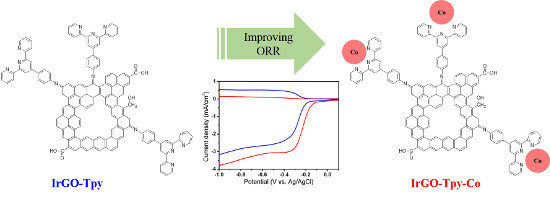
Terpyridine-Containing Imine-Rich Graphene for the Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results
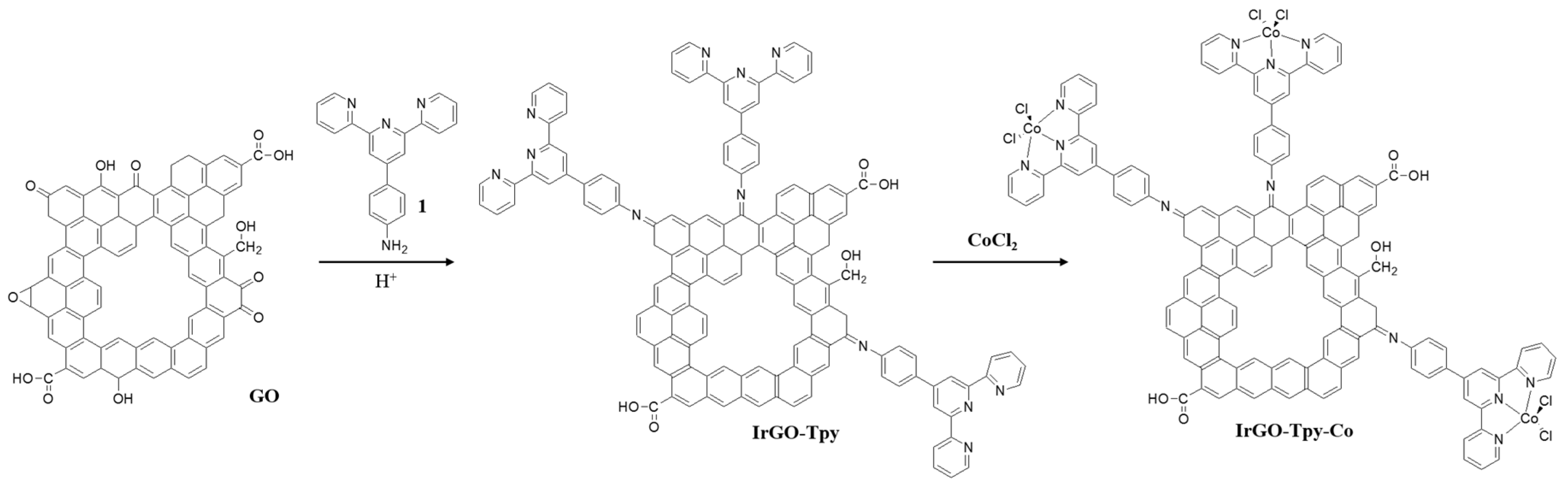
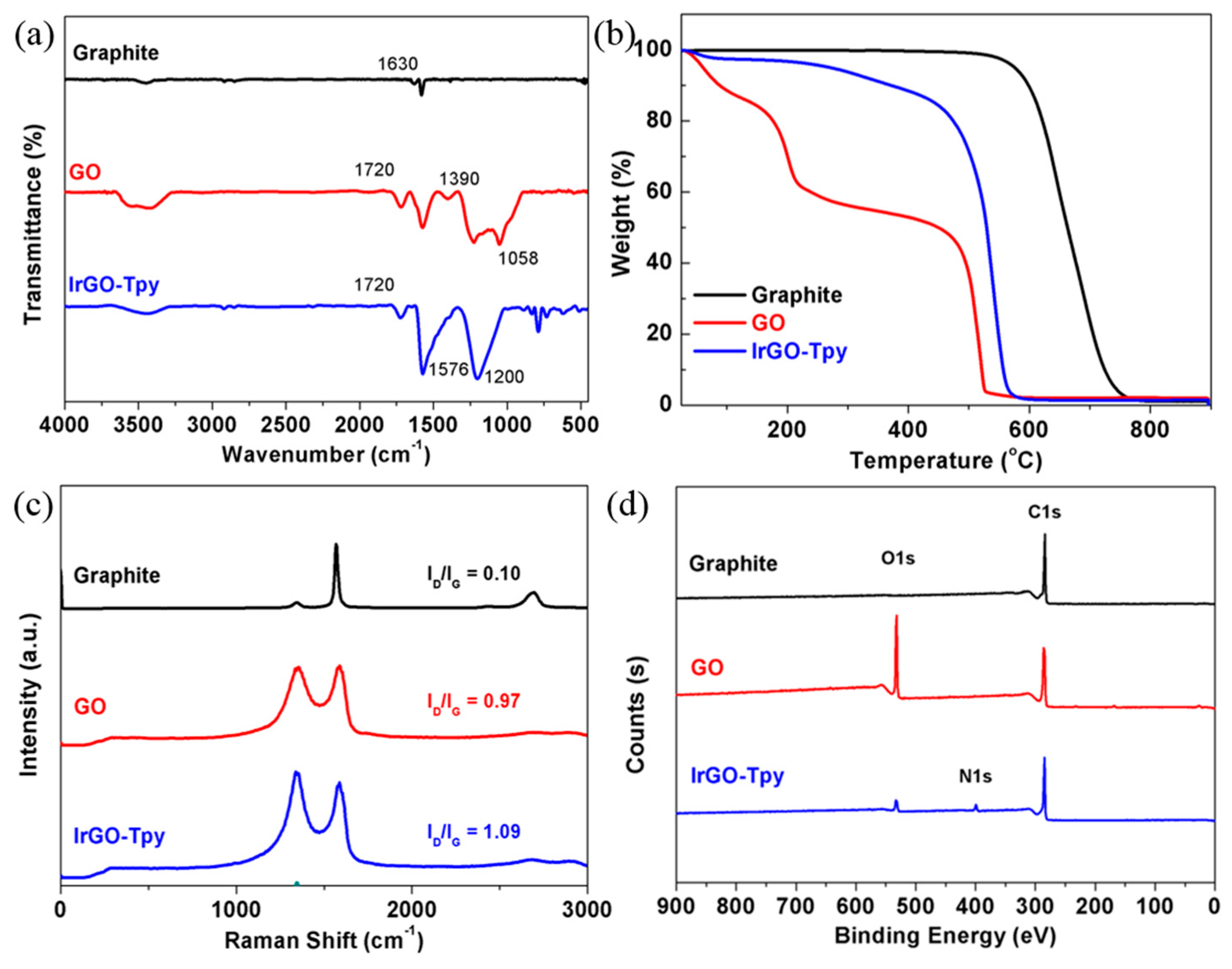
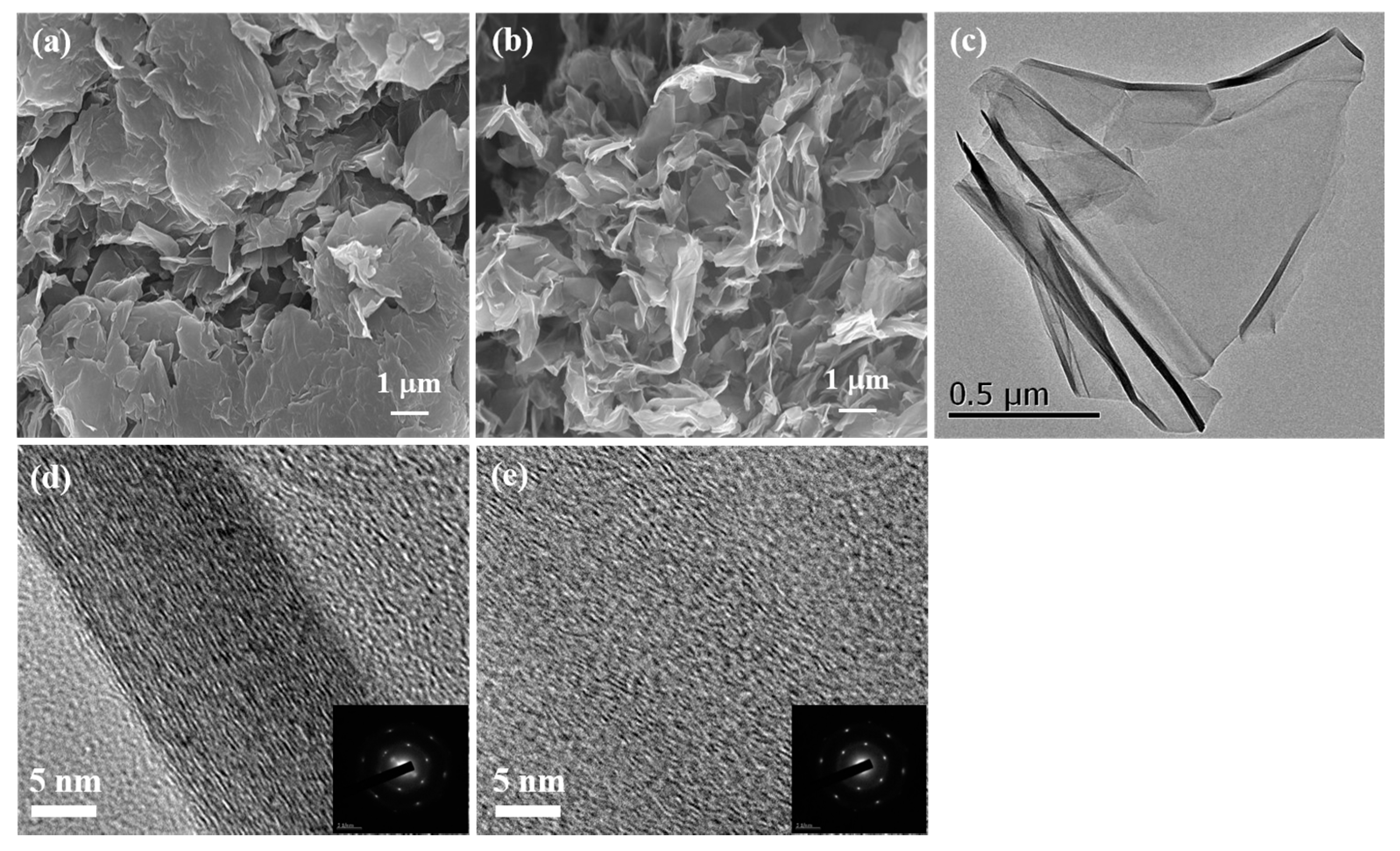
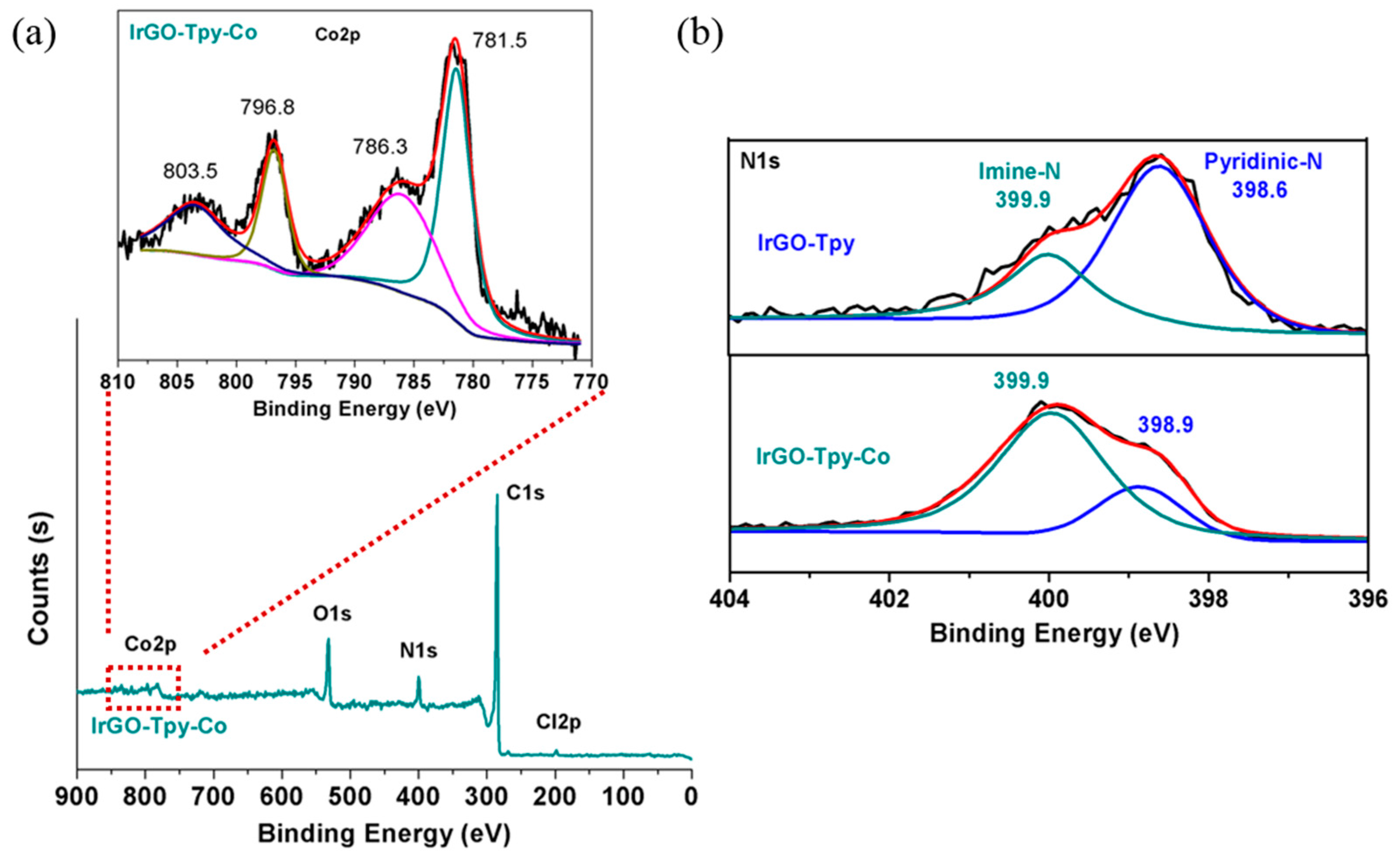
2.1. Synthesis and Characterization
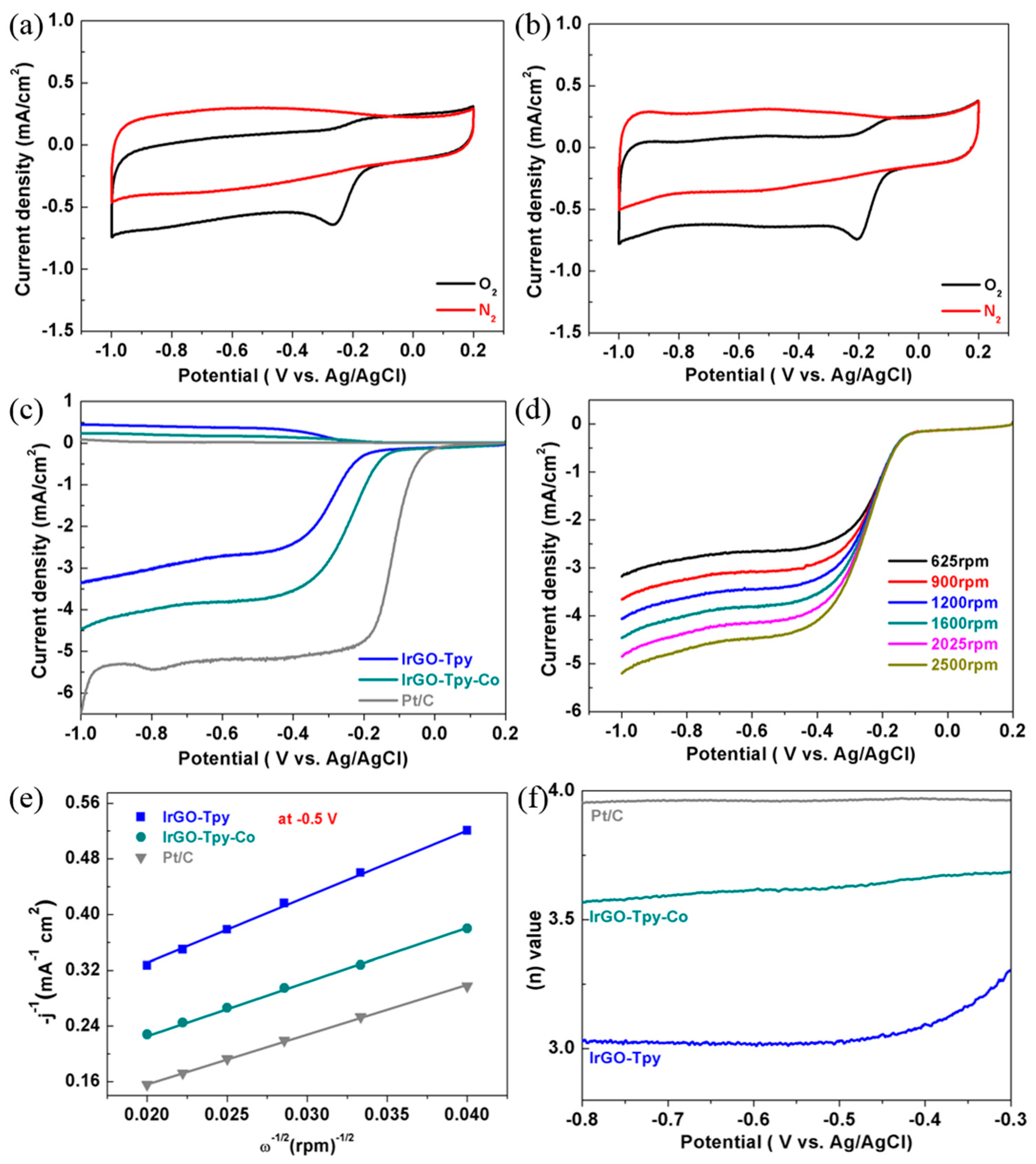
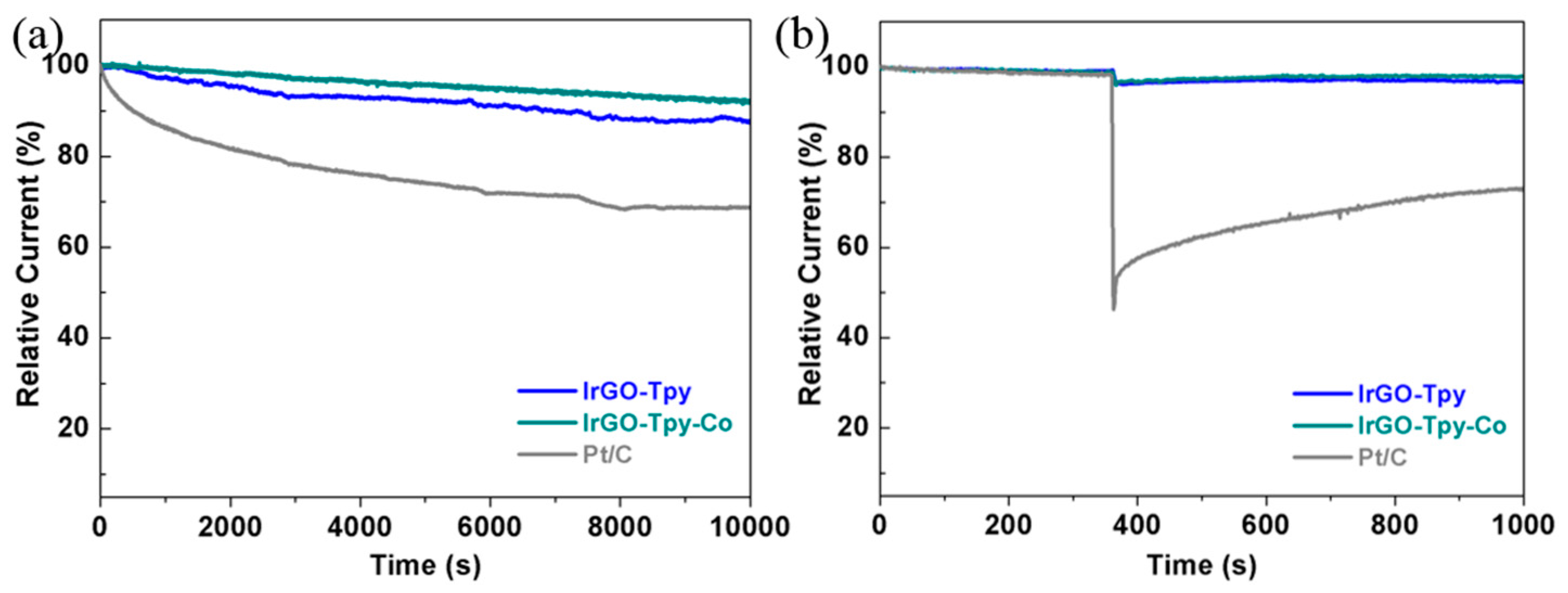
2.2. Electrochemical and ORR Characteristics
3. Materials and Methods
3.1. Synthesis of IrGO-Tpy and IrGO-Tpy-Co
3.2. Characterization and Electrochemical Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Porter, N.S.; Wu, H.; Quan, Z.; Fang, J. Shape-control and electrocatalytic activity-enhancement of pt-based bimetallic nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Acres, G.J. Recent advances in fuel cell technology and its applications. J. Power Sources 2001, 100, 60–66. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Sealy, C. The problem with platinum. Mater. Today 2008, 11, 65–68. