Visible-Light Photocatalytic E to Z Isomerization of Activated Olefins and Its Application for the Syntheses of Coumarins
Abstract
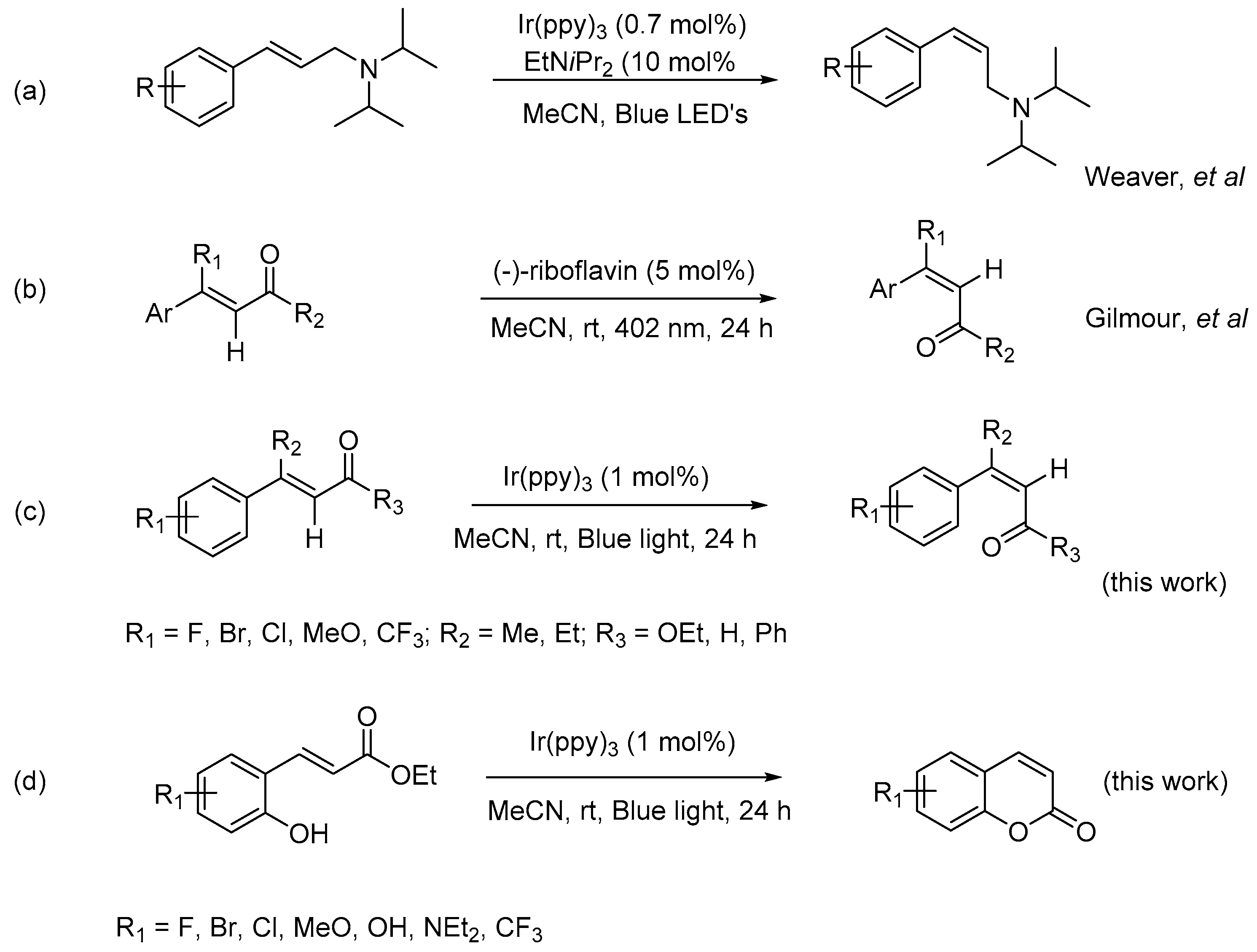
:1. Introduction
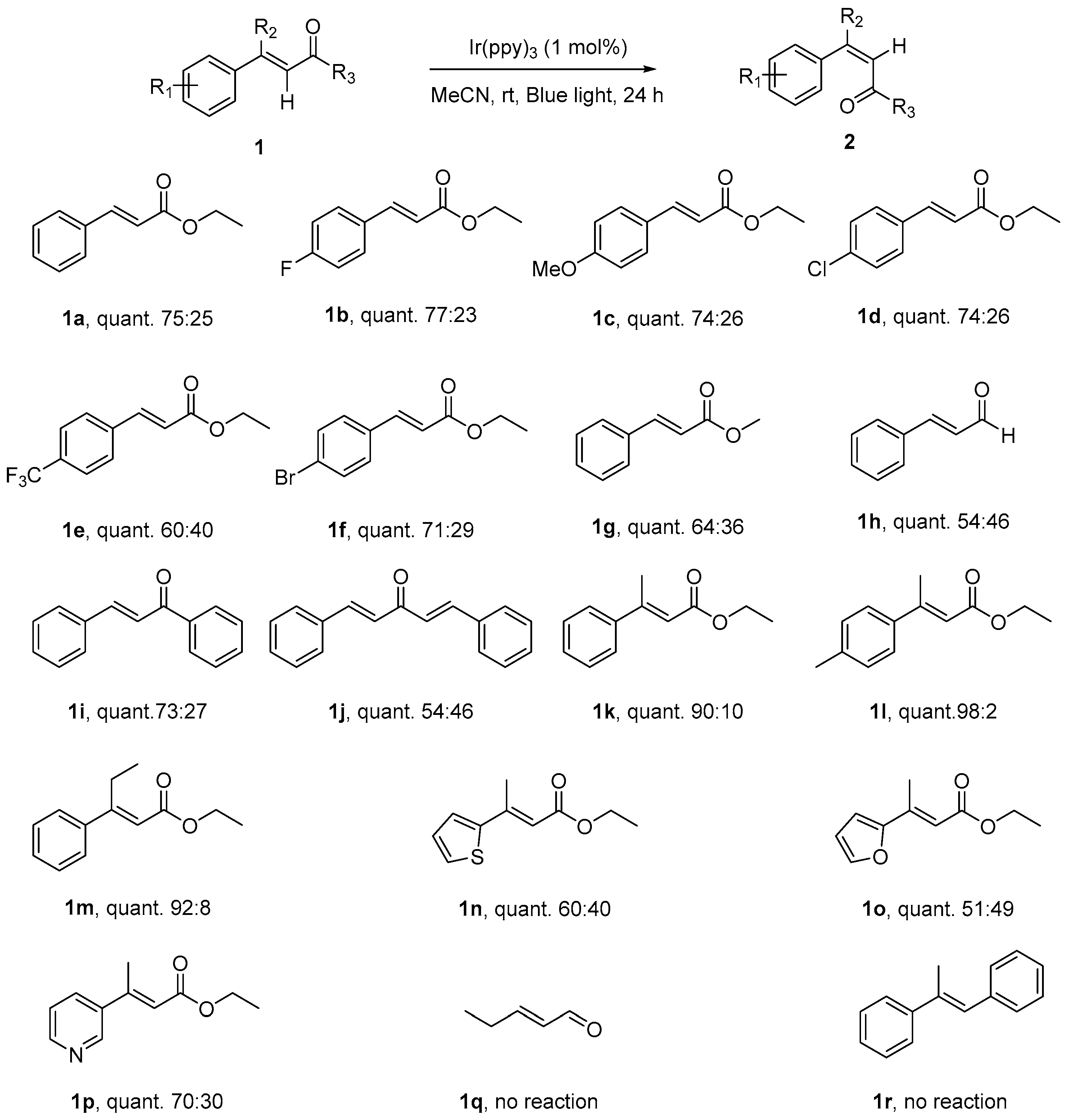
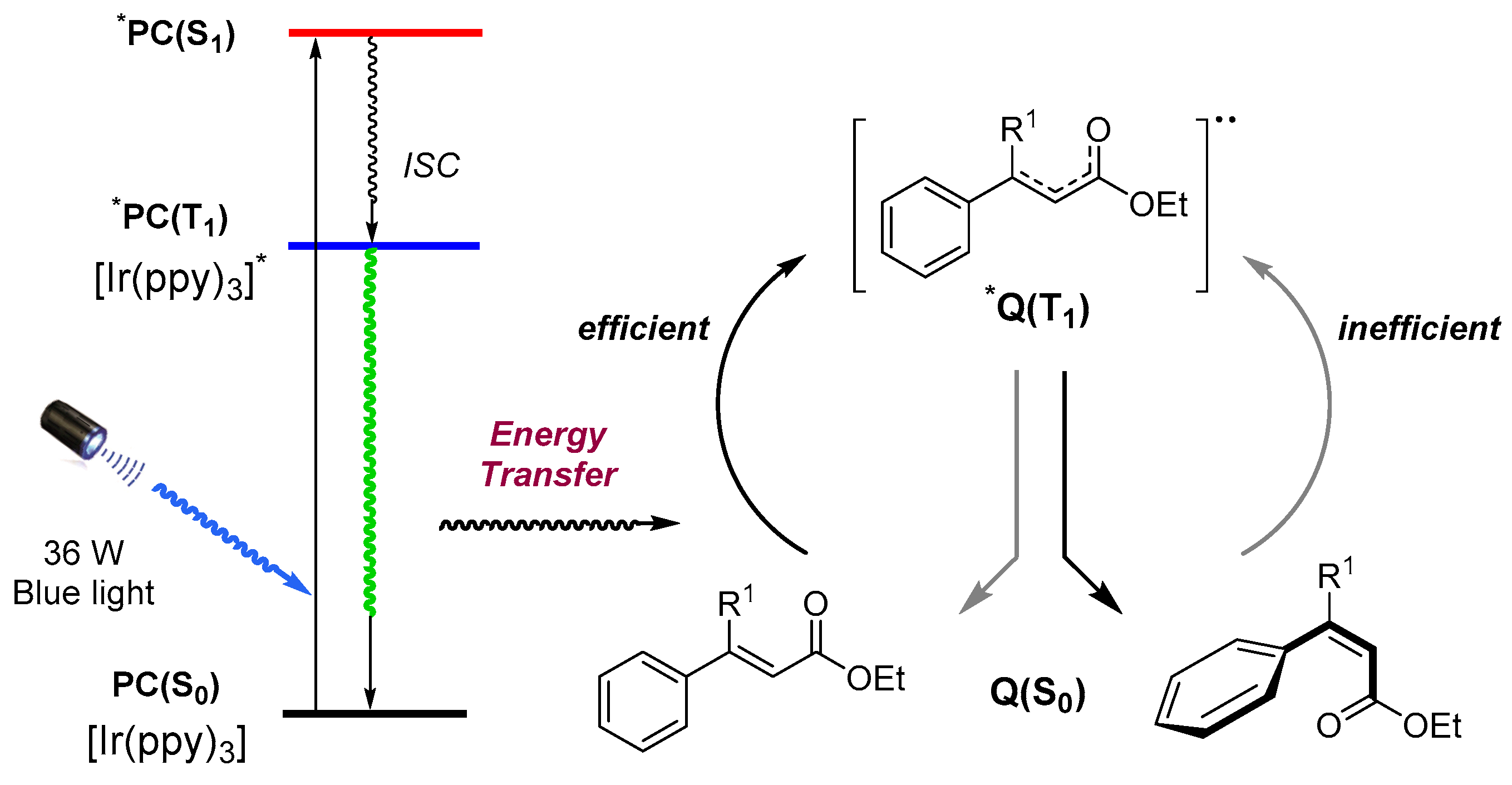
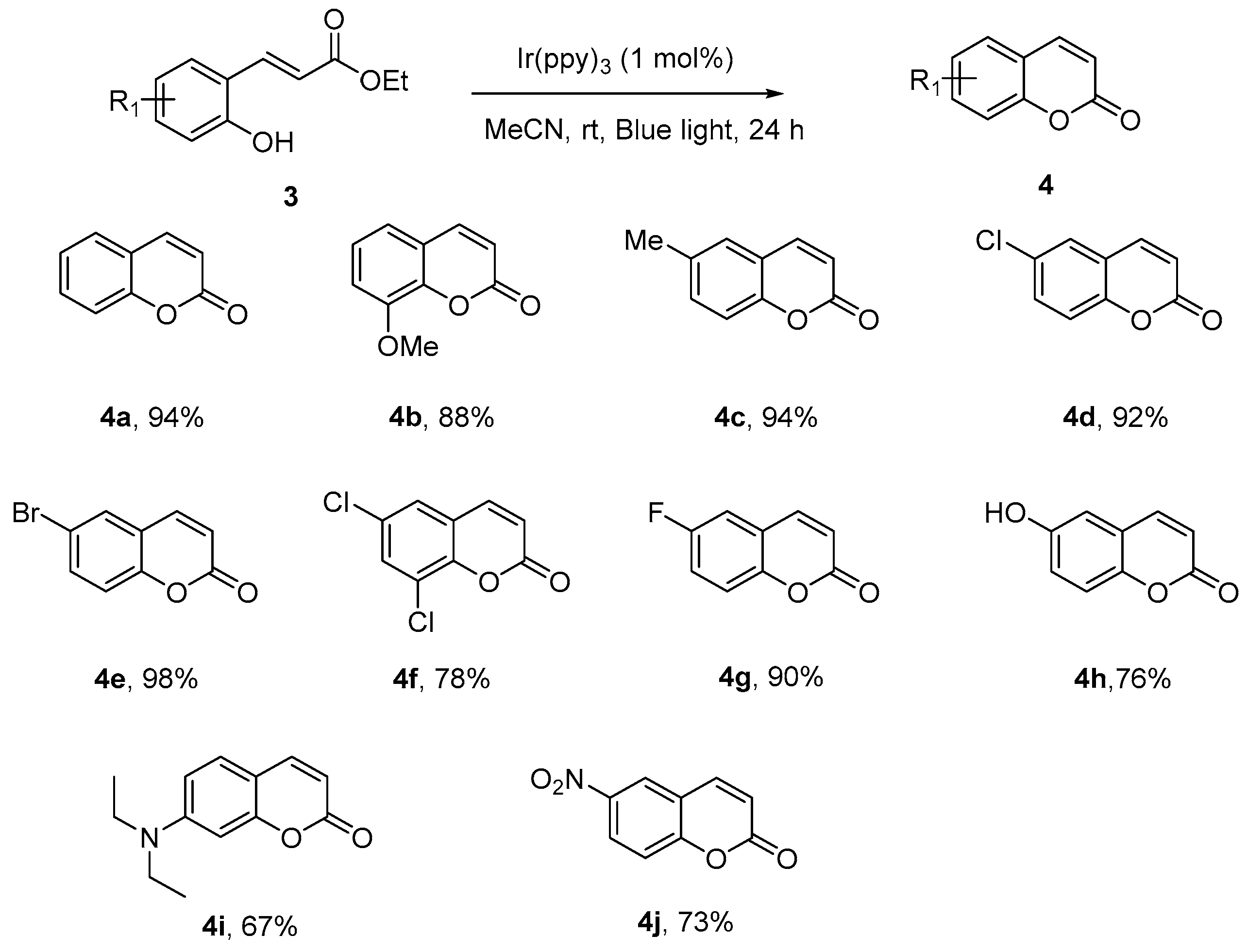
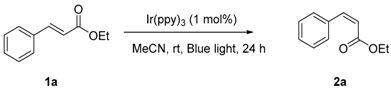
2. Results and Discussion
3. Experimental Details
3.1. General Procedure for the Isomerization of α,β-Unsaturated Carbonyl Compounds
3.2. General Procedure for the Synthesis Ortho-Hydroxycinnamates (3)
3.3. General Procedure for the Synthesis of Coumarin (4)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Snyder, J.J.; Tise, F.P.; Davis, R.D.; Kropp, P.J. Photochemistry of alkenes 7. E-reversible Z isomerization of alkenes sensitized with benzene and derivatives. J. Org. Chem. 1981, 46, 3609–3611. [Google Scholar]
- Dugave, C.; Demange, L. Cis-trans isomerization of organic molecules and biomolecules: Implications and applications. Chem. Rev. 2003, 103, 2475–2532. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Akita, M. Fine design of photoredox systems for catalytic fluoromethylation of carbon-carbon multiple bonds. Acc. Chem. Res. 2016, 49, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, K. Photoredox catalysis with visible light. Angew. Chem. Int. Ed. Engl. 2009, 48, 9785–9789. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.U.; Strakova, K.; Slanina, T.; Konig, B. Eosin Y (EY) photoredox-catalyzed sulfonylation of alkenes: Scope and mechanism. Chem. Eur. J. 2016, 22, 8694–8699. [Google Scholar] [CrossRef] [PubMed]
