Intrinsic Activity of MnOx-CeO2 Catalysts in Ethanol Oxidation
Abstract
:1. Introduction
2. Results
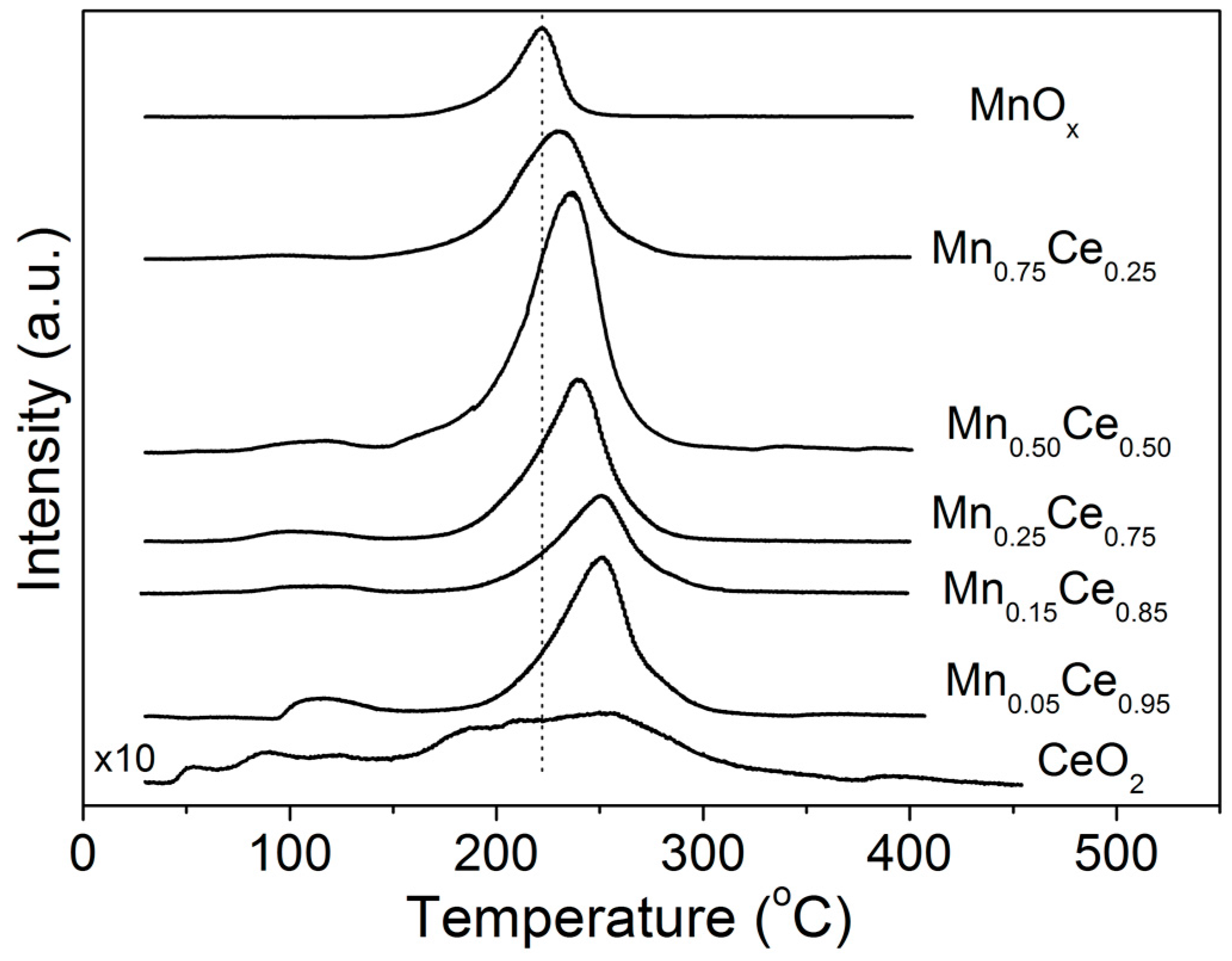
2.1. TPD of Ethanol
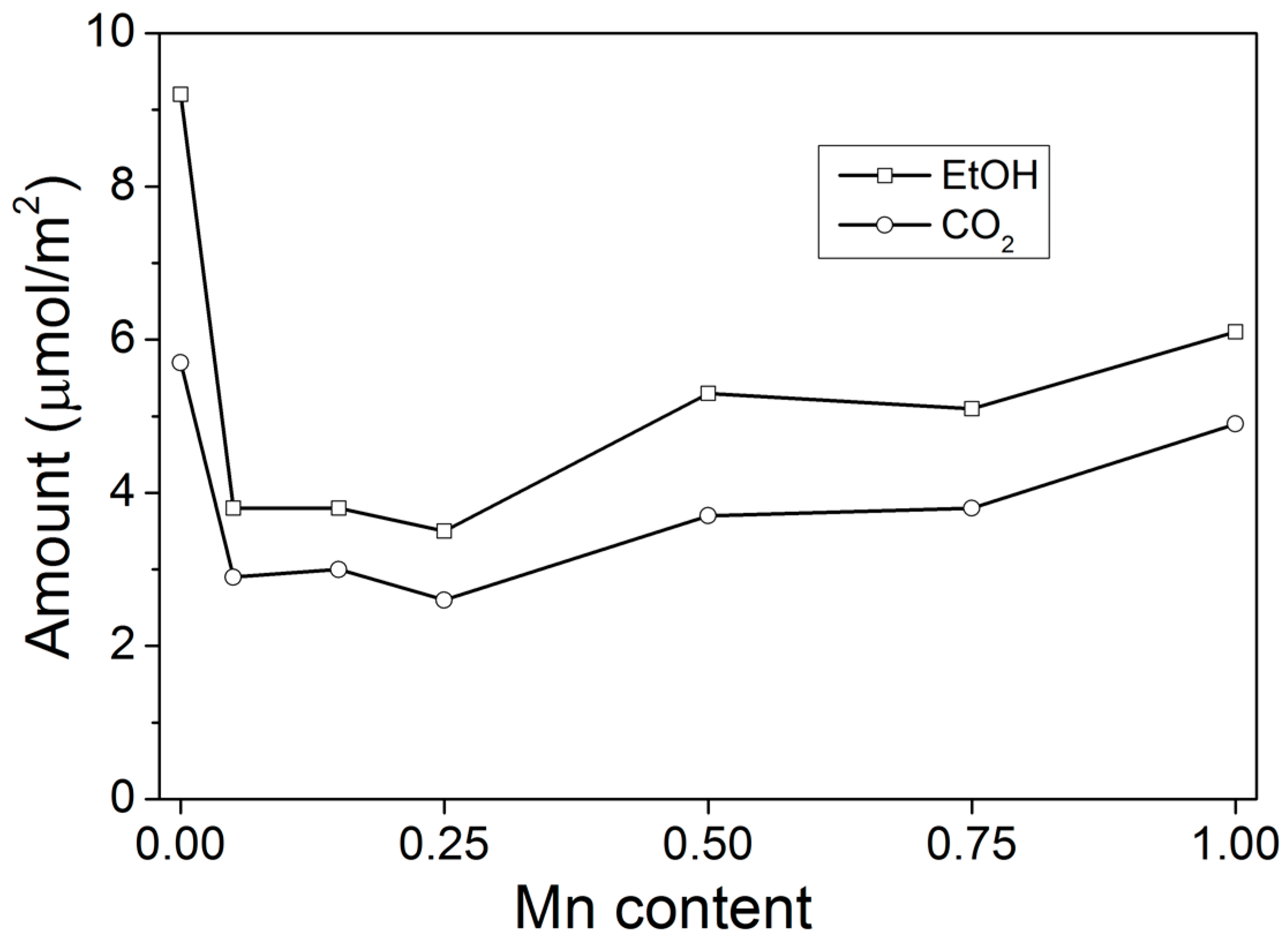
2.2. Temperature-Programmed Oxidation (TPO) of Ethanol
3. Discussion
4. Materials and Methods
5. Conclusions
- A decrease of the concentration of active sites, on the hypothesis that the amount of adsorbed ethanol and produced CO2 during TPO are proper probes for counting these sites
- Doubling of TOF for ethanol oxidation.
- A decrease of the concentration of active sites
- A decrease of TOF for ethanol oxidation by ~50%.
