Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation
Abstract
:1. Introduction
2. Monoliths
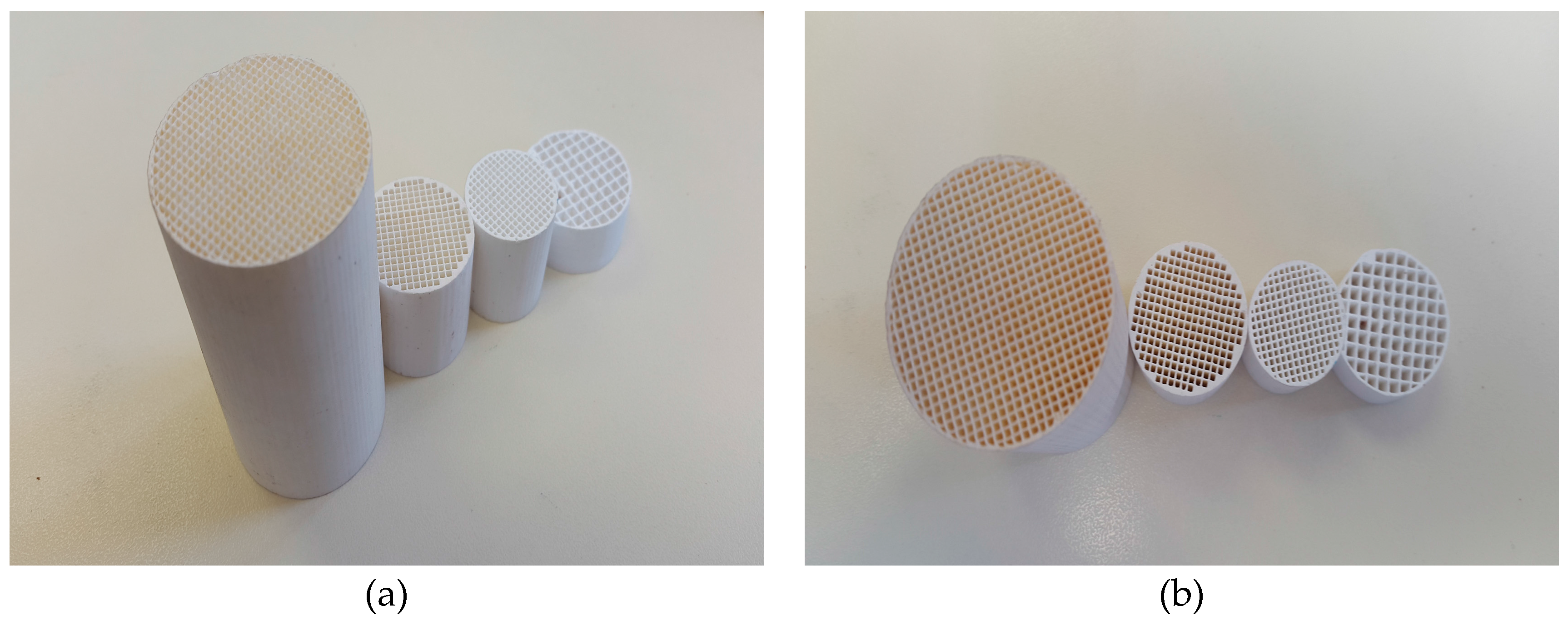
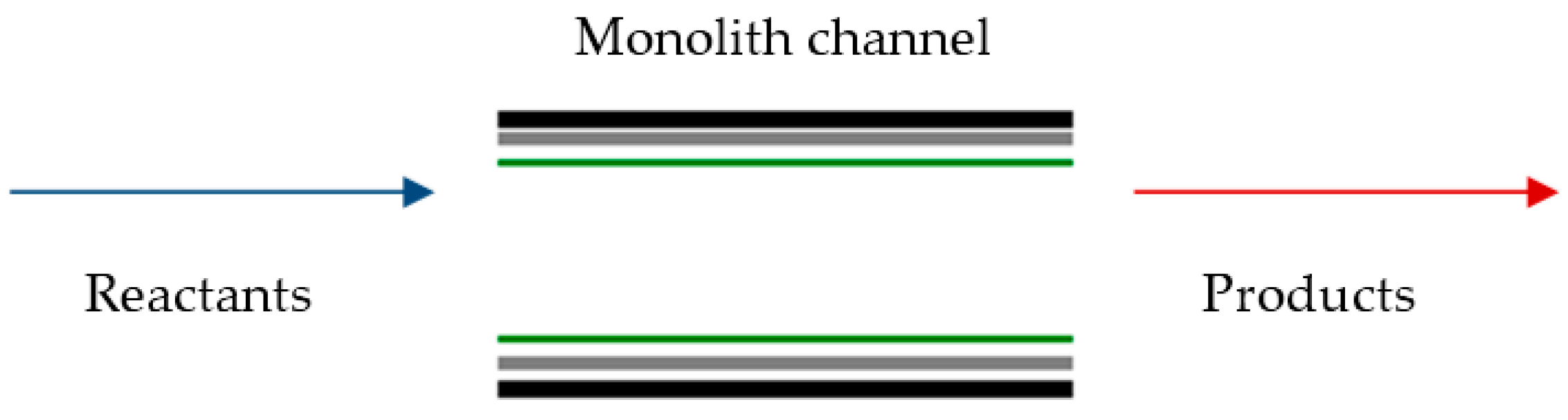
2.1. Monolith Basics
2.2. Preparation of Monoliths
2.2.1. Ceramic Monoliths
2.2.2. Metallic Monoliths
2.3. Preparation of Monolithic Catalysts
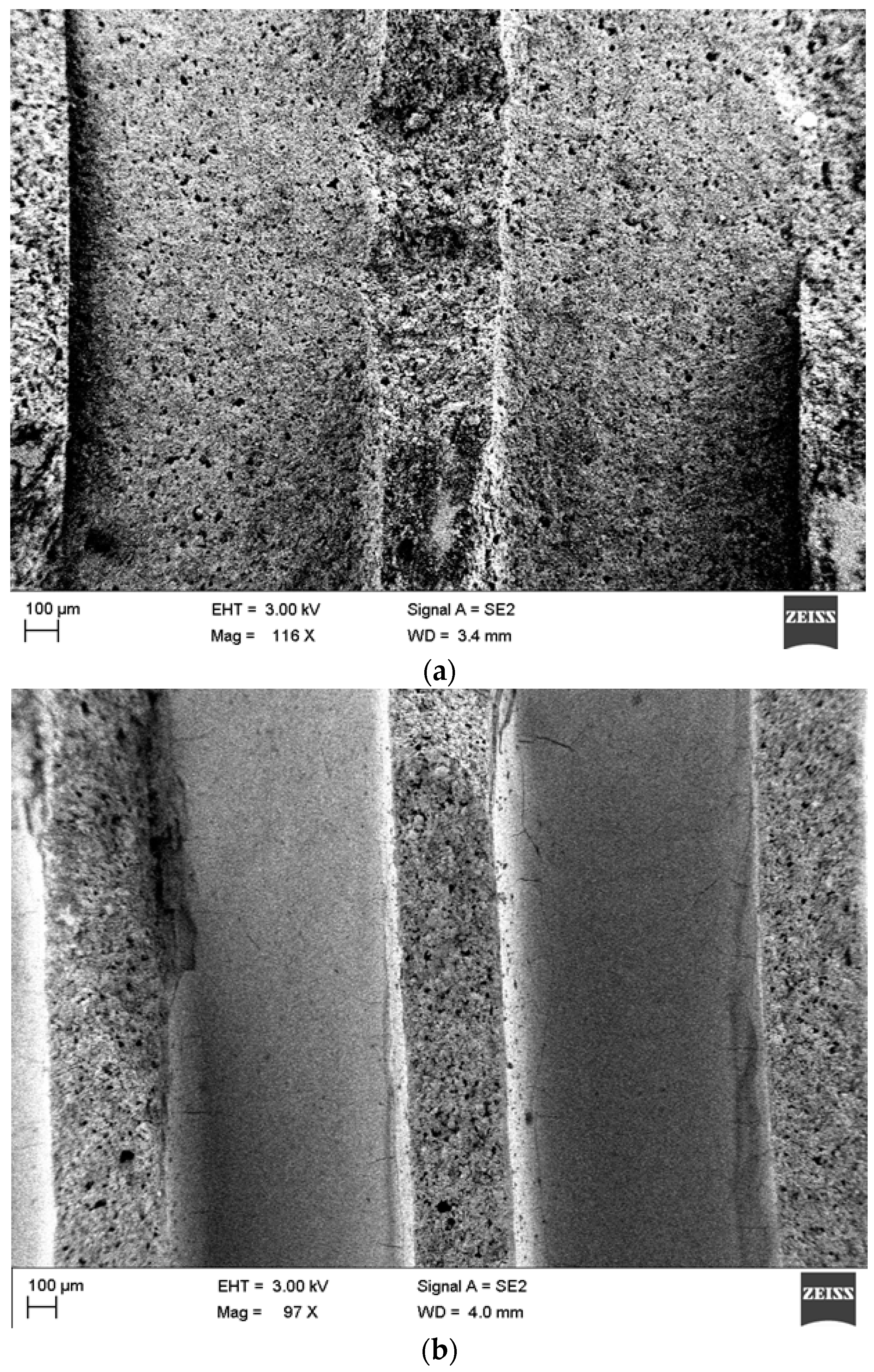
2.3.1. Coating γ-Alumina as a Secondary Support
Colloidal Coating
Sol-Gel
Slurry Coating
Coating Alumina on a Metallic Surface
2.3.2. Depositing Metals onto Monoliths
2.3.3. Zeolite-Based Monoliths
Coating Zeolites onto Monoliths
Extruding Zeolites into Monoliths
2.3.4. Carbon-Based Monoliths
Coating Carbon Materials onto Monoliths
Extruding Carbon into Monoliths
2.4. Monolithic Catalysts in Oxidation Catalysis
2.4.1. The Oxidation of Alkanes
2.4.2. Catalytic Combustion
2.4.3. Preferential Oxidation of Carbon Monoxide
3. Conclusions and Perspective
Acknowledgments
Conflicts of Interest
References
- Avila, P.; Montes, M.; Miro, E.E. Monolithic reactors for environmental applications: A review on preparation technologies. Chem. Eng. J. 2005, 109, 11–36. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Heck, R.M. Catalytic converters: State of the art and perspectives. Catal. Today 1999, 51, 351–360. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J. Automobile exhaust catalysts. Appl. Catal. A 2001, 221, 443–457. [Google Scholar] [CrossRef]
- Heck, R.M. Catalytic abatement of nitrogen oxides–stationary applications. Catal. Today 1999, 53, 519–523. [Google Scholar] [CrossRef]
- Heck, R.M.; Gulati, S.; Farruto, R.J. The application of monoliths for gas phase catalytic reactions. Chem. Eng. J. 2001, 82, 149–156. [Google Scholar] [CrossRef]
- Geus, J.W.; van Giezen, J.C. Monoliths in catalytic oxidation. Catal. Today 1999, 47, 169–180. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Monoliths in heterogeneous catalysis. Catal. Rev. 1994, 36, 179–270. [Google Scholar] [CrossRef]
