Strategies to Enhance the Catalytic Performance of ZSM-5 Zeolite in Hydrocarbon Cracking: A Review
Abstract
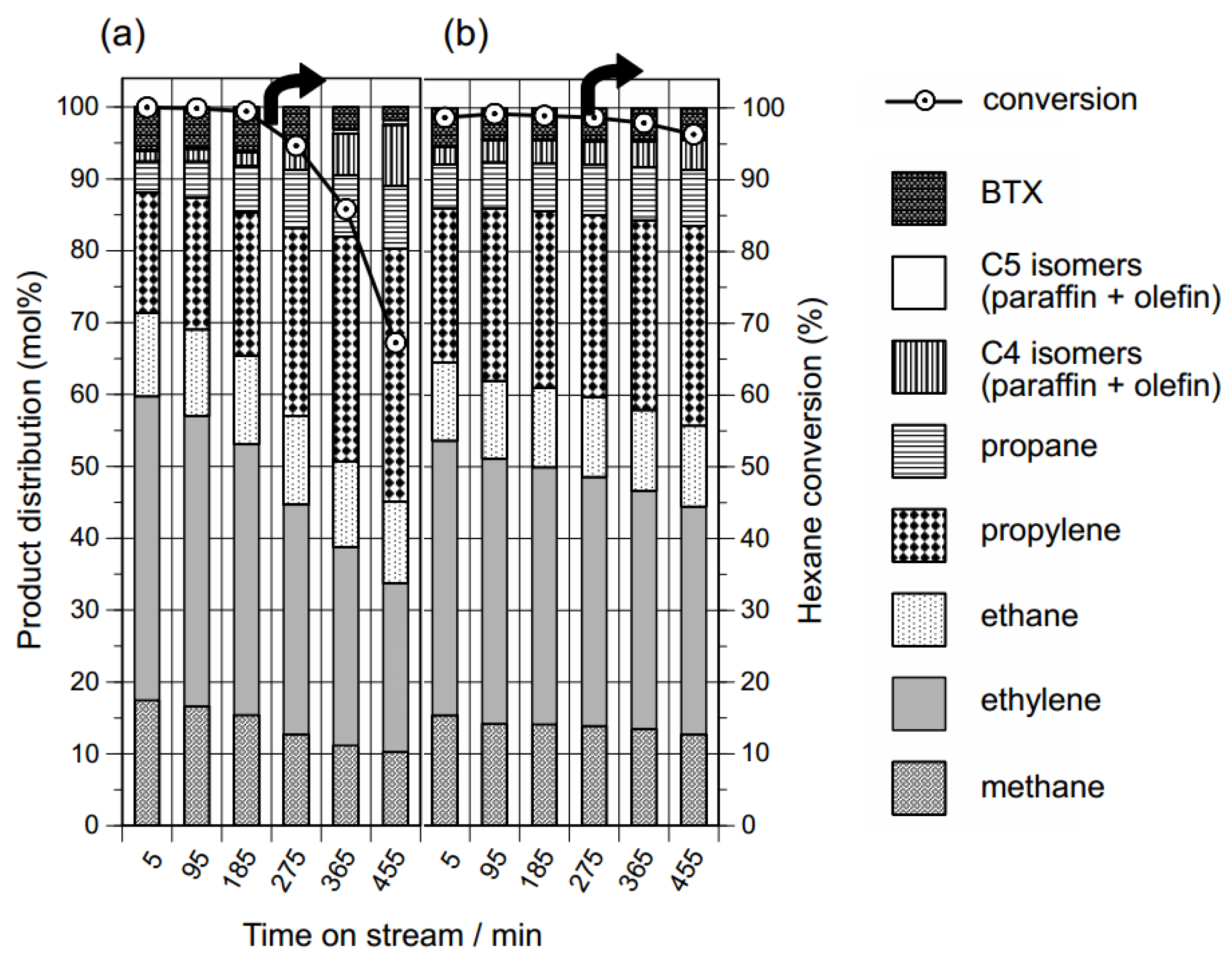
:1. Introduction
- (1)
- Synthesis of ZSM-5 zeolite with special morphology to decrease the diffusion pathway or increase the diffusion efficiency;
- (2)
- Synthesis of hierarchical ZSM-5 zeolite with extra mesopores or macropores to increase the diffusion efficiency of molecules and the accessibility of acid sites;
- (3)
- Synthesis of nano-sized ZSM-5 zeolite with short diffusion length to promote the diffusion of molecules and;
- (4)
- Optimization of the acid properties of ZSM-5 zeolite to enhance the catalytic activity.
2. ZSM-5 Zeolite with Special Morphology
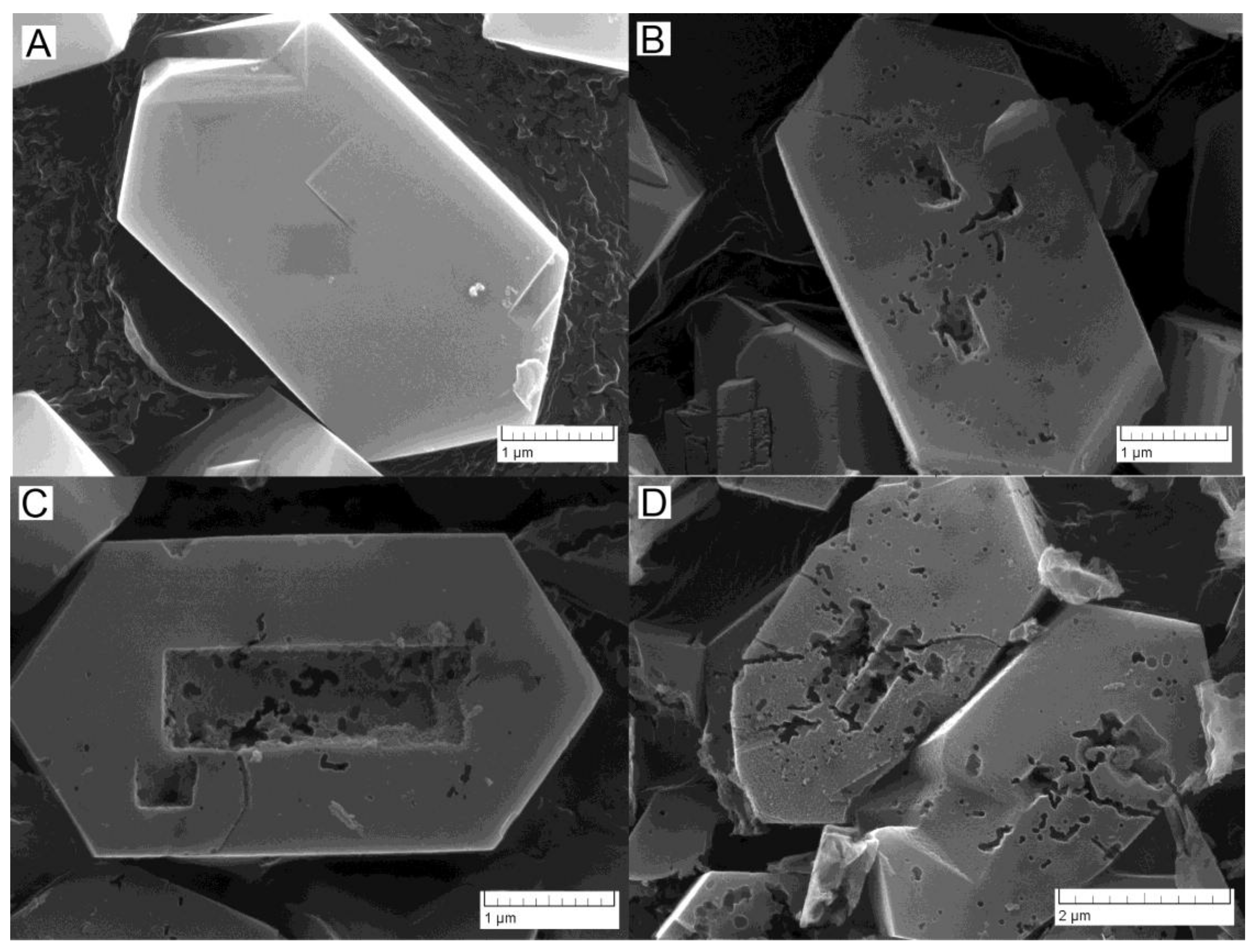
2.1. Hollow ZSM-5 Zeolite
2.2. Zeolitic Composites of ZSM-5 and Other Materials
2.3. Nanosheet MFI Zeolite
2.4. b-Oriented MFI Zeolite
2.5. Other Zeolite with Special Morphology
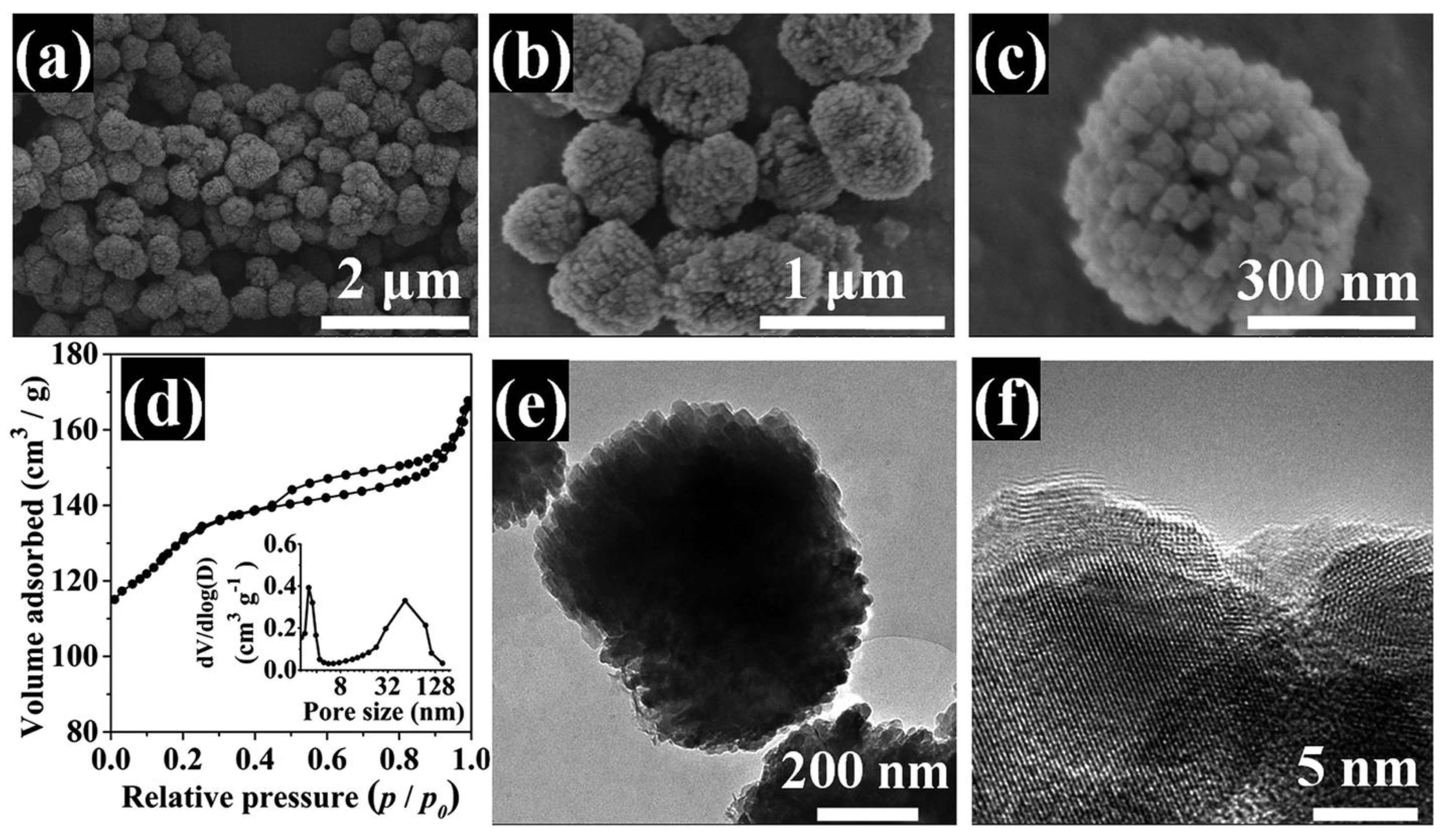
3. Hierarchical ZSM-5 Zeolite
3.1. Double Templating with Hard-Template Method
3.2. Double Templating with Soft-Template Method
3.3. Single Templating Method
3.4. Post-Treatment Method
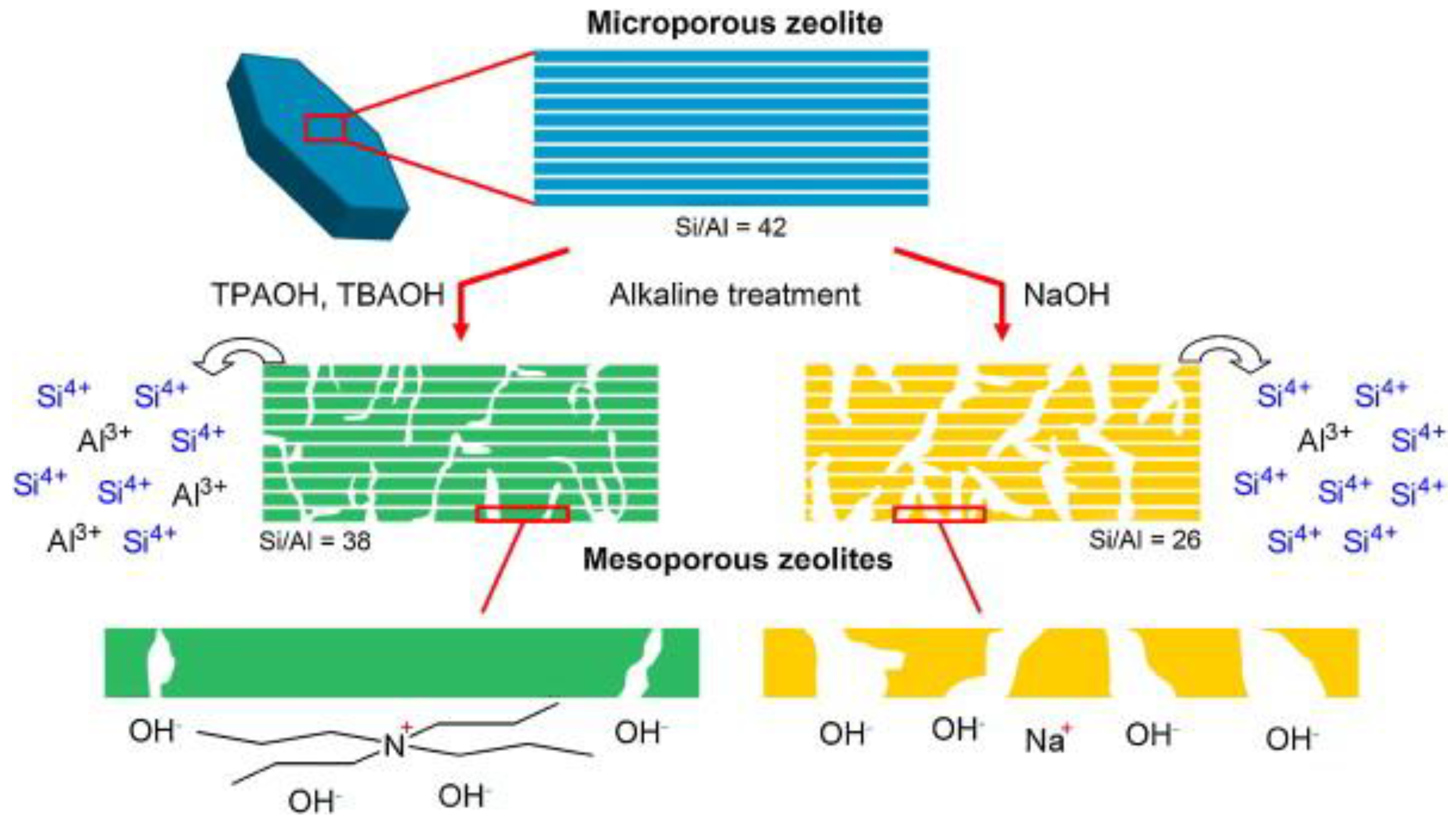
3.4.1. Alkaline Etching
3.4.2. Fluoride Etching
4. Nano-Sized ZSM-5 Zeolite
4.1. Synthesis of Nano-Sized ZSM-5 Zeolite by Hydrothermal Method
4.2. Synthesis of Nano-Sized ZSM-5 Zeolite by Bead Milling Method
5. Adjustment of Acid Properties
5.1. Acidity Strength
5.2. Acid Amount
5.3. Surface Acid Sites
6. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Emori, E.Y.; Hirashima, F.H.; Zandonai, C.H.; Ortiz-Bravo, C.A.; Fernandes-Machado, N.R.C.; Olsen-Scaliante, M.H.N. Catalytic cracking of soybean oil using ZSM5 zeolite. Catal. Today 2017, 279, 168–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.; Wang, L.; Zhang, X.; Li, G. Controllable Decomposition of Methanol for Active Fuel Cooling Technology. Energey Fuels 2014, 28, 4431–4439. [Google Scholar] [CrossRef]
- Li, W.; Li, G.; Jin, C.; Liu, X.; Wang, J. One-step synthesis of nanorod-aggregated functional hierarchical iron-containing MFI zeolite microspheres. J. Mater. Chem. A 2015, 3, 14786–14793. [Google Scholar] [CrossRef]
- Aziz, A.; Kim, K.S. Investigation of tertiary butyl alcohol as template for the synthesis of ZSM-5 zeolite. J. Porous Mater. 2015, 22, 1401–1406. [Google Scholar] [CrossRef]
- Xu, D.; Swindlehurst, G.R.; Wu, H.; Olson, D.H.; Zhang, X.; Tsapatsis, M. On the Synthesis and Adsorption Properties of Single-Unit-Cell Hierarchical Zeolites Made by Rotational Intergrowths. Adv. Funct. Mater. 2014, 24, 201–208. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites history and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–701. [Google Scholar] [CrossRef] [PubMed]
- Sadrameli, S.M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: A state-of-the-art review II: Catalytic cracking review. Fuel 2016, 173, 285–297. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A. Martineztriguero, J. Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Xue, N.; Nie, L.; Fang, D.; Guo, X.; Shen, J.; Ding, W.; Chen, Y. Synergistic effects of tungsten and phosphorus on catalytic cracking of butene to propene over HZSM-5. Appl. Catal. A 2009, 352, 87–94. [Google Scholar] [CrossRef]
- Luo, J.; Bhaskar, B.V.; Yeh, Y.-H.; Gorte, R.J. n-Hexane cracking at high pressures on H-ZSM-5, H-BEA, H-MOR, and USY for endothermic reforming. Appl. Catal. A 2014, 478, 228–233. [Google Scholar] [CrossRef]
- Pagis, C.; Morgado Prates, A.R.; Farrusseng, D.; Bats, N.; Tuel, A. Hollow Zeolite Structures: An Overview of Synthesis Methods. Chem. Mater. 2016, 28, 5205–5223. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Chal, R.; Gérardin, C.; Bulut, M.; Van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Mintova, S.; Jaber, M.; Valtchev, V. Nanosized microporous crystals: Emerging applications. Chem. Soc. Rev. 2015, 44, 7207–7233. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Tao, H.; Li, C.; Ren, J.; Wang, Y.; Lu, G. Synthesis of mesoporous zeolite single crystals with cheap porogens. J. Solid State Chem. 2011, 184, 1820–1827. [Google Scholar] [CrossRef]
