Base Catalysis by Mono- and Polyoxometalates
Abstract
:1. Introduction
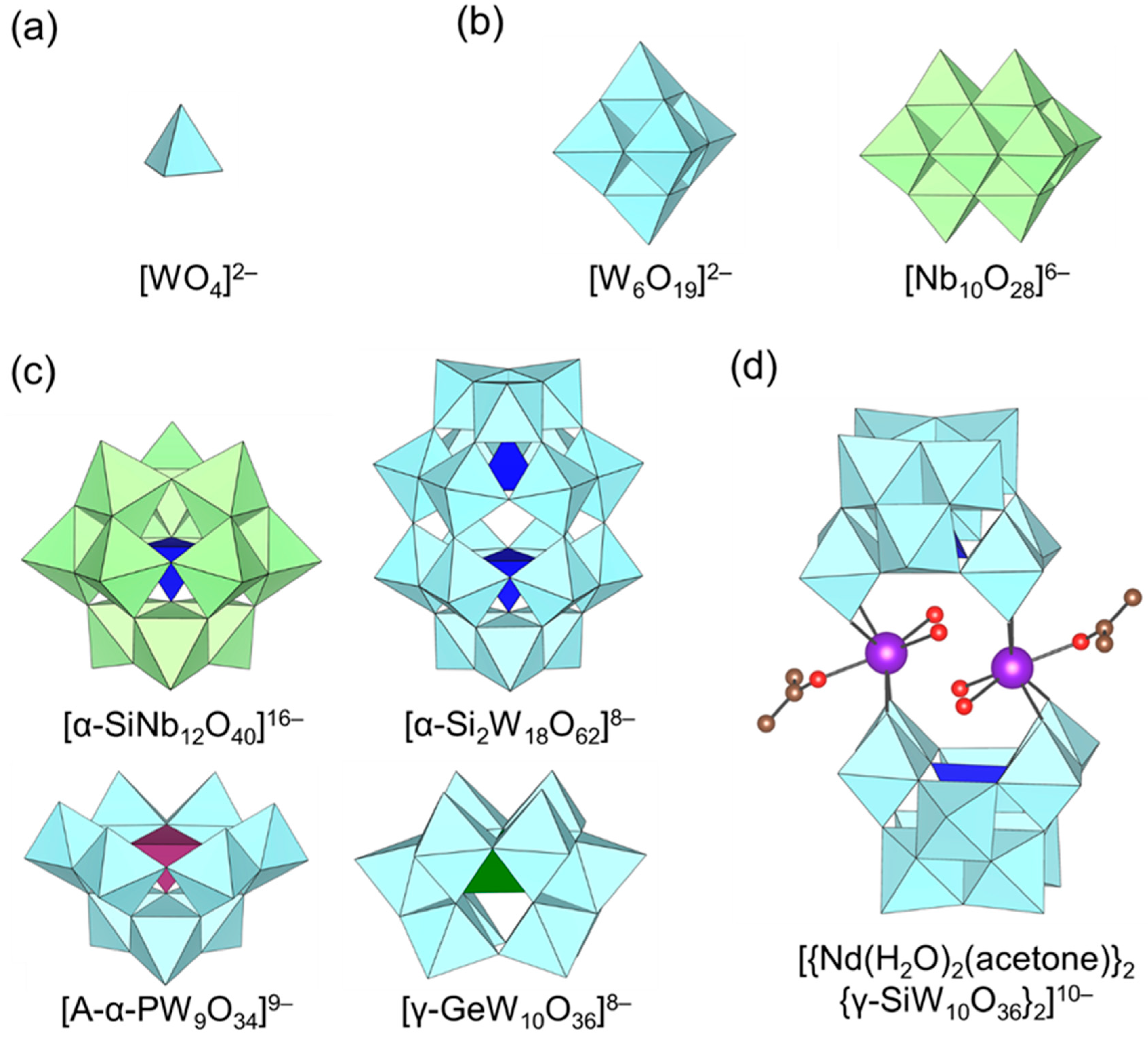
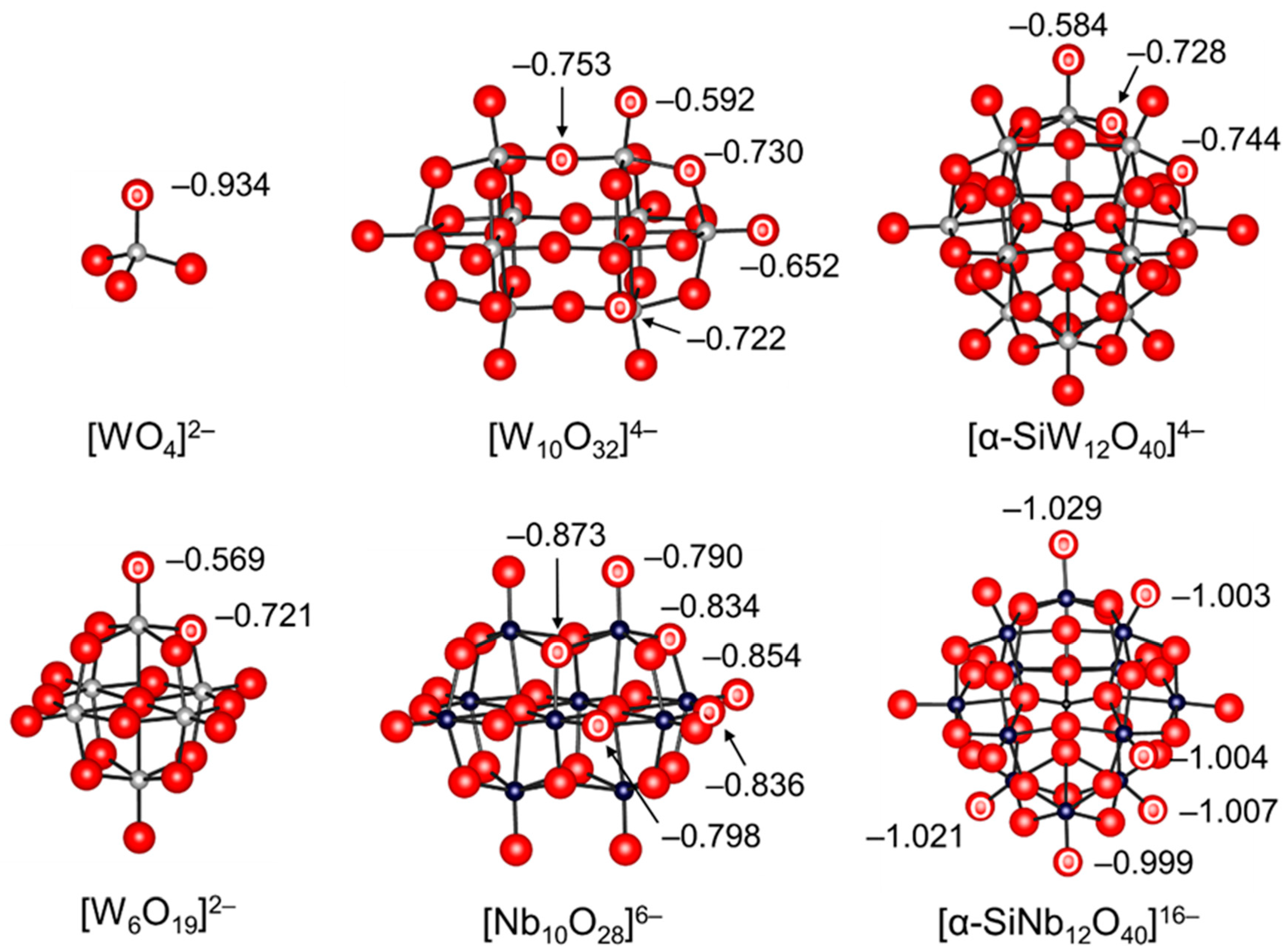
2. Structure and Basic Property of Mono- and Polyoxometalates
2.1. Monomeric Metalates
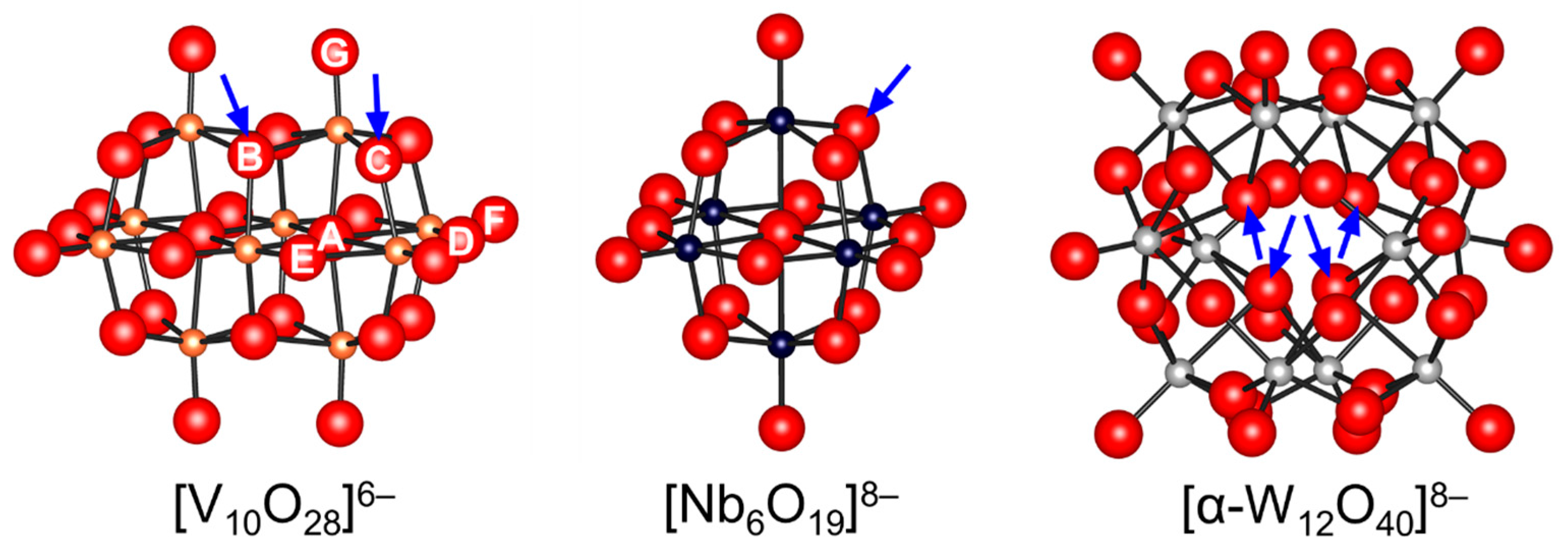
2.2. Isopolyoxometalates
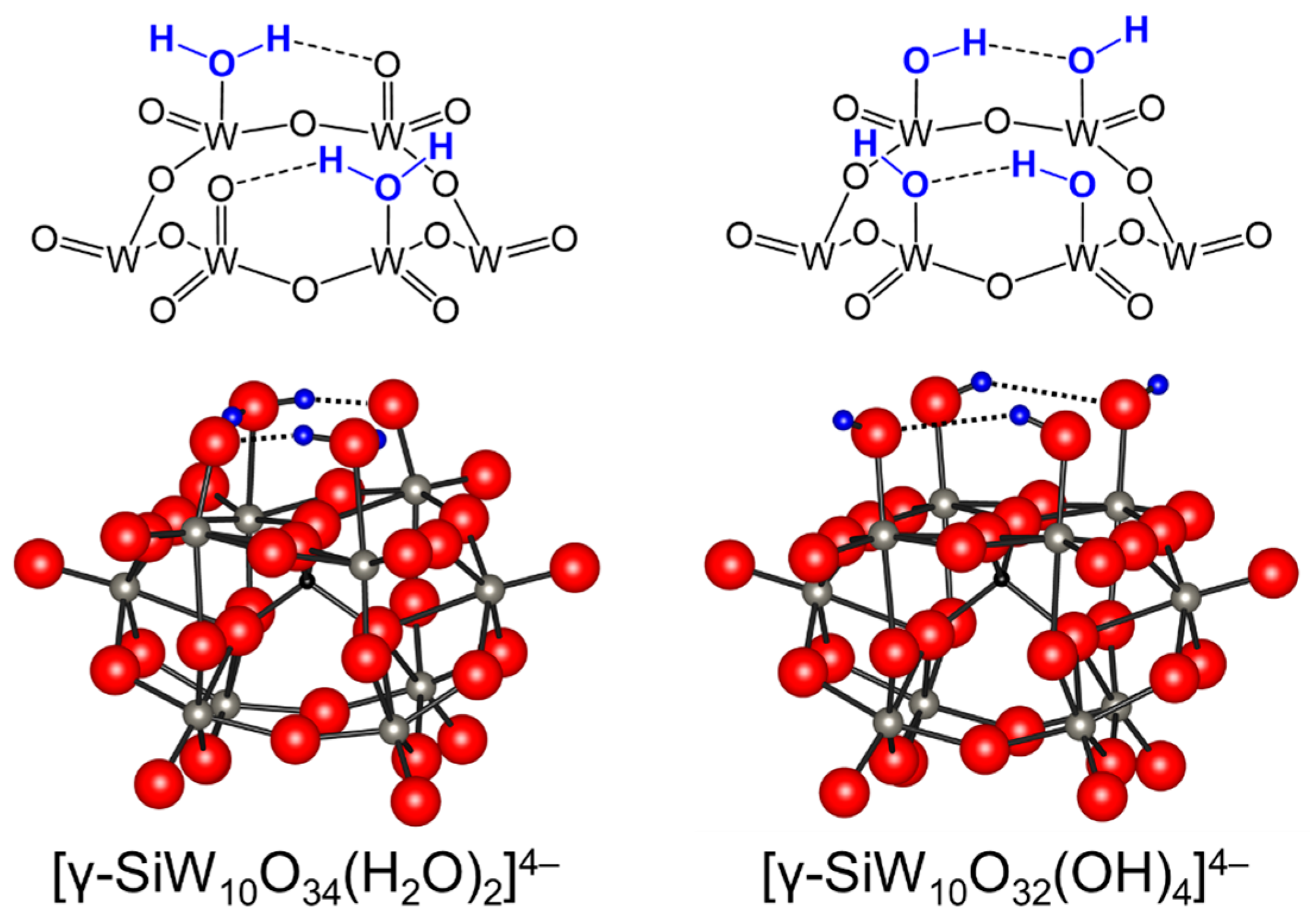
2.3. Heteropolyoxometalates
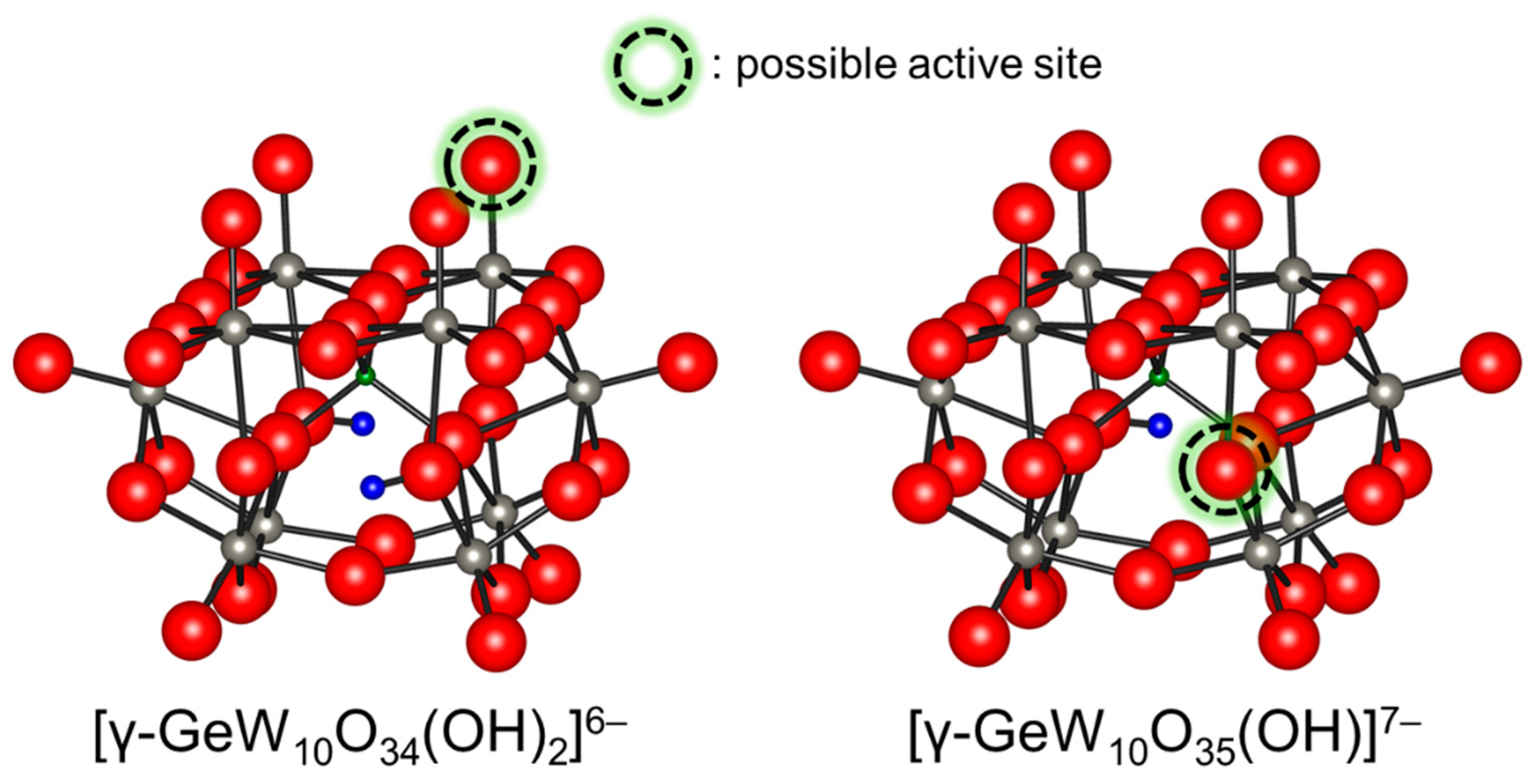
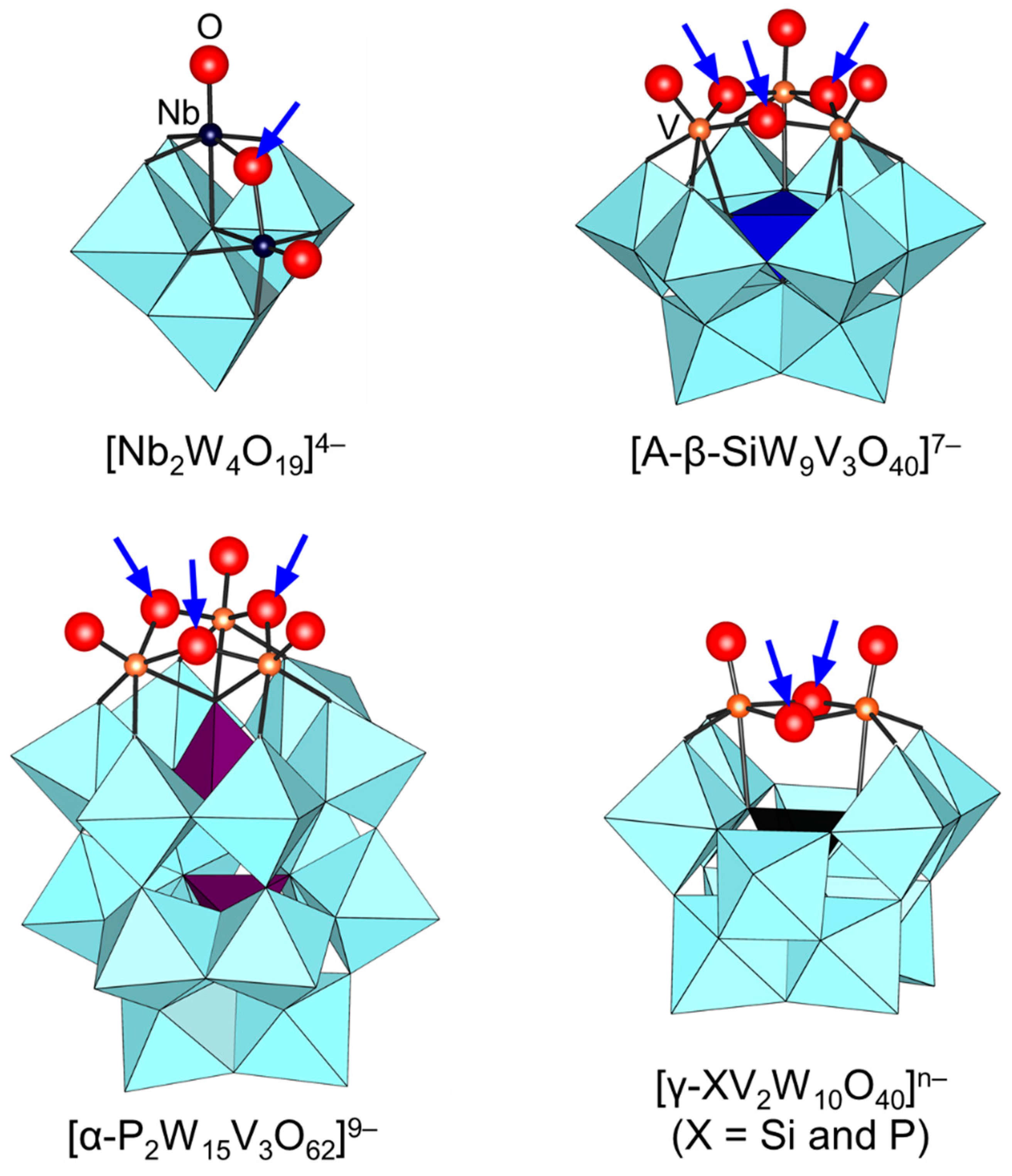
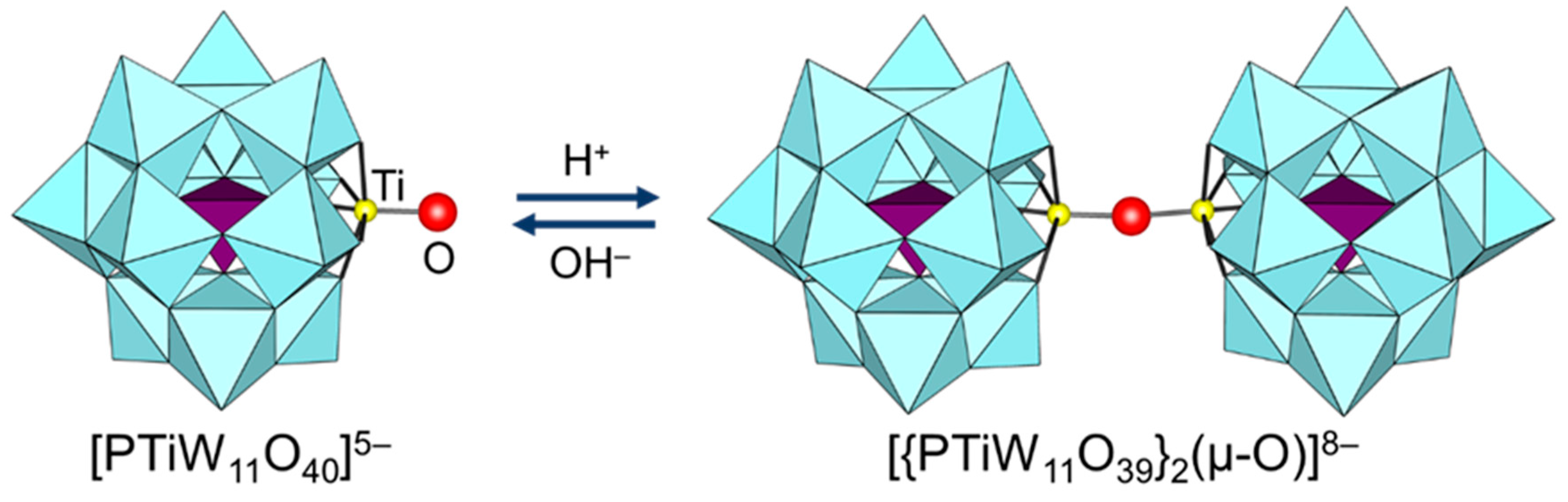
2.4. Transition-Metal-Substituted Polyoxometalates
3. Base-Catalyzed Reactions by Mono- and Polyoxometalates
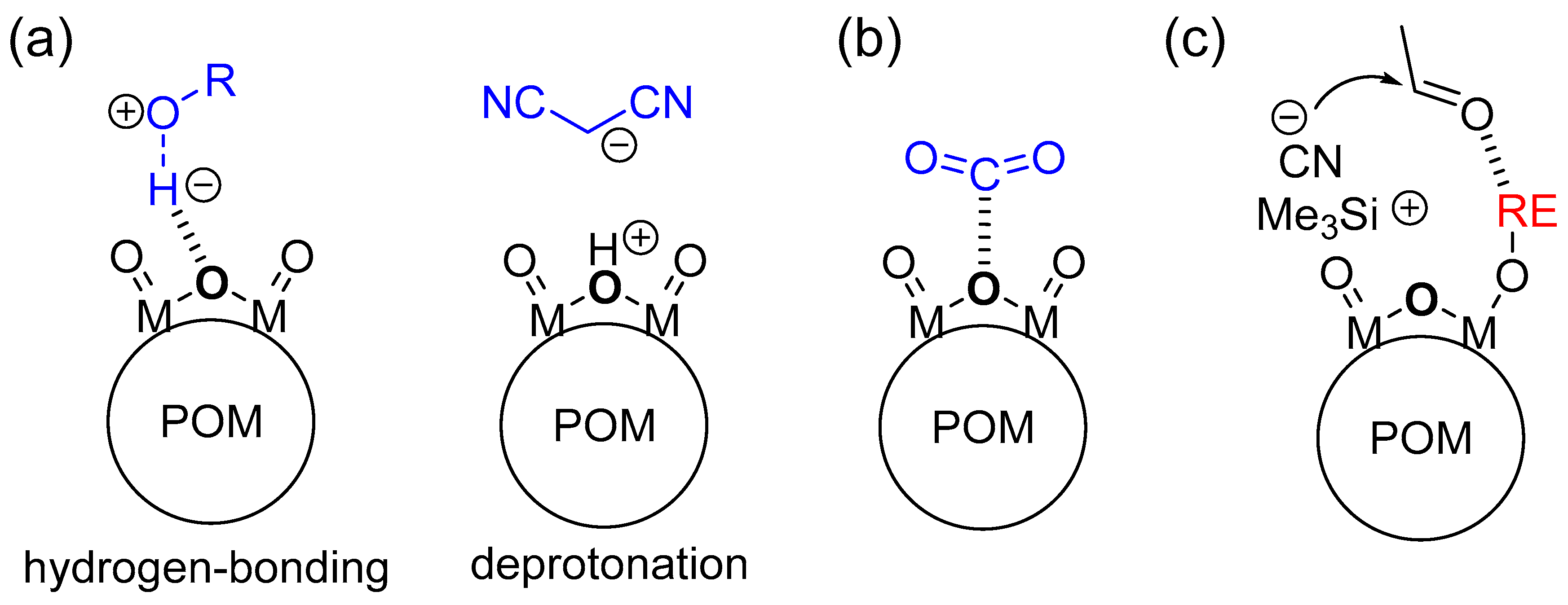
3.1. Chemical Fixation of CO2
3.2. Knoevenagel Condensation
3.3. Cyanosilylation
3.4. Other Reactions
4. Conclusions and Future Opportunities
Acknowledgments
Conflicts of Interest
References
- Climent, M.J.; Corma, A.; Iborra, S.; Primo, J. Base catalysis for fine chemicals production: Claisen-schmidt condensation on zeolites and hydrotalcites for the production of chalcones and flavanones of pharmaceutical interest. J. Catal. 1995, 151, 60–66. [Google Scholar] [CrossRef]
- Tanabe, K. Industrial application of solid acid–base catalysts. Appl. Catal. A Gen. 1999, 181, 399–434. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, H. Solid Base Catalysis; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ono, Y. Solid base catalysts for the synthesis of fine chemicals. J. Catal. 2003, 216, 406–415. [Google Scholar] [CrossRef]
- Hattori, H. Solid base catalysts: Fundamentals and their applications in organic reactions. Appl. Catal. A Gen. 2015, 504, 103–109. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Kim, J.; Fernando, W.J.N. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, Germany, 1983. [Google Scholar]
- Dickman, M.H.; Pope, M.T. Peroxo and superoxo complexes of chromium, molybdenum, and tungsten. Chem. Rev. 1994, 94, 569–584. [Google Scholar] [CrossRef]
- Hill, C.L. Thematic issue on “Polyoxometalates”. Chem. Rev. 1998, 98, 1–389. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Kögerler, P.; Dress, A.W.M. Giant metal-oxide-based spheres and their topology: From pentagonal building blocks to keplerates and unusual spin systems. Coord. Chem. Rev. 2001, 222, 193–218. [Google Scholar] [CrossRef]
- Pope, M.T. Polyoxoanions: Synthesis and structure. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Pergamon: Amsterdam, The Netherlands, 2004; Volume 4, pp. 635–678. [Google Scholar]
- Uchida, S.; Mizuno, N. Design and syntheses of nano-structured ionic crystals with selective sorption properties. Coord. Chem. Rev. 2007, 251, 2537–2546. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Bassil, B.S.; Kortz, U. Divacant polyoxotungstates: Reactivity of the gamma-decatungstates [γ-XW10O36]8− (X = Si, Ge). Dalton Trans. 2011, 40, 9649–9661. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Mialane, P.; Sécheresse, F.; Keita, B.; Nadjo, L. Functionalized polyoxometalates with covalently linked bisphosphonate, n-donor or carboxylate ligands: From electrocatalytic to optical properties. Chem. Commun. 2012, 48, 8299–8316. [Google Scholar] [CrossRef] [PubMed]
- Miras, H.N.; Vilà-Nadal, L.; Cronin, L. Polyoxometalate based open-frameworks (POM-OFs). Chem. Soc. Rev. 2014, 43, 5679–5699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-W.; Li, Y.-Z.; Chen, L.-J.; Yang, G.-Y. Research progress on polyoxometalate-based transition-metal-rare-earth heterometallic derived materials: Synthetic strategies, structural overview and functional applications. Chem. Commun. 2016, 52, 4418–4445. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Rompel, A. Ten good reasons for the use of the tellurium-centered Anderson-Evans polyoxotungstate in protein crystallography. Acc. Chem. Res. 2017, 50, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalytic chemistry of heteropoly compounds. Adv. Catal. 1996, 41, 113–252. [Google Scholar]
- Kozhevnikov, I.V. Catalysts for Fine Chemical Synthesis, Volume 2, Catalysis by Polyoxometalates; John Wiley & Sons: Chichester, UK, 2002. [Google Scholar]
