Saturated Resin Ectopic Regeneration by Non-Thermal Dielectric Barrier Discharge Plasma
Abstract
:1. Introduction
2. Results
Degradation Pathway Process
3. Discussion
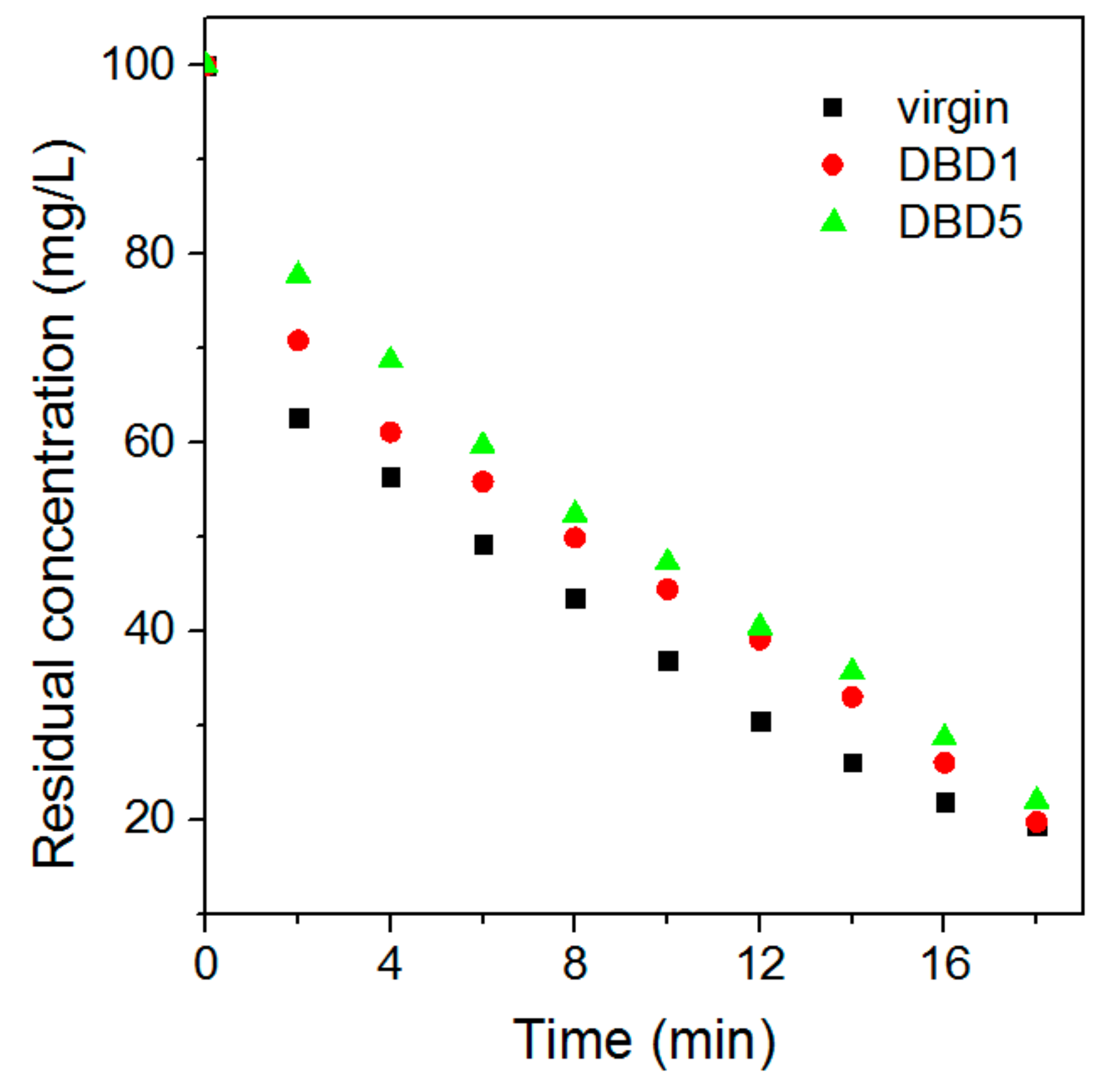
3.1. Effect of Regeneration on Adsorption Capacity and Kinetics of Resin
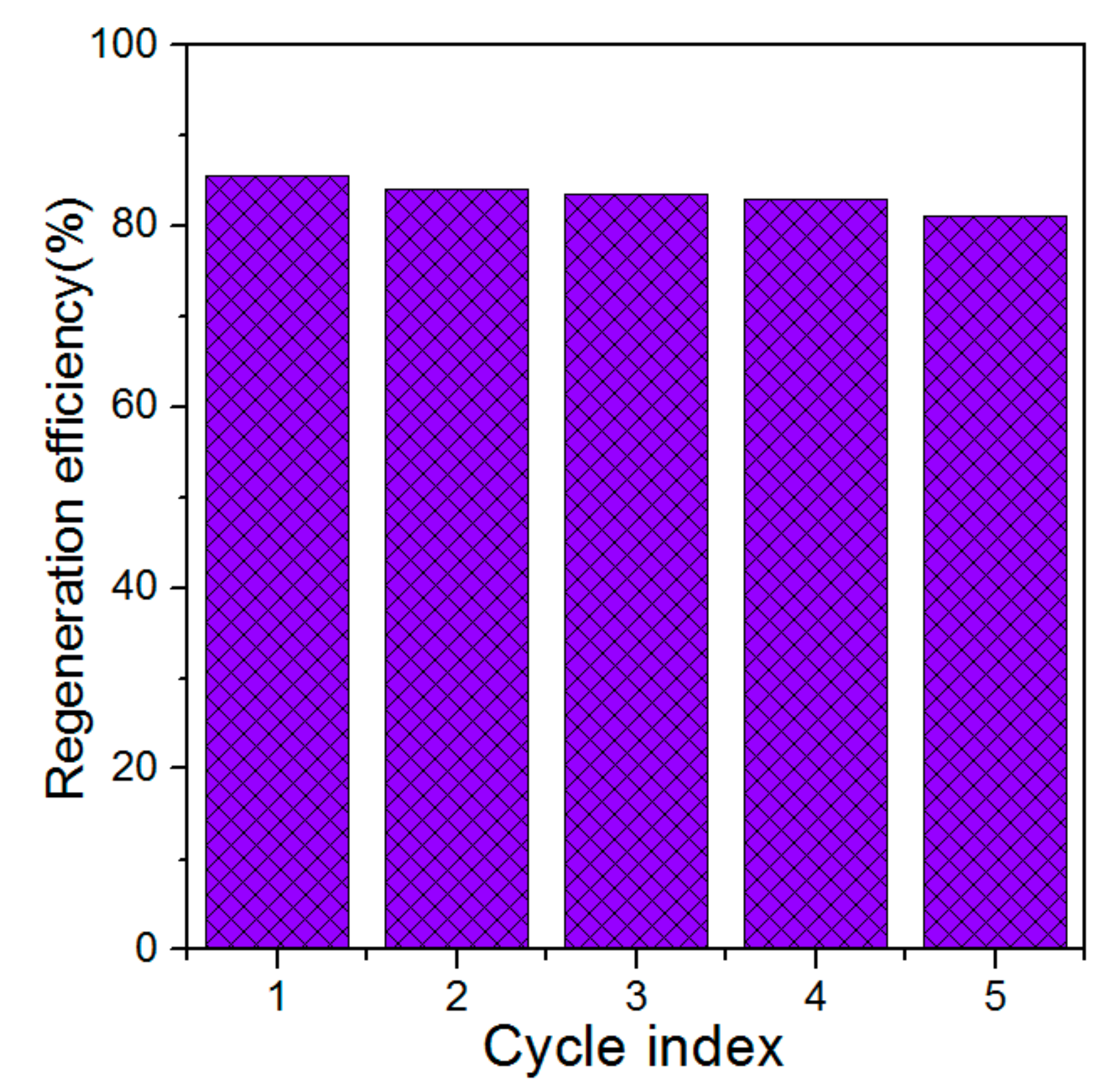
3.2. Effect of Regeneration on the Regeneration Efficiency
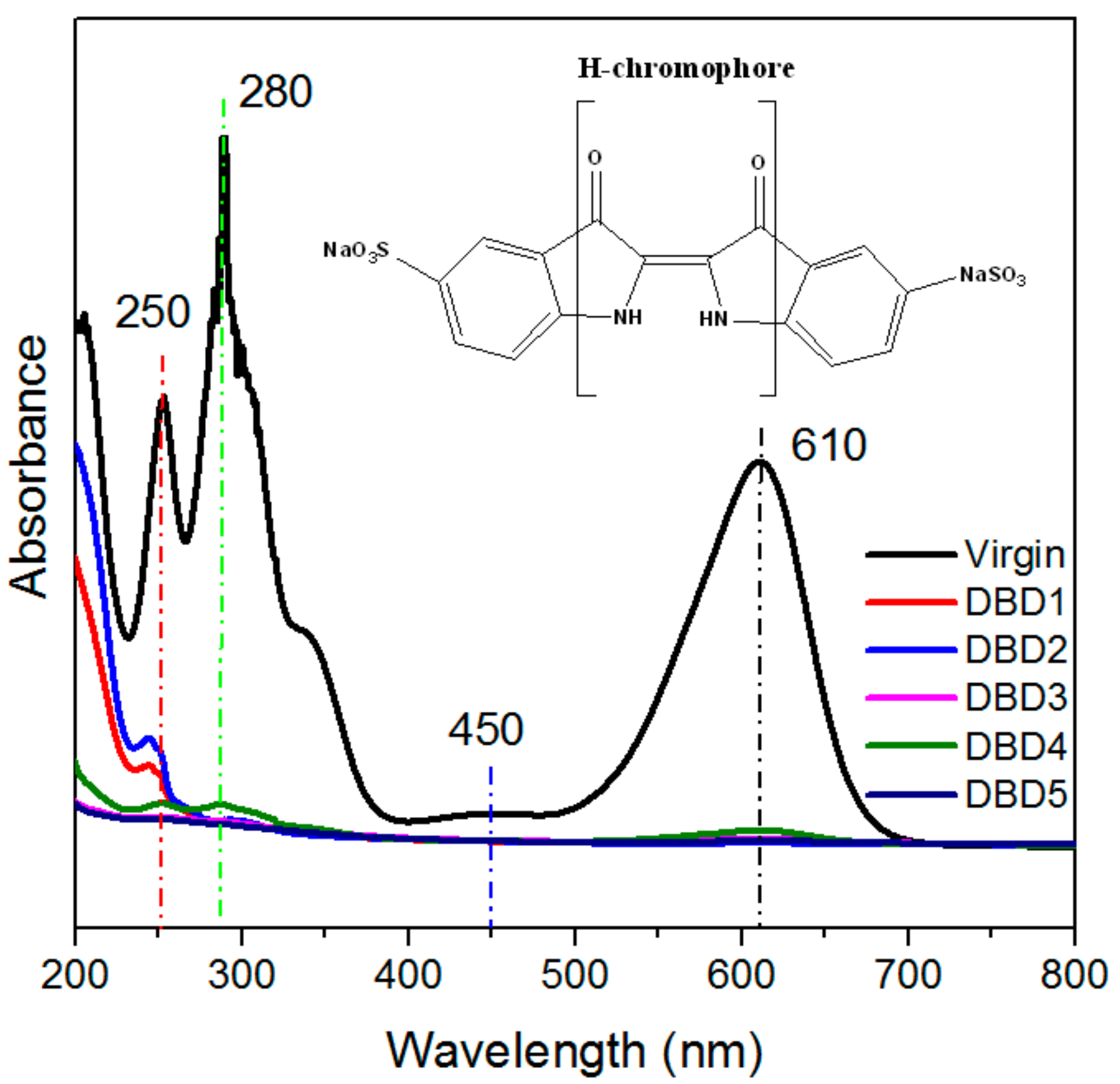
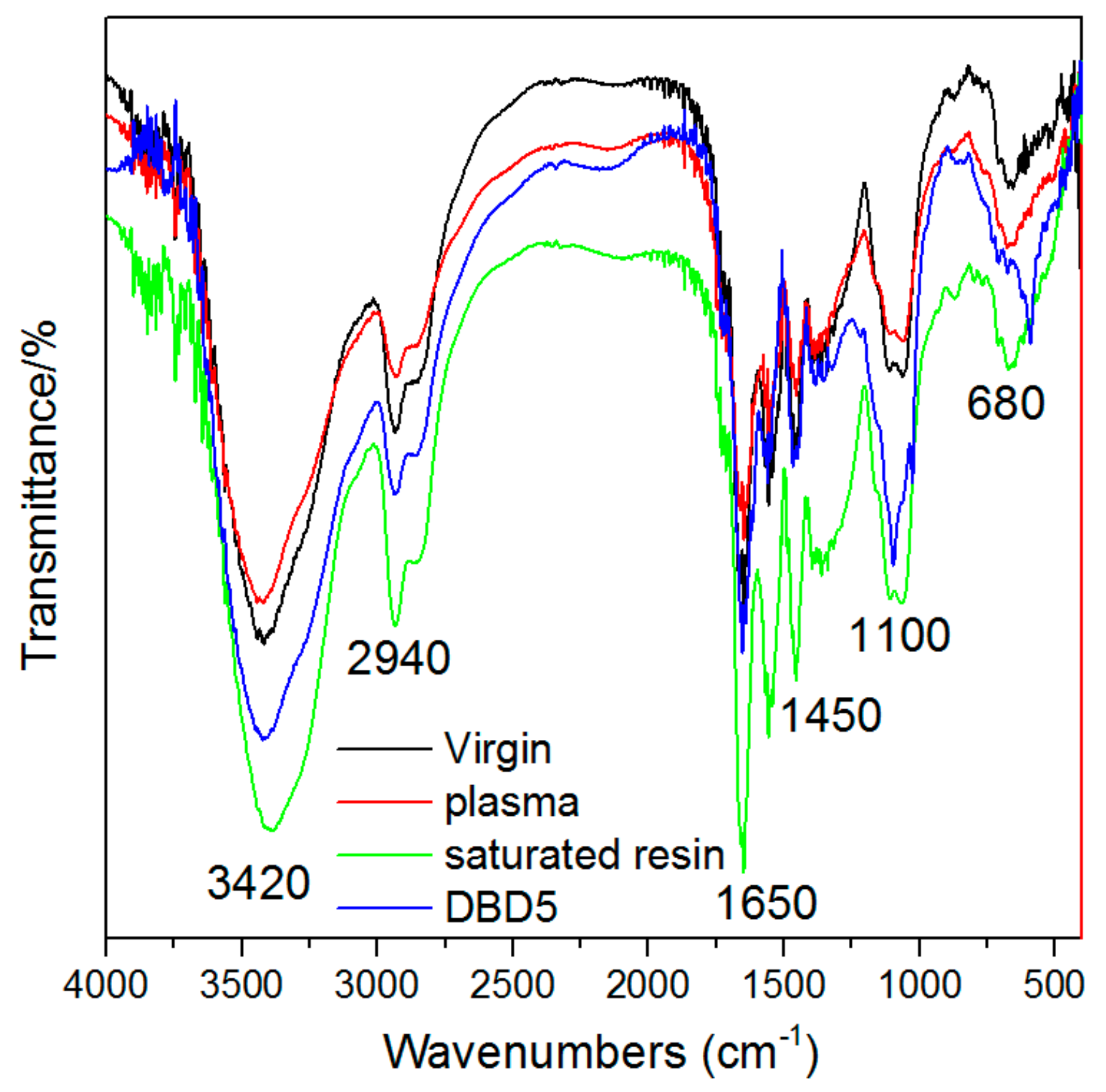
3.3. Changes in the Structural Properties of Resins
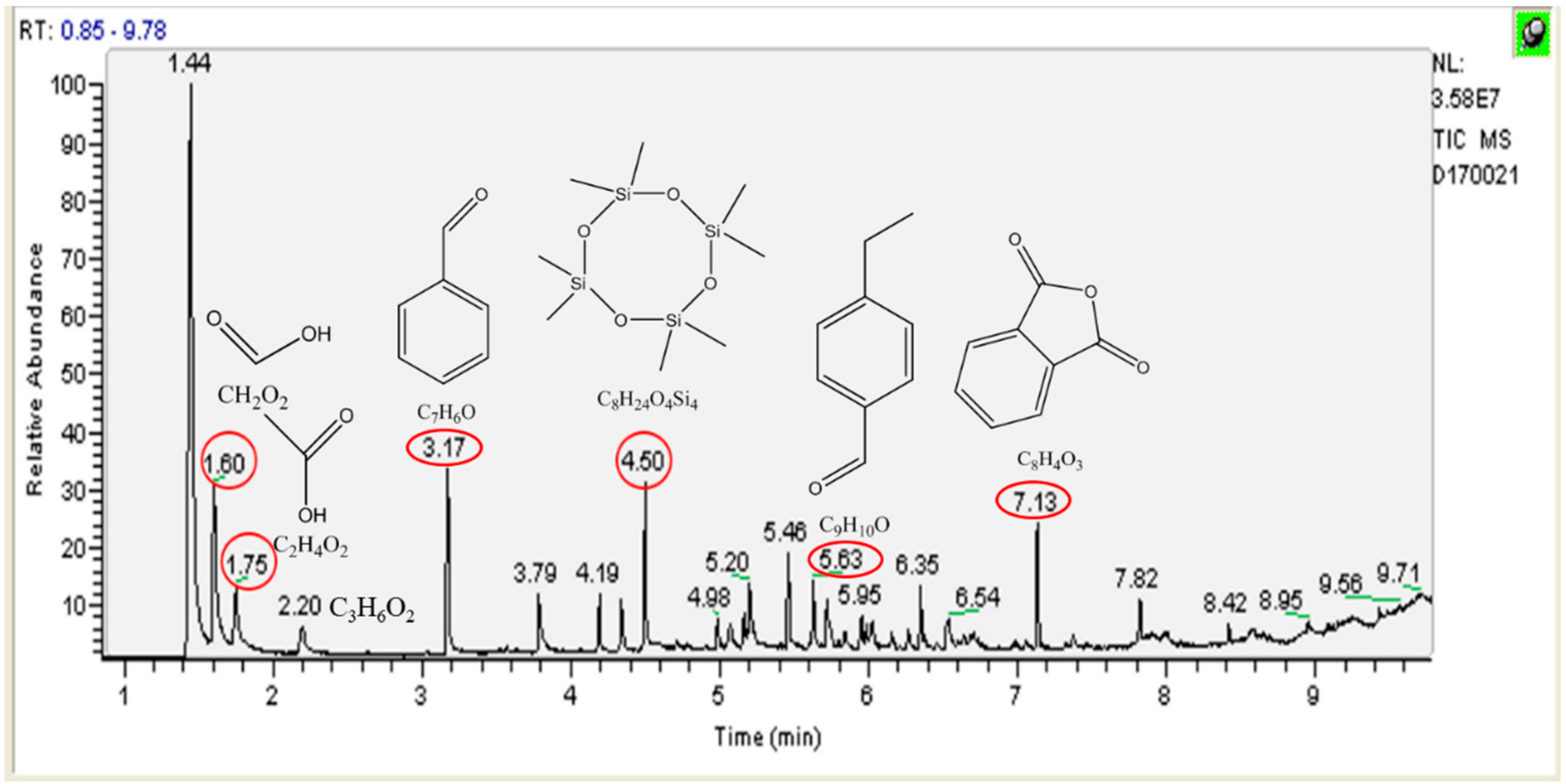
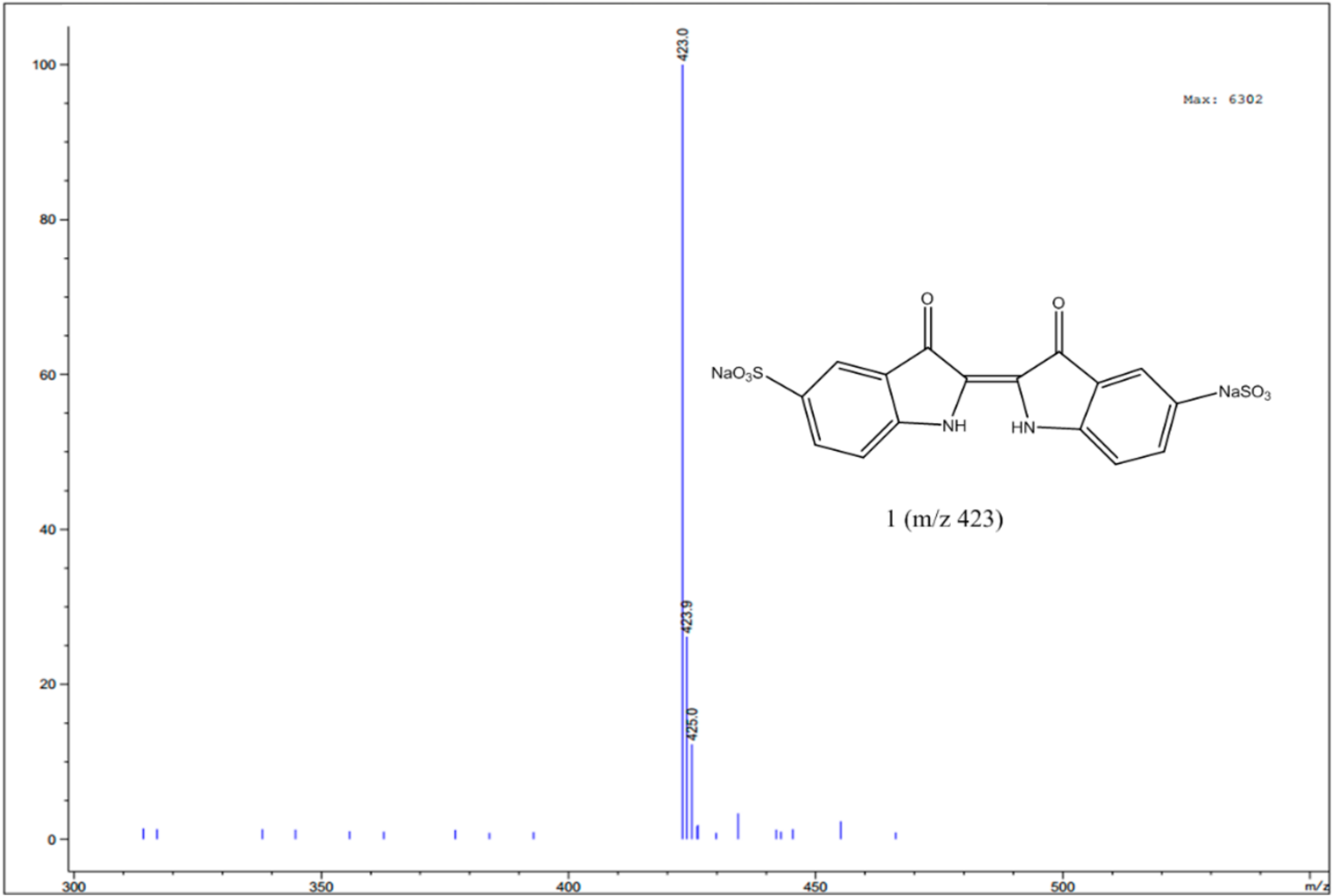
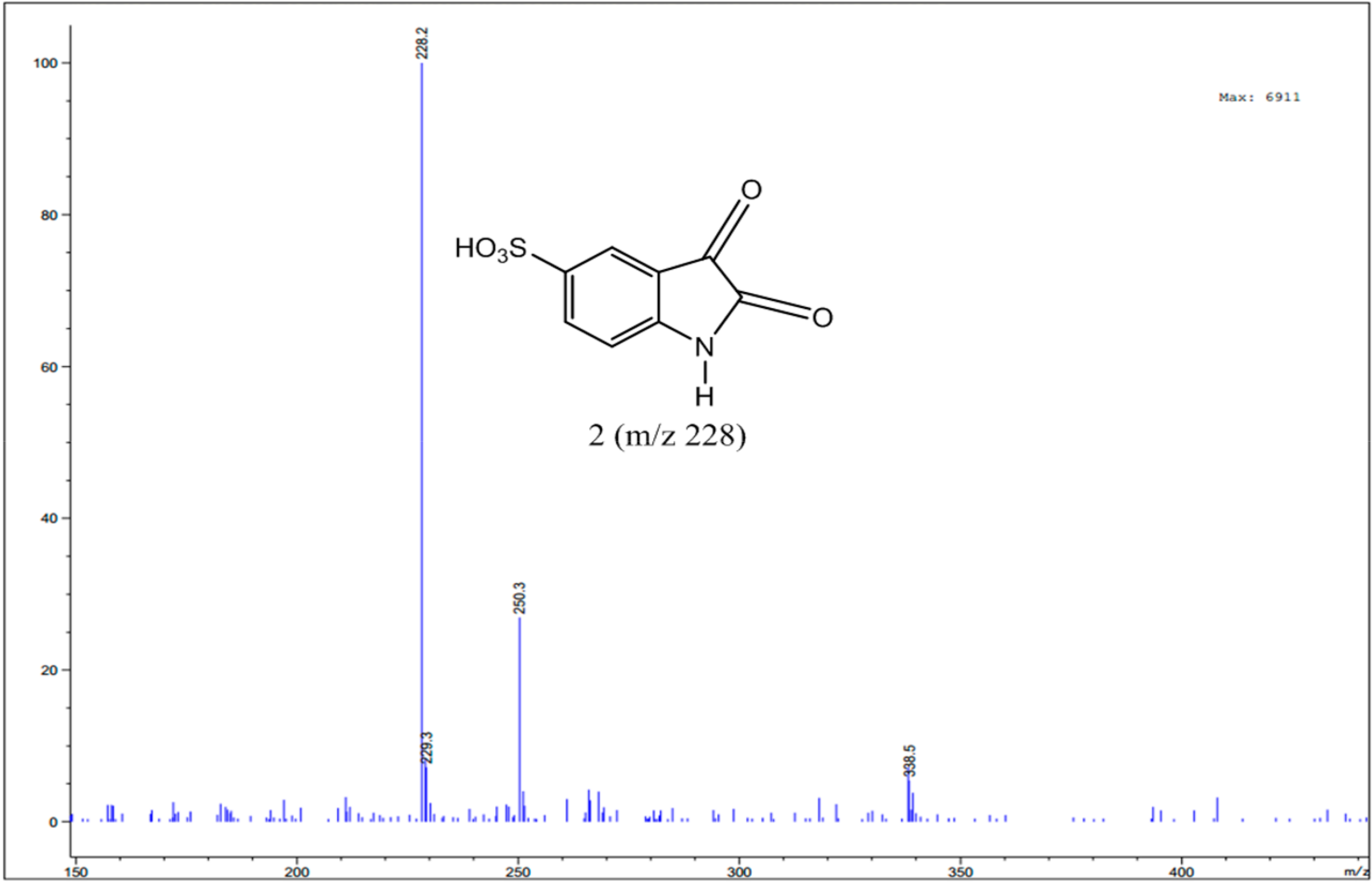
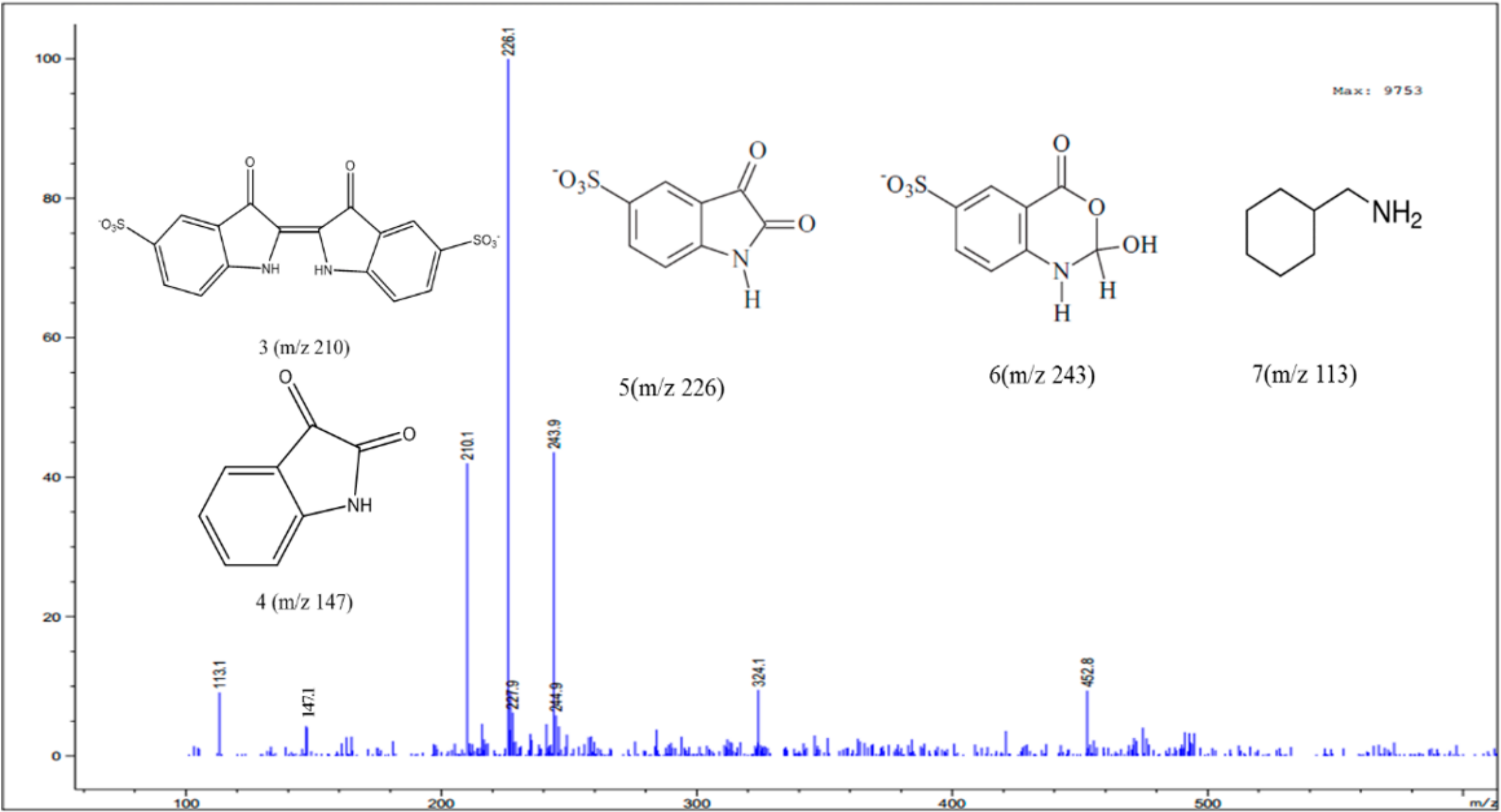
3.4. Identification of Intermediates by GC-MS and LC-MS
4. Materials and Analysis Methods
4.1. Materials
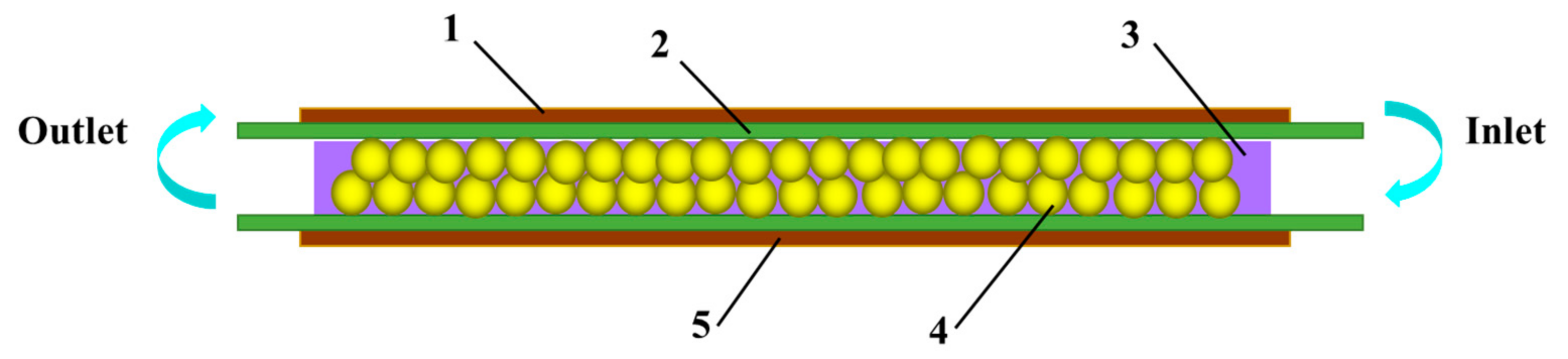
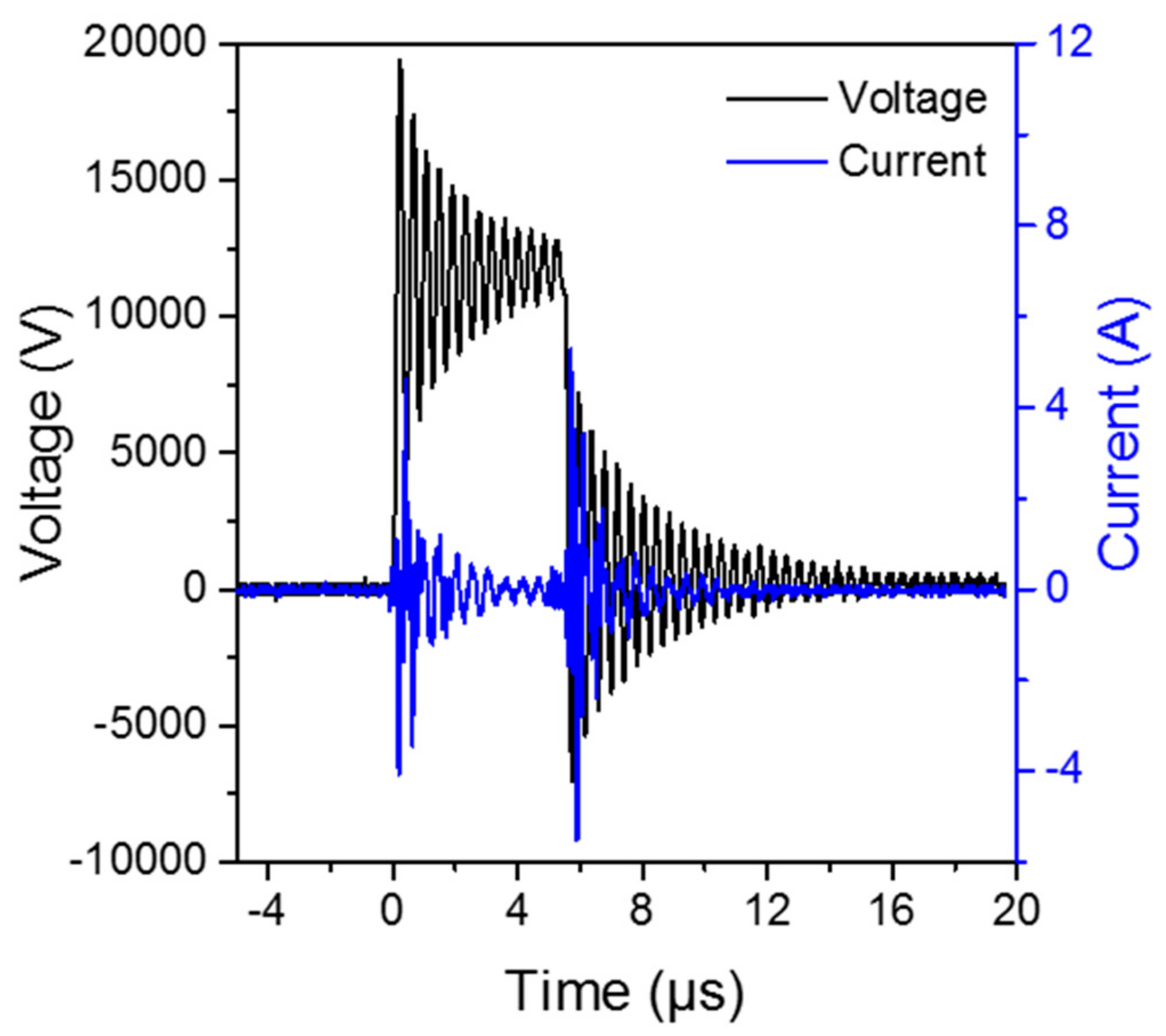
4.2. The DBD Regeneration Reaction System
4.3. Analytical Device
4.4. The Regeneration of Indigo Carmine Saturated Resin
4.5. Kinetics Adsorption
4.6. Adsorption Equilibrium Isotherms