- Ochola, J.R.; Wolf, M.O. The effect of photocatalyst excited state lifetime on the rate of photoredox catalysis. Org. Biomol. Chem. 2016, 14, 9088–9092. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Chen, J.R.; Xiao, W.J. Controllable remote C–H bond functionalization by visible-light photocatalysis. Angew. Chem. Int. Ed. Engl. 2017, 56, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Goralski, C.T.; Singaram, B.; Rangaishenvi, M.V.; Brown, H.C. Stereospecific synthesis of pure [Z]-alkenes and [E]-alkenes from enamines via hydroboration. Abstr. Pap. Am. Chem. Soc. 1988, 195, 242. [Google Scholar]
- Hull, C.; Mortlock, S.V.; Thomas, E.J. Stereoselective synthesis of Z-alkenes from alpha-methylcrotylstannanes and aldehydes. Tetrahedron Lett. 1987, 28, 5343–5346. [Google Scholar] [CrossRef]
- Sano, S.; Takehisa, T.; Ogawa, S.; Yokoyama, K.; Nagao, Y. Stereoselective synthesis of tetrasubstituted (Z)-alkenes from aryl alkyl ketones utilizing the Horner-Wadsworth-Emmons reaction. Chem. Pharm. Bull. 2002, 50, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Siau, W.Y.; Zhang, Y.; Zhao, Y. Stereoselective synthesis of Z-alkenes. Top. Curr. Chem. 2012, 327, 33–58. [Google Scholar] [PubMed]
- Pelter, A.; Buss, D.; Colclough, E. Stereoselective synthesis of E-alkene and Z-alkene by the boron-wittig reaction. J. Chem. Soc. Chem. Commun. 1987, 297–299. [Google Scholar] [CrossRef]