- Hill, C.L. Polyoxometalates: Reactivity. In Comprehensive Coordination Chemistry II, Vol. 4; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Science: New York, NY, USA, 2004; Volume 4, pp. 679–759. [Google Scholar]
- Mizuno, N.; Yamaguchi, K.; Kamata, K. Epoxidation of olefins with hydrogen peroxide catalyzed by polyoxometalates. Coord. Chem. Rev. 2005, 249, 1944–1956. [Google Scholar] [CrossRef]
- Neumann, R.; Khenkin, A.M. Molecular oxygen and oxidation catalysis by phosphovanadomolybdates. Chem. Commun. 2006, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Kamata, K. Catalytic oxidation of hydrocarbons with hydrogen peroxide by vanadium-based polyoxometalates. Coord. Chem. Rev. 2011, 255, 2358–2370. [Google Scholar] [CrossRef]
- Mizuno, N.; Kamata, K.; Yamaguchi, K. Liquid-Phase Selective Oxidation by Multimetallic Active Sites of Polyoxometalate-Based Molecular Catalysts in Topics in Organometallic Chemistry: Chemistry of Bifunctional Molecular Catalysis; Ikariya, T., Shibasaki, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 37, pp. 127–160. [Google Scholar]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, J.; Putaj, P.; Caps, V.; Lefebvre, F.; Pelletier, J.; Basset, J.-M. Catalytic oxidation of light alkanes (C1–C4) by heteropoly compounds. Chem. Rev. 2014, 114, 981–1019. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K. Design of highly functionalized polyoxometalate-based catalysts. Bull. Chem. Soc. Jpn. 2015, 88, 1017–1028. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Z.; Hou, W.; Wang, Q.; Wang, J. Polyoxometalate-based phase transfer catalysis for liquid-solid organic reactions: A review. Catal. Sci. Technol. 2015, 5, 4324–4335. [Google Scholar] [CrossRef]
- Narkhede, N.; Singh, S.; Patel, A. Recent progress on supported polyoxometalates for biodiesel synthesis via esterification and transesterification. Green Chem. 2015, 17, 89–107. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Thomas, H.J.; Climent, M.J.; Romanelli, G.P.; Iborra, S. Heteropolycompounds as catalysts for biomass product transformations. Catal. Rev. Sci. Eng. 2016, 58, 497–586. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Decatungstate anion for photocatalyzed “window ledge” reactions. Acc. Chem. Res. 2016, 49, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Hikichi, S.; Mizuno, N. Acid–base catalyses by dimeric disilicoicosatungstates and divacant γ-Keggin-type silicodecatungstate parent: Reactivity of the polyoxometalate compounds controlled by step-by-step protonation of lacunary W=O sites. J. Organomet. Chem. 2007, 692, 455–459. [Google Scholar] [CrossRef]
- Kikukawa, Y.; Suzuki, K.; Sugawa, M.; Hirano, T.; Kamata, K.; Yamaguchi, K.; Mizuno, N. Cyanosilylation of carbonyl compounds with trimethylsilyl cyanide catalyzed by an yttrium-pillared silicotungstate dimer. Angew. Chem. Int. Ed. 2012, 51, 3686–3690. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kamata, K.; Mizuno, N. A bifunctional tungstate catalyst for chemical fixation of CO2 at atmospheric pressure. Angew. Chem. Int. Ed. 2012, 51, 6700–6703. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Kimura, T.; Kamata, K.; Yamaguchi, K.; Mizuno, N. A highly negatively charged γ-Keggin germanodecatungstate efficient for knoevenagel condensation. Chem. Commun. 2012, 48, 8422–8424. [Google Scholar] [CrossRef] [PubMed]
- Davoodnia, A. An efficient method for knoevenagel condensation catalyzed by tetrabutylammonium hexatungstate [TBA]2[W6O19] as novel and reusable heterogeneous catalyst. Synth. React. Inorg. Met.-Org. Chem. 2012, 42, 1022–1026. [Google Scholar] [CrossRef]
- Suzuki, K.; Sugawa, M.; Kikukawa, Y.; Kamata, K.; Yamaguchi, K.; Mizuno, N. Strategic design and refinement of Lewis acid-base catalysis by rare-earth-metal-containing polyoxometalates. Inorg. Chem. 2012, 51, 6953–6961. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Sunaba, H.; Kamata, K.; Mizuno, N. Efficient [WO4]2−-catalyzed chemical fixation of carbon dioxide with 2-aminobenzonitriles to quinazoline-2,4(1H,3H)-diones. Inorg. Chem. 2012, 51, 13001–13008. [Google Scholar] [CrossRef] [PubMed]