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Spivey, J.J. Complete catalytic oxidation of volatile organics. Ind. Eng. Chem. Res. 1987, 27, 2165–2180. [Google Scholar] [CrossRef]
- Cordi, E.M.; Falconer, J.L. Oxidation of volatile organic compounds on Al2O3, Pd/Al2O3, and PdO/Al2O3 catalysts. J. Catal. 1996, 162, 104–117. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Oikonomopoulos, E.; Kanistras, D.; Ioannides, T. Complete oxidation of ethanol over alkali-promoted Pt/Al2O3 catalysts. Appl. Catal. B Environ. 2006, 65, 62–69. [Google Scholar] [CrossRef]
- Larsson, P.O.; Andersson, A. Complete oxidation of CO, ethanol, and ethyl acetate over copper oxide supported on Titania and ceria modified Titania. J. Catal. 1998, 179, 72–89. [Google Scholar] [CrossRef]
- Kamal, M.S.; Shaikh, A.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Xu, H.; Yan, N.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.; Zhao, S. Gaseous heterogeneous catalytic reactions over Mn-Based Oxides for environmental applications: A critical review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef] [PubMed]
- Delimaris, D.; Ioannides, T. VOC oxidation over MnOx-CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2008, 84, 303–312. [Google Scholar] [CrossRef]
