1. Introduction
Sunlight-driven photoelectrochemical water splitting is one of the promising methods for converting solar energy to chemical energy without emission of CO
[
1,
2,
3]. To achieve this goal with high efficiency, materials used in water splitting devices should be naturally abundant, have high absorbance for solar radiation, possess proper energy level positions and fast kinetics for oxygen evolution reaction or hydrogen evolution reaction, and must be stable under the harsh working conditions. Although Groups III–V semiconductors exhibit good photoelectrochemical energy conversion efficiency [
4], transition-metal oxide, such as BiVO
[
5,
6,
7,
8,
9], Cu
O [
10,
11,
12], Fe
O
[
13,
14,
15,
16,
17,
18,
19], and WO
[
20,
21] have attracted great attention as the photoanode due to their good stability and Earth-abundance. Among them, hematite (
-Fe
O
) has the potential for achieving high energy conversion efficiency because of its suitable energy bandgap of ≈2.2 eV. Further improvement of the efficiency of hematite photoanode by nanostructuring and intrinsic doping [
13,
19] or extrinsic doping [
15,
16,
17,
18,
19] has been actively pursued.
Although nanostructured hematite holds great promise in many aspects, its inadequate surface state energy level results in a low photovoltage and thus a high onset potential for water splitting. Therefore, many works have been done to deposit various materials such as IrO
[
22], Al
O
[
23,
24], Co-Pi [
25], Co(OH)
/Co
O
[
26], Ga
O
[
24], In
O
[
24], and Ni
xFe
1−xO
y [
27,
28,
29] on hematite as an electrocatalyst for better energetics or faster kinetics. Among them, Ni
xFe
1−xO
y shows great promise because it can greatly reduce the onset potential. Du et al. [
27] used photochemical metal-organic deposition (PMOD) to deposit a 200-nm thick NiFeO
film on hematite, and the decorated hematite photoanodes showed a photovoltage of 0.61 V, an onset potential of 0.61 V (vs. reversible hydrogen electrode (RHE)), and a current density of 0.6 mA/cm
at 1.4 V (vs. RHE). Morales-Guio et al. [
28] used electrochemical and photoelectrochemical deposition to deposit a thin (<10 nm), optically transparent FeNiO
overlayer on hematite, and the FeNiO
/Fe
O
samples showed an onset potential of 0.9 V (vs. RHE) and a current density of about 0.55 mA/cm
at 1.4 V (vs. RHE). With nanostructured Fe
O
, the onset potential remained the same, but the current density increased to 3 mA/cm
at 1.4 V (vs. RHE). Jang et al. [
29] utilized a regrowth method to produce a hematite layer without surface disorder followed by deposition of a NiFeO
overlayer by PMOD to fabricate a photoanode with a photovoltage of 0.8 V and an onset potential of 0.45 V (vs. RHE), which enabled unbiased solar water splitting with efficiency of 0.91% when used in conjunction with a Pt-decorated Si photocathode. Although all of these works revealed the great potential of Ni
xFe
1−xO
y as electrocatalyst, they used different Ni-to-Fe ratios in the precursor, and the degree of reduction in the onset potential varied significantly. Therefore, a systematic study of the dependence of the electrochemical and photoelectrochemical characteristics of the photoanode on the condition of the Ni
xFe
1−xO
y electrocatalyst is needed.
