Catalytic Deoxygenation of Hexadecyl Palmitate as a Model Compound of Euglena Oil in H2 and N2 Atmospheres
Abstract
:1. Introduction
2. Results and Discussion
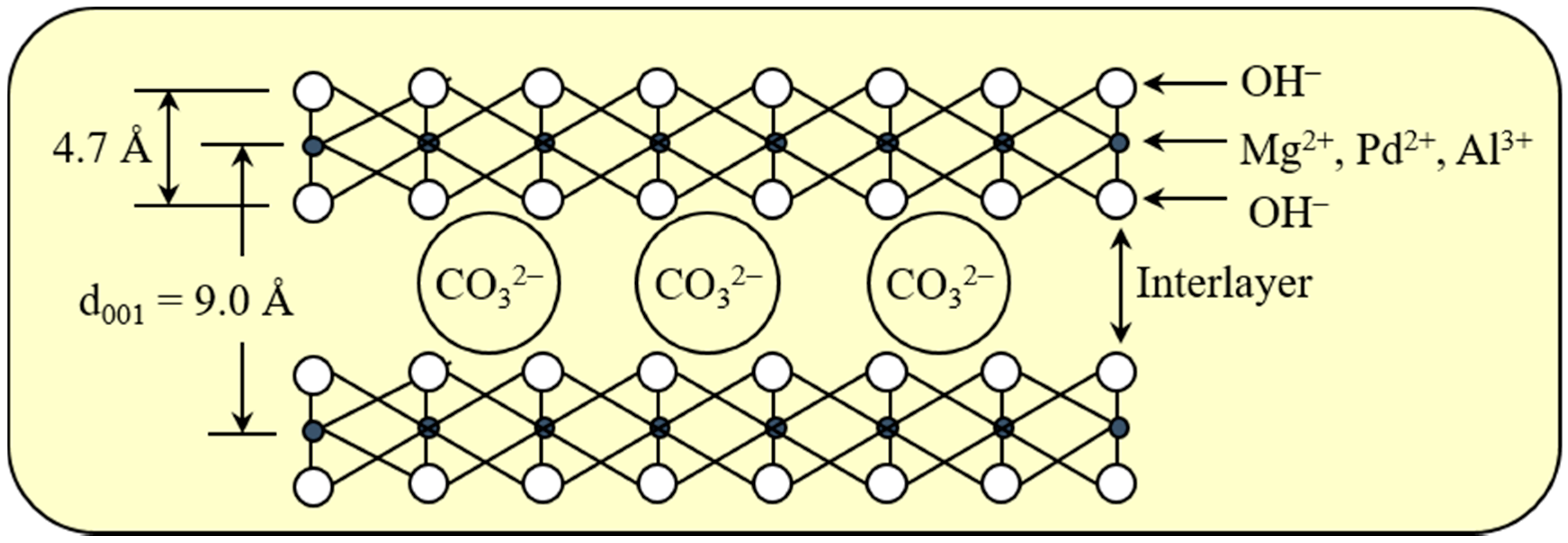
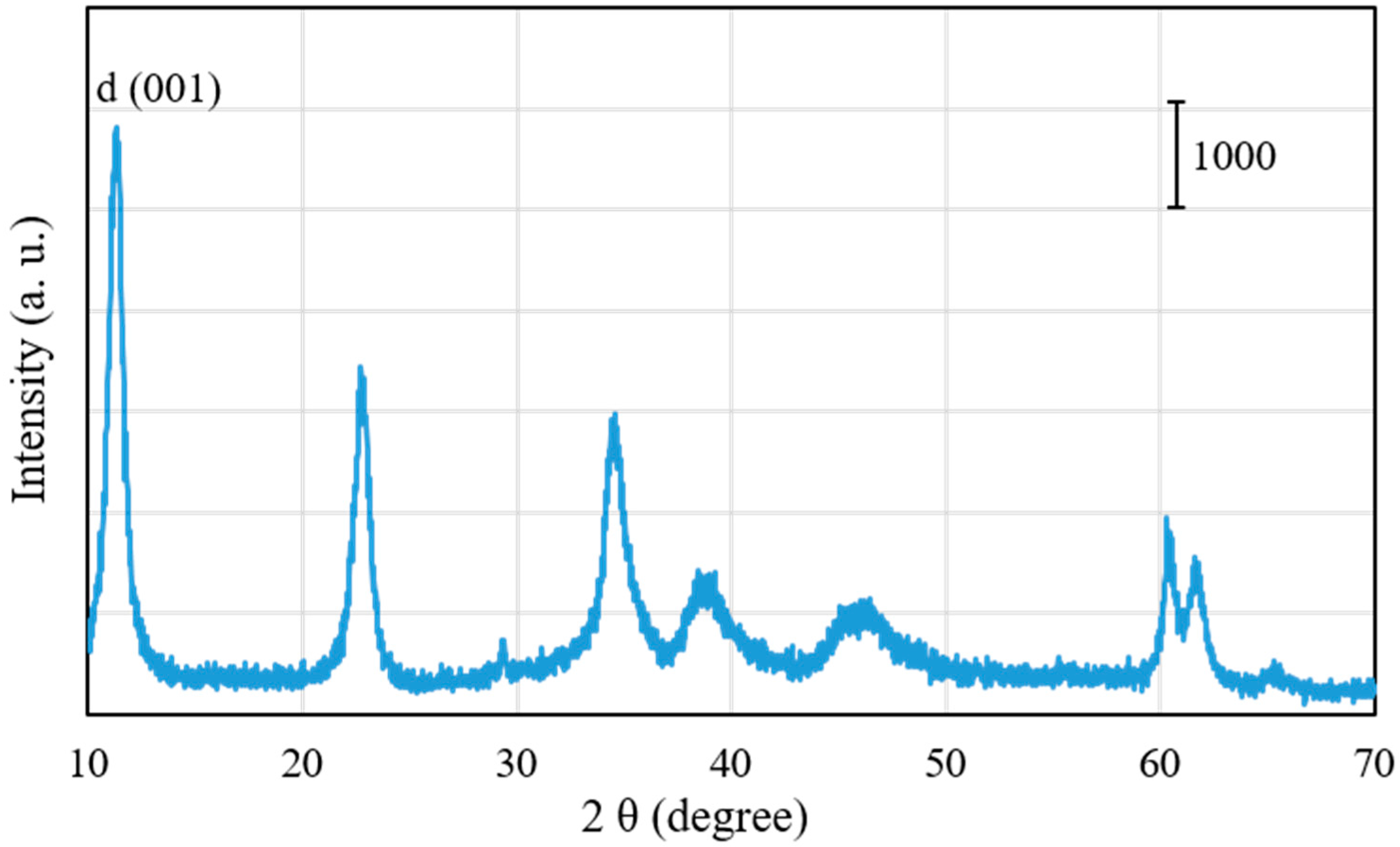
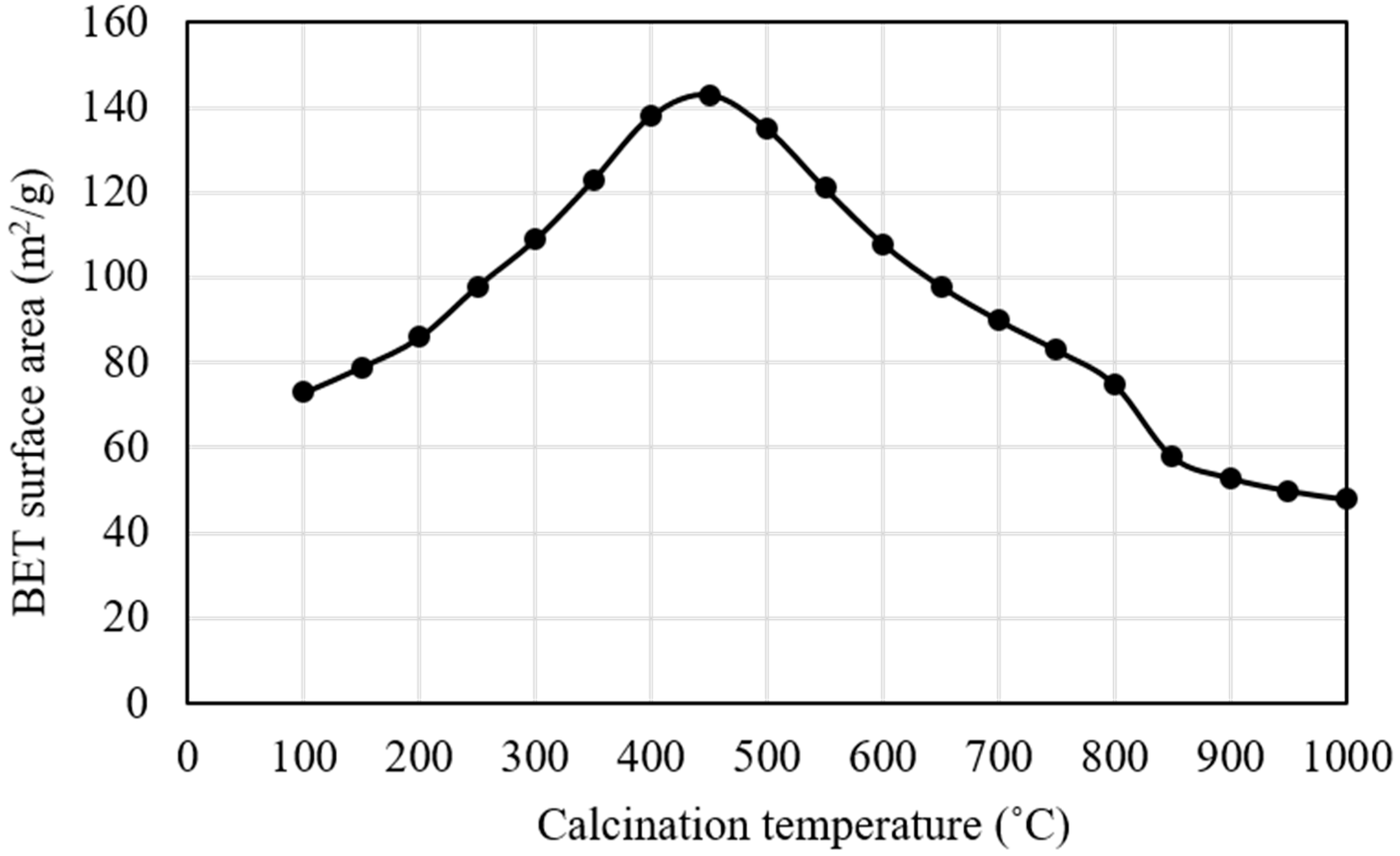
2.1. Catalyst Characterization
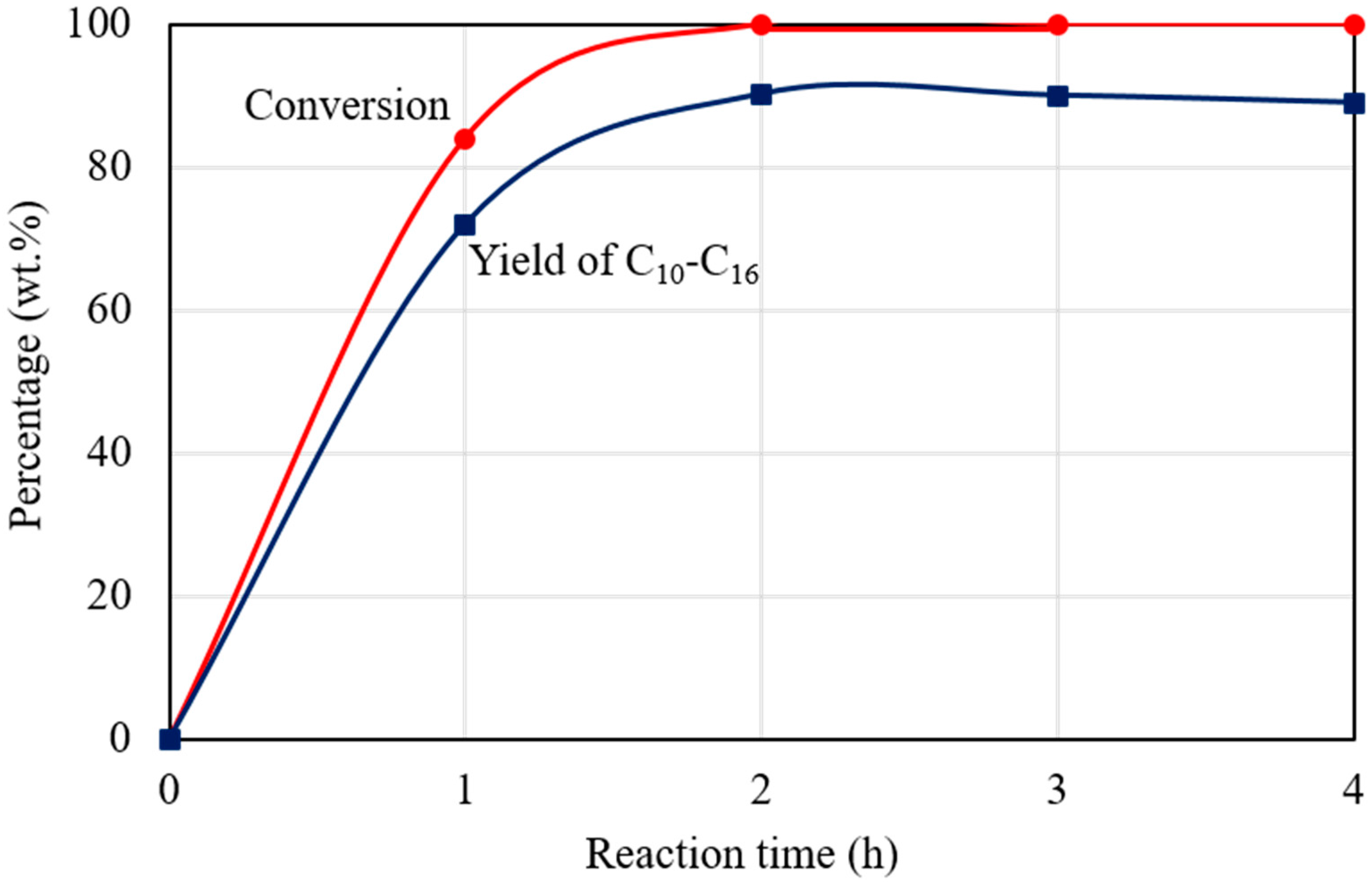
2.2. Catalytic Deoxygenation of C15H31COOC16H33 in H2 Atmosphere
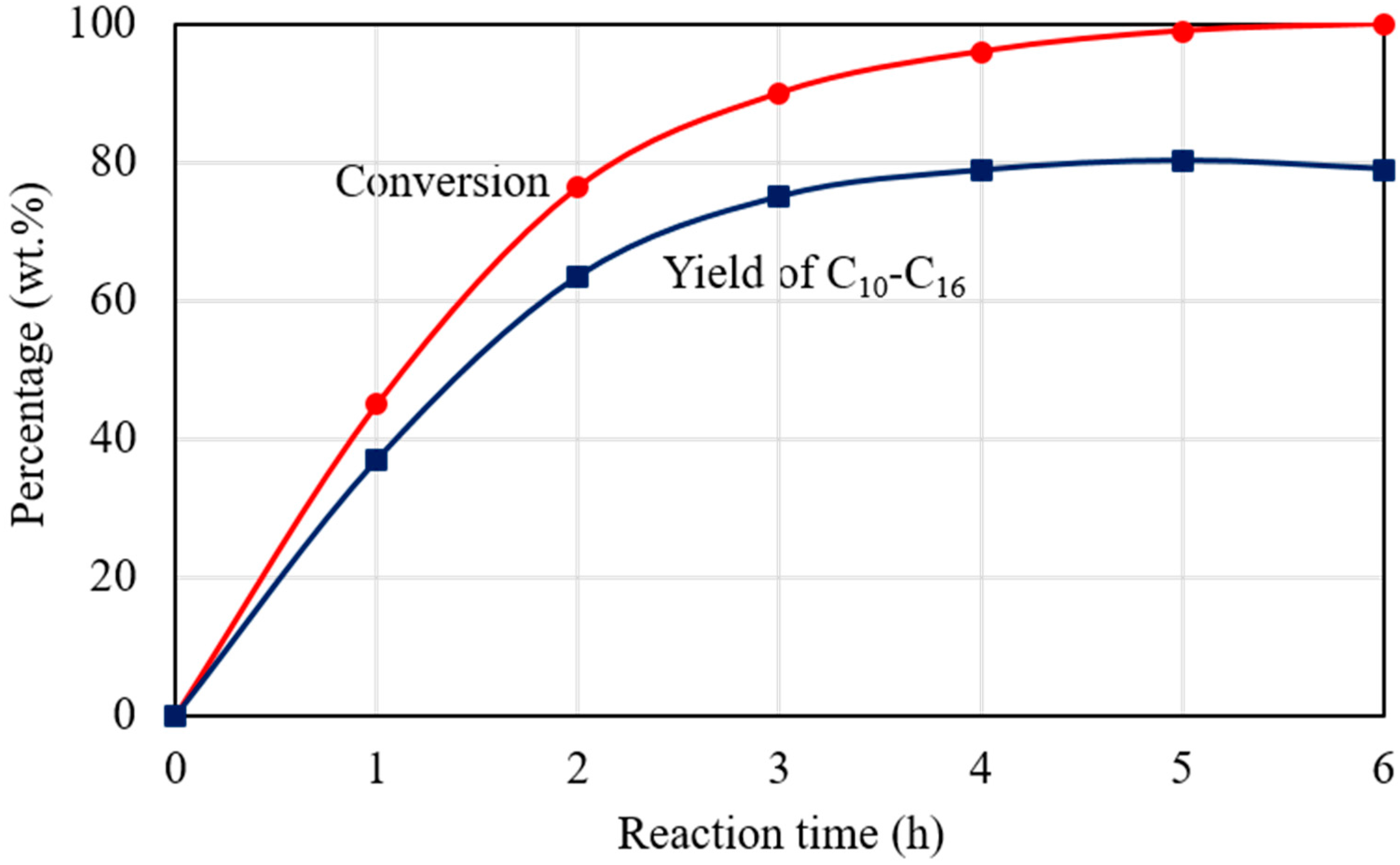
2.3. Catalytic Deoxygenation of C15H31COOC16H33 in N2 Atmosphere
3. Experimental Section
3.1. Reagents
3.2. Catalysts
3.3. Instruments
3.4. Reactions
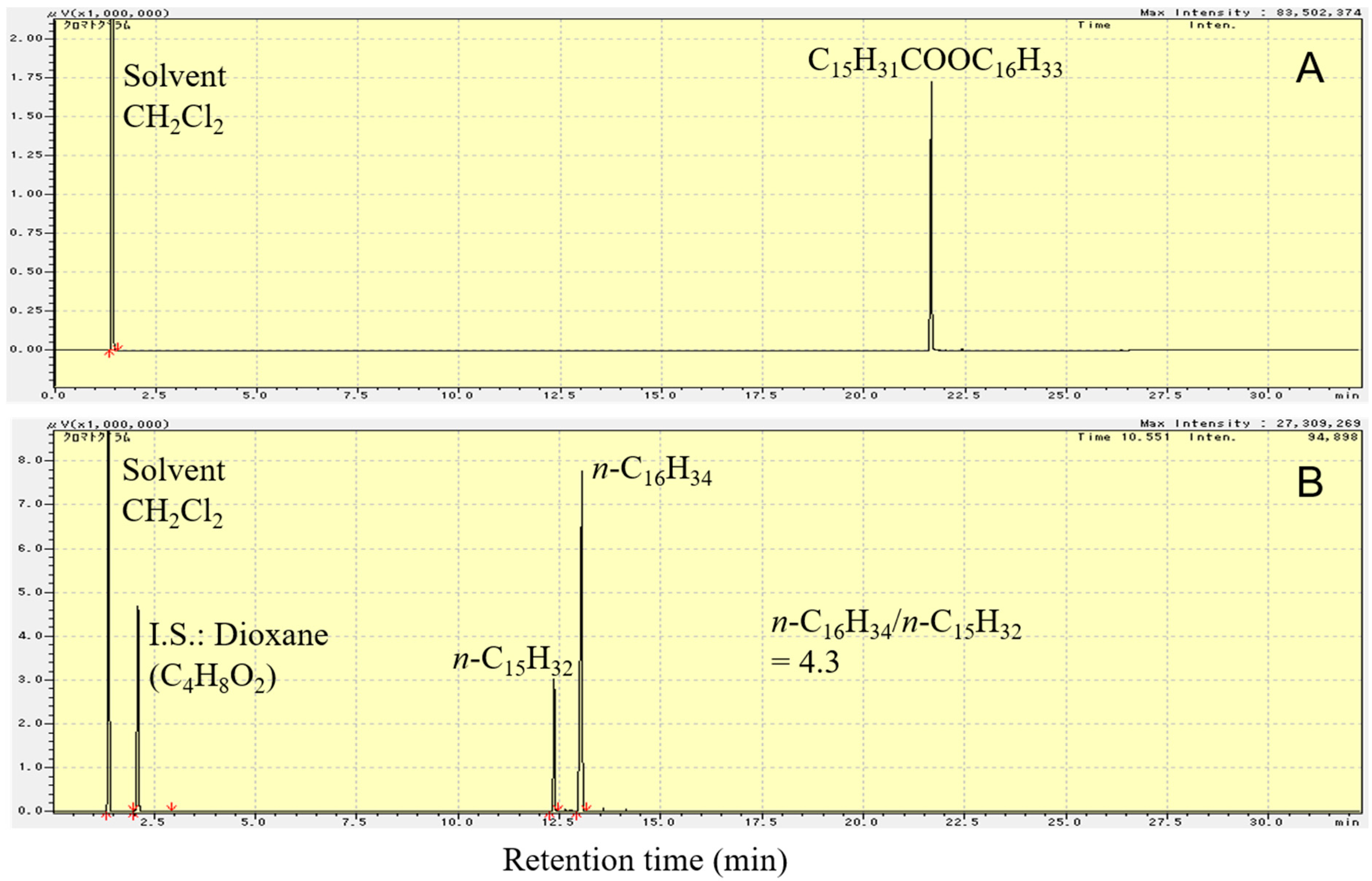
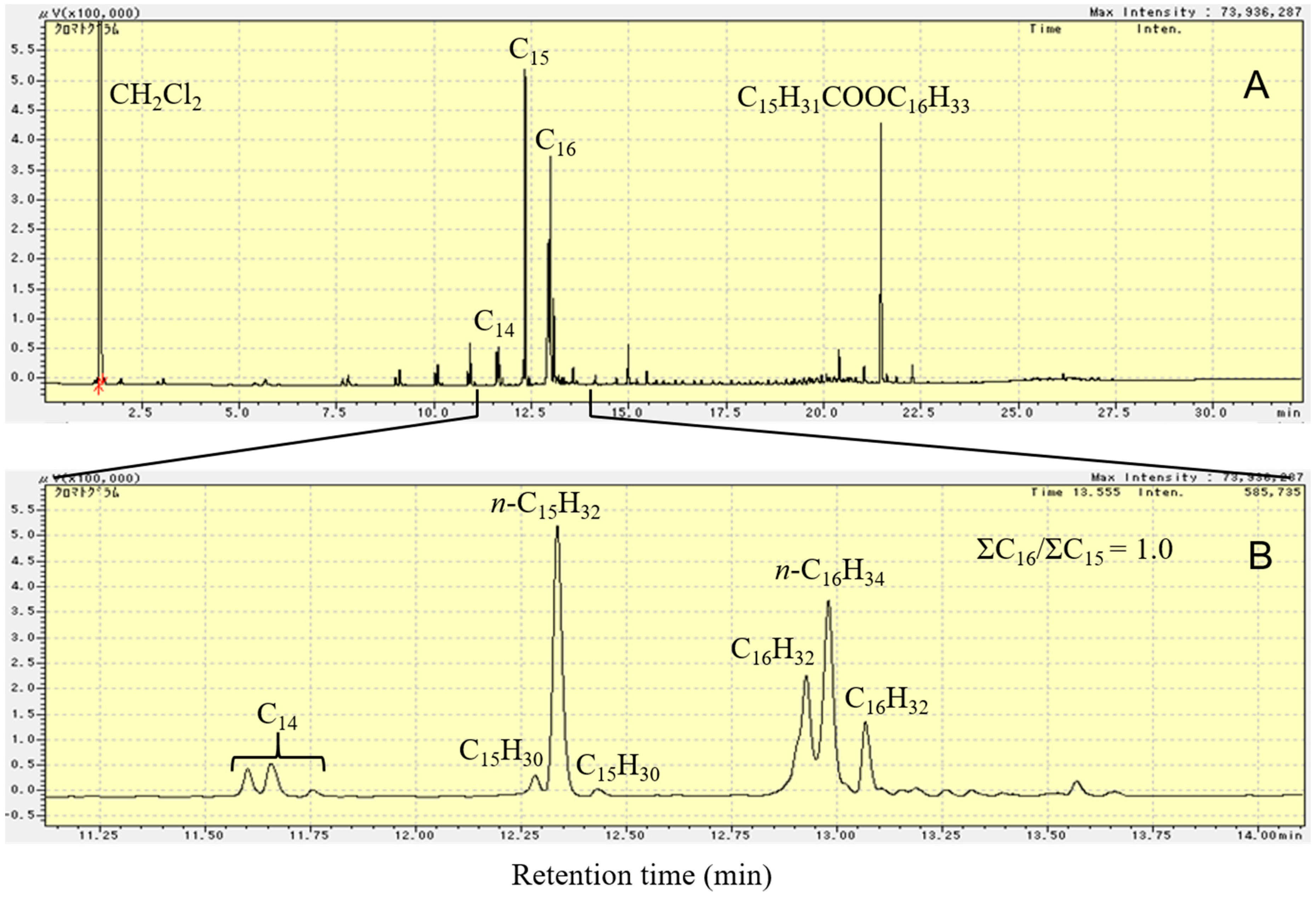
3.5. Analyses
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Cesaro, A.; Belgiorno, V. Combined biogas and bioethanol production: Opportunities and challenge for industrial application. Energies 2015, 8, 8121–8144. [Google Scholar] [CrossRef]
- Hanaoka, T.; Liu, Y.; Matsunaga, K.; Miyazawa, T.; Hirata, S.; Sakanishi, K. Bench-scale production of liquid fuel from woody biomass via gasification. Fuel Process. Technol. 2010, 91, 859–865. [Google Scholar] [CrossRef]
