Effects of Preparation Method on the Structure and Catalytic Activity of Ag–Fe2O3 Catalysts Derived from MOFs
Abstract
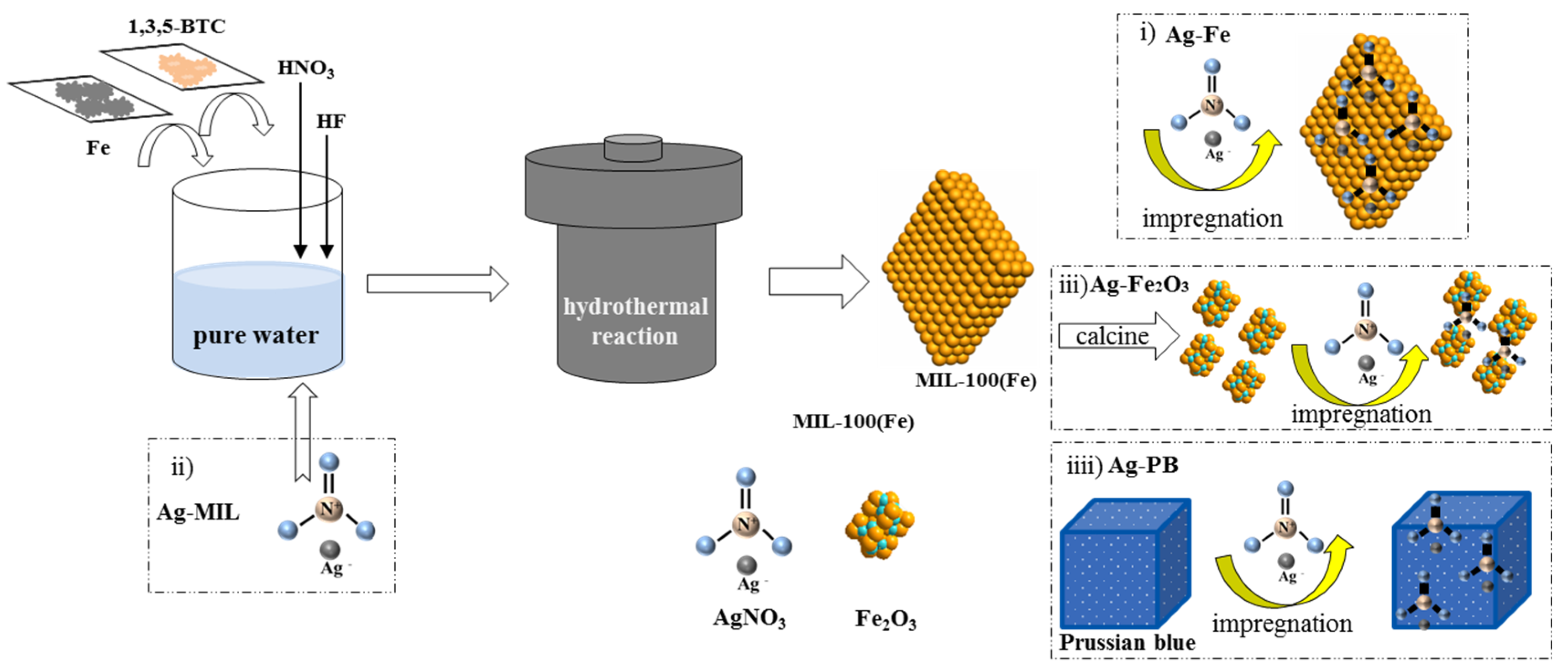
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalysts Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gardner, S.D.; Upchurch, B.T.; Schryer, D.R.; Kielin, E.J.; Schryer, J. Comparison of the performance characteristics of Pt-SnOx and Au-MnOx catalysts for low-temperature CO oxidation. J. Catal. 1991, 129, 114–120. [Google Scholar] [CrossRef]
- Zhang, X.D.; Qu, Z.P.; Li, X.Y.; Wen, M.; Quan, X.; Ma, D.; Wu, J.J. Studies of silver species for low-temperature CO oxidation on Ag/SiO2 catalysts. Sep. Purif. Technol. 2010, 72, 395–400. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.F.; Chen, C.W.; He, C.; Hao, Z.P.; Liu, H.X.; Shen, Z.X. Nanosphere-shaped Mn3O4 catalyst with remarkable for low-temperature activity for methyl-ethyl-ketone catalytic combustion of Methyl-ethyl-ketone. Environ. Sci. Technol. 2017, 51, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, L.Y.; Sun, N.N.; Wang, H.; Zhong, L.S.; He, C.; Wei, W.; Sun, Y.H. Hollow MnOx/CeO2 mixed oxides as highly efficient catalysts in NO oxidation. Chem. Eng. J. 2017, 322, 46–55. [Google Scholar] [CrossRef]
- Yu, Y.K.; Miao, J.F.; Wang, J.X.; He, C.; Chen, J.S. Facile synthesis of CuSO4/TiO2 catalysts with broad temperature window, superior activity and SO2 tolerance for NH3-SCR: Physicochemical property and reaction mechanism. Catal. Sci. Technol. 2017, 7, 1590–1601. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, H.X.; Yang, Y.; Zhang, T.T.; Wen, X.; Liu, N.; Wang, D.J. Facile synthesis of new efficient Cu/MnO2 catalysts from used battery for CO oxidation. J. Environ. Chem. Eng. 2017, 5, 5179–5186. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Dong, H.; Wang, Y.; Wang, Y.X.; Liu, N.; Wang, D.J.; Zhang, X.D. A facile synthesis for porous CuO/Cu2O composites derived from MOFs and their superior catalytic performance for CO oxidation. Inorg. Chem. Commun. 2017, 86, 74–77. [Google Scholar] [CrossRef]