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vandevivere, P.C.; Bianchi, R.; Verstraete, W. Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J. Chem. Technol. Biotechnol. 1998, 72, 289–302. [Google Scholar] [CrossRef]
- Minamitani, Y.; Shoji, S.; Ohba, Y.; Higashiyama, Y. Decomposition of dye in water solution by pulsed power discharge in a water droplet spray. IEEE Trans. Plasma Sci. 2008, 36, 2586–2591. [Google Scholar] [CrossRef]
- Jiang, S.; Wen, Y.; Liu, K. Treatments of dye wastewater by water spout in the pulsed DBD. In Proceedings of the IEEE Internatyional Conference on Plasma Science (ICOPS), Antalya, Turkey, 24–28 May 2015. [Google Scholar]
- Khraisheh, M.A.M.; Al-Ghouti, M.A.; Allen, S.J.; Ahmad, M.N. Effect of OH and silanol groups in the removal of dyes from aqueous solution using diatomite. Water Res. 2005, 39, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hui, K.N.; Hui, K.S.; Lee, S.K.; Zhou, W.; Chen, R.; Hwang, D.H.; Cho, Y.R.; Son, Y.G. Adsorption of basic yellow 87 from aqueous solution onto two different mesoporous adsorbents. Chem. Eng. J. 2012, 180, 91–98. