1. Introduction
Anthropogenic emission of CO
2 is closely linked to serious global warming issues. The large contribution in total CO
2 emission originates from coal or natural gas power plants [
1,
2,
3,
4,
5,
6]. To reducing CO
2 concentrations, two proposed methods have been developed and implemented: carbon capture and storage (CCS) and carbon capture and utilization (CCU) [
7,
8,
9,
10]. Converting CO
2 into valuable intermediate chemicals and products (such as methanol, carbonates, formic acid, methane or kerosene) is economically of interest as can potentially recoups the costs of CO
2 capture and conversion [
10,
11,
12]. Methanol is one of the possible fuel candidates that can be made by CO
2 hydrogenation [
13,
14,
15]. Methanol production and demands have shown a substantial increasing trend in the coming years. It is regarded as an excellent alternative energy resource due to its excellent combustion properties [
16,
17,
18,
19,
20]. Methanol is also a very important primary raw material for production of various chemicals such as acetic acid, dimethyl ether (DME), methyl tert-butyl ether (MTBE) and formaldehyde [
21,
22,
23,
24].
To reduce the life cycle CO
2 emissions in methanol production process, hydrogen must be provided in a sustainable way. There are various technologies for H
2 production from renewable raw materials such as water, biomass or biogas [
25]. Water electrolysis by a renewable source of electricity (solar radiation, biomass and wind) is one of the simplest technologies for hydrogen production without any by-products [
26,
27]. Although extremely pure hydrogen can be produced through water electrolysis technology, the cost of electricity is relatively high and plays a significant role in the price of hydrogen yield [
28]. In the current electrolytic processes, the applied electrodes are usually coated with precious platinum, which makes commercialization of the technology much further away [
29]. However, the eco-friendly source of the feedstock makes the methanol production emerge as an attractive green process [
27,
30,
31]. There are two different synthesis routes for methanol production from CO
2 and H
2: direct and indirect routes. In the first route, methanol is directly produced from CO
2 and hydrogen, while, in the second, CO
2 is first converted into CO via reverse water-gas shift (RWGS) reaction to produce syngas and then the syngas is transported to a reactor to convert into methanol [
32].
As the environmental concerns have been becoming more serious, numerous studies regarding catalyst development for methanol production from CO
2 have been conducted. Fujita et al. conducted an experimental study on CuO/ZnO catalyst for the reaction of CO
2 dehydrogenation to methanol. They investigated different structures and a wide range of Cu/Zn ratios and concluded that this type of catalyst exhibited an acceptable performance [
33]. Subsequently, Arena et al. synthesized CuO/ZnO/ZrO
2 catalyst by means of co-precipitation under ultrasound irradiation and evaluated its impact on the reaction rate and conversion [
34]. Newly, some researchers have been focusing on the impact of active metals like Pd, Au, Ag on various catalysts to improve CO
2 hydrogenation reactions. Hartadi et al. [
35] investigated the methanol formation rate on Au/ZnO catalysts and found that the activity of this catalyst for CO
2 hydrogenation in CO
2/H
2 is remarkably higher than that for CO hydrogenation in CO/H
2 synthesis gas. Rodriguez et al. [
36] conducted an experimental investigation on CO
2 hydrogenation on Au/TiC, Cu/TiC, and Ni/TiC catalysts. It was concluded that, although the major product over these catalysts was CO, a substantial amount of methanol was also produced over Au/TiC and Cu/TiC catalysts. Different Pd:Cu:Zn molar compositions in PdCuZn/SiC catalysts were examined by Ramírez et al. The Pd active sites favored carbon monoxide synthesis via reverse water–gas–shift, whereas the PdZn alloys catalyzed methanol formation. In their work, 37.5:12.5:50 Pd:Cu:Zn (mol %) was chosen as the most active catalyst for methanol production [
37]. The effect of adding Ag to CuO-ZrO
2 catalysts was studied by Tada et al. The addition of Ag to a CuO-ZrO
2 catalyst offered higher methanol selectivity than CuO-ZrO
2 and Ag/ZrO
2 catalysts. This contributed to the fact that the Ag/CuO-ZrO
2 catalysts contained special active sites for CO
2 conversion to methanol [
38].
More recently, Pontzen et al. [
39] researched the feasibility of methanol production over the commercial catalyst, CuO/ZnO/Al
2O
3. Their study proved the stability of the conventional catalyst. Afterwards, Meyer and coworkers [
40] modeled methanol synthesis process on the commercial catalyst and compared Graaf and Bussche and Froment kinetic models for CO
2 hydrogenation reactions. Fortes et al. [
27] evaluated carbon capture and utilization as raw material in the methanol production process. The cost of production as well as the net reduction of CO
2 emissions were compared with the conventional synthesis process.
However, low conversion of carbon dioxide over the commercial catalyst [
32] and its deactivation due to water production during the reaction [
41,
42] are the main concerns of the researchers for industrial development of this process. It was proved that the conventional catalyst (CuO/ZnO/Al
2O
3) results in a high conversion in a process with a syngas feedstock (containing CO, CO
2, and H
2); however, if pure CO
2 is substituted, it does not offer a good efficiency [
43,
44]. To overcome these obstacles, an efficient process consists of three stage heat exchanger reactors connected in series was proposed for direct CO
2 hydrogenation. This novel configuration increases CO
2 conversion remarkably compared to one stage reactor. Also to reduce water production, a hydroxy sodalite (H-SOD) membrane was assisted in each reactor for separation of water from the reaction side. H-SOD as a zeolite-like material has high selectivity of H
2O on the basis of molecular sieving in hybrid processes. The maximum water permeation of this membrane is reported 10
−6 mol/(s m
2 Pa) for an ideal case [
45,
46,
47,
48,
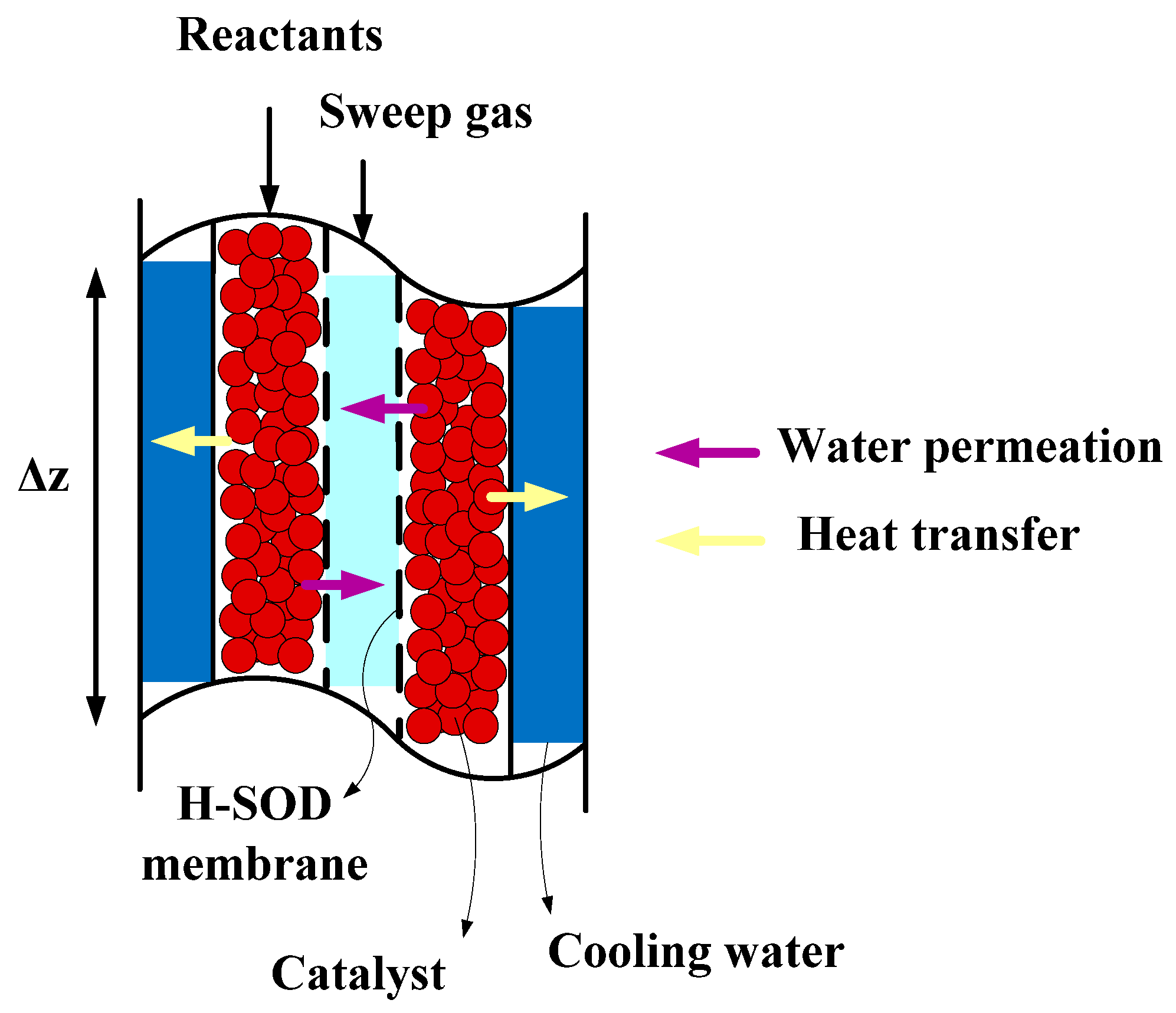
49]. The permeated water is swept with a sweep gas at low pressure to create a high driving force across the membrane. Then, this process was compared with one-stage reactor and conventional methanol synthesis reactor from coal and natural gas.
