Fine-Tuning Synthesis and Characterization of Mono-Sized H-Beta Zeolite-Supported Palladium-Iridium Nanoparticles and Application in the Selective Hydrogenation of Acetylene
Abstract
:1. Introduction
2. Results and Discussion
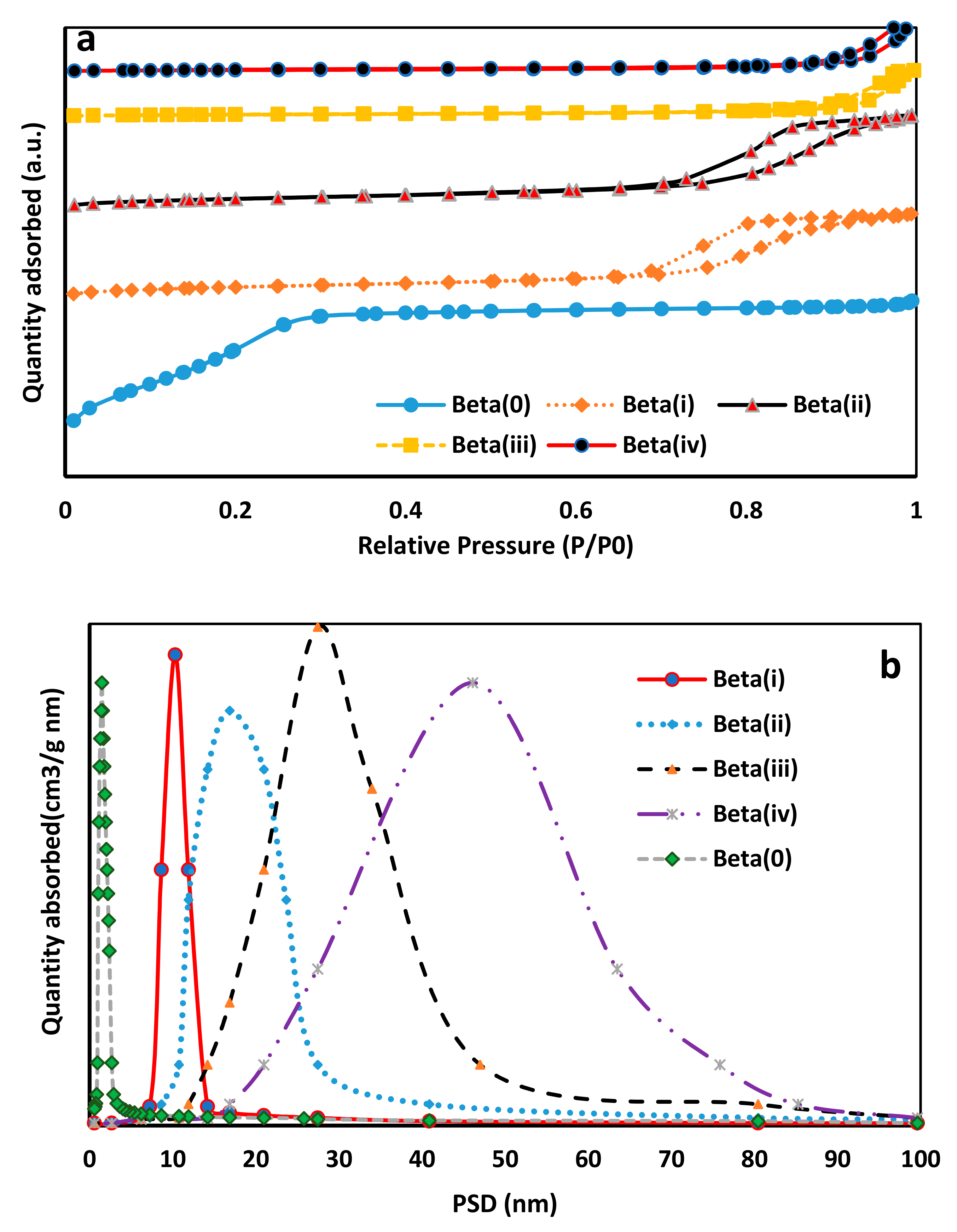
2.1. BET and DLS Characterization
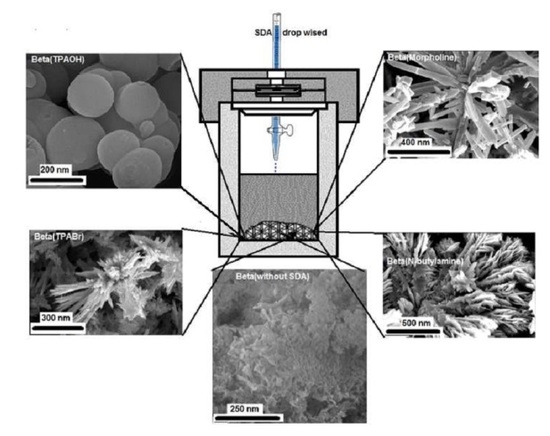
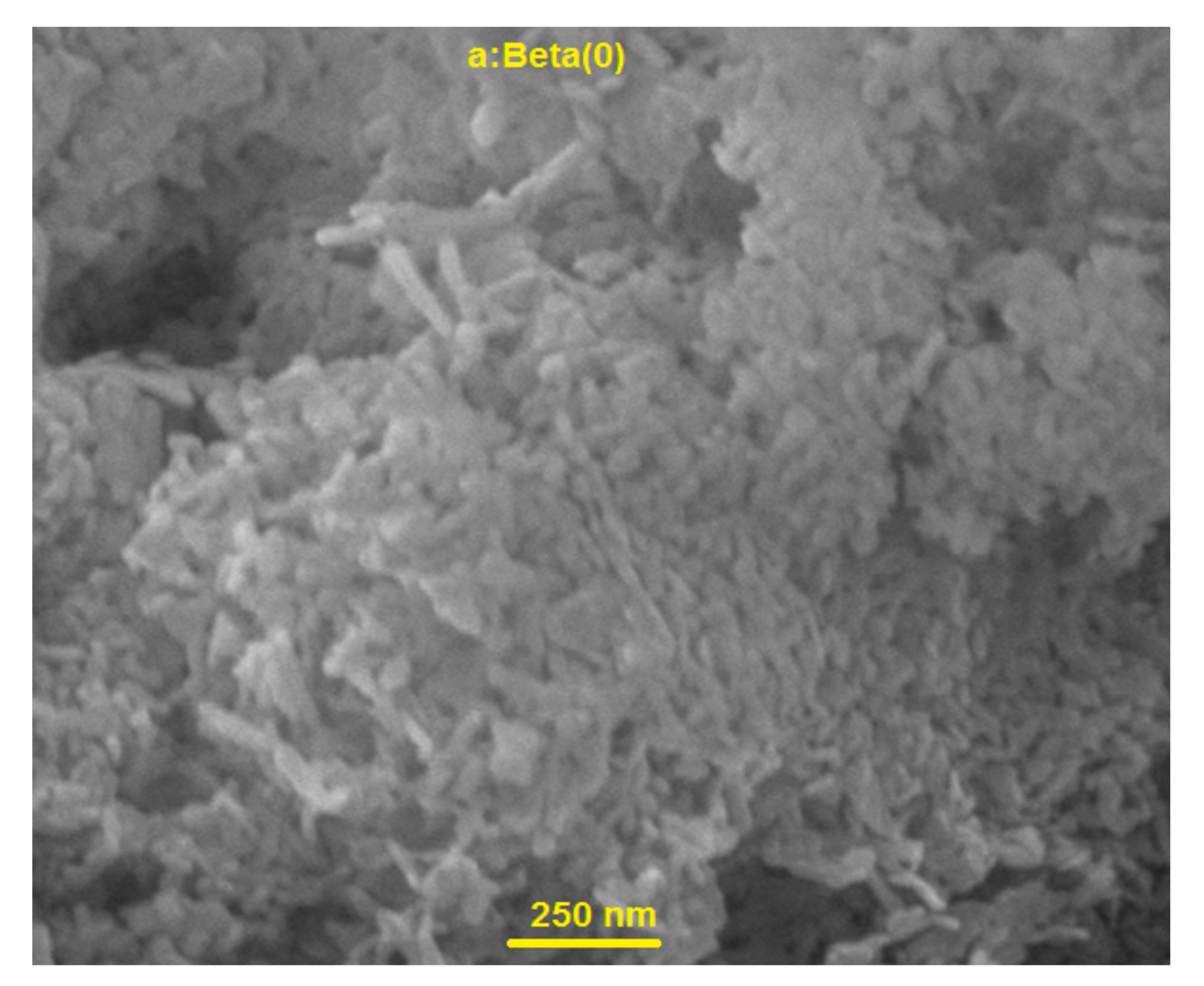
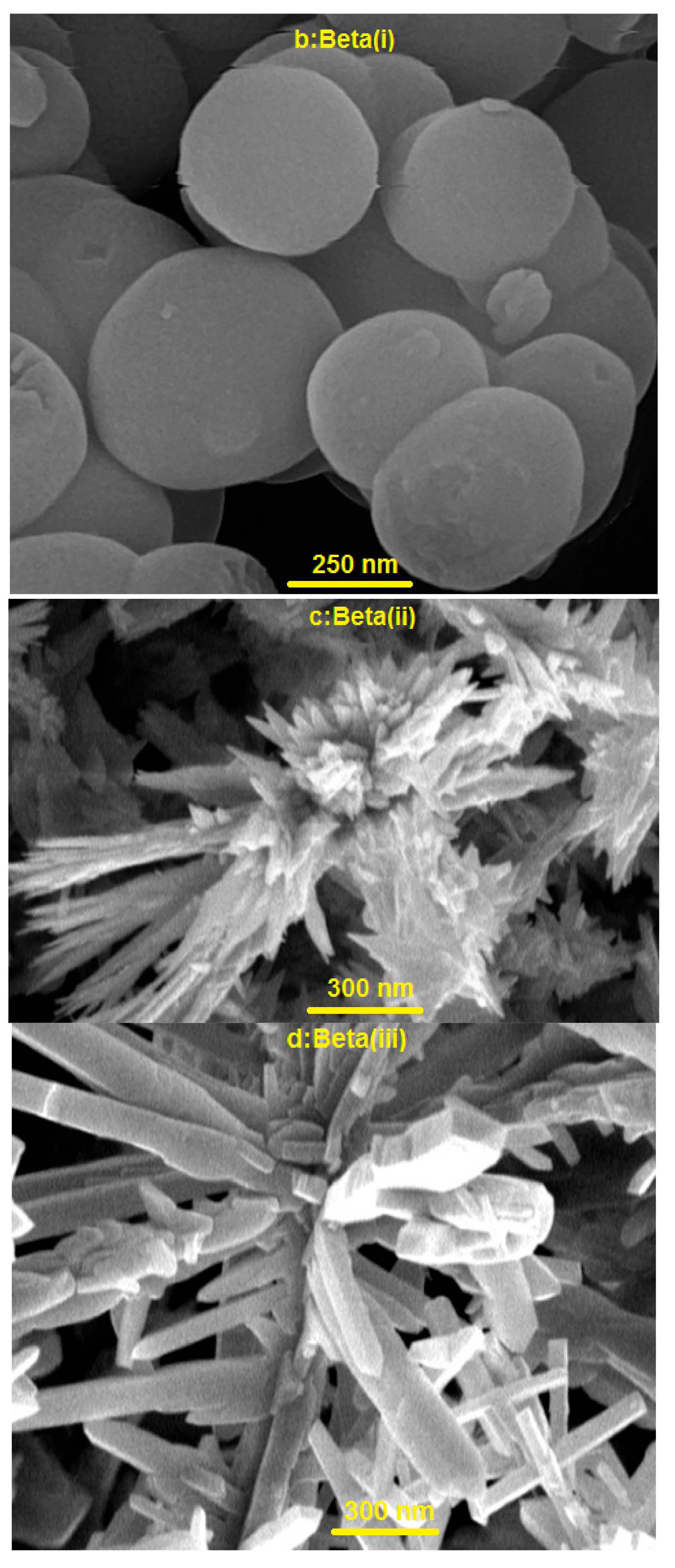
2.2. TEM and FESEM Characterization
2.3. XRD Patterns
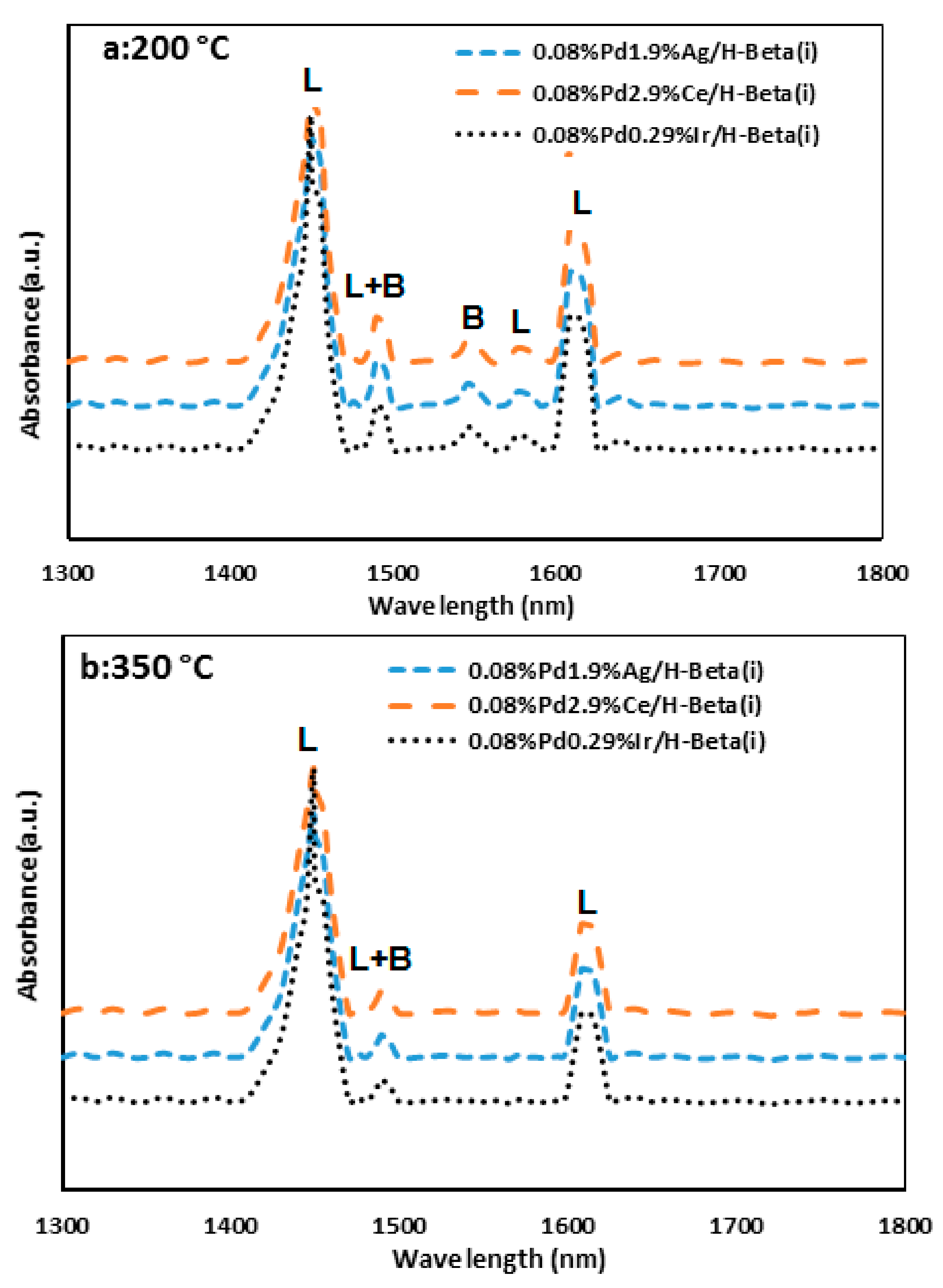
2.4. FT-IR Spectroscopy
2.5. Surfac Acidity Meassurment
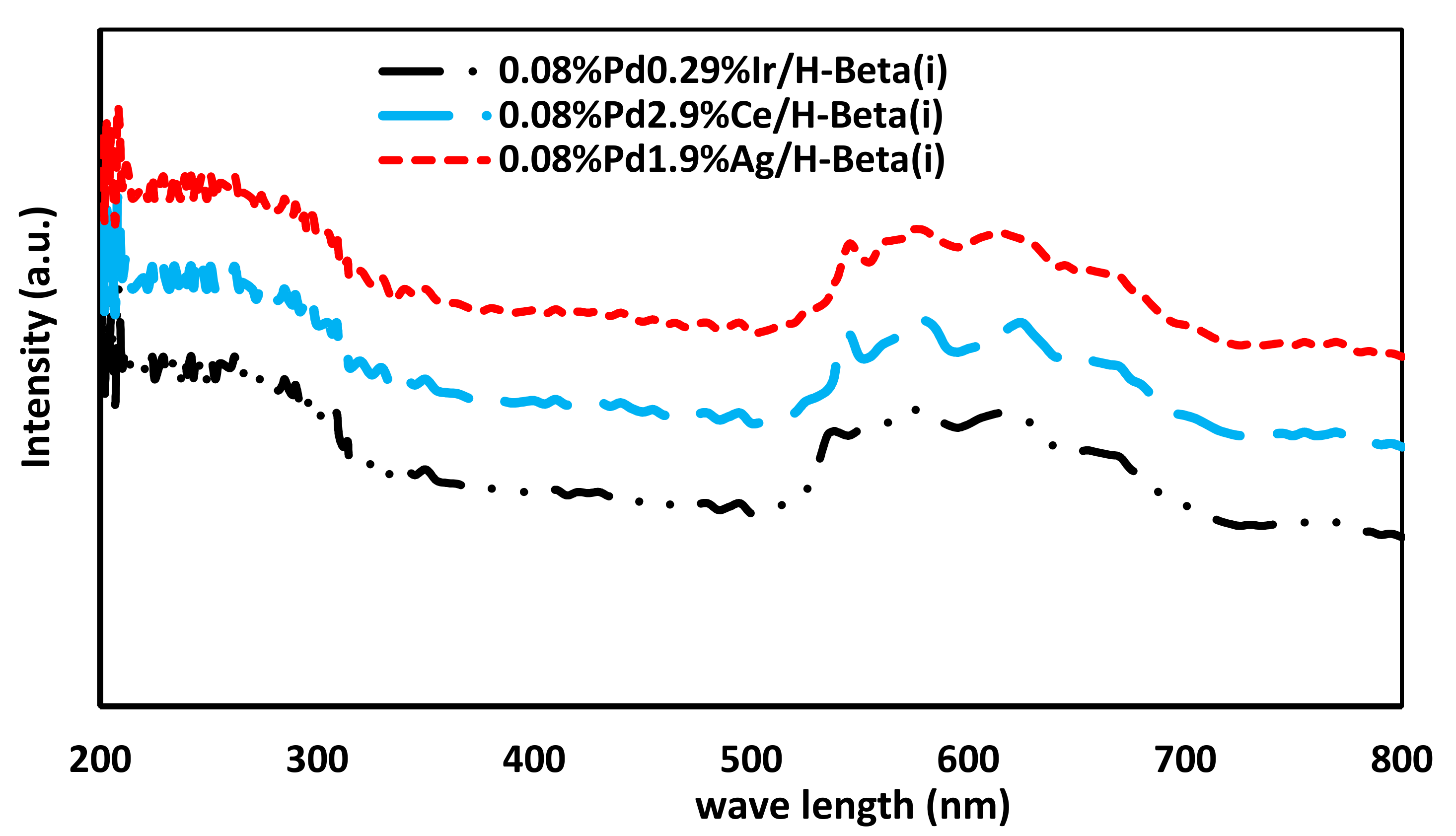
2.6. UV–Visible Spectroscopy Specification
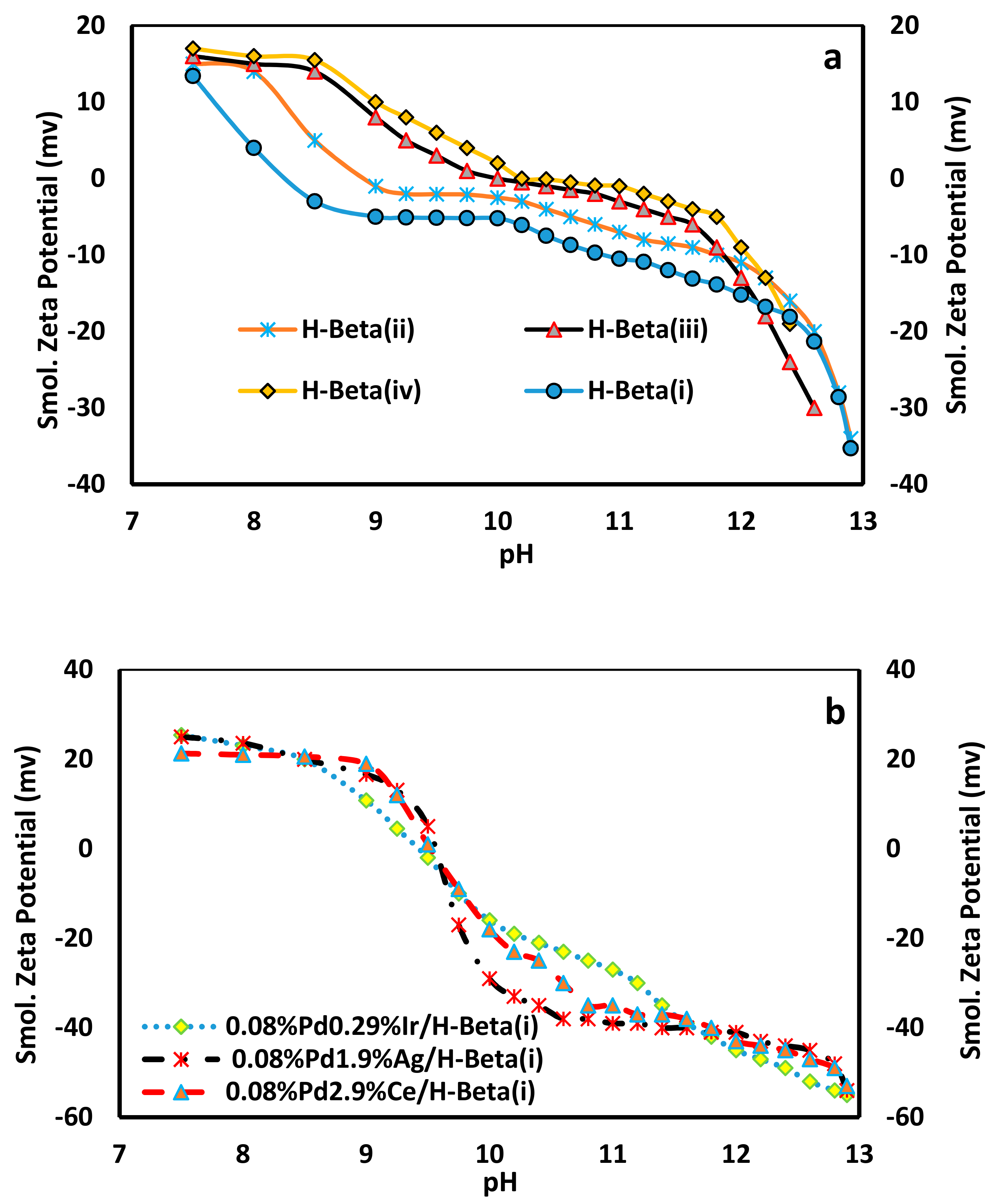
2.7. Zeta (ξ) Potentiail Analysis
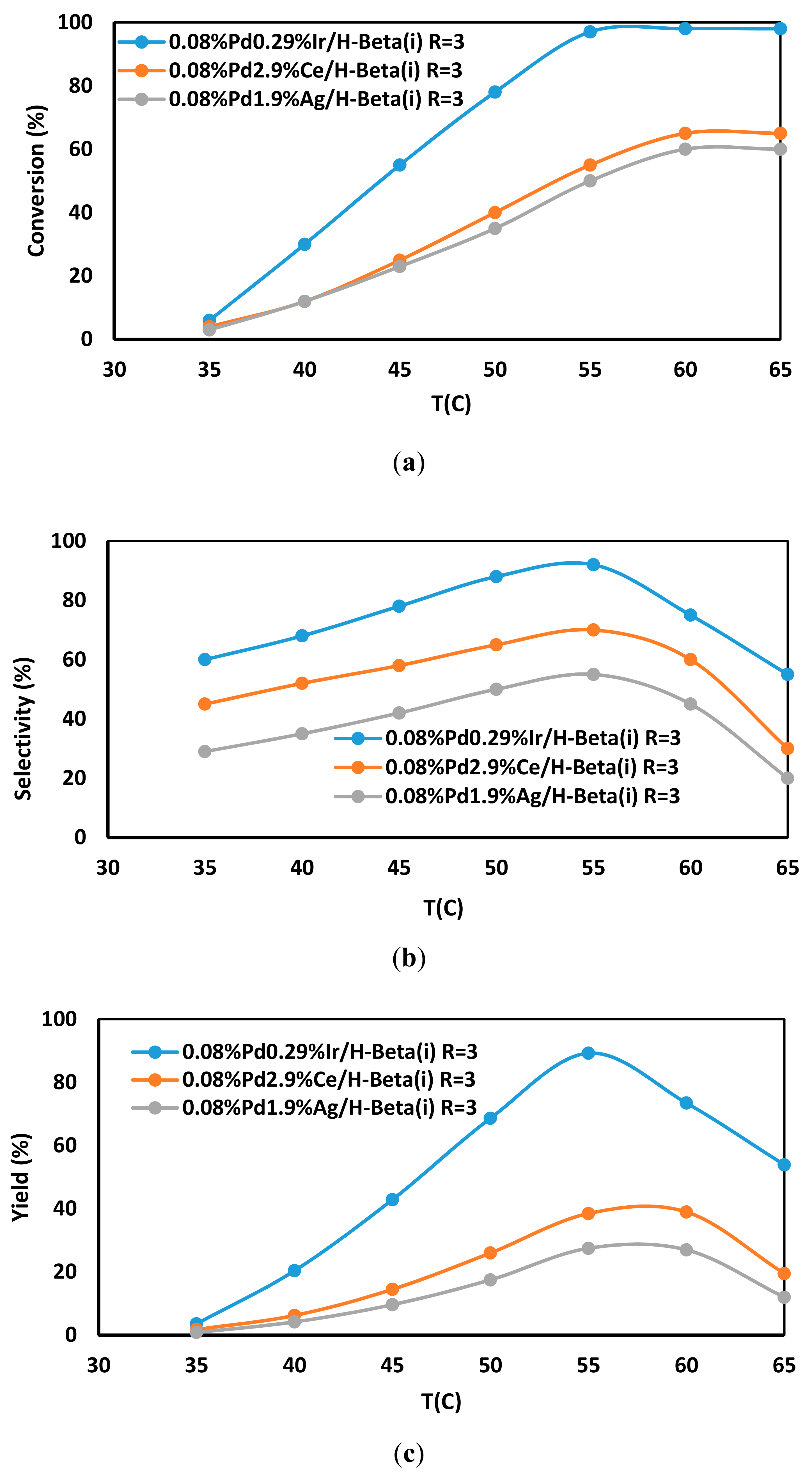
2.8. Catalytic Performance in the Selective Hydrogenation of Acetylene
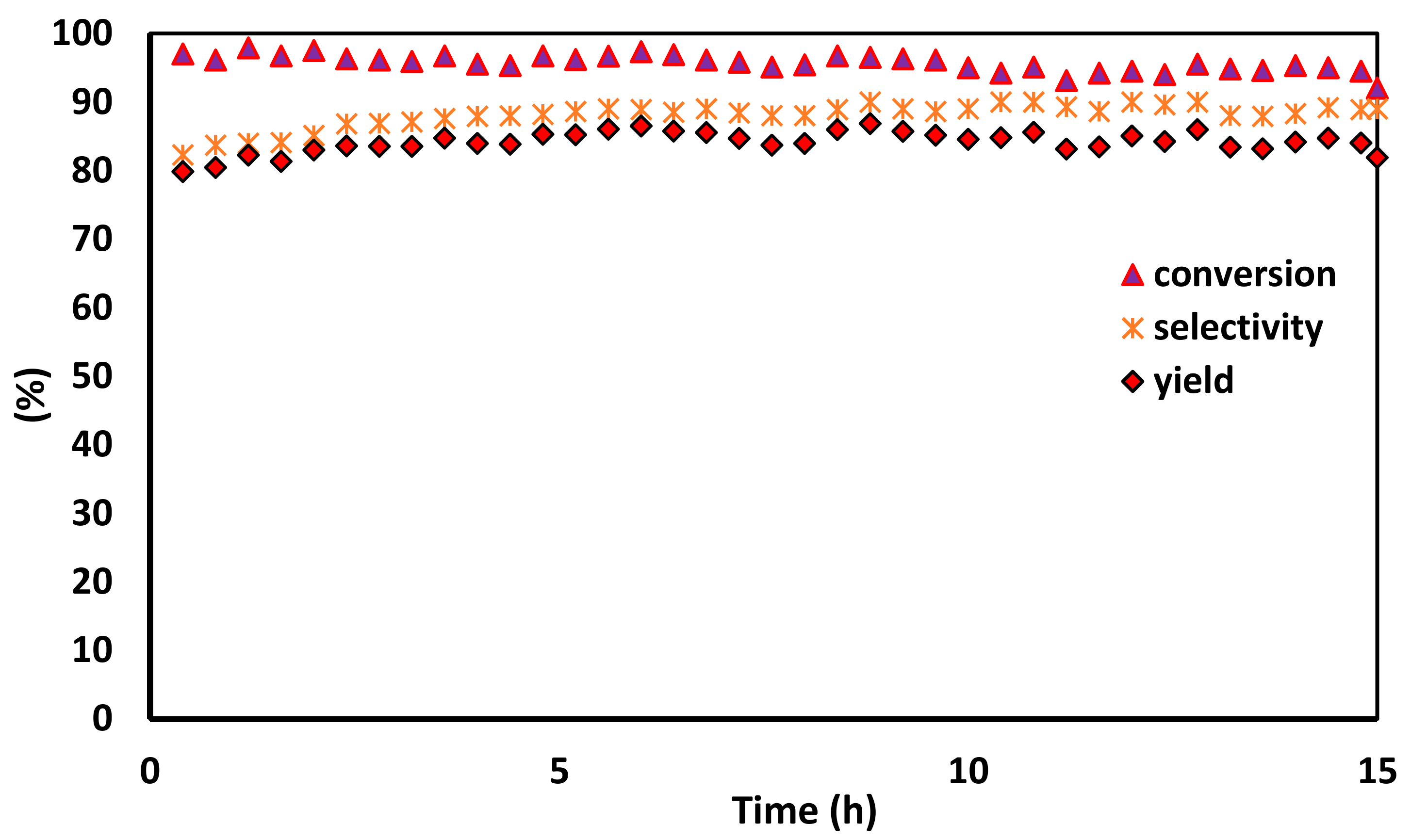
2.9. Stability
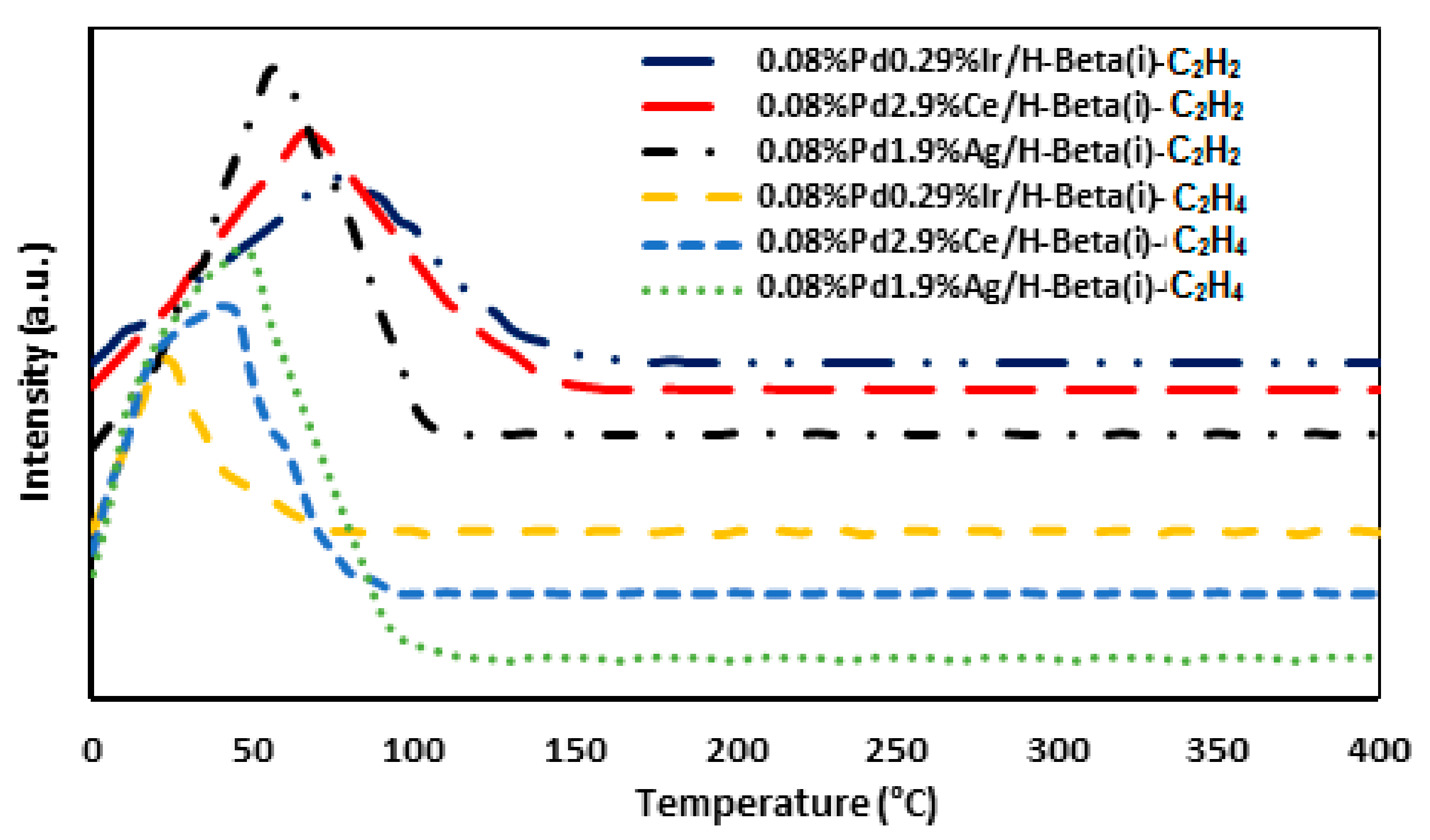
2.10. TPD–C2H2 and TPD–C2H4
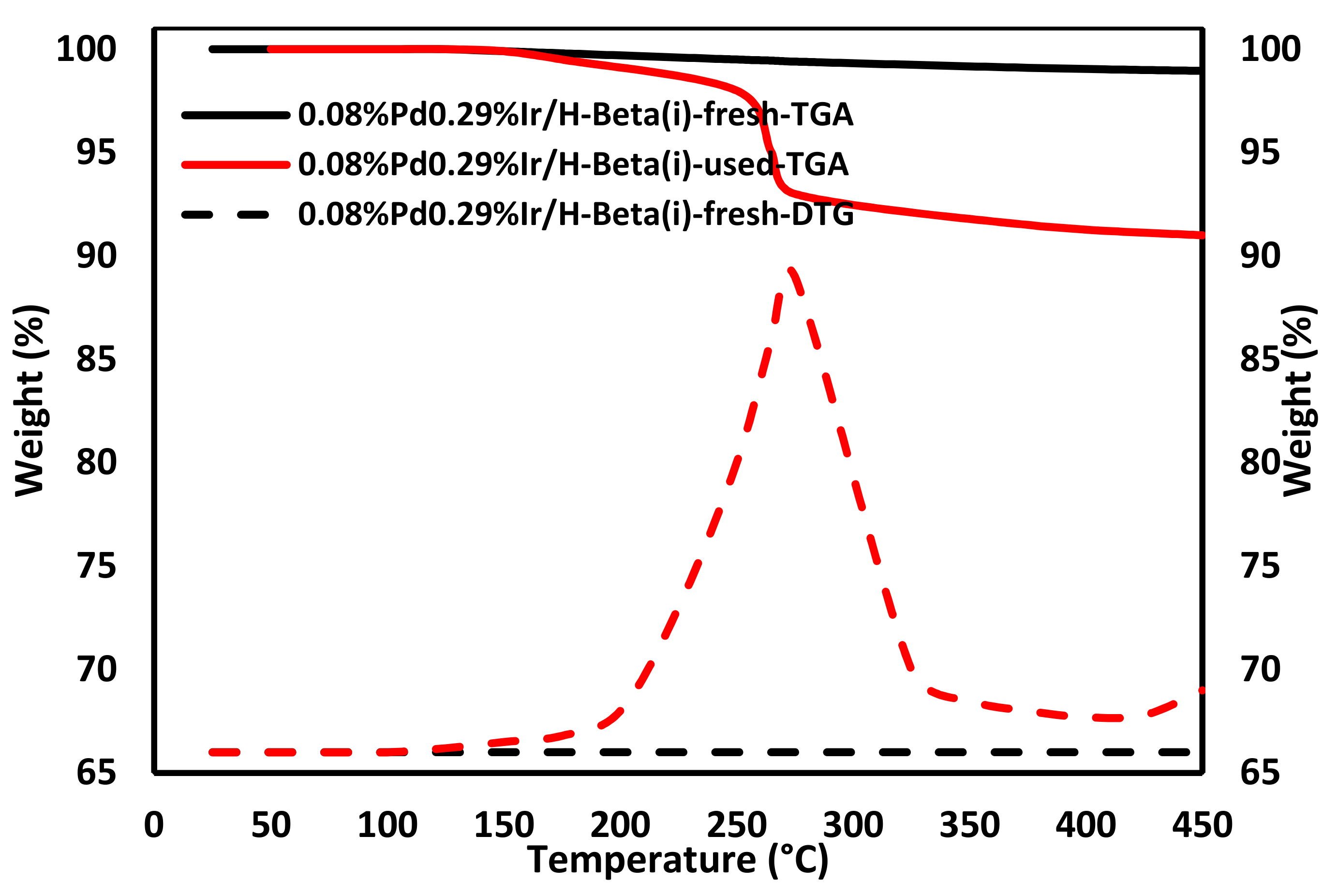
2.11. Coke Analysis
3. Experimental Methods
3.1. Materials
3.2. Synthesis of H-Beta-Based Catalyst
3.3. Synthesis of Different Catalysts by the Incipient Wetness Co-Impregnation Method
3.4. Catalysts and Supports Characterizations
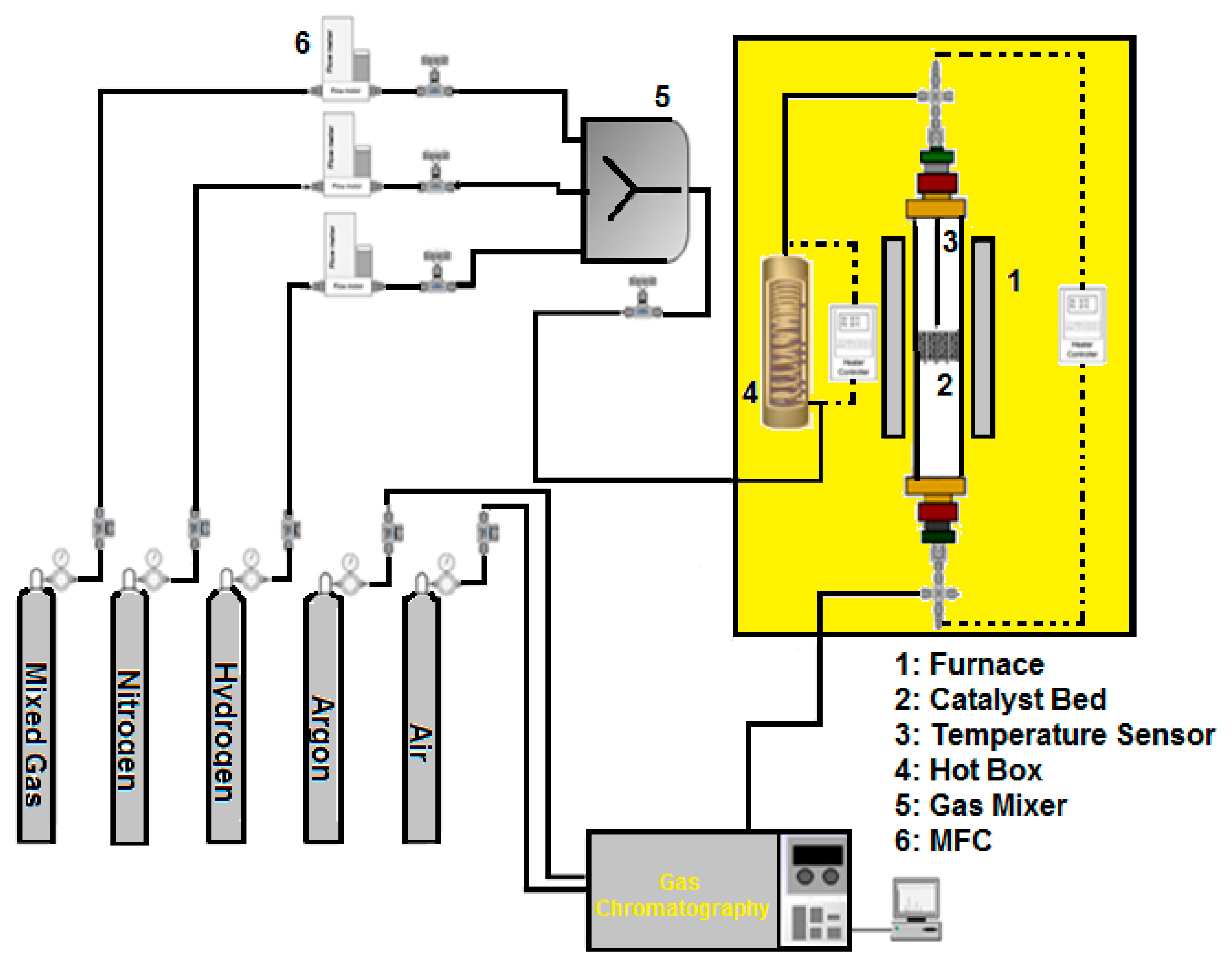
3.5. Catalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Sharma, P.; Song, J.S.; Han, M.H.; Cho, C.H. Gis-nap1 zeolite microspheres as potential water adsorption material: Influence of initial silica concentration on adsorptive and physical/topological properties. Sci. Rep. 2016, 6, 22734. [Google Scholar] [CrossRef] [PubMed]
- Awala, H.; Gilson, J.P.; Retoux, R.; Boullay, P.; Goupil, J.M.; Valtchev, V.; Mintova, S. Template-free nanosized faujasite-type zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium nanocatalysts confined in mesoporous silica for heterogeneous reduction of nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Crivelli, P.; Cooke, D.; Pérez-Ramírez, J. Mesopore quality determines the lifetime of hierarchically structured zeolite catalysts. Nat. Commun. 2014. [Google Scholar] [CrossRef]
- Kim, K.; Lee, T.; Kwon, Y.; Seo, Y.; Song, J.; Park, J.K.; Lee, H.; Park, J.Y.; Ihee, H.; Cho, S.J.; et al. Lanthanum-catalysed synthesis of microporous 3d graphene-like carbons in a zeolite template. Nature 2016, 535, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Humplik, T.; Bevilacqua, A.; Tsapatsis, M.; Chiavazzo, E.; Wang, E.N.; Asinari, P. Interplay between hydrophilicity and surface barriers on water transport in zeolite membranes. Nat. Commun. 2016, 7, 12762. [Google Scholar] [CrossRef] [PubMed]