- Weng, X.L.; Zeng, Q.S.; Zhang, Y.L.; Dong, F.; Wu, Z.B. Facile Approach for the Syntheses of Ultrafine TiO2 Nanocrystallites with Defects and C Heterojunction for Photocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2016, 4, 4314–4320. [Google Scholar] [CrossRef]
- Sun, P.F.; Wang, W.L.; Dai, X.X.; Weng, X.L.; Wu, Z.B. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Yang, X.Q.; Yu, X.L.; Lin, M.Y.; Ge, M.F.; Zhao, Y.; Wang, F.Y. Interface effect of mixed phase Pt/ZrO2 catalysts for HCHO oxidation at ambient temperature. J. Mater. Chem. A 2017, 5, 13799–13806. [Google Scholar] [CrossRef]
- Meng, Q.J.; Wang, W.J.; Weng, X.L.; Liu, Y.; Wang, H.Q.; Wu, Z.B. Active oxygen species in Lan+1NinO3n+1 layered perovskites for catalytic oxidation of toluene and methane. J. Phys. Chem. C 2016, 120, 3259–3266. [Google Scholar] [CrossRef]
- Wang, W.L.; Meng, Q.J.; Weng, X.L.; Wu, Z.B. Rapid syntheses of ultrafine LaMnO3 nano-crystallites with superior activity for catalytic oxidation of toluene. Catal. Commun. 2016, 84, 167–170. [Google Scholar] [CrossRef]
- Weng, X.L.; Dai, X.X.; Zeng, Q.S.; Liu, Y.; Wu, Z.B. DRIFT studies on promotion mechanism of H3PW12O40 in selective catalytic reduction of NO with NH3. J. Colloid Interface Sci. 2016, 461, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.J.; Dong, Y.Y.; Zhang, X.D.; Cui, Y.B.; Qu, X.X.; Qi, X.H. The highly enhanced visible light photocatalytic degradation of gaseous o-dichlorobenzene through fabricating like-flowers BiPO4/BiOBr p-n heterojunction composites. Appl. Surf. Sci. 2017, 391, 525–534. [Google Scholar] [CrossRef]
- Wang, Y.X.; Yang, Y.Q.; Xi, L.M.; Zhang, X.D.; Jia, M.H.; Xu, H.M.; Wu, H.G. A simple hydrothermal synthesis of flower-like ZnO microspheres and their improved photocatalytic activity. Mater. Lett. 2016, 180, 55–58. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wang, Y.X.; Hou, F.L.; Li, H.X.; Yang, Y.; Yang, Y.Q.; Wang, Y. Effects of Ag loading on structural and photocatalytic properties of flower-like ZnO microspheres. Appl. Surf. Sci. 2017, 391, 476–483. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Li, H.X.; Hou, F.L.; Hu, J.Y.; Zhang, X.D.; Wang, Y.X. Facile synthesis of ZnO/Ag nanocomposites with enhanced photocatalytic properties under visible light. Mater. Lett. 2016, 180, 97–100. [Google Scholar] [CrossRef]