- Yin, C.; Wei, Y.; Wang, F.; Chen, Y. Introduction of mesopority in zeolite ZSM-5 using resin as templates. Mater. Lett. 2013, 98, 194–196. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.; Xiao, C.; Cychosz, K.A.; Li, K.; Wan, W.; Zou, X.; Thommes, M. Evidence of Intracrystalline Mesostructured Porosity in Zeolites by Advanced Gas Sorption, Electron Tomography and Rotation Electron Diffraction. ChemCatChem 2014, 6, 3110–3115. [Google Scholar] [CrossRef]
- Mei, C.; Liu, Z.; Wen, P.; Xie, Z.; Hua, W.; Gao, Z. Regular HZSM-5 microboxes prepared via a mild alkaline treatment. J. Mater. Chem. 2008, 18, 3496–3500. [Google Scholar] [CrossRef]
- Fodor, D.; Krumeich, F.; Hauert, R.; Van Bokhoven, J.A. Differences between individual ZSM-5 crystals in forming hollow single crystals and mesopores during base leaching. Chemistry 2015, 21, 6272–6277. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, A.; Liu, M.; Guo, X.; Song, C. Hollow ZSM-5 with Silicon-Rich Surface, Double Shells, and Functionalized Interior with Metallic Nanoparticles and Carbon Nanotubes. Adv. Funct. Mater. 2015, 25, 7479–7487. [Google Scholar] [CrossRef]
- Li, H.; He, S.; Ma, K.; Wu, Q.; Jiao, Q.; Sun, K. Micro-mesoporous composite molecular sieves H-ZSM-5/MCM-41 for methanol dehydration to dimethyl ether: Effect of SiO2/Al2O3 ratio in H-ZSM-5. Appl. Catal. A 2013, 450, 152–159. [Google Scholar] [CrossRef]
- Sang, Y.; Jiao, Q.; Li, H.; Wu, Q.; Zhao, Y.; Sun, K. HZSM-5/MCM-41 composite molecular sieves for the catalytic cracking of endothermic hydrocarbon fuels: Nano-ZSM-5 zeolites as the source. J. Nanopart. Res. 2014, 16, 2755–2765. [Google Scholar] [CrossRef]
- Peng, P.; Wang, Y.; Zhang, Z.; Qiao, K.; Liu, X.; Yan, Z.; Subhan, F.; Komarneni, S. ZSM-5-based mesostructures by combined alkali dissolution and re-assembly: Process controlling and scale-up. Chem. Eng. J. 2016, 302, 323–333. [Google Scholar] [CrossRef]
- Diao, Z.; Wang, L.; Zhang, X.; Liu, G. Catalytic cracking of supercritical n-dodecane over meso-HZSM-5@Al-MCM-41 zeolites. Chem. Eng. Sci. 2015, 135, 452–460. [Google Scholar] [CrossRef]
- Wang, D.; Xu, L.; Wu, P. Hierarchical, core–shell meso-ZSM-5@mesoporous aluminosilicate-supported Pt nanoparticles for bifunctional hydrocracking. J. Mater. Chem. A 2014, 2, 15535–15545. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, G.; Pan, M.; Guo, D.; Zhao, Q.; Li, B.; Li, R. Hierarchical core–shell zeolite composite ZSM-5@SAPO-34 fabricated by using ZSM-5 as the nutrients for the growth of SAPO-34. Microporous Mesoporous Mater. 2015, 206, 114–120. [Google Scholar] [CrossRef]
- Vu, X.H.; Nguyen, S.; Dang, T.T.; Phan, B.M.Q.; Nguyen, D.A.; Armbruster, U.; Martin, A. Catalytic Cracking of Triglyceride-Rich Biomass toward Lower Olefins over a Nano-ZSM-5/SBA-15 Analog Composite. Catalysts 2015, 5, 1692–1703. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, Y.; Duanmu, C.; Xu, Y.; Feng, L.; Gu, X.; Chen, J. Preparation of hollow ZSM-5 crystals in the presence of polyacrylamide. Microporous Mesoporous Mater. 2012, 163, 11–20. [Google Scholar] [CrossRef]
- Tao, H.; Ren, J.; Liu, X.; Wang, Y.; Lu, G. Facile synthesis of hollow zeolite microspheres through dissolution–recrystallization procedure in the presence of organosilanes. J. Solid State Chem. 2013, 200, 179–188. [Google Scholar] [CrossRef]
- Pashkova, V.; Tokarova, V.; Brabec, L.; Dedecek, J. Self-templating synthesis of hollow spheres of zeolite ZSM-5 from spray-dried aluminosilicate precursor. Microporous Mesoporous Mater. 2016, 228, 59–63. [Google Scholar] [CrossRef]
- Groen, J.C.; Bach, T.; Ziese, U.; Donk, A.M.P.-V.; Jong, K.P.D.; Moulijn, J.A.; Pérez-Ramírez, J. Creation of Hollow Zeolite Architectures by Controlled Desilication of Al-Zoned ZSM-5 Crystals. J. Am. Chem. Soc. 2005, 127, 10792–10793. [Google Scholar] [CrossRef] [PubMed]
- Vu, X.; Armbruster, U.; Martin, A. Micro/Mesoporous Zeolitic Composites: Recent Developments in Synthesis and Catalytic Applications. Catalysts 2016, 6, 183. [Google Scholar] [CrossRef]
- Li, G.; Diao, Z.; Na, J.; Wang, L. Exploring suitable ZSM-5/MCM-41 zeolites for catalytic cracking of n-dodecane: Effect of initial particle size and Si/Al ratio. Chin. J. Chem. Eng. 2015, 23, 1655–1661. [Google Scholar] [CrossRef]
- Boukoussa, B.; Aouad, N.; Hamacha, R.; Bengueddach, A. Key factor affecting the structural and textural properties of ZSM-5/MCM-41 composite. J. Phys. Chem. Solids 2015, 78, 78–83. [Google Scholar] [CrossRef]