In this work, we demonstrated a novel and facile chemical bath deposition method for depositing NixFe1−xOy electrocatalyst on hematite as photoanode for solar hydrogen production. By varying the Ni-to-Fe ratio of the precursor and immersion time, the composition and the average thickness of the NixFe1−xOy overlayer could be easily controlled to minimize the onset potential for water splitting. With a Ni percentage of >80% and an immersion time of 2 min, the optimal performance with a photovoltage of 0.70 V, an onset potential of 0.70 V (vs. RHE), and a current density of 0.6 mA/cm at 1.4 V (vs. RHE) under simulated AM (Air Mass) 1.5 G global solar irradiation was attained. The current density at 1.0 V vs. RHE was enhanced by 30-fold with the deposition of NixFe1−xOy overlayer. The dependence of the polarization curve and the open-circuit voltage on the composition and the average thickness of the NixFe1−xOy overlayer was investigated and explained based on the energetics and kinetics of the hematite layer and the NixFe1−xOy layer.
2. Results and Discussion
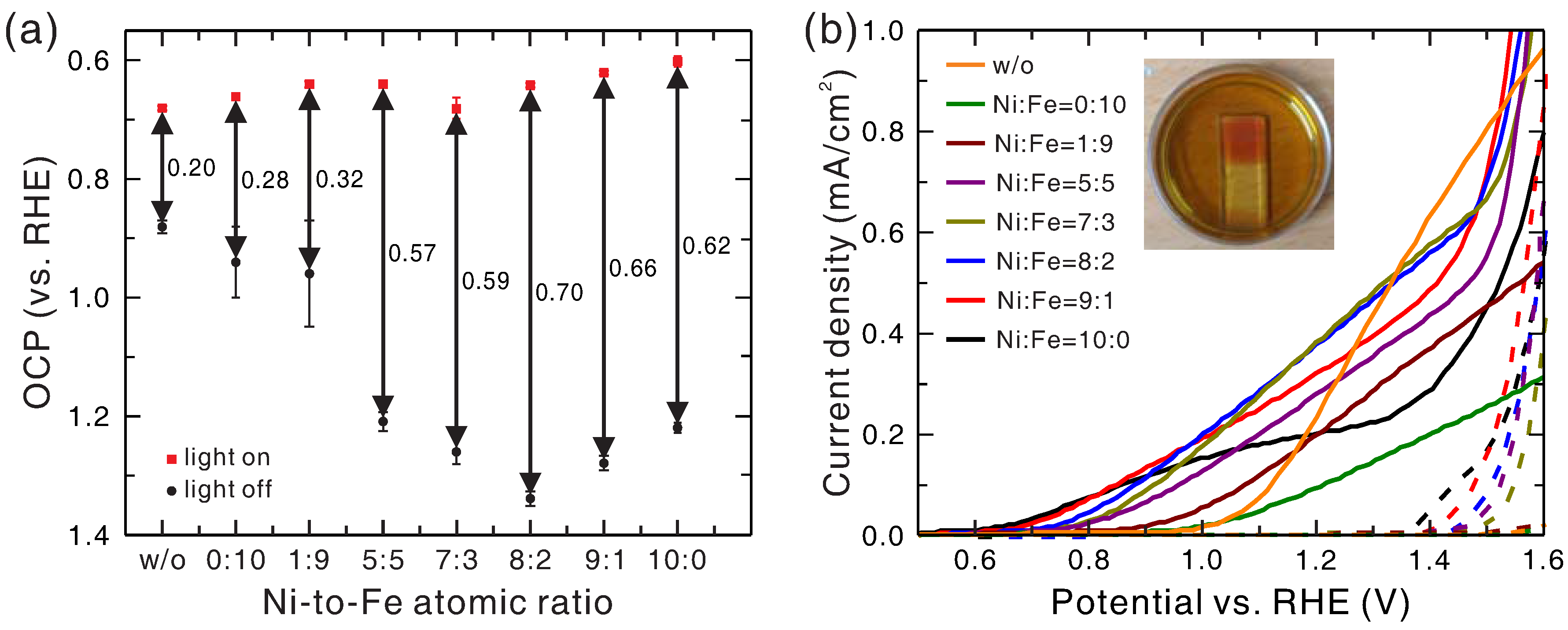
The open-circuit potentials (OCP) of Ni
xFe
1−xO
y/hematite photoanodes for various Ni-to-Fe ratios of the precursor at the light-on and light-off conditions are shown in
Figure 1a. Without light irradiation, the OCP increased when the hematite was coated with Ni
xFe
1−xO
y. The increase in OCP became larger for higher Ni-to-Fe ratio and reached the maximum of 1.34 V at a Ni-to-Fe ratio of 8:2. Further increase of Ni-to-Fe ratio resulted in a slight reduction of the OCP. With light irradiation the OCP for each photoanode was lowered, and its value stayed roughly the same (between 0.6 V and 0.7 V vs. RHE) for all Ni-to-Fe ratios. The difference between the OCPs under light-on and light-off conditions is defined as the photovoltage (explained below). As shown, the photovoltage of bare hematite photoanode was only 0.20 V, and it was raised by the addition of the Ni
xFe
1−xO
y electrocatalyst, reaching the maximum of 0.70 V at the Ni-to-Fe ratio of 8:2. The photovoltage represents the capability of the photoanode to perform photo-assisted water splitting, and thus the increase in photovoltage normally results in a corresponding reduction in the onset potential for water splitting.
Figure 1b shows the current density-to-potential (J–V) curves of Ni
xFe
1−xO
y/hematite photoanode for various Ni-to-Fe ratios at the light-on and light-off conditions, in comparison with that of bare hematite photoanode. The onset potential of bare hematite photoanode was about 1.08 V vs. RHE, and the deposition of Ni
xFe
1−xO
y on hematite lowered the onset potential significantly. The onset potential decreased with increasing Ni-to-Fe ratio; from about 1.0 V for the Ni-to-Fe ratio of 0:10 sample to 0.6 V for the Ni-to-Fe ratio of 10:0 sample. The changing trend of the onset potential was roughly consistent with the changing trend of the photovoltage both qualitatively and quantitatively. For Ni-to-Fe ratio larger than 8:2, the onset potential continued dropping, but the current density began to decrease. For water splitting without light irradiation, the onset potentials were about 1.6 V (vs. RHE) and 1.4 V (vs. RHE) for the bare hematite photoanode and the Ni
xFe
1−xO
y/hematite photoanode, respectively. These numbers are consistent with those obtained in the previous work using PMOD with the same precursors [
30].
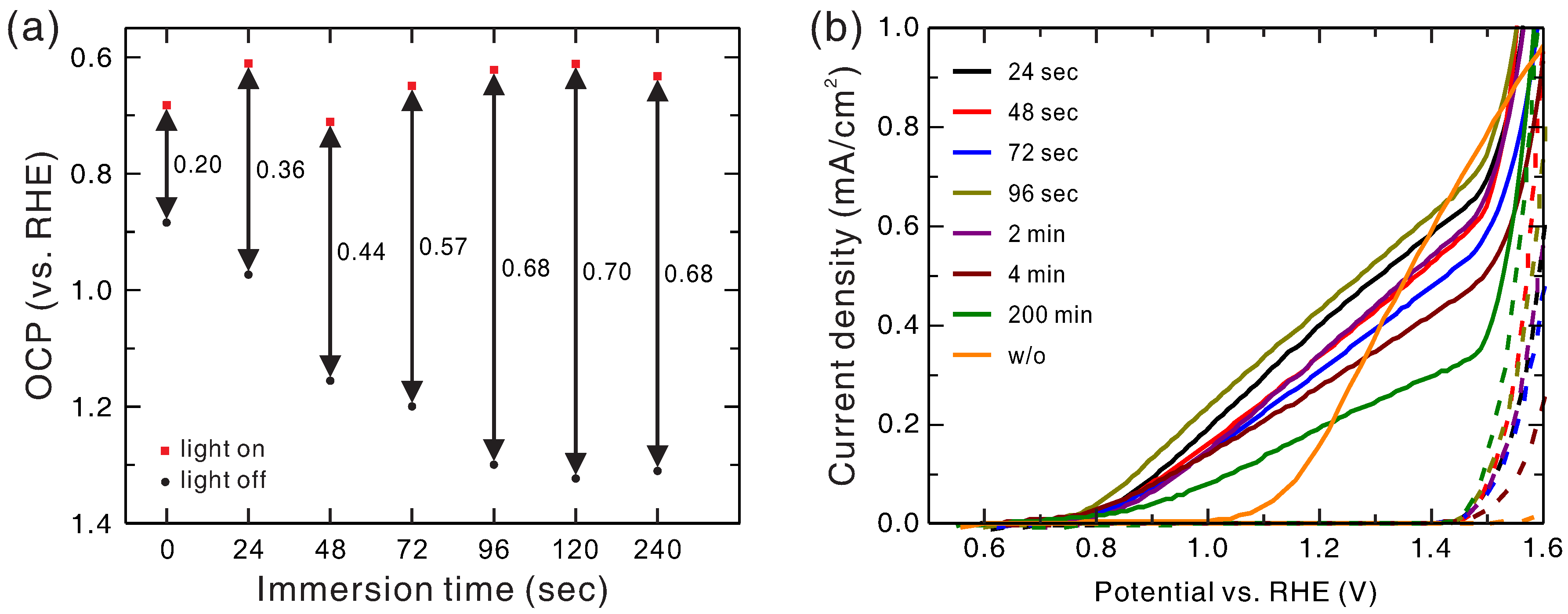
Figure 2a shows the OCP of Ni
xFe
1−xO
y/hematite photoanode as a function of immersion time of Ni
xFe
1−xO
y deposition at the light-on and light-off conditions. The Ni-to-Fe ratio of the precursor was fixed at 8:2. Without light irradiation, the OCP increased with increasing immersion time, reached the maximum of 1.32 V at 2 min, and saturated with further increase of immersion time. With light irradiation, the OCP stayed roughly constant with immersion time. As a result, the photovoltage became larger with longer immersion time, reached the maximum of 0.70 V at 2 min, and then became saturated.