- Liu, Y.; Hanaoka, T.; Miyazawa, T.; Murata, K.; Okabe, K.; Sakanishi, K. Fischer-Tropsch synthesis in slurry-phase reactors over Mn- and Zr-modified Co/SiO2 catalysts. Fuel Process. Technol. 2009, 90, 901–908. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic technologies for biodiesel fuel production and utilization of glycerol: A review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J. Review of heterogeneous catalysts for catalytically upgrading vegetable oils into hydrocarbon biofuels. Catalysts 2017, 7, 83. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo-Boyas, R.; Murata, K.; Minowa, T.; Sakanishi, K. Production of bio-hydrogenated diesel by hydrotreatment of high-acid-value waste cooking oil over ruthenium catalyst supported on Al-polyoxocation-pillared pontmorillonite. Catalysts 2012, 2, 171–190. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo-Boyas, R.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of vegetable oils to produce bio-hydrogenated diesel and liquefied petroleum gas fuel over catalysts containing sulfided Ni-Mo and solid Acids. Energy Fuels 2011, 25, 4675–4685. [Google Scholar] [CrossRef]
- Yoshida, M.; Tanabe, Y.; Yonezawa, N.; Watanabe, M.M. Energy innovation potential of oleaginous microalgae. Biofuels 2012, 3, 761–781. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Mayfield, S.P. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-derived biofuel: A review of method of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef] [Green Version]
