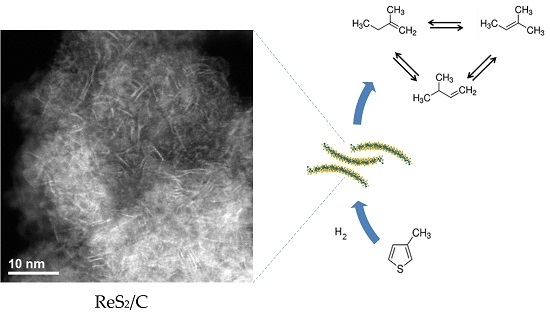
Low-Dimensional ReS2/C Composite as Effective Hydrodesulfurization Catalyst
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Catalyst
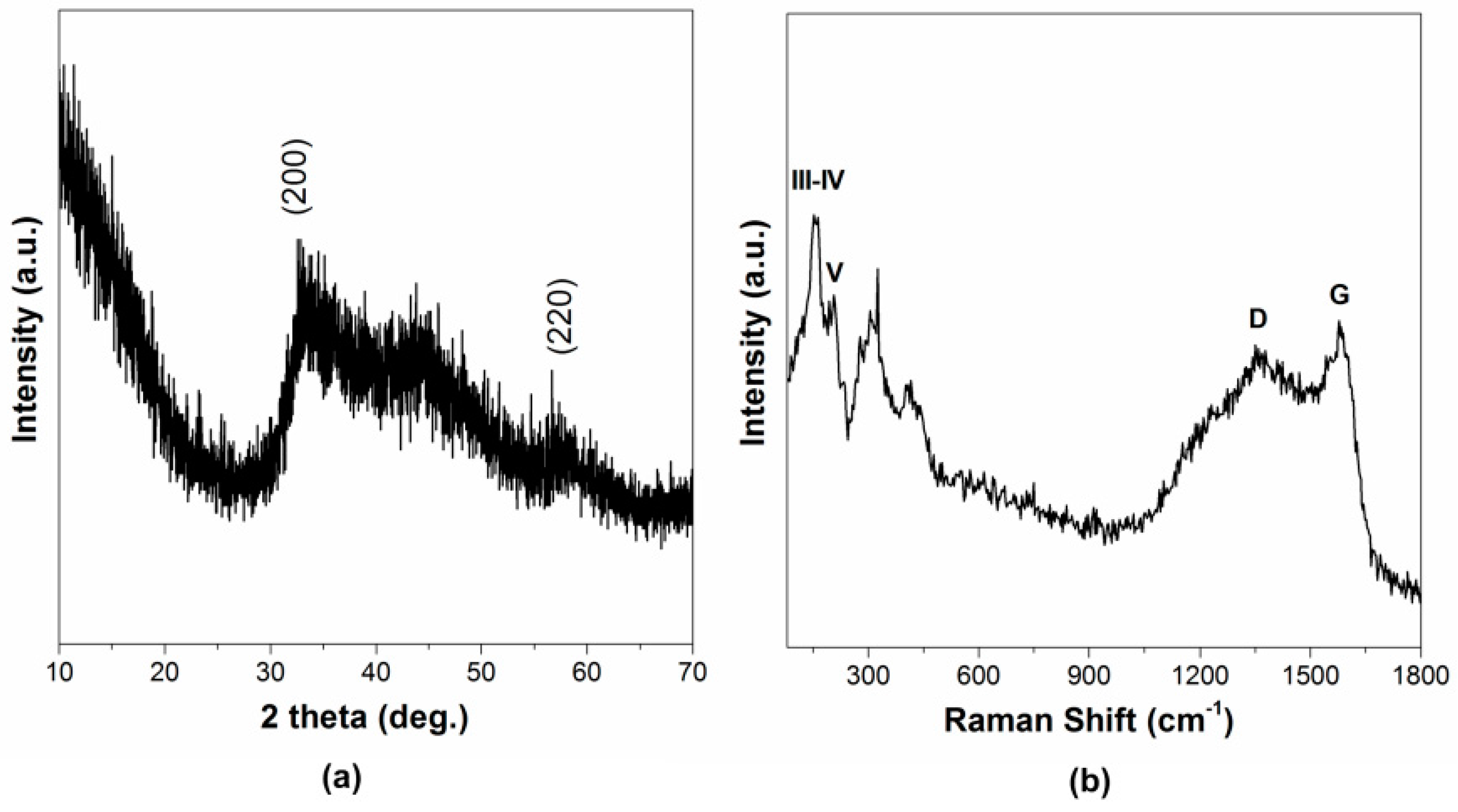
2.1.1. X-ray Diffraction and Raman Spectroscopy Analysis
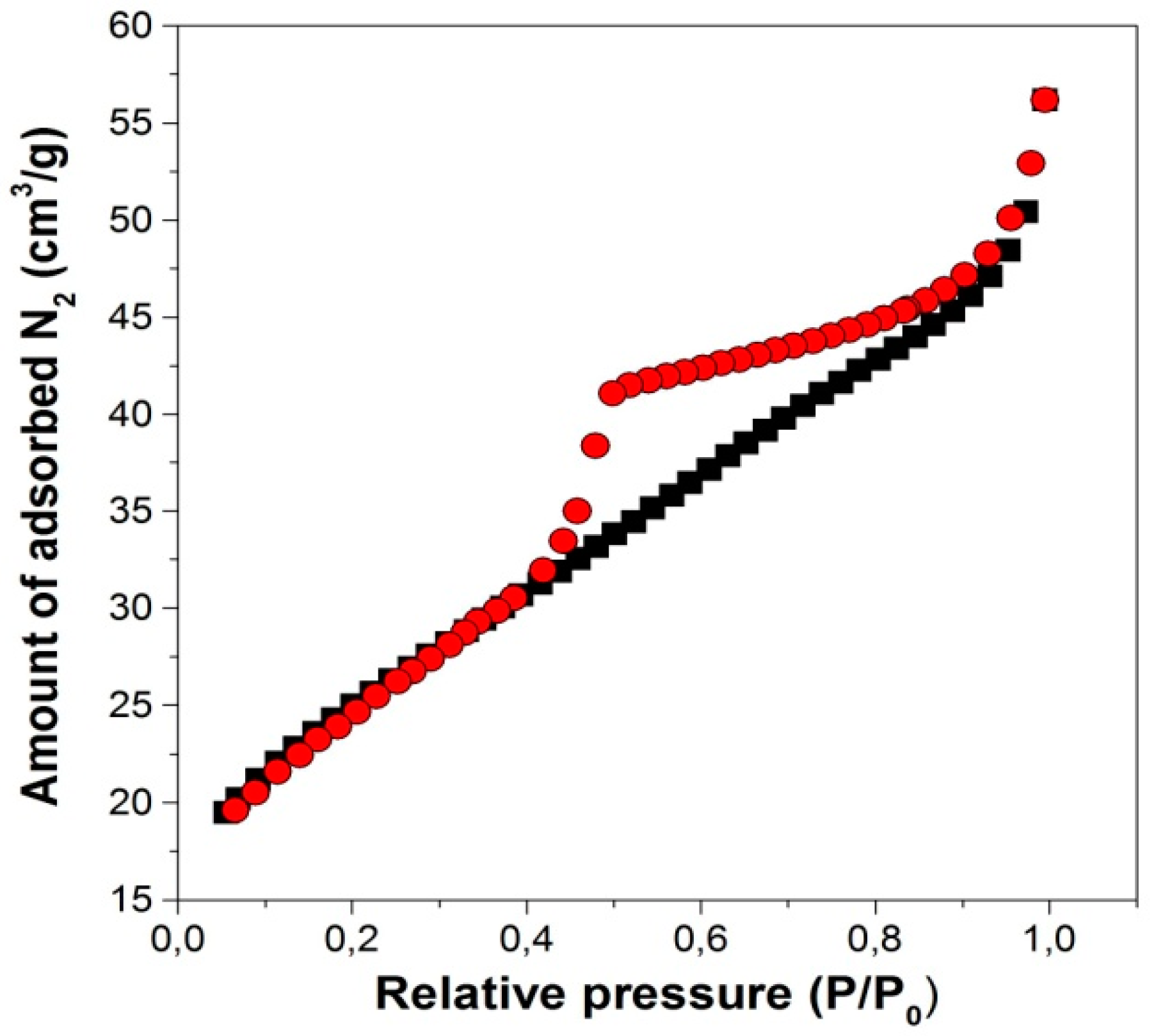
2.1.2. Surface Area Analysis
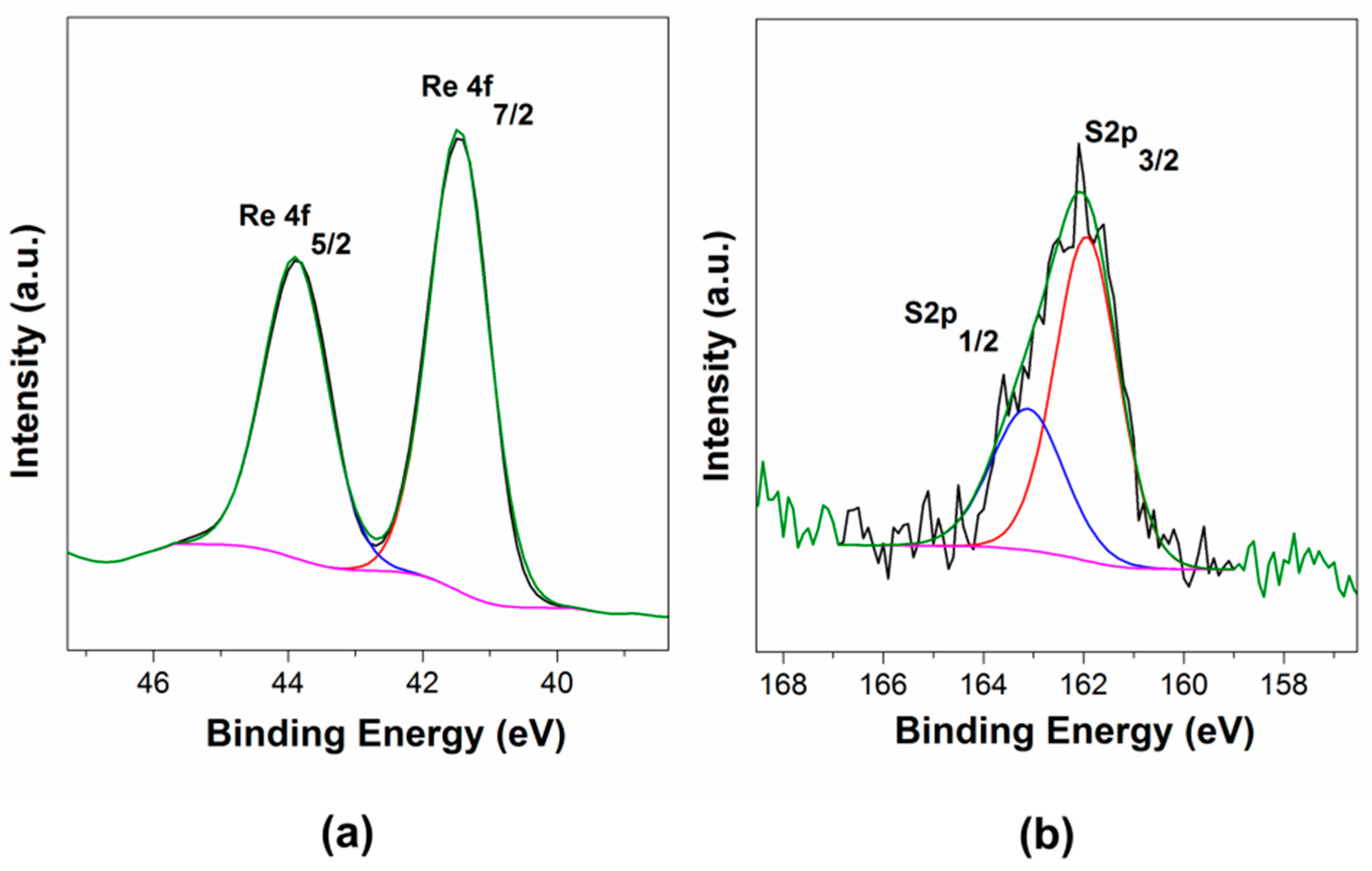
2.1.3. X-ray Photoelectron and Energy-Dispersive X-ray Spectroscopy
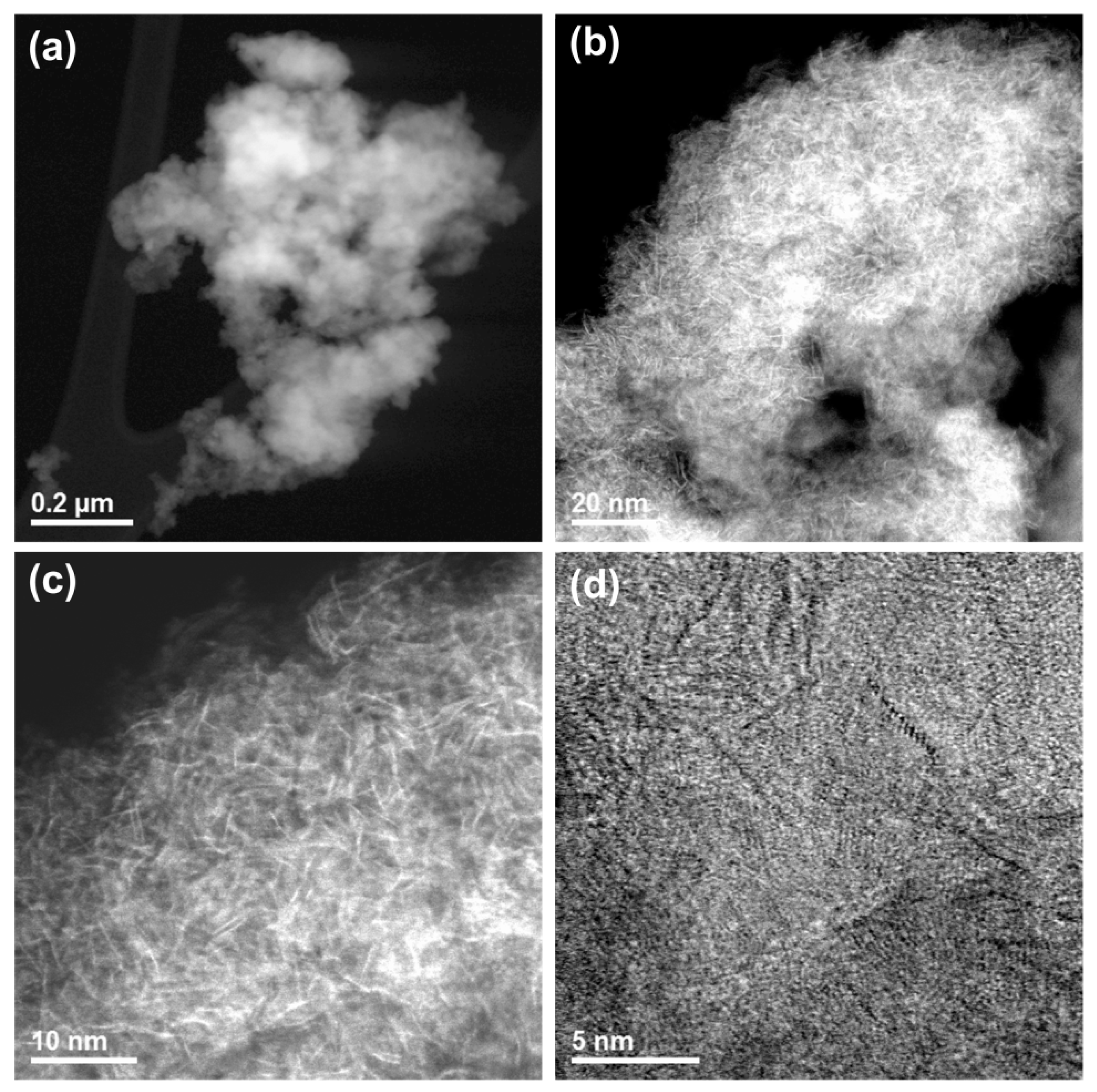
2.1.4. Scanning Transmission Electron Microscopy Analysis
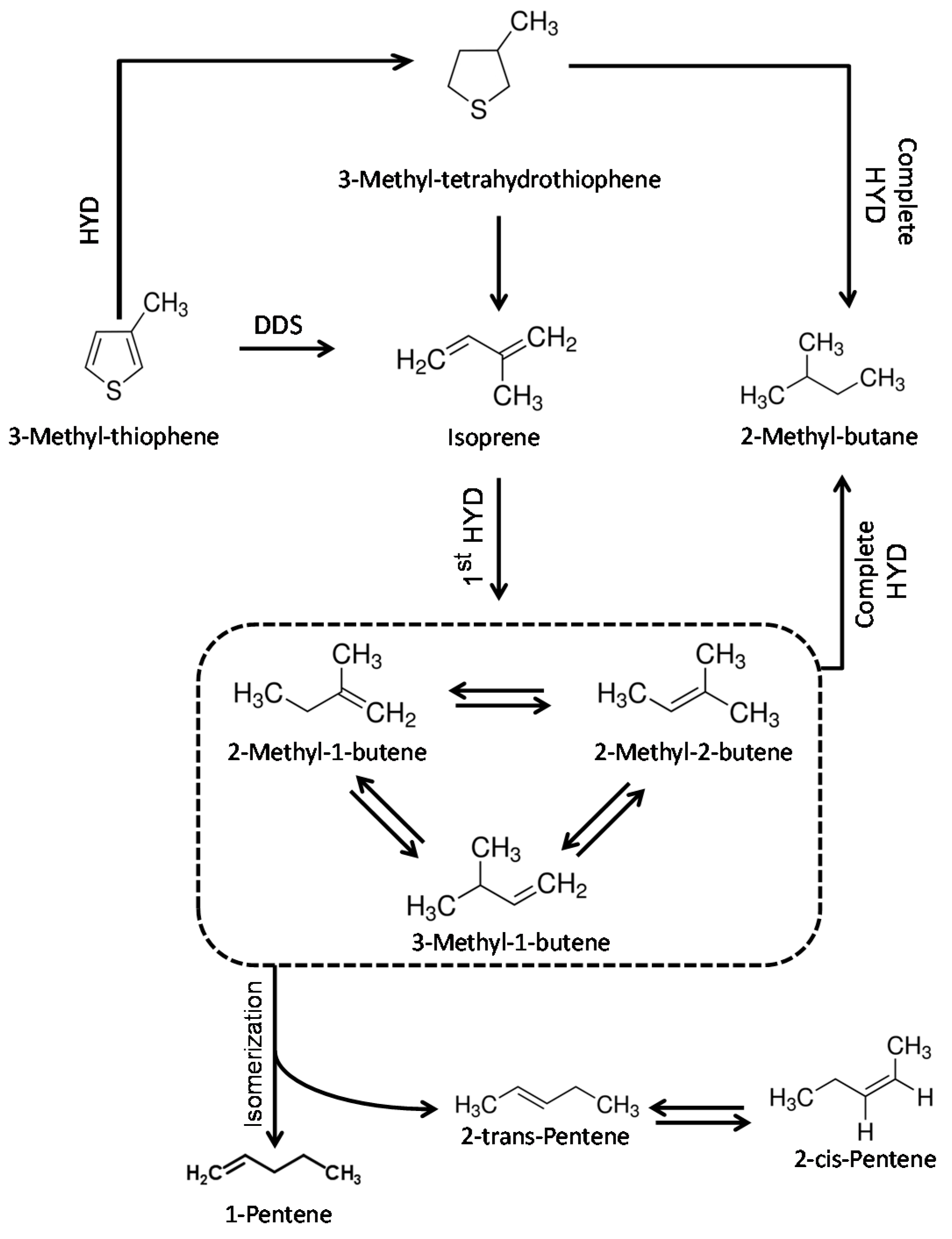
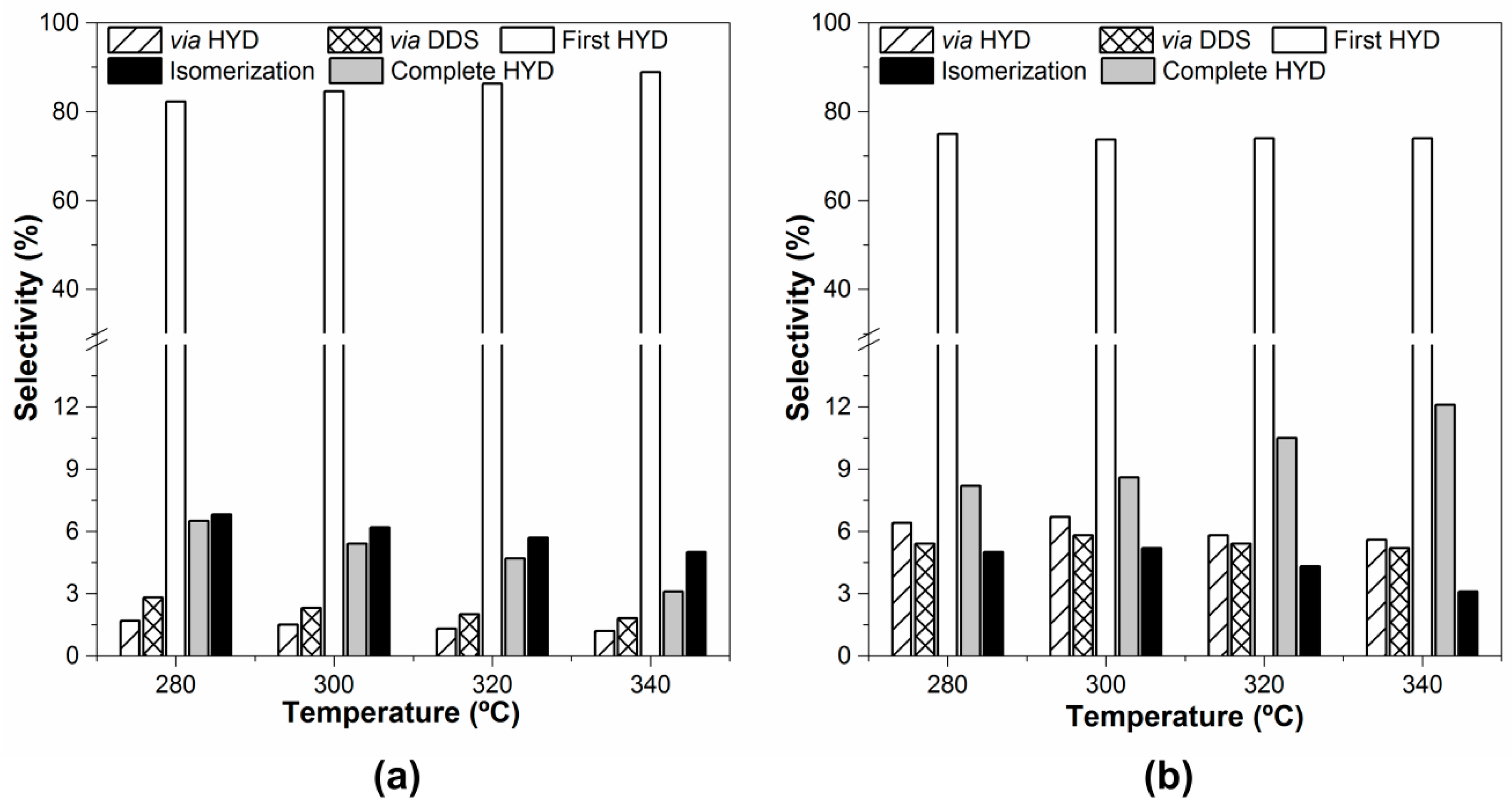
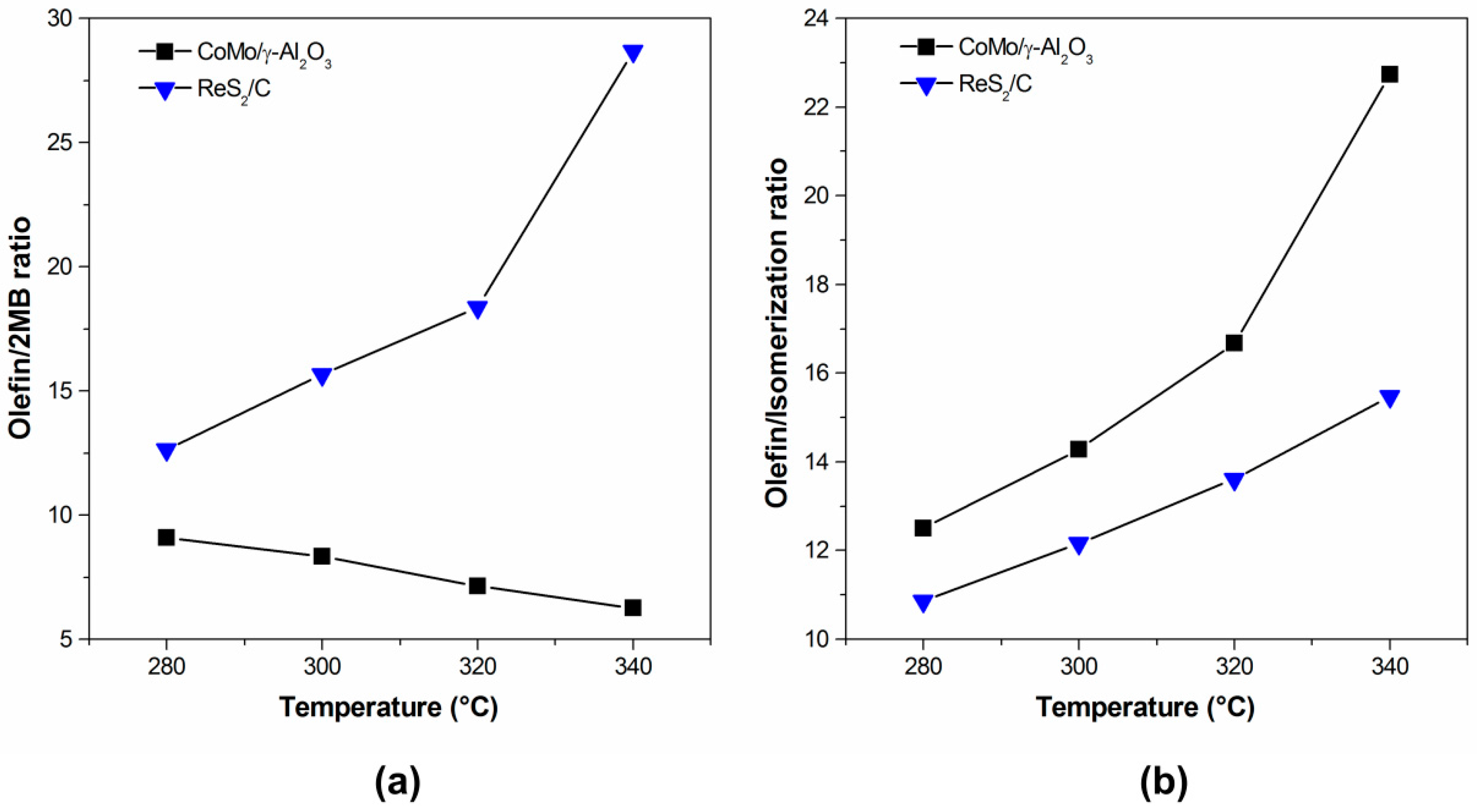
2.2. Hydrodesulfurization Activity
3. Materials and Methods
3.1. Preparation of Catalyst
3.2. Characterization and Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wildervanck, J.; Jellinek, F. The dichalcogenides of technetium and rhenium. J. Less Common Met. 1971, 24, 73–81. [Google Scholar] [CrossRef]
- Lamfers, H.J.; Meetsma, A.; Wiegers, G.; De Boer, J. The crystal structure of some rhenium and technetium dichalcogenides. J. Alloy Compd. 1996, 241, 34–39. [Google Scholar] [CrossRef]
