A Non-Precious Metal Promoting the Synthesis of 5-Hydroxymethylfurfural
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Cu-K-OMS-2 Catalysts
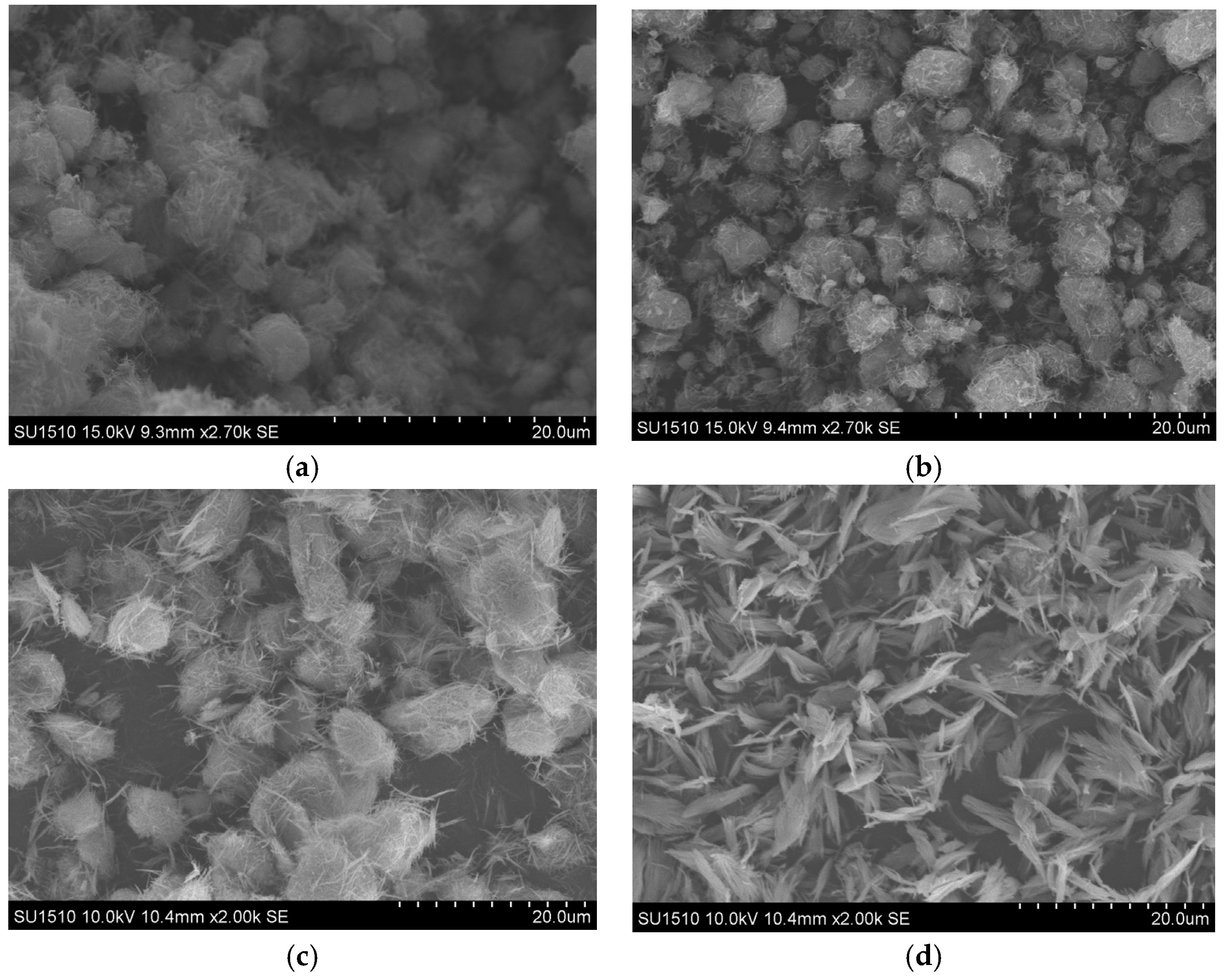
2.1.1. Scanning Electron Microscope Images
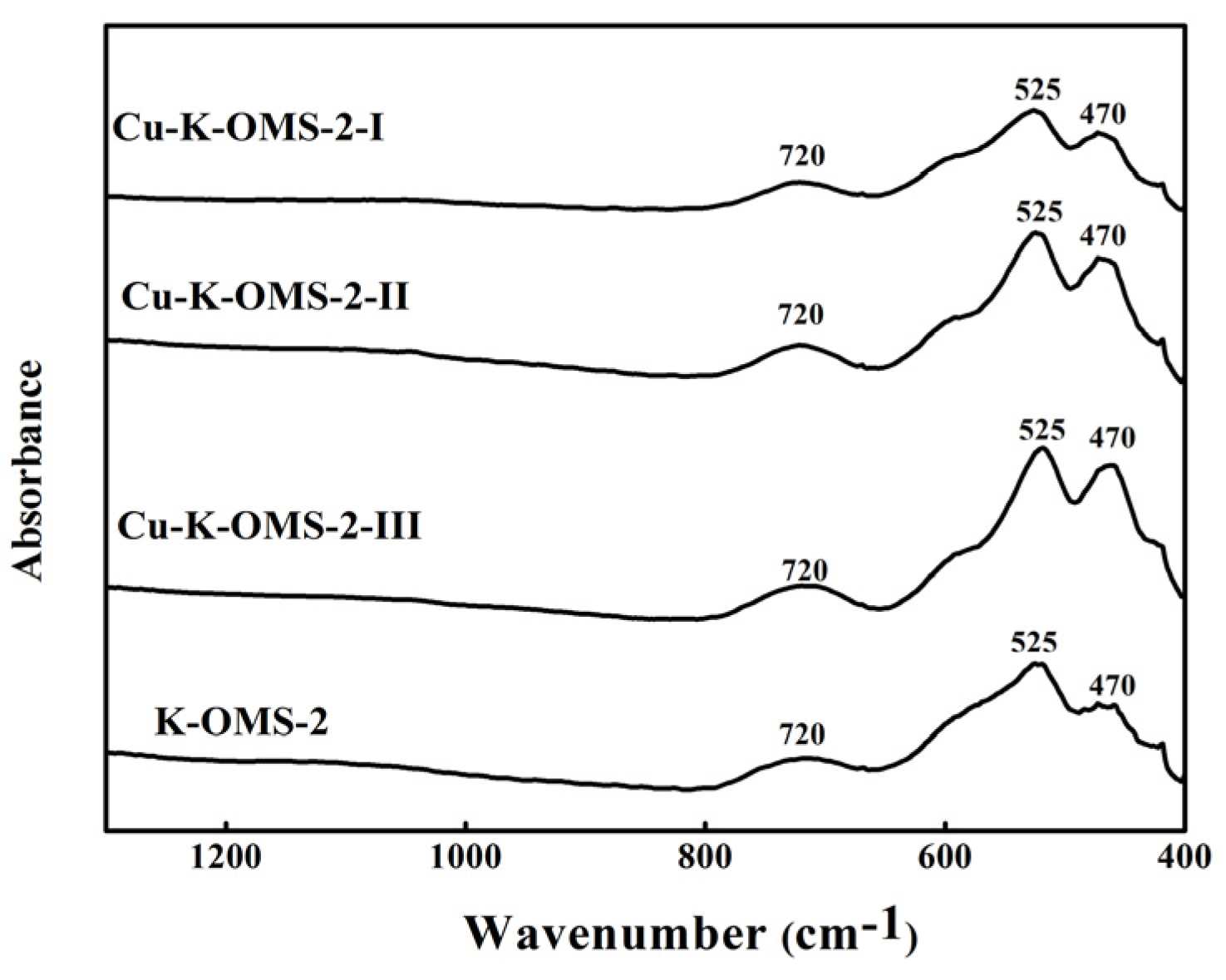
2.1.2. Spectra of Fourier Transform Infrared Spectoscopy
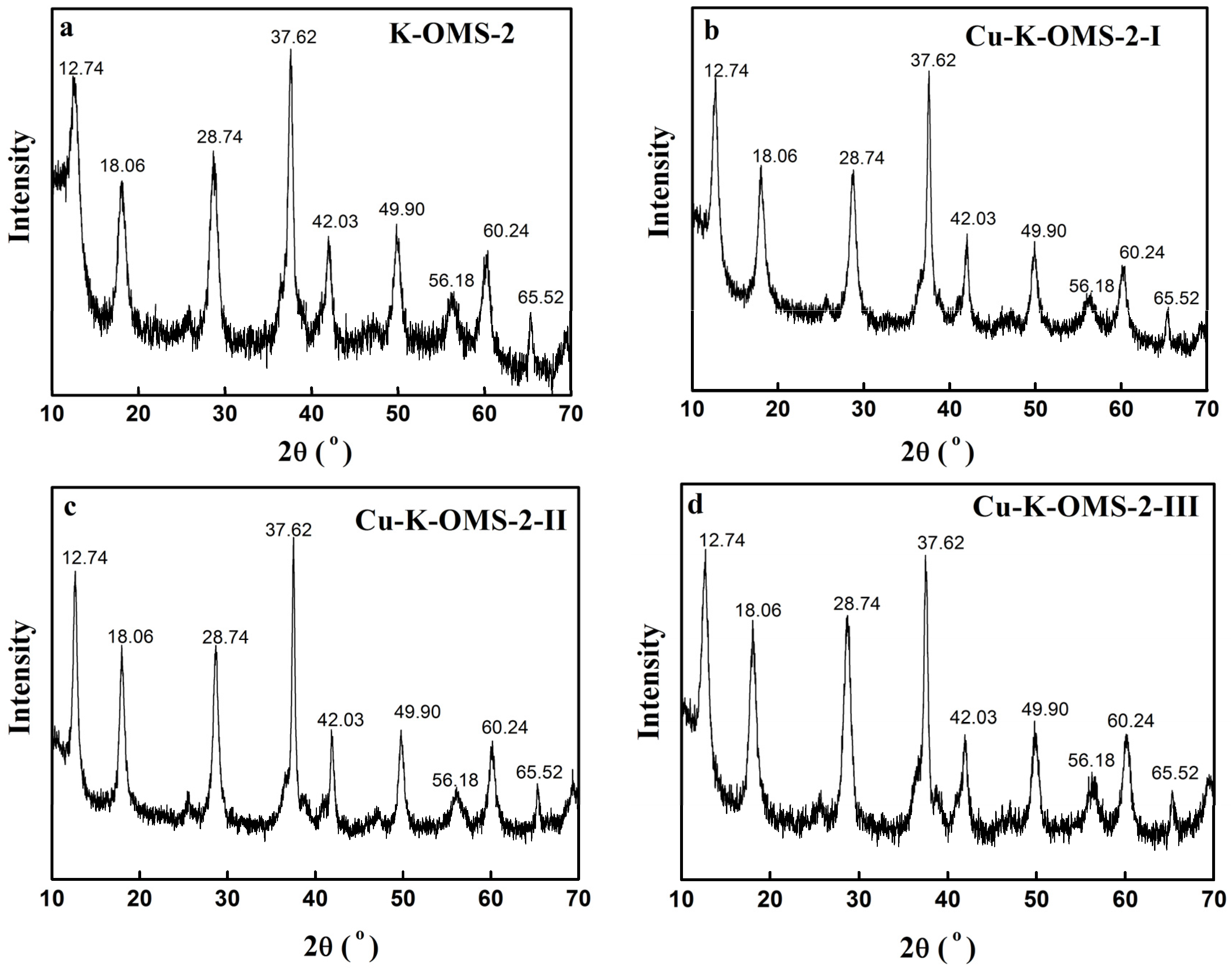
2.1.3. X-ray Diffraction (XRD) Patterns
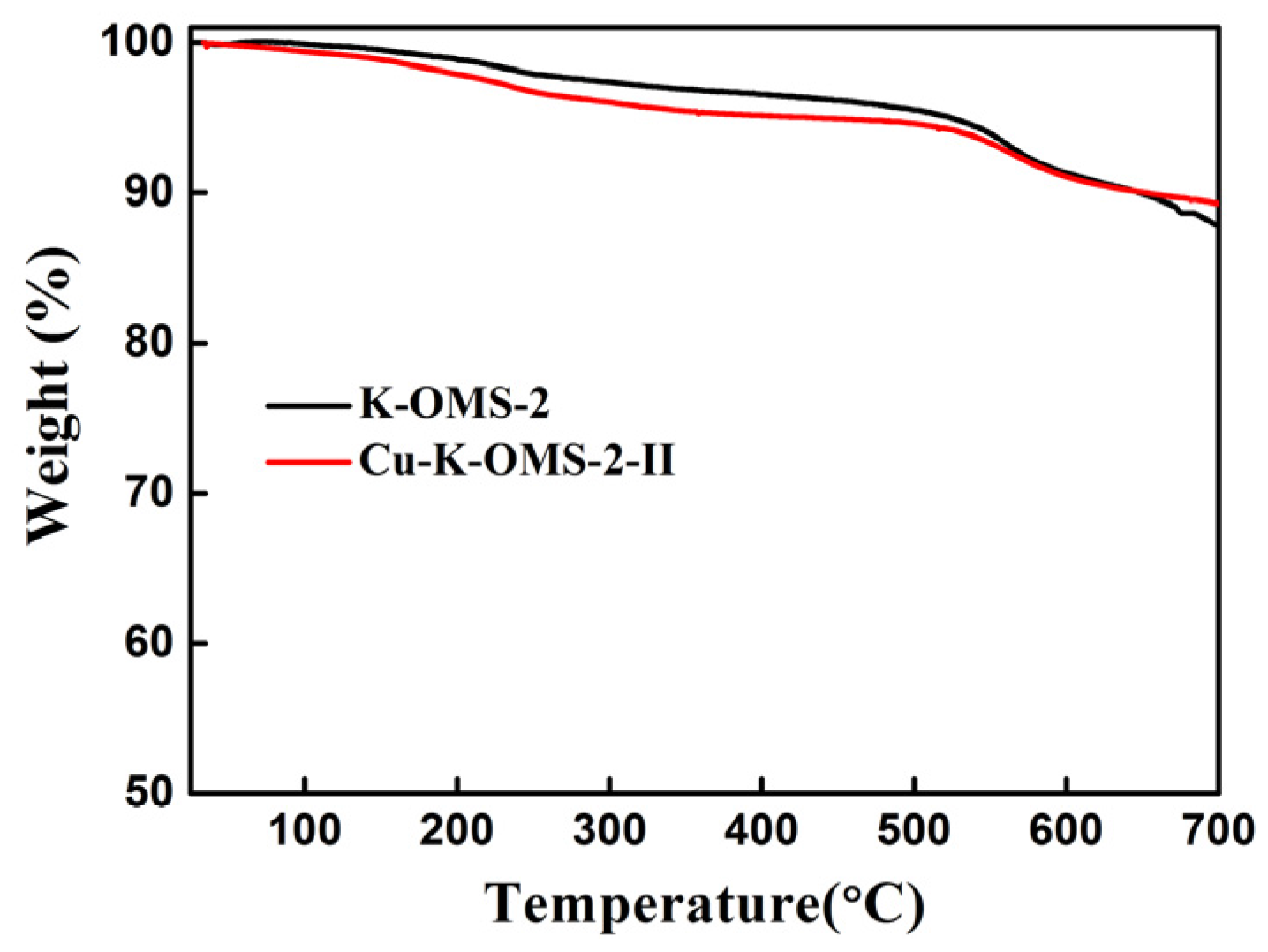
2.1.4. Thermogravimetric Analysis
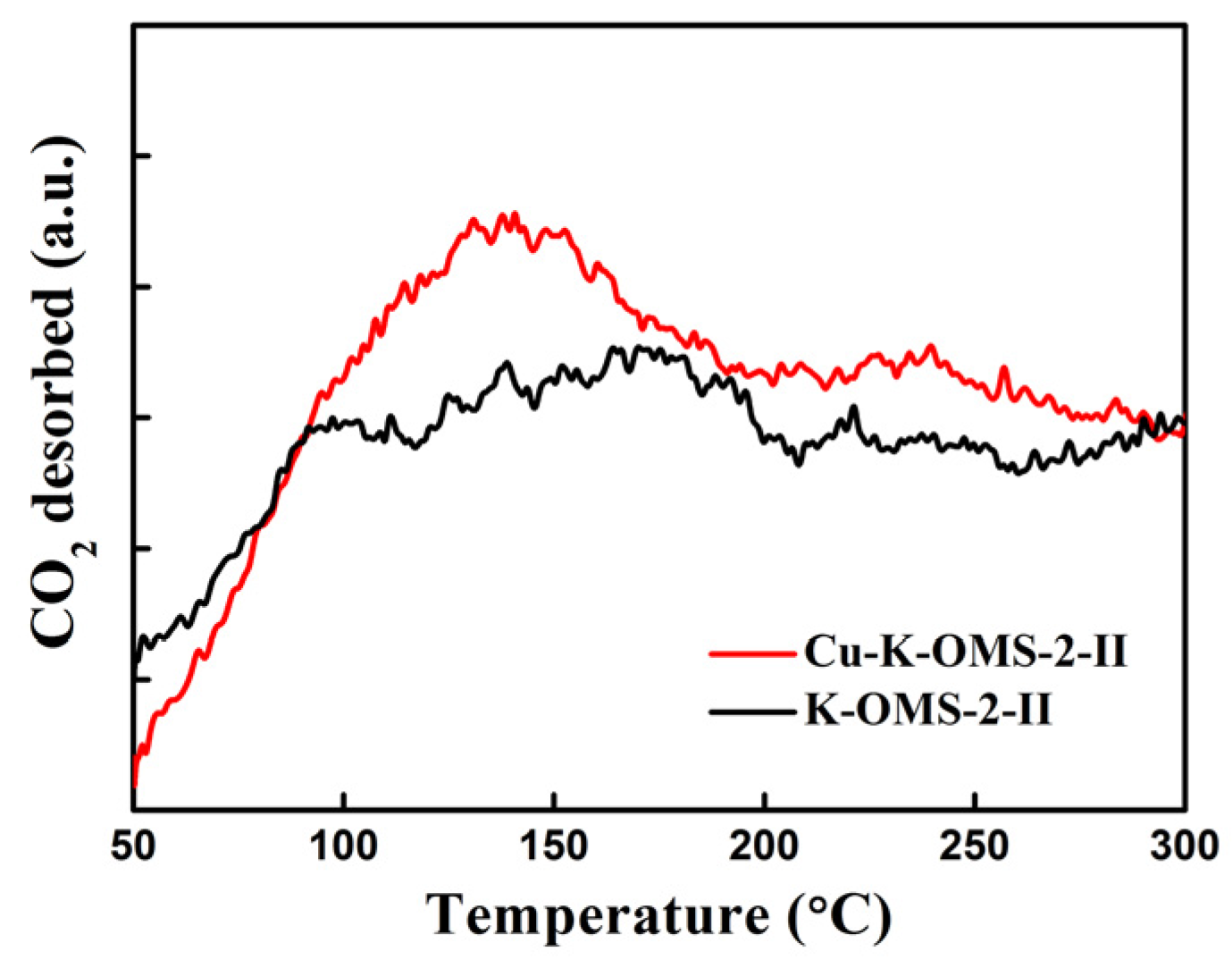
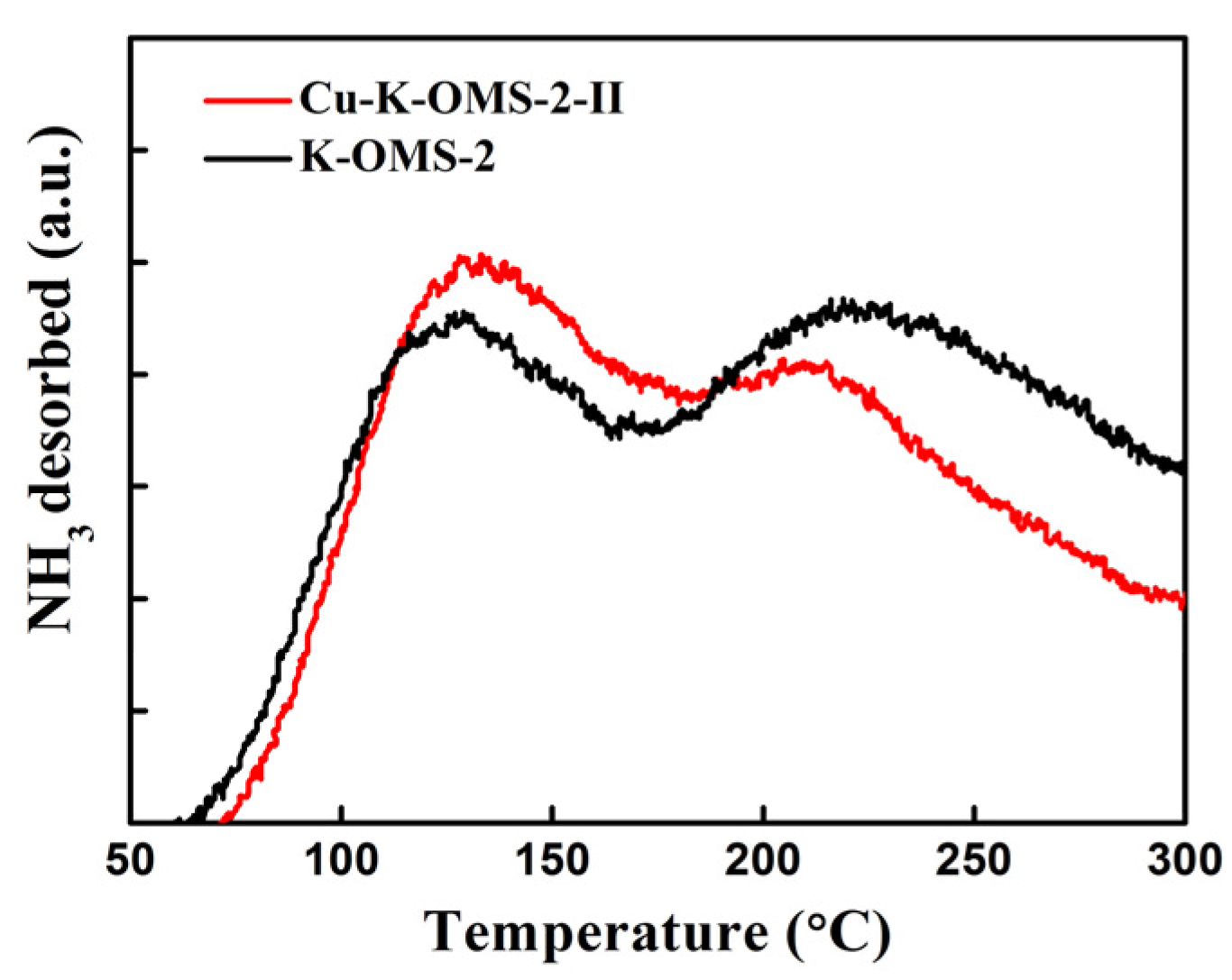
2.1.5. Temperature-Programmed Desorption
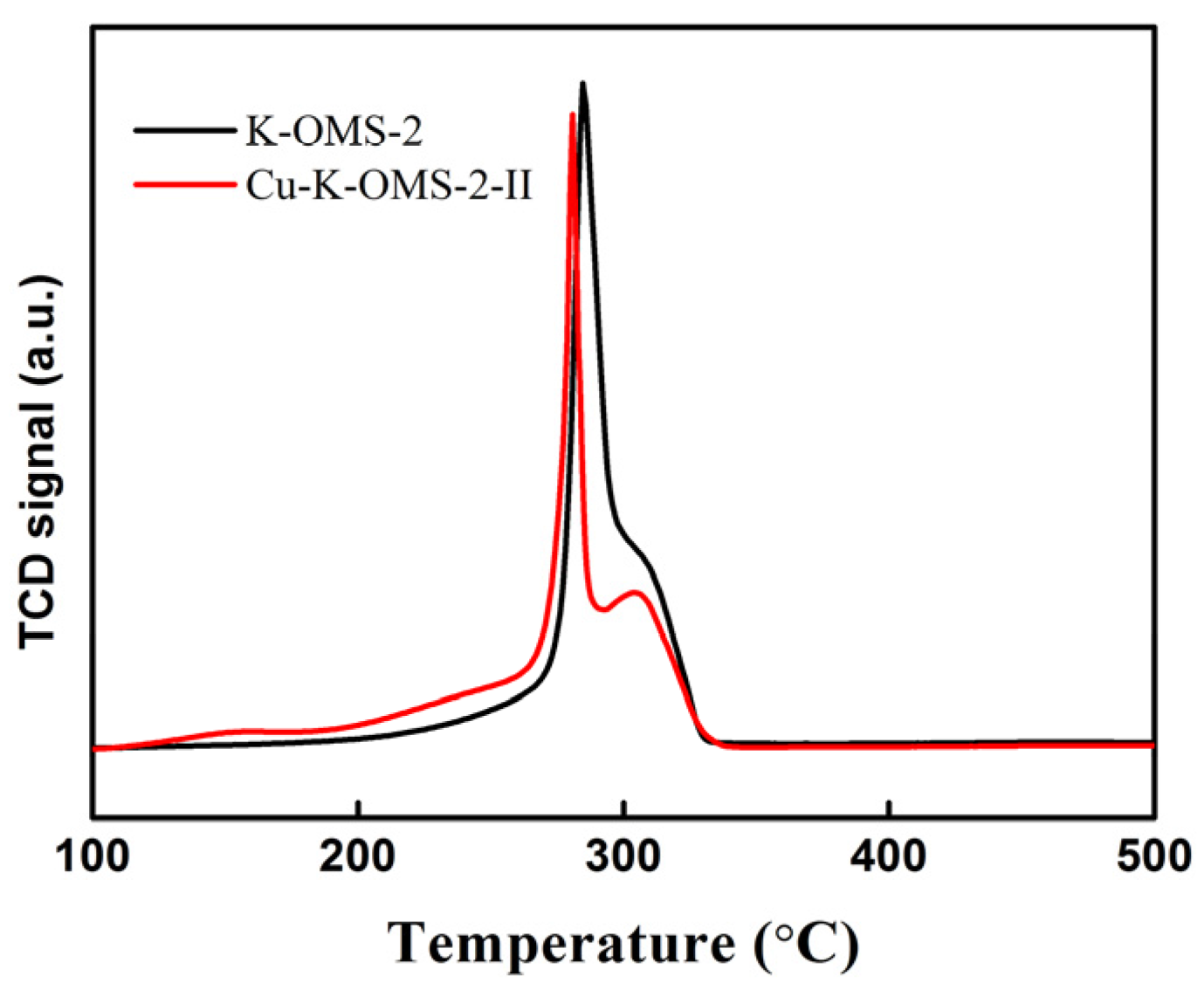
2.1.6. Temperature-Programmed Reduction
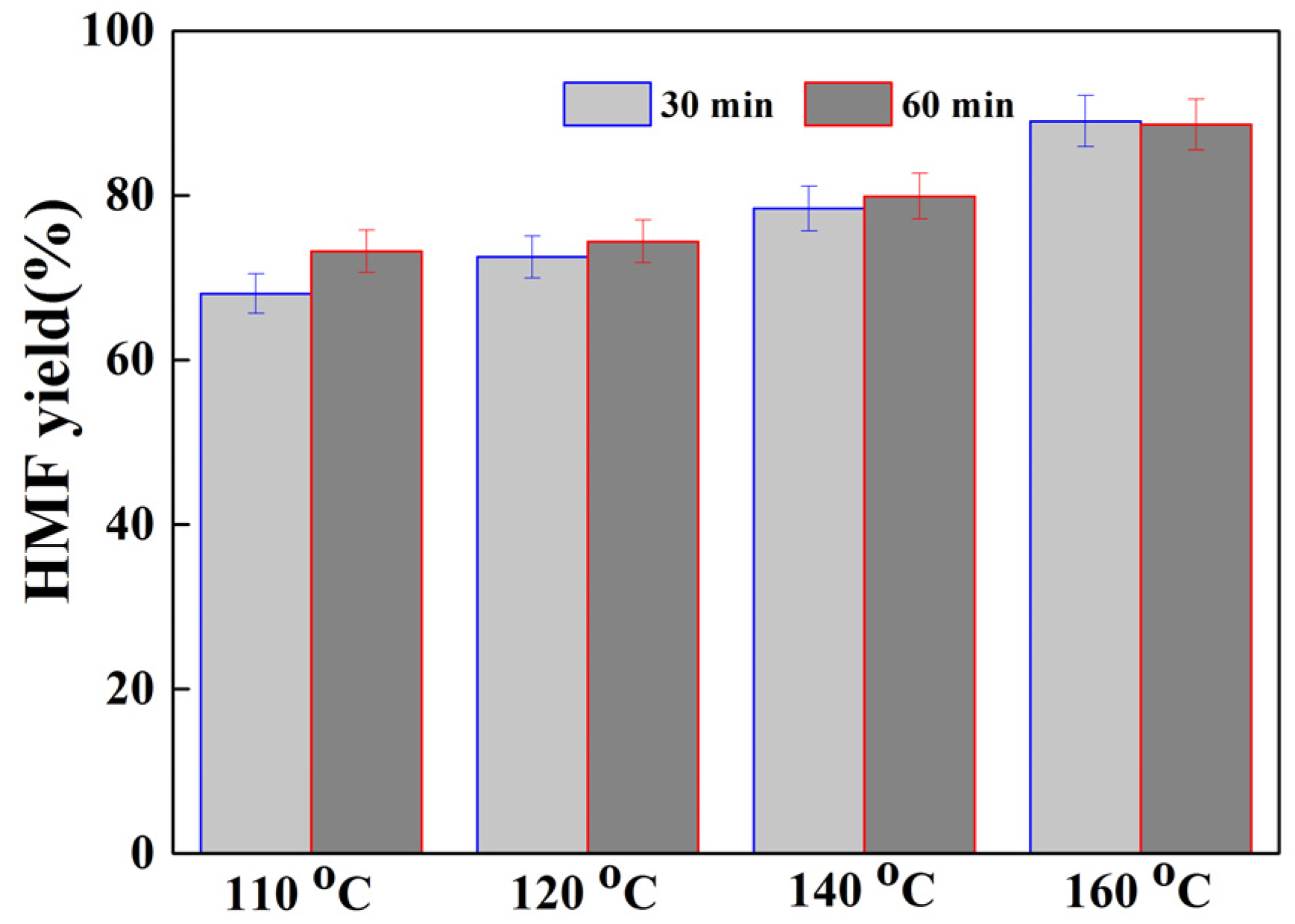
2.2. DFF Synthesis
3. Experimental Section
3.1. Materials
3.2. Catalyst Synthesis
3.3. Aerobic Oxidation of HMF