- Xue, H.; He, Z.; Zhao, Y.; Jiao, Q.; Wu, Q.; Li, H. Controllable synthesis of composites of ZSM-5 and KIT-1 using an ionic liquid as template. Solid State Sci. 2017, 64, 29–33. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Gumidyala, A.; Grabow, L.C.; Crossley, S.P.; Rime, J.D. Epitaxial Growth of ZSM-5@Silicalite-1: A Core-Shell Zeolite Designed with Passivated Surface Acidity. ACS Nano 2015, 9, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.-Y.; Chen, L.; Au, C.-T.; Yin, S.-F. Synthesis and application of HZSM-5@silicalite-1 core–shell composites for the generation of light olefins from CH3Br. Microporous Mesoporous Mater. 2016, 227, 76–80. [Google Scholar] [CrossRef]
- Vu, X.H.; Bentrup, U.; Hunger, M.; Kraehnert, R.; Armbruster, U.; Martin, A. Direct synthesis of nanosized-ZSM-5/SBA-15 analog composites from preformed ZSM-5 precursors for improved catalytic performance as cracking catalyst. J. Mater. Sci. 2014, 49, 5676–5689. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, L.; Magusin, P.C.M.M.; Mezari, B.; Hensen, E.J.M. On the synthesis of highly acidic nanolayered ZSM-5. J. Catal. 2015, 327, 10–21. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Jing, Z.; Han, L.; Singh, B.; Feng, J.; Shen, X.; Cao, F.; Oleynikov, P.; Sun, H.; et al. π-π interaction of aromatic groups in amphiphilic molecules directing for single-crystalline mesostructured zeolite nanosheets. Nat. Commun. 2014, 5, 4262–4270. [Google Scholar] [CrossRef] [PubMed]
- Keoh, S.H.; Chaikittisilp, W.; Muraoka, K.; Mukti, R.R.; Shimojima, A.; Kumar, P.; Tsapatsis, M.; Okubo, T. Factors Governing the Formation of Hierarchically and Sequentially Intergrown MFI Zeolites by Using Simple Diquaternary Ammonium Structure-Directing Agents. Chem. Mater. 2016, 28, 8997–9007. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, B.; Yang, H.; Yan, W. Synthesis of isomorphous MFI nanosheet zeolites for supercritical catalytic cracking of n-dodecane. Appl. Catal. A 2017, 533, 90–98. [Google Scholar] [CrossRef]
- Emdadi, L.; Wu, Y.; Zhu, G.; Chang, C.-C.; Fan, W.; Pham, T.; Lobo, R.F.; Liu, D. Dual Template Synthesis of Meso- and Microporous MFI Zeolite Nanosheet Assemblies with Tailored Activity in Catalytic Reactions. Chem. Mater. 2014, 26, 1345–1355. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz, K.A.; Agrawal, K.V.; Al Wahedi, Y.; Bhan, A.; Al Hashimi, S.; Terasaki, O.; et al. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 2012, 336, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Srivastava, R.; Satpati, B. One-Step Dual Template Mediated Synthesis of Nanocrystalline Zeolites of Different Framework Structures. Cryst. Growth Des. 2016, 16, 3323–3333. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Seo, Y.; Kim, J.-C.; Ryoo, R. n-Heptane hydroisomerization over Pt/MFI zeolite nanosheets: Effects of zeolite crystal thickness and platinum location. J. Catal. 2013, 301, 187–197. [Google Scholar] [CrossRef]
- Park, W.; Yu, D.; Na, K.; Jelfs, K.E.; Slater, B.; Sakamoto, Y.; Ryoo, R. Hierarchically Structure-Directing Effect of Multi-Ammonium Surfactants for the Generation of MFI Zeolite Nanosheets. Chem. Mater. 2011, 23, 5131–5137. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.J.; Chmelka, B.F.; Ryoo, R. Directing zeolite structures into hierarchically nanoporous architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, A.J.J.; Kim, W.; Degirmenci, V.; Xin, H.; Ryoo, R.; Hensen, E.J.M. Catalytic performance of sheet-like Fe/ZSM-5 zeolites for the selective oxidation of benzene with nitrous oxide. J. Catal. 2013, 299, 81–89. [Google Scholar] [CrossRef]
- Jo, C.; Cho, K.; Kim, J.; Ryoo, R. MFI zeolite nanosponges possessing uniform mesopores generated by bulk crystal seeding in the hierarchical surfactant-directed synthesis. Chem. Commun. 2014, 50, 4175–4177. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, L.; Zhu, Z.; Zhang, K.; Xi, H.; Qian, Y. Effect of synthesis conditions on the structural and catalytic properties of hierarchically structured ZSM-5 zeolites. RSC Adv. 2014, 4, 13831–13838. [Google Scholar] [CrossRef]
- Wu, L.; Magusin, P.C.M.M.; Degirmenci, V.; Li, M.; Almutairi, S.M.T.; Zhu, X.; Mezari, B.; Hensen, E.J.M. Acidic properties of nanolayered ZSM-5 zeolites. Microporous Mesoporous Mater. 2014, 189, 144–157. [Google Scholar] [CrossRef]