- Tongay, S.; Sahin, H.; Ko, C.; Luce, A.; Fan, W.; Liu, K.; Zhou, J.; Huang, Y.-S.; Ho, C.-H.; Yan, J.; et al. Monolayer behaviour in bulk ReS2 due to electronic and vibrational decoupling. Nat. Commun. 2014, 5, 3252–3257. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Shen, Q.; McConnachie, J.; Kliewer, C. Kinetic characterization of unsupported ReS2 as hydroprocessing catalyst. J. Catal. 2010, 276, 114–122. [Google Scholar] [CrossRef]
- Aliaga, J.; Zepeda, T.; Pawelec, B.; Araya, J.; Antúnez-García, J.; Farías, M.; Fuentes, S.; Galván, D.; Alonso-Núñez, G.; González, G. Microspherical ReS2 as a High-Performance Hydrodesulfurization Catalyst. Catal. Lett. 2017, 147, 1243–1251. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Luxa, J.; Sedmidubsky, D.; Ambrosi, A.; Pumera, M. Layered rhenium sulfide on free-standing three-dimensional electrodes is highly catalytic for the hydrogen evolution reaction: Experimental and theoretical study. Electrochem. Commun. 2016, 63, 39–43. [Google Scholar] [CrossRef]
- Gao, J.; Li, L.; Tan, J.W.; Sun, H.; Li, B.C.; Idrobo, J.C.; Singh, C.V.; Lu, T.M.; Koratkar, N. Vertically Oriented Arrays of ReS2 Nanosheets for Electrochemical Energy Storage and Electrocatalysis. Nano Lett. 2016, 16, 3780–3787. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Gao, J.; Li, B.; Tan, J.; Xiang, Y.; Gupta, T.; Li, L.; Suresh, S.; Idrobo, J.C.; Lu, T.