- Lin, P.; Luo, M.-F.; Xin, Q.; Sun, G.-Q. The mechanism studies of ethanol oxidation on PdO catalysts by TPSR techniques. Catal. Lett. 2004, 93, 139–144. [Google Scholar] [CrossRef]
- Idriss, H.; Diagne, C.; Hindermann, J.P.; Kiennemann, A.; Barteau, M.A. Reactions of acetaldehyde on CeO2 and CeO2-Supported catalysts. J. Catal. 1995, 155, 219–237. [Google Scholar] [CrossRef]
- Yee, A.; Morrison, S.J.; Idriss, H. A study of the reactions of ethanol on CeO2 and Pd/CeO2 by steady state reactions, temperature programmed desorption, and in situ FT-IR. J. Catal. 1999, 186, 279–295. [Google Scholar] [CrossRef]
- Yee, A.; Morrison, S.J.; Idriss, H. A study of ethanol reactions over Pt/CeO2 by temperature-programmed desorption and in situ FT-IR spectroscopy: Evidence of benzene formation. J. Catal. 2000, 191, 30–45. [Google Scholar] [CrossRef]
- De Lima, S.M.; da Silva, A.M.; da Costa, L.O.O.; Graham, U.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Study of catalyst deactivation and reaction mechanism of steam reforming, partial oxidation, and oxidative steam reforming of ethanol over Co/CeO2 catalyst. J. Catal. 2009, 268, 268–281. [Google Scholar] [CrossRef]
- Li, H.; Qi, G.; Tana; Zhang, X.; Li, W.; Shen, W. Morphological impact of manganese–cerium oxides on ethanol oxidation. Catal. Sci. Technol. 2011, 1, 1677–1682. [Google Scholar] [CrossRef]