- Gizzatov, A.; Key, J.; Aryal, S.; Ananta, J.; Cervadoro, A.; Palange, A.L.; Fasano, M.; Stigliano, C.; Zhong, M.; Di Mascolo, D. Hierarchically structured magnetic nanoconstructs with enhanced relaxivity and cooperative tumor accumulation. Adv. Funct. Mater. 2014, 24, 4584–4594. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Wittering, K.E.; Chen, T.-H.; Kaveevivitchai, W.; Popov, I.; Butler, K.T.; Wilson, C.C.; Cruickshank, D.L.; Miljanić, O.S.; Walsh, A. Absorbate-induced piezochromism in a porous molecular crystal. Nano Lett. 2015, 15, 2149–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, K.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Metal nanocrystals embedded in single nanocrystals of mofs give unusual selectivity as heterogeneous catalysts. Nano Lett. 2014, 14, 5979–5983. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Pinar, A.B.; Kenvin, J.; Crivelli, P.; Kärger, J.; Pérez-Ramírez, J. Structural analysis of hierarchically organized zeolites. Nat. Commun. 2015, 6, 8633. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Jeon, M.Y.; Ren, L.; Knight, C.; Deem, M.W.; Tsapatsis, M.; Siepmann, J.I. Discovery of optimal zeolites for challenging separations and chemical transformations using predictive materials modeling. Nat. Commun. 2015, 6, 5912. [Google Scholar] [CrossRef] [PubMed]
- Linares, M.; Vargas, C.; García, A.; Ochoa-Hernández, C.; Čejka, J.; García-Muñoz, R.A.; Serrano, D.P. Effect of hierarchical porosity in beta zeolites on the beckmann rearrangement of oximes. Catal. Sci. Technol. 2017, 7, 181–190. [Google Scholar] [CrossRef]
- Van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, characterization, and impact of mesopores in zeolite catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Breck, D.; Eversole, W.; Milton, R.; Reed, T.; Thomas, T. Crystalline zeolites. I. The properties of a new synthetic zeolite, type A. J. Am. Chem. Soc. 1956, 78, 5963–5972. [Google Scholar] [CrossRef]
- Kuznicki, S.M.; Bell, V.A.; Nair, S.; Hillhouse, H.W.; Jacubinas, R.M.; Braunbarth, C.M.; Toby, B.H.; Tsapatsis, M. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules. Nature 2001, 412, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Čejka, J. Exploiting chemically selective weakness in solids as a route to new porous materials. Nat. Chem. 2015, 7, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Choi, M.; Ryoo, R. Cyclic diquaternary ammoniums for nanocrystalline bea, mtw and mfi zeolites with intercrystalline mesoporosity. J. Mater. Chem. 2009, 19, 6713–6719. [Google Scholar] [CrossRef]
- Kresge, C.; Leonowicz, M.; Roth, W.; Vartuli, J.; Beck, J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Hould, N.D.; Foster, A.; Lobo, R.F. Zeolite beta mechanisms of nucleation and growth. Microporous Mesoporous Mater. 2011, 142, 104–115. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite mfi as active and long-lived catalysts. Nature 2009, 461, 246. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, J.-C.; Kim, J.; Seo, Y.; Ryoo, R. External surface catalytic sites of surfactant-tailored nanomorphic zeolites for benzene isopropylation to cumene. ACS Catal. 2013, 3, 192–195. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, H.; Jiang, J.; Wu, H.-H.; Wu, P. Hydrophobic nanosized all-silica beta zeolite: Efficient synthesis and adsorption application. ACS Appl. Mater. Interfaces 2017, 9, 27273–27283. [Google Scholar] [CrossRef] [PubMed]