- Wang, K.K.; Chu, K.H. Preparation of (Z)-alkenes, ketones, and alkynes via organoboranes—Stereoselective synthesis of the sex-pheromones of the douglas-fir tussock moth, the gypsy-moth, and the wild silkmoth antheraea polyphemus. Abstr. Pap. Am. Chem. Soc. 1984, 188, 21. [Google Scholar]
- Wang, C.B.; Yu, M.; Kyle, A.F.; Jakubec, P.; Dixon, D.J.; Schrock, R.R.; Hoveyda, A.H. Efficient and selective formation of macrocyclic disubstituted z alkenes by ring-closing metathesis (RCM) reactions catalyzed by Mo- or W-based monoaryloxide pyrrolide (MAP) complexes: Applications to total syntheses of epilachnene, yuzu lactone, ambrettolide, epothilone c, and nakadomarin a. Chem. Eur. J. 2013, 19, 2726–2740. [Google Scholar] [PubMed]
- Hepperle, S.S.; Li, Q.B.; East, A.L.L. Mechanism of cis/trans equilibration of alkenes via iodine catalysis. J. Phys. Chem. A 2005, 109, 10975–10981. [Google Scholar] [CrossRef] [PubMed]
- Guignard, R.F.; Petit, L.; Zard, S.Z. A method for the net contra-thermodynamic isomerization of cyclic trisubstituted alkenes. Org. Lett. 2013, 15, 4178–4181. [Google Scholar] [CrossRef] [PubMed]
- Metternich, J.B.; Gilmour, R. A bio-inspired, catalytic E→Z isomerization of activated olefins. J. Am. Chem. Soc. 2015, 137, 11254–11257. [Google Scholar] [CrossRef] [PubMed]
- Metternich, J.B.; Gilmour, R. Photocatalytic E to Z isomerization of alkenes. Synlett 2016, 27, 2541–2552. [Google Scholar]