- Li, H.; Qi, G.; Tana; Zhang, X.; Huang, X.; Li, W.; Shen, W. Low-temperature oxidation of ethanol over a Mn0.6Ce0.4O2 mixed oxide. Appl. Catal. B Environ. 2011, 103, 54–61. [Google Scholar] [CrossRef]
- Liu, G.; Yue, R.; Jia, Y.; Ni, Y.; Yang, J.; Liu, H.; Wang, Z.; Wu, X.; Chen, Y. Catalytic oxidation of benzene over Ce–Mn oxides synthesized by flame spray pyrolysis. Particuology 2013, 11, 454–459. [Google Scholar] [CrossRef]
- Liao, Y.; Fu, M.; Chen, L.; Wu, J.; Huang, B.; Ye, D. Catalytic oxidation of toluene over nanorod-structured Mn–Ce mixed oxides. Catal. Today 2013, 216, 220–228. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Liu, G.; Li, S.; Li, D.; Li, W.; Chen, Y. Preparation of hierarchical layer-stacking Mn-Ce composite oxide for catalytic total oxidation of VOCs. J. Rare Earths 2015, 33, 62–69. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Li, W.; Chen, Y. Porous Mn-Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun. 2014, 56, 134–138. [Google Scholar] [CrossRef]
- Picasso, G.; Cruz, R.; del Rosario Sun Kou, M. Preparation by co-precipitation of Ce-Mn based catalysts for combustion of n-hexane. Mater. Res. Bull. 2015, 70, 621–632. [Google Scholar] [CrossRef]