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Zhang, M. Decolorization and biodegradability of dyeing wastewater treated by a TiO2-sensitized photo-oxidation process. Water Sci. Technol. 1996, 34, 49–55. [Google Scholar]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Raghu, S.; Basha, C.A. Chemical or electrochemical techniques, followed by ion exchange, for recycle of textile dye wastewater. J. Hazard. Mater. 2007, 149, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montano, J.; Ruiz, N.; Munoz, I.; Domenech, X.; Garcia-Hortal, J.A.; Torrades, F.; Peral, J. Environmental assessment of different photo-Fenton approaches for commercial reactive dye removal. J. Hazard. Mater. 2006, 138, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Fongsatitkul, P.; Elefsiniotis, P.; Yamasmit, A.; Yamasmit, N. Use of sequencing batch reactors and Fenton’s reagent to treat a wastewater from a textile industry. Biochem. Eng. J. 2004, 21, 213–220. [Google Scholar] [CrossRef]
- Pouran, S.R.; Aziz, A.R.A.; Daud, W.M.A.W. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Clements, J.S.; Sato, M.; Davis, R.H. Preliminary investigation of pre-breakdown phenomena and chemical-reactions using a pulsed high voltage discharge in water. IEEE Trans. Ind. Appl. 1987, 23, 224–235. [Google Scholar] [CrossRef]
- Eliasson, B.; Kogelschatz, U. Modeling and applications of silent discharge plasmas. IEEE Trans. Ind. Appl. 1991, 19, 309–323. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Vergara Sanchez, J.; Torres Segundo, C.; Montiel Palacios, E.; Gomez Diaz, A.; Reyes Romero, P.G.; Martinez Valencia, H. Degradation of textile dye AB 52 in an aqueous solution by applying a plasma at atmospheric pressure. IEEE Trans. Plasma Sci. 2017, 45, 479–484. [Google Scholar] [CrossRef]
- Kuroki, T.; Fujioka, T.; Kawabata, R.; Okubo, M.; Yamamoto, T. Regeneration of honeycomb zeolite by nonthermal plasma desorption of toluene. IEEE Trans. Ind. Appl. 2009, 45, 10–15. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Lu, X.; Liu, Q.; Wu, M.; Yan, Z.; Qiu, S.; Xue, Q.; Wei, Z.; Xiao, H.; et al. Degradation of organic dye by pulsed discharge non-thermal plasma technology assisted with modified activated carbon fibers. Chem. Eng. J. 2013, 215, 969–978. [Google Scholar] [CrossRef]