- Zou, X.J.; Dong, Y.Y.; Zhang, X.D.; Cui, Y.B. Synthesize and characterize of Ag3VO4/TiO2 nanorods photocatalysts and its photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2016, 366, 173–180. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.N.; Li, W.Y.; Gou, X.L. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.L.; Wang, S.R.; Guo, X.Z.; Zhang, J.; Xia, H.J.; Zhang, S.M.; Wu, S.H. Facile Synthesis of Porous alpha-Fe2O3 Nanorods and Their Application in Ethanol Sensors. J. Phys. Chem. C 2008, 112, 17804–17808. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Qin, W.; Zheng, Z.M.; Xiao, X.B.; Dong, C.Q. Activity of Fe2O3 and its effect on CO oxidation in the chemical looping combustion: A theoretical account. Adv. Environ. Technol. 2013, 726–731, 2040–2044. [Google Scholar]
- Liu, X.J.; Liu, J.F.; Chang, Z.; Sun, X.M.; Li, Y.D. Crystal plane effect of Fe2O3 with various morphologies on CO catalytic oxidation. Catal. Commun. 2011, 12, 530–534. [Google Scholar] [CrossRef]
- Gao, Q.X.; Wang, X.F.; Di, J.L.; Wu, X.C.; Tao, Y.R. Enhanced catalytic activity of α-Fe2O3 nanorods enclosed with {110} and {001} planes for methane combustion and CO oxidation. Catal. Sci. Technol. 2011, 1, 574–577. [Google Scholar] [CrossRef]
- Zhang, X.D.; Hou, F.L.; Li, H.X.; Yang, Y.; Wang, Y.X.; Liu, N.; Yang, Y.Q. A strawsheave-like metal organic framework Ce-BTC derivative containing high specific surface area for improving the catalytic activity of CO oxidation reaction. Microporous Mesoporous Mater. 2017. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.Y.; Zhang, X.D.; Tang, L.; Wang, L.; Wang, Y.X.; Wu, M.H. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB. Appl. Catal. B 2017, 221, 119–128. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, Y.; Huang, W.Y.; Yang, Y.Q.; Wang, Y.X.; He, C.; Liu, N.; Wu, M.H.; Tang, L. g-C3N4/UiO-66 nanohybrids with enhanced photocatalytic activities for the oxidation of dye under visible light irradiation. Mater. Res. Bull. 2017. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, H.X.; Hou, F.L.; Yang, Y.; Dong, H.; Liu, N.; Wang, Y.; Cui, L.F. Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. Appl. Surf. Sci. 2017, 411, 27–33. [Google Scholar] [CrossRef]
- Zhang, R.R.; Hu, L.; Bao, S.X.; Li, R.; Gao, L.; Li, R.; Chen, Q.W. Surface polarization enhancement: High catalytic performance of Cu/CuOx/C nanocomposites derived from Cu-BTC for CO oxidation. J. Mater. Chem. A 2016, 4, 8412–8420. [Google Scholar] [CrossRef]
- Zhang, X.D.; Hou, F.L.; Yang, Y.; Wang, Y.; Liu, N.; Chen, D.; Yang, Y.Q. A facile synthesis for cauliflower like CeO2 catalysts from Ce-BTC precursor and their catalytic performance for CO oxidation. Appl. Surf. Sci. 2017, 423, 771–779. [Google Scholar] [CrossRef]
- Cui, L.F.; Zhao, D.; Yang, Y.; Wang, Y.G.; Zhang, X.D. Synthesis of highly efficient α-Fe2O3 catalysts for CO oxidation derived from MIL-100(Fe). J. Solid State Chem. 2017, 247, 168–172. [Google Scholar] [CrossRef]
- Abney, C.W.; Patterson, J.T.; Gilhula, J.C.; Wang, L.; Hensley, D.K.; Chen, J.H.; Foo, G.S.; Wu, Z.L.; Dai, S. Controlling Interfacial Properties in Supported Metal Oxide Catalysts through Metal-Organic Framework Templating. J. Mater. Chem. A 2017, 26, 13565–13572. [Google Scholar] [CrossRef]
- Das, R.; Pachfule, P.; Banerjee, R.; Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides. Nanoscale 2012, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, J.L.; Odoom-Wubah, T.; Hong, Y.L.; Du, M.M.; Sun, D.H.; Jia, L.S.; Li, Q.B. Efficient Ag/CeO2 catalysts for CO oxidation prepared with microwave-assisted biosynthesis. Chem. Eng. J. 2015, 269, 105–112. [Google Scholar] [CrossRef]