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for pem fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Tong, X.; Wei, Q.; Zhan, X.; Zhang, G.; Sun, S. The new graphene family materials: Synthesis and applications in oxygen reduction reaction. Catalysts 2017, 7, 1. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-doped carbon nanotube and graphene materials for oxygen reduction reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Zhang, S.; Zhang, L.; Choi, H.J.; Seo, J.M.; Xia, Z.; Dai, L.; Baek, J.B. Edge-selectively sulfurized graphene nanoplatelets as efficient metal-free electrocatalysts for oxygen reduction reaction: The electron spin effect. Adv. Mater. 2013, 25, 6138–6145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Lee, M.S.; Whang, D.R.; Choi, H.-J.; Yang, M.H.; Kim, B.-G.; Baek, J.-B.; Chang, D.W. A facile approach to tailoring electrocatalytic activities of imine-rich nitrogen-doped graphene for oxygen reduction reaction. Carbon 2017, 122, 515–523. [Google Scholar] [CrossRef]
- Chang, D.W.; Choi, H.-J.; Baek, J.-B. Wet-chemical nitrogen-doping of graphene nanoplatelets as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. 2015, 3, 7659–7665. [Google Scholar] [CrossRef]
- Zhan, Y.; Huang, J.; Lin, Z.; Yu, X.; Zeng, D.; Zhang, X.; Xie, F.; Zhang, W.; Chen, J.; Meng, H. Iodine/nitrogen co-doped graphene as metal free catalyst for oxygen reduction reaction. Carbon 2015, 95, 930–939. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, S.H.; Woo, S.I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 2012, 6, 7084–7091. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, A.; Nabae, Y.; Okajima, T.; Ohsaka, T. Kinetic approach to investigate the mechanistic pathways of oxygen reduction reaction on Fe-containing n-doped carbon catalysts. ACS Catal. 2015, 5, 5194–5202. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Q.; Lv, Z.; Dong, H.; Yu, J.; Dong, L. Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions. Int. J. Hydrogen Energy 2013, 38, 1413–1418. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, J.P.; Collin, J.P.; Chambron, J.C.; Guillerez, S.; Coudret, C.; Balzani, V.; Barigelletti, F.; De Cola, L.; Flamigni, L. Ruthenium(II) and osmium(II) bis (terpyridine) complexes in covalently-linked multicomponent systems: Synthesis, electrochemical behavior, absorption spectra, and photochemical and photophysical properties. Chem. Rev. 1994, 94, 993–1019. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Song, S.; Xue, Y.; Feng, L.; Elbatal, H.; Wang, P.; Moorefield, C.N.; Newkome, G.R.; Dai, L. Reversible self-assembly of terpyridine-functionalized graphene oxide for energy conversion. Angew. Chem. Int. Ed. 2014, 53, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Andres, P.R.; Schubert, U.S. New functional polymers and materials based on 2, 2′: 6′, 2″-terpyridine metal complexes. Adv. Mater. 2004, 16, 1043–1068. [Google Scholar] [CrossRef]
- Schubert, U.S.; Alexeev, A.; Andres, P.R. Terpyridine-functionalized tentagel microbeads: Synthesis, metal chelation and first sequential complexation. Macromol. Mater. Eng. 2003, 288, 852–860. [Google Scholar] [CrossRef]
- Chang, D.W.; Lee, E.K.; Park, E.Y.; Yu, H.; Choi, H.-J.; Jeon, I.-Y.; Sohn, G.-J.; Shin, D.; Park, N.; Oh, J.H. Nitrogen-doped graphene nanoplatelets from simple solution edge-functionalization for n-type field-effect transistors. J. Am. Chem. Soc. 2013, 135, 8981–8988. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of n-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, D.; Wang, Y.; Hou, B. Electrocatalytic activity of nitrogen-doped graphene synthesized via a one-pot hydrothermal process towards oxygen reduction reaction. J. Power Sources 2013, 227, 185–190. [Google Scholar] [CrossRef]
- Yang, J.; Jo, M.R.; Kang, M.; Huh, Y.S.; Jung, H.; Kang, Y.-M. Rapid and controllable synthesis of nitrogen doped reduced graphene oxide using microwave-assisted hydrothermal reaction for high power-density supercapacitors. Carbon 2014, 73, 106–113. [Google Scholar] [CrossRef]
- Zhang, H.; Kuila, T.; Kim, N.H.; Yu, D.S.; Lee, J.H. Simultaneous reduction, exfoliation, and nitrogen doping of graphene oxide via a hydrothermal reaction for energy storage electrode materials. Carbon 2014, 69, 66–78. [Google Scholar] [CrossRef]
- Lee, M.S.; Choi, H.-J.; Baek, J.-B.; Chang, D.W. Simple solution-based synthesis of pyridinic-rich nitrogen-doped graphene nanoplatelets for supercapacitors. Appl. Energy 2017, 195, 1071–1078. [Google Scholar] [CrossRef]
- Schubert, U.S.; Eschbaumer, C. Macromolecules containing bipyridine and terpyridine metal complexes: Towards metallosupramolecular polymers. Angew. Chem. Int. Ed. 2002, 41, 2892–2926. [Google Scholar] [CrossRef]