-M. Humidity sensing using vertically oriented arrays of ReS2 nanosheets deposited on an interdigitated gold electrode. 2D Mater. 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Yang, S.; Kang, J.; Yue, Q.; Coey, J.; Jiang, C. Defect-Modulated Transistors and Gas-Enhanced Photodetectors on ReS2 Nanosheets. Adv. Mater. Interfaces. 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, S.; Mendes, R.G.; Sun, Z.; Chen, Y.; Kong, X.; Xue, Y.; Rümmeli, M.H.; Wu, X.; Chen, S. Extremely Weak van der Waals Coupling in Vertical ReS2 Nanowalls for High-Current-Density Lithium-Ion Batteries. Adv. Mater. 2016, 28, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Chen, Y.; Zheng, B.; He, J.; Li, Q.; Wang, X.; Yu, B.; Lin, J.; Zhou, J.; Li, P. 3D chrysanthemum-like ReS2 microspheres composed of curly few-layered nanosheets with enhanced electrochemical properties for lithium-ion batteries. J. Mater. Sci. 2017, 52, 3622–3629. [Google Scholar] [CrossRef]
- Qi, F.; He, J.; Chen, Y.; Zheng, B.; Li, Q.; Wang, X.; Yu, B.; Lin, J.; Zhou, J.; Li, P. Few-layered ReS2 nanosheets grown on carbon nanotubes: A highly efficient anode for high-performance lithium-ion batteries. Chem. Eng. J. 2017, 315, 10–17. [Google Scholar] [CrossRef]