- Li, C.; Ren, Y.; Gou, J.; Liu, B.; Xi, H. Facile synthesis of mesostructured ZSM-5 zeolite with enhanced mass transport and catalytic performances. Appl. Surf. Sci. 2017, 392, 785–794. [Google Scholar] [CrossRef]
- Peral, A.; Escola, J.M.; Serrano, D.P.; Přech, J.; Ochoa-Hernández, C.; Čejka, J. Bidimensional ZSM-5 zeolites probed as catalysts for polyethylene cracking. Catal. Sci. Technol. 2016, 6, 2754–2765. [Google Scholar] [CrossRef]
- Ji, M.; Liu, G.; Wang, L.; Zhang, X. Layer by layer fabrication of b-oriented HZSM-5 coatings for supercritical catalytic cracking of n-dodecane. Fuel 2014, 134, 180–188. [Google Scholar] [CrossRef]
- Ji, M.; Liu, G.; Chen, C.; Wang, L.; Zhang, X.; Hu, S.; Ma, X. Catalytic performances of b-oriented bi-layered HZSM-5 coatings for cracking of hydrocarbon fuels. Appl. Catal. A 2014, 482, 8–15. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, X.; Wang, Z.; Yan, Y. Fabrication of b-Oriented MFI Zeolite Films under Neutral Conditions without the Use of Hydrogen Fluoride. Angew. Chem. Int. Ed. Engl. 2015, 54, 5709–5712. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Peng, Y.; Wang, Z.; Yan, Y. Rapid fabrication of highly b-oriented zeolite MFI thin films using ammonium salts as crystallization-mediating agents. Chem. Commun. 2015, 51, 11076–11079. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Peng, Y.; Wang, Z.; Yan, Y. A facile fabrication of highly b-oriented MFI zeolite films in the TEOS-TPAOH-H2O system without additives. Microporous Mesoporous Mater. 2016, 230, 49–57. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, H.; Liu, Y.; Wang, G.; Kong, Q.; Pan, M.; Tian, H.; Li, R. Synthesis of Wool-Ball-Like ZSM-5 with Enlarged External Surfaces and Improved Diffusion: A Potential Highly-Efficient FCC Catalyst Component for Elevating Pre-cracking of Large Molecules and Catalytic Longevity. Catal. Lett. 2016, 146, 1457–1469. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Jalil, A.A.; Triwahyono, S.; Hamdan, H.; Salleh, M.M.; Ahmad, W.F.W.; Kadja, G.T.M. Synthesis and characterization of fibrous silica ZSM-5 for cumene hydrocracking. Catal. Sci. Technol. 2016, 6, 5178–5182. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, G.; Liu, Y.; Di, J.; Wang, Y.; Zhao, Z.; Sun, Q.; Xu, C.; Gao, J.; Duan, A.; et al. Hierarchical macro-meso-microporous ZSM-5 zeolite hollow fibers with highly efficient catalytic cracking capability. Sci. Rep. 2014, 4, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jiang, G.; Han, S.; Liu, J.; Zhang, Y.; Liu, Y.; Wang, R.; Zhao, Z.; Xu, C.; Wang, Y.; et al. The Fabrication of Ga2O3/ZSM-5 Hollow Fibers for Efficient Catalytic Conversion of n-Butane into Light Olefins and Aromatics. Catalysts 2016, 6, 13. [Google Scholar] [CrossRef]
- Fu, T.; Zhou, H.; Li, Z. Effect of Particle Morphology for ZSM-5 Zeolite on the Catalytic Conversion of Methanol to Gasoline-Range Hydrocarbons. Catal. Lett. 2016, 146, 1973–1983. [Google Scholar] [CrossRef]
- Zhang, K.; Ostraat, M.L. Innovations in hierarchical zeolite synthesis. Catal. Today 2016, 264, 3–15. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Rooke, J.C.; Zhang, Y.; Yang, X.; Tang, Y.; Xiao, F.; Su, B. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, S.; Wang, L.; Zhang, X.; Liu, G. Preparation and performance of hierarchical HZSM-5 coatings on stainless-steeled microchannels for catalytic cracking of hydrocarbons. Catal. Today 2013, 216, 64–70. [Google Scholar] [CrossRef]
- Miyake, K.; Hirota, Y.; Uchida, Y.; Nishiyama, N. Synthesis of mesoporous MFI zeolite using PVA as a secondary template. J. Porous Mater. 2016, 23, 1395–1399. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, L.; Zhang, X.; Liu, G. Different roles of CNTs in hierarchical HZSM-5 synthesis with hydrothermal and steam-assisted crystallization. RSC Adv. 2015, 5, 78238–78246. [Google Scholar] [CrossRef]
- Bai, P.; Wu, P.; Xing, W.; Liu, D.; Zhao, L.; Wang, Y.; Xu, B.; Yan, Z.; Zhao, X.S. Synthesis and catalytic properties of ZSM-5 zeolite with hierarchical pores prepared in the presence of n-hexyltrimethylammonium bromide. J. Mater. Chem. A 2015, 3, 18586–18597. [Google Scholar] [CrossRef]