- Chojnacka, A.; Molenda, M.; Chmielarz, L.; Piwowarska, Z.; Gajewska, M.; Dudek, B.; Dziembaj, R. Ceria based novel nanocomposites catalysts MnxCe1−x O2/α-Al2O3 for low-temperature combustion of methanol. Catal. Today 2015, 257, 104–110. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.; Wang, M.; Xie, Z.; Chen, H.; Shi, J. Highly active MnOx–CeO2 catalyst for diesel soot combustion. RSC Adv. 2017, 7, 3233–3239. [Google Scholar] [CrossRef]
- Colman-Lerner, E.; Peluso, M.A.; Sambeth, J.; Thomas, H. Cerium, manganese and cerium/manganese ceramic monolithic catalysts. Study of VOCs and PM removal. J. Rare Earths 2016, 34, 675–682. [Google Scholar] [CrossRef]
- Pintos, D.G.; Juan, A.; Irigoyen, B. Mn-Doped CeO2: DFT+U Study of a Catalyst for Oxidation Reactions. J. Phys. Chem. C 2013, 117, 18063–18073. [Google Scholar] [CrossRef]
- Vannice, M.A. Kinetics of Catalytic Reactions, 1st ed.; Springer: New York, NY, USA, 2005; pp. 6–8. ISBN 978-1-4419-3758-2. [Google Scholar]
- Sheng, P.-Y.; Bowmaker, G.A.; Idriss, H. The Reactions of Ethanol over Au/CeO2. Appl. Catal. A Gen. 2004, 261, 171–181. [Google Scholar] [CrossRef]
- Machida, M.; Uto, M.; Kurogi, D.; Kijima, T. MnOx-CeO2 Binary Oxides for Catalytic NOx Sorption at Low Temperatures. Sorptive Removal of NOx. Chem. Mater. 2000, 12, 3158–3164. [Google Scholar] [CrossRef]