- Carty, W.M.; Lednor, P.W. Monolithic ceramics and heterogeneous catalysts: Honeycombs and foams. Curr. Opin. Solid State Mater. Sci. 1996, 1, 88–95. [Google Scholar] [CrossRef]
- Forzatti, P.; Ballardini, D.; Sighicelli, L. Preparation and characterization of extruded monolithic ceramic catalysts. Catal. Today 1998, 41, 87–94. [Google Scholar] [CrossRef]
- Xiaoding, X.; Vonk, H.; Cybulski, A.; Moulijn, J.A. Alumina washcoating and metal deposition of ceramic monoliths. In Studies in Surface Science and Catalysis; Poncelet, G., Martens, J., Delmon, B., Jacobs, P.A., Grange, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 91, pp. 1069–1078. [Google Scholar]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Carbon coating of ceramic monolithic substrates. In Studies in Surface Science and Catalysis; Delmon, B., Jacobs, P.A., Maggi, R., Martens, J.A., Grange, P., Poncelet, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 118, pp. 175–183. [Google Scholar]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. Sci. Eng. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. Mass and heat transfer effects in catalytic monolith reactors. Chem. Eng. Sci. 1994, 49, 3587–3599. [Google Scholar] [CrossRef]
- Tomasic, V.; Jovic, F. State-of-the-art in the monolithic catalyst/reactors. Appl. Catal. A 2006, 311, 112–121. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E.; Forzatti, P. Modelling op catalytic combustors for gas turbine applications. Catal. Today 1993, 17, 237–249. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E. Theoretical analysis of mass and heat transfer in monolith catalysts with triangular channels. Chem. Eng. Sci. 1997, 52, 3521–3526. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Structured Catalysts and Reactors, 2nd ed.; CRC Press, Taylor and Francis Group: London, UK, 2005; p. 856. [Google Scholar]