Figure 2b shows the J–V curves of Ni
xFe
1−xO
y/hematite photoanodes grown with various immersion times at the light-on and light-off conditions. It was found that the variation of the photovoltage and the onset potential with varying immersion time did not show similar correspondence as that observed with varying Ni-to-Fe ratio. For an immersion time of 2 min or less, the onset potential dropped to around 0.76 V regardless of the immersion time. With further increase of immersion time, the onset potential increased and the current density decreased very slowly with increasing immersion time.
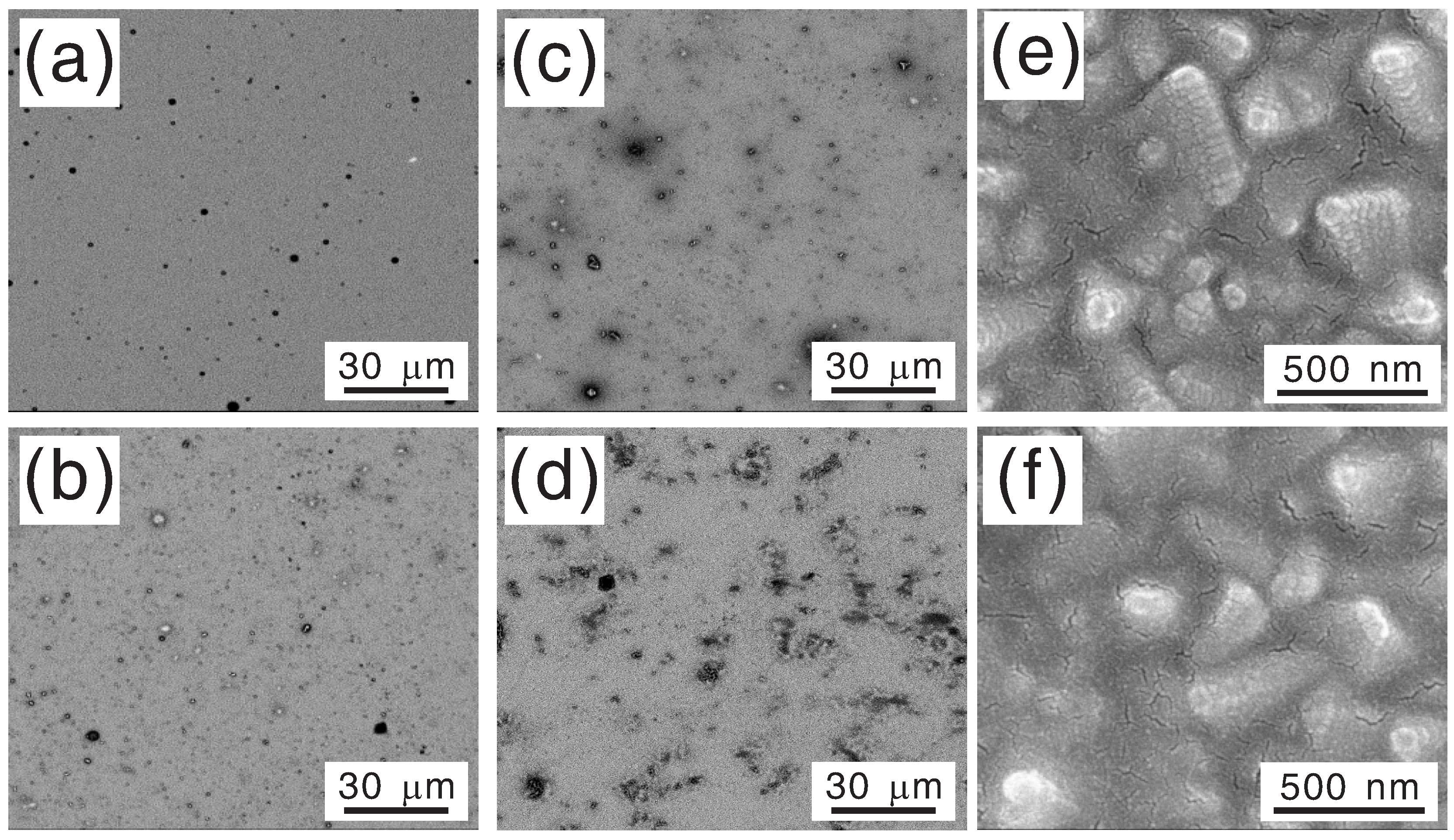
To investigate the dependencies of the thickness and the morphology of as-prepared Ni
xFe
1−xO
y layer on the immersion time, top-view SEM images of the photoanodes grown with various immersion times were taken after photoelectrochemical test, as shown in
Figure 3. Energy-dispersive spectroscopy (EDS) examination showed that the darker region corresponded to thicker Ni
xFe
1−xO
y. The increase of the fraction of darker region shown in
Figure 3a–d implies that, with an immersion time of less than 2 min, the hematite layer could be only partially covered by Ni
xFe
1−xO
y, and the coverage increased with increasing immersion time. Full coverage was reached at around 2 min according to EDS examination, and then the average thickness continued increasing with increasing immersion time as shown in
Figure 3e,f. This observation could be used to explain the difference between the dependencies of the photovoltage and the onset potential on the immersion time. With an immersion time of <2 min, the photoanode was a hematite layer partially covered by Ni
xFe
1−xO
y. Thus, it could be considered as a parallel connection of a bare hematite photoanode and a Ni
xFe
1−xO
y/hematite photoanode. In the measurement of J–V curve, because the Ni
xFe
1−xO
y/hematite part had a lower onset potential, it determined the overall onset potential of the combination. In the measurement of OCP, since the measurement was done in an open-circuit condition, the two parts equilibrated to determine a common Fermi level for the whole photoanode. Therefore, the equilibrated Fermi level, and thus the OCP without light irradiation, should increase with increasing coverage by Ni
xFe
1−xO
y. For an immersion time longer than 2 min, a complete coverage was obtained, and both the photovoltage and the onset potential were determined only by the energy levels of the Ni
xFe
1−xO
y layer and the hematite layer. Therefore, there was no significant change of photovoltage and onset potential with increasing immersion time (increasing Ni
xFe
1−xO
y layer thickness), but a gradual decrease of current density due to presumably increased charge transport resistance in a thicker Ni
xFe
1−xO
y layer. It was also noted that there were traces of the precursor on as-prepared samples, revealed by an EDS peak of carbon, and it was quickly removed by the highly alkaline electrolyte during the photoelectrochemical test.