- Chen, H.; Sayari, A.; Adnot, A.; Larachi, F. Composition–activity effects of Mn-Ce-O composites on phenol catalytic wet oxidation. Appl. Catal. B Environ. 2001, 32, 195–204. [Google Scholar] [CrossRef]
- Picasso, G.; Gutiérrez, M.; Pina, M.P.; Herguido, J. Preparation and characterization of Ce-Zr and Ce-Mn based oxides for n-hexane combustion: Application to catalytic membrane reactors. Chem. Eng. J. 2007, 126, 119–130. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Yang, C.; Yu, X.; Heißler, S.; Nefedov, A.; Colussi, S.; Llorca, J.; Trovarelli, A.; Wang, Y.; Wöll, C. Surface Faceting and Reconstruction of Ceria Nanoparticles. Angew. Chem. Int. Ed. 2017, 56, 375–379. [Google Scholar] [CrossRef] [PubMed]
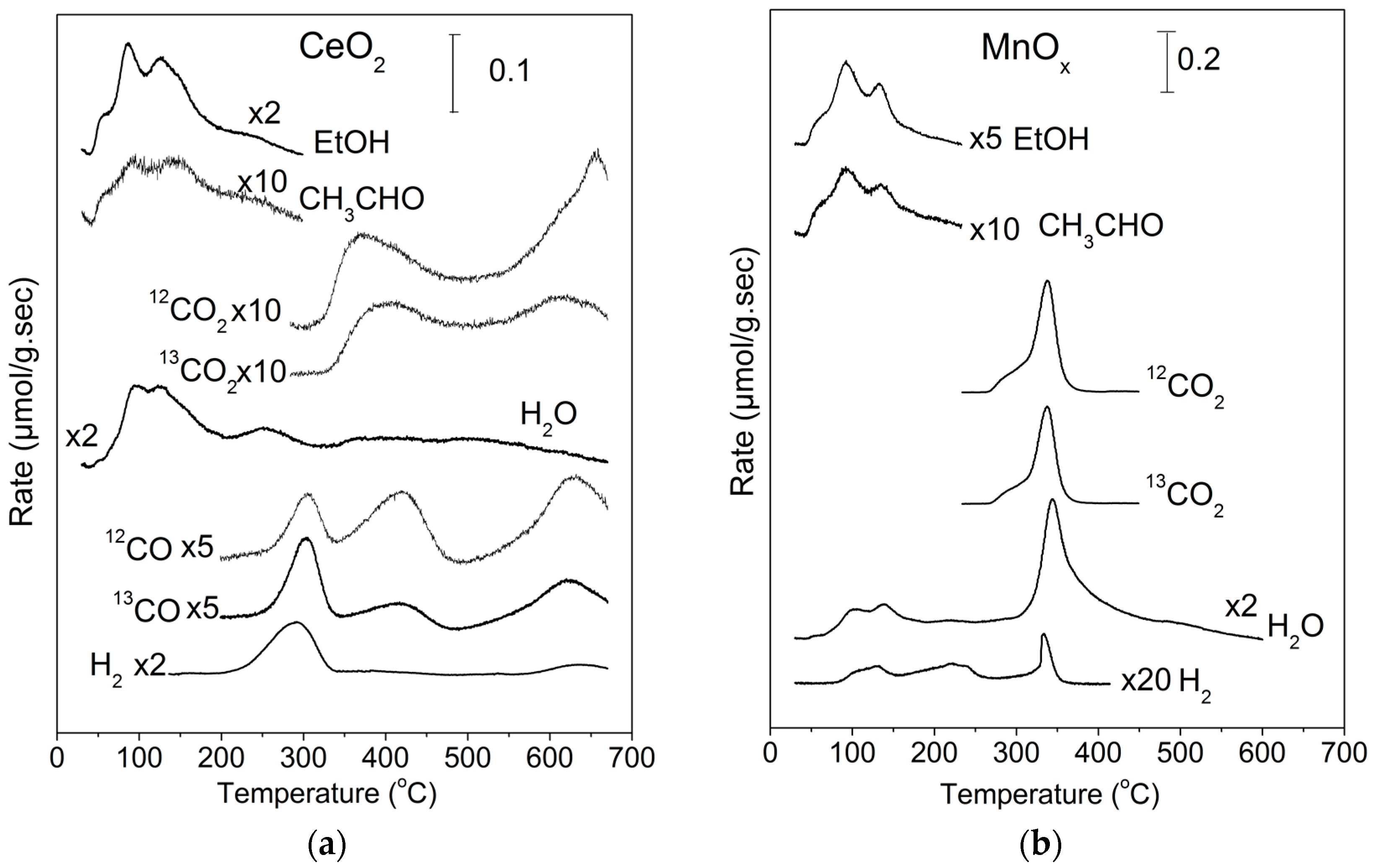
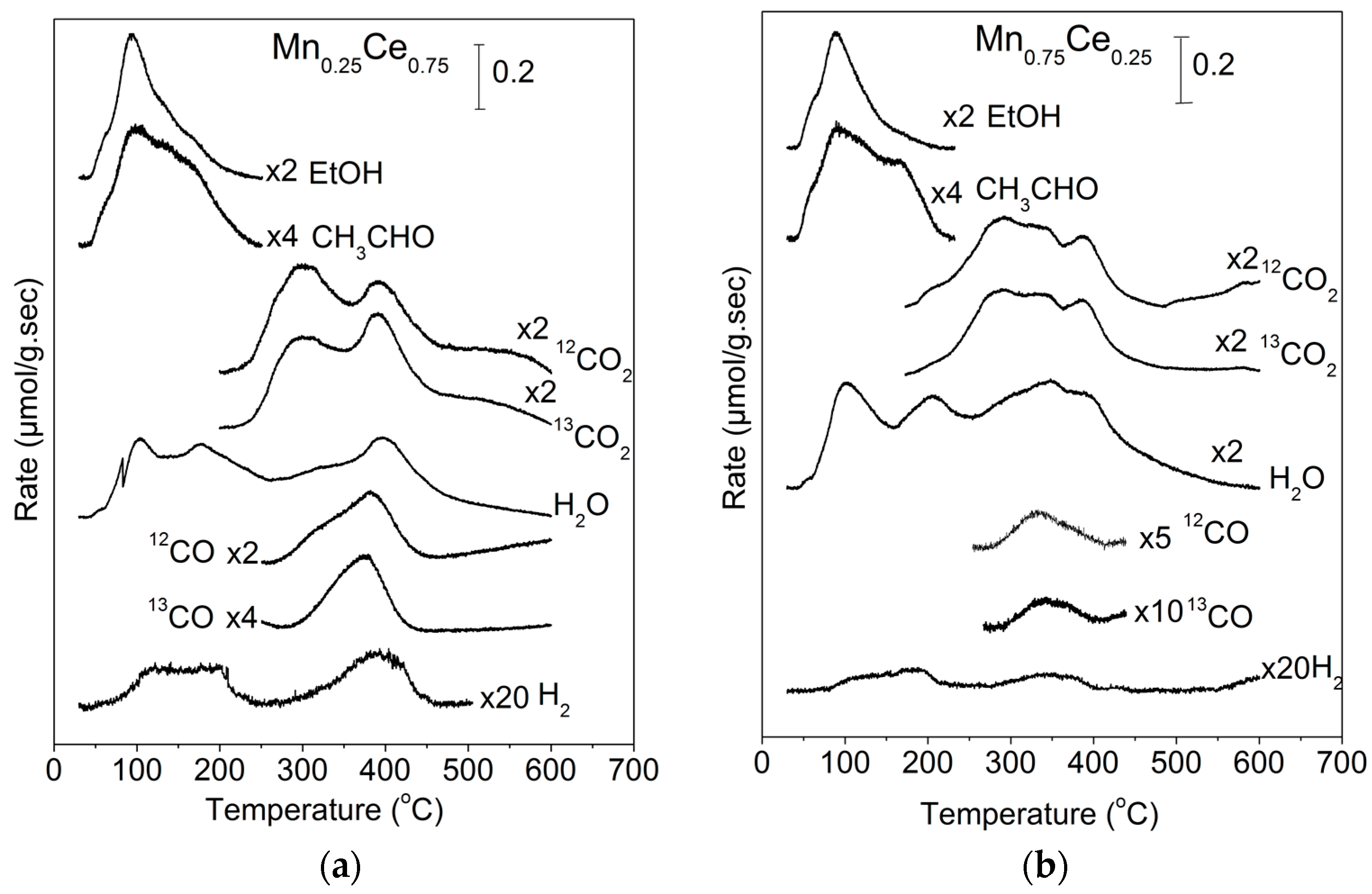
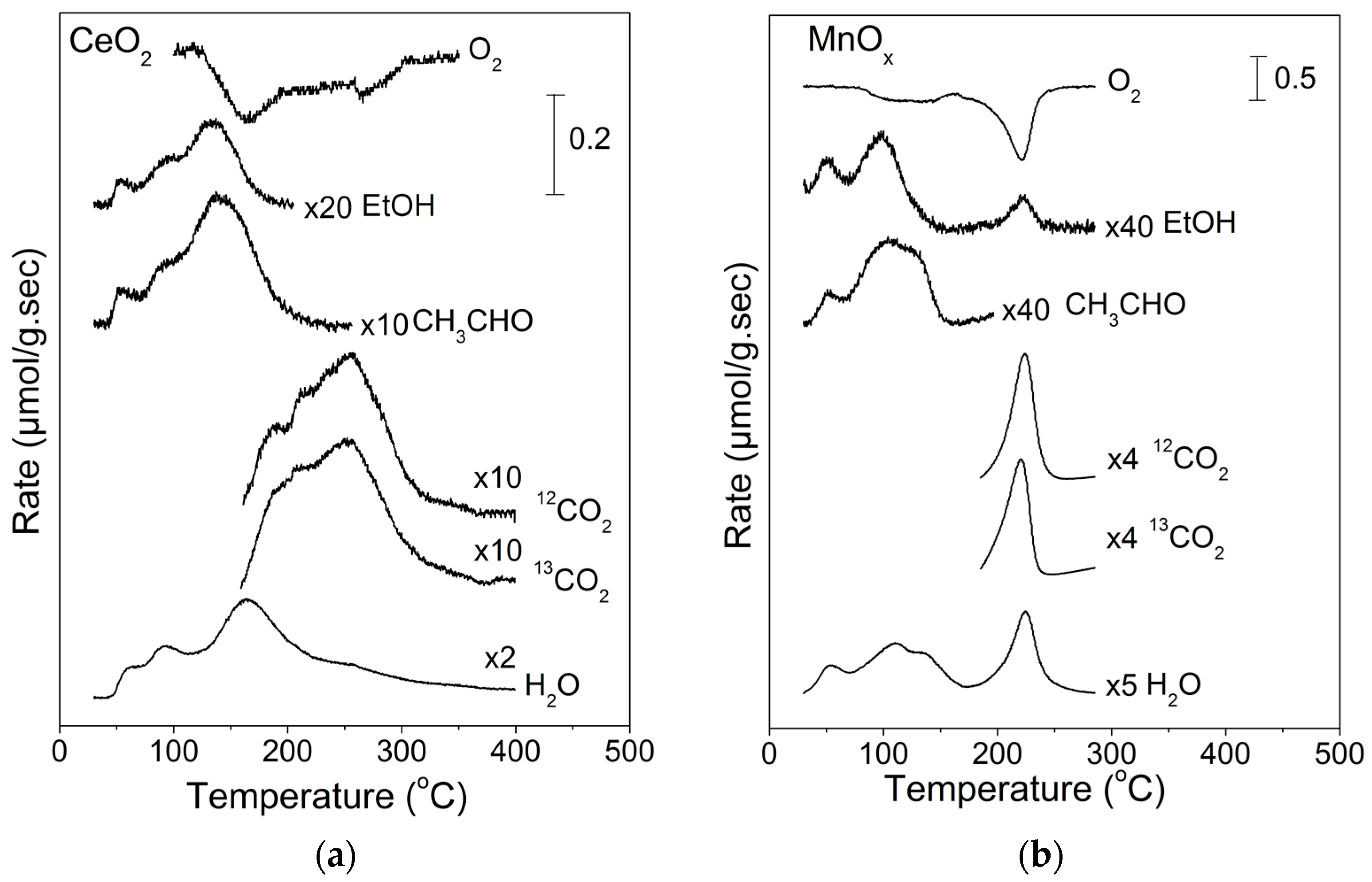
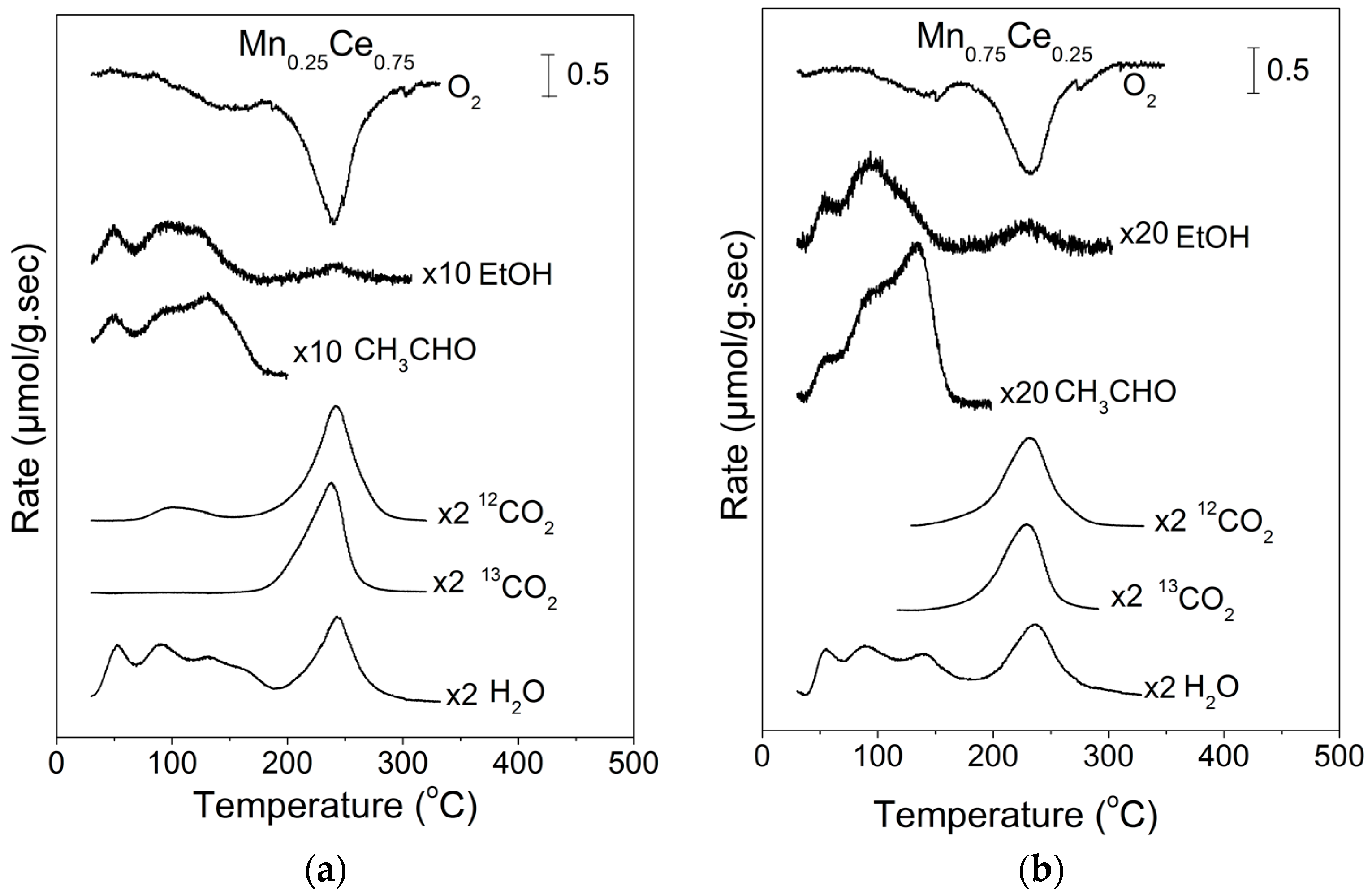
| EtOH (%) | CH3CHO (%) | CO2 (%) | CO (%) | H2 a (%) | CO2 b (%) | |
|---|---|---|---|---|---|---|
| CeO2 | 28.1 | 22.7 | 28.1 | 21.9 | 6.8 | 3.1 |
| Mn0.05Ce0.95 | 17.7 | 19.0 | 47.2 | 15 | 1.6 | 2.6 |
| Mn0.15Ce0.85 | 15.5 | 15.8 | 53.1 | 14.1 | 1.4 | 0.6 |
| Mn0.25Ce0.75 | 14.6 | 15.9 | 59.6 | 7.9 | 0.8 | - |
| Mn0.50Ce0.50 | 13.0 | 15.3 | 60.3 | 7.9 | - | 0.3 |
| Mn0.75Ce0.25 | 13.0 | 21.5 | 62.5 | 2.8 | 0.6 | 0.3 |