- Williams, J.L. Monolith structures, materials, properties and uses. Catal. Today 2001, 69, 3–9. [Google Scholar] [CrossRef]
- Roy, S.; Heibel, K.A.; Liu, W.; Boger, T. Design of monolithic catalysts for multiphase reactions. Chem. Eng. Sci. 2004, 59, 957–966. [Google Scholar] [CrossRef]
- Boger, T.; Heibel, A.K.; Sorensen, C.M. Monolithic catalysts for the chemical industry. Ind. Eng. Chem. Res. 2004, 43, 4602–4611. [Google Scholar] [CrossRef]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Optimization of geometric properties of a monolithic catalyst for the selective hydrogenation of phenylacetylene. Ind. Eng. Chem. Res. 2001, 40, 2801–2809. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Heiszwolf, J.J. Multiphase monolith reactors: Chemical reaction engineering of segmented flow in microchannels. Chem. Eng. Sci. 2005, 60, 5895–5916. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A. Shouldn’t catalysts shape up?: Structured reactors in general and gas–liquid monolith reactors in particular. Catal. Today 2006, 111, 111–118. [Google Scholar] [CrossRef]
- Roy, S.; Bauer, T.; Al-Dahhan, M.; Lehner, P.; Turek, T. Monoliths as multiphase reactors: A review. AIChE J. 2004, 50, 2918–2938. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Kleijn, C.R.; Heiszwolf, J.J. Inertial and interfacial effects on pressure drop of Taylor flow in capillaries. AIChE J. 2005, 51, 2428–2440. [Google Scholar] [CrossRef]
- Hayes, R.E.; Kolaczkowski, S.T. A study of Nusselt and Sherwood numbers in a monolith reactor. Catal. Today 1999, 47, 295–303. [Google Scholar] [CrossRef]
- Hayes, R.E.; Liu, B.; Moxom, R.; Votsmeier, M. The effect of washcoat geometry on mass transfer in monolith reactors. Chem. Eng. Sci. 2004, 59, 3169–3181. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Mass-transfer characterization of metallic foams as supports for structured catalysts. Ind. Eng. Chem. Res. 2005, 44, 4993–5002. [Google Scholar] [CrossRef]