- McGuire, R., Jr.; Dogutan, D.K.; Teets, T.S.; Suntivich, J.; Shao-Horn, Y.; Nocera, D.G. Oxygen reduction reactivity of cobalt(II) hangman porphyrins. Chem. Sci. 2010, 1, 411–414. [Google Scholar] [CrossRef]
- Tang, H.; Yin, H.; Wang, J.; Yang, N.; Wang, D.; Tang, Z. Molecular architecture of cobalt porphyrin multilayers on reduced graphene oxide sheets for high-performance oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 125, 5695–5699. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Vignesh, R.H.; Vasylechko, L.; Lee, Y.; Selvan, R.K. Synthesis and electrochemical performance of Co2TiO4 and its core–shell structure of Co2TiO4@C as negative electrodes for li-ion batteries. RSC Adv. 2016, 6, 69016–69026. [Google Scholar] [CrossRef]
- Jin, S.; Yang, G.; Song, H.; Cui, H.; Wang, C. Ultrathin hexagonal 2D Co2GeO4 nanosheets: Excellent Li-storage performance and ex situ investigation of electrochemical mechanism. ACS Appl. Mater. Interfaces 2015, 7, 24932–24943. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Sakamoto, R.; Yi, S.-T.; Katagiri, S.; Kambe, T.; Nishihara, H. Electrochromic bis (terpyridine) metal complex nanosheets. J. Am. Chem. Soc. 2015, 137, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Higgins, D.; Chen, Z. Nitrogen doped carbon nanotubes and their impact on the oxygen reduction reaction in fuel cells. Carbon 2010, 48, 3057–3065. [Google Scholar] [CrossRef]
- Koohmareh, G.A.; Sharifi, M. Synthesis, characterization, and coordination behavior of copoly (styrene-maleimide) functionalized with terpyridine. J. Appl. Polym. Sci. 2010, 116, 179–183. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.S.; Yang, M.H.; Park, J.S.; Chang, D.W. Terpyridine-Containing Imine-Rich Graphene for the Oxygen Reduction Reaction. Catalysts 2017, 7, 338. https://doi.org/10.3390/catal7110338
Lee MS, Yang MH, Park JS, Chang DW. Terpyridine-Containing Imine-Rich Graphene for the Oxygen Reduction Reaction. Catalysts. 2017; 7(11):338. https://doi.org/10.3390/catal7110338
Chicago/Turabian StyleLee, Min Seok, Mun Ho Yang, Jong S. Park, and Dong Wook Chang. 2017. "Terpyridine-Containing Imine-Rich Graphene for the Oxygen Reduction Reaction" Catalysts 7, no. 11: 338. https://doi.org/10.3390/catal7110338