- Liu, E.; Long, M.; Zeng, J.; Luo, W.; Wang, Y.; Pan, Y.; Zhou, W.; Wang, B.; Hu, W.; Ni, Z. High Responsivity Phototransistors Based on Few-Layer ReS2 for Weak Signal Detection. Adv. Funct. Mater. 2016, 26, 1938–1944. [Google Scholar] [CrossRef]
- Hafeez, M.; Gan, L.; Li, H.; Ma, Y.; Zhai, T. Large-Area Bilayer ReS2 Film/Multilayer ReS2 Flakes Synthesized by Chemical Vapor Deposition for High Performance Photodetectors. Adv. Funct. Mater. 2016, 26, 4551–4560. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Kong, X.; Mendes, R.G.; Fang, L.; Xue, Y.; Xiao, Y.; Rümmeli, M.H.; Chen, S.; Fu, L. Edge-to-edge oriented self-assembly of ReS2nanoflakes. J. Am. Chem. Soc. 2016, 138, 11101–11104. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Davey, K.; Qiao, S.Z. Advent of 2D Rhenium Disulfide (ReS2): Fundamentals to Applications. Adv. Funct. Mater. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Keyshar, K.; Gong, Y.; Ye, G.; Brunetto, G.; Zhou, W.; Cole, D.P.; Hackenberg, K.; He, Y.; Machado, L.; Kabbani, M. Chemical vapor deposition of monolayer rhenium disulfide (ReS2). Adv. Mater. 2015, 27, 4640–4648. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaimi, N.; Lewis, D.J.; Zhong, X.L.; Malik, M.A.; O’Brien, P. Chemical vapour deposition of rhenium disulfide and rhenium-doped molybdenum disulfide thin films using single-source precursors. J. Mater. Chem. C. 2016, 4, 2312–2318. [Google Scholar] [CrossRef]