- Hao, X.L.; Zhang, X.W.; Lei, L.C. Degradation characteristics of toxic contaminant with modified activated carbons in aqueous pulsed discharge plasma process. Carbon 2009, 47, 153–161. [Google Scholar] [CrossRef]
- Berenguer, R.; Marco-Lozar, J.P.; Quijada, C.; Cazorla-Amoros, D.; Morallon, E. Electrochemical regeneration and porosity recovery of phenol-saturated granular activated carbon in an alkaline medium. Carbon 2010, 48, 2734–2745. [Google Scholar] [CrossRef]
- Dabrowski, A.; Podkoscielny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Juang, R. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Iyim, T.B.; Acar, I.; Oezguemues, S. Removal of basic dyes from aqueous solutions with sulfonated phenol-formaldehyde resin. J. Appl. Polym. Sci. 2008, 109, 2774–2780. [Google Scholar] [CrossRef]
- Fan, J.; Li, H.; Shuang, C.; Li, W.; Li, A. Dissolved organic matter removal using magnetic anion exchange resin treatment on biological effluent of textile dyeing wastewater. J. Environ. Sci. 2014, 26, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lippincott, L.; Meng, X. Feasibility and kinetics study on the direct bio-regeneration of perchlorate laden anion-exchange resin. Water Res. 2008, 42, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Anand, B.; Meganathan, C.; Joshua, B.D. FT-IR, FT-Raman spectra and ab initio HF, DFT vibrational analysis of 2,3-difluoro phenol. Spectrochim. Acta A 2007, 68, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Brick, A.A.; Mohamed, A.A. An efficient adsorption of indigo carmine dye from aqueous solution on mesoporous Mg/Fe layered double hydroxide nanoparticles prepared by controlled sol-gel route. Chemosphere 2017, 174, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.M.K.; Raju, B.R.; Karuppiah, J.; Reddy, E.L.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Feng, F. Improving the indigo carmine decolorization ability of a bacillus amyloliquefaciens laccase by site-directed mutagenesis. Catalysts 2017, 7, 275. [Google Scholar] [CrossRef]