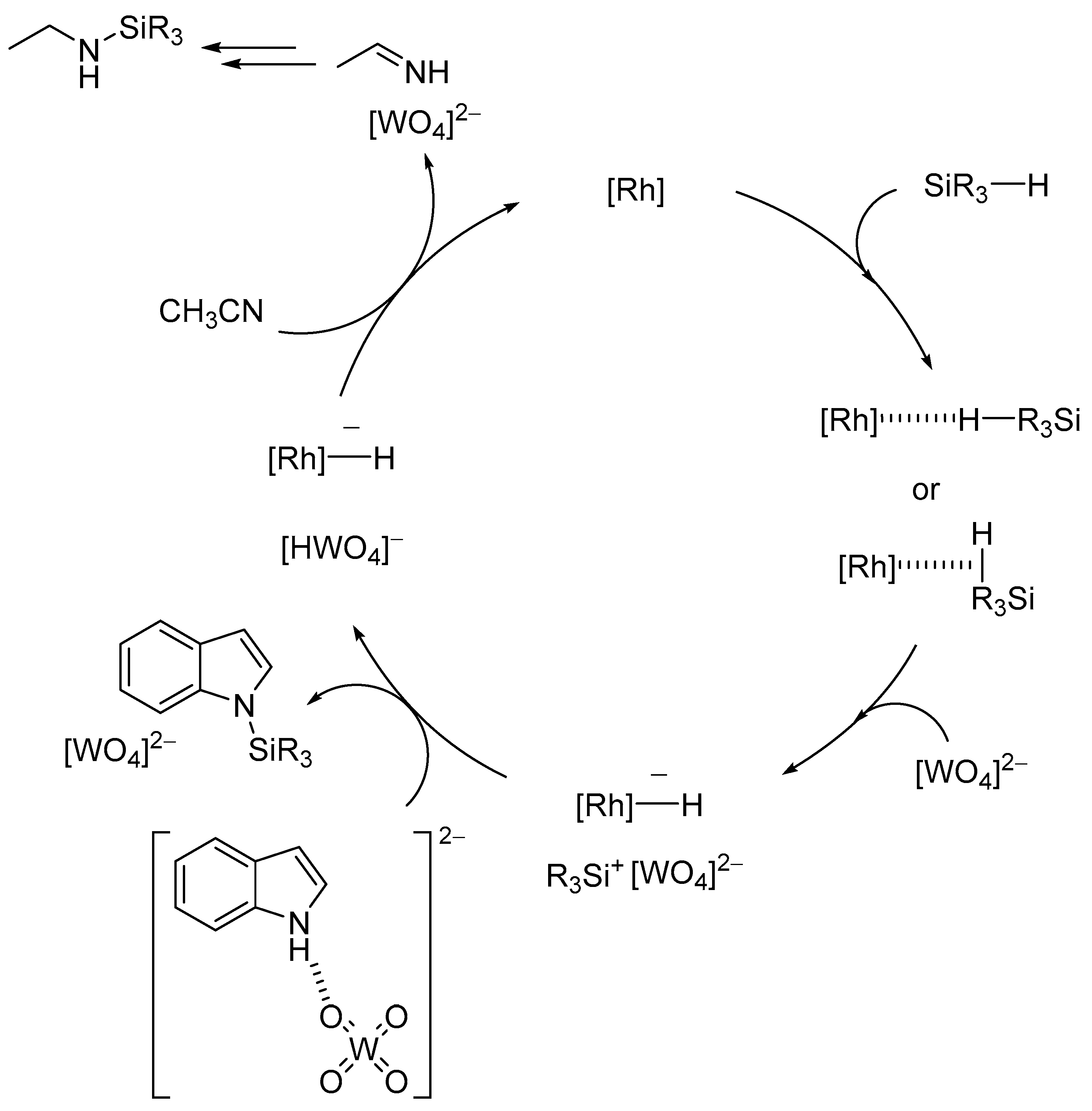
- Itagaki, S.; Kamata, K.; Yamaguchi, K.; Mizuno, N. Rhodium acetate/base-catalyzed N-silylation of indole derivatives with hydrosilanes. Chem. Commun. 2012, 48, 9269–9271. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; Sunaba, H.; Kamata, K.; Yamaguchi, K.; Mizuno, N. Hydrosilylation of various multiple bonds by a simple combined catalyst of a tungstate monomer and rhodium acetate. Chem. Lett. 2013, 42, 980–982. [Google Scholar] [CrossRef]
- Kamata, K.; Kimura, T.; Sunaba, H.; Mizuno, N. Scope of chemical fixation of carbon dioxide catalyzed by a bifunctional monomeric tungstate. Catal. Today 2014, 226, 160–166. [Google Scholar] [CrossRef]
- Sugahara, K.; Satake, N.; Kamata, K.; Nakajima, T.; Mizuno, N. A basic germanodecatungstate with a –7 charge: Efficient chemoselective acylation of primary alcohols. Angew. Chem. Int. Ed. 2014, 53, 13248–13252. [Google Scholar] [CrossRef] [PubMed]
- Minato, T.; Suzuki, K.; Kamata, K.; Mizuno, N. Synthesis of α-Dawson-type silicotungstate [α-Si2W18O62]8− and protonation and deprotonation inside the aperture through intramolecular hydrogen bonds. Chem. Eur. J. 2014, 20, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-W.; Yu, B.; Li, X.-D.; Ma, R.; Diao, Z.-F.; Li, R.-G.; Li, W.; He, L.-N. Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system. Green Chem. 2014, 16, 1633–1638. [Google Scholar] [CrossRef]
- Knopf, I.; Ono, T.; Temprado, M.; Tofan, D.; Cummins, C.C. Uptake of one and two molecules of CO2 by the molybdate dianion: A soluble, molecular oxide model system for carbon dioxide fixation. Chem. Sci. 2014, 5, 1772–1776. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Y.; Song, Y.-F. Tri-lacunary polyoxometalates of Na8H[PW9O34] as heterogeneous Lewis base catalysts for Knoevenagel condensation, cyanosilylation and the synthesis of benzoxazole derivatives. Appl. Catal. A Gen. 2014, 475, 140–146. [Google Scholar] [CrossRef]
- Fei, F.; An, H.; Meng, C.; Wang, L.; Wang, H. Lanthanide-supported molybdenum–vanadium oxide clusters: Syntheses, structures and catalytic properties. RSC Adv. 2015, 5, 18796–18805. [Google Scholar] [CrossRef]
- An, H.; Wang, L.; Hu, Y.; Fei, F. Temperature-induced racemic compounds and chiral conglomerates based on polyoxometalates and lanthanides: Syntheses, structures and catalytic properties. CrystEngComm 2015, 17, 1531–1540. [Google Scholar] [CrossRef]
- Chen, A.; Chen, C.; Xiu, Y.; Liu, X.; Chen, J.; Guo, L.; Zhang, R.; Hou, Z. Niobate salts of organic base catalyzed chemical fixation of carbon dioxide with epoxides to form cyclic carbonates. Green Chem. 2015, 17, 1842–1852. [Google Scholar] [CrossRef]
- Guo, C.-X.; Yu, B.; Xie, J.-N.; He, L.-N. Silver tungstate: A single-component bifunctional catalyst for carboxylation of terminal alkynes with CO2 in ambient conditions. Green Chem. 2015, 17, 474–479. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamazoe, S.; Koyasu, K.; Tsukuda, T. Application of group V polyoxometalate as an efficient base catalyst: A case study of decaniobate clusters. RSC Adv. 2016, 6, 16239–16242. [Google Scholar] [CrossRef]
- Ge, W.; Wang, X.; Zhang, L.; Du, L.; Zhou, Y.; Wang, J. Fully-occupied Keggin type polyoxometalate as solid base for catalyzing CO2 cycloaddition and Knoevenagel condensation. Catal. Sci. Technol. 2016, 6, 460–467. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamazoe, S.; Koyasu, K.; Tsukuda, T. Lewis base catalytic properties of [Nb10O28]6− for CO2 fixation to epoxide: Kinetic and theoretical studies. Chem. Asian J. 2017, 12, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; An, H.; Ding, B.; Li, Y. Evans-showell-type polyoxometalate constructing novel 3d inorganic architectures with alkaline earth metal linkers: Syntheses, structures and catalytic properties. Dalton Trans. 2017, 46, 8439–8450. [Google Scholar] [CrossRef] [PubMed]