- Arranz, M.; Pérez-Pariente, J.; Blasco, T. Fluorine-containing organic molecules as structure-directing agents in the synthesis of crystalline microporous materials. Part ii: Synthesis of all-silica zeolites from fluorine-containing derivatives of 1-benzyl-1-methyl-hexamethylenammonium cations. Microporous Mesoporous Mater. 2006, 89, 235–245. [Google Scholar] [CrossRef]
- Stelzer, J.; Paulus, M.; Hunger, M.; Weitkamp, J. Hydrophobic properties of all-silica zeolite beta. Microporous Mesoporous Mater. 1998, 22, 1–8. [Google Scholar] [CrossRef]
- Karimi, B.; Abedi, S.; Clark, J.H.; Budarin, V. Highly efficient aerobic oxidation of alcohols using a recoverable catalyst: The role of mesoporous channels of sba-15 in stabilizing palladium nanoparticles. Angew. Chem. 2006, 118, 4894–4897. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, P.; Cui, C. The pore confinement effect of fdu-12 mesochannels on mos2active phases and their hydrodesulfurization performance. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Zang, W.; Li, G.; Wang, L.; Zhang, X. Catalytic hydrogenation by noble-metal nanocrystals with well-defined facets: A review. Catal. Sci. Technol. 2015, 5, 2532–2553. [Google Scholar] [CrossRef]
- McCue, A.J.; Anderson, J.A. Recent advances in selective acetylene hydrogenation using palladium containing catalysts. Front. Chem. Sci. Eng. 2015, 9, 142–153. [Google Scholar] [CrossRef]
- Borodziński, A.; Bond, G.C. Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts, part 2: Steady-state kinetics and effects of palladium particle size, carbon monoxide, and promoters. Catal. Rev. 2008, 50, 379–469. [Google Scholar] [CrossRef]
- Benavidez, A.D.; Burton, P.D.; Nogales, J.L.; Jenkins, A.R.; Ivanov, S.A.; Miller, J.T.; Karim, A.M.; Datye, A.K. Improved selectivity of carbon-supported palladium catalysts for the hydrogenation of acetylene in excess ethylene. Appl. Catal. A 2014, 482, 108–115. [Google Scholar] [CrossRef]
- Burton, P.D.; Boyle, T.J.; Datye, A.K. Facile, surfactant-free synthesis of pd nanoparticles for heterogeneous catalysts. J. Catal. 2011, 280, 145–149. [Google Scholar] [CrossRef]
- Khan, N.A.; Shaikhutdinov, S.; Freund, H.-J. Acetylene and ethylene hydrogenation on alumina supported pd-ag model catalysts. Catal. Lett. 2006, 108, 159–164. [Google Scholar] [CrossRef]
- Ziaei-Azad, H.; Semagina, N. Iridium addition enhances hydrodesulfurization selectivity in 4,6-dimethyldibenzothiophene conversion on palladium. Appl. Catal. B 2016, 191, 138–146. [Google Scholar] [CrossRef]