To measure the thicknesses of the hematite layer and the Ni
xFe
1−xO
y layer, cross-sectional TEM images and cross-sectional EDS maps of Fe, Sn, and Ni were obtained. The results for Ni
xFe
1−xO
y/hematite photoanode with a Ni-to-Fe ratio of 8:2 and an immersion time of 2 min are shown in
Figure 4. The average thicknesses of the hematite layer and the Ni
xFe
1−xO
y layer were both around 30 nm.
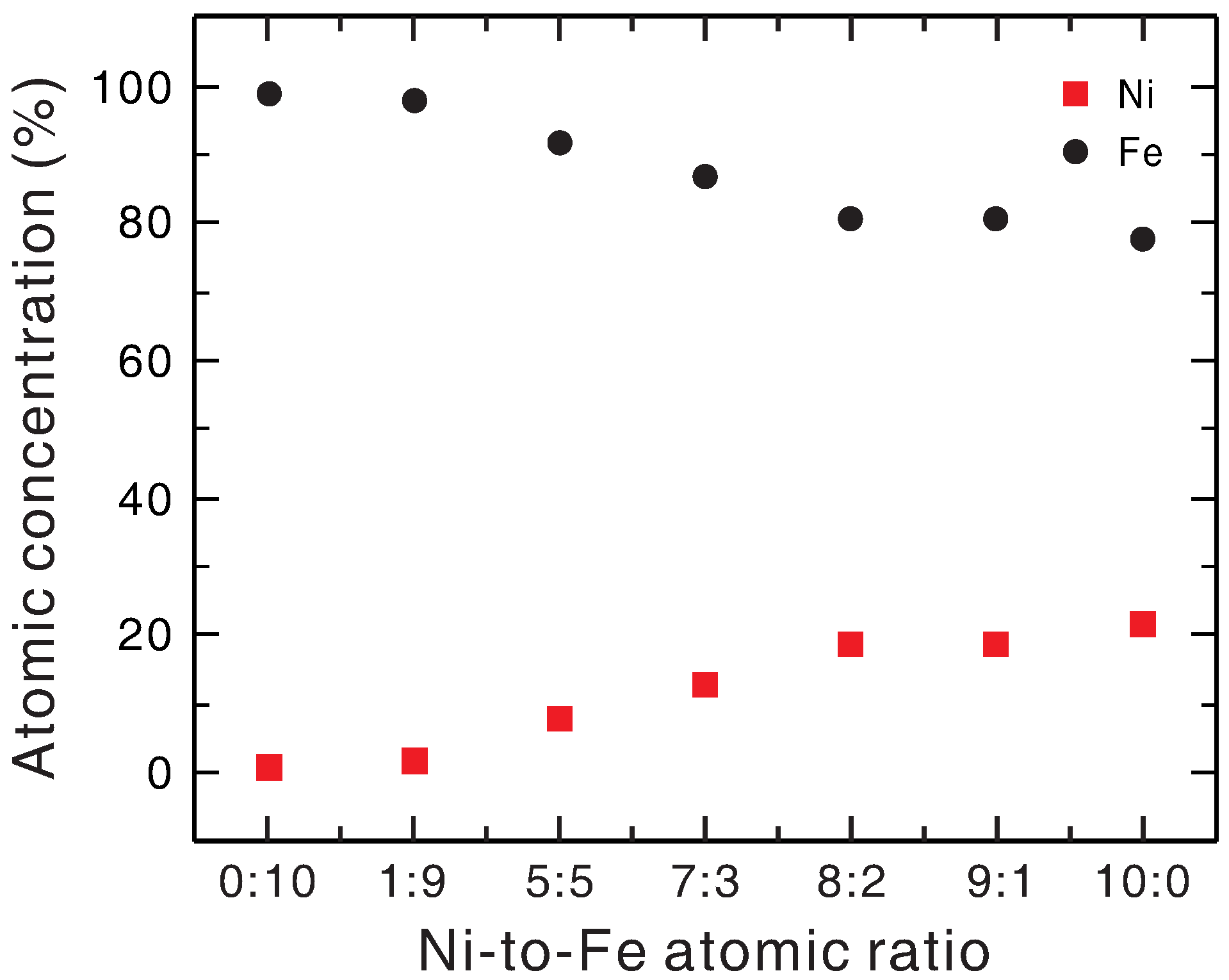
The Ni-to-Fe ratio in all of the data shown here refers to that in the precursor. To examine the actual composition of the as-prepared Ni
xFe
1−xO
y layer, EDS was used to measure the Ni and Fe atomic concentrations of Ni
xFe
1−xO
y/hematite photoanodes with various Ni-to-Fe ratios of the precursor after the photoelectrochemical test. The results are shown in
Figure 5. As can be seen, the Ni percentage indeed increased with increasing Ni-to-Fe ratio in the precursor. Although the EDS data showed that the absolute values of concentration were not as expected for the Ni
xFe
1−xO
y layer and the dependence was not in strictly linear proportion, these did not reflect the true content of the Ni
xFe
1−xO
y layer. The lower Ni-to-Fe ratio readings, as compared to the actual ratios in the precursor, resulted from the fact that the EDS measurement also included the contribution from the underlying hematite layer, as revealed by that the Fe atomic concentration still took up 78% even when the as-prepared electrocatalyst did not contain Fe in its precursor.