- Giani, L.; Groppi, G.; Tronconi, E. Heat transfer characterization of metallic foams. Ind. Eng. Chem. Res. 2005, 44, 9078–9085. [Google Scholar] [CrossRef]
- Groppi, G.; Giani, L.; Tronconi, E. Generalized correlation for gas/solid mass-transfer coefficients in metallic and ceramic foams. Ind. Eng. Chem. Res. 2007, 46, 3955–3958. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E. Simulation of structured catalytic reactors with enhanced thermal conductivity for selective oxidation reactions. Catal. Today 2001, 69, 63–73. [Google Scholar] [CrossRef]
- Votruba, J.; Mikuš, O.; Nguen, K.; Hlaváček, V.; Skřivánek, J. Heat and mass transfer in honeycomb catalysts—II. Chem. Eng. Sci. 1975, 30, 201–206. [Google Scholar] [CrossRef]
- Votruba, J.; Sinkule, J.; Hlaváček, V.; Skřivánek, J. Heat and mass transfer in monolithic honeycomb catalysts—I. Chem. Eng. Sci. 1975, 30, 117–123. [Google Scholar] [CrossRef]
- Sinkule, J.; Hlaváček, V. Heat and mass transfer in monolithic honeycomb catalysts—III. Chem. Eng. Sci. 1978, 33, 839–845. [Google Scholar] [CrossRef]
- Kapteijn, F.; Nijhuis, T.A.; Heiszwolf, J.J.; Mouljin, J.A. New non-traditional multiphase catalytic reactors based on monolithic structures. Catal. Today 2001, 66, 133–144. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Air Pollution Aspects of Emission Sources: Nitric Acid Manufacturing—A Bibliography with Abstracts; U.S. Air Pollution Control Office, Ed.; U.S. Environmental Protection Agency: Raleigh, NC, USA, 1971.
- Keith, C.D.; Schreuders, T.; Cunningham, C.E. Apparatus for Purifying Exhaust Gases of an Internal Combustion Engine. U.S. Patent 3,441,381, 29 April 1969. [Google Scholar]
- Keith, C.D.; Mooney, J.J.; Blamble, K.W. Catalytic Exhaust Purifier. U.S. Patent 3,597,165, 3 August 1971. [Google Scholar]
- Scheitlin, G.E.; Vautaw, R.L. Catalytic Converter. U.S. Patent 3,915,658, 28 October 1975. [Google Scholar]