- Qi, F.; Chen, Y.; Zheng, B.; Zhou, J.; Wang, X.; Li, P.; Zhang, W. Facile growth of large-area and high-quality few-layer ReS2 by physical vapour deposition. Mater. Lett. 2016, 184, 324–327. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Slabon, A.; Hu, P.; Feng, S.; Zhang, K.; Prabhakar, R.R.; Kloc, C. Rapid synthesis of transition metal dichalcogenide few-layer thin crystals by the microwave-induced-plasma assisted method. J. Cryst. Growth 2016, 450, 140–147. [Google Scholar] [CrossRef]
- Afanasiev, P. Synthetic approaches to the molybdenum sulfide materials. C. R. Chim. 2008, 11, 159–182. [Google Scholar] [CrossRef]
- Tang, G.; Sun, J.; Wei, C.; Wu, K.; Ji, X.; Liu, S.; Tang, H.; Li, C. Synthesis and characterization of flowerlike MoS2 nanostructures through CTAB-assisted hydrothermal process. Mater. Lett. 2012, 86, 9–12. [Google Scholar] [CrossRef]
- Li, N.; Chai, Y.; Li, Y.; Tang, Z.; Dong, B.; Liu, Y.; Liu, C. Ionic liquid assisted hydrothermal synthesis of hollow vesicle-like MoS2 microspheres. Mater. Lett. 2012, 66, 236–238. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.D.