- Qu, G.-Z.; Li, J.; Wu, Y.; Li, G.-F.; Li, D. Regeneration of acid orange 7-exhausted granular activated carbon with dielectric barrier discharge plasma. Chem. Eng. J. 2009, 146, 168–173. [Google Scholar] [CrossRef]
- Mangun, C.L.; Benak, K.R.; Economy, J.; Foster, K.L. Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 2001, 39, 1809–1820. [Google Scholar] [CrossRef]

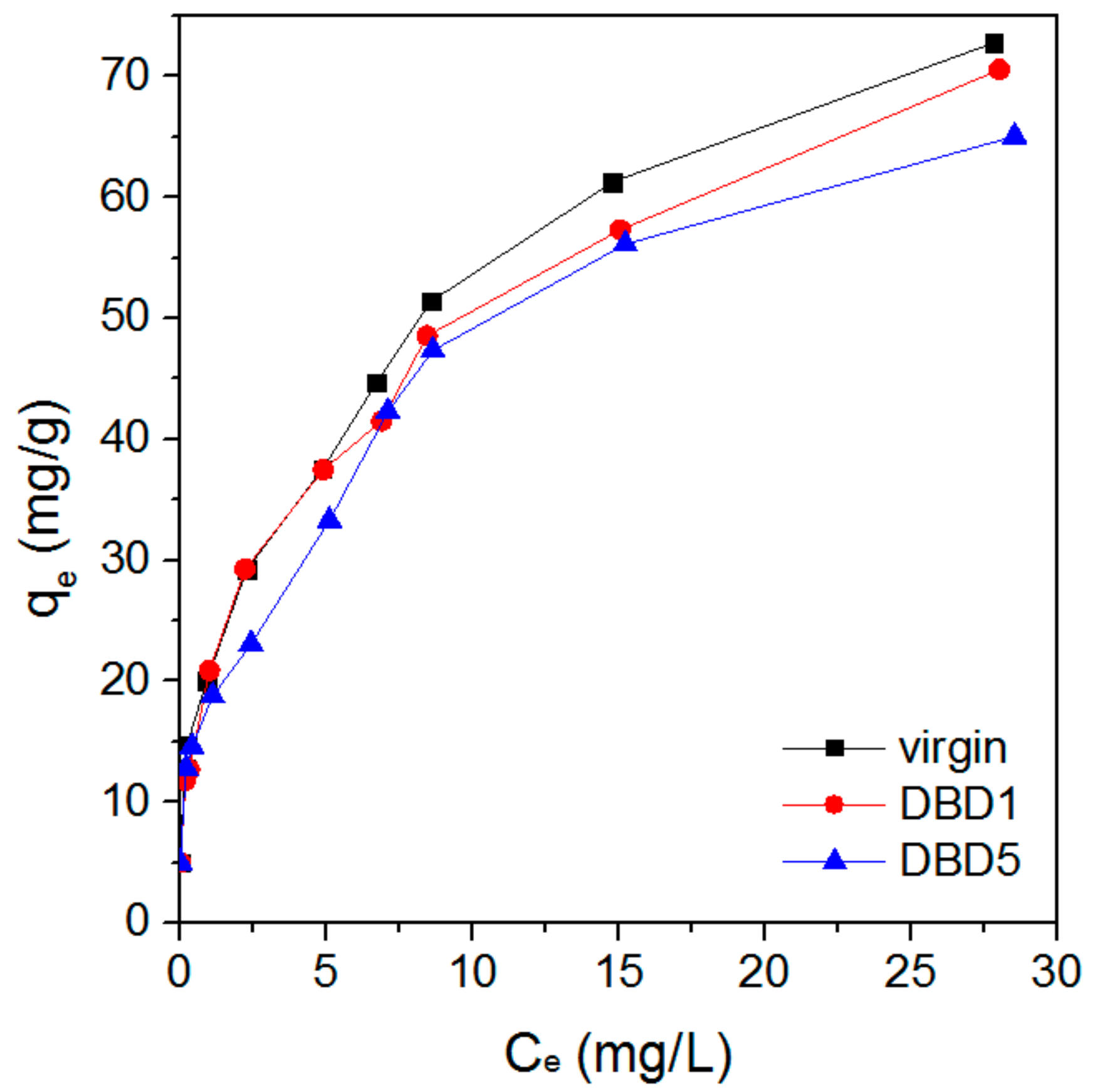

| Sample | KF (L/g) | 1/n | R2 |
|---|---|---|---|
| Virgin | 2.31 | 0.33 | 0.989 |
| DBD1 | 2.39 | 0.30 | 0.979 |
| DBD5 | 2.29 | 0.30 | 0.989 |
| Sample | SBET (m2/g) | VTotal pore (cm3/g) | Pore Size (nm) | Adsorption Capacity (mg/g) |
|---|---|---|---|---|
| Virgin | 163 | 0.3210 | 43.05 | - |
| Saturated | 74.6 | 0.1630 | 23.94 | 70.58 |
| DBD5 | 157.8 | 0.299 | 46.33 | 65.06 |
| Compound | Structure | Molecular Formula | Retention Time (min) |
|---|---|---|---|
| Formic acid |  | CH2O2 | 1.6 |
| Acetic acid |  | C2H4O2 | 1.75 |
| Propanoic acid |  | C3H6O2 | 2.2 |
| Benzaldehyde |  | C7H6O | 3.17 |
| Octamethyl-cyclotetrasiloxane |  | C8H24O4Si4 | 4.5 |
| 4-ethyl-benzaldehyde |  | C9H10O | 5.63 |
| Phthalic anhydride |  | C8H4O3 | 7.13 |