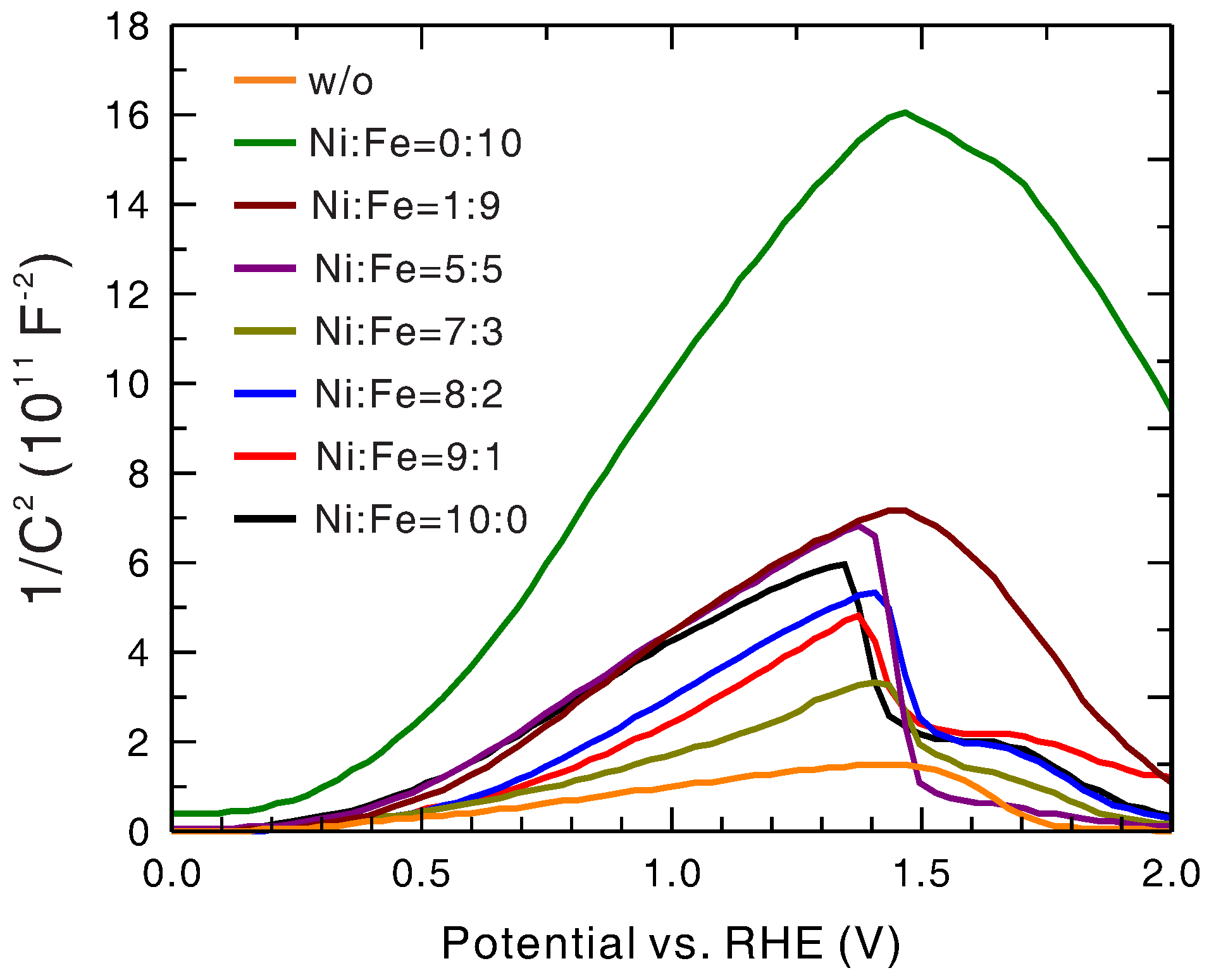
To measure the type and density of the intrinsic doping (nonstoichiometric doping) and the flat-band potential of the hematite layer, Mott–Schottky analysis [
15,
16] were conducted for the bare hematite photoanode and the Ni
xFe
1−xO
y/hematite photoanodes with various Ni-to-Fe ratios of the precursor. The results are shown in
Figure 6 and
Table 1. The positive slopes of the curves in the low-potential region indicate that these photoanodes were all
n-type semiconductors as expected for the general tendency of nonstoichiometric doping of hematite, justifying their use as the anode. The flat-band potential was roughly constant regardless of the presence and compositional variation of the Ni
xFe
1−xO
y layer. This could be ascribed to the fact that, since the flat-band potential was determined by the original Fermi level of the hematite layer, it should not be affected by the Ni
xFe
1−xO
y layer. This was also supported by the fact that the values of the flat-band potentials were close to the OCPs with light irradiation. The doping densities of hematite in these photoanodes were all on the order of 10
cm
, signifying a high doping level that was beneficial for photoanode performance (better charge separation and higher electron conductivity). The variation could be attributed to from-sample-to-sample fluctuation in the pulsed-laser deposition (PLD) process of the hematite layer. This is based on the observations that the doping density of several bare hematite photoanodes made in the same production batch displayed a variation of more than a factor of 2 (data not shown) and that the doping density of the Ni
xFe
1−xO
y-coated hematite did not show a systematic trend with an increasing Ni-to-Fe ratio. It was found that the presence of an Ni
xFe
1−xO
y layer with a Ni-to-Fe ratio of >5:5 led to a steep drop in the Mott–Schottky curve at 1.35–1.45 V vs. RHE. This could be ascribed to the onset of charge transfer at the photoanode/electrolyte interface because the numbers matched the onset potentials for water splitting without light irradiation, as shown in
Figure 1b. For the bare hematite photoanode and the Ni
xFe
1−xO
y/hematite photoanodes with low Ni fraction, the linearly rising region of the Mott–Schottky curve terminated at some potential before reaching the onset potential. This could be ascribed to the complete depletion of the hematite layer. The width of the depletion layer calculated for the termination point was about 30 nm, consistent with the thickness of the hematite layer measured with TEM.