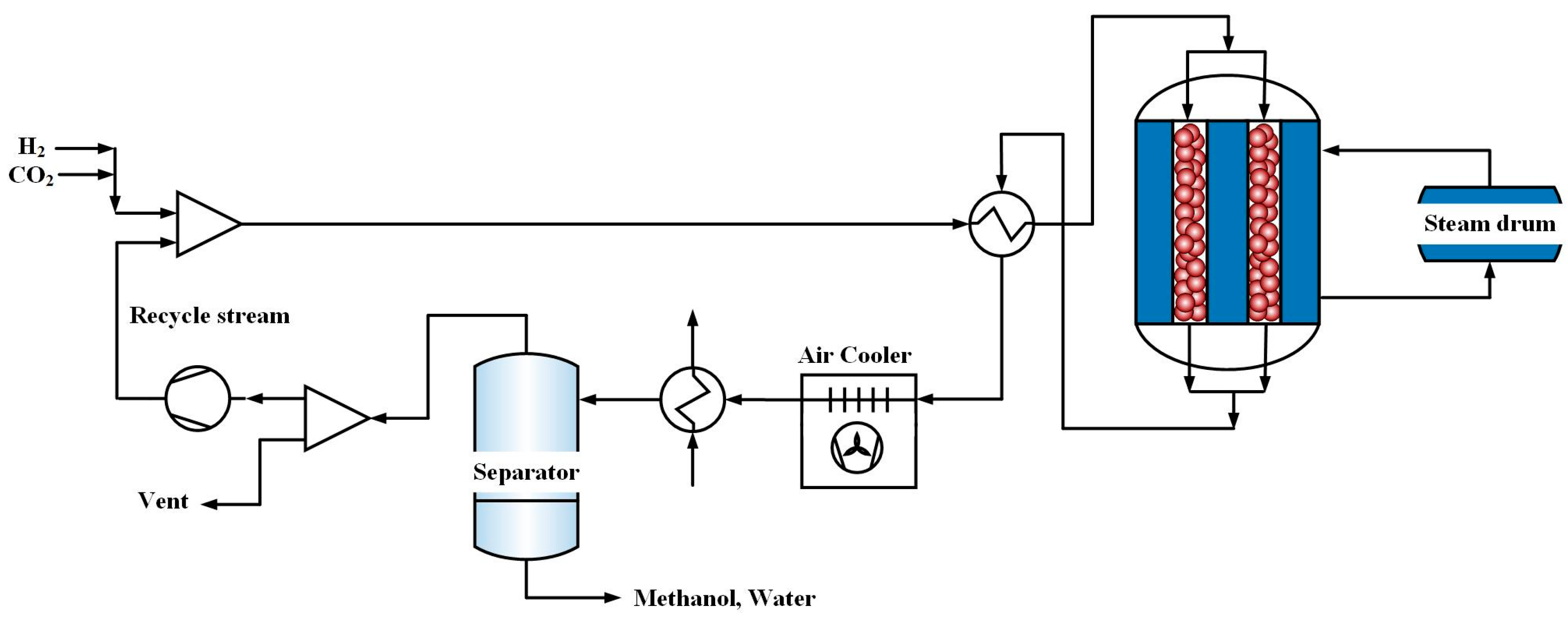
6. Results and Discussion
In this work, CO
2 hydrogenation over the commercial catalyst (CuO/ZnO/Al
2O
3) was investigated. Because of low conversion of carbon dioxide over this catalyst, a three-stage heat exchanger reactors connected in series was proposed for direct CO
2 hydrogenation. Stoichiometric number (SN) and H
2O concentration of feedstock to the methanol production process are the two main specifications that should be considered. The desired stoichiometric number in a process consisting of CO, CO
2, and H
2 in the feedstock is equal to 2. Stoichiometric number higher than 2 shows an excess value of H
2 in the feedstock, while a lower value indicates an excess value of carbon. As illustrated in
Figure 1 and
Figure 2, the feedstock of the unit; containing H
2 and CO
2; is combined with a recycle stream in a mixer. Therefore, H
2/CO
2 ratio equal to 3, guarantees that SN = 2.
H2O concentration in the feedstock should also be lower than 1% vol because it is a poison for the commercial methanol synthesis catalyst. To ensure this requirement, the water produced in each reactor is removed by a flash drum and then the syngas free of water is fed to the next reactor.
6.1. The Effect of Operating Conditions on the Performance of Three-Stage Reactors
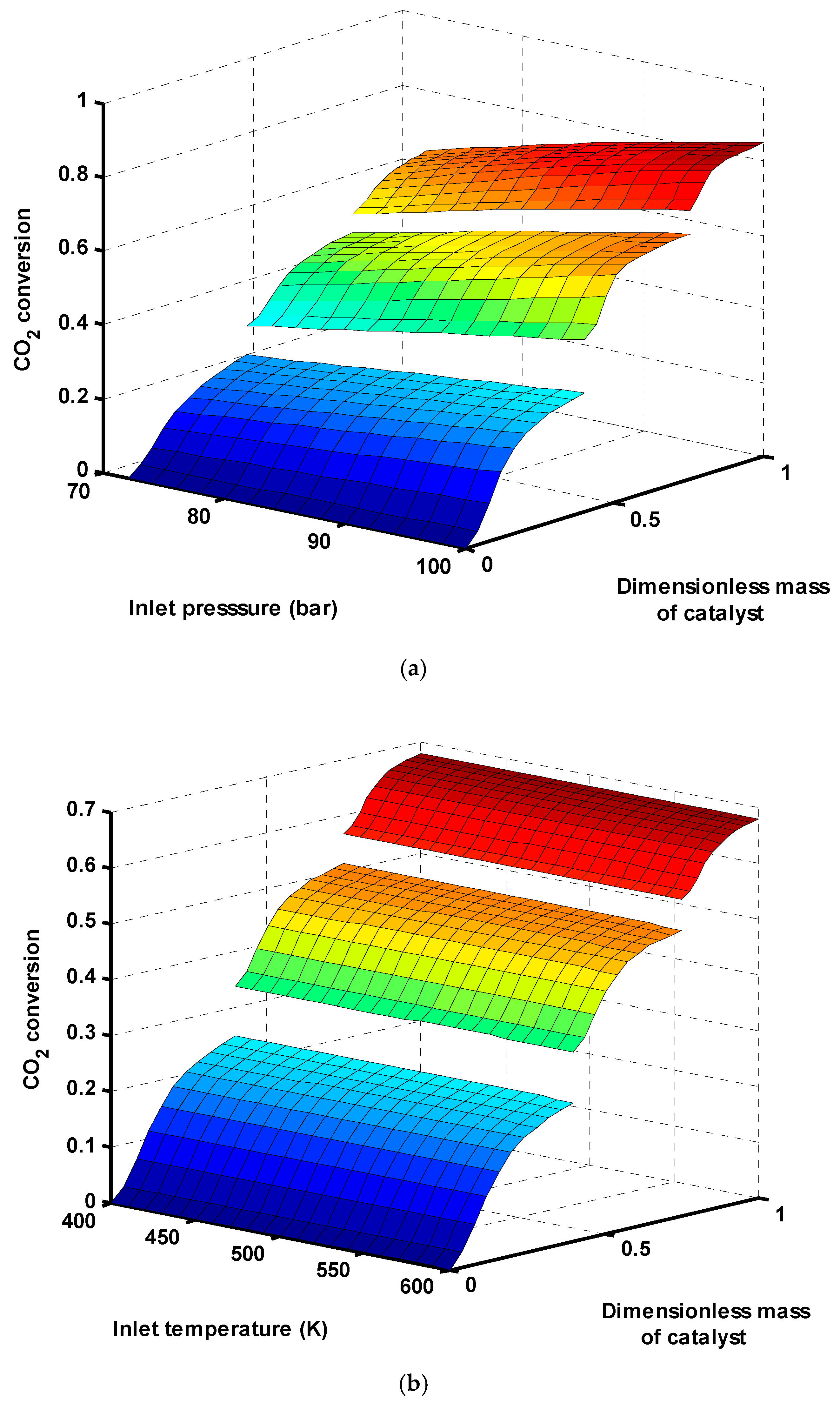
CO
2 conversion in three-stage reactors as a function of inlet pressure and temperature of the first reactor was depicted in
Figure 4a,b, respectively. CO
2 conversion is defined as Equation (30). At a fixed inlet temperature (T
0 = 503 K), CO
2 conversion increases in all three reactors as the inlet pressure is increased. This is attributed to the fact that, in CO
2 hydrogenation, reactions (2) and (3) occur predominantly in the reactor (the feedstock contains mainly H
2 and CO
2) and reaction (1) is performed at a much lower rate because of CO content in the recycle stream. Since the number of total moles is reduced in reaction (3), thus it is thermodynamically favored operating at high pressures. As shown in
Figure 4b, there is no considerable difference in CO
2 conversion by inlet temperature changing:
Since the inlet temperature plays a more significant role in adiabatic or isothermal reactors, only the effect of inlet pressure was investigated on the performance of this heat exchanger reactor.
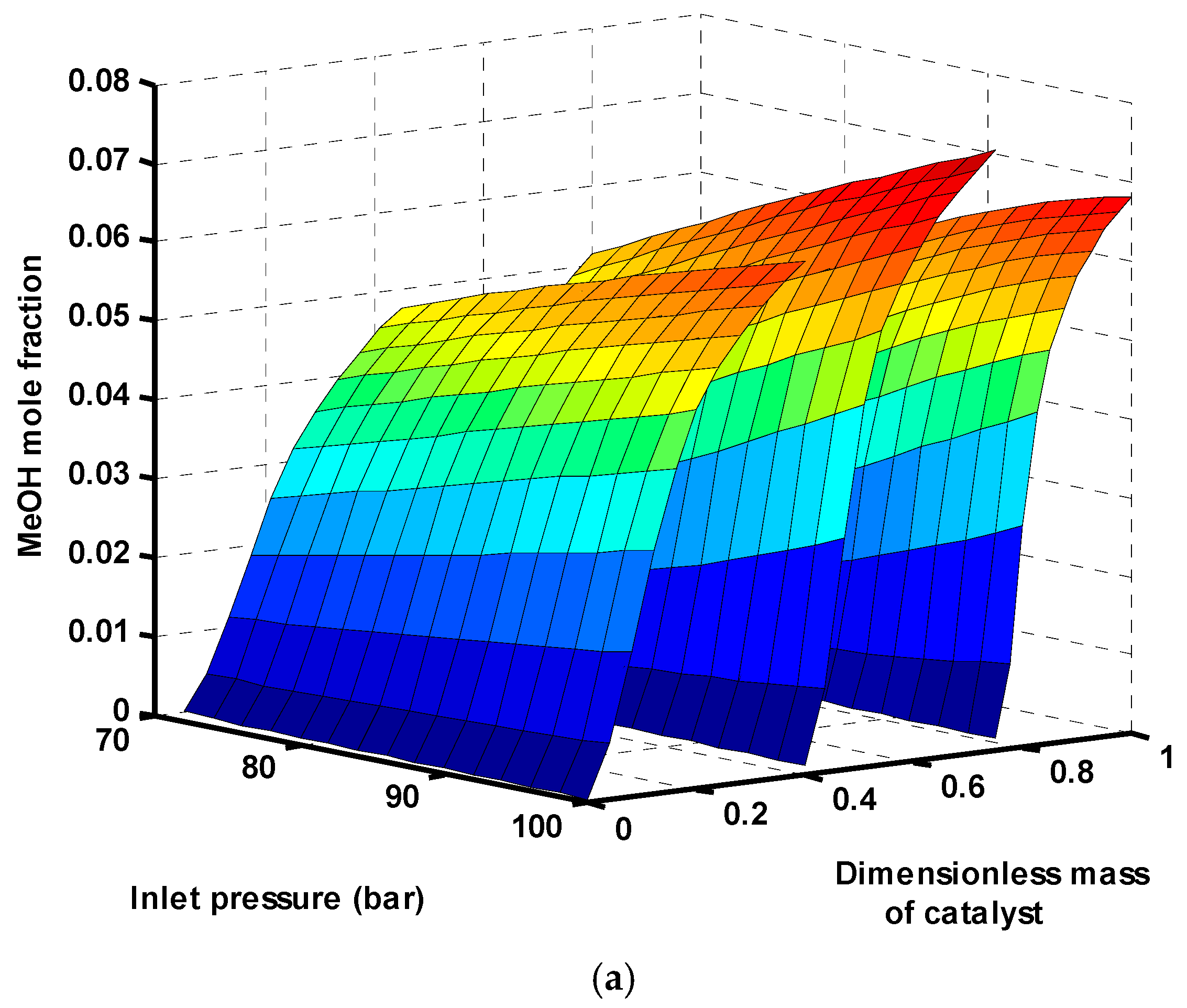
Figure 5 demonstrates the changes in the mole fractions of different components of the three-stage reactors as a function of inlet pressure of the first reactor. Methanol is produced along the reactors through reaction (3) and also reaction (1). Both these reactions are accompanied with a reduction of total moles; thus, based on the Le Chatelier principle, the reactions are shifted towards the production side at higher pressures. The produced methanol from the first reactor is mainly separated from the unreacted gases in a flash drum and in the second and third reactors is produced again. The changes in mole fraction of water as the unwanted product are depicted in
Figure 5b. Water is produced by both CO
2 hydrogenation (Equation (3)) and reverse water gas shift (RWGS) reactions (Equation (2)). More water is produced in higher pressures, which is not desirable. This is because, in the commercial methanol synthesis catalyst (CuO/ZnO/Al
2O
3), alumina is a very hydrophilic substance and consequently adsorbs the generated water. This phenomenon causes catalyst deactivation [
56,
57], and, therefore, it reduces the methanol yield over the time. Therefore, lower pressures are favored for reduction of water.