- Metternich, J.B.; Artiukhin, D.G.; Holland, M.C.; von Bremen-Kühne, M.; Neugebauer, J.; Gilmour, R. Photocatalytic E→Z isomerization of polarized alkenes inspired by the visual cycle: Mechanistic dichotomy and origin of selectivity. J. Org. Chem. 2017, 82, 9955–9977. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.D.; Singh, K.; Staig, S. Facile synthesis of Z-alkenes via uphill catalysis. J. Am. Chem. Soc. 2014, 136, 5275–5278. [Google Scholar]
- Chen, X.; Qiu, S.; Wang, S.; Wang, H.; Zhai, H. Blue-light-promoted carbon-carbon double bond isomerization and its application in the syntheses of quinolines. Org. Biomol. Chem. 2017, 15, 6349–6352. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.B.; Patel, N.R.; Primer, D.N.; Jouffroy, M.; Tellis, J.C.; Molander, G.A. Preparation of visible-light-activated metal complexes and their use in photoredox/nickel dual catalysis. Nat. Protoc. 2017, 12, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Oderinde, M.S.; Varela-Alvarez, A.; Aquila, B.; Robbins, D.W.; Johannes, J.W. Effects of molecular oxygen, solvent, and light on iridium-photoredox/nickel dual-catalyzed cross-coupling reactions. J. Org. Chem. 2015, 80, 7642–7651. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.R.; Umapathy, S. Solvent effects on the structure of the triplet excited state of xanthone: A time-resolved resonance raman study. J. Raman Spectrosc. 2016, 47, 1220–1230. [Google Scholar] [CrossRef]
- Narra, S.; Shigeto, S. Direct observation of the solvent effects on the low-lying nπ and ππ* excited triplet states of acetophenone derivatives in thermal equilibrium. J. Phys. Chem. B 2015, 119, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.T.; Thomas, J.K. Laser and flash photolytic studies on effects of various solvents and solutes on excited singlet and triplet states of N,N,N’N’, tetramethyl paraphenylene diamine (TMPD). Radiat. Res. 1969, 39, 535. [Google Scholar]