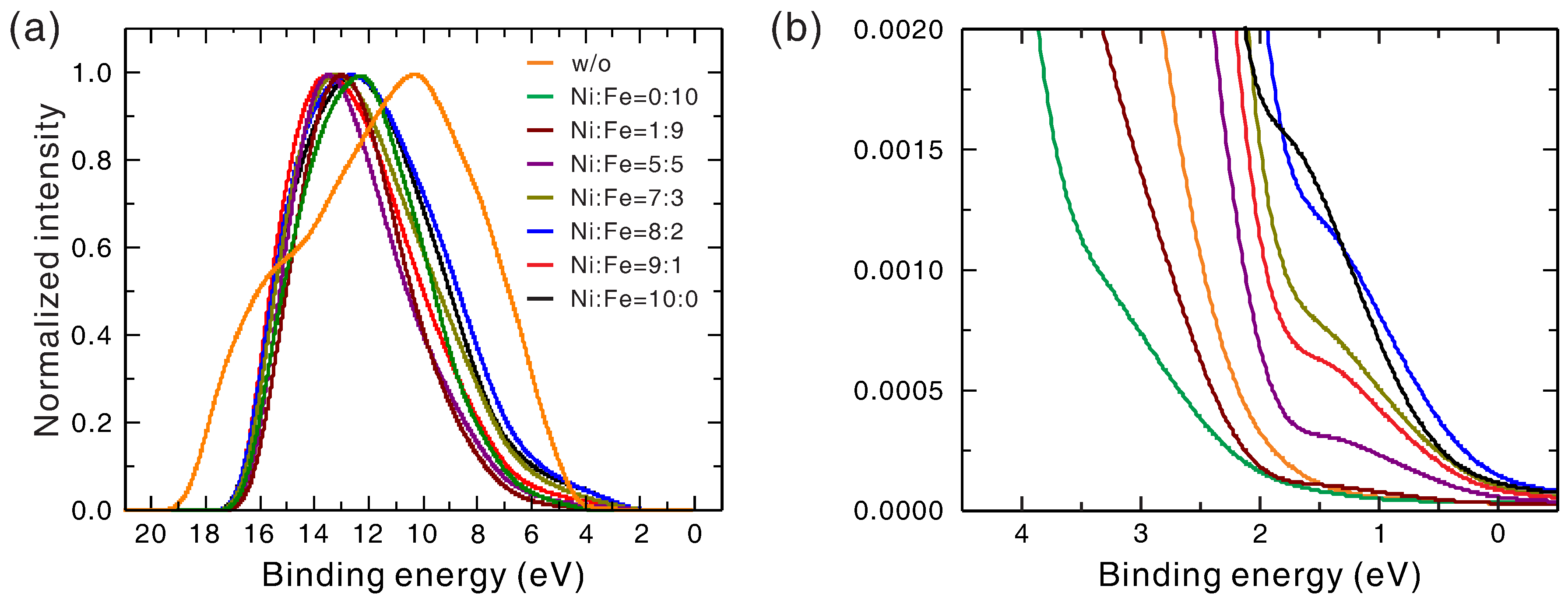
In order to build an energy level diagram that could be used to explain the dependence of the OCP and the onset potential on the Ni-to-Fe ratio, ultraviolet photoelectron spectroscopy (UPS) was used to measure the positions of the Fermi level and the valence band maximum (VBM) of the bare hematite and the Ni
xFe
1−xO
y covered on hematite. The UPS spectra are shown in
Figure 7, and the energies of Fermi level and VBM for each sample are shown in
Table 2. From
Figure 7, all Ni
xFe
1−xO
y covered samples displayed a shift in the high binding-energy cutoff and an extension in the low binding-energy cutoff. The large low bind-energy cutoff of bare hematite indicated a large separation between its Fermi level and VBM (as large as its bandgap), revealing its
n-type nature. The small low binding-energy cutoffs of the Ni
xFe
1−xO
y electrocatalysts indicated a small separation between their Fermi level and VBM, showing that the electrocatalysts grown with this method were all of
p-type intrinsic doping with similar doping density regardless of their Ni-to-Fe ratios. Furthermore, it seems that the Fermi level and the VBM of the Ni
xFe
1−xO
y electrocatalyst down-shifted more with increasing Ni fraction, although an accurate determination of their values for quantitative comparison with OCPs are difficult due to the uncertainty in the definition of the cutoffs in UPS spectra. The VBM of the hematite layer was about −6.5 eV, and thus its conduction band minimum was about −4.3 eV based on the bandgap energy of 2.2 eV. Both numbers are consistent with those reported previously [
1], considering the measurement uncertainly of a few tenth eV. The VBM of the NiO
was about −5.2 eV, which is consistent with that reported previously (−5.4 eV) [
31]. These matches justify the definitions of the high cutoff and the low cutoff used in our UPS data analysis. The different VBM energy and intrinsic doping type of the hematite layer and the FeO
electrocatalyst layer indicated that the latter was likely of FeO or Fe
O
nature, distinct from that of the hematite layer (Fe
O
).