The profile of CO mole fraction was shown in
Figure 5c. In low pressures, CO increases along the reactor, because of RWGS reaction (Equation (2)), while, in high pressures, reaction (3) is favored and more H
2O is produced. Augmentation of H
2O in the reactor shifts the RWGS reaction toward the consumption of CO and H
2O (known as WGS reaction). Thus, in higher pressures, a maximum is observed for CO mole fraction, and, after that, CO is reduced along the reactor. In addition, it can be deduced from the figure that, in high inlet pressures, a lower concentration of CO results in the feedstock of each reactor. This is because, in high pressures, CO consumes considerably in each reactor and then, with lower concentration in the reactor outlet, is fed to the next reactor.
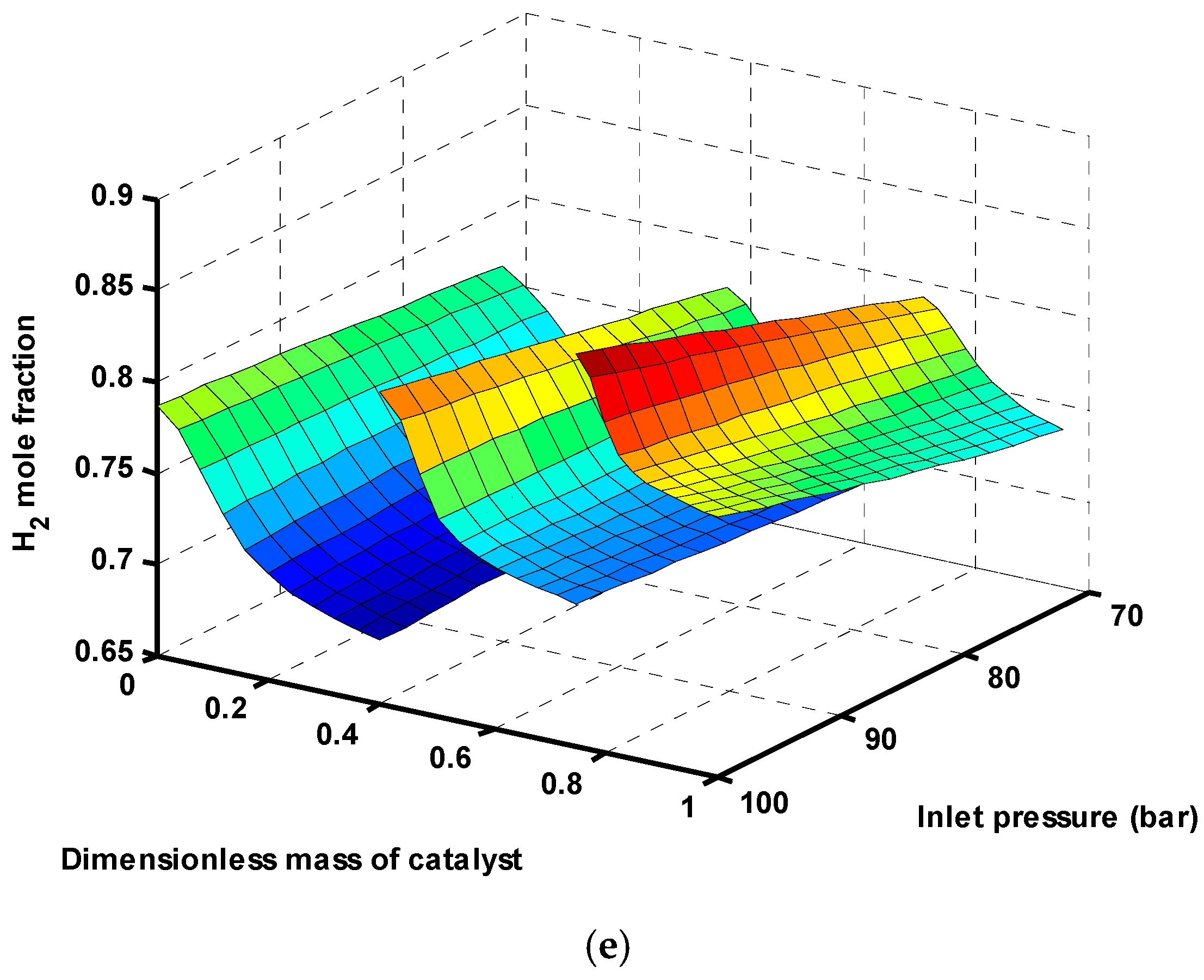
Figure 5d,e show that CO
2 and H
2 are consumed more in high pressures (reactions (1) and (3) are favored at high pressures), which confirms
Figure 4a.
Figure 6a displays the profile of temperature along the reactor. As seen, there is a hot spot in the beginning of the reactors because of the occurrence of the exothermic reactions, which causes temperature to increase. As the fluid goes through the reactor, the influence of cooling water becomes more significant, and it dominates the released heat, and, as a result, the temperature diminishes. This figure also reflects that a rise in the inlet pressure contributes to higher temperature in the reactor. This phenomenon can be attributed to two reasons; first, the augmentation of inlet pressure favors the exothermic reactions (Equations (1) and (3)), contributing to more heat generation and higher temperature in the reactor. Second, increasing the H
2O concentration leads to occurrence of the exothermic WGS reaction (the reverse of Equation (2)), and, consequently, more heat is generated by the reactions. Therefore, higher inlet pressures make the reactions highly exothermic and, thus, controlling the reactor temperature becomes difficult.
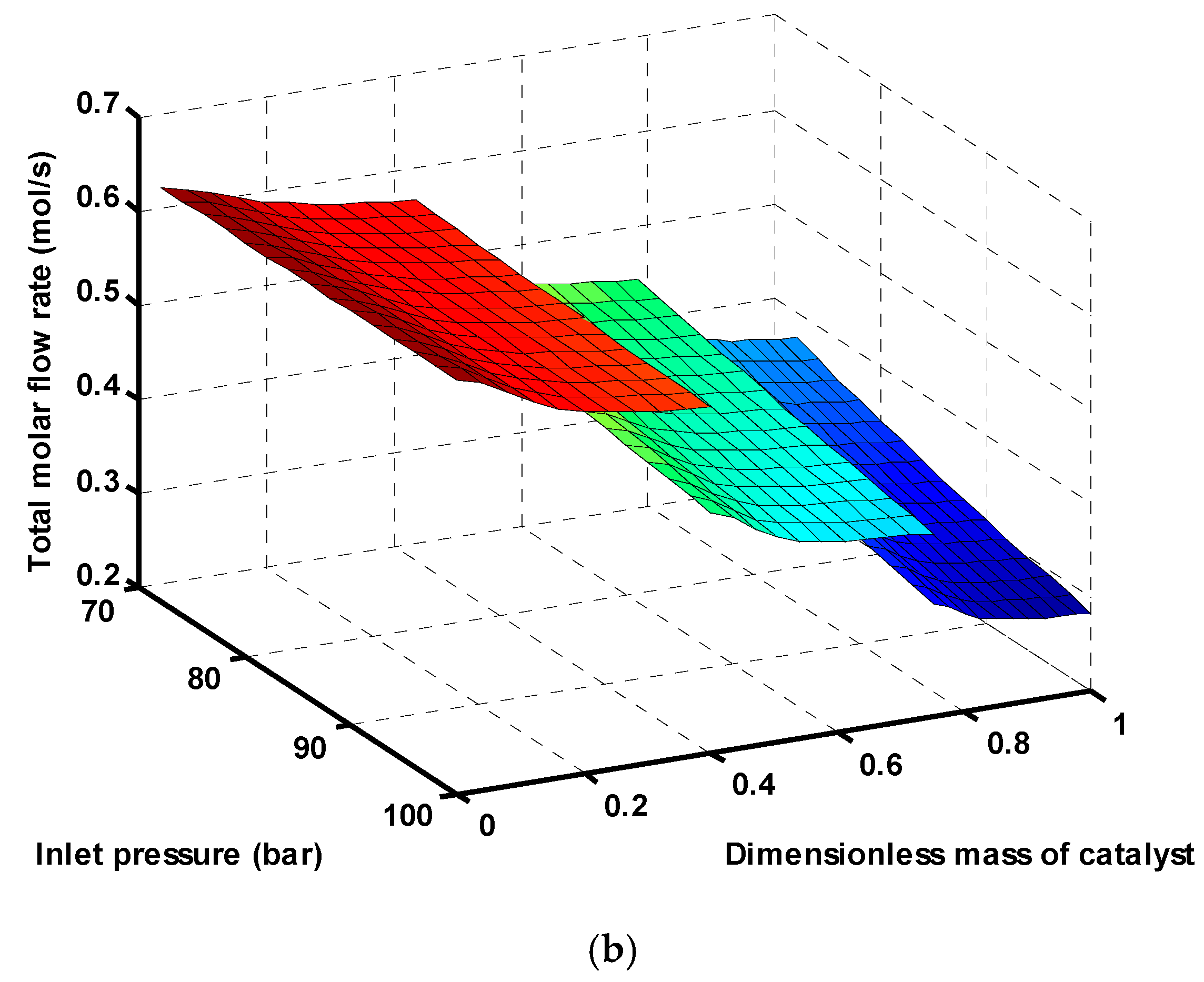
Figure 6b demonstrates reduction of total molar flow rate in all three reactors by increasing the inlet pressure. As mentioned before, in reactions (1) and (3), the total moles are reduced, so, based on the Le Chatelier principle, the reactions are shifted toward the right side at higher pressures, and, subsequently, the total molar flow rate decreases.
Although methanol production favors high inlet pressures, high amounts of water as the catalyst poisoning is also produced during the reaction. In addition, the pressure should be kept as low as possible to reduce capital costs. Moreover, higher inlet pressures result in a higher hot spot in methanol synthesis reactor, which may lead to catalyst sintering and increasing the risk of coke deposition. Thus, it seems to be necessary operating at a pressure in which high methanol yield with a reasonable amount of water and hot spot along the reactor result. In this work, the inlet pressure equals to 76.98 bar as the designed pressure of conventional methanol synthesis reactor is considered in the proposed process model.
