Hydrodeoxygenation of Methyl Laurate over Ni Catalysts Supported on Hierarchical HZSM-5 Zeolite
Abstract
:1. Introduction
2. Results
2.1. Nitrogen Physisorption Characterization
2.2. Structural XRD Characterization
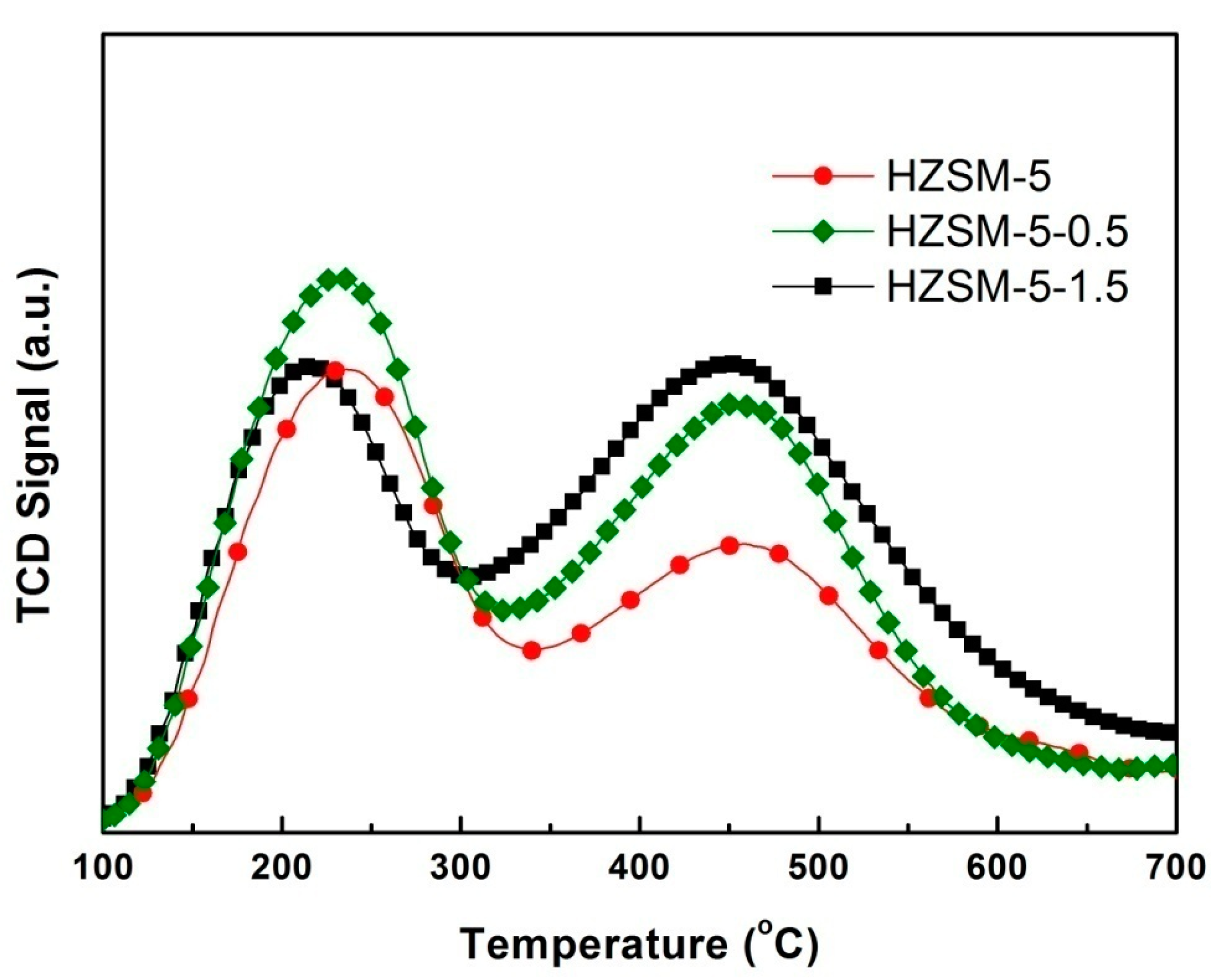
2.3. NH3-TPD Characterization
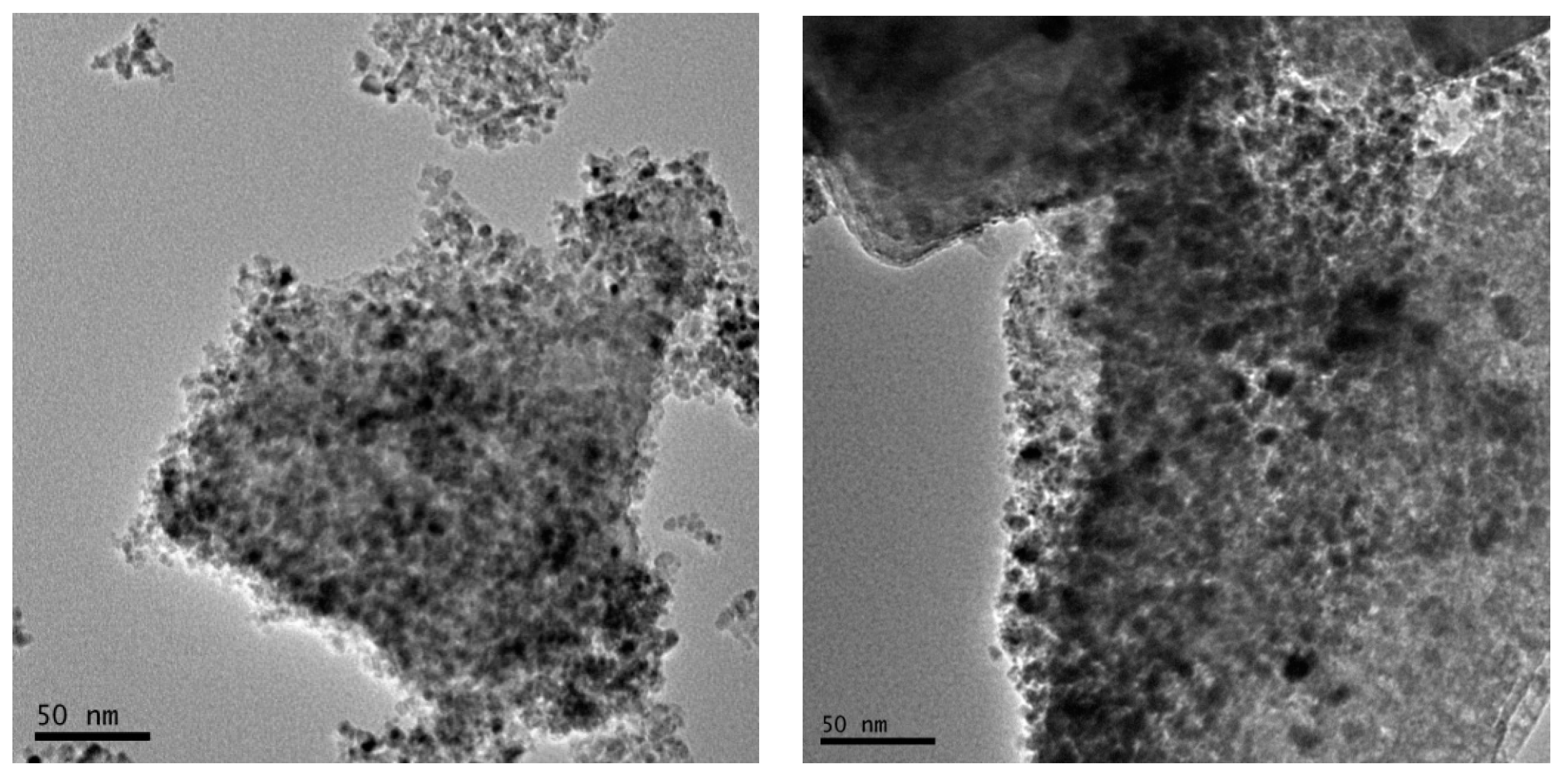
2.4. Transmission Electron Microscopic Observation
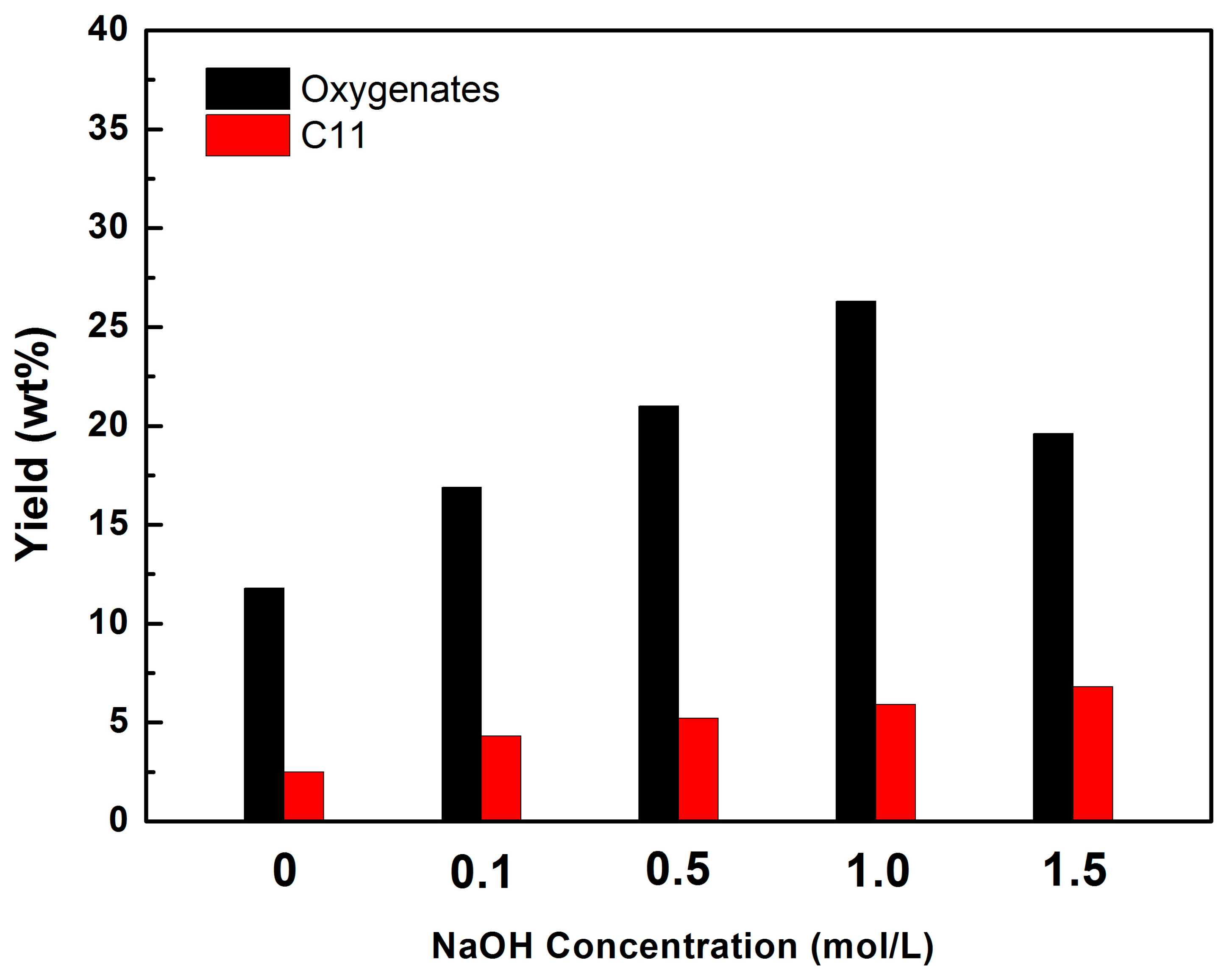
2.5. Deoxygenation of Methyl Laurate over Ni HZSM-5 Catalysts in N2
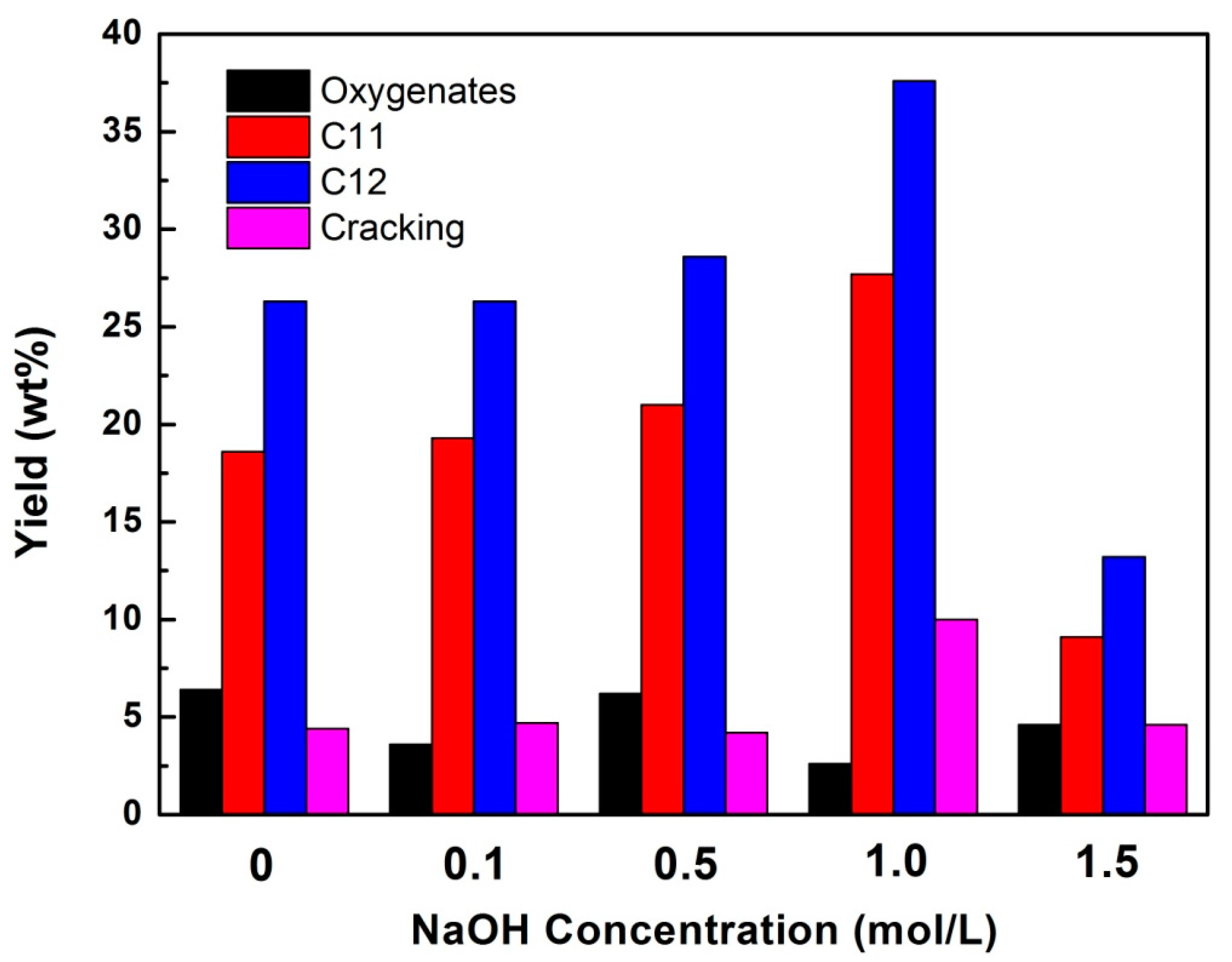
2.6. Hydrodeoxygenation of Methyl Laurate over Ni/HZSM-5 Catalysts in H2
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Catalyst Testing
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Zhao, C.; Bruck, T.; Lercher, J.A. Catalytic deoxygenation of microalgae oil to green hydrocarbons. Green Chem. 2013, 15, 1720–1739. [Google Scholar] [CrossRef]
- Guo, J.H.; Xu, G.Y.; Shen, F.; Fu, Y.; Zhang, Y.; Guo, Q.X. Catalytic conversion of Jatropha oil to alkanes under mild conditions with a Ru/La(OH)3 catalyst. Green Chem. 2015, 17, 2888–2895. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Hollak, S.A.; Chang, S.W.; Van, H.J.; de Jong, K.P.; Bitter, J.H.; van Es, D.S. Reaction pathways for the deoxygenation of vegetable oils and related model compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Suarez, P.A.Z.; Meneghetti, M.R.; Meneghetti, S.M.P. Catalytic production of biodiesel and diesel-like hydrocarbons from triglycerides. Energy Environ. Sci. 2009, 2, 1258–1265. [Google Scholar] [CrossRef]
- Smith, B.; Greenwell, H.C.; Whiting, A. Catalytic upgrading of tri-glycerides and fatty acids to transport biofuels. Energy Environ. Sci. 2009, 2, 262–271. [Google Scholar] [CrossRef]
- Lestari, S.; Mäkiarvela, P.; Beltramini, J.; Lu, G.Q.; Murzin, D.Y. Transforming triglycerides and fatty acids into biofuels. ChemSusChem 2009, 2, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.W.; Stellwagen, D.R.; Bitter, J.H. Tungsten-Based Catalysts for Selective Deoxygenation. Angew. Chem. Int. Ed. 2013, 52, 5089–5092. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Bie, Y.W.; Qiu, S.B.; Zhang, Q.; Sainio, J.; Wang, T.J.; Ma, L.L.; Lehtonen, J. Hydrogenolysis of methyl heptanoate over Co based catalysts: Mediation of support property on activity and product distribution. Appl. Catal. B-Environ. 2014, 147, 236–245. [Google Scholar] [CrossRef]
- Han, J.X.; Duan, J.Z.; Chen, P.; Lou, H.; Zheng, X.M. Molybdenum Carbide-Catalyzed Conversion of Renewable Oils into Diesel-like Hydrocarbons. Adv. Synth. Catal. 2011, 353, 2577–2583. [Google Scholar] [CrossRef]
- Hollak, S.A.W.; Gosselink, R.W.; van Es, D.S.; Bitter, J.H. Comparison of Tungsten and Molybdenum Carbide Catalysts for the Hydrodeoxygenation of Oleic Acid. ACS Catal. 2013, 3, 2837–2844. [Google Scholar] [CrossRef]
- Kandel, K.; Anderegg, J.W.; Nelson, N.C.; Chaudhary, U.; Slowing, I.I. Supported iron nanoparticles for the hydrodeoxygenation of microalgal oil to green diesel. J. Catal. 2014, 314, 142–148. [Google Scholar] [CrossRef]
- Yang, L.Q.; Tate, K.L.; Jasinski, J.B.; Carreon, M.A. Decarboxylation of Oleic Acid to Heptadecane over Pt Supported on Zeolite 5A Beads. ACS Catal. 2015, 5, 6497–6502. [Google Scholar] [CrossRef]