| MnOx | 13.2 | 11.8 | 70.6 | - | 0.8 | 4.2 |
| EtOH (%) | CH3CHO (%) | CO2 (%) | |
|---|---|---|---|
| CeO2 | 12.5 | 22.7 | 61.6 |
| Mn0.05Ce0.95 | 11.1 | 28.6 | 75.8 |
| Mn0.15Ce0.85 | 4.8 | 18.0 | 79.2 |
| Mn0.25Ce0.75 | 9.5 | 21.9 | 72.9 |
| Mn0.50Ce0.50 | 10.9 | 26.1 | 70.1 |
| Mn0.75Ce0.25 | 6.6 | 15.3 | 74.4 |
| MnOx | 6.3 | 15.9 | 80.8 |
| Catalyst | SSA (m2 g−1) | Csites (μmol m−2) | R (μmol s−1 g−1) | TOF (×10−3 s−1) |
|---|---|---|---|---|
| CeO2 | 4.8 | 9.2 | 0.07 | 1.52 |
| Mn0.05Ce0.95 | 59.4 | 3.8 | 0.35 | 1.57 |
| Mn0.15Ce0.85 | 50.0 | 3.8 | 0.56 | 2.95 |
| Mn0.25Ce0.75 | 47.3 | 3.5 | 0.50 | 3.03 |
| Mn0.50Ce0.50 | 48.8 | 5.3 | 0.73 | 2.81 |
| Mn0.75Ce0.25 | 38.2 | 5.1 | 0.75 | 3.86 |
| MnOx | 10.3 | 6.1 | 0.37 | 5.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delimaris, D.; Ioannides, T. Intrinsic Activity of MnOx-CeO2 Catalysts in Ethanol Oxidation. Catalysts 2017, 7, 339. https://doi.org/10.3390/catal7110339
Delimaris D, Ioannides T. Intrinsic Activity of MnOx-CeO2 Catalysts in Ethanol Oxidation. Catalysts. 2017; 7(11):339. https://doi.org/10.3390/catal7110339
Chicago/Turabian StyleDelimaris, Dimitrios, and Theophilos Ioannides. 2017. "Intrinsic Activity of MnOx-CeO2 Catalysts in Ethanol Oxidation" Catalysts 7, no. 11: 339. https://doi.org/10.3390/catal7110339