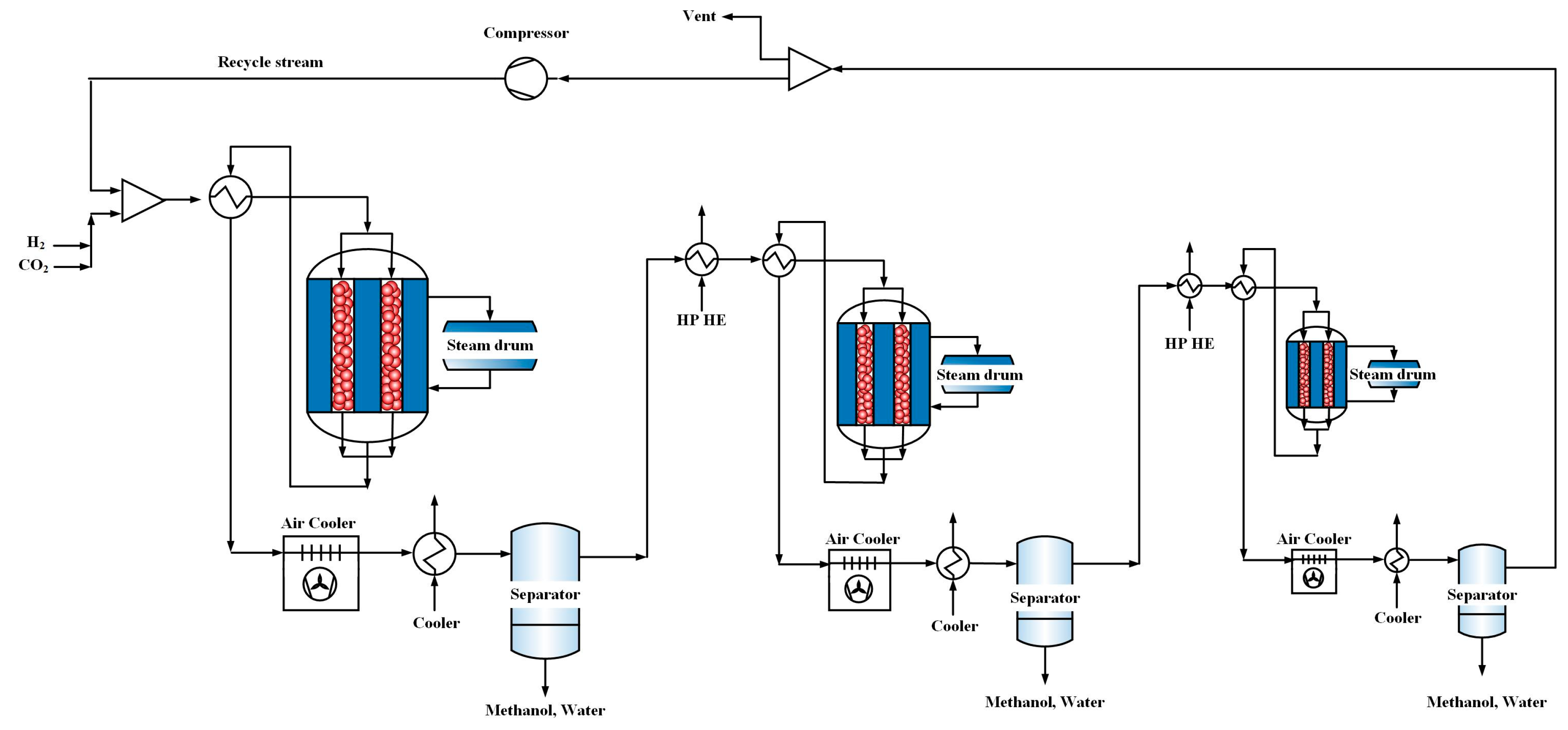
6.2. A Comparison between One-Stage, Three-Stage and Three-Stage Membrane Reactors (MR)
As mentioned, low conversion of carbon dioxide over the commercial catalyst and its deactivation due to water production during the reaction are the two main concerns in CO2 hydrogenation process. To overcome low conversion of carbon dioxide, a three-stage heat exchanger reactors connected in series was proposed for direct CO2 hydrogenation and the results in the previous section indicate significant augmentation in CO2 conversion. To solve the latter problem, an H-SOD membrane was applied in each reactor of the proposed process for separation of water from the reaction side. In this section, the performance of these three-stage membrane reactors (MR) was compared with one-stage and three-stage reactors.
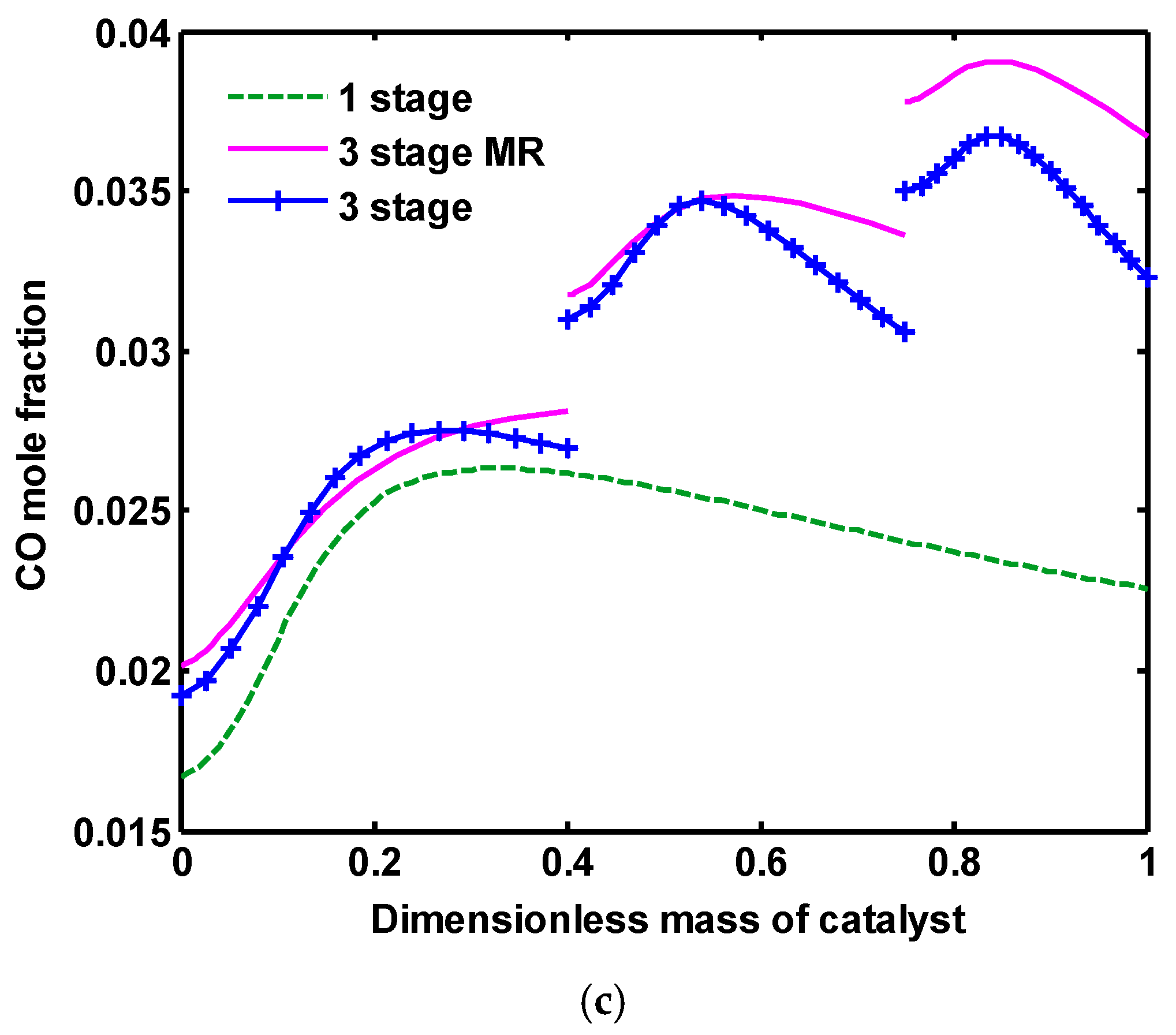
In
Figure 7, the profiles of H
2O, MeOH and CO mole fractions are compared for these three configurations. Water produced during RWGS reaction (Equation (2)) greatly reduces the methanol production rate by suppressing reaction (3). Furthermore, the produced water accelerates the crystallization of Cu and ZnO contained in the commercial catalyst, leading to the catalyst deactivation [
41,
42]. In addition, alumina presented in the catalyst is a very hydrophilic substance and consequently adsorbs the generated water, resulting in catalyst poisoning. Therefore, to improve the CO
2 hydrogenation process, in situ removal of water during the reaction is crucial. In, a one-stage reactor, as the common process for CO
2 hydrogenation, a remarkable amount of H
2O is produced during RWGS and CO
2 hydrogenation reactions (Equations (2) and (3)). In a three-stage configuration where the volume of catalysts is divided into three reactors connected in series, lower amounts of water are produced compared to one-stage because of lower catalyst volume in each reactor. By applying water perm-selective membrane, the concentration of water as the undesirable product is reduced considerably in all three reactors. In the membrane concept, water vapor is withdrawn from the reaction to the permeation side by a water perm-selective membrane. In
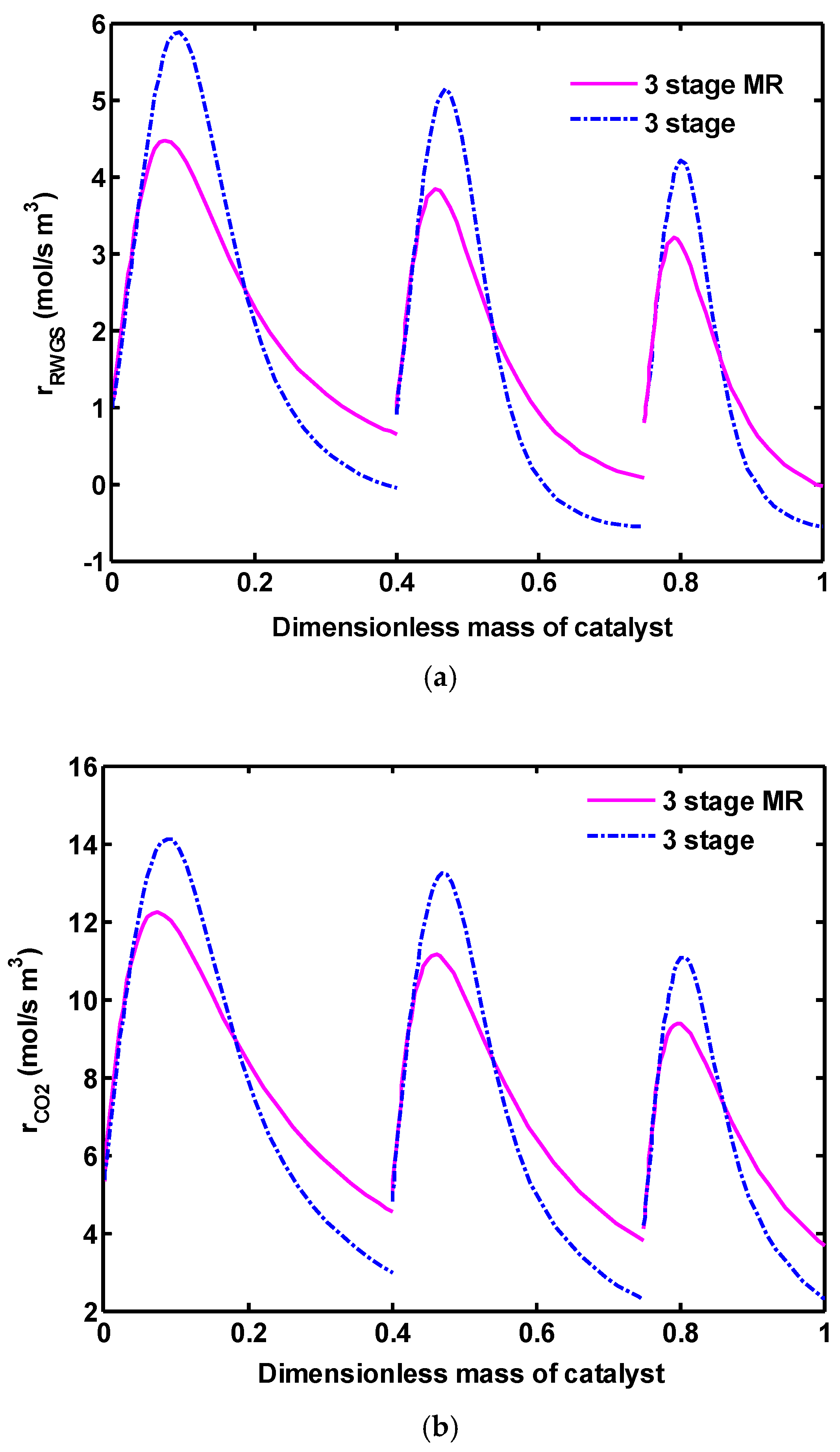
Figure 7b, methanol mole fraction distribution along the reactors was demonstrated. Because of lower catalyst volume in each reactor of three-stage and three-stage MR, lower amounts of methanol are produced compared to one-stage. The produced methanol from each reactor is mainly separated from the unreacted gases in a flash drum and is produced again in the next reactor. Thus, the total methanol production rate increases extremely in this novel configuration. There is no significant difference in the methanol concentration in three-stage and three-stage MR. Although more methanol is expected to be produced by eliminating water from the reaction side (according to the Le Chatelier principle, see Equation (3)), a lower amount of methanol was produced in three-stage MR compared with three-stage. This observation can be justified by taking a close look at the reaction rates profiles as seen in
Figure 8a–c. These figures reveals that all reaction rates have lower values in three-stage MR, consequently the conversion of CO
2 and H
2 (see
Figure 9) is decreased and finally less methanol is produced. The reason of reduction of all reaction rates in three-stage MR is that, in this configuration, a lower temperature profile is observed during the reaction, due to further heat transferring with the permeation side and, as it is quite clear, lower reactor bed temperature results in lower reaction rate. In one-stage reactor, CO is first produced during RWGS reaction, and then, by increasing water production, the RWGS reaction is shifted towards CO consumption (WGS reaction occurs). This trend is exactly repeated in three-stage reactors, while in three-stage MR, by removing H
2O from the reaction side, based on the Le Chatelier principle, RWGS reaction is shifted towards more CO production. Therefore, the profile of CO mole fraction is slightly different in this configuration.