- Rozmyslowicz, B.; Maki-Arvela, P.; Tokarev, A.; Leino, A.R.; Eranen, K.; Murzin, D.Y. Influence of Hydrogen in Catalytic Deoxygenation of Fatty Acids and Their Derivatives over Pd/C. Ind. Eng. Chem. Res. 2012, 51, 8922–8927. [Google Scholar] [CrossRef]
- Verma, D.; Kumar, R.; Rana, B.S.; Sinha, A.K. Aviation fuel production from lipids by a single-step route using hierarchical mesoporous zeolites. Energy Environ. Sci. 2011, 4, 1667–1671. [Google Scholar] [CrossRef]
- Wang, C.X.; Tian, Z.J.; Wang, L.; Xu, R.S.; Liu, Q.H.; Qu, W.; Ma, H.J.; Wang, B.C. One-Step Hydrotreatment of Vegetable Oil to Produce High Quality Diesel-Range Alkanes. ChemSusChem 2012, 5, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Gong, S.F.; Qian, E.W. Effect of reduction temperature of NiMoO3−x/SAPO-11 on its catalytic activity in hydrodeoxygenation of methyl laurate. Appl. Catal. B-Environ. 2015, 174, 253–263. [Google Scholar] [CrossRef]
- Ma, B.; Hu, J.B.; Wang, Y.M.; Zhao, C. Ni nanoparticles encapsulated into mesoporous single-crystalline HBEA: Application for drainage oil hydrodeoxygenation to diesel. Green Chem. 2015, 17, 4610–4617. [Google Scholar] [CrossRef]