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, K.; Molla, R.A.; Asif Iqubal, M.; Safikul Islam, S.; Manirul Islam, S. Ruthenium nanoparticles supported on N-containing mesoporous polymer catalyzed aerobic oxidation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF). Appl. Catal. A 2016, 520, 44–52. [Google Scholar] [CrossRef]
- Jérôme, F.; Vigier, K.D.O. catalytic conversion of carbohydrates to furanic derivatives in the presence of choline chloride. Catalysts 2017, 7, 218. [Google Scholar] [CrossRef]
- Baruah, D.; Hussain, F.L.; Suri, M.; Saikia, U.P.; Sengupta, P.; Dutta, D.K.; Konwar, D. Bi(NO3)3·5H2O and cellulose mediated Cu-NPs—A highly efficient and novel catalytic system for aerobic oxidation of alcohols to carbonyls and synthesis of DFF from HMF. Catal. Commun. 2016, 77, 9–12. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Z.; Liu, B.; Chen, S.; Zhang, Z. Catalytic oxidation of biomass derived 5-hydroxymethylfurfural (HMF) over RuIII-incorporated zirconium phosphate catalyst. J. Ind. Eng. Chem. 2016, 38, 181–185. [Google Scholar] [CrossRef]
- Biswas, S.; Dutta, B.; Mannodi-Kanakkithodi, A.; Clarke, R.; Song, W.; Ramprasad, R.; Suib, S.L. Heterogeneous mesoporous manganese/cobalt oxide catalysts for selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Chem. Commun. 2017, 53, 11751–11754. [Google Scholar] [CrossRef] [PubMed]
- Grasset, F.L.; Katryniok, B.; Paul, S.; Nardello-Rataj, V.; Pera-Titus, M.; Clacens, J.M.; De Campof, F.; Dumeignil, F. Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over intercalated vanadium phosphate oxides. RSC Adv. 2013, 3, 9942–9948. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Jiang, L.; Wang, J.; Zhang, Z. Catalytic conversion of fructose and 5-hydroxymethylfurfural into 2,5-diformylfuran over SBA-15 supported ruthenium catalysts. Energy Fuels 2016, 30, 5885–5892. [Google Scholar] [CrossRef]
- Takagaki, A.; Takahashi, M.; Nishimura, S.; Ebitani, K. One-pot synthesis of 2,5-diformylfuran from carbohydrate derivatives by sulfonated resin and hydrotalcite-supported ruthenium catalysts. ACS Catal. 2011, 1, 1562–1565. [Google Scholar] [CrossRef]
- Cui, M.; Huang, R.; Qi, W.; Su, R.; He, Z. Cascade catalysis via dehydration and oxidation: One-pot synthesis of 2,5-diformylfuran from fructose using acid and V2O5/ceramic catalysts. RSC Adv. 2017, 7, 7560–7566. [Google Scholar] [CrossRef]
- Mittal, N.; Nisola, G.M.; Malihan, L.B.; Seo, J.G.; Kim, H.; Lee, S.P.; Chung, W.J. One-pot synthesis of 2,5-diformylfuran from fructose using a magnetic bi-functional catalyst. RSC Adv. 2016, 6, 25678–25688. [Google Scholar] [CrossRef]
- Halliday, G.A.; Young, R.J.; Grushi, V. One-pot, two-step, practical catalytic synthesis of 2,5-diformylfuran from fructose. Org. Lett. 2003, 5, 2003–2005. [Google Scholar] [CrossRef] [PubMed]
- Shyam, P.; Chaturvedi, S.; Karmakar, K.; Bhattacharya, A.; Singh, S.; Kulkarni, S. Structural and magnetic investigations on a wet chemically synthesized nanoscale S = 1/2 spin chain compound—CuSe2O5. J. Mater. Chem. C 2016, 4, 611–621. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Taylor, N.J.; Nazar, L.F. Structure and ion exchange properties of a new cobalt borate with a tunnel structure “templated” by Na+. J. Am. Chem. Soc. 2002, 124, 6522–6523. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, J. Effects of precursor and sulfation on OMS-2 catalyst for oxidation of ethanol and acetaldehyde at low temperatures. Environ. Sci. Technol. 2010, 44, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Kumara, R.; Sithambarama, S.; Suibabc, S.L. Cyclohexane oxidation catalyzed by manganese oxide octahedral molecular sieves—Effect of acidity of the catalyst. J. Catal. 2009, 262, 304–313. [Google Scholar] [CrossRef]
- Iyer, A.; Galindo, H.; Sithambaram, S.; Merker, T.; Schleid, T.; Hasse, H.; Gläser, R. Nanoscale manganese oxide octahedral molecular sieves (OMS-2) as efficient photocatalysts in 2-propanol oxidation. Appl. Catal. A 2010, 375, 295–302. [Google Scholar] [CrossRef]
- Schurz, F.; Bauchert, J.M.; Merker, T.; Schleid, T.; Hasse, H.; Gläser, R. Octahedral molecular sieves of the type K-OMS-2 with different particle sizes and morphologies: Impact on the catalytic properties in the aerobic partial oxidation of benzyl alcohol. Appl. Catal. A 2009, 355, 42–49. [Google Scholar] [CrossRef]
- Deguzman, R.N.; Shen, Y.; Neth, E.J.; Suib, S.L.; O’Young, C.; Levine, S.; Newsam, J.M. Synthesis and characterization of octahedral molecular sieves (OMS-2) having the hollandite structure. Chem. Mater. 1994, 6, 815–821. [Google Scholar] [CrossRef]
- Gong, W.; Zheng, K.; Ji, P. Platinum deposited on cerium coordination polymer for catalytic oxidation of hydroxymethylfurfural producing 2,5-furandicarboxylic acid. RSC Adv. 2017, 7, 34776–34782. [Google Scholar] [CrossRef]
- Glover, D.; Schumm, B., Jr.; Kozowa, A. (Eds.) Handbook of Manganese Dioxides Battery Grade; International Battery Materials Association: Paris, France, 1989. [Google Scholar]