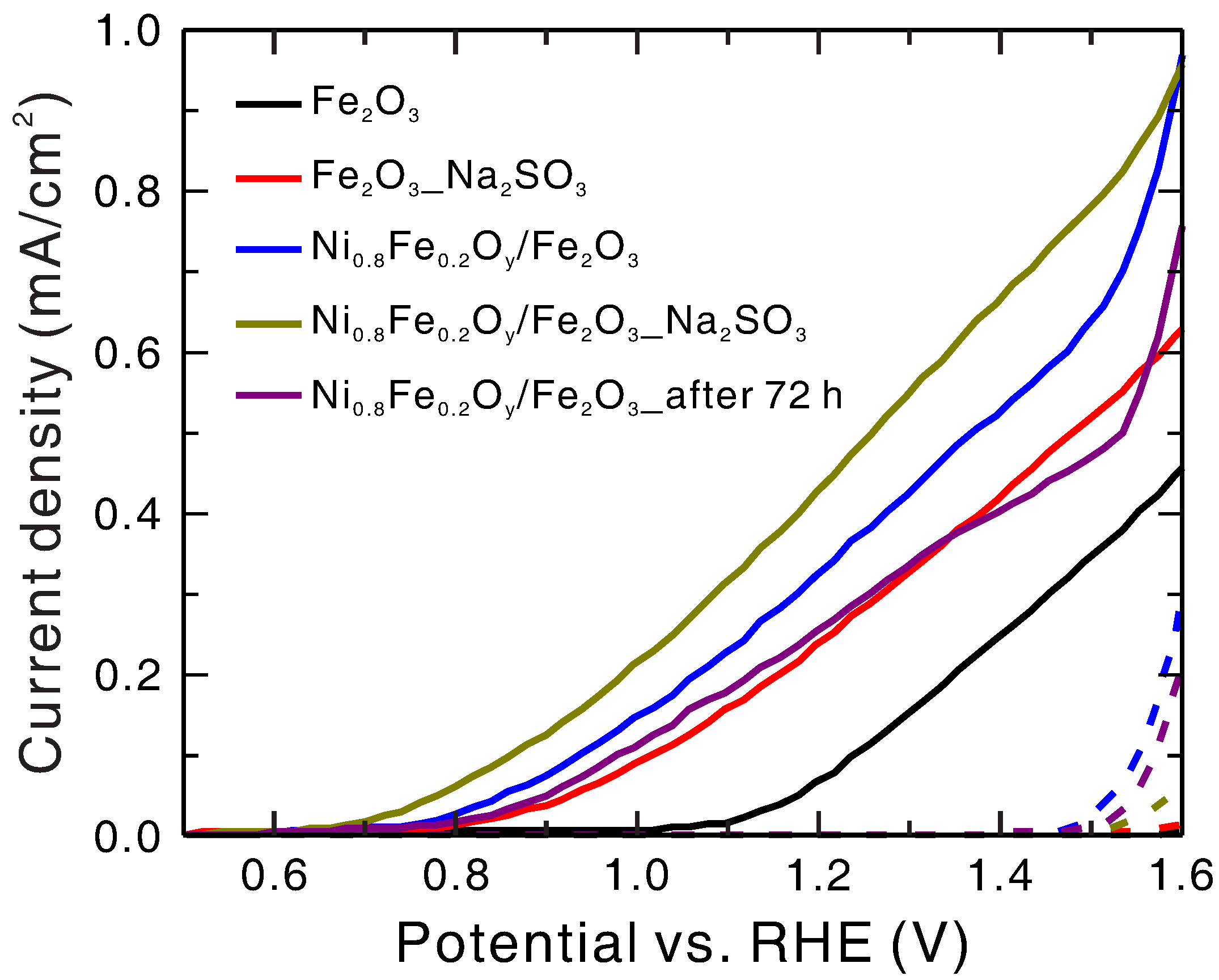
To study the kinetics, the J–V curves of the Ni
Fe
O
/hematite photoanode and the bare hematite photoanode with and without the presence of 0.1 M Na
SO
as the hole scavenger (sacrificial reagent) in the electrolyte were measured [
32], as shown in
Figure 8. The water photo-oxidation current can be expressed as
, where
is the water oxidation photocurrent density,
is the photocurrent density determined by the photon absorption that will be used to generate photocurrent,
is the charge separation efficiency, and
is the interfacial charge transfer efficiency for water oxidation [
32]. Using
, where
is the current density measured with the presence of the hole scavenger (thus
= 100%) [
32], the charge transfer efficiencies at the surfaces of the Ni
Fe
O
/hematite photoanode and the bare hematite photoanode were obtained as 76% and 31%, respectively, based on the current densities at 1.23 V vs. RHE (following Ref. [
32]). In addition, by comparing the current density of the Ni
Fe
O
/hematite photoanode with that of the bare hematite photoanode, both measured with the presence of the hole scavenger, it was found that the charge separation efficiency of the Ni
Fe
O
/hematite photoanode was 1.75 times that of the bare hematite photoanode, assuming the two samples had the same
. That is, the deposition of the Ni
Fe
O
electrocatalyst on hematite enhanced both the charge transfer efficiency and the charge separation efficiency of the photoanode.
To characterize the stability of the Ni
xFe
1−xO
y/hematite photoanode, the J–V curve of the Ni
Fe
O
/hematite photoanode after 72 h continuous light-on operation at 1.23 V vs. RHE was measured, as shown in
Figure 8. The current density at 1.23 V vs. RHE dropped to about 80% of its initial value after 72 h operation, while the onset potential remained roughly the same. To investigate the reason for this drop in current density, the Ni
Fe
O
/hematite photoanode were characterized using SEM and EDS before and after 72 h operation. It was found that both the morphology and the elemental composition did not exhibit any significant changes after 72 h operation. On the other hand, it was noted that the epoxy used to seal the uncoated fluorine-doped tin oxide (FTO) regions and the contact lead displayed a change in its color (turning yellow) after 72 h operation. This observation and that there were no significant changes in morphology and elemental composition indicate that the drop in current density could be caused by contamination of the electrolyte by the degraded epoxy, not by degradation of the Ni
Fe
O
/hematite itself.