- Ma, B.; Yi, X.F.; Chen, L.; Zheng, A.M.; Zhao, C. Interconnected hierarchical HUSY zeolite-loaded Ni nano-particles probed for hydrodeoxygenation of fatty acids, fatty esters, and palm oil. J. Mater. Chem. 2016, 4, 11330–11341. [Google Scholar] [CrossRef]
- Peng, B.X.; Yuan, X.G.; Zhao, C.; Lercher, J.A. Stabilizing Catalytic Pathways via Redundancy: Selective Reduction of Microalgae Oil to Alkanes. J. Am. Chem. Soc. 2012, 134, 9400–9405. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhao, C. High-grade diesel production by hydrodeoxygenation of palm oil over a hierarchically structured Ni/HBEA catalyst. Green Chem. 2015, 17, 1692–1701. [Google Scholar] [CrossRef]
- Van Aelst, J.; Verboekend, D.; Philippaerts, A.; Nuttens, N.; Kurttepeli, M.; Gobechiya, E.; Haouas, M.; Sree, S.P.; Denayer, J.F.M.; Martens, J.A. Catalyst Design by NH4OH Treatment of USY Zeolite. Adv. Funct. Mater. 2015, 25, 7130–7144. [Google Scholar] [CrossRef]
- Kang, J.C.; Cheng, K.; Zhang, L.; Zhang, Q.H.; Ding, J.S.; Hua, W.Q.; Lou, Y.C.; Zhai, Q.G.; Wang, Y. Mesoporous Zeolite-Supported Ruthenium Nanoparticles as Highly Selective Fischer-Tropsch Catalysts for the Production of C5–C11 Isoparaffins. Angew. Chem. Int. Ed. 2011, 50, 5200–5203. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi, M.; Toshiyuki, Y.; Hiroyuki, I.; Seitaro, N.; Junko, N.K.; Takashi, T. Effect of desilication of H-ZSM-5 by alkali treatment on catalytic performance in hexane cracking. Appl. Catal. A Gen. 2012, 449, 188–197. [Google Scholar] [CrossRef]
- Peng, B.X.; Zhao, C.; Kasakov, S.; Foraita, S.; Lercher, J.A. Manipulating Catalytic Pathways: Deoxygenation of Palmitic Acid on Multifunctional Catalysts. Chem. Eur. J. 2013, 19, 4732–4741. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Shi, H.; Li, L.; Li, K.L. Deoxygenation of methyl laurate as a model compound to hydrocarbons on transition metal phosphide catalysts. Appl. Catal. B-Environ. 2014, 144, 870–884. [Google Scholar] [CrossRef]
- Peng, B.X.; Yao, Y.; Zhao, C.; Lercher, J.A. Towards Quantitative Conversion of Microalgae Oil to Diesel-Range Alkanes with Bifunctional Catalysts. Angew. Chem. Int. Ed. 2012, 51, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