- Hedman, B. Multicomponent polyanions. 18. A neutron diffraction study of Na3Mo9PO31(OH2)3.12–13H2O, a compound containing 9-molybdomonophosphate anions with molybdenum-coordinated water molecules. Acta Chem. Scand. A 1978, 32, 439–446. [Google Scholar] [CrossRef]
- Che, T.M.; Day, V.W.; Francesconi, L.C.; Fredrich, M.F.; Klemperer, W.G.; Shum, W. Synthesis and structure of the [(η5-C5H5)Ti(Mo5O18)]3− and [(η5-C5H5)Ti(W5O18)]3− anions. Inorg. Chem. 1985, 24, 4055–4062. [Google Scholar] [CrossRef]
- Day, V.W.; Klemperer, W.G.; Maltbie, D.J. Where are the protons in H3V10O283−? J. Am. Chem. Soc. 1987, 109, 2991–3002. [Google Scholar] [CrossRef]
- Day, V.W.; Klemperer, W.G.; Schwartz, C. Synthesis, characterization, and interconversion of the niobotungstic acid Nb2W4O19H3− and its anhydride and alkyl/silyl esters. J. Am. Chem. Soc. 1987, 109, 6030–6044. [Google Scholar] [CrossRef]
- Che, T.M.; Day, V.W.; Francesconi, L.C.; Klemperer, W.G.; Main, D.J.; Yagasaki, A.; Yaghi, O.M. Mono- and diprotonation of the [(η5-C5H5)Ti(W5O18)]3− and [(η5-C5Me5)Ti(W5O18)]3− anions. Inorg. Chem. 1992, 31, 2920–2928. [Google Scholar] [CrossRef]
- Ozeki, T.; Yamase, T.; Naruke, H.; Sasaki, Y. X-ray structural characterization of the protonation sites in the dihydrogenhexaniobate anion. Bull. Chem. Soc. Jpn. 1994, 67, 3249–3253. [Google Scholar] [CrossRef]
- Nakamura, S.; Ozeki, T. Hydrogen-bonded aggregates of protonated decavanadate anions in their tetraalkylammonium salts. J. Chem. Soc. Dalton Trans. 2001, 472–480. [Google Scholar] [CrossRef]
- Kamata, K.; Yonehara, K.; Sumida, Y.; Yamaguchi, K.; Hikichi, S.; Mizuno, N. Efficient epoxidation of olefins with ≥99% selectivity and use of hydrogen peroxide. Science 2003, 300, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Leclerc-Laronze, N.; Haouas, M.; Marrot, J.; Taulelle, F.; Hervé, G. Step-by-step assembly of trivacant tungstosilicates: Synthesis and characterization of tetrameric anions. Angew. Chem. Int. Ed. 2005, 45, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Yoshimura, M.; Uehara, K.; Hikichi, S.; Mizuno, N. Formation of S-shaped disilicoicosatungstate and efficient baeyer-villiger oxidation with hydrogen peroxide. Angew. Chem. Int. Ed. 2006, 45, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Hill, C.L. Multiple reversible protonation of polyoxoanion surfaces: Direct observation of dynamic structural effects from proton transfer. Angew. Chem. Int. Ed. 2007, 46, 3877–3880. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ozeki, T. Guest driven rearrangements of protonation and hydrogen bonding in decavanadate anions as their tetraalkylammonium salts. Dalton Trans. 2008, 6135–6140. [Google Scholar] [CrossRef] [PubMed]
- Klemperer, W.G.; Shum, W. Charge distribution in large polyoxoanions: Determination of protonation sites in vanadate (V10O286−) by 17O nuclear magnetic resonance. J. Am. Chem. Soc. 1977, 99, 3544–3545. [Google Scholar] [CrossRef]
- Klemperer, W.G.; Shum, W. Isomerism and charge distribution in mixed-metal polyoxoanion clusters: 17O nuclear magnetic resonance structure determinations of cis-V2W4O194− and cis-HV2W4O193−. J. Am. Chem. Soc. 1978, 100, 4891–4893. [Google Scholar] [CrossRef]
- Filowitz, M.; Ho, R.K.C.; Klemperer, W.G.; Shum, W. 17O nuclear magnetic resonance spectroscopy of polyoxometalates. 1. Sensitivity and resolution. Inorg. Chem. 1979, 18, 93–103. [Google Scholar] [CrossRef]
- Harrison, A.T.; Howarth, O.W. Oxygen exchange and protonation of polyanions: A multinuclear magnetic resonance study of tetradecavanadophosphate(9–) and decavanadate(6–). J. Chem. Soc. Dalton Trans. 1985, 1953–1957. [Google Scholar] [CrossRef]
- Finke, R.G.; Rapko, B.; Saxton, R.J.; Domaille, P.J. Trisubstituted heteropolytungstates as soluble metal oxide analogs. 3. Synthesis, characterization, 31P, 29Si, 51V, 31P, and 1-and 2-D 183W NMR, deprotonation, and proton mobility studies of organic solvent solute forms of HxSiW9V3O40x−7 and HxP2W15V3O62x−9. J. Am. Chem. Soc. 1986, 108, 2947–2960. [Google Scholar]