- Soghrati, E.; Kazemeini, M.; Rashidi, A.M.; Jozani, K.J. Preparation and characterization of Co-Mo Catalyst supported on CNT coated cordierite monoliths utilized for naphta HDS process. Procedia Eng. 2012, 42, 1484–1492. [Google Scholar] [CrossRef]
- Mohino, F.; Martin, A.B.; Salerno, P.; Bahamonde, A.; Mendioroz, S. High surface area monoliths based on pillared clay materials as carriers for catalytic processes. Appl. Clay Sci. 2005, 29, 125–136. [Google Scholar] [CrossRef]
- Lundsager, C.B.; Murch, R.M. Ceramic Firing Process. U.S. Patent 3,985,846, 12 October 1976. [Google Scholar]
- Ohtaka, M.; Miyahara, K. Process for Firing Ceramic Honeycomb Structural Bodies. U.S. Patent 4,927,577, 22 May 1990. [Google Scholar]
- Coblenz, W.S. Fibrous Monolithic Ceramic and Method for Production. U.S. Patent 4,772,524, 20 September 1988. [Google Scholar]
- Popovic, D.; Halloran, J.W.; Hilmas, G.E.; Brady, G.A.; Somers, S.; Barda, A.; Zywicki, G. Process for Preparing Textured Ceramic Composites. U.S. Patent 5,645,781, 8 July 1997. [Google Scholar]
- Matsuhisa, T.; Soejima, S.; Yamamoto, N. Cordierite Ceramic Honeycomb and a Method for Producing the Same. U.S. Patent 4,295,892, 20 October 1981. [Google Scholar]
- Murtagh, M.J.; Limited, N.I. Method for Producing Cordierite Articles. U.S. Patent 5,141,686, 25 August 1992. [Google Scholar]
- Lundsager, C.B. Porous Ceramic Structure. U.S. Patent 3,963,504, 15 June 1976. [Google Scholar]
- Corbin, N.D.; Miller, B.J.; Sawicki, K.; Lucek, J.W.; Hannoosh, J.G. High Temperature Ceramic Composite. U.S. Patent 5,306,565, 26 April 1994. [Google Scholar]
- De Luca, J.P.; Campbell, L.E. Monolithic catalyst supports. In Advanced Materials in Catalysis; Burton, J.J., Garten, K.L., Eds.; Academic Press: London, UK, 1977. [Google Scholar]