- Xue, T.; Liu, H.; Zhang, Y.; Wu, H.; Wu, P.; He, M. Synthesis of ZSM-5 with hierarchical porosity: In-situ conversion of the mesoporous silica-alumina species to hierarchical zeolite. Microporous Mesoporous Mater. 2017, 242, 190–199. [Google Scholar] [CrossRef]
- Ding, J.; Hu, J.; Xue, T.; Wang, Y.; Wu, H.; Wu, P.; He, M. Direct synthesis of self-assembled ZSM-5 microsphere with controllable mesoporosity and its enhanced LDPE cracking properties. RSC Adv. 2016, 6, 38671–38679. [Google Scholar] [CrossRef]
- Wan, Z.; Wu, W.; Chen, W.; Yang, H.; Zhang, D. Direct Synthesis of Hierarchical ZSM-5 Zeolite and Its Performance in Catalyzing Methanol to Gasoline Conversion. Ind. Eng. Chem. Res. 2014, 53, 19471–19478. [Google Scholar] [CrossRef]
- Ge, T.; Hua, Z.; He, X.; Zhu, Y.; Ren, W.; Chen, L.; Zhang, L.; Chen, H.; Lin, C.; Yao, H.; et al. One-pot synthesis of hierarchically structured ZSM-5 zeolites using single micropore-template. Chin. J. Catal. 2015, 36, 866–873. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Yang, X. Effect of alkaline treatment on pore structure and acidity of HZSM-5 in the synthesis of ethyl mercaptan. Catal. Commun. 2015, 60, 32–36. [Google Scholar] [CrossRef]
- Shahid, A.; Lopez-Orozco, S.; Marthala, V.R.; Hartmann, M.; Schwieger, W. Direct oxidation of benzene to phenol over hierarchical ZSM-5 zeolites prepared by sequential post synthesis modification. Microporous Mesoporous Mater. 2017, 237, 151–159. [Google Scholar] [CrossRef]
- Shahid, A.; Ahmed, N.; Saleh, T.; Al-Thabaiti, S.; Basahel, S.; Schwieger, W.; Mokhtar, M. Solvent-Free Biginelli Reactions Catalyzed by Hierarchical Zeolite Utilizing a Ball Mill Technique: A Green Sustainable Process. Catalysts 2017, 7, 84. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Chen, L.; Wu, H.; Wu, P. Postsynthesis of mesoporous ZSM-5 zeolite by piperidine-assisted desilication and its superior catalytic properties in hydrocarbon cracking. J. Mater. Chem. A 2015, 3, 3511–3521. [Google Scholar] [CrossRef]
- Nandan, D.; Saxena, S.K.; Viswanadham, N. Synthesis of hierarchical ZSM-5 using glucose as a templating precursor. J. Mater. Chem. A 2014, 2, 1054–1059. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Y.; Zhu, K.; Qian, G.; Zhou, X. Carbon nanotubes as transient inhibitors in steam-assisted crystallization of hierarchical ZSM-5 zeolites. Mater. Lett. 2015, 159, 466–469. [Google Scholar] [CrossRef]
- Han, S.; Wang, Z.; Meng, L.; Jiang, N. Synthesis of uniform mesoporous ZSM-5 using hydrophilic carbon as a hard template. Mater. Chem. Phys. 2016, 177, 112–117. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, D.; Ren, S.; Chen, X.; Qiu, M.; Liu, G.; Zeng, G.; Sun, Y. Facile one-pot solvent-free synthesis of hierarchical ZSM-5 for methanol to gasoline conversion. RSC Adv. 2016, 6, 15816–15820. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.-H.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Rohling, R.; Filonenko, G.; Mezari, B.; Hofmann, J.P.; Asahina, S.; Hensen, E.J. Synthesis of hierarchical zeolites using an inexpensive mono-quaternary ammonium surfactant as mesoporogen. Chem. Commun. 2014, 50, 14658–14661. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Dong, M.; Zhao, H.; Huang, W. Production of gasoline range hydrocarbons from methanol on hierarchical ZSM-5 and Zn/ZSM-5 catalyst prepared with soft second template. J. Energy Chem. 2015, 24, 490–496. [Google Scholar] [CrossRef]
- Zhou, F.; Gao, Y.; Wu, G.; Ma, F.; Liu, C. Improved catalytic performance and decreased coke formation in post-treated ZSM-5 zeolites for methanol aromatization. Microporous Mesoporous Mater. 2017, 240, 96–107. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, F.; Xiao, G. Performance of hierarchical HZSM-5 zeolites prepared by NaOH treatments in the aromatization of glycerol. RSC Adv. 2015, 5, 63697–63704. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Effect of desilication of H-ZSM-5 by alkali treatment on catalytic performance in hexane cracking. Appl. Catal. A 2012, 449, 188–197. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Desilication: On the controlled generation of mesoporosity in MFI zeolites. J. Mater. Chem. 2006, 16, 2121–2131. [Google Scholar] [CrossRef]
- Yuan, E.; Tang, Z.; Mo, Z.; Lu, G. A new method to construct hierarchical ZSM-5 zeolites with excellent catalytic activity. J. Porous Mater. 2014, 21, 957–965. [Google Scholar] [CrossRef]