- Ziaei-azad, H.; Yin, C.-X.; Shen, J.; Hu, Y.; Karpuzov, D.; Semagina, N. Size- and structure-controlled mono- and bimetallic ir–pd nanoparticles in selective ring opening of indan. J. Catal. 2013, 300, 113–124. [Google Scholar] [CrossRef]
- Meku, E.; Du, C.; Sun, Y.; Du, L.; Wang, Y.; Kong, F.; Yin, G. Composition optimization of ternary palladium–iridium–iron alloy catalysts for oxygen reduction reaction in acid medium. RSC Adv. 2016, 6, 22754–22763. [Google Scholar] [CrossRef]
- Yang, T.; Cao, G.; Huang, Q.; Ma, Y.; Wan, S.; Zhao, H.; Li, N.; Yin, F.; Sun, X.; Zhang, D.; et al. Truncated octahedral platinum–nickel–iridium ternary electro-catalyst for oxygen reduction reaction. J. Power Sources 2015, 291, 201–208. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsu, H.-C.; Wang, K.-C. Iridium-decorated palladium–platinum core–shell catalysts for oxygen reduction reaction in proton exchange membrane fuel cell. J. Coll. Interface Sci. 2014, 427, 91–97. [Google Scholar] [CrossRef] [PubMed]
- You, D.J.; Jin, S.-A.; Lee, K.H.; Pak, C.; Choi, K.H.; Chang, H. Improvement of activity for oxygen reduction reaction by decoration of ir on pdcu/c catalyst. Catal. Today 2012, 185, 138–142. [Google Scholar] [CrossRef]
- Mei, D.; Neurock, M.; Smith, C.M. Hydrogenation of acetylene–ethylene mixtures over pd and pd–ag alloys: First-principles-based kinetic monte carlo simulations. J. Catal. 2009, 268, 181–195. [Google Scholar] [CrossRef]
- Han, Y.; Peng, D.; Xu, Z.; Wan, H.; Zheng, S.; Zhu, D. TiO2 supported pd@ ag as highly selective catalysts for hydrogenation of acetylene in excess ethylene. Chem. Commun. 2013, 49, 8350–8352. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, B.; Song, Q. Kinetics of acetylene hydrochlorination over bimetallic au–cu/c catalyst. Catal. Lett. 2010, 134, 102–109. [Google Scholar] [CrossRef]
- Song, Q.; Wang, S.; Shen, B.; Zhao, J. Palladium-based catalysts for the hydrochlorination of acetylene: Reasons for deactivation and its regeneration. Pet. Sci. Technol. 2010, 28, 1825–1833. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Wang, J.; Zhao, Y.; Wang, Y.; Yang, D. Bimetallic pd-k/y-zeolite catalyst in acetylene hydrochlorination for pvc production. React. Kinet. Mech. Catal. 2014, 114, 725–734. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Xu, L.; Zhu, M. Hydrochlorination of acetylene to vinyl chloride monomer over bimetallic au–la/sac catalysts. J. Ind. Eng. Chem. 2012, 18, 49–54. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Li, W.; Han, Y.; Gu, J.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over bimetallic au–co(iii)/sac catalysts for vinyl chloride monomer production. Green Chem. 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 2008, 320, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Bridier, B.; López, N.; Pérez-Ramírez, J. Molecular understanding of alkyne hydrogenation for the design of selective catalysts. Dalton Trans. 2010, 39, 8412–8419. [Google Scholar] [CrossRef] [PubMed]