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, J.A.; Alonso-Núñez, G.; Zepeda, T.; Araya, J.F.; Rubio, P.F.; Bedolla-Valdez, Z.; Paraguay-Delgado, F.; Farías, M.; Fuentes, S.; González, G. Synthesis of highly destacked ReS2 layers embedded in amorphous carbon from a metal-organic precursor. J. Non-Cryst. Solids 2016, 447, 29–34. [Google Scholar] [CrossRef]
- Tao, H.; Yanagisawa, K.; Zhang, C.; Ueda, T.; Onda, A.; Li, N.; Shou, T.; Kamiya, S.; Tao, J. Synthesis and growth mechanism of monodispersed MoS2 sheets/carbon microspheres. CrystEngComm 2012, 14, 3027–3032. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, W.; Gong, Q.; Liu, C.; Liu, Z.; Li, Y.; Dai, H. Ultrathin WS2 nanoflakes as a high-performance electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 7860–7863. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Sangwan, V.K.; Wood, J.D.; Liu, X.; Balla, I.; Lam, D.; Hersam, M.C. Layer-by-Layer Sorting of Rhenium Disulfide via High-Density Isopycnic Density Gradient Ultracentrifugation. Nano Lett. 2016, 16, 7216–7223. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.; Del Valle, M.; Cruz, J.; Petranovskii, V.; Licea-Claverie, A.; Fuentes, S. Preparation of MoS2 catalysts by in situ decomposition of tetraalkylammoniumthiomolybdates. Catal. Today 1998, 43, 117–122. [Google Scholar] [CrossRef]