RWGS reaction rate profile was shown in
Figure 8a. A close look reveals that the RWGS reaction in three-stage configuration is shifted towards the left side (WGS reaction) at the end of each reactor, while in three-stage MR, only the forward reaction takes place. As mentioned, this is because of the fact that, in the membrane concepts, H
2O removal pushes the RWGS reaction to the right side. Although CO and CO
2 hydrogenation reaction rates follow a similar trend in both configurations (see
Figure 8b,c), three-stage MR predicts lower values compared to the other one, because of its slightly lower reactor bed temperature profiles.
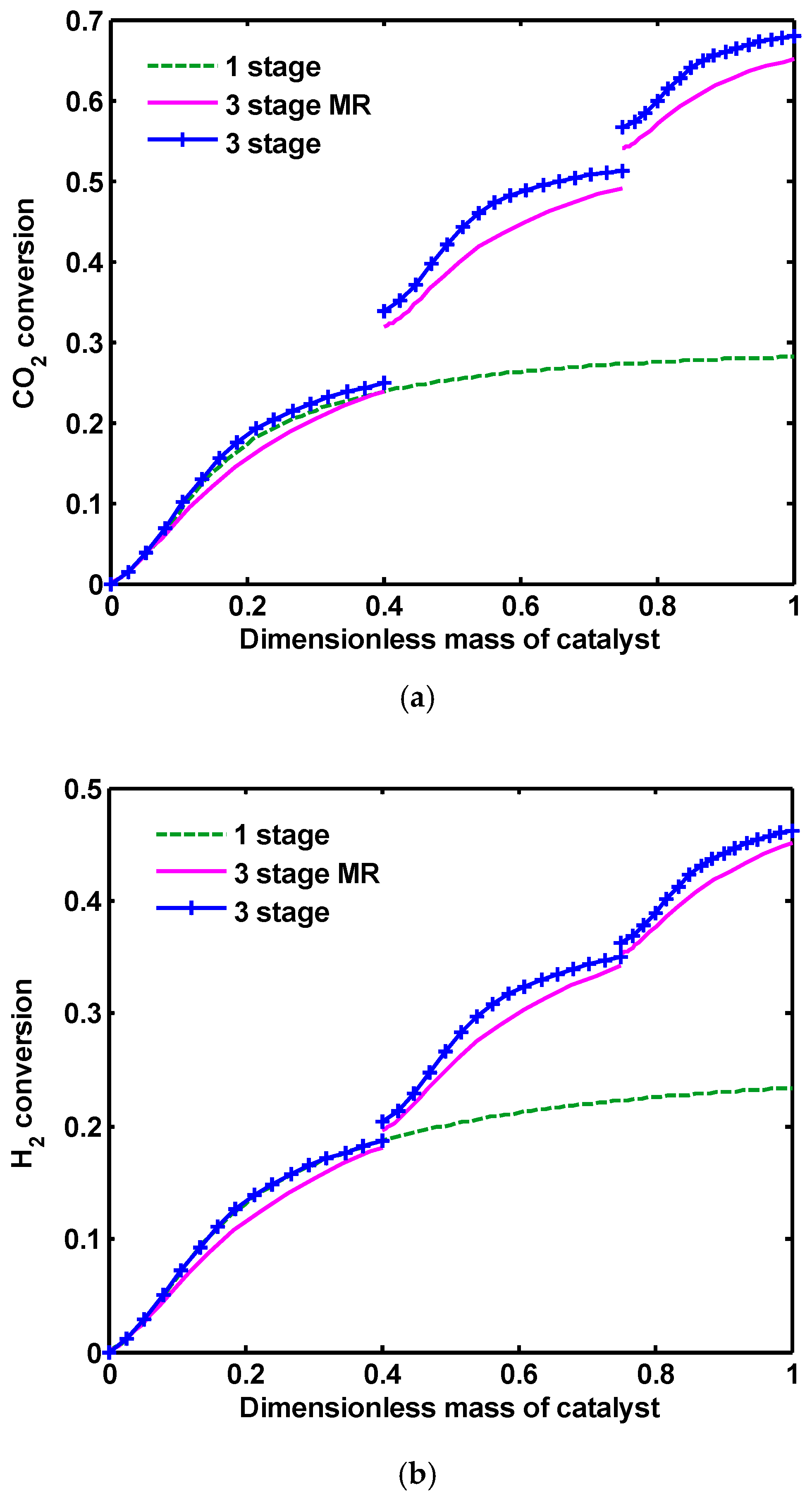
Figure 9 shows considerably higher CO
2 and H
2 conversion in three-stage reactor compared to 1 one stage reactor. CO
2 and H
2 conversions are approximately increases twice higher than the common process. This is due to using the unreacted gases in the second and third reactor. In three-stage MR, the rates of CO, CO
2 hydrogenation and RWGS reactions are reduced in the presence of H-SOD membrane, consequently H
2 and CO
2 conversions have lower values in comparison to three-stage reactors.