- Kanda, H.; Li, P.; Goto, M.; Makio, H. Energy-saving lipid extraction from wet Euglena gracilis by the low-boiling-point solvent dimethyl ether. Energies 2015, 8, 610–620. [Google Scholar] [CrossRef]
- Tani, Y.; Okumura, M.; Ii, S. Liquid wax ester production by euglena gracilis. Agric. Biol. Chem. 1987, 51, 225–230. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Kubickova, I.; Snare, M.; Eranen, K.; Murzin, D.Y. Catalytic deoxygenation of fatty acids and their derivatives. Energy Fuels 2007, 21, 30–41. [Google Scholar] [CrossRef]
- Sotelo-Boyas, R.; Liu, Y.; Minowa, T. Renewable diesel production from the hydrotreating of rapeseed oil with Pt/Zeolite and NiMo/Al2O3 catalysts. Ind. Eng. Chem. Res. 2011, 50, 2791–2799. [Google Scholar] [CrossRef]
- Murata, K.; Liu, Y.; Watanabe, M.M.; Inaba, M.; Takahara, I. Hydrocracking of algae oil into aviation fuel-range hydrocarbons using a Pt-Re catalyst. Energy Fuels 2014, 28, 6999–7006. [Google Scholar] [CrossRef]
- Sanchez-Cardenas, M.; Medina-Valtierra, J.; Kamaraj, S.-K.; Ramirez, R.R.M.; Sanchez-Olmos, L.A. Effect of size and distribution of Ni nanoparticles on γ-Al2O3 in oleic acid hydrodeoxygenation to produce n-alkanes. Catalysts 2016, 6, 156. [Google Scholar] [CrossRef]