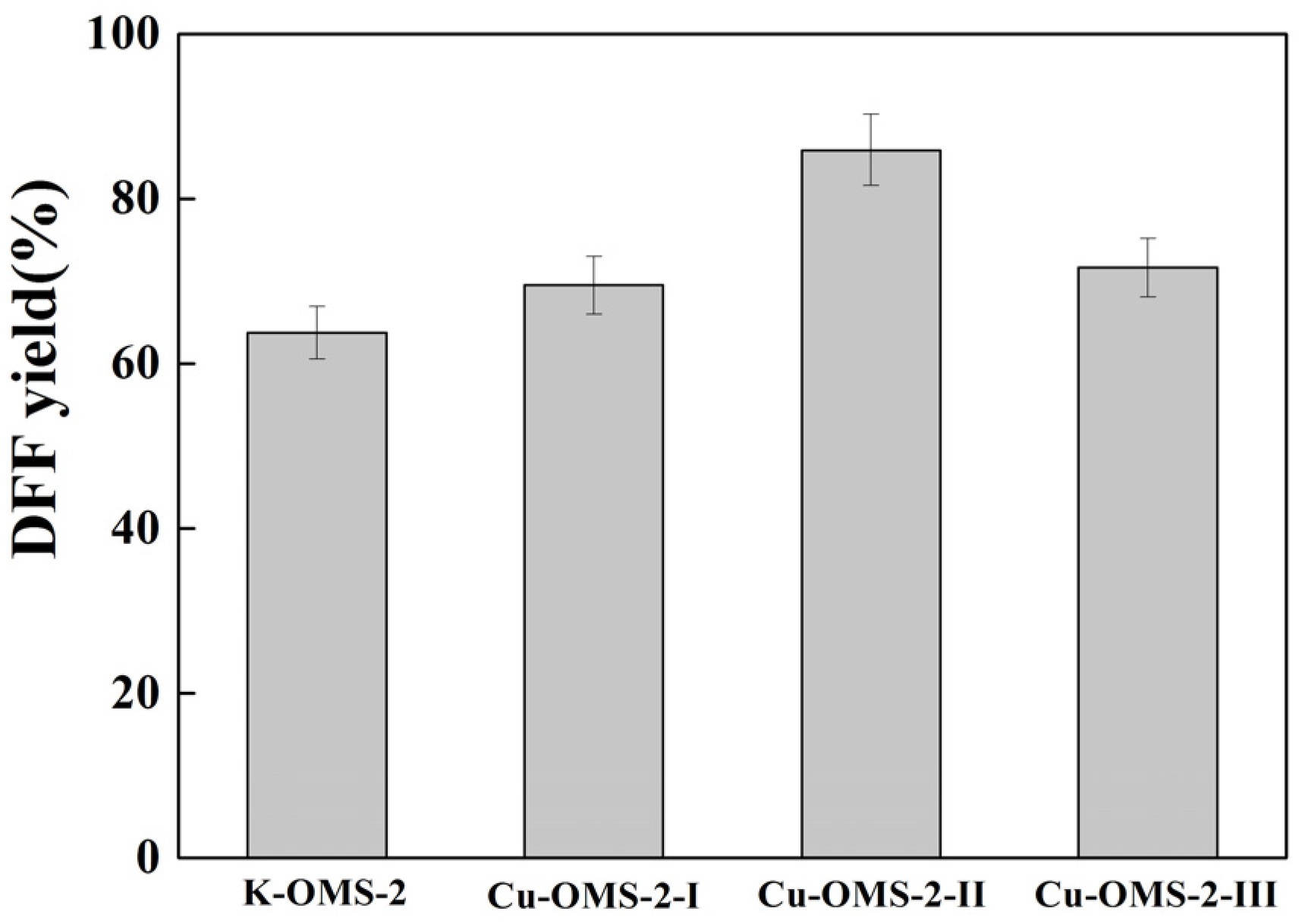


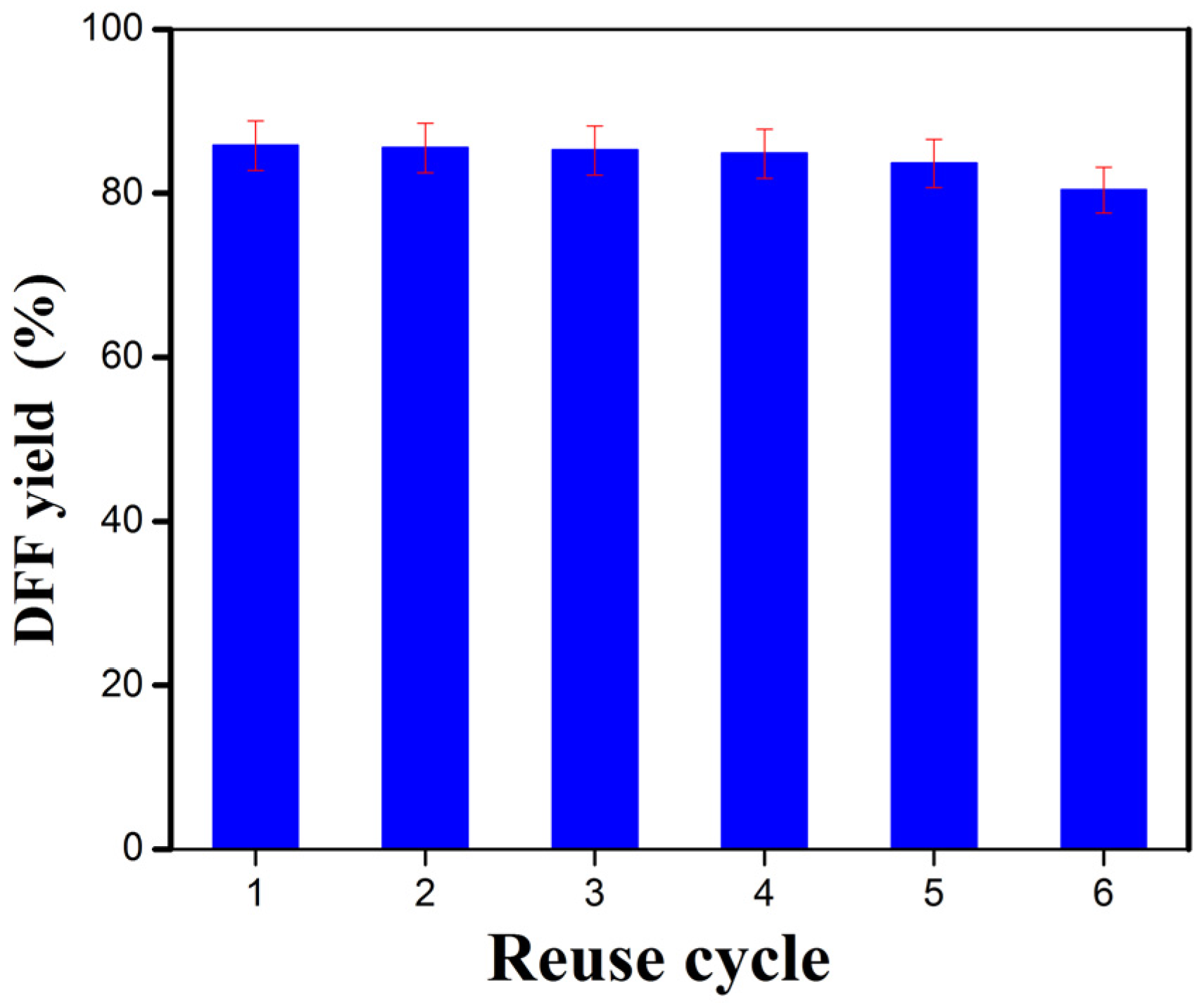
| Catalysts | Cu/Mn (Atomic Ratio) |
|---|---|
| K-OMS-2 | 0 |
| Cu-K-OMS-2-I | 0.019 |
| Cu-K-OMS-2-II | 0.024 |
| Cu-K-OMS-2-III | 0.031 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Zhao, H.; Feng, W.; Ji, P. A Non-Precious Metal Promoting the Synthesis of 5-Hydroxymethylfurfural. Catalysts 2017, 7, 330. https://doi.org/10.3390/catal7110330
Lu X, Zhao H, Feng W, Ji P. A Non-Precious Metal Promoting the Synthesis of 5-Hydroxymethylfurfural. Catalysts. 2017; 7(11):330. https://doi.org/10.3390/catal7110330
Chicago/Turabian StyleLu, Xinyuan, Hongjie Zhao, Wei Feng, and Peijun Ji. 2017. "A Non-Precious Metal Promoting the Synthesis of 5-Hydroxymethylfurfural" Catalysts 7, no. 11: 330. https://doi.org/10.3390/catal7110330