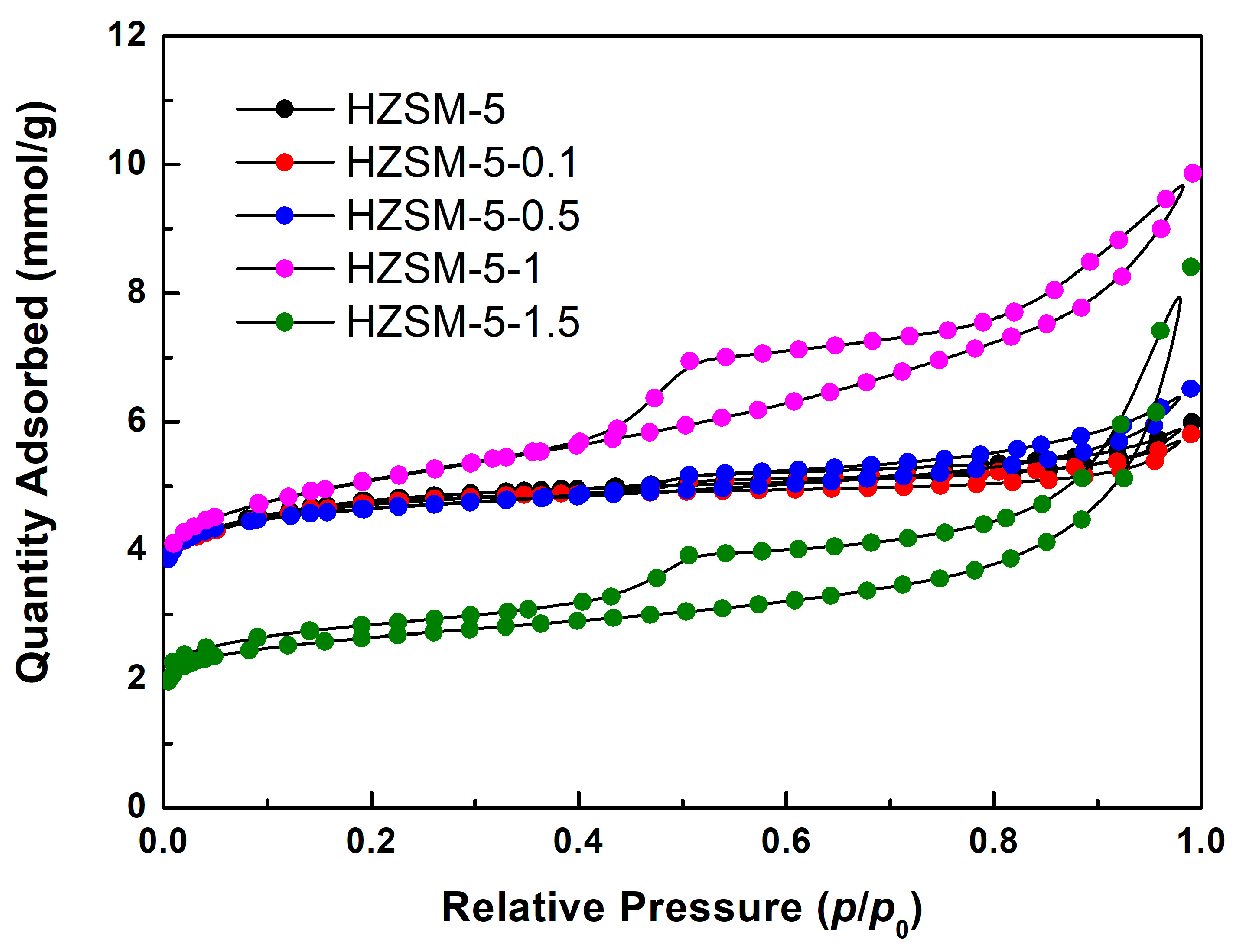
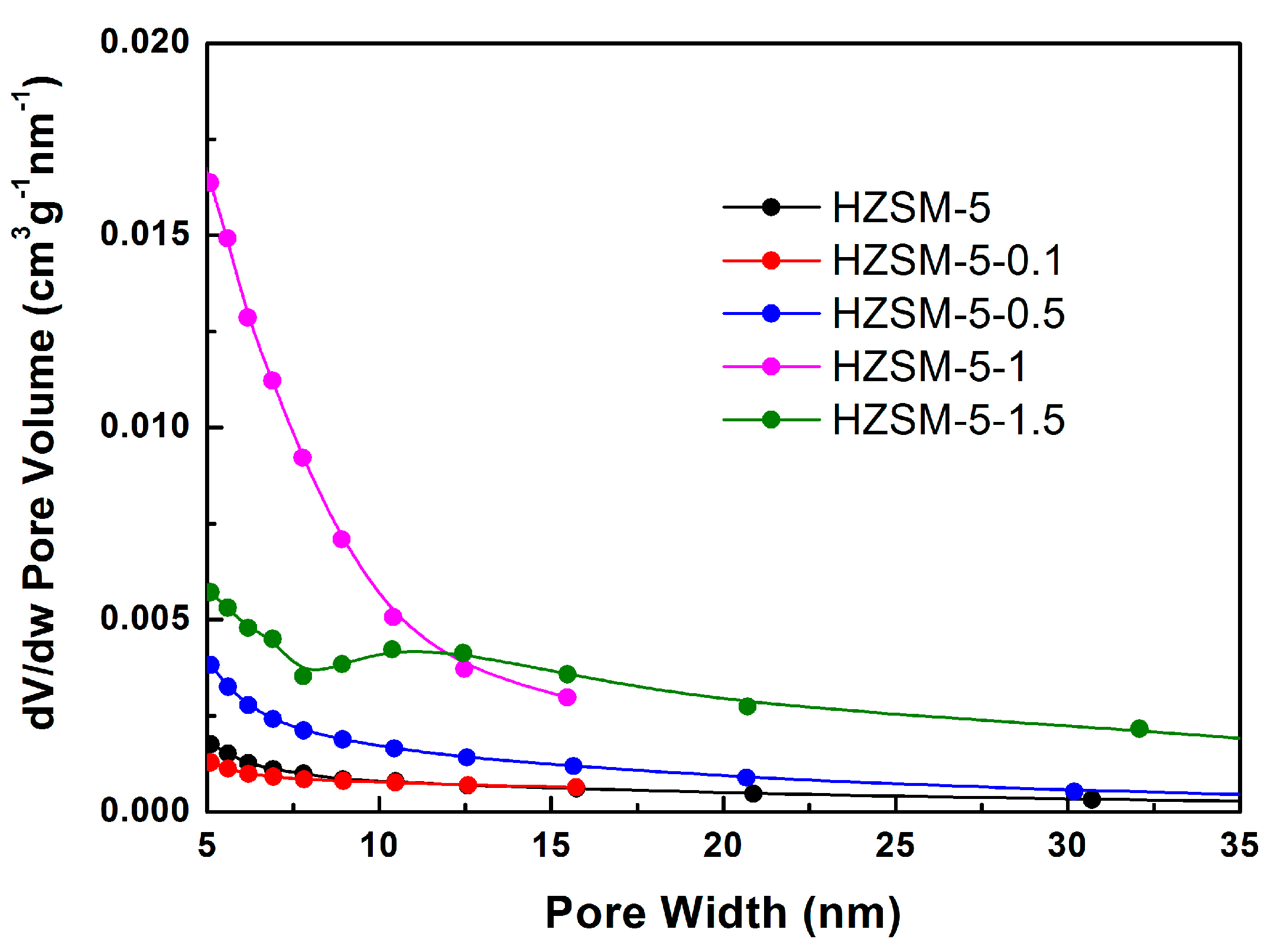
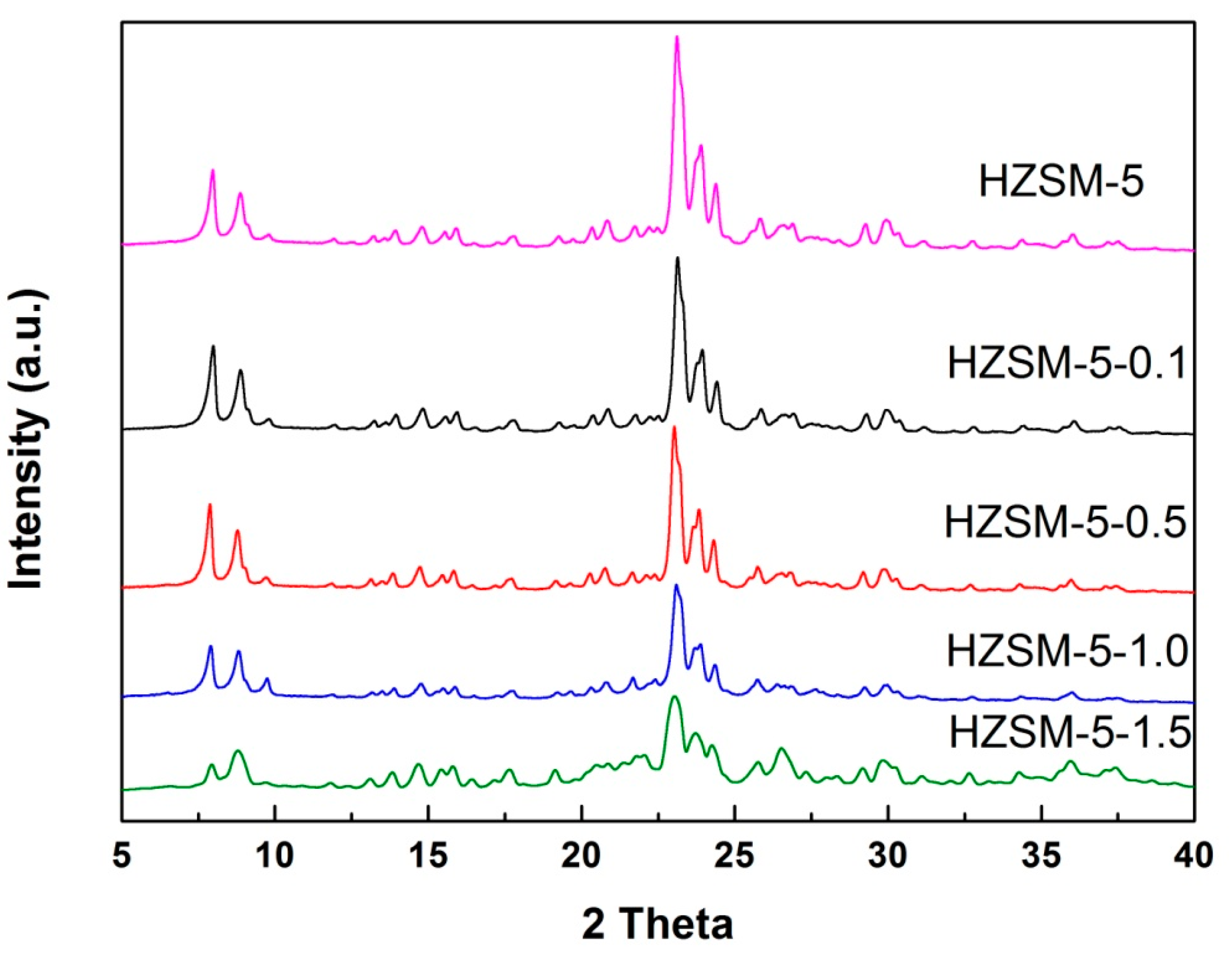
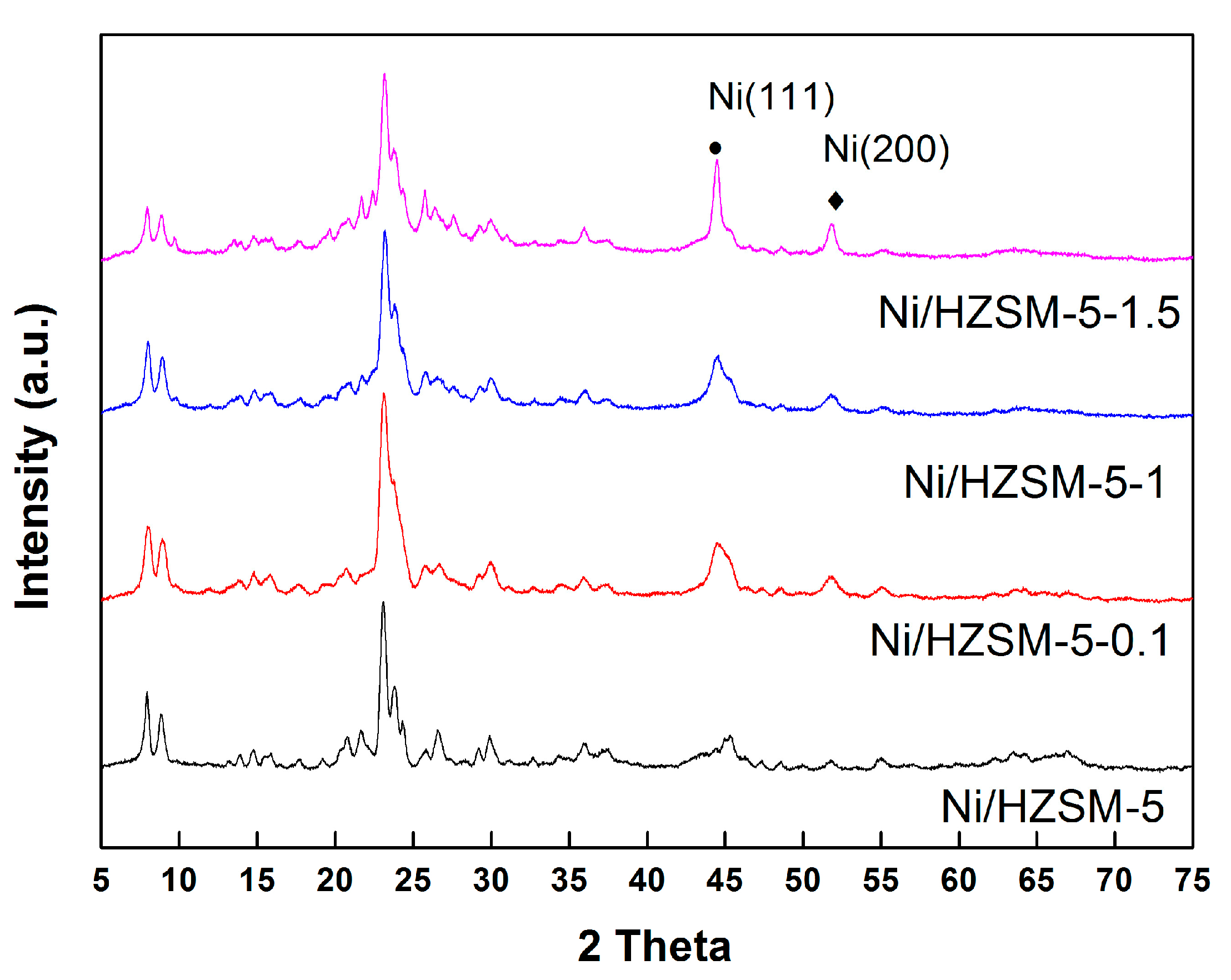

| Sample | NaOH Concentration (mol/L) | SMeso (m2/g) | SMicro (m2/g) | VMeso (cm3/g) | VMicro (cm3/g) | Rel. Crystal. (%) |
|---|---|---|---|---|---|---|
| HZSM-5 | 0 | 19 | 387 | 0.05 | 0.16 | 100 |
| HZSM-5-0.1 | 0.1 | 13 | 388 | 0.04 | 0.16 | 83 |
| HZSM-5-0.5 | 0.5 | 35 | 371 | 0.08 | 0.15 | 78 |
| HZSM-5-1 | 1 | 126 | 300 | 0.21 | 0.12 | 54 |
| HZSM-5-1.5 | 1.5 | 63 | 161 | 0.22 | 0.06 | 45 |
| Catalyst | Si/Al (mol/mol) a | dNi (nm) b | SBET (m2/g) |
|---|---|---|---|
| Ni/HZSM-5 | 22 | 8–11 | 259 |
| Ni/HZSM-5-0.1 | - | - | 284 |
| Ni/HZSM-5-1 | 14.2 | 8–11 | 232 |
| Ni/HZSM-1.5 | - | - | 286 |
| Catalyst | Atmosphere | Conversion (%) | Yield (wt %) | |||
|---|---|---|---|---|---|---|
| Oxygenates | C12 | C11 | Cracking | |||
| Ni/HZSM-5 | N2 | 25.4 | 11.8 | - | 2.5 | - |
| Ni/HZSM-5-0.1 | N2 | 39.6 | 16.9 | - | 4.3 | - |