- Vilé, G.; Bridier, B.; Wichert, J.; Pérez-Ramírez, J. Ceria in hydrogenation catalysis: High selectivity in the conversion of alkynes to olefins. Angew. Chem. Int. Ed. 2012, 51, 8620–8623. [Google Scholar] [CrossRef] [PubMed]
- Segura, Y.; López, N.; Pérez-Ramírez, J. Origin of the superior hydrogenation selectivity of gold nanoparticles in alkyne+ alkene mixtures: Triple-versus double-bond activation. J. Catal. 2007, 247, 383–386. [Google Scholar] [CrossRef]
- Yan, X.; Wheeler, J.; Jang, B.; Lin, W.-Y.; Zhao, B. Stable au catalysts for selective hydrogenation of acetylene in ethylene. Appl. Catal. A 2014, 487, 36–44. [Google Scholar] [CrossRef]
- Vilé, G.; Baudouin, D.; Remediakis, I.N.; Copéret, C.; López, N.; Pérez-Ramírez, J. Silver nanoparticles for olefin production: New insights into the mechanistic description of propyne hydrogenation. ChemCatChem 2013, 5, 3750–3759. [Google Scholar] [CrossRef]
- Bridier, B.; López, N.; Pérez-Ramírez, J. Partial hydrogenation of propyne over copper-based catalysts and comparison with nickel-based analogues. J. Catal. 2010, 269, 80–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Diao, W.; Monnier, J.R.; Williams, C.T. Pd–Ag/SiO2 bimetallic catalysts prepared by galvanic displacement for selective hydrogenation of acetylene in excess ethylene. Catal. Sci. Technol. 2015, 5, 4123–4132. [Google Scholar] [CrossRef]
- Kuhn, M.; Lucas, M.; Claus, P. Long-time stability vs deactivation of pd–Ag/Al2O3 egg-shell catalysts in selective hydrogenation of acetylene. Ind. Eng. Chem. Res. 2015, 54, 6683–6691. [Google Scholar] [CrossRef]
- Gulyaeva, Y.K.; Kaichev, V.V.; Zaikovskii, V.I.; Suknev, A.P.; Bal’zhinimaev, B.S. Selective hydrogenation of acetylene over pd/fiberglass catalysts: Kinetic and isotopic studies. Appl. Catal. A 2015, 506, 197–205. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J.; Tang, X.; Zhao, Y.; Yang, D.; Jia, F.; Hao, T. Hydrochlorination of acetylene to vinyl chloride over pd supported on zeolite y. React. Kinet. Mech. Catal. 2013, 110, 187–194. [Google Scholar] [CrossRef]
- Azizi, Y.; Petit, C.; Pitchon, V. Formation of polymer-grade ethylene by selective hydrogenation of acetylene over Au/CeO2 catalyst. J. Catal. 2008, 256, 338–344. [Google Scholar] [CrossRef]
- Delgado, J.A.; Benkirane, O.; Claver, C.; Curulla-Ferre, D.; Godard, C. Advances in the preparation of highly selective nanocatalysts for the semi-hydrogenation of alkynes using colloidal approaches. Dalton Trans. 2017, 46, 12381–12403. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zheng, P.; Song, S.; Wang, X.; Zhang, M.; Chi, K.; Xu, C.; Duan, A.; Zhao, Z. Synthesis of a novel micro/mesoporous composite material beta-fdu-12 and its hydro-upgrading performance for fcc gasoline. RSC Adv. 2016, 6, 1018–1026. [Google Scholar] [CrossRef]