- Abelló, S.; Bonilla, A.; Pérez-Ramírez, J. Mesoporous ZSM-5 zeolite catalysts prepared by desilication with organic hydroxides and comparison with NaOH leaching. Appl. Catal. A 2009, 364, 191–198. [Google Scholar] [CrossRef]
- Meng, F.; Wang, Y.; Wang, S. Methanol to gasoline over zeolite ZSM-5: Improved catalyst performance by treatment with HF. RSC Adv. 2016, 6, 58586–58593. [Google Scholar] [CrossRef]
- Qin, Z.; Lakiss, L.; Gilson, J.P.; Thomas, K.; Goupil, J.M.; Fernandez, C.; Valtchev, V. Chemical Equilibrium Controlled Etching of MFI-Type Zeolite and Its Influence on Zeolite Structure, Acidity, and Catalytic Activity. Chem. Mater. 2013, 25, 2759–2766. [Google Scholar] [CrossRef]
- Qin, Z.; Gilson, J.-P.; Valtchev, V. Mesoporous zeolites by fluoride etching. Curr. Opin. Chem. Eng. 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Y.; Ju, C.; Fang, Y. Remarkable increasing of ZSM-5 lifetime in methanol to hydrocarbon reaction by post engineering in fluoride media. Appl. Catal. A 2016, 512, 1–8. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Watanabe, R.; Namba, S.; Kondo, J.N.; Tatsumi, T. Facile control of crystallite size of ZSM-5 catalyst for cracking of hexane. Microporous Mesoporous Mater. 2011, 145, 165–171. [Google Scholar] [CrossRef]
- Popov, A.G.; Pavlov, V.S.; Ivanova, I.I. Effect of crystal size on butenes oligomerization over MFI catalysts. J. Catal. 2016, 335, 155–164. [Google Scholar] [CrossRef]
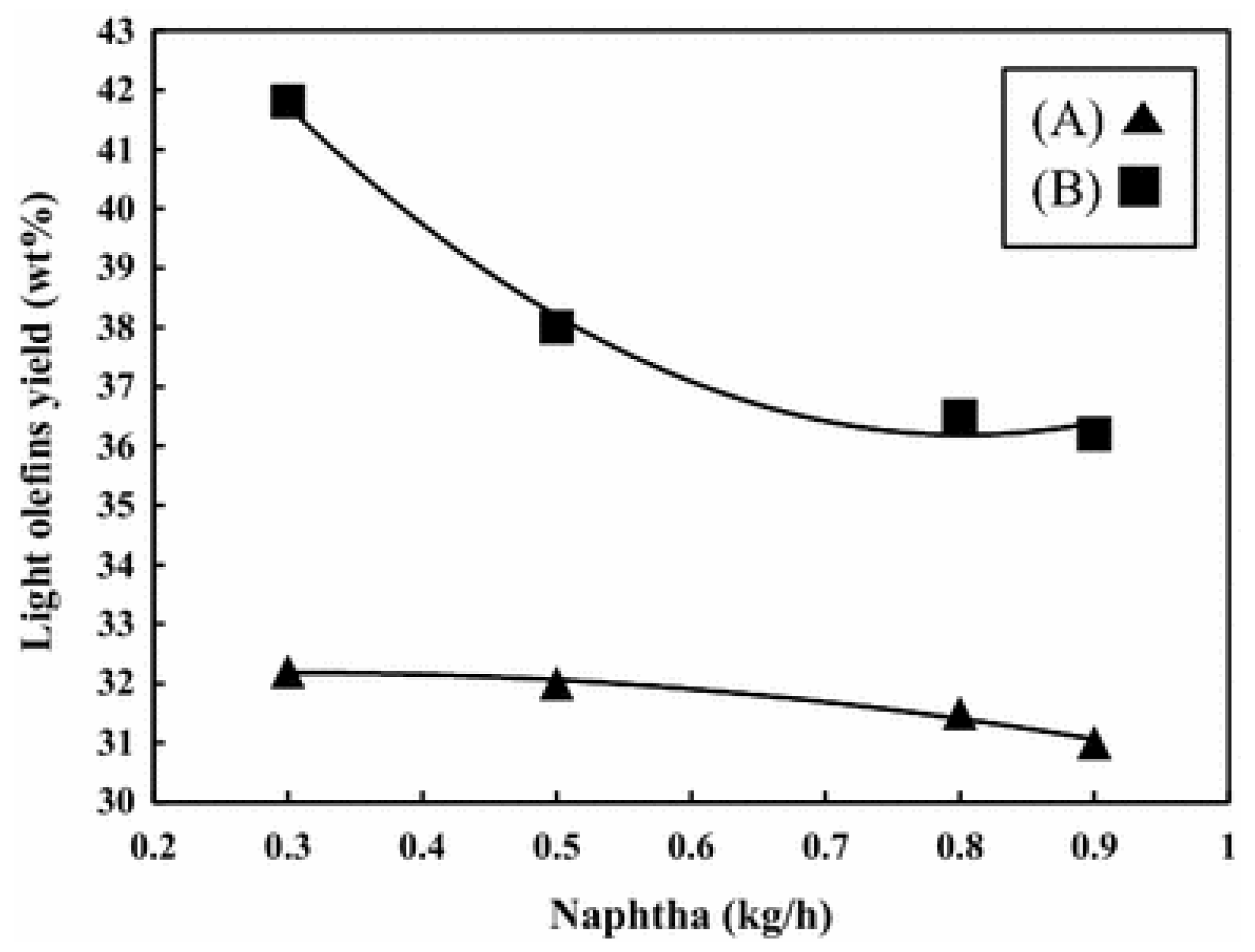
- Alipour, S.M. Recent advances in naphtha catalytic cracking by nano ZSM-5: A review. Chin. J. Catal. 2016, 37, 671–680. [Google Scholar] [CrossRef]
- Konno, H.; Tago, T.; Nakasaka, Y.; Ohnaka, R.; Nishimura, J.-I.; Masuda, T. Effectiveness of nano-scale ZSM-5 zeolite and its deactivation mechanism on catalytic cracking of representative hydrocarbons of naphtha. Microporous Mesoporous Mater. 2013, 175, 25–33. [Google Scholar] [CrossRef]
- Nakasaka, Y.; Okamura, T.; Konno, H.; Tago, T.; Masuda, T. Crystal size of MFI-type zeolites for catalytic cracking of n-hexane under reaction-control conditions. Microporous Mesoporous Mater. 2013, 182, 244–249. [Google Scholar] [CrossRef]
- Javaid, R.; Urata, K.; Furukawa, S.; Komatsu, T. Factors affecting coke formation on H-ZSM-5 in naphtha cracking. Appl. Catal. A 2015, 491, 100–105. [Google Scholar] [CrossRef]
- Lakiss, L.; Ngoye, F.; Canaff, C.; Laforge, S.; Pouilloux, Y.; Qin, Z.; Tarighi, M.; Thomas, K.; Valtchev, V.; Vicente, A.; et al. On the remarkable resistance to coke formation of nanometer-sized and hierarchical MFI zeolites during ethanol to hydrocarbons transformation. J. Catal. 2015, 328, 165–172. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Meng, F.; Li, H.; Wang, S.; Sun, C.; Wang, S.; Wang, X. Conversion of methanol to propylene over nano-sized ZSM-5 zeolite aggregates synthesized by a modified seed-induced method with CTAB. RSC Adv. 2016, 6, 76642–76651. [Google Scholar] [CrossRef]
- Kang, N.Y.; Woo, S.I.; Lee, Y.J.; Bae, J.; Choi, W.C.; Park, Y.-K. Enhanced hydrothermal stability of ZSM-5 formed from nanocrystalline seeds for naphtha catalytic cracking. J. Mater. Sci. 2016, 51, 3735–3749. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Li, N.; Lin, S. Direct synthesis of monolithic nano-sized ZSM-5 aggregates possessing ordered mesoporosity by controlling arrangement of nanoparticles. J. Alloys Compd. 2017, 695, 2488–2498. [Google Scholar] [CrossRef]
- Wakihara, T.; Sato, K.; Inagaki, S.; Tatami, J.; Komeya, K.; Meguro, T.; Kubota, Y. Fabrication of Fine Zeolite with Improved Catalytic Properties by Bead Milling and Alkali Treatment. ACS Appl. Mater. Interfaces 2010, 2, 2715–2718. [Google Scholar] [CrossRef]
- Rosilda, S.T.; Chiang, A.S. Some Observations on the Synthesis of Fully-Dispersible Nanocrystalline Zeolite ZSM-5. J. Nanosci. Nanotechnol. 2014, 14, 7351–7359. [Google Scholar]
- Nada, M.H.; Larsen, S.C. Insight into seed-assisted template free synthesis of ZSM-5 zeolites. Microporous Mesoporous Mater. 2017, 239, 444–452. [Google Scholar] [CrossRef]
- Inagaki, S.; Shinoda, S.; Hayashi, S.; Wakihara, T.; Yamazaki, H.; Kondo, J.N.; Kubota, Y. Improvement in the catalytic properties of ZSM-5 zeolite nanoparticles via mechanochemical and chemical modifications. Catal. Sci. Technol. 2016, 6, 2598–2604. [Google Scholar] [CrossRef]