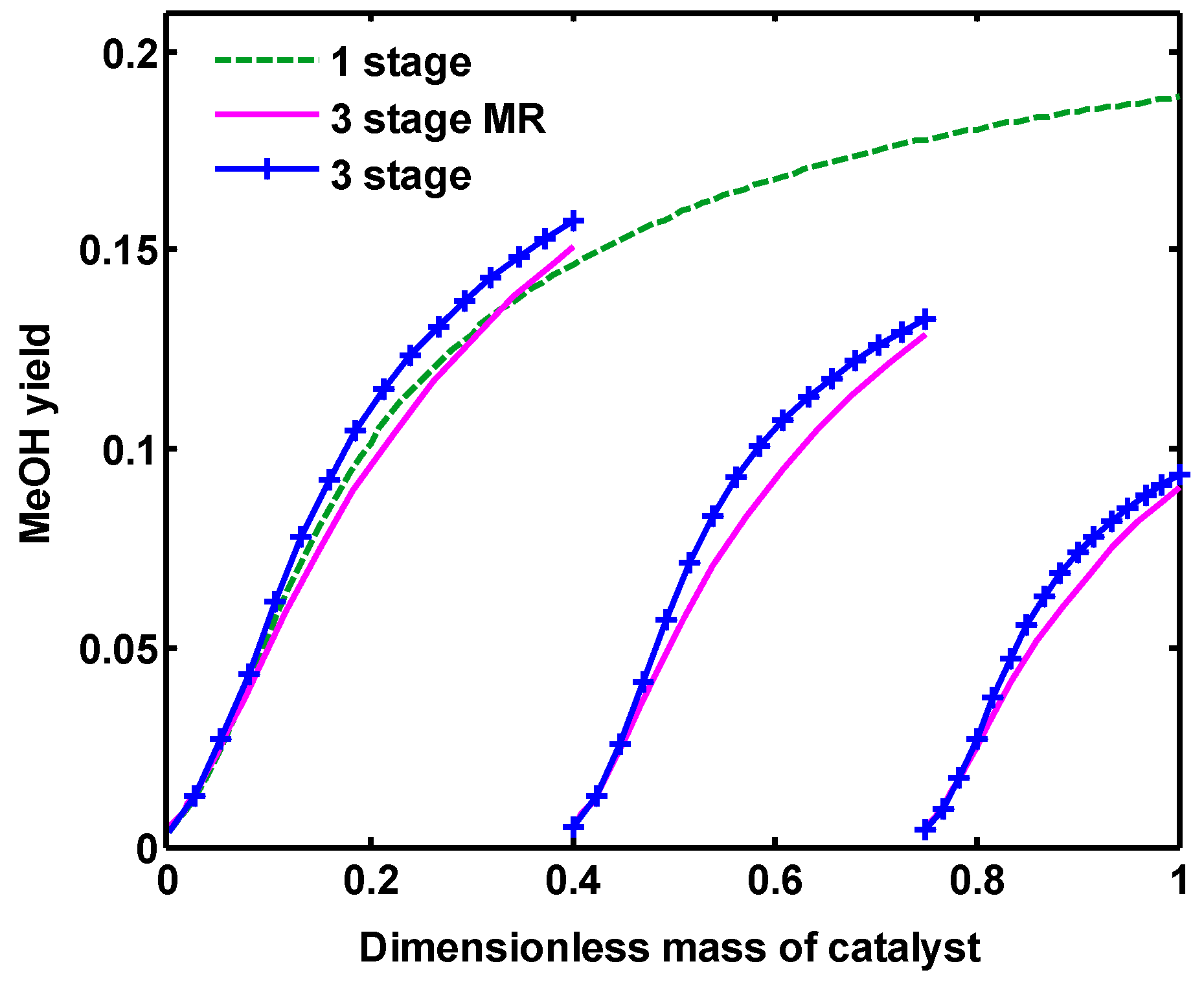
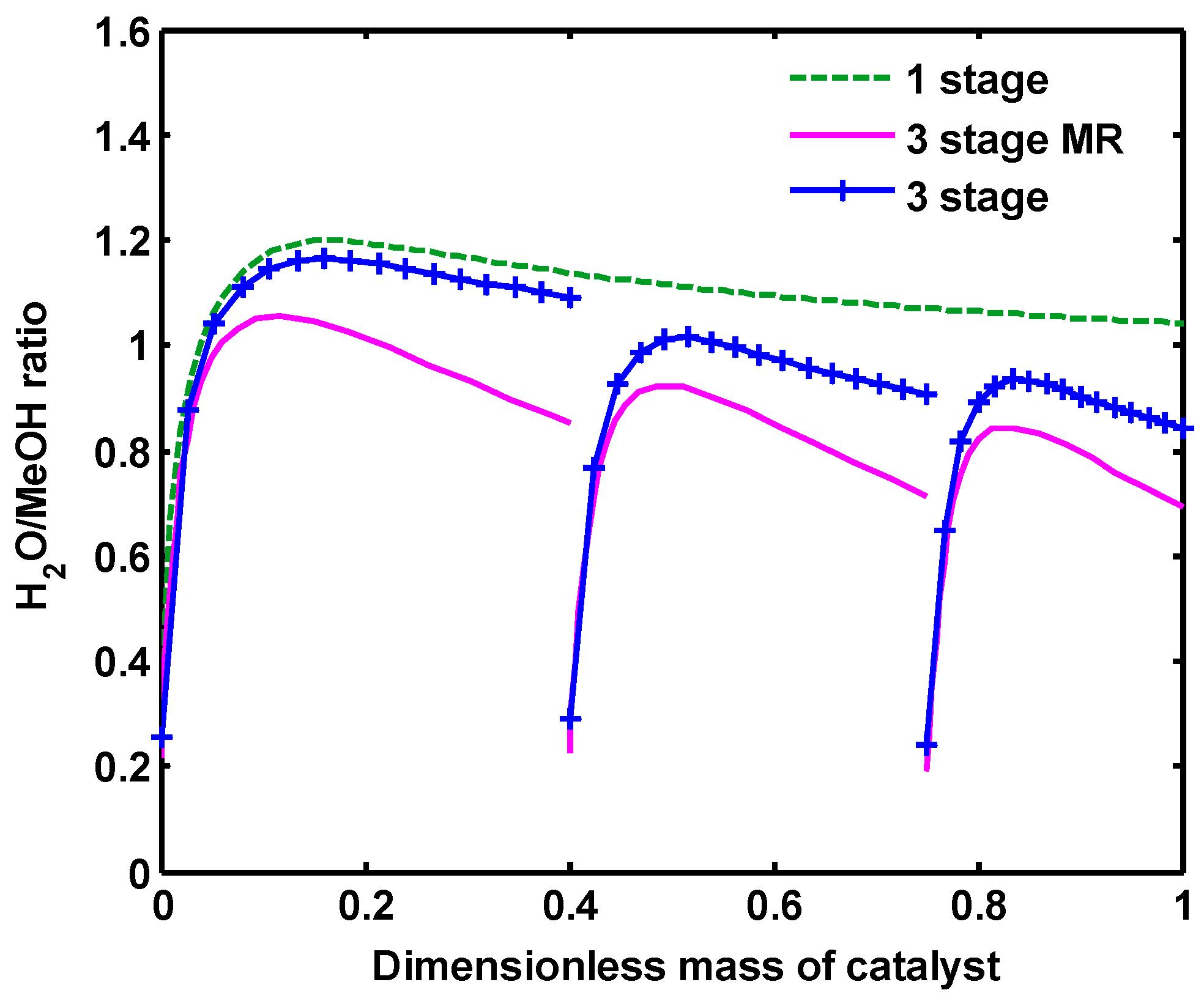
Figure 10 demonstrates MeOH yield along all configurations, which has a similar trend to the MeOH mole fraction. MeOH yield is described as Equation (31). Water to MeOH ratio is also shown in
Figure 11. As clearly shown, H
2O/MeOH ratio increases in all configurations, even reaching more than one. This can be attributed to the fact that, in the CO
2 hydrogenation process, water is produced through reactions (2) and (3), while MeOH is mainly produced by reaction (3) and a little by reaction (3). However, from the middle to the end of the each reactor in one-stage and three-stage configurations, this ratio follows a constant trend, due to thermodynamic limitations, while in three-stage MR, this ratio diminishes up to the end of the reactor because of H
2O permeation during the reaction.
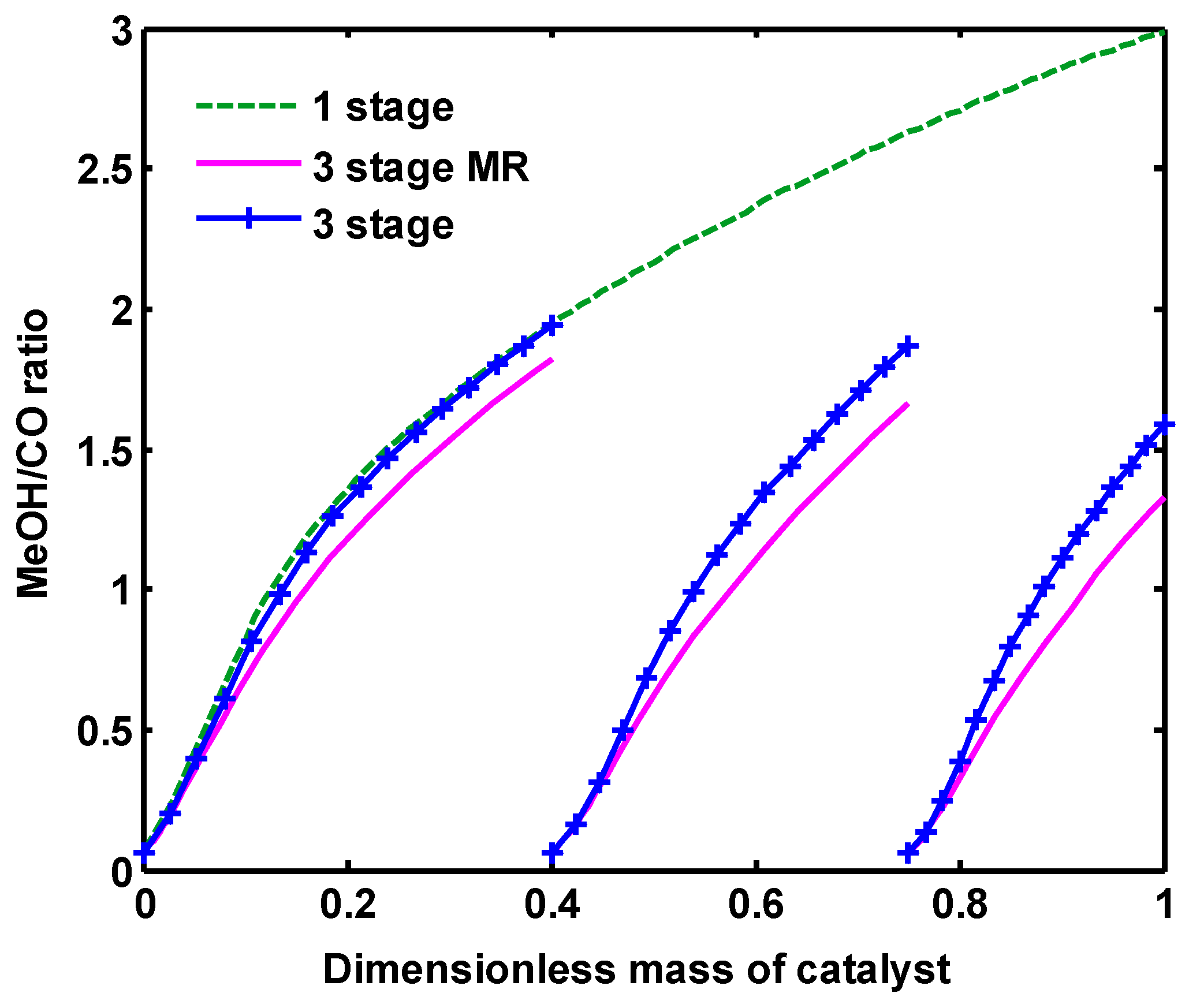
Figure 12 depicts MeOH to CO ratio for all configurations. This ratio increases as the gas flowing through the reactor. The reason is that methanol is produced by reactions (1) and (3), while CO is formed through reaction (2) and consumed by reaction (1). Consequently, more MeOH will be gainedcompared to CO:
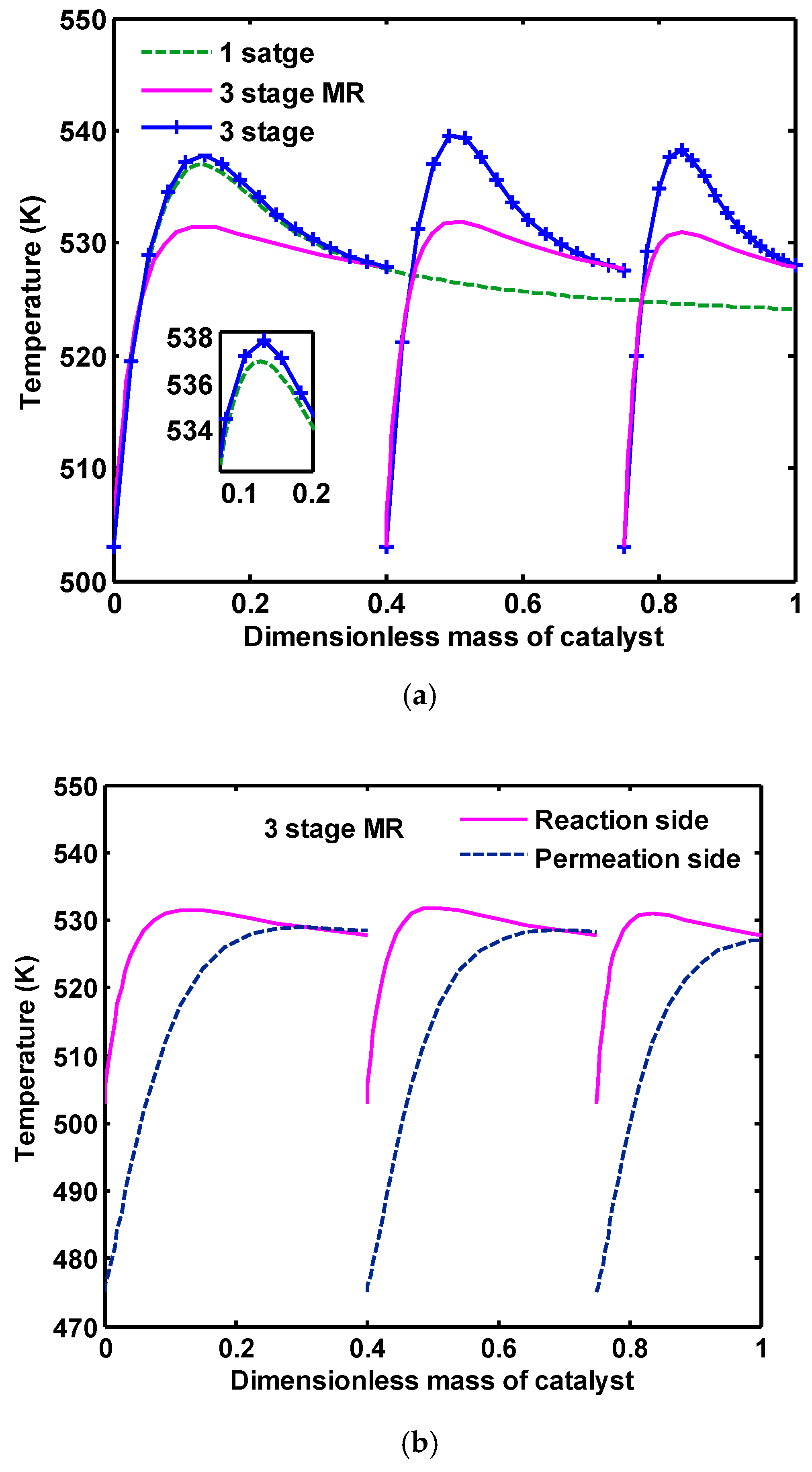
The temperature distribution along the reactors is depicted in
Figure 13a. The temperature profile in the first reactor of three-stage configuration has the same trend as one-stage reactors. This is due to the same conditions of these two reactors, except in their catalyst volume and a small difference in their inlet compositions. The temperature profile in the second and third reactors in three-stage configuration are accompanied with a higher hot spot because of higher concentrations of CO in the inlet of these two reactors, which results in CO hydrogenation being a highly exothermic reaction. In three-stage MR configuration, a more favorable profile of temperature is observed because of the heat transfer between the reaction and the permeation sides in this configuration (see
Figure 13b, the inlet temperature of the permeation side of each reactor is 475 K, while the inlet temperature of the reaction side is 503 K).