- Jin, Q.; He, Y.; Miao, M.; Guan, C.; Du, Y.; Feng, J.; Li, D. Highly selective and stable pdni catalyst derived from layered double hydroxides for partial hydrogenation of acetylene. Appl. Catal. A 2015, 500, 3–11. [Google Scholar] [CrossRef]
- Li, C.; Fujimoto, K. Synthesis gas conversion to isobutane-rich hydrocarbons over a hybrid catalyst containing beta zeolite—Role of doped palladium and influence of the SiO2/Al2O3 ratio. Catal. Sci. Technol. 2015, 5, 4501–4510. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, J.; Liu, J.; Wang, L.; Wan, H.; Hu, J.; Wang, Y.; Peden, C.H.F.; Nie, Z. Solvent evaporation assisted preparation of oriented nanocrystalline mesoporous mfi zeolites. ACS Catal. 2011, 1, 682–690. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.; Liu, J.; Li, C.; Cui, Q. A fast organic template-free, ZSM-11 seed-assisted synthesis of zsm-5 with good performance in methanol-to-olefin. Microporous Mesoporous Mater. 2013, 181, 192–200. [Google Scholar] [CrossRef]
- Zaera, F. Cheminform abstract: New advances in the use of infrared absorption spectroscopy for the characterization of heterogeneous catalytic reactions. ChemInform 2015, 46. [Google Scholar] [CrossRef]
- Pirngruber, G.; Seshan, K.; Lercher, J. Dehydroisomerization of n-butane over Pt–ZSM5 (i): Effect of the metal loading and acid site concentration. J. Catal. 1999, 186, 188–200. [Google Scholar] [CrossRef]
- McKenna, F.; Mantarosie, L.; Wells, R.; Hardacre, C.; Anderson, J. Selective hydrogenation of acetylene in ethylene rich feed streams at high pressure over ligand modified Pd/TiO2. Catal. Sci. Technol. 2012, 2, 632–638. [Google Scholar] [CrossRef]
- Zhang, H.; Han, L.; Duan, A.; Xu, C.; Zhao, Z.; Wei, Y.; Jiang, G.; Liu, J.; Wang, D.; Xia, Z. Synthesis of micro-mesoporous materials Zsm-5/Fdu-12 and the performance of dibenzothiophene hydrodesulfurization. RSC Adv. 2017, 7, 28038–28047. [Google Scholar] [CrossRef]
- Kataoka, T.; Dumesic, J.A. Cheminform abstract: Acidity of unsupported and silica-supported vanadia, molybdena, and titania as studied by pyridine adsorption. ChemInform 1988, 19. [Google Scholar] [CrossRef]
- Klimova, T.; Peña, L.; Lizama, L.; Salcedo, C.; Gutiérrez, O.Y. Modification of activity and selectivity of nimo/sba-15 hds catalysts by grafting of different metal oxides on the support surface. Ind. Eng. Chem. Res. 2008, 48, 1126–1133. [Google Scholar] [CrossRef]
- Weber, R.S. Effect of local structure on the uv-visible absorption edges of molybdenum oxide clusters and supported molybdenum oxides. J. Catal. 1995, 151, 470–474. [Google Scholar] [CrossRef]
- Soled, S.; Wachter, W.; Wo, H. Use of zeta potential measurements in catalyst preparation. Stud. Surf. Sci. Catal. 2010, 175, 101–107. [Google Scholar]