- Zhang, X.D.; Dong, H.; Zhao, D.; Wang, Y.; Wang, Y.G.; Cui, L.F. Effect of support calcination temperature on Ag structure and catalytic activity for CO oxidation. Chem. Res. Chin. Univ. 2016, 32, 455–460. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Hou, F.L.; Li, H.X.; Liu, N.; Wang, Y.; Zhang, X.D. Facile synthesis of Ag/KIT-6 catalyst via a simple one pot method and application in the CO oxidation. J. Porous Mater. 2017, 24, 1661–1665. [Google Scholar] [CrossRef]
- Somodi, F.; Borbath, I.; Hegedus, M.; Tompos, A.; Sajo, I.E.; Szegedi, A.; Rojas, S.; Garcia Fierro, J.L.; Margitfalvi, J.L. Modified preparation method for highly active Au/SiO2 catalysts used in CO oxidation. Appl. Catal. A 2008, 347, 216–222. [Google Scholar] [CrossRef]
- Daniells, S.T.; Overweg, A.R.; Makkee, M.; Moulijn, J.A. The mechanism of low-temperature CO oxidation with Au/Fe2O3 catalysts: A combined Mossbauer, FT-IR, and TAP reactor study. J. Catal. 2005, 230, 52–65. [Google Scholar] [CrossRef]
- Jiang, X.C.; Yu, A.B. Synthesis of Pd/alpha-Fe2O3 nanocomposites for catalytic CO oxidation. J. Mater. Process. Technol. 2009, 209, 4558–4562. [Google Scholar] [CrossRef]
- Pan, H.; Jian, Y.F.; Yu, Y.K.; He, C.; Shen, Z.X.; Liu, H.X. Regeneration and sulfur poisoning of In/H-BEA catalyst for NOx reduction by CH4. Appl. Surf. Sci. 2017, 401, 120–126. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Bahrami, M.; Roushani, M. High CO tolerance of Pt/Fe/Fe2O3 nanohybrid thin film suitable for methanol oxidation in alkaline medium. RSC Adv. 2014, 4, 46992–46999. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yu, X.L.; Yang, X.Q.; Li, K.Z.; Ge, M.F.; Li, J.H. Highly active and stable interface derived from Pt supported on Ni/Fe layered double oxides for HCHO oxidation. Catal. Sci. Technol. 2017, 7, 1573–1580. [Google Scholar] [CrossRef]
- He, H.; Yu, Y.B. Selective catalytic reduction of NOx over Ag/Al2O3 catalyst: From reaction mechanism to diesel engine test. Catal. Today 2005, 100, 37–47. [Google Scholar] [CrossRef]