- Pohl, M.; Finke, R.G. Polyoxoanion-supported, atomically dispersed transition-metal precatalyst [(1,5-COD)Ir·P2W15Nb3O62]8−: Direct 17O NMR evidence for Ir-ONb2 bonding and for a C3V average symmetry, iridium-to-polyoxoanion support interaction. Organometallics 1993, 12, 1453–1457. [Google Scholar] [CrossRef]
- Weiner, H.; Aiken, J.D.; Finke, R.G. Polyoxometalate catalyst precursors. Improved synthesis, H+-titration procedure, and evidence for 31P NMR as a highly sensitive support-site indicator for the prototype polyoxoanion−organometallic-support system [(n-C4H9)4]9P2W15Nb3O62. Inorg. Chem. 1996, 35, 7905–7913. [Google Scholar] [CrossRef]
- Sprangers, C.R.; Marmon, J.K.; Duncan, D.C. Where are the protons in α-[HxW12O40](8−x)− (x = 2–4)? Inorg. Chem. 2006, 45, 9628–9630. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Anderson, T.M.; Rustad, J.R.; Nyman, M.; Casey, W.H. Rates of oxygen-isotope exchange between sites in the [HxTa6O19](8−x)−(aq) Lindqvist ion and aqueous solutions: Comparisons to [HxNb6O19](8−x)−(aq). Inorg. Chem. 2007, 46, 7032–7039. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Guirauden, A.; Fourmigué, M.; Boubekeur, K.; Batail, P.; Rohmer, M.-M.; Bénard, M.; Coulon, C.; Sallé, M.; Blanchard, P. Relative basicities of the oxygen atoms of the Linquist polyoxometalate [Mo6O19]2− and their recognition by hydroxyl groups in radical cation salts based on functionalized tetrathiafulvalene π donors. J. Chem. Soc. Dalton Trans. 1999, 1241–1248. [Google Scholar] [CrossRef]
- López, X.; Bo, C.; Poblet, J.M. Electronic properties of polyoxometalates: Electron and proton affinity of mixed-addenda Keggin and Wells−Dawson anions. J. Am. Chem. Soc. 2002, 124, 12574–12582. [Google Scholar] [CrossRef] [PubMed]
- Musaev, D.G.; Morokuma, K.; Geletii, Y.V.; Hill, C.L. Computational modeling of di-transition-metal-substituted γ-Keggin polyoxometalate anions. Structural refinement of the protonated divacant lacunary silicodecatungstate. Inorg. Chem. 2004, 43, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Sartorel, A.; Carraro, M.; Bagno, A.; Scorrano, G.; Bonchio, M. Asymmetric tetraprotonation of γ-[(SiO4)W10O32]8− triggers a catalytic epoxidation reaction: Perspectives in the assignment of the active catalyst. Angew. Chem. Int. Ed. 2007, 46, 3255–3258. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, I.; Neumann, R. Protonation of phosphovanadomolybdates H3+xPVxMo12−xO40: Computational insight into reactivity. J. Phys. Chem. A 2011, 115, 4811–4826. [Google Scholar] [CrossRef] [PubMed]
- Himeno, S.; Takamoto, M.; Santo, R.; Ichimura, A. Redox properties and basicity of Keggin-type polyoxometalate complexes. Bull. Chem. Soc. Jpn. 2005, 78, 95–100. [Google Scholar] [CrossRef]
- Nakajima, K.; Eda, K.; Himeno, S. Effect of the central oxoanion size on the voltammetric properties of Keggin-type [XW12O40]n− (n = 2–6) complexes. Inorg. Chem. 2010, 49, 5212–5215. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Sandei, L.; Sartorel, A.; Scorrano, G.; Bonchio, M. Hybrid polyoxotungstates as second-generation POM-based catalysts for microwave-assisted H2O2 activation. Org. Lett. 2006, 8, 3671–3674. [Google Scholar] [CrossRef] [PubMed]
- Sartorel, A.; Carraro, M.; Bagno, A.; Scorrano, G.; Bonchio, M. H2O2 activation by heteropolyacids with defect structures: The case of γ-[(XO4)W10O32]n− (X = Si, Ge, n = 8; X = P, n = 7). J. Phys. Org. Chem. 2008, 21, 596–602. [Google Scholar] [CrossRef]
- Phan, T.D.; Kinch, M.A.; Barker, J.E.; Ren, T. Highly efficient utilization of H2O2 for oxygenation of organic sulfides catalyzed by [γ-SiW10O34(H2O)2]4−. Tetrahedron Lett. 2005, 46, 397–400. [Google Scholar] [CrossRef]
- Berardi, S.; Bonchio, M.; Carraro, M.; Conte, V.; Sartorel, A.; Scorrano, G. Fast catalytic epoxidation with H2O2 and [γ-SiW10O36(PhPO)2]4− in ionic liquids under microwave irradiation. J. Org. Chem. 2007, 72, 8954–8957. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Kuzuya, S.; Hirano, T.; Kamata, K.; Mizuno, N. Reversible deprotonation and protonation behaviors of a tetra-protonated γ-Keggin silicodecatungstate. Inorg. Chem. 2012, 51, 7932–7939. [Google Scholar] [CrossRef] [PubMed]