- Alonso, G.; Berhault, G.; Paraguay, F.; Rivera, E.; Fuentes, S.; Chianelli, R.R. Mesoporous carbon-containing MoS2 materials formed from the in situ decomposition of tetraalkylammoniumthiomolybdates. Mater. Res. Bull. 2003, 38, 1045–1055. [Google Scholar] [CrossRef]
- Lu, X.; Lin, Y.; Dong, H.; Dai, W.; Chen, X.; Qu, X.; Zhang, X. One-step hydrothermal fabrication of three-dimensional MoS2 nanoflower using polypyrrole as template for efficient hydrogen evolution reaction. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Devers, E.; Afanasiev, P.; Jouguet, B.; Vrinat, M. Hydrothermal syntheses and catalytic properties of dispersed molybdenum sulfides. Catal. Lett. 2002, 82, 13–17. [Google Scholar] [CrossRef]
- Jacobsen, C.J.; Törnqvist, E.; Topsøe, H. HDS, HDN and HYD activities and temperature-programmed reduction of unsupported transition metal sulfides. Catal. Lett. 1999, 63, 179–183. [Google Scholar] [CrossRef]
- Romero-Rivera, R.; Berhault, G.; Alonso-Nunez, G.; Del Valle, M.; Paraguay-Delgado, F.; Fuentes, S.; Salazar, S.; Aguilar, A.; Cruz-Reyes, J. Tungsten disulfide catalysts from tetraalkylammoniumthiotungstates by ex situ activation, their properties and HDS activity. Appl. Catal. A 2012, 433, 115–121. [Google Scholar] [CrossRef]
- Davis, S. Photoemission studies of rhenium disulfide oxidation: Altered core-level structure and reactivity of defect sites. Catal. Lett. 1989, 2, 1–7. [Google Scholar] [CrossRef]