- Ohashi, Y.; Kobayashi, T. Study on electronic excited-states of iridium(iii) complexes containing bipyridine and phenanthroline ligands—Solvent effect on triplet-triplet absorption-spectra. Bull. Chem. Soc. Jpn. 1979, 52, 2214–2217. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Akita, M. Visible-light radical reaction designed by Ru- and Ir-based photoredox catalysis. Inorg. Chem. Front. 2014, 1, 562–576. [Google Scholar] [CrossRef]
- Boeck, F.; Blazejak, M.; Anneser, M.R.; Hintermann, L. Cyclization of ortho-hydroxycinnamates to coumarins under mild conditions: A nucleophilic organocatalysis approach. Beilstein J. Org. Chem. 2012, 8, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, Z.; Li, Y.; Wu, X.F. Iridium-catalyzed and ligand-controlled carbonylative synthesis of flavones from simple phenols and internal alkynes. Chem. Eur. J. 2017, 23, 3276–3279. [Google Scholar] [CrossRef] [PubMed]
- Metternich, J.B.; Gilmour, R. One photocatalyst, n activation modes strategy for cascade catalysis: Emulating coumarin biosynthesis with (–)-riboflavin. J. Am. Chem. Soc. 2016, 138, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Horaguchi, T.; Hosokawa, N.; Tanemura, K.; Suzuki, T. Photocyclization reactions. Part 8 [1]. Synthesis of 2-quinolone, quinoline and cournarin derivatives using trans-cis isomerization by photoreaction. J. Heterocycl. Chem. 2002, 39, 61–67. [Google Scholar] [CrossRef]