- Lachman, I.M.; McNally, R.N. High-Temperature Monolithic Supports for Automobile Exhaust Catalysis, 2nd ed.; John Wiley and Sons Incorporated: Singapore, 1981; Volume 2. [Google Scholar]
- Lachman, I.M.; McNally, R.M. Monolithic honeycomb supports for catalysis. Chem. Eng. J. 1985, 81, 29–31. [Google Scholar]
- Lachman, I.M. Ceramic honeycombs for catalysis and industrial applications. Sprechsaal 1986, 119, 1116–1119. [Google Scholar]
- Lachman, I.M.; Williams, J.L. Extruded monolithic catalyst supports. Catal. Today 1992, 14, 317–329. [Google Scholar] [CrossRef]
- Brockmeyer, J.W. Ceramic Foam Filter. U.S. Patent 4,343,704, 10 August 1982. [Google Scholar]
- Schmidt, H.; Zievers, J.F.; Eggerstedt, P. Composite for Filtering Hot Gas and Method of Its Manufacture. U.S. Patent 5,071,457, 10 December 1991. [Google Scholar]
- Buciuman, F.C.; Kraushaar-Czarnetzki, B. Ceramic foam monoliths as catalyst carriers. 1. Adjustment and description of the morphology. Ind. Eng. Chem. Res. 2003, 42, 1863–1869. [Google Scholar] [CrossRef]
- Richardson, J.T.; Peng, Y.; Remue, D. Properties of ceramic foam catalyst supports: Pressure drop. Appl. Catal. A 2000, 204, 19–32. [Google Scholar] [CrossRef]
- Twigg, M.V.; Richardson, J.T. Theory and applications of ceramic foam catalysts. Chem. Eng. Res. Des. 2002, 80, 183–189. [Google Scholar] [CrossRef]
- Schwartzwalder, K.; Somers, A.V. Method of Making Porous Ceramic Articles. U.S. Patent 3,090,094, 21 May 1963. [Google Scholar]
- Winkler, J. Method of Producing an Inorganic Foam and Product. U.S. Patent 3,408,180, 29 October 1968. [Google Scholar]
- Kesten, N.P.; Schwartzwalder, K. Ceramic Foam. U.S. Patent 3,451,841, 24 June 1969. [Google Scholar]
- Twigg, M.V.; Sengelow, W.M. Foam Catalysts, Method of Manufacture and Method of Using. U.S. Patent 4,810,685, 7 March 1989. [Google Scholar]
- Ceramic Functional Structures for Tailored Chemical Reactors. Available online: https://www.hzdr.de/db/Cms?pOid=42526&pNid=3367 (accessed on 2 October 2016).