- Christopher, P.; Linic, S. Engineering selectivity in heterogeneous catalysis: Ag nanowires as selective ethylene epoxidation catalysts. J. Am. Chem. Soc. 2008, 130, 11264–11265. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, S.; Zafeiratos, S.; Stampfl, C.; Hansen, T.W.; Haevecker, M.; Teschner, D.V.; Bukhtiyarov, I.; Girgsdies, F.; Knop-Gericke, A.; Schloegl, R.; et al. Alloy Catalyst in a Reactive Environment: The Example of Ag-Cu Particles for Ethylene Epoxidation. Phys. Rev. Lett. 2010, 104. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, L.; Flytzani-Stephanopoulos, M. Deep oxidation of methane over zirconia supported Ag catalysts. Appl. Catal. A 1999, 183, 35–51. [Google Scholar] [CrossRef]
- Zhang, X.D.; Qu, Z.P.; Jia, J.X.; Wang, Y. Ag nanoparticles supported on wormhole HMS material as catalysts for CO oxidation: Effects of preparation methods. Powder Technol. 2012, 230, 212–218. [Google Scholar] [CrossRef]
- Shiau, C.Y.; Ma, M.W.; Chuang, C.S. CO oxidation over CeO2-promoted Cu/gamma-Al2O3 catalyst: Effect of preparation method. Appl. Catal. A 2006, 301, 89–95. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Matralis, H. Influence of the preparation method on the performance of CuO-CeO2 catalysts for the selective oxidation of CO. Appl. Catal. B 2005, 56, 87–93. [Google Scholar] [CrossRef]
- Zhang, X.D.; Qu, Z.P.; Li, X.Y.; Zhao, Q.D.; Wang, Y.; Quan, X. Low temperature CO oxidation over Ag/SBA-15 nanocomposites prepared via in-situ “pH-adjusting” method. Catal. Commun. 2011, 16, 11–14. [Google Scholar] [CrossRef]
- Biabani-Ravandi, A.; Rezaei, M.; Fattah, Z. Catalytic performance of Ag/Fe2O3 for the low temperature oxidation of carbon monoxide. Chem. Eng. J. 2013, 219, 124–130. [Google Scholar] [CrossRef]
- Narasimharao, K.; Al-Shehri, A.; Al-Thabaiti, S. Al-Thabaiti, Porous Ag-Fe2O3 nanocomposite catalysts for the oxidation of carbon monoxide. Appl. Catal. A 2015, 505, 431–440. [Google Scholar] [CrossRef]
- Bao, S.X.; Yan, N.; Shi, X.H.; Li, R.; Chen, Q.W. High and stable catalytic activity of porous Ag/Co3O4 nanocomposites derived from MOFs for CO oxidation. Appl. Catal. A 2014, 487, 189–194. [Google Scholar] [CrossRef]