- Charusiri, W.; Vitidsant, T. Catalytic cracking of used cooking oil to liquid fuels over HZSM-5. J. Energy 2003, 5, 58–68. [Google Scholar]
- Na, J.G.; Han, J.K.; Oh, Y.K.; Park, J.H.; Jung, T.S.; Han, S.S.; Yoon, H.C.; Chung, S.H.; Kim, J.N.; Ko, C.H. Decarboxylation of microalgal oil without hydrogen into hydrocarbon for the production of transportation fuel. Catal. Today 2012, 185, 313–317. [Google Scholar] [CrossRef]
- Tani, H.; Hasegawa, T.; Shimouchi, M.; Asami, K.; Fujimoto, K. Selective catalytic decarboxy-cracking of triglyceride to middle-distillate hydrocarbon. Catal. Today 2011, 164, 410–414. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Rozmyszowicz, B.; Lestari, S.; Simakova, O.; Eranen, K.; Salmi, T.; Murzin, D.Y. Catalytic deoxygenation of Tall oil fatty acid over palladium supported on mesoporous carbon. Energy Fuels 2011, 25, 2815–2825. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Basile, F.; Basini, L.; Fornasari, G.; Gazzano, M.; Trifiro, F.; Vaccari, A. New hydrotalcite-type anionic clays containing noble metals. Chem. Commun. 1996, 2435–2436. [Google Scholar] [CrossRef]
- Narayanan, S.; Krishna, K. Structural influence of hydrotalcite on Pd dispersion and phenol hydrogenation. Chem. Commun. 1997, 1991–1992. [Google Scholar] [CrossRef]