Figure 14 shows H
2O permeation rate in the permeation sides of three-stage MR configuration.
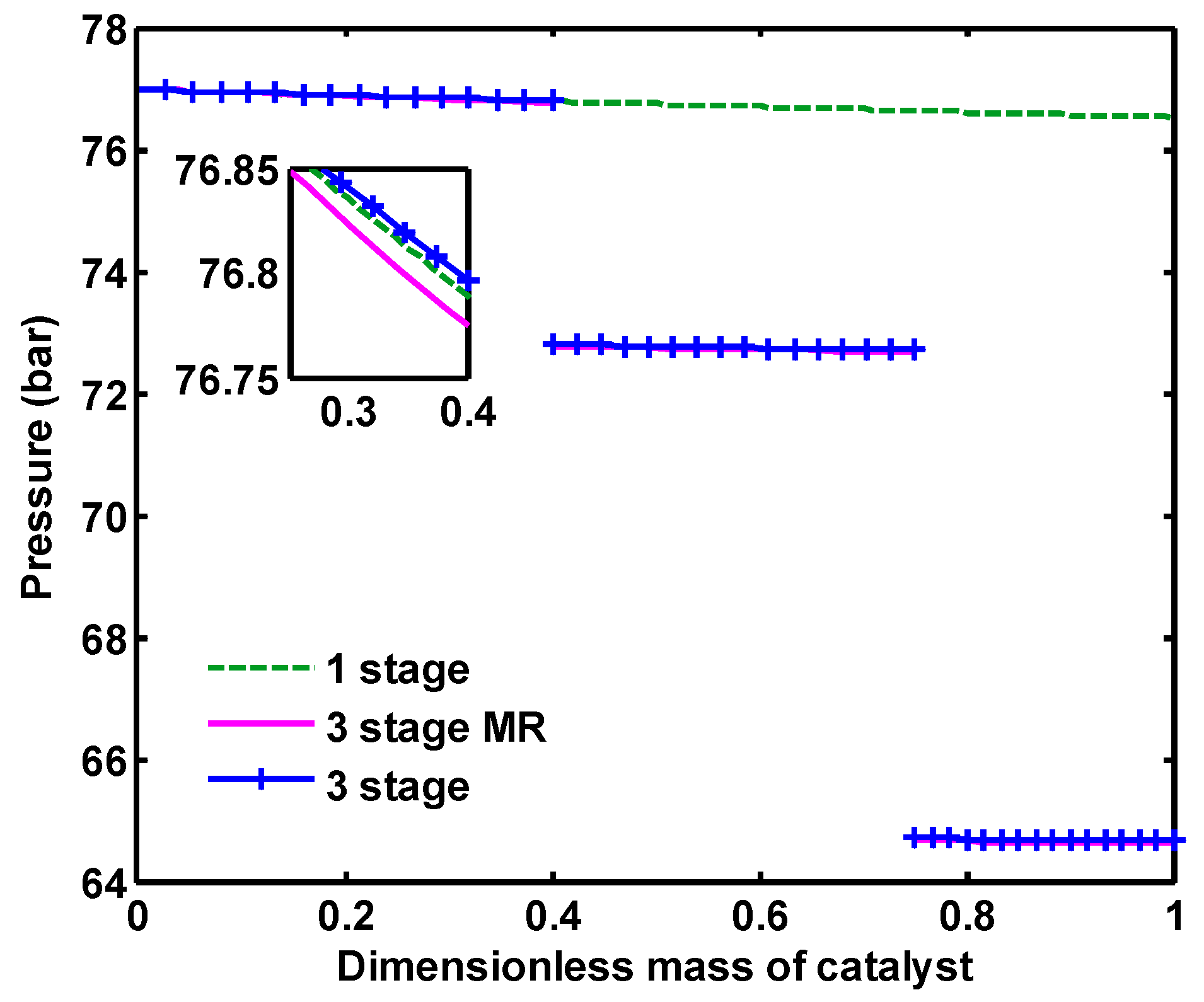
Pressure distribution along one-stage, three-stage and three-stage membrane reactors are presented in
Figure 15. In one-stage configuration, 0.46 bar pressure drop occurs for the gas flowing through the reactor, while in multi stage configurations, approximately, 0.18 bar pressure drop takes places for each of reactors. It should be noted that a total of 4 bar pressure drop was considered between the reactors of multi stage configurations, which means that the inlet pressure of the second and third reactors are 4 bar lower than that of the previous one.
6.3. A Comparison between Three-Stage Membrane Reactors (MR) and the Conventional Methanol Synthesis Reactor (CR)
As proved in the previous sections, the problems of low conversion of CO2 and a high amount of water production in direct CO2 hydrogenation can be solved in three-stage MR configuration. To investigate the plausibility of methanol production from a mixture of CO2 and H2, a comparison was performed between this novel configuration and the traditional process from coal and natural gas.
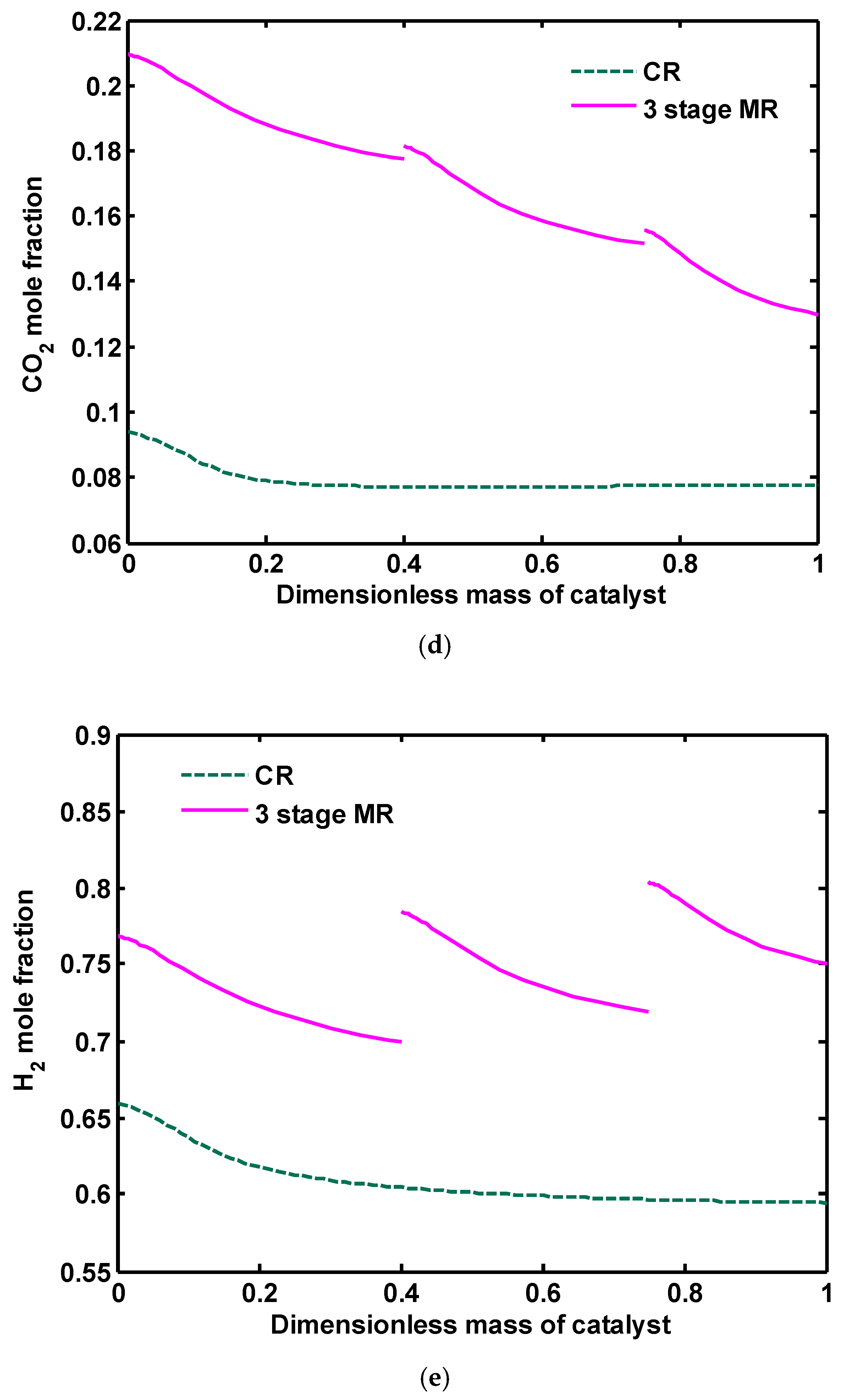
Figure 16a reflects that, although conventional reactor (CR) results in a higher methanol mole fraction compared to each rector in three-stage MR, the total methanol production rate is remarkably higher than CR. It can be deduced from
Figure 16b that, in the green methanol synthesis process, H
2O production is increased because more CO
2 content in the feedstock favors the RWGS reaction, and, therefore, more H
2O is produced compared to CR. In CR, WGS reaction (the reverse of Equation (2)) consumes the produced water during the reaction, thus the water mole fraction reaches its equilibrium and then follows a constant trend. As discussed in the previous section, in three-stage MR, the rate of water production is reduced significantly compared to one-stage and three-stage reactors. However, the amount of water is still higher compared to CR but is fairly acceptable. There is a clear difference in the profile of CO mole fraction as seen in
Figure 16c. In CR, CO is consumed through CO hydrogenation and WGS reaction, and so follows a decreasing trend along the reactor. In the green methanol production process, there is a lower CO content in the feedstock resulting from the recycle stream, and, as demonstrated, this component is produced as a result of the RWGS reaction. As the amount of CO increases, its generation rate in the reactor reduces. By further increasing of the CO level in the feed, CO is consumed through WGS reaction and its hydrogenation to methanol. Consequently, it shows a decreasing trend in the second and third reactors. CO
2 and H
2 mole fractions have the same increasing trend. In CR, CO
2 and H