| Compound | Structure | Molecular Formula | MS Fragments (m/z) |
|---|---|---|---|
| Indigo carmine |  | C16H8N2Na2O8S2 | 423/425 |
 | C16H8N2O8S2 | 210 | |
| 5-Isatinsulfonic acid sodium salt |  | C8H4NO5S·Na | 250 |
| Isatin 5-sulfonic acid |  | C8H5NO5S | 228/229 |
 | C8H4NO5S | 226/227 | |
 | C8H6NO6S | 243/244 | |
| Isatin |  | C8H5NO2 | 147 |
| Cyclohexanemethylamine |  | C7H15N | 113 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, C.; Xiao, Z.; Xu, D.; Zhang, C.; Qiu, J.; Liu, K. Saturated Resin Ectopic Regeneration by Non-Thermal Dielectric Barrier Discharge Plasma. Catalysts 2017, 7, 362. https://doi.org/10.3390/catal7120362
Hao C, Xiao Z, Xu D, Zhang C, Qiu J, Liu K. Saturated Resin Ectopic Regeneration by Non-Thermal Dielectric Barrier Discharge Plasma. Catalysts. 2017; 7(12):362. https://doi.org/10.3390/catal7120362
Chicago/Turabian StyleHao, Chunjing, Zehua Xiao, Di Xu, Chengbo Zhang, Jian Qiu, and Kefu Liu. 2017. "Saturated Resin Ectopic Regeneration by Non-Thermal Dielectric Barrier Discharge Plasma" Catalysts 7, no. 12: 362. https://doi.org/10.3390/catal7120362