| Ni/HZSM-5-0.5 | N2 | 54.2 | 21.0 | - | 5.2 | - |
| Ni/HZSM-5-1 | N2 | 61.5 | 26.3 | - | 5.9 | - |
| Ni/HZSM-1.5 | N2 | 50.5 | 19.6 | - | 6.8 | - |
| Ni/HZSM-5 | H2 | 86.2 | 6.4 | 26.3 | 18.6 | 4.4 |
| Ni/HZSM-5-0.1 | H2 | 87.6 | 3.6 | 26.3 | 19.3 | 4.7 |
| Ni/HZSM-5-0.5 | H2 | 87.8 | 6.2 | 28.6 | 21.0 | 4.2 |
| Ni/HZSM-5-1 | H2 | 89.8 | 2.6 | 37.6 | 27.7 | 10 |
| Ni/HZSM-1.5 | H2 | 68.7 | 4.6 | 13.2 | 9.1 | 4.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Bi, Y.; Xia, X.; Chen, H.; Hu, J. Hydrodeoxygenation of Methyl Laurate over Ni Catalysts Supported on Hierarchical HZSM-5 Zeolite. Catalysts 2017, 7, 383. https://doi.org/10.3390/catal7120383
Li N, Bi Y, Xia X, Chen H, Hu J. Hydrodeoxygenation of Methyl Laurate over Ni Catalysts Supported on Hierarchical HZSM-5 Zeolite. Catalysts. 2017; 7(12):383. https://doi.org/10.3390/catal7120383
Chicago/Turabian StyleLi, Nana, Yadong Bi, Xiaoqiang Xia, Hui Chen, and Jianli Hu. 2017. "Hydrodeoxygenation of Methyl Laurate over Ni Catalysts Supported on Hierarchical HZSM-5 Zeolite" Catalysts 7, no. 12: 383. https://doi.org/10.3390/catal7120383