- Lin, L.F.; Zhao, S.F.; Zhang, D.W.; Fan, H.; Liu, Y.M.; He, M.Y. Acid Strength Controlled Reaction Pathways for the Catalytic Cracking of 1-Pentene to Propene over ZSM-5. ACS Catal. 2015, 5, 4048–4059. [Google Scholar] [CrossRef]
- Hodoshima, S.; Motomiya, A.; Wakamatsu, S.; Kanai, R.; Yagi, F. Catalytic conversion of light hydrocarbons to propylene over MFI-zeolite/metal-oxide composites. Microporous Mesoporous Mater. 2016, 233, 125–132. [Google Scholar] [CrossRef]
- Furumoto, Y.; Harada, Y.; Tsunoji, N.; Takahashi, A.; Fujitani, T.; Ide, Y.; Sadakane, M.; Sano, T. Effect of acidity of ZSM-5 zeolite on conversion of ethanol to propylene. Appl. Catal. A 2011, 399, 262–267. [Google Scholar] [CrossRef]
- Wan, Z.; Wu, W.; Li, G.; Wang, C.; Yang, H.; Zhang, D. Effect of SiO2/Al2O3 ratio on the performance of nanocrystal ZSM-5 zeolite catalysts in methanol to gasoline conversion. Appl. Catal. A 2016, 523, 312–320. [Google Scholar] [CrossRef]
- Qi, C.; Wang, Y.; Ding, X.; Su, H. Catalytic cracking of light diesel over Au/ZSM-5 catalyst for increasing propylene production. Chin. J. Catal. 2016, 37, 1747–1754. [Google Scholar] [CrossRef]
- Epelde, E.; Santos, J.I.; Florian, P.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J.; Castaño, P. Controlling coke deactivation and cracking selectivity of MFI zeolite by H3PO4 or KOH modification. Appl. Catal. A 2015, 505, 105–115. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Zhang, Q.; Yan, W. Phosphorus modification increases catalytic activity and stability of ZSM-5 zeolite on supercritical catalytic cracking of n-dodecane. J. Solid State Chem. 2017, 251, 7–13. [Google Scholar] [CrossRef]
- Tsunoji, N.; Sonoda, T.; Furumoto, Y.; Sadakane, M.; Sano, T. Recreation of Brønsted acid sites in phosphorus-modified HZSM-5(Ga) by modification with various metal cations. Appl. Catal. A 2014, 481, 161–168. [Google Scholar] [CrossRef]
- Lee, J.; Hong, U.G.; Hwang, S.; Youn, M.H.; Song, I.K. Catalytic cracking of C5 raffinate to light olefins over lanthanum-containing phosphorous-modified porous ZSM-5: Effect of lanthanum content. Fuel Process. Technol. 2013, 109, 189–195. [Google Scholar] [CrossRef]
- Rahimi, N.; Moradi, D.; Sheibak, M.; Moosavi, E.; Karimzadeh, R. The influence of modification methods on the catalytic cracking of LPG over lanthanum and phosphorus modified HZSM-5 catalysts. Microporous Mesoporous Mater. 2016, 234, 215–223. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Ma, H.; Sun, Q.; Ying, W.; Fang, D. Effect of Impregnating Fe into P-Modified HZSM-5 in the Coupling Cracking of Butene and Pentene. Ind. Eng. Chem. Res. 2015, 54, 1796–1805. [Google Scholar] [CrossRef]
- Li, J.-W.; Li, T.; Ma, H.-F.; Sun, Q.-W.; Ying, W.-Y.; Fang, D.-Y. Effect of nickel on phosphorus modified HZSM-5 in catalytic cracking of butene and pentene. Fuel Process. Technol. 2017, 159, 31–37. [Google Scholar] [CrossRef]
- Inagaki, S.; Shinoda, S.; Kaneko, Y.; Takechi, K.; Komatsu, R.; Tsuboi, Y.; Yamazaki, H.; Kondo, J.N.; Kubota, Y. Facile fabrication of ZSM-5 zeolite catalyst with high durability to coke formation during catalytic cracking of paraffins. ACS Catal. 2013, 3, 74–78. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Xie, Q.; Chen, P.; Ruan, R. Reducing coke formation in the catalytic fast pyrolysis of bio-derived furan with surface modified HZSM-5 catalysts. RSC Adv. 2015, 5, 56286–56292. [Google Scholar] [CrossRef]
- Inagaki, S.; Sato, K.; Hayashi, S.; Tatami, J.; Kubota, Y.; Wakihara, T. Mechanochemical approach for selective deactivation of external surface acidity of ZSM-5 zeolite catalyst. ACS Appl. Mater. Interfaces 2015, 7, 4488–4493. [Google Scholar] [CrossRef] [PubMed]

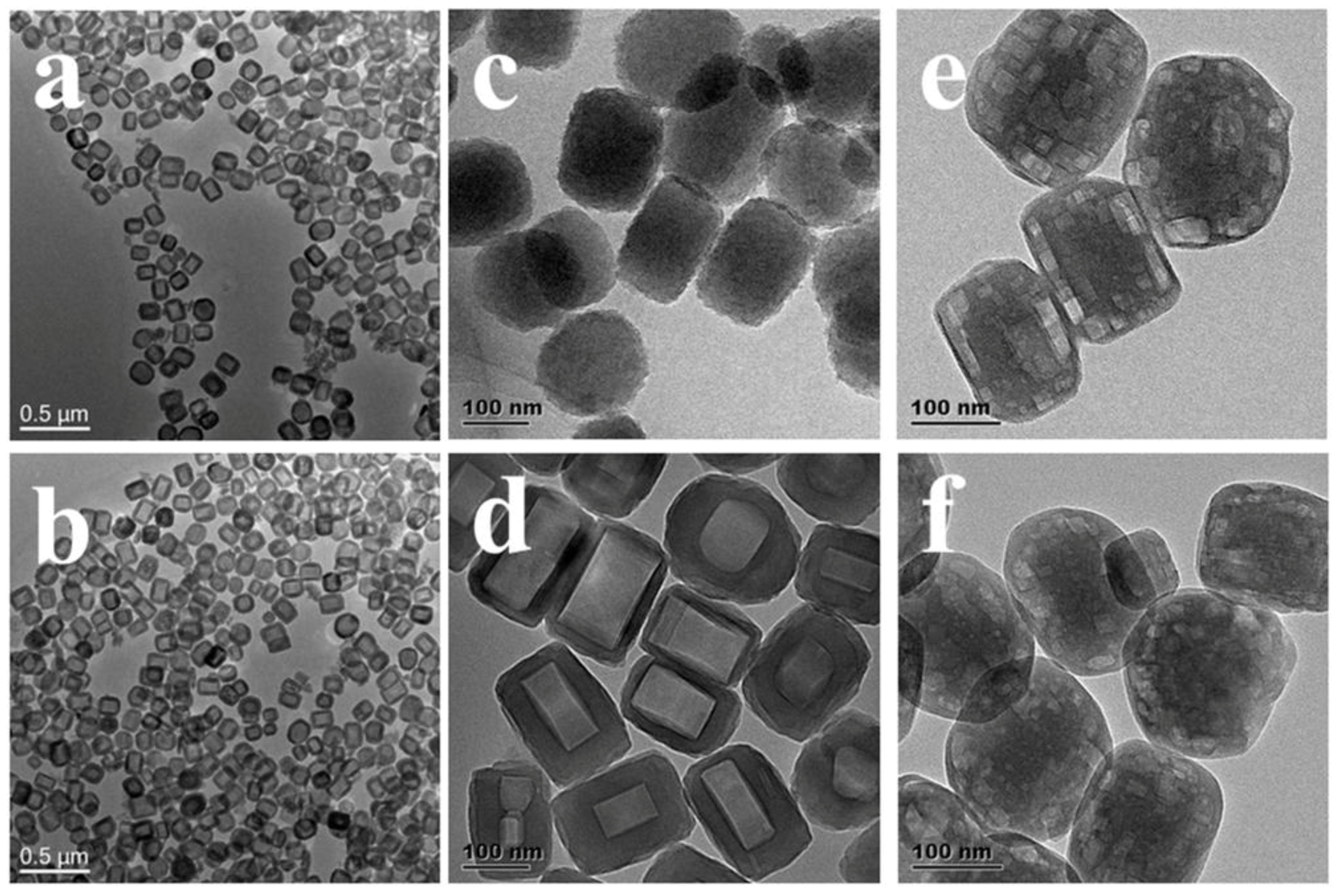
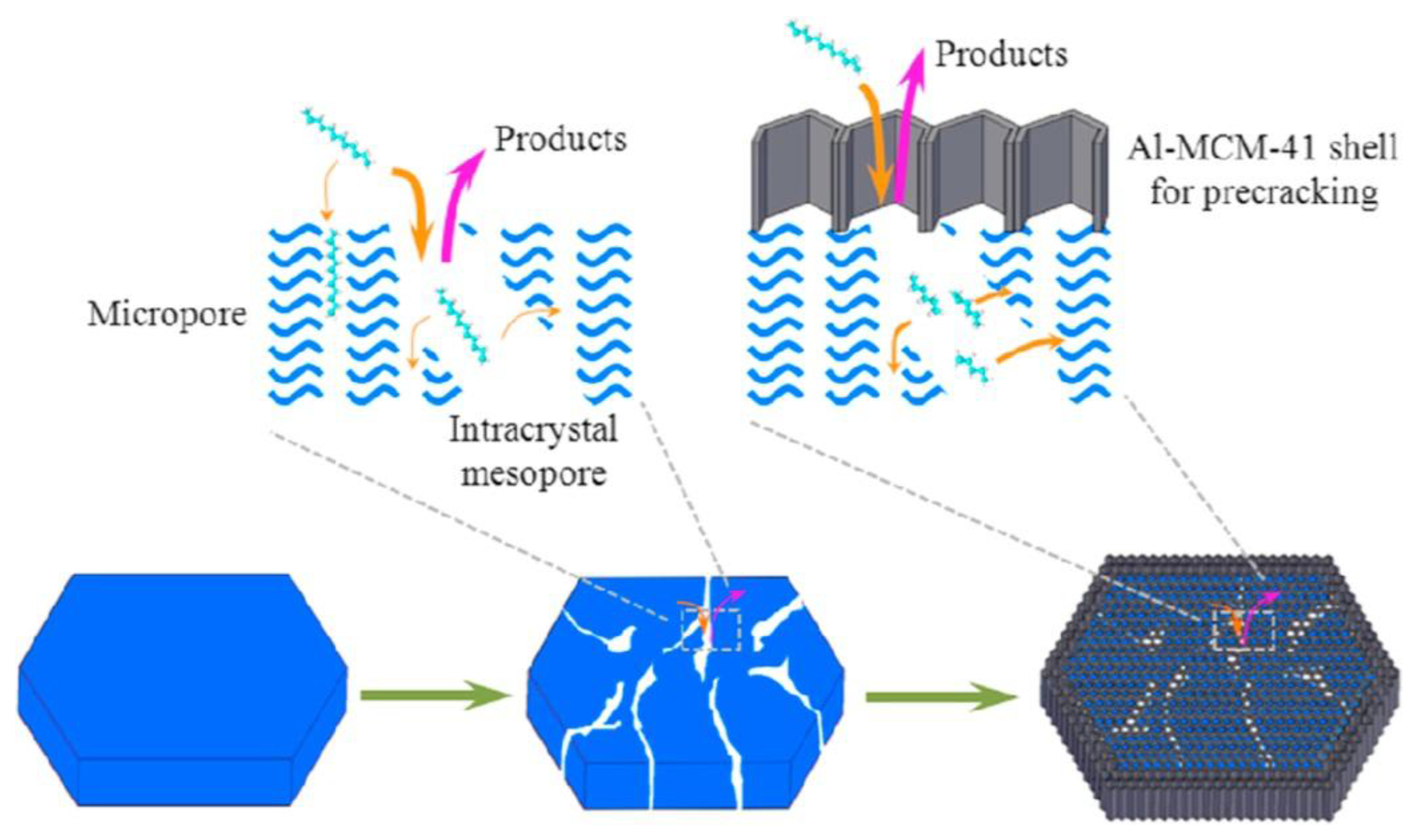
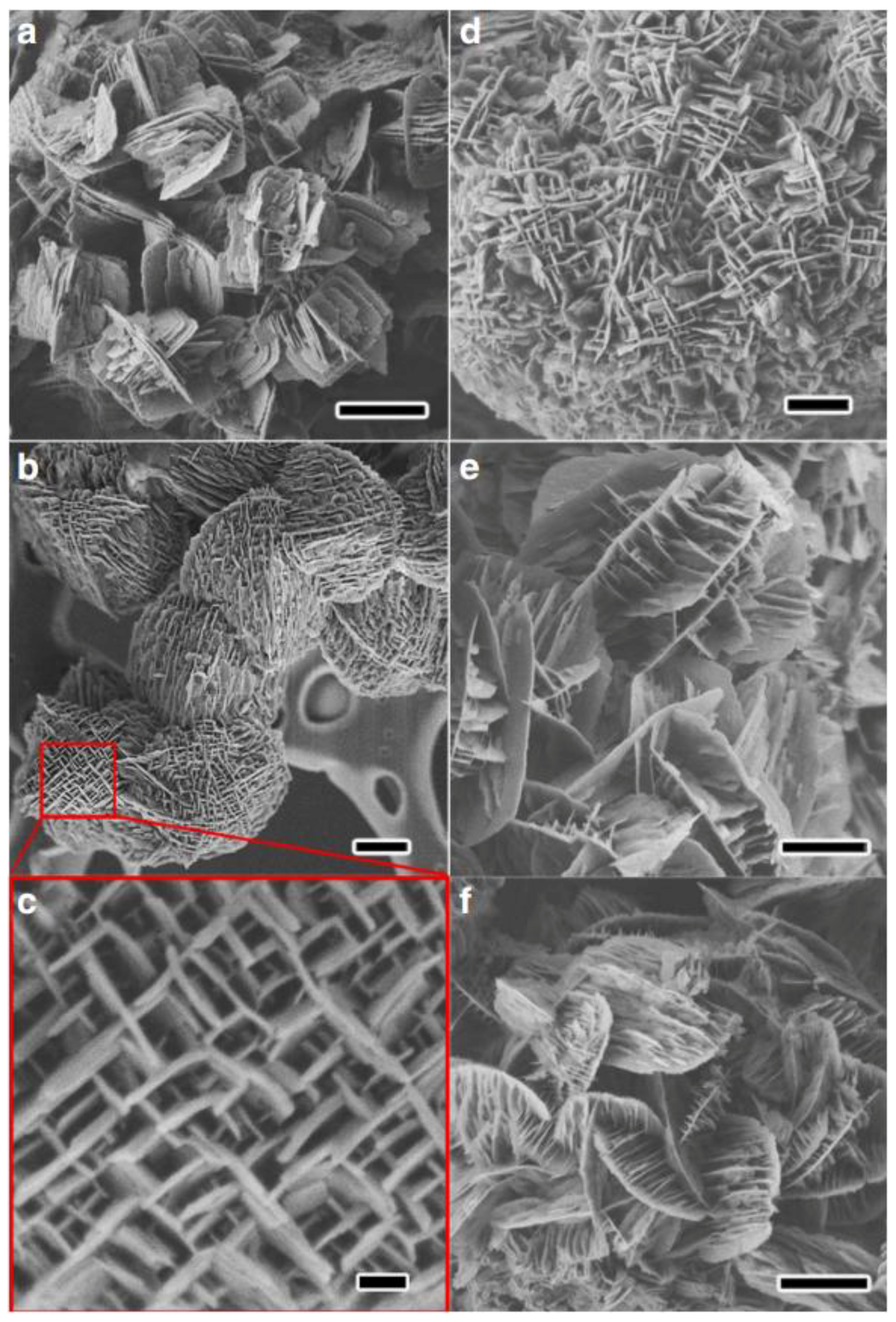
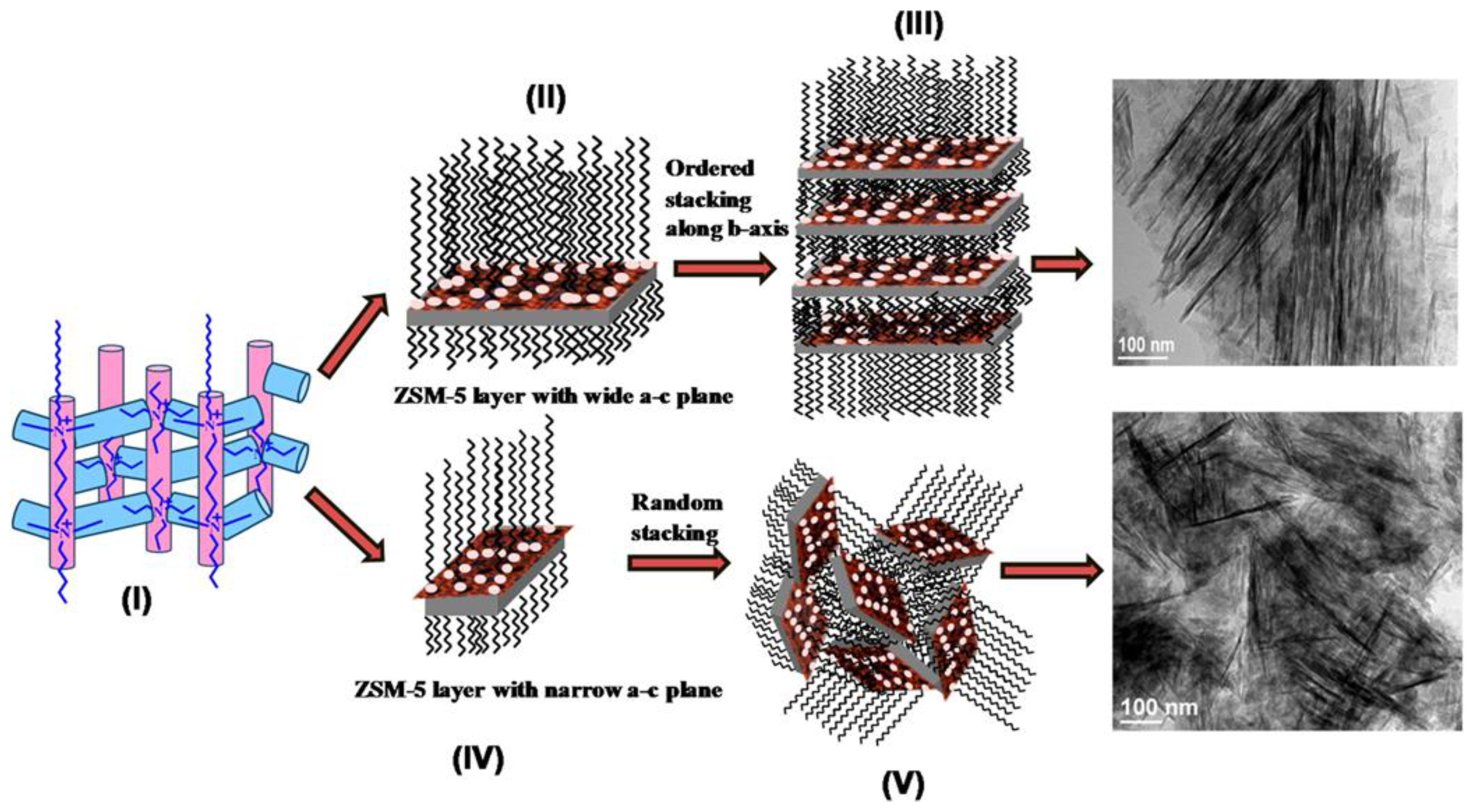


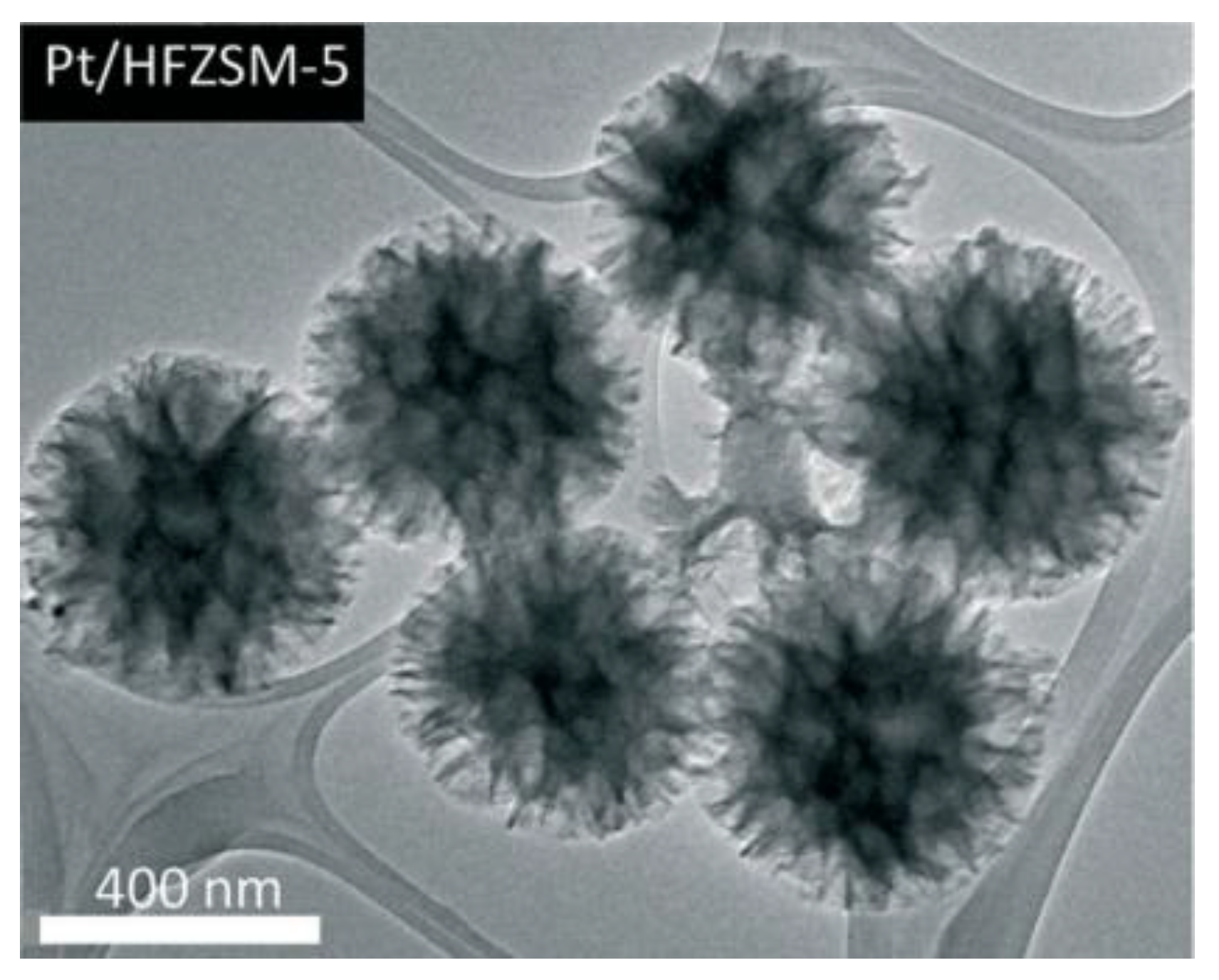
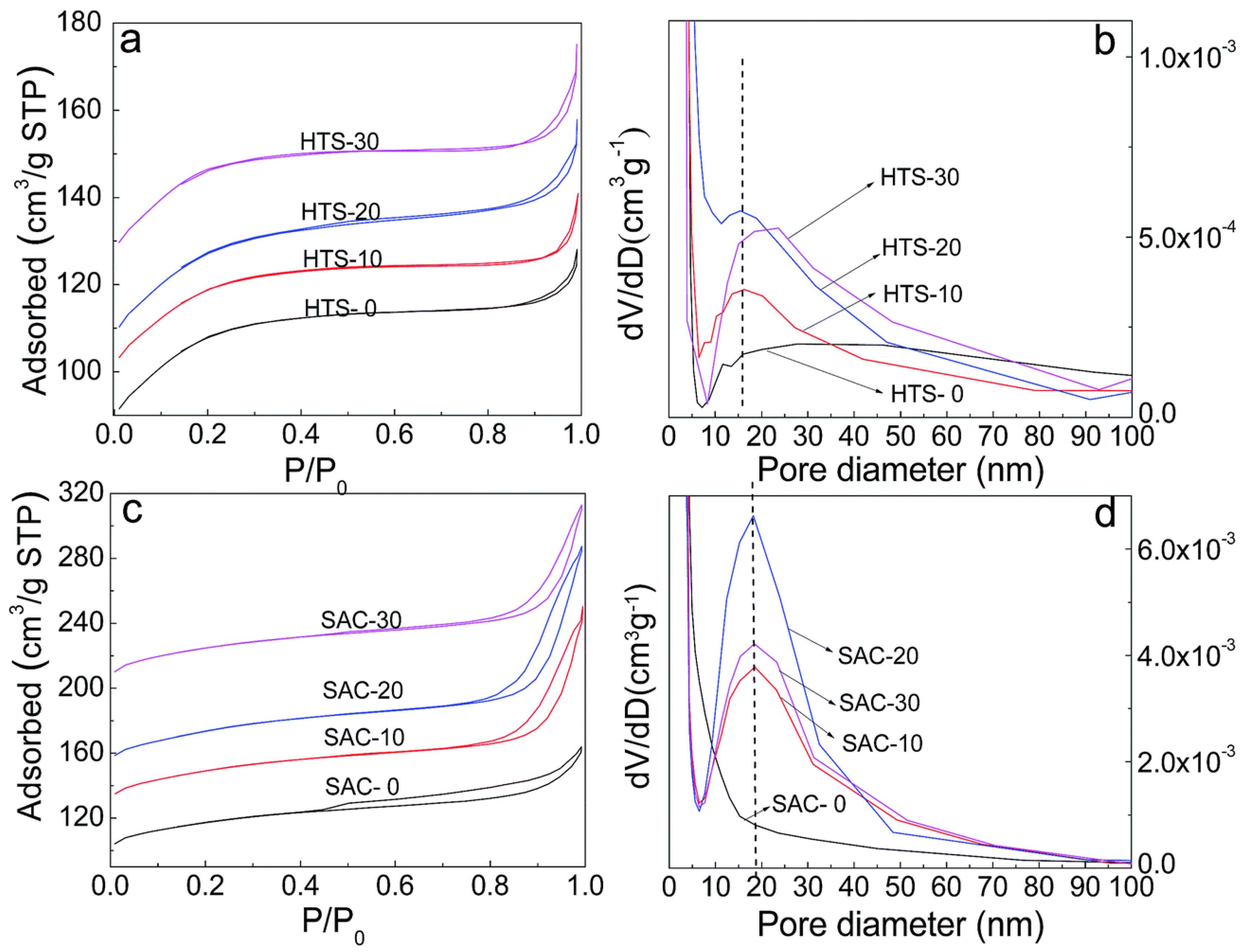
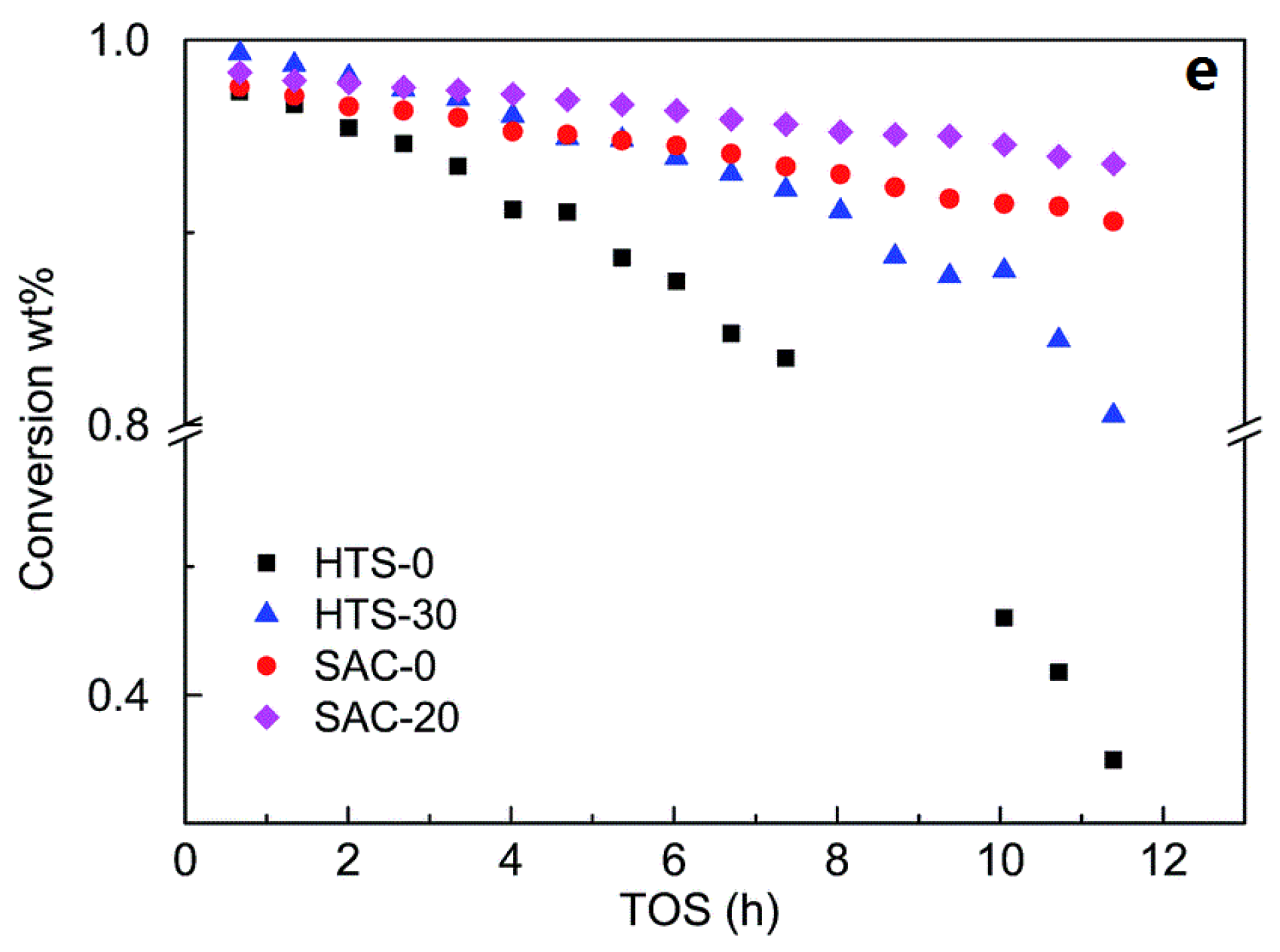
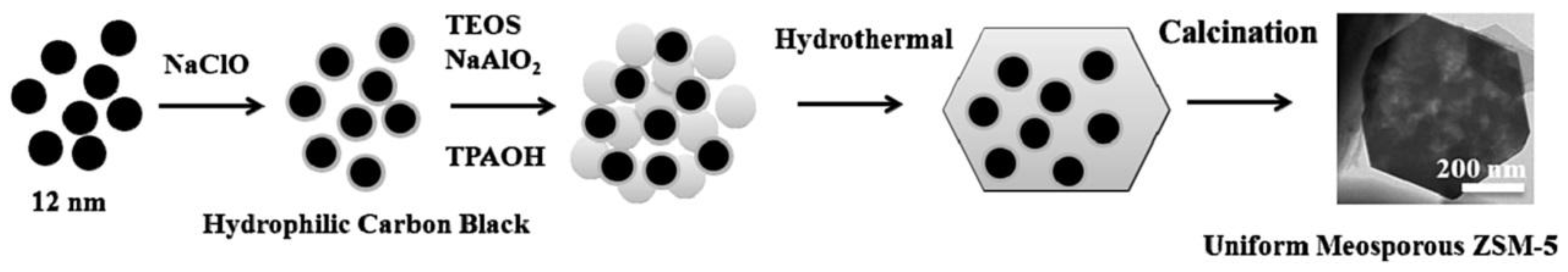

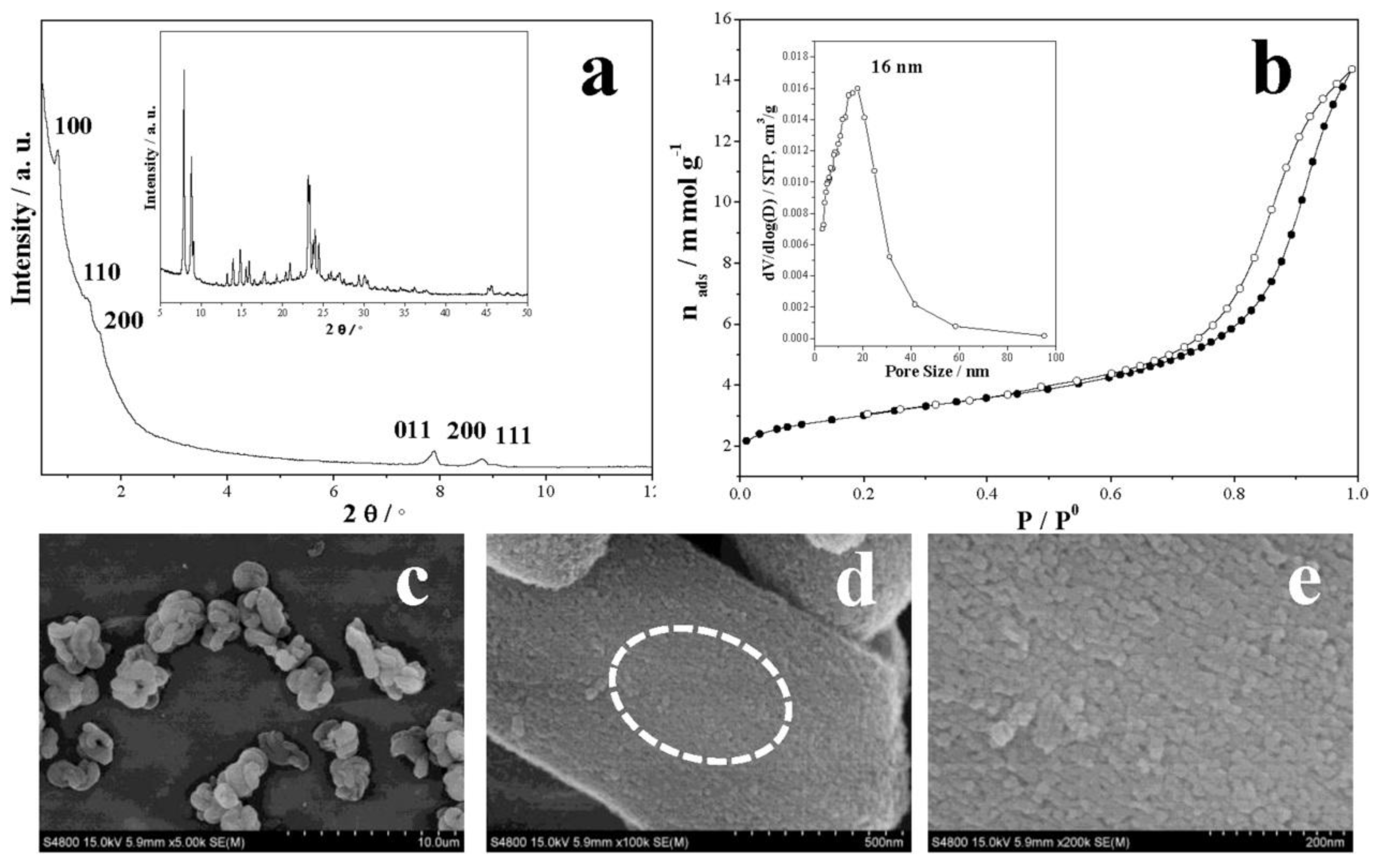
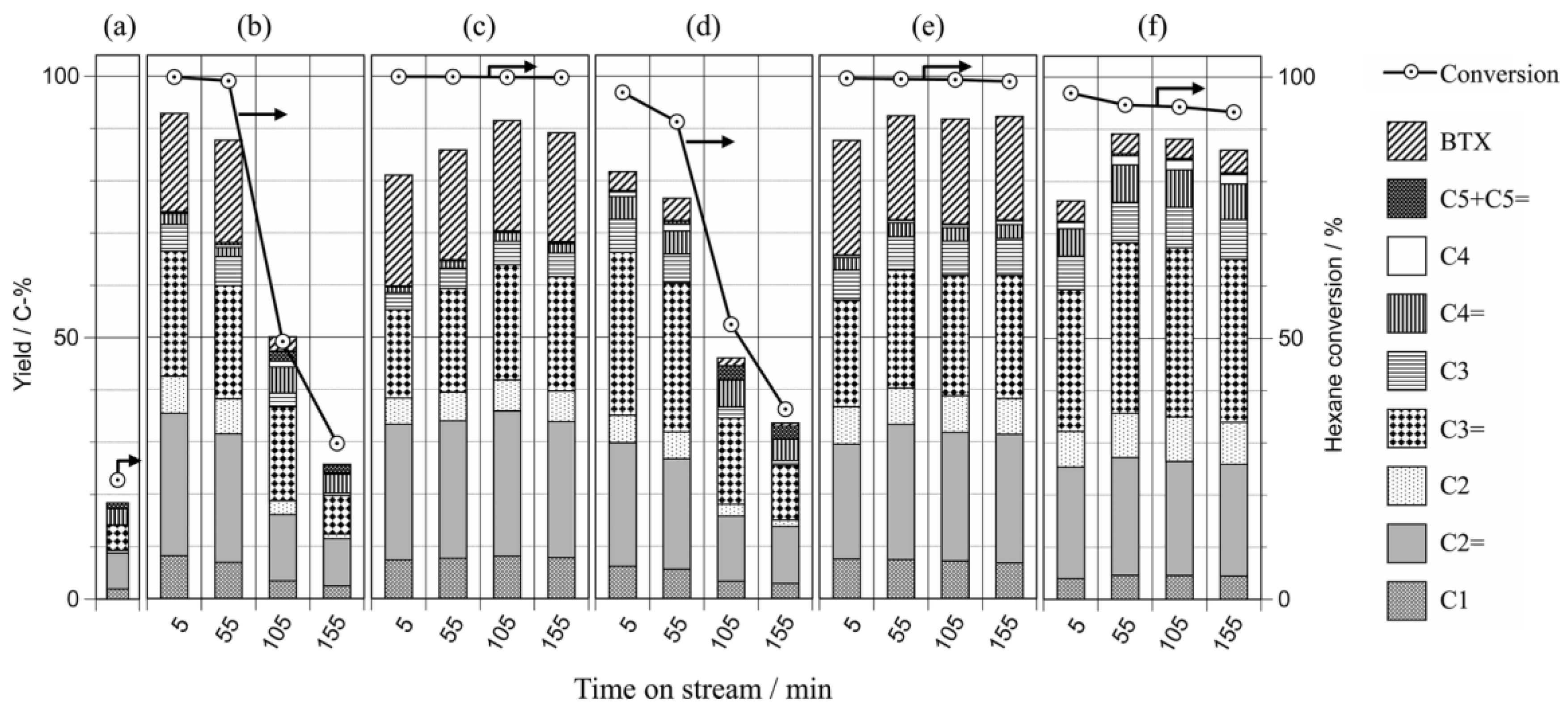
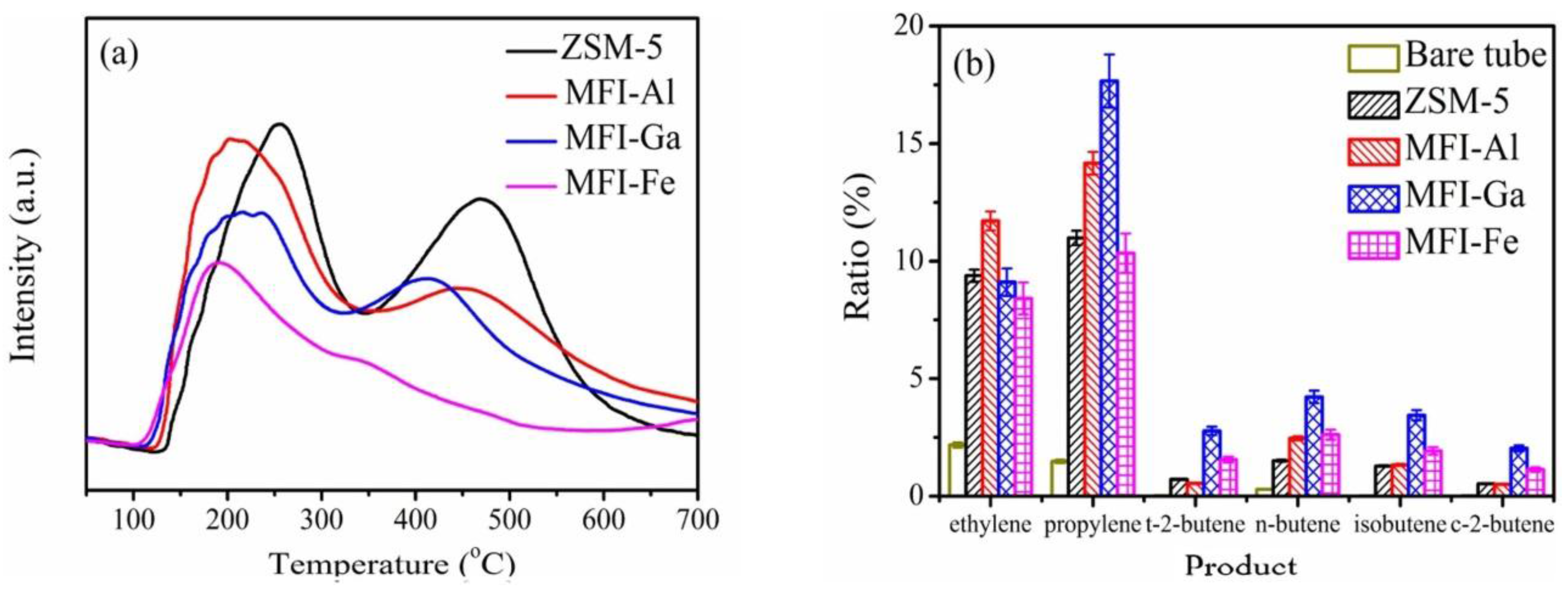
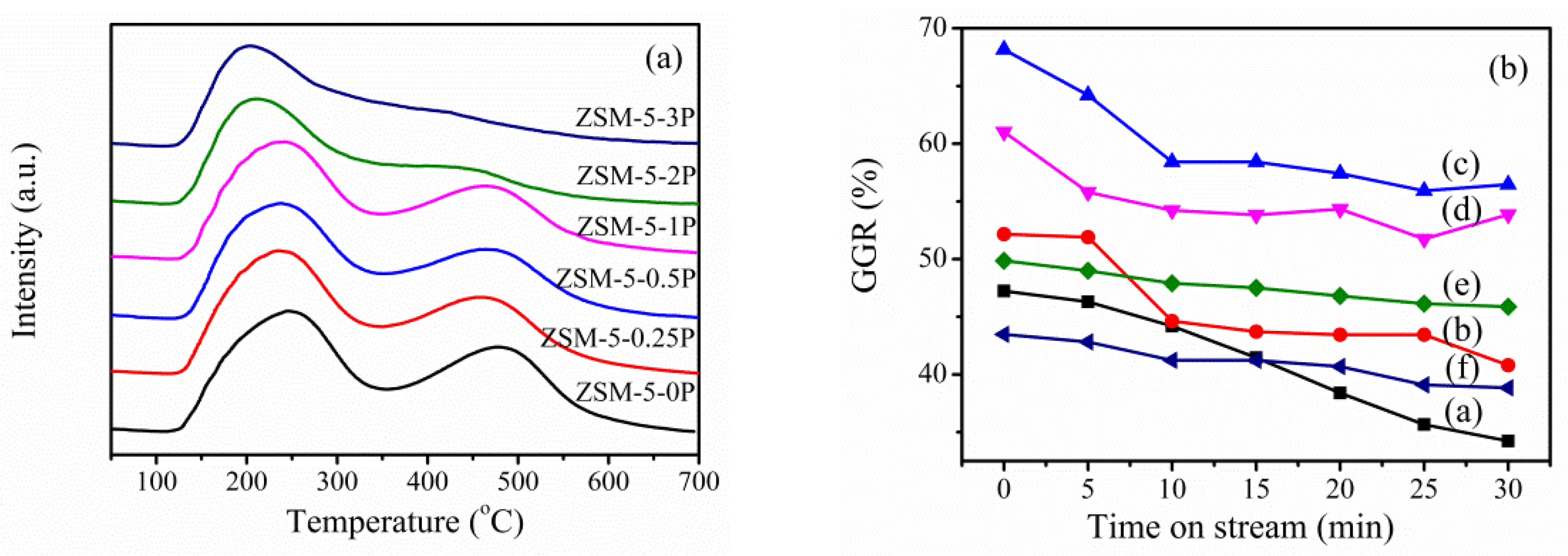