- Pan, L.; Tang, J.; Chen, Y.H. Synthesis of Fe3O4, Fe2O3, Ag/Fe3O4 and Ag/Fe2O3 nanoparticles and their electrocatalytic properties. Sci. China Chem. 2012, 56, 362–369. [Google Scholar] [CrossRef]
- Bai, B.Y.; Qiao, Q.; Arandiyan, H.; Li, J.H.; Hao, J.M. Three-Dimensional Ordered Mesoporous MnO2-Supported Ag Nanoparticles for Catalytic Removal of Formaldehyde. Environ. Sci. Technol. 2016, 50, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.S.; Slavinskaya, E.M.; Stonkus, O.A.; Gulyaev, R.V.; Glazneva, T.S.; Noskova, A.S.; Boronin, A.I. Highly active and durable Pd/Fe2O3 catalysts for wet CO oxidation under ambient conditions. Catal. Sci. Technol. 2016, 6, 3918–3928. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Iriarte-Velasco, U.; Gurbani, A.; Gutierrez-Ortiz, M.A. Investigation of the calcination temperature effect on the interaction between Au nanoparticles and the catalytic support Au/Fe2O3 for the low temperature CO oxidation. J. Taiwan Inst. Chem. Eng. 2017, 75, 18–28. [Google Scholar] [CrossRef]
- Wang, F.; Dai, H.; Deng, J.; Bai, G.; Ji, K.; Liu, Y. Manganese oxides with rod-, wire-, tube-, and flower-like morphologies: Highly effective catalysts for the removal of toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Guldur, C.; Balikci, F. Selective carbon monoxide oxidation over Ag-based composite oxides. Int. J. Hydrog. Energy 2002, 27, 219–224. [Google Scholar] [CrossRef]
- Horcajada, P.; Surble, S.; Serre, C.; Hong, D.Y.; Seo, Y.K.; Chang, J.S.; Greneche, J.M.; Margiolaki, I.; Ferey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. [Google Scholar] [CrossRef] [PubMed]
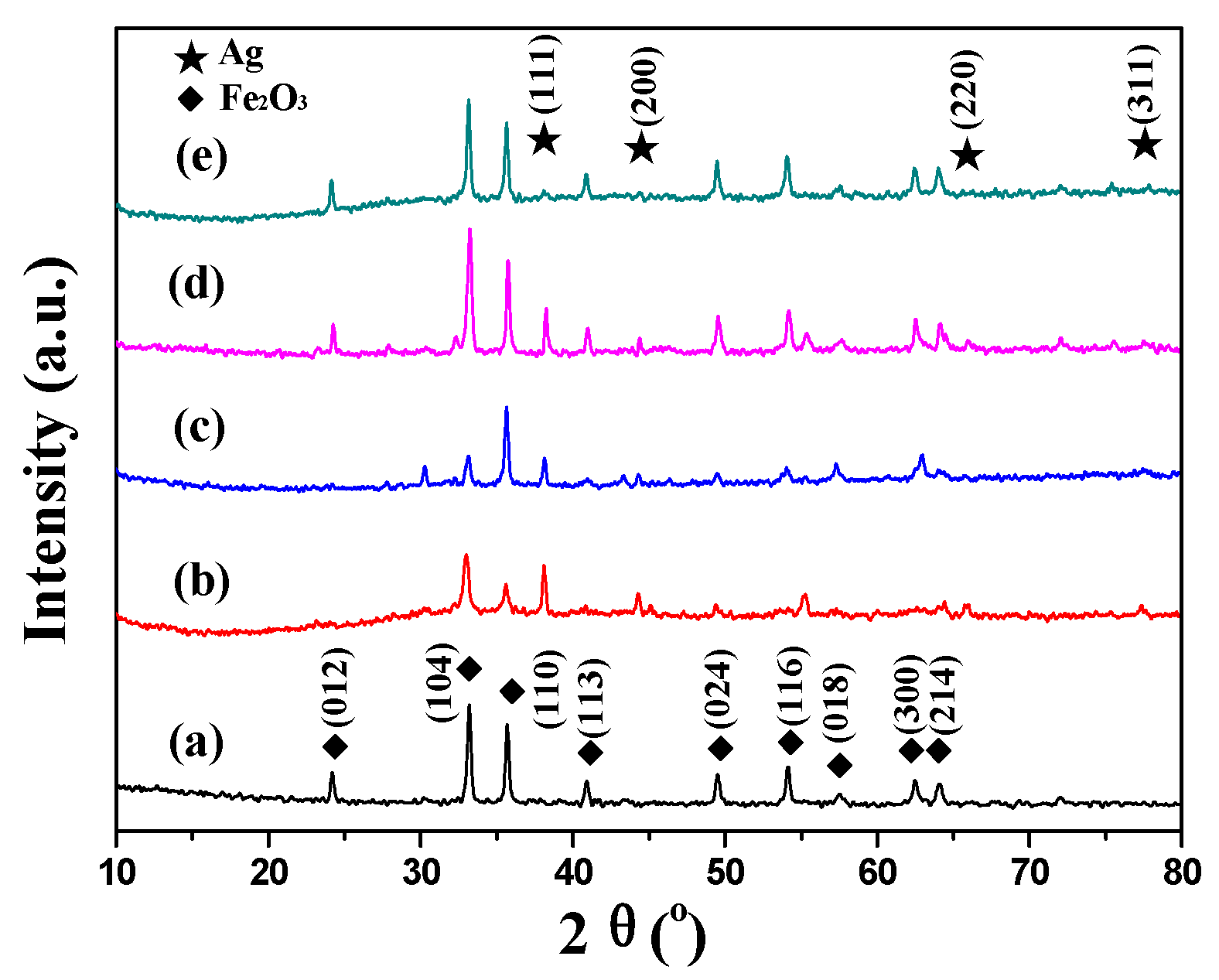
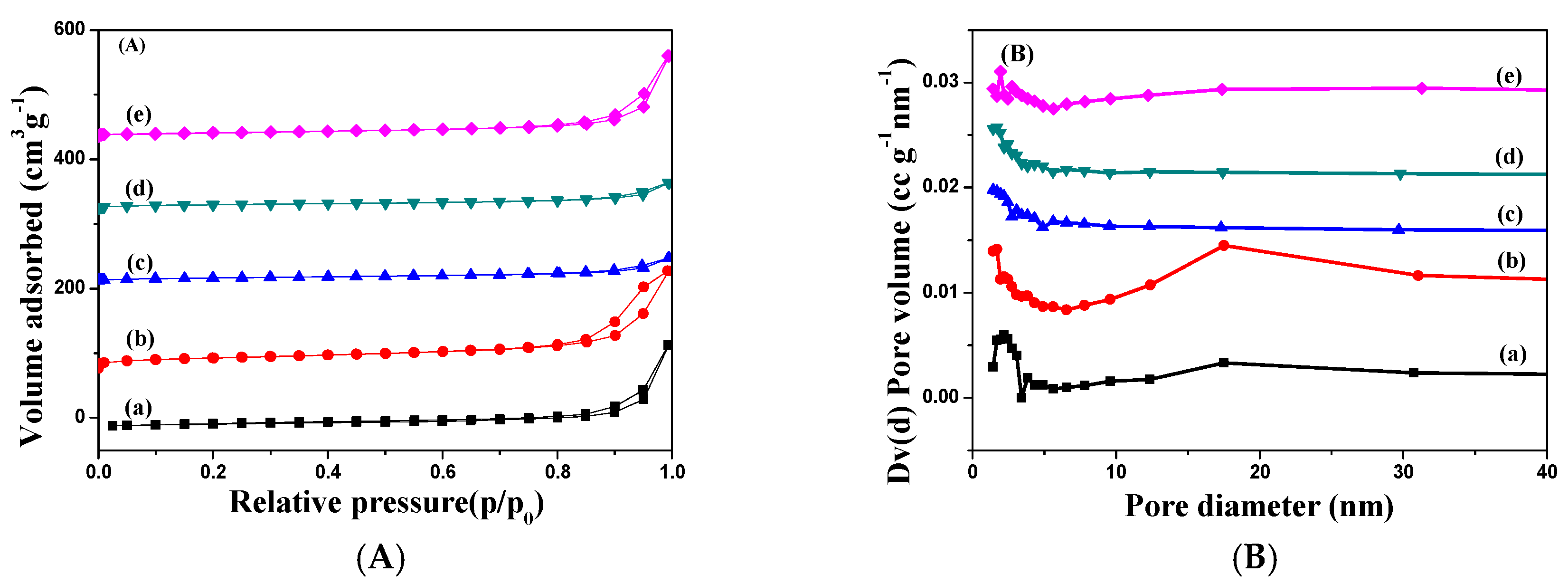
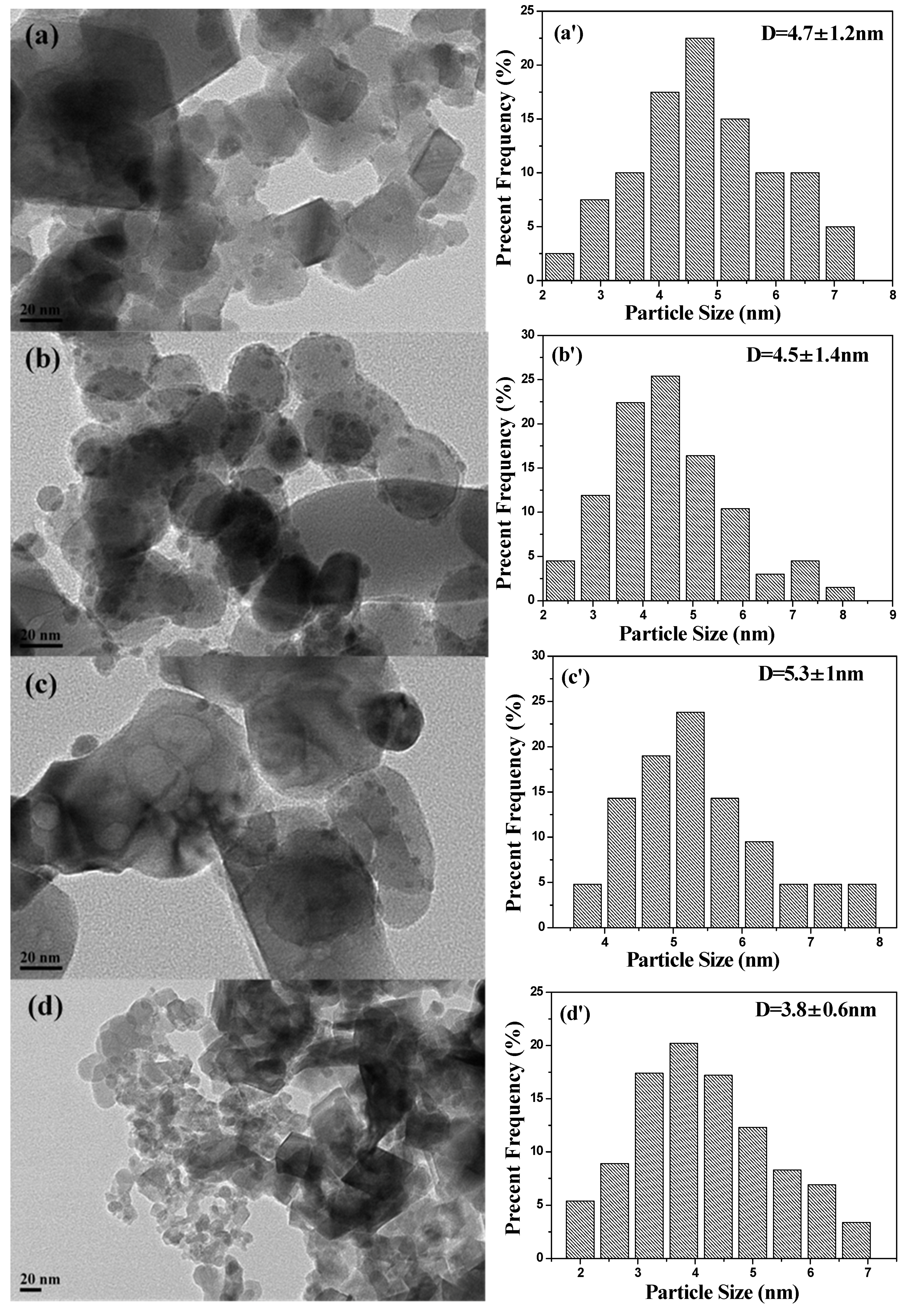
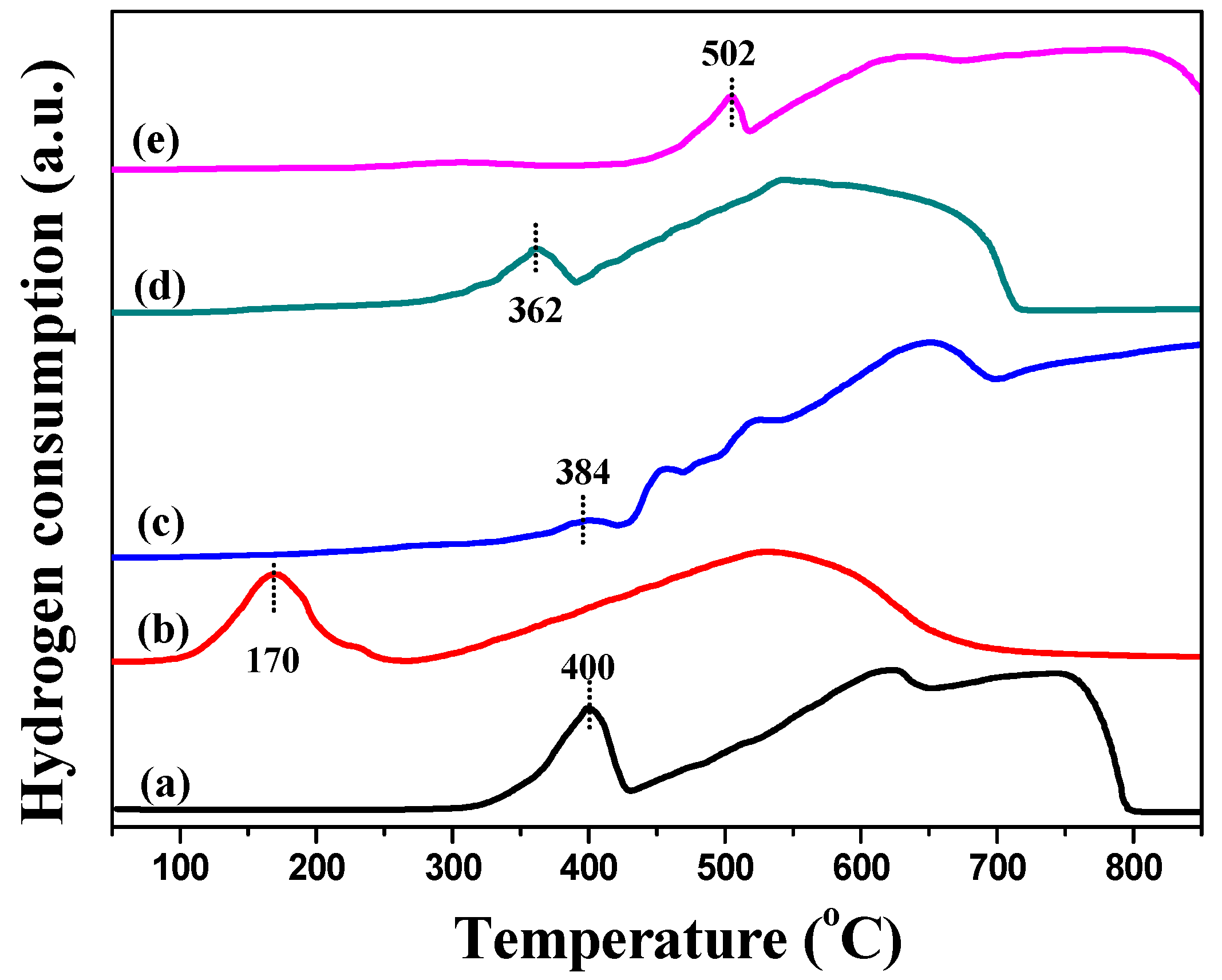


| Catalysts | Textural Properties | Crystallite Size (nm) | Chemical Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBET (m2 g−1) a | V (cc g−1) b | D (nm) c | XRD (nm) d | TEM (nm) e | ICP-OES (%) f | XPS(%) g | ||||||
| Fe | Ag | Ag | Fe | Ag | Ag–Fe2O3 | Fe | Ag | Ag–Fe | ||||