- Daage, M.; Chianelli, R. Structure-function relations in molybdenum sulfide catalysts: The “rim-edge” model. J. Catal. 1994, 149, 414–427. [Google Scholar] [CrossRef]
| Sample | Rate (10−8 mol3-MT g−1·Cat·s−1) | Ea (kJ mol−1) | |||
|---|---|---|---|---|---|
| 280 °C | 300 °C | 320 °C | 340 °C | ||
| ReS2/C | 35 | 74 | 148 | 251 | 94 |
| CoMo/Al2O3 | 38 | 67 | 121 | 191 | 75 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaga, J.A.; Zepeda, T.; Araya, J.F.; Paraguay-Delgado, F.; Benavente, E.; Alonso-Núñez, G.; Fuentes, S.; González, G. Low-Dimensional ReS2/C Composite as Effective Hydrodesulfurization Catalyst. Catalysts 2017, 7, 377. https://doi.org/10.3390/catal7120377
Aliaga JA, Zepeda T, Araya JF, Paraguay-Delgado F, Benavente E, Alonso-Núñez G, Fuentes S, González G. Low-Dimensional ReS2/C Composite as Effective Hydrodesulfurization Catalyst. Catalysts. 2017; 7(12):377. https://doi.org/10.3390/catal7120377
Chicago/Turabian StyleAliaga, Juan Antonio, Trino Zepeda, Juan Francisco Araya, Francisco Paraguay-Delgado, Eglantina Benavente, Gabriel Alonso-Núñez, Sergio Fuentes, and Guillermo González. 2017. "Low-Dimensional ReS2/C Composite as Effective Hydrodesulfurization Catalyst" Catalysts 7, no. 12: 377. https://doi.org/10.3390/catal7120377