| Sample | SBET a (m2∙g−1) | Sext b (m2∙g−1) | VTot c (cm3∙g−1) | Vmic d (cm3∙g−1) | Vmes e (cm3∙g−1) | R f |
|---|---|---|---|---|---|---|
| Z50_10 h | 383 | 71 | 0.37 | 0.15 | 0.22 | [21] |
| Hollow ZSM-5 | 443 | 105 | 0.74 | 0.15 | 0.59 | [22] |
| H-ZSM-5/MCM-41 | 548 | - | 0.49 | 0.10 | 0.39 | [23] |
| HZM-N(50) | 562 | 256 | 0.80 | 0.34 | 0.46 | [24] |
| 50L-1st | 918 | - | 0.94 | - | - | [25] |
| 50L-2nd | 913 | - | 0.94 | - | - | [25] |
| HZ-ER | 572 | - | 0.40 | 0.05 | 0.35 | [26] |
| MZAT0.2-PI0.02@MSA | 539 | 358 | 0.61 | 0.09 | 0.52 | [27] |
| ZSM-5@SAPO-34(6) | 474 | 148 | 0.48 | 0.13 | 0.35 | [28] |
| ZSC-24 | 361 | 233 | 0.84 | 0.06 | 0.78 | [29] |
| Sample | SDA | SBET a (m2∙g−1) | Sext b (m2 g−1) | VTot c (cm3 g−1) | Vmic d (cm3∙g−1) | Vmes e (cm3∙g−1) | Tthic f (nm) | T g (d) | R h |
|---|---|---|---|---|---|---|---|---|---|
| ZSM-5-bulk | TPAOH | 373 | 91 | 0.21 | 0.17 | 0.04 | - | 3 | [41] |
| ZSM-5(6, 20, 423) | C22-6-6(Br)2 | 685 | 826 | 0.75 | 0.09 | 0.66 | - | 5 | [41] |
| MFI nanosheets | BCPh-6-6-6 | 658 | - | 0.62 | 0.11 | 0.51 | 4.8 | - | [42] |
| MFI nanosheets | BCPh-8-6-6 | 621 | - | 0.58 | 0.10 | 0.48 | 4.9 | - | [42] |
| MFI-Al | C22-6-6(OH)2, C2H5OH | 479 | - | 1.05 | 0.22 | 0.83 | - | 5 | [44] |
| MFI-10/3 | C22−6−6(Br)2, TPAOH | 517 | 376 | 0.50 | 0.07 | 0.43 | - | 5 | [45] |
| ZSM-5 | C18−6−6(Br)2, TPABr | 612 | 260 | 0.80 | - | - | - | 5 | [47] |
| MFI-like | 18–N3–18 | 1190 | - | 1.58 | - | - | 1.7 | - | [50] |
| MFI-like | 18–N4–18 | 1060 | - | 1.48 | - | - | 2.3 | - | [50] |
| Fe/ZSM-5-sheet | C16-6-6(Br)2 | 508 | - | 0.57 | 0.11 | 0.45 | 2–3 | 9 | [51] |
| Fe/ZSM-5-sheet | C16-6-6(OH)2 | 522 | - | 0.65 | 0.12 | 0.53 | 2–3 | 9 | [51] |
| MFI nanosheets | C18-6-6(Br)2 | 530 | - | 0.64 | 0.13 | 0.51 | 2.5 | 11 | [52] |
| ZSM-5-S | C22-N4-C22(Br)4 | 630 | 439 | 1.18 | 0.15 | 1.03 | - | 5 | [53] |
| ZSM-5-R | C22-N4-C22(Br)4 | 518 | 355 | 1.32 | 0.13 | 1.19 | - | 5 | [53] |
| MesoMFI(2, OH) | C22-6-6(OH)2 | 457 | 393 | 0.85 | 0.08 | 0.77 | - | 12 | [54] |
| MesoMFI(3, OH) | C22-6-6-6(OH)3 | 532 | 429 | 0.85 | 0.12 | 0.73 | - | 12 | [54] |
| MesoMFI(4, OH) | C22-6-6-6-6(OH)4 | 527 | 535 | 1.15 | 0.10 | 1.05 | - | 12 | [54] |
| MLMFI | Cbiphen-8-6-6 | 519 | 221 | 0.27 | 0.12 | 0.15 | 3.5 | 5 | [55] |
| PI-ZSM-5 | C22-6-6Br2 | 698 | 498 | 0.61 | 0.12 | 0.49 | - | 7 | [56] |
| Sample | SBET a (m2∙g−1) | Sext b (m2∙g−1) | VTot c (cm3∙g−1) | Vmic d (cm3∙g−1) | Vmes e (cm3∙g−1) | R f |
|---|---|---|---|---|---|---|
| MFI-PVA(3.0) | 345 | 140 | 0.33 | - | - | [70] |
| SAC-20 | 395 | 132 | 0.35 | 0.12 | 0.23 | [71] |
| HZ-32-170C-2d-13 | 682 | - | 0.84 | 0.17 | 0.67 | [72] |
| MZ5-FS-0.1-60 | 426 | 112 | 0.46 | 0.14 | 0.32 | [73] |
| ZM-3 | 377 | 119 | 0.24 | 0.12 | 0.12 | [74] |
| Hi-ZSM-5 | 433 | 133 | 0.30 | 0.12 | 0.18 | [75] |
| HSZs-120 | 427 | 158 | 0.53 | 0.12 | 0.41 | [76] |
| DS-2 | 391 | 135 | 0.33 | 0.11 | 0.22 | [77] |
| HM27_AcT_AT | 450 | 136 | 0.42 | 0.13 | 0.29 | [78] |
| MFI27_6 | 363 | - | 0.38 | 0.08 | 0.3 | [79] |
| AT 0.2-PI 0.01 | 487 | - | 0.80 | 0.12 | 0.68 | [80] |
| Sample | P a | SBET b (m2∙g−1) | Sext c (m2∙g−1) | VTot d (cm3∙g−1) | Vmic e (cm3∙g−1) | Vmes f (cm3∙g−1) | R g |
|---|---|---|---|---|---|---|---|
| H2O/Si = 30 | 253 | 400 | 40 | 0.28 | 0.17 | 0.11 | [98] |
| H2O/Si = 8.3 | 106 | 403 | 49 | 0.56 | 0.17 | 0.39 | [98] |
| H-MFI(1) | 40–45 | - | - | 0.30 | 0.12 | 0.18 | [99] |
| Z-0.02C-0.08S | 20–50 | 416 | 271 | 0.30 | 0.07 | 0.13 | [105] |
| B-2 | - | 424 | 42 | 0.21 | 0.13 | 0.08 | [106] |
| ZNA-373 | 20–40 | 536 | 403 | 0.89 | 0.05 | 0.84 | [107] |
| Z60-AT | 30–500 | 430 | 79 | - | - | - | [108] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Yang, H.; Yan, W. Strategies to Enhance the Catalytic Performance of ZSM-5 Zeolite in Hydrocarbon Cracking: A Review. Catalysts 2017, 7, 367. https://doi.org/10.3390/catal7120367
Ji Y, Yang H, Yan W. Strategies to Enhance the Catalytic Performance of ZSM-5 Zeolite in Hydrocarbon Cracking: A Review. Catalysts. 2017; 7(12):367. https://doi.org/10.3390/catal7120367
Chicago/Turabian StyleJi, Yajun, Honghui Yang, and Wei Yan. 2017. "Strategies to Enhance the Catalytic Performance of ZSM-5 Zeolite in Hydrocarbon Cracking: A Review" Catalysts 7, no. 12: 367. https://doi.org/10.3390/catal7120367