- Boglio, C.; Micoine, K.; Derat, É.; Thouvenot, R.; Hasenknopf, B.; Thorimbert, S.; Lacôte, E.; Malacria, M. Regioselective activation of oxo ligands in functionalized Dawson polyoxotungstates. J. Am. Chem. Soc. 2008, 130, 4553–4561. [Google Scholar] [CrossRef] [PubMed]
- Thorimbert, S.; Hasenknopf, B.; Lacôte, E. Cross-linking organic and polyoxometalate chemistries. Isr. J. Chem. 2011, 51, 275–280. [Google Scholar] [CrossRef]
- Micoine, K.; Malacria, M.; Lacôte, E.; Thorimbert, S.; Hasenknopf, B. Regioselective double organic functionalization of polyoxotungstates through electrophilic addition of aromatic isocyanates to [P2W17O61(SnR)]7−. Eur. J. Inorg. Chem. 2013, 2013, 1737–1741. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kamata, K.; Kotani, M.; Yamaguchi, K.; Mizuno, N. Polyoxovanadometalate-catalyzed selective epoxidation of alkenes with hydrogen peroxide. Angew. Chem. Int. Ed. 2005, 44, 5136–5141. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Mizuno, N. Mechanism of [γ-H2SiV2W10O40]4−-catalyzed epoxidation of alkenes with hydrogen peroxide. Inorg. Chem. 2007, 46, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Yonehara, K.; Nakagawa, Y.; Uehara, K.; Mizuno, N. Efficient stereo- and regioselective hydroxylation of alkanes catalysed by a bulky polyoxometalate. Nat. Chem. 2010, 2, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Yonehara, K.; Kamata, K.; Yamaguchi, K.; Mizuno, N. An efficient H2O2-based oxidative bromination of alkenes, alkynes, and aromatics by a divanadium-substituted phosphotungstate. Chem. Commun. 2011, 47, 1692–1694. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Sugahara, K.; Yonehara, K.; Ishimoto, R.; Mizuno, N. Efficient epoxidation of electron-deficient alkenes with hydrogen peroxide catalyzed by [γ-PW10O38V2(μ-OH)2]3−. Chem. Eur. J. 2011, 17, 7549–7559. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Yamaura, T.; Mizuno, N. Chemo- and regioselective direct hydroxylation of arenes with hydrogen peroxide catalyzed by a divanadium-substituted phosphotungstate. Angew. Chem. Int. Ed. 2012, 51, 7275–7278. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Miyachi, T.; Mizuno, N. Amphiprotic properties of a bis(μ-hydroxo)divanadium(IV)-substituted γ-Keggin-type silicodecatungstate containing two different kinds of hydroxyl moieties. Inorg. Chem. 2014, 53, 5341–5347. [Google Scholar] [CrossRef] [PubMed]
- Kholdeeva, O.A.; Maksimov, G.M.; Maksimovskaya, R.I.; Kovaleva, L.A.; Fedotov, M.A.; Grigoriev, V.A.; Hill, C.L. A dimeric titanium-containing polyoxometalate. Synthesis, characterization, and catalysis of H2O2-based thioether oxidation. Inorg. Chem. 2000, 39, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Kholdeeva, O.A.; Maksimov, G.M.; Maksimovskaya, R.I.; Vanina, M.P.; Trubitsina, T.A.; Naumov, D.Y.; Kolesov, B.A.; Antonova, N.S.; Carbó, J.J.; Poblet, J.M. Zr(IV)-monosubstituted keggin-type dimeric polyoxometalates: Synthesis, characterization, catalysis of H2O2-based oxidations, and theoretical study. Inorg. Chem. 2006, 45, 7224–7234. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Murakami, H.; Sakai, Y.; Nomiya, K. Syntheses, molecular structures and pH-dependent monomer–dimer equilibria of dawson α2-monotitanium(IV)-substituted polyoxometalates. Dalton Trans. 2008, 4630–4638. [Google Scholar] [CrossRef]
- Xuan, W.-J.; Botuha, C.; Hasenknopf, B.; Thorimbert, S. Addition of carbon nucleophiles to hemiaminals promoted by a Lewis acidic polyoxotungstate. Org. Chem. Front. 2014, 1, 1091–1095. [Google Scholar] [CrossRef]
- Dupré, N.; Rémy, P.; Micoine, K.; Boglio, C.; Thorimbert, S.; Lacôte, E.; Hasenknopf, B.; Malacria, M. Chemoselective catalysis with organosoluble lewis acidic polyoxotungstates. Chem. Eur. J. 2010, 16, 7256–7264. [Google Scholar] [CrossRef] [PubMed]