- Limousy, L.; Mahzoul, H.; Hamon, L.; Siffert, B. Determination by zetametry and ‘streaming induced potential’ measurements of the amounts of catalytic precursors necessary to saturate a support. Coll. Surf. A 2001, 181, 91–97. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Baek, S.-C.; Jun, K.-W. Methanol conversion on sapo-34 catalysts prepared by mixed template method. Appl. Catal. A 2007, 329, 130–136. [Google Scholar] [CrossRef]
- Zaera, F. Tuning selectivity in hydrocarbon conversion catalysis. J. Mol. Catal. A 2005, 228, 21–26. [Google Scholar] [CrossRef]
- He, Y.-F.; Feng, J.-T.; Du, Y.-Y.; Li, D.-Q. Controllable synthesis and acetylene hydrogenation performance of supported pd nanowire and cuboctahedron catalysts. ACS Catal. 2012, 2, 1703–1710. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.H.; Ahn, I.Y.; Kim, W.-J.; Moon, S.H. Performance of cu-promoted pd catalysts prepared by adding cu using a surface redox method in acetylene hydrogenation. Appl. Catal. A 2011, 401, 12–19. [Google Scholar] [CrossRef]
- Rafiaei, S.M.; Kim, A.; Shokouhimehr, M. Gadolinium triflate immobilized on magnetic nanocomposites as recyclable lewis acid catalyst for acetylation of phenols. Nanosci. Nanotechnol. Lett. 2014, 6, 309–313. [Google Scholar] [CrossRef]
- Hickman, D.; Schmidt, L. Production of syngas by direct catalytic oxidation of methane. Science 1993, 259, 343. [Google Scholar] [CrossRef] [PubMed]
- Prinz, D.; Riekert, L. Formation of ethene and propene from methanol on zeolite zsm-5. Appl. Catal. 1988, 37, 139–154. [Google Scholar] [CrossRef]
- Gil, B.; Zones, S.I.; Hwang, S.-J.; Bejblová, M.; Čejka, J. Acidic properties of ssz-33 and ssz-35 novel zeolites: A complex infrared and mas nmr study. J. Phys. Chem. C 2008, 112, 2997–3007. [Google Scholar] [CrossRef]
- Saidi, M.; Rahimpour, M.R.; Raeissi, S. Upgrading process of 4-methylanisole as a lignin-derived bio-oil catalyzed by Pt/γ-Al2O3: Kinetic investigation and reaction network development. Energy Fuels 2015, 29, 3335–3344. [Google Scholar] [CrossRef]
- Rahimpour, H.R.; Saidi, M.; Rostami, P.; Gates, B.C.; Rahimpour, M.R. Experimental investigation on upgrading of lignin-derived bio-oils: Kinetic analysis of anisole conversion on sulfided como/Al2O3 catalyst. Int. J. Chem. Kinet. 2016, 48, 702–713. [Google Scholar] [CrossRef]
- Saidi, M.; Rostami, P.; Rahimpour, H.R.; Roshanfekr Fallah, M.A.; Rahimpour, M.R.; Gates, B.C.; Raeissi, S. Kinetics of upgrading of anisole with hydrogen catalyzed by platinum supported on alumina. Energy Fuels 2015, 29, 4990–4997. [Google Scholar] [CrossRef]
- Saidi, M.; Rostami, P.; Rahimpour, M.R.; Gates, B.C.; Raeissi, S. Upgrading of lignin-derived bio-oil components catalyzed by pt/γ-Al2O3: Kinetics and reaction pathways characterizing conversion of cyclohexanone with H2. Energy Fuels 2015, 29, 191–199. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Hesami, M.; Saidi, M.; Jahanmiri, A.; Farniaei, M.; Abbasi, M. Methane steam reforming thermally coupled with fuel combustion: Application of chemical looping concept as a novel technology. Energy Fuels 2013, 27, 2351–2362. [Google Scholar] [CrossRef]