- Basile, F.; Fornasari, G.; Gazzano, M.; Vaccari, A. Synthesis and thermal evolution of hydrotalcite-type compounds containing noble metals. Appl. Clay Sci. 2000, 16, 185–200. [Google Scholar] [CrossRef]
- Davis, R.J.; Derouane, E.G. A non-porous supported-platinum catalyst for aromatization of n-hexane. Nature 1991, 349, 313–315. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Hanaoka, T.; Inaba, M.; Sakanishi, K. Syntheses of new peroxo-polyoxometalates intercalated layered double hydroxides for propene epoxidation by molecular oxygen in methanol. J. Catal. 2007, 248, 277–287. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Direct epoxidation of proptlene by molecular oxygen over catalyst system containing palladium and peroxo-Heteropoly compound in methanol. Chem. Commun. 2004, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Suzuki, K.; Hamakawa, S.; Hayakawa, T.; Murata, K.; Ishii, T.; Kumagai, M. Highly active methanol decomposition catalyst derived from Pd-hydrotalcite dispersed on mesoporous silica. Catal. Lett. 2000, 66, 205–213. [Google Scholar] [CrossRef]
- Liu, Y.; Suzuki, K.; Hamakawa, S.; Hayakawa, T.; Murata, K.; Ishii, T.; Kumagai, M. Catalytic methanol decomposition at low temperature over Pd catalyst derived from mesoporous silica carried Pd-hydrotalcite. Chem. Lett. 2000, 486–487. [Google Scholar] [CrossRef]