| Entry | Catalyst | Loading of Cat. (mol %) | Solvent | Other Conditions | Z/E Ratio 2 |
|---|---|---|---|---|---|
| 1 | fac-Ir(ppy)3 | 5 | MeCN | - | 65:35 |
| 2 | none | 5 | MeCN | - | 1:99 |
| 3 | Ru(bpy)3Cl2 | 5 | MeCN | - | 18:82 |
| 4 | Ir(ppy)2(dtbbpy)+ | 5 | MeCN | - | 31:69 |
| 5 | (−)-riboflavin | 5 | MeCN | - | 8:92 |
| 6 | Rose bengal | 5 | MeCN | - | 6:94 |
| 7 | fac-Ir(ppy)3 | 5 | acetone | - | 37:63 |
| 8 | fac-Ir(ppy)3 | 5 | DMF | - | 2:98 |
| 9 | fac-Ir(ppy)3 | 5 | DCM | - | 2:98 |
| 10 | fac-Ir(ppy)3 | 5 | THF | - | 2:98 |
| 11 | fac-Ir(ppy)3 | 3 | MeCN | - | 68:32 |
| 12 | fac-Ir(ppy)3 | 1 | MeCN | - | 75:25 |
| 13 | fac-Ir(ppy)3 | 1 | MeCN | DIPEA | 72:28 |
| 14 | fac-Ir(ppy)3 | 1 | MeCN | degassed | 73:27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, K.; Li, Y. Visible-Light Photocatalytic E to Z Isomerization of Activated Olefins and Its Application for the Syntheses of Coumarins. Catalysts 2017, 7, 337. https://doi.org/10.3390/catal7110337
Zhan K, Li Y. Visible-Light Photocatalytic E to Z Isomerization of Activated Olefins and Its Application for the Syntheses of Coumarins. Catalysts. 2017; 7(11):337. https://doi.org/10.3390/catal7110337
Chicago/Turabian StyleZhan, Kun, and Yi Li. 2017. "Visible-Light Photocatalytic E to Z Isomerization of Activated Olefins and Its Application for the Syntheses of Coumarins" Catalysts 7, no. 11: 337. https://doi.org/10.3390/catal7110337