2 mole fractions are reduced at the beginning of the reactor and then it shows a monotonous profile, indicating the fact that the reactions approach their equilibrium state. The WGS reaction is shifted towards H
2 and CO
2 production, and CO and CO
2 hydrogenation reactions consume H
2 and CO
2. This competing effect results in an approximately constant trend in CR. CO
2 conversion is also compared in both configurations in
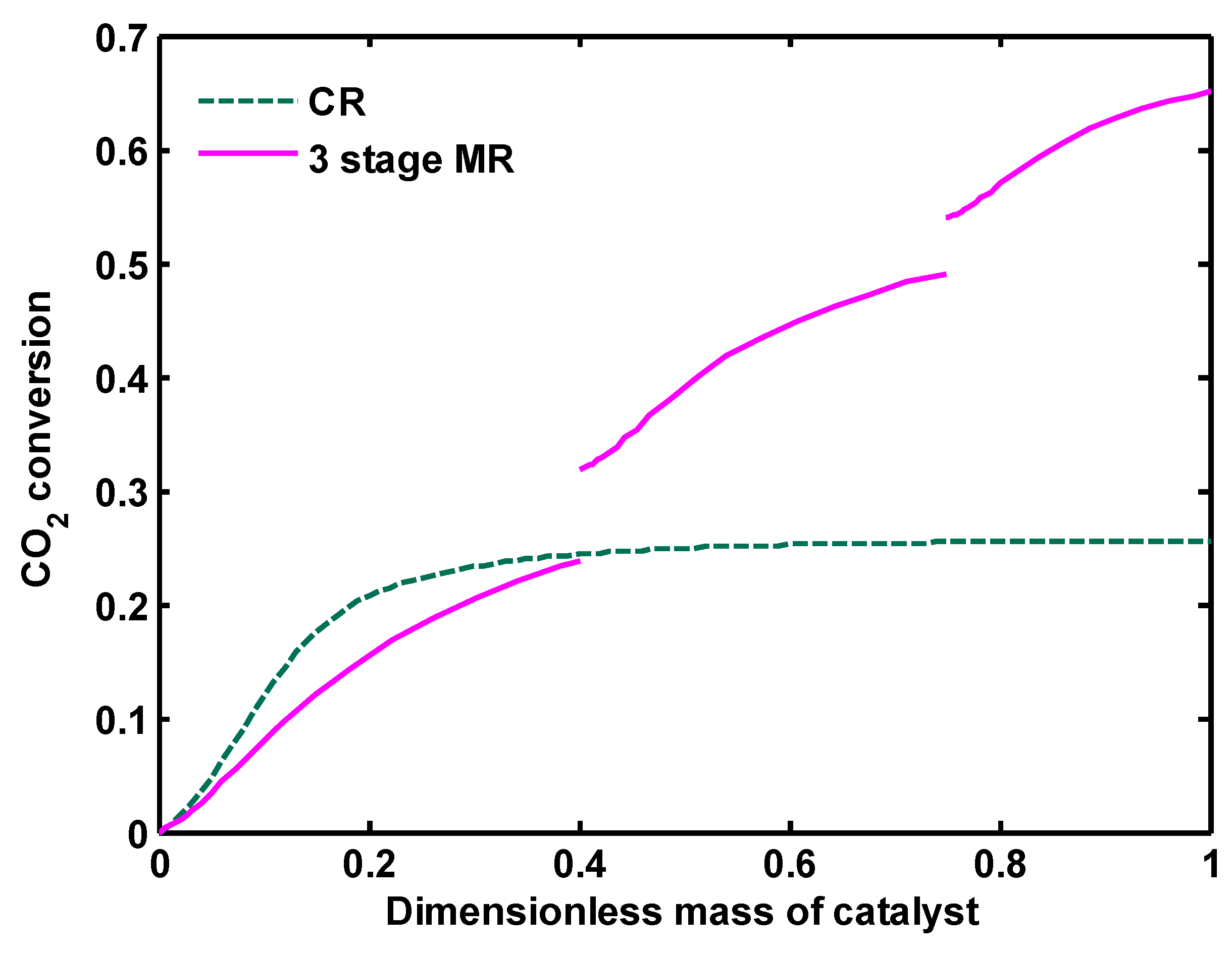
Figure 17. As depicted, CO
2 conversion increases 50% in three-stage MR compared to CR.
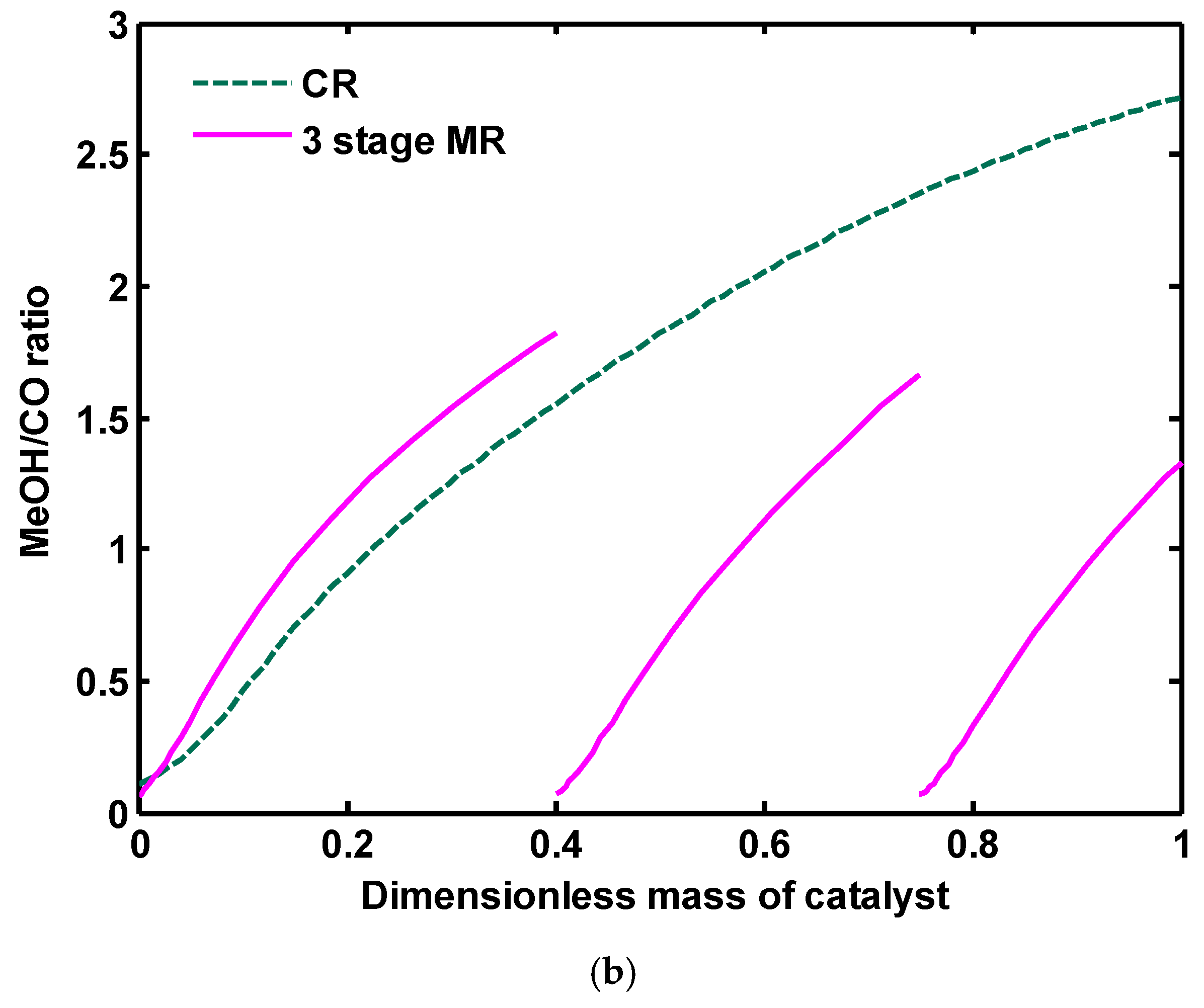
Figure 18a,b show H
2O/MeOH ratio and MeOH/CO ratio along CR and three-stage MR, respectively. Water to MeOH ratio in CR configuration increases up to 0.6 and then a little is reduced, after that, becomes constant up to end of the reactor. Because in methanol production by synthesis gas feedstock, reaction (2) is shifted to the left side and some parts of the produced water is consumed through this reaction. Thus, the volume of produced water is much lower than when CO
2 is used as the reactor feedstock. MeOH to CO ratio is increased along the reactor for both configurations. In CR, methanol is predominantly produced by reactions (1) and (3), while CO is consumed by reaction (1) and WGS reaction.
Methanol production rates are compared in CR and direct CO
2 hydrogenation (one-stage, three-stage and three-stage MR). Methanol is produced 288, 305, 586 and 569 ton/day in CR, one-stage, three-stage and three-stage MR, respectively, as presented in
Figure 19. Methanol production rate in CO
2 hydrogenation process is higher than the conventional route from syngas. This can be attributed to two reasons: first, in methanol production by CO
2 hydrogenation, the feedstock considered in this study only contains CO
2 and H
2, without any inert gas, while, in the conventional process from synthesis gas, approximately 20% of the feedstock consists of CH
4 and N
2 as the inert gases. The presence of the inert gases in the feed stream reduces the partial pressure of the reactants and consequently the reaction rates are decreased, resulting in a lower production rate. Second, in the CO
2 hydrogenation process considered here, the unreacted gases are recycled to the reactor to provide a higher reactor yield. It is noteworthy to mention that the CO content in the recycle stream compensates a portion of the absence effects of this component in the initial feedstock. In a three-stage reactor configuration, the methanol production rate increases 281 ton/day (92% increasing) compared to a one-stage reactor. To reduce the water content in the reactor, three-stage MR was suggested, although methanol production rate in this configuration is a bit less than three-stage reactors, the produced water as the cause of catalyst poisoning is notably reduced. The results show that the proposed process is feasible and beneficial for CO
2 hydrogenation to produce green methanol and can be competitive with a traditional methanol synthesis process.