| Fe2O3 [30] | 27 | 0.21 | 2.2 | 26 | - | - | 69.1 | 0 | 0 | - | - | - |
| Ag–Fe | 68 | 0.26 | 1.5–4, 12–25 | 21 | 10 | 4.7 ± 1.2 | 65.5 | 3.41 | 3.64 | 6.81 | 1.78 | 0.26 |
| Ag–MIL | 17 | 0.06 | 1.4 | 26 | 9 | 4.5 ± 1.4 | 62.2 | 8.26 | 9.29 | 6.07 | 1.72 | 0.28 |
| Ag–Fe2O3 | 21 | 0.06 | 1.7 | 26 | 12 | 5.3 ± 1.0 | 60.6 | 5.03 | 5.81 | 7.27 | 1.35 | 0.19 |
| Ag–PB | 21 | 0.19 | 1.9 | 27 | 8 | 3.8 ± 0.6 | 53.2 | 5.09 | 6.70 | 7.76 | 2.38 | 0.31 |
| Catalysts | Ag0/Agδ+ | Fe2+/Fe3+ | Olatt/Oads |
|---|---|---|---|
| Fe2O3 | - | 1.24 | 1.27 |
| Ag–Fe | 2.00 | 1.38 | 2.78 |
| Ag–MIL | 0.97 | 0.71 | 6.05 |
| Ag–Fe2O3 | 1.17 | 0.51 | 1.32 |
| Ag–PB | 1.37 | 0.68 | 1.15 |
| Catalysts | Ag Loading (wt %) | Flow Rate (mL min−1) | T10 (°C) | T50 (°C) | T98 (°C) | References |
|---|---|---|---|---|---|---|
| Fe2O3 | 0 | 30 | 110 | 230 | 253 | [30] |
| Ag–Fe | 3.64 | 30 | <100 | 132 | 157 | This work |
| Ag–MIL | 9.29 | 30 | 190 | 230 | 275 | This work |
| Ag–Fe2O3 | 5.81 | 30 | 120 | ≈180 | 215 | This work |
| Ag–PB | 6.70 | 30 | 290 | >350 | >350 | This work |
| Ag–Fe2O3 | ≈20 | 100 | ≈110 | ≈150 | ≈270 | [50] |
| Ag–Fe2O3 | 6.7 | 30 | <50 | 80 | ≈200 | [51] |
| Ag–Co3O4 | - | 30 | ≈50 | ≈85 | 100 | [52] |
| Pd/α–Fe2O3 | 1.04 | 200 | - | - | 20 | [55] |
| Au/α–Fe2O3 | 0.74 | 200 | ≈50 | >75 | >75 | [56] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, Y.; Lv, X.; Wang, Y.; Cui, L. Effects of Preparation Method on the Structure and Catalytic Activity of Ag–Fe2O3 Catalysts Derived from MOFs. Catalysts 2017, 7, 382. https://doi.org/10.3390/catal7120382
Zhang X, Yang Y, Lv X, Wang Y, Cui L. Effects of Preparation Method on the Structure and Catalytic Activity of Ag–Fe2O3 Catalysts Derived from MOFs. Catalysts. 2017; 7(12):382. https://doi.org/10.3390/catal7120382
Chicago/Turabian StyleZhang, Xiaodong, Yang Yang, Xutian Lv, Yuxin Wang, and Lifeng Cui. 2017. "Effects of Preparation Method on the Structure and Catalytic Activity of Ag–Fe2O3 Catalysts Derived from MOFs" Catalysts 7, no. 12: 382. https://doi.org/10.3390/catal7120382