- Liu, Y.; Suzuki, K.; Hayakawa, T.; Tsunoda, T.; Suzuki, K.; Hamakawa, S.; Murata, K.; Shiozaki, R.; Ishii, T.; Kumagai, M. Steam reforming of methanol over Cu/CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts. Top. Catal. 2003, 22, 205–213. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Suzuki, K.; Hamakawa, S.; Tsunoda, T.; Ishii, T.; Kumagai, M. High active copper/ceria catalysts for the steam reforming of methanol. Appl. Catal. A Gen. 2002, 223, 137–145. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Ishii, T.; Kumagai, M.; Yasuda, H.; Suzuki, K.; Hamakawa, S.; Murata, K. Methanol decomposition to synthesis gas at low temperature over palladium support on ceria-zirconia solid solutions. Appl. Catal. A Gen. 2001, 210, 301–314. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M.; Takahara, I.; Okabe, K. Mixed synthesis of mixed alcohols from syngas over Cs- and Ni-modified Cu/CeO2 catalysts. Fuel 2013, 104, 62–69. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M.; Takahara, I.; Okabe, K. Synthesis of ethanol from syngas over Rh/Ce1−xZrxO2 catalysts. Catal. Today 2011, 164, 308–314. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Sakanishi, K. Hydroisomerization-cracking of gasoline distillate from Fischer–Tropsch synthesis over bifunctional catalysts containing Pt and heteropolyacids. Fuel 2011, 90, 3056–3065. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo-Boyás, R.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of Jatropha oil to produce green diesel over trifunctional Ni-Mo/SiO2-Al2O3 catalyst. Chem. Lett. 2009, 38, 552–553. [Google Scholar] [CrossRef]
- Liu, Y.; Koyano, G.; Misono, M. Hydroisomerization of n-hexane and n-heptane over platinum-promoted Cs2.5H0.5PW12O40 (Cs2.5) studied in comparison with several other solid acids. Top. Catal. 2000, 11, 239–246. [Google Scholar] [CrossRef]
- Liu, Y.; Na, K.; Misono, M. Skeletal isomerization of n-pentane over Pt-promoted cesium hydrogen salts of 12-tungstophosphoric acid. J. Mol. Catal. A Chem. 1999, 141, 145–153. [Google Scholar] [CrossRef]
- Liu, Y.; Misono, M. Hydroisomerization of n-butane over platinum-promoted cesium hydrogen salt of 12-tungstophosphoric acid. Materials 2009, 2, 2319–2336. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Okabe, K.; Inaba, M.; Takahara, I.; Hanaoka, T.; Sakanishi, K. Selective hydrocracking of Fischer-Tropsch waxes to high-quality diesel fuel over Pt-promoted polyoxocation-pillared montmorillonites. Top. Catal. 2009, 52, 597–608. [Google Scholar] [CrossRef]