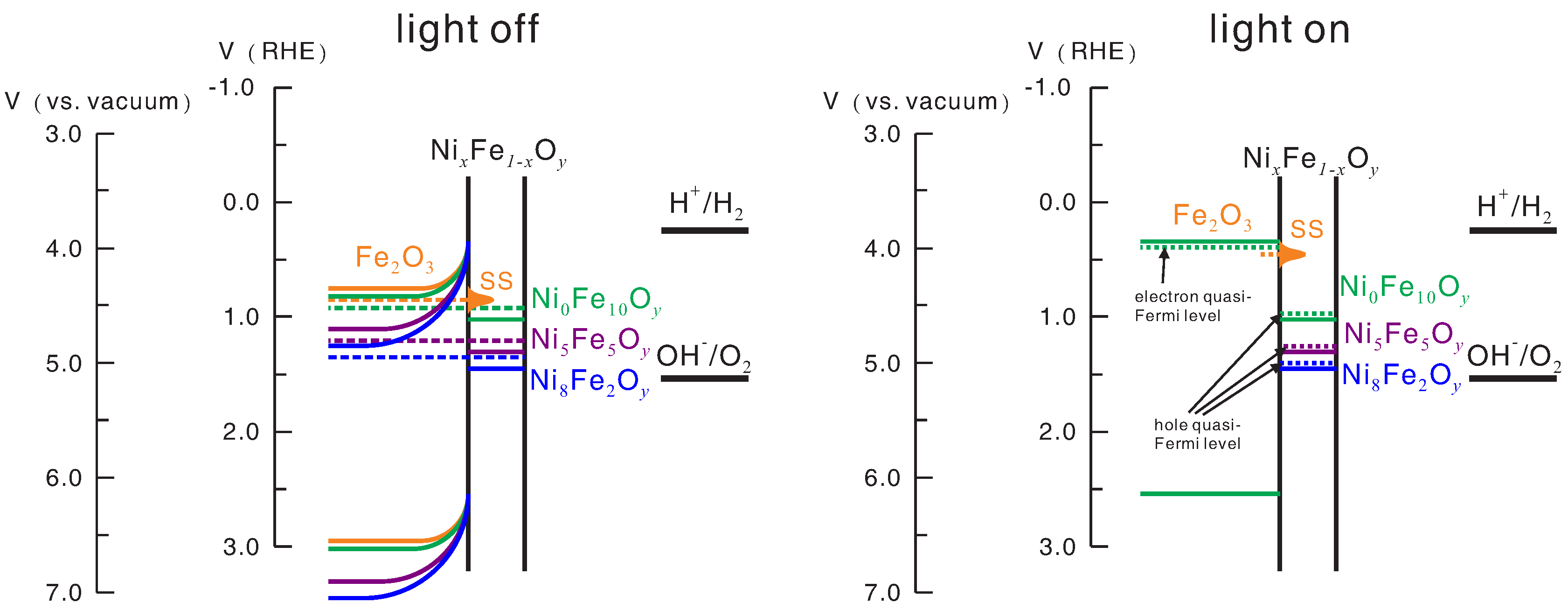
Figure 9 shows the energy level diagrams of the bare hematite photoanode and the Ni
xFe
1−xO
y/hematite photoanodes, based on the results of UPS measurement, under the open-circuit condition with and without light irradiation. Under open-circuit condition and without light irradiation, the Fermi level of the hematite layer is shifted to match the Fermi level of the Ni
xFe
1−xO
y layer, so the OCP is determined by the Fermi level of the Ni
xFe
1−xO
y layer. This explains the increase in OCP with increasing Ni-to-Fe ratio under the condition of no light irradiation. For water splitting, the potential of the photoanode needs to be raised to down-shift the Fermi level to the position which permits transfer of holes from the electrocatalyst to the water oxidation energy level, so the onset potential for water splitting is lower for lower electrocatalyst Fermi level associated with higher Ni-to-Fe ratio. With light irradiation the electron quasi-Fermi level shifts to near the conduction band minimum of the hematite layer, so the OCPs for all cases are roughly the same. Likewise, with light irradiation, the hole quasi-Fermi level shifts to near the valence band maximum of the Ni
xFe
1−xO
y layer, so the onset potential for hole transfer exhibits a dependence on the Ni-to-Fe ratio similar to that of OCP. Therefore, the difference between electron quasi-Fermi level and hole quasi-Fermi level, referred to as photovoltage, can be approximately obtained from the difference between the OCP without light irradiation (close to hole quasi-Fermi level) and the OCP with light irradiation (electron quasi-Fermi level). Note that, although for simplicity it is assumed in
Figure 9 that the
p-type doping densities of the electrocatalysts are much higher than the
n-type doping density of the hematite so that no obvious shift of Fermi level and band bending takes place upon thermal equilibration of the two sides, all the trends described above still hold when this assumption is abrogated, except for slight changes in the values. The low photovoltage (and thus high onset potential) of the bare hematite was believed to be due to the presence of surface states [
27], which affect the result in a reason similar to the presence of the electrocatalyst.
Table 3 shows the photovoltages extracted from J–V curves compared with that extracted from OCPs. It can be seen that, for the photoanodes with a Ni-to-Fe ratio higher than 8:2, the photovoltages extracted from these two methods are about the same. However, for the bare hematite and the photoanodes with lower Ni-to-Fe ratios, the photovoltage extracted from OCP is smaller than that extracted from the J–V curve. Such discrepancy may be ascribed to two reasons. Firstly, OCP is actually a measure of the thermodynamic potential of electrocatalyst or surface state, whereas the onset potential in the J–V curve is determined by not only the thermodynamic potential but also the overpotential required by charge-transfer kinetics, which also varies with different electrocatalysts [
33]. Secondly, the photovoltage extracted from OCP for bare hematite in our case (0.20 V) is close to that reported by Jang et al. [
29] (0.24 V) but significantly different from that reported by Morales-Guio et al. [
28] (0.57 V, which is consistent with the photovoltage extracted from the J–V curve in their case). This indicates that, in our case and Jang’s case, two different kinds of surface states could be involved in determining the outcomes of these two different measurements. The variation among the results of different groups could be caused by the difference in the deposition methods. Morales-Guio et al. [
28] used electrochemical impedance spectroscopy to measure charge-transfer resistance at the photoanode/electrolyte interface and found that the charge–transfer resistance at 0.8 V vs. RHE was lowered by two orders of magnitude upon FeNiO
electrocatalyst deposition. This indicates that, in their case, the reduction in onset potential was due to faster water oxidation kinetics. Further investigation is necessary to clarify the relative contributions from these two factors and the discrepancy between the photovoltages extracted with the two different methods.
The chemical bath deposition (immersion) with metal-organic complex as the precursor used in this work provides a simple method for depositing Ni
xFe
1−xO
y onto hematite as an effective photoanode for solar water splitting. The reason why this process can occur spontaneously at room temperature could be attributed to the similarity in the chemical properties of Ni
xFe
1−xO
y to that of hematite (Fe
O
). This is supported by the observation that deposition of Ni
xFe
1−xO
y could not take place on a bare FTO substrate and the dish. Although the deposition is not conformal (forming a thin layer covering all the surfaces with uniform thickness) on a nanometer scale, the improvement in onset potential and the current density attained are comparable to all of the previous reports on Ni
xFe
1−xO
y/hematite grown by much more complicated methods. It is believed that the current density could be raised dramatically by increasing the porosity and the thickness of the hematite layer and depositing thin electrocatalyst conformally in the pores to increase both the absorbance and the electrochemical surface area of the photoanode [
28]. In our experiment, the increase in porosity could be accomplished by increasing the total gas pressure and/or tilting the substrate in pulsed laser deposition. The thickness could be increased by using higher laser pulse energy and more suitable on-target laser spot size. The conformal deposition of thin electrocatalyst overlayer might be achieved by reducing the precursor concentration to inhibit spontaneous deposition and using a 532 nm continuous-wave laser beam to heat the nanoporous hematite layer transiently to drive and control the deposition of Ni
xFe
1−xO
y. The experiments for realizing these strategies are undergoing.