- Boglio, C.; Lemière, G.; Hasenknopf, B.; Thorimbert, S.; Lacôte, E.; Malacria, M. Lanthanide complexes of the monovacant dawson polyoxotungstate [α1-P2W17O61]10− as selective and recoverable Lewis acid catalysts. Angew. Chem. Int. Ed. 2006, 45, 3324–3327. [Google Scholar] [CrossRef] [PubMed]
- El Moll, H.; Nohra, B.; Mialane, P.; Marrot, J.; Dupré, N.; Riflade, B.; Malacria, M.; Thorimbert, S.; Hasenknopf, B.; Lacôte, E.; et al. Lanthanide polyoxocationic complexes: Experimental and theoretical stability studies and Lewis acid catalysis. Chem. Eur. J. 2011, 17, 14129–14138. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M. Carbon Dioxide as Chemical Feedstock; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Shi, F.; Deng, Y.; SiMa, T.; Peng, J.; Gu, Y.; Qiao, B. Alternatives to phosgene and carbon monoxide: Synthesis of symmetric urea derivatives with carbon dioxide in ionic liquids. Angew. Chem. Int. Ed. 2003, 42, 3257–3260. [Google Scholar] [CrossRef] [PubMed]
- Ion, A.; Parvulescu, V.; Jacobs, P.; Vos, D.D. Synthesis of symmetrical or asymmetrical urea compounds from CO2 via base catalysis. Green Chem. 2007, 9, 158–161. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, X.; Zhou, Y.; Liang, S.; Zhang, J.; Han, B. Solvent-free synthesis of substituted ureas from CO2 and amines with a functional ionic liquid as the catalyst. Green Chem. 2008, 10, 465–469. [Google Scholar] [CrossRef]
- Kayaki, Y.; Yamamoto, M.; Ikariya, T. N-heterocyclic carbenes as efficient organocatalysts for CO2 fixation reactions. Angew. Chem. Int. Ed. 2009, 48, 4194–4197. [Google Scholar] [CrossRef] [PubMed]
- Lalrempuia, R.; Iglesias, M.; Polo, V.; Sanz Miguel, P.J.; Fernández-Alvarez, F.J.; Pérez-Torrente, J.J.; Oro, L.A. Effective fixation of CO2 by iridium-catalyzed hydrosilylation. Angew. Chem. Int. Ed. 2012, 51, 12824–12827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyzavi, M.H.; Klet, R.C.; Tussupbayev, S.; Borycz, J.; Vermeulen, N.A.; Cramer, C.J.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. A hafnium-based metal-organic framework as an efficient and multifunctional catalyst for facile CO2 fixation and regioselective and enantioretentive epoxide activation. J. Am. Chem. Soc. 2014, 136, 15861–15864. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Z.; Wang, X.J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y. A triazole-containing metal-organic framework as a highly effective and substrate size-dependent catalyst for CO2 conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.W.; Boebel, T.A.; Hartwig, J.F. Iridium-catalyzed, silyl-directed borylation of nitrogen-containing heterocycles. J. Am. Chem. Soc. 2010, 132, 4068–4069. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimoto, T.; Iketani, Y.; Sekine, M. Zinc-catalyzed dehydrogenative N-silylation of indoles with hydrosilanes. Chem. Eur. J. 2012, 18, 9500–9504. [Google Scholar] [CrossRef] [PubMed]
- Dassonville, A.; Lardic, M.; Breteche, A.; Nourrisson, M.R.; Le Baut, G.; Grimaud, N.; Petit, J.Y.; Duflos, M. N-pyridinyl(methyl)-indole-1- or 3-propanamides and propenamides acting as topical and systemic inflammation inhibitors. J. Enzyme Inhib. Med. Chem. 2008, 23, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Ferorelli, S.; Abate, C.; Pedone, M.P.; Colabufo, N.A.; Contino, M.; Perrone, R.; Berardi, F. Synthesis and binding assays of novel 3,3-dimethylpiperidine derivatives with various lipophilicities as sigma(1) receptor ligands. Bioorg. Med. Chem. 2011, 19, 7612–7622. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.; Acher, F.C.; McCort-Tranchepain, I. Microwave-promoted michael addition of azaheterocycles to α,β-unsaturated esters and acid under solvent-free conditions. Synlett 2012, 23, 791–795. [Google Scholar] [CrossRef]
- Sevov, C.S.; Zhou, J.S.; Hartwig, J.F. Iridium-catalyzed, intermolecular hydroamination of unactivated alkenes with indoles. J. Am. Chem. Soc. 2014, 136, 3200–3207. [Google Scholar] [CrossRef] [PubMed]
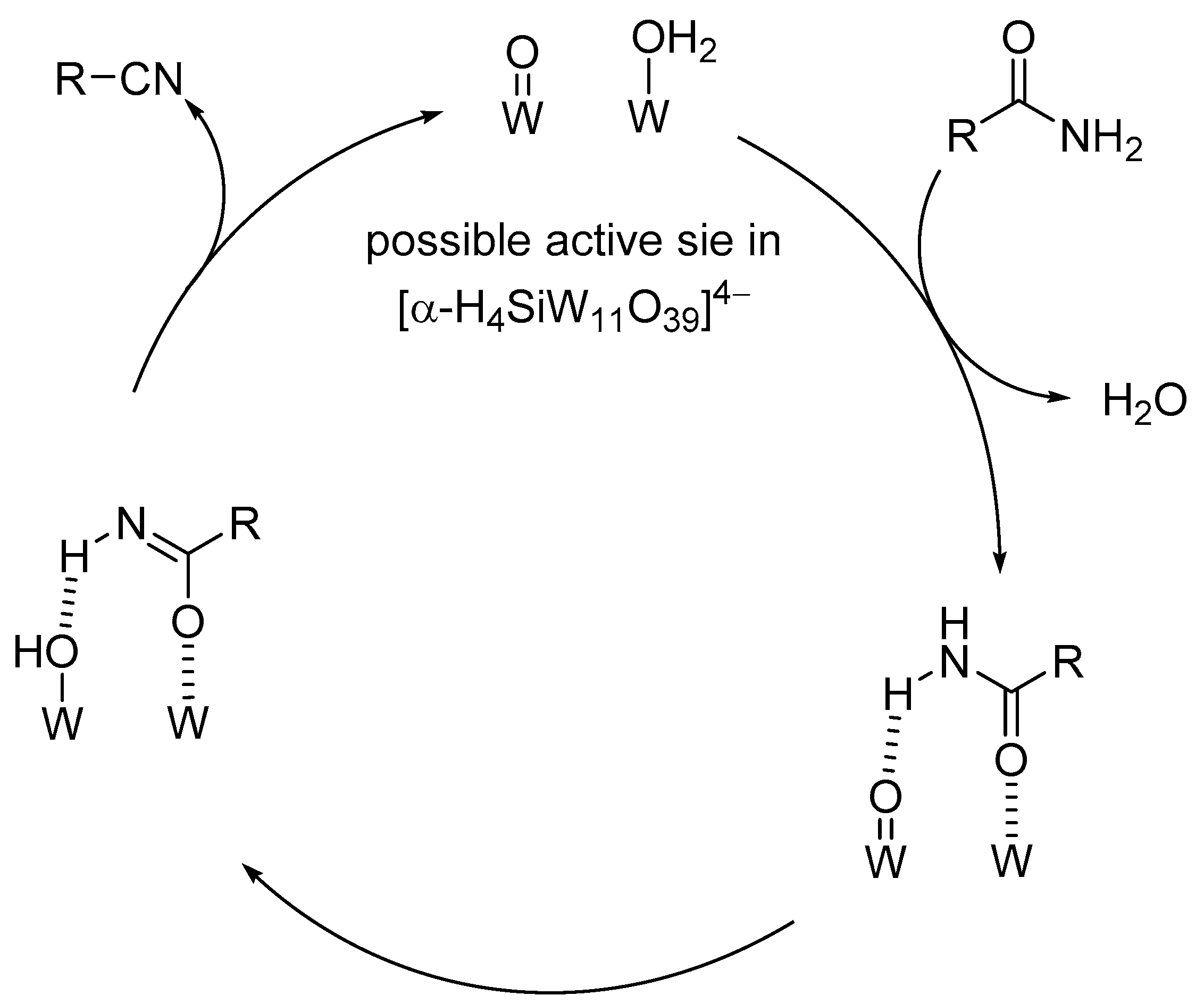
