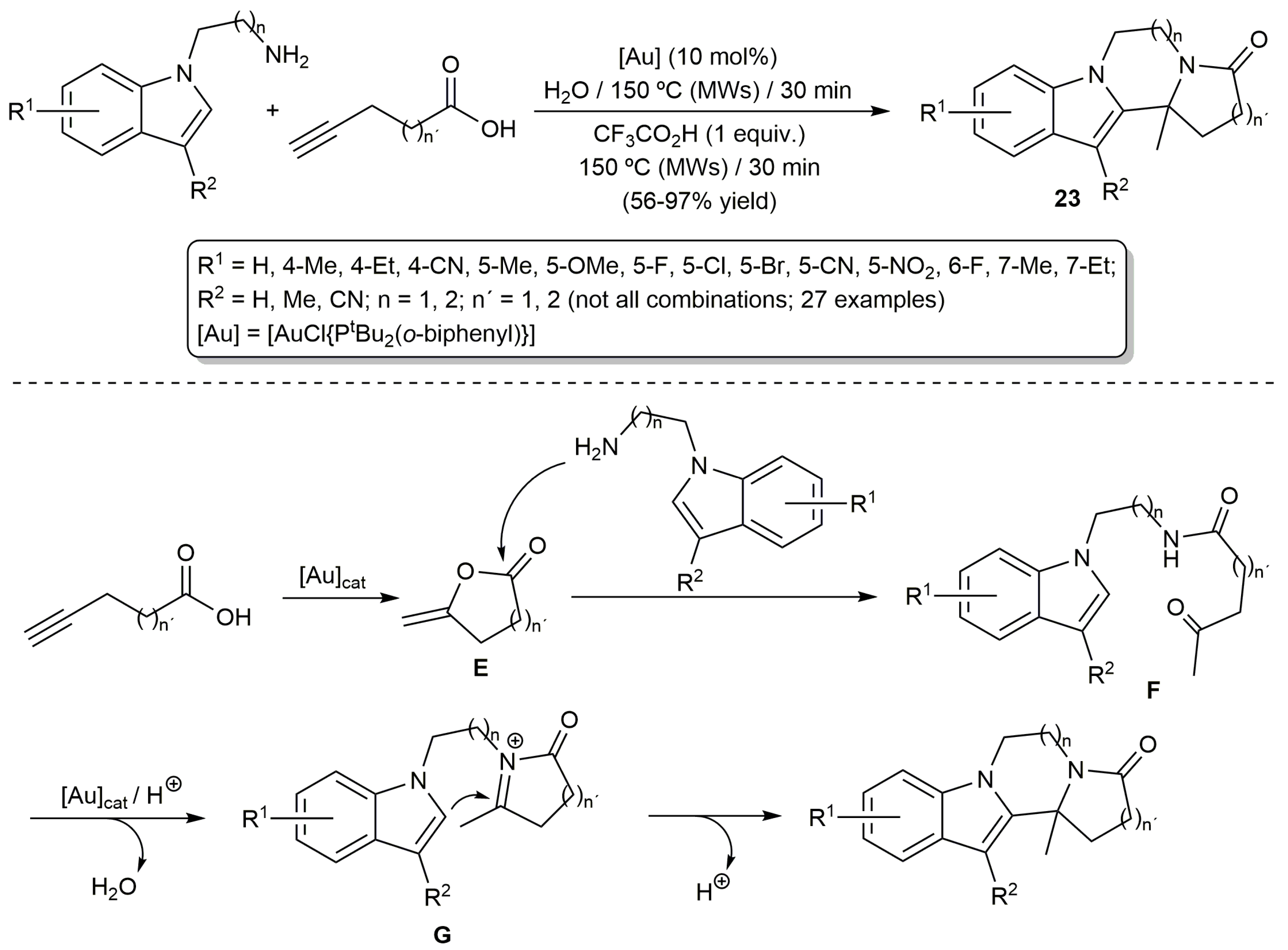
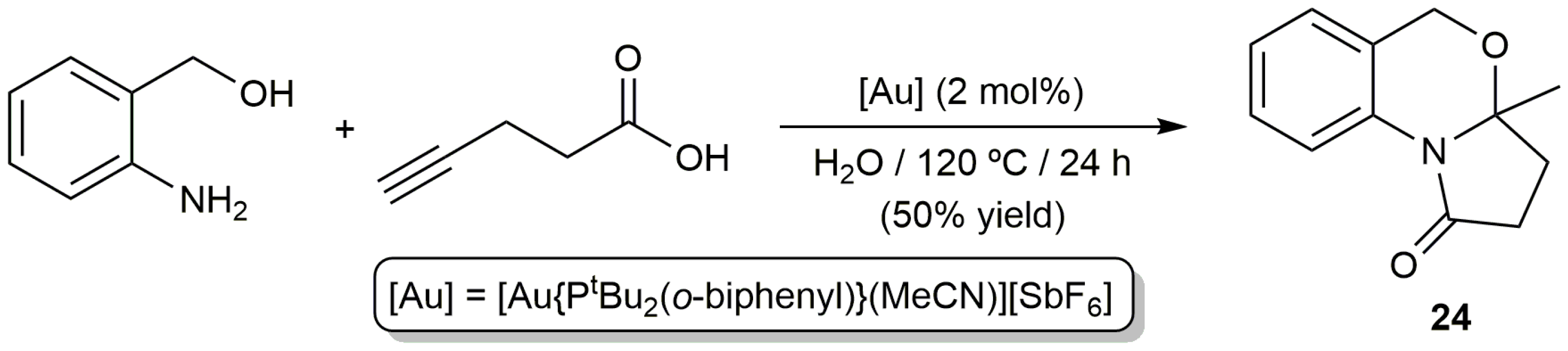
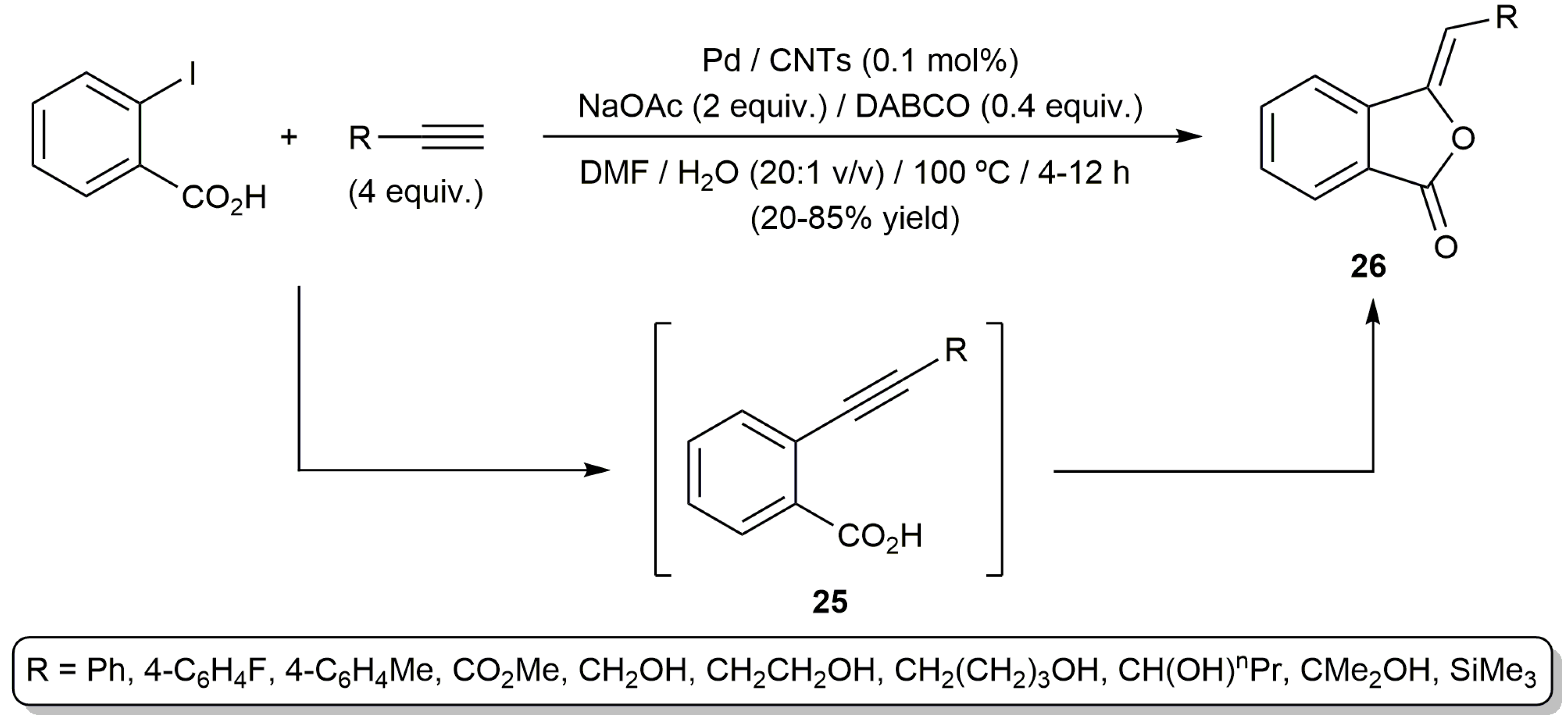
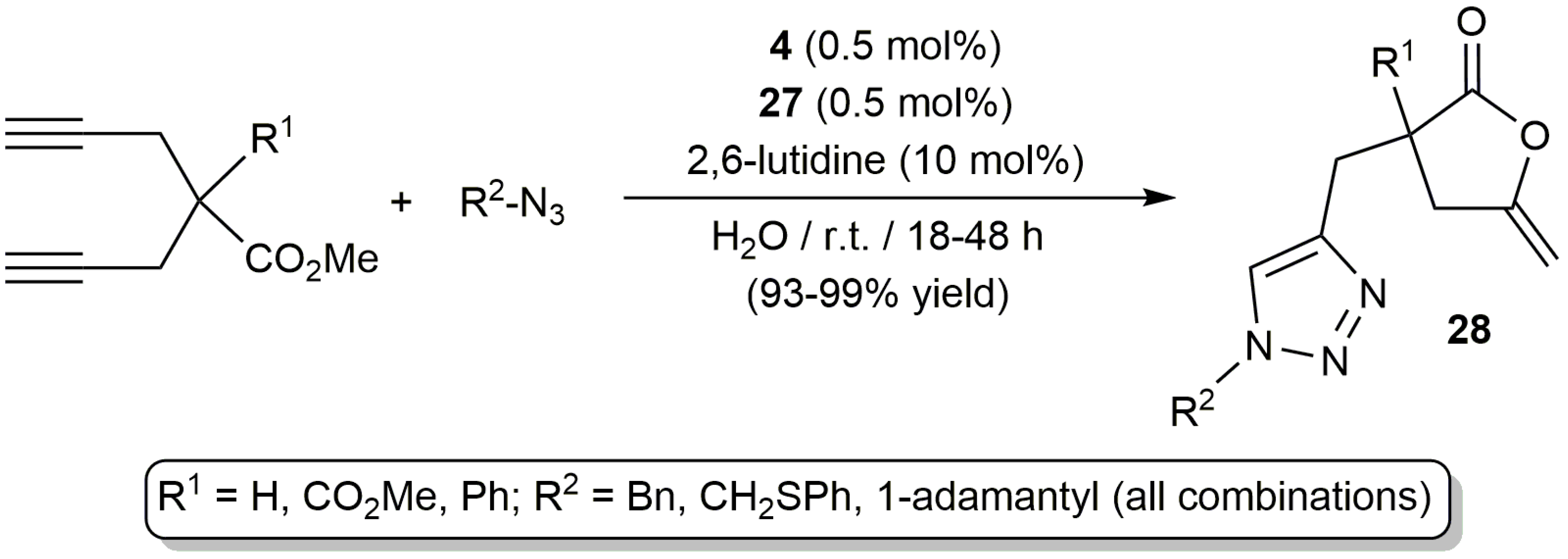

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francos, J.; Cadierno, V. Metal-Catalyzed Intra- and Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: A Review. Catalysts 2017, 7, 328. https://doi.org/10.3390/catal7110328
Francos J, Cadierno V. Metal-Catalyzed Intra- and Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: A Review. Catalysts. 2017; 7(11):328. https://doi.org/10.3390/catal7110328
Chicago/Turabian StyleFrancos, Javier, and Victorio Cadierno. 2017. "Metal-Catalyzed Intra- and Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: A Review" Catalysts 7, no. 11: 328. https://doi.org/10.3390/catal7110328