- Liu, Y.; Koyano, G.; Na, K.; Misono, M. Isomerization of n-pentane and n-hexane over cesium hydrogen salt of 12-tungstophosphoric acid promoted by platinum. Appl. Catal. A Gen. 1998, 166, L263–L265. [Google Scholar] [CrossRef]
- Hattori, H. Heterogenous basic catalysis. Chem. Rev. 1995, 95, 537–558. [Google Scholar] [CrossRef]



| Catalyst | Conversion (%) | Yield (%) | |||
|---|---|---|---|---|---|
| C1‒C4 | C5‒C9 | C10‒C16 | CO2 | ||
| Pd/Mg(Al)O b | 76.4 | 1.4 | 3.6 | 63.5 | 4.8 |
| Mg(Al)O | 23.3 | 0.5 | 1.7 | 15.1 | 1.6 |
| H-ZSM-5 | 55.6 | 5.8 | 26.3 | 8.3 | 3.1 |
| Pd/C b | 41.7 | 1.2 | 4.1 | 26.2 | 2.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Inaba, M.; Matsuoka, K. Catalytic Deoxygenation of Hexadecyl Palmitate as a Model Compound of Euglena Oil in H2 and N2 Atmospheres. Catalysts 2017, 7, 333. https://doi.org/10.3390/catal7110333
Liu Y, Inaba M, Matsuoka K. Catalytic Deoxygenation of Hexadecyl Palmitate as a Model Compound of Euglena Oil in H2 and N2 Atmospheres. Catalysts. 2017; 7(11):333. https://doi.org/10.3390/catal7110333
Chicago/Turabian StyleLiu, Yanyong, Megumu Inaba, and Koichi Matsuoka. 2017. "Catalytic Deoxygenation of Hexadecyl Palmitate as a Model Compound of Euglena Oil in H2 and N2 Atmospheres" Catalysts 7, no. 11: 333. https://doi.org/10.3390/catal7110333