- Itagaki, S.; Kamata, K.; Yamaguchi, K.; Mizuno, N. A monovacant lacunary silicotungstate as an efficient heterogeneous catalyst for dehydration of primary amides to nitriles. ChemCatChem 2013, 5, 1725–1728. [Google Scholar] [CrossRef]


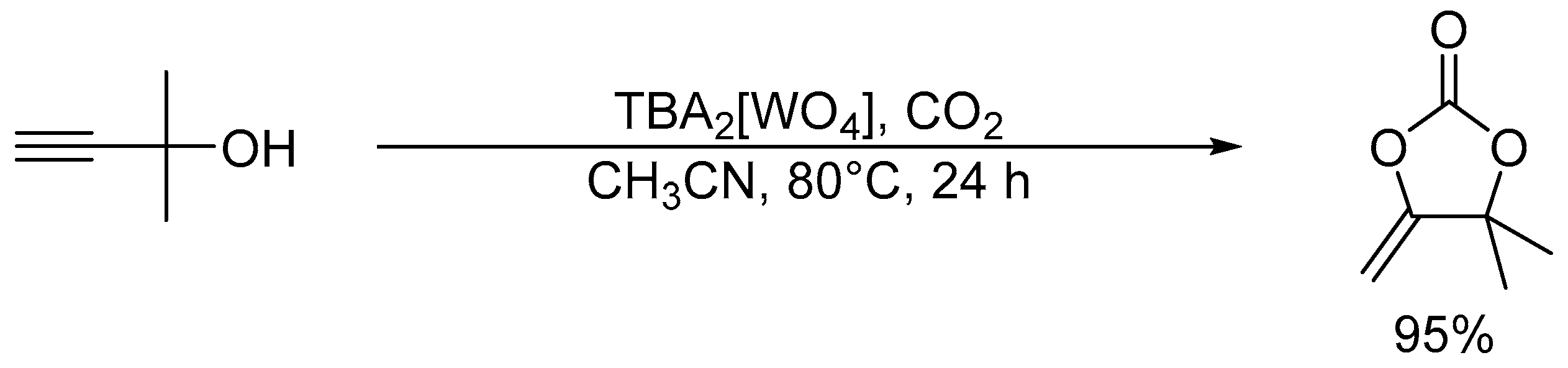
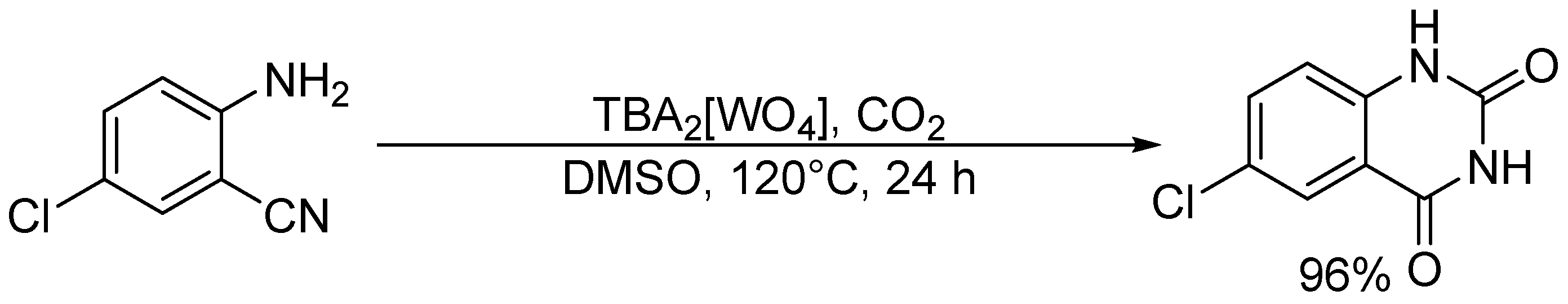
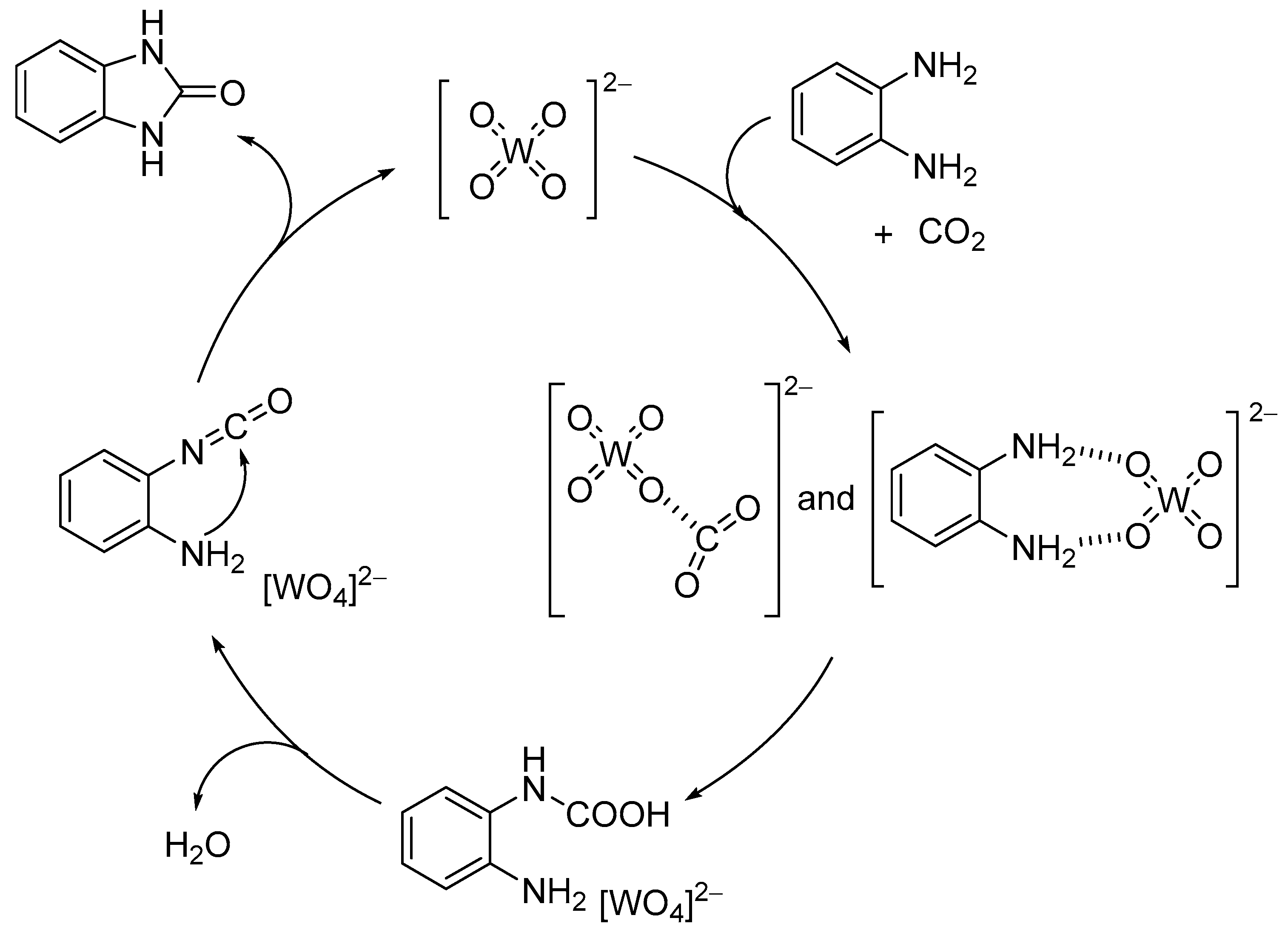
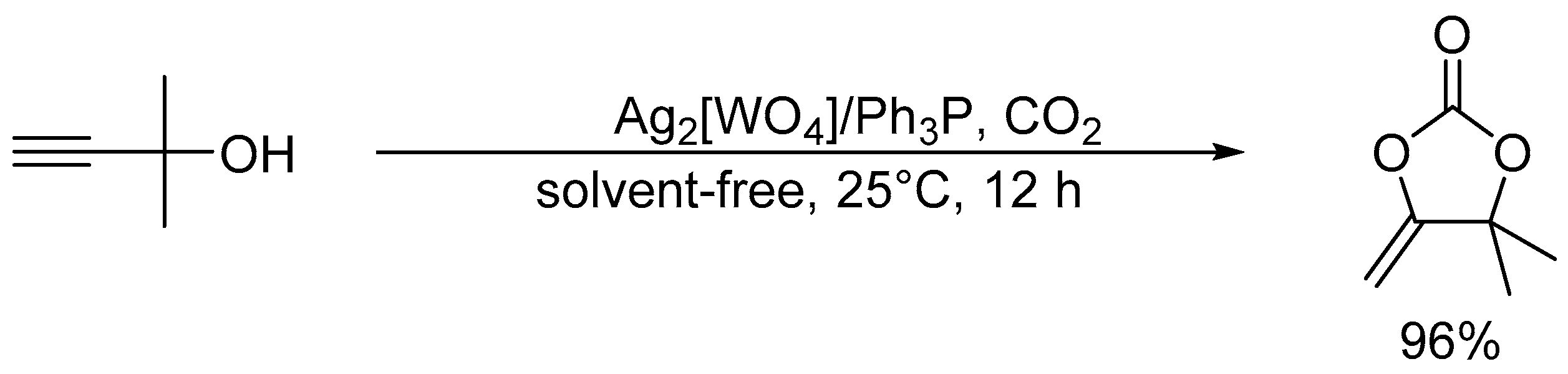
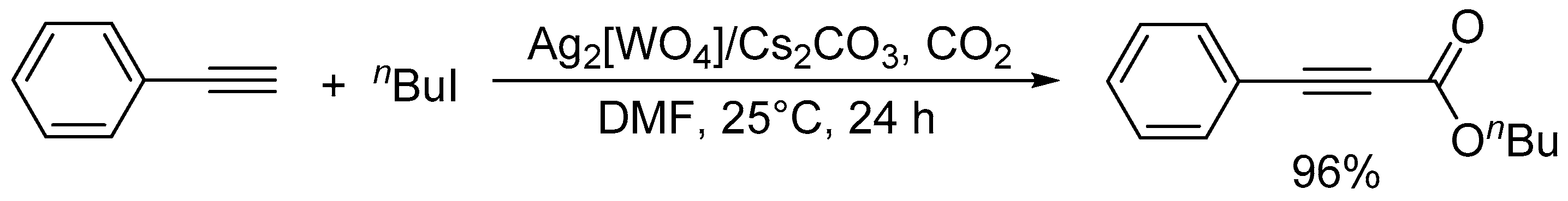

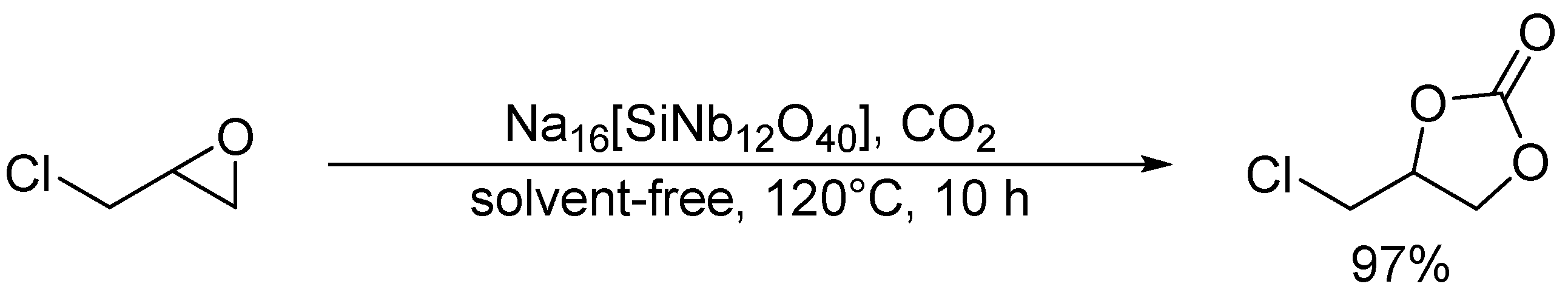
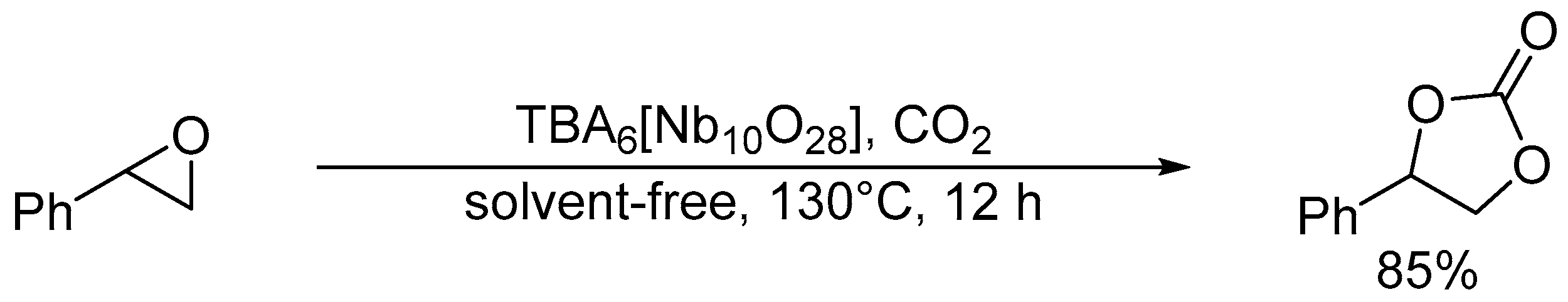
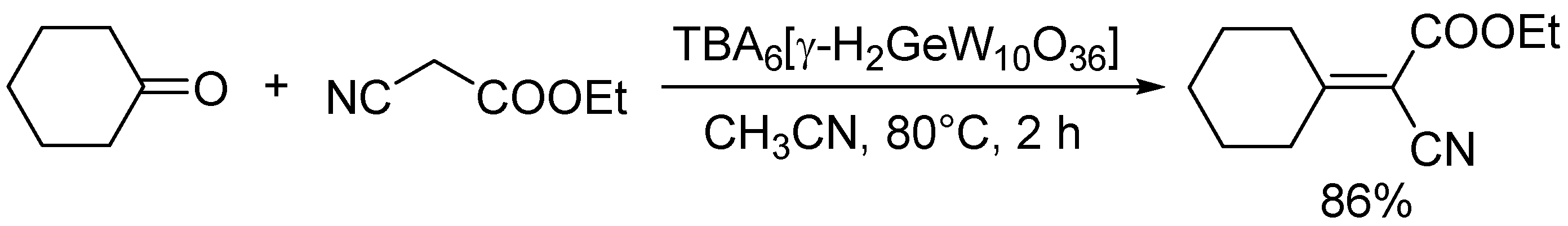
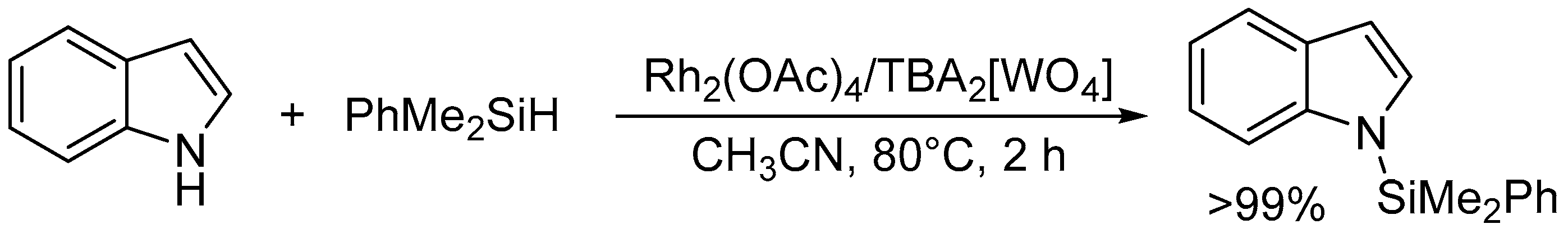
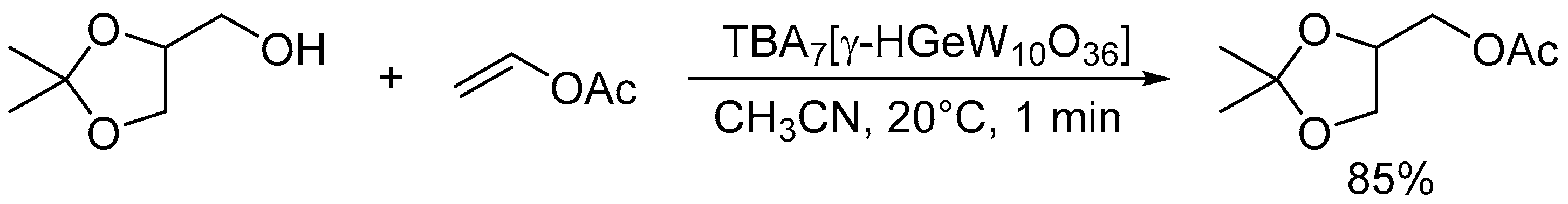
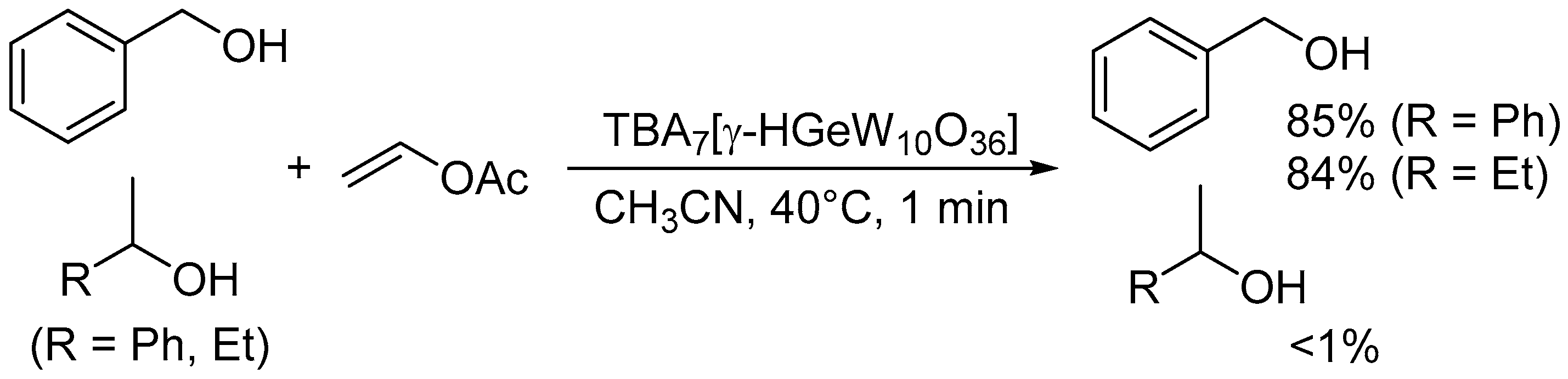


| Compound | pKa | Refs. |
|---|---|---|
| [WO4]2− | 3.5 | [7] |
| [MoO4]2− | 3.9 | [7] |
| [VO4]3− | 13.2 | [7] |
| [CO3]2− | 10.3 | [38] |
| 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) | 12.0 | [38] |
| 1,1,3,3-Tetramethylguanidine (TMG) | 13.6 | [38] |

| Catalyst (mol %) | Donor (Equiv.) | Solvent | Temp. (°C) | Time | Yield (%) | TON [a] | TOF [b] (h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| TBA6[γ-H2GeW10O36] (0.5) | Malononitrile (1.5) | CH3CN | 32 | 0.5 h | 99 | 198 | 396 | [39] |
| TBA6[γ-H2GeW10O36] (1) | Ethyl cyanoacetate (1.5) | CH3CN | 32 | 2 h | 98 | 98 | 49 | [39] |
| TBA6[γ-H2GeW10O36] (1) | Ethyl cyanoacetate (1.5) | – | 40 | 5 min | 85 | 85 | 1020 (>3000) [c] | [39] |
| TBA6[γ-H2GeW10O36] (5) | Phenylacetonitirle (1.5) | CH3CN | 80 | 2 h | 96 | 19 | 10 | [39] |
| TBA2[W6O19] (3.7) | Malononitrile (1) | EtOH | reflux | 7 min | 92 | 25 | 214 | [40] |
| TBA8[α-Si2W18O62] (0.5) | Ethyl cyanoacetate (1.5) | CH3CN | 32 | 3 h | 96 | 192 | 64 | [47] |
| TMA6[Nb10O28] (1) | Phenylacetonitirle (1) | CH3OH | 70 | 24 h | 35 | 35 | 1.5 | [55] |
| TBA7[γ-HGeW10O36] (2) | Phenylacetonitirle (1.5) | CH3CN | 80 | 5 min | 83 | 42 | 504 | [46] |
| Na16[SiNb12O40] (0.23) | Ethyl cyanoacetate (1) | – | 70 | 2 h | 67 | 288 | 144 | [56] |
| TBA4[γ-SiW10O34(H2O)2] (0.5) | Malononitrile (0.67) | CH3CN | 32 | 2.5 h | 90 | 180 | 72 | [36] |
| Na8H[PW9O34] (0.25) | Malononitrile (1.5) | CH3OH | 25 | 6 h | 92 | 368 | 61 | [50] |
| Na8H[PW9O34] (0.25) | Ethyl cyanoacetate (1.5) | CH3OH | 25 | 6 h | 80 | 320 | 53 | [50] |

| Catalyst (mol %) | Substrate | TMSCN (Equiv.) | Solvent | Temp. (°C) | Time | Yield (%) | TON [c] | TOF [d] (h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| TBA4[γ-SiW10O34(H2O)2] (1) | 2-Adamantanone | 2 | DCE [a] | 32 | 72 h | 30 | 30 | 0.4 | [36] |
| TBA8H2[{Y(H2O)2}2(γ-SiW10O36)2] (1) | 2-Adamantanone | 1.5 | DCE [a] | 30 | 1.5 h | 91 | 91 | 61 | [37] |
| TBA8H2[{Y(H2O)2}2(γ-SiW10O36)2] (0.01) | Benzaldehyde | 1.5 | DCE [a] | 30 | 15 min | 94 | 9400 | 37,600 | [37] |
| TBA6[γ-H2GeW10O36] (0.1) | Acetophenone | 1.5 | CH3CN | 30 | 30 min | 99 | 990 | 1980 | [39] |
| TBA8H2[{Nd(H2O)2}2(γ-SiW10O36)2] (0.5) | 2-Adamantanone | 1.5 | DCE [a] | 30 | 7 min | 98 | 196 | 1680 | [41] |
| TBA8H2[{Nd(H2O)2}2(γ-SiW10O36)2] (0.1) | Benzaldehyde | 1.5 | DCE [a] | 30 | 2 min | 99 | 990 | 29,700 | [41] |
| Na8H[PW9O34] (0.25) | 2-Adamantanone | 1.5 | EtOAc [b] | 25 | 6 h | 92 | 368 | 61 | [50] |
| Na8H[PW9O34] (0.25) | Benzaldehyde | 1.5 | EtOAc [b] | 25 | 6 h | 99 | 396 | 66 | [50] |
| [Nd(H2O)5]2MoV2O26 (1) | Benzaldehyde | 3 | – | 25 | 7 h | 96 | 96 | 14 | [51] |
| [Sm(H2O)5][Sm(H2O)7][Co2Mo10H4O38] (2) | Benzaldehyde | 3 | – | 25 | 5 h | 98 | 49 | 10 | [52] |
| (C2N2H12)[Ba(H2O)3][Co2Mo10H4O38] (2) | Benzaldehyde | 3 | – | 25 | 6 h | 99 | 50 | 8 | [52] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamata, K.; Sugahara, K. Base Catalysis by Mono- and Polyoxometalates. Catalysts 2017, 7, 345. https://doi.org/10.3390/catal7110345
Kamata K, Sugahara K. Base Catalysis by Mono- and Polyoxometalates. Catalysts. 2017; 7(11):345. https://doi.org/10.3390/catal7110345
Chicago/Turabian StyleKamata, Keigo, and Kosei Sugahara. 2017. "Base Catalysis by Mono- and Polyoxometalates" Catalysts 7, no. 11: 345. https://doi.org/10.3390/catal7110345