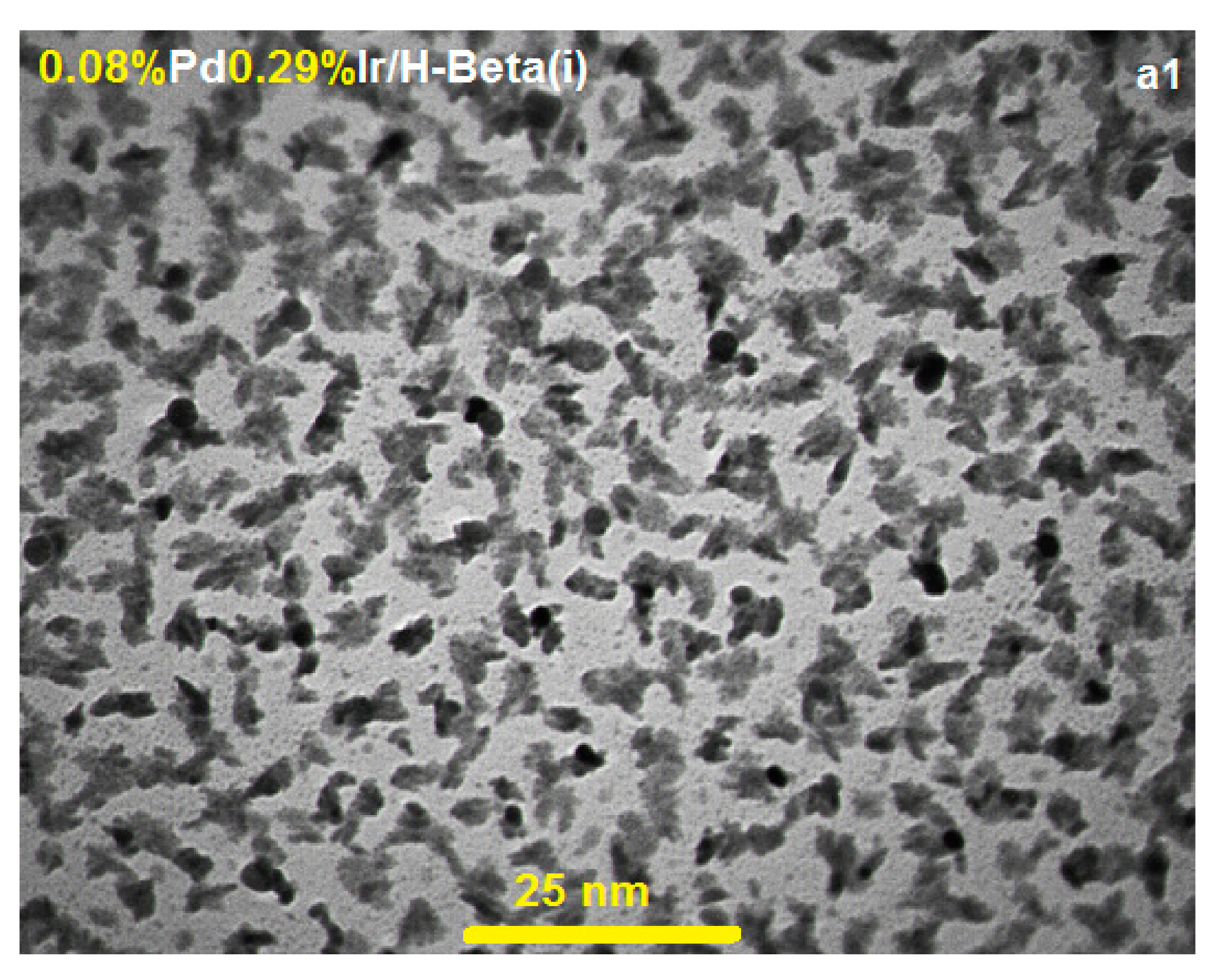
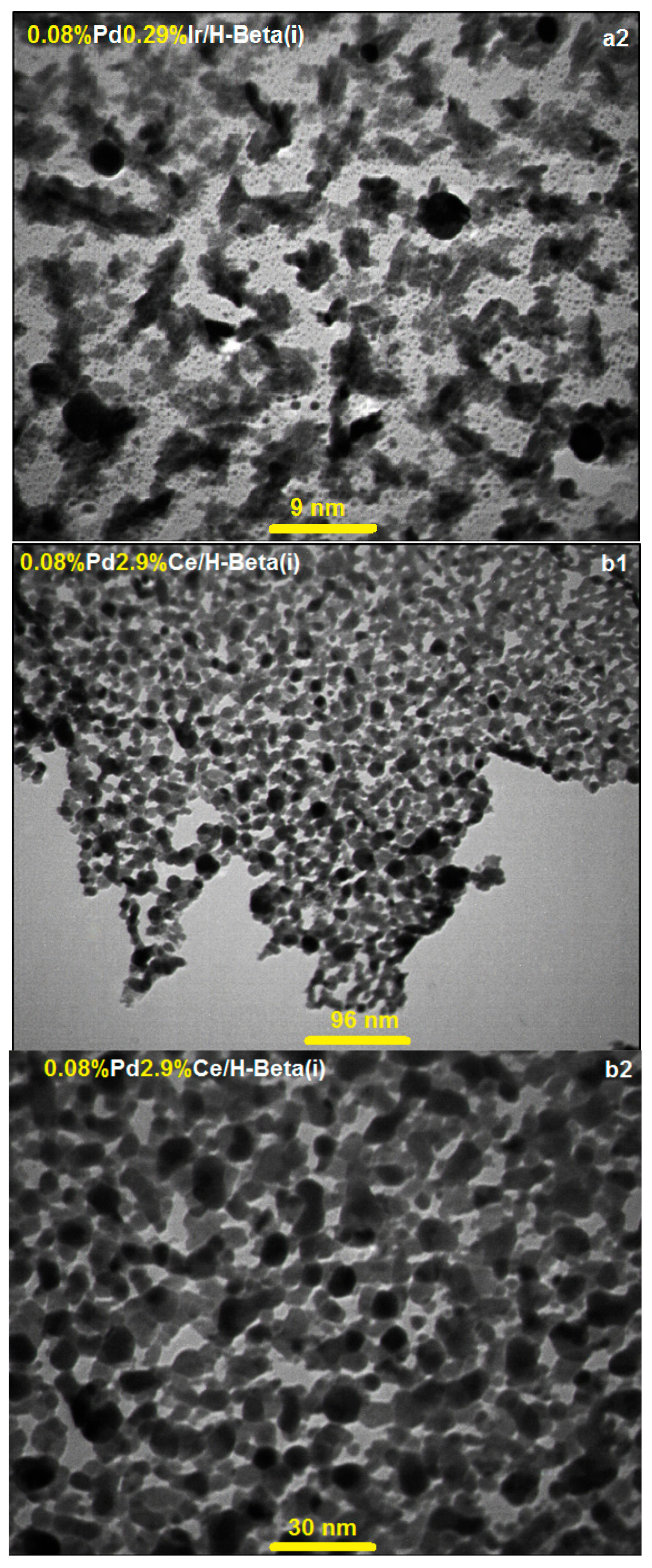




| Supports | i (mM) | ii (mM) | iii (mM) | iv (mM) | BET (m2·g−1) | PSD (nm) | DLS (nm) | |
|---|---|---|---|---|---|---|---|---|
| H-Beta(0) | - | - | - | - | 28.57 | 1474.78 | 1.40 | 3 |
| H-Beta(i) | 35.00 | - | - | - | 19.05 | 1358.30 | 10.3 | 12 |
| H-Beta(ii) | - | 26.73 | - | - | 20.67 | 1054.20 | 16.7 | 18 |
| H-Beta(iii) | - | - | 81.67 | - | 13.18 | 754.900 | 27.9 | 40 |
| H-Beta(iv) | - | - | - | 97.31 | 11.95 | 587.400 | 46.1 | 67 |
| Catalysts | Fresh Mono-Pd/H-Beta(i) | Fresh Pd-Ir/H-Beta(i) | Fresh Pd-Ce/H-Beta(i) | Fresh Pd-Ag/H-Beta(i) |
|---|---|---|---|---|
| Pd (wt%) a | 0.083 | 0.081 | 0.081 | 0.081 |
| Ir (wt%) a | 0 | 0.294 | 0 | 0 |
| Ce (wt%) a | 0 | 0 | 2.93 | 0 |
| Ag (wt%) a | 0 | 0 | 0 | 1.92 |
| BET (m2 g−1) b | - | 1108.9 | 973.7 | 850.3 |
| PSD (nm) c | - | 7.6 | 5.9 | 6.3 |
| Vt (cm3 g−1) d | - | 0.45 | 0.41 | 0.37 |
| Vmes (cm3 g−1) c | - | 0.44 | 0.38 | 0.33 |
| Vmic (cm3 g−1) e | - | 0.01 | 0.02 | 0.02 |
| Crystal size (nm) f | - | 12 | 15 | 21 |
| Amount of Acid Sites () | ||||||||
|---|---|---|---|---|---|---|---|---|
| Catalysts | 200 °C | 350 °C | ||||||
| L | B | L + B | B/L | L | B | L + B | B/L | |
| 0.08%Pd1.9%Ag/H-Beta(i) | 87.1 | 8.3 | 95.40 | 0.095 | 39.2 | 0 | 39.2 | 0 |
| 0.08%Pd2.9%Ce/H-Beta(i) | 91.6 | 7.9 | 99.50 | 0.086 | 40.5 | 0 | 40.5 | 0 |
| 0.08%Pd0.29%Ir/H-Beta(i) | 97.4 | 6.1 | 103.5 | 0.063 | 43.1 | 0 | 43.1 | 0 |
| Component | Value (mol%) |
|---|---|
| Ethylene | 65.5307 |
| Ethane | 33.6406 |
| Acetylene | 0.7591 |
| Propylene | 0.0596 |
| Methane | 0.0077 |
| Propane | 0.0007 |
| Other C4s | 0.0015 |
| 1,3-butadiene | <0.0001 |
| C5 + hydrocarbons | <0.0001 |
| Cyclopropane | <0.0001 |
| Methylacetylene-Propadiene (MAPD) | <0.0001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahooli, M.; Rahimpour, M.R.; Khorram, M. Fine-Tuning Synthesis and Characterization of Mono-Sized H-Beta Zeolite-Supported Palladium-Iridium Nanoparticles and Application in the Selective Hydrogenation of Acetylene. Catalysts 2017, 7, 343. https://doi.org/10.3390/catal7110343
Sahooli M, Rahimpour MR, Khorram M. Fine-Tuning Synthesis and Characterization of Mono-Sized H-Beta Zeolite-Supported Palladium-Iridium Nanoparticles and Application in the Selective Hydrogenation of Acetylene. Catalysts. 2017; 7(11):343. https://doi.org/10.3390/catal7110343
Chicago/Turabian StyleSahooli, Masood, Mohammad Reza Rahimpour, and Mohammad Khorram. 2017. "Fine-Tuning Synthesis and Characterization of Mono-Sized H-Beta Zeolite-Supported Palladium-Iridium Nanoparticles and Application in the Selective Hydrogenation of Acetylene" Catalysts 7, no. 11: 343. https://doi.org/10.3390/catal7110343