- Pugh, S.F. Supports for Catalyst Materials. U.S. Patent 3,920,583, 18 November 1975. [Google Scholar]
- Webster, D.E.; Rouse, I.M. Production of Formaldehyde. U.S. Patent 4,208,353, 17 June 1980. [Google Scholar]
- Xu, X.; Moulijin, J.A. Transformation of a structured carrier into structured catalyst. In Structured Catalysts and Reactors; Cybulski, A., Moulijn, J.A., Eds.; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Noakes, M.L.; Caesar, W.G.; Lloyd, H. Fabricating Bodies. U.S. Patent 3,966,646, 29 June 1976. [Google Scholar]
- Li, Y.; Wang, Y.; Hong, X.; Zhang, Z.; Fang, Z.; Pan, Y.; Lu, Y.; Han, Z. Partial oxidation of methane to syngas over nickel monolithic catalysts. AIChE J. 2006, 52, 4276–4279. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Z.; Hong, X.; Liu, Y. Oxidative reformings of methane to syngas with steam and CO2 catalyzed by metallic Ni based monolithic catalysts. Catal. Commun. 2008, 9, 1040–1044. [Google Scholar] [CrossRef]
- Almeida, L.C.; González, O.; Sanz, O.; Paul, A.; Centeno, M.A.; Odriozola, J.A.; Montes, M. Fischer-tropsch catalyst deposition on metallic structured supports. In Studies in Surface Science and Catalysis; Fábio Bellot Noronha, M.S., Eduardo Falabella, S.-A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 167, pp. 79–84. [Google Scholar]
- Martínez, T.L.M.; Frías, D.M.; Centeno, M.A.; Paúl, A.; Montes, M.; Odriozola, J.A. Preparation of Au-CeO2 and Au-Al2O3/AISI 304 austenitic stainless steel monoliths and their performance in the catalytic oxidation of CO. Chem. Eng. J. 2008, 136, 390–397. [Google Scholar] [CrossRef]
- Martínez T, L.M.; Sanz, O.; Centeno, M.A.; Odriozola, J.A. AISI 304 austenitic stainless steel monoliths: Modification of the oxidation layer and catalytic coatings after deposition and its catalytic implications. Chem. Eng. J. 2010, 162, 1082–1090. [Google Scholar] [CrossRef]
- Martínez T, L.M.; Sanz, O.; Domínguez, M.I.; Centeno, M.A.; Odriozola, J.A. AISI 304 austenitic stainless steels monoliths for catalytic applications. Chem. Eng. J. 2009, 148, 191–200. [Google Scholar] [CrossRef]
- Sanz, O.; Almeida, L.C.; Zamaro, J.M.; Ulla, M.A.; Miró, E.E.; Montes, M. Washcoating of Pt-ZSM5 onto aluminium foams. Appl. Catal. B 2008, 78, 166–175. [Google Scholar] [CrossRef]
- Sanz, O.; Javier Echave, F.; Sánchez, M.; Monzón, A.; Montes, M. Aluminium foams as structured supports for volatile organic compounds (VOCs) oxidation. Appl. Catal. A 2008, 340, 125–132. [Google Scholar] [CrossRef]
- Aguero, F.N.; Barbero, B.P.; Sanz, O.; Lozano, F.J.E.; Montes, M.; Cadus, L.E. Influence of the support on MnOx metallic monoliths for the combustion of volatile organic compounds. Ind. Eng. Chem. Res. 2010, 49, 1663–1668. [Google Scholar] [CrossRef]
- Serres, T.; Dreibine, L.; Schuurman, Y. Synthesis of enamel-protected catalysts for microchannel reactors: Application to methane oxidative coupling. Chem. Eng. J. 2012, 213, 31–40. [Google Scholar] [CrossRef]
- Domínguez, M.I.; Pérez, A.; Centeno, M.A.; Odriozola, J.A. Metallic structured catalysts: Influence of the substrate on the catalytic activity. Appl. Catal. A 2014, 478, 45–57. [Google Scholar] [CrossRef]
- Sheller, D.T. Process for Welding a Stack of Thin Metal Sheets. U.S. Patent 5,532,453, 2 July 1996. [Google Scholar]
- Wieres, L. Method and Apparatus for Applying Brazing Material to a Metal Honeycomb Body. U.S. Patent 5,431,330, 11 July 1995. [Google Scholar]
- Wolfgang, M.; Wieres, L. Process for the Production of a Honeycomb Body from Two Differently Constructed Kinds of Sheet Metal Layers. U.S. Patent 6,095,406, 1 August 2000. [Google Scholar]
- High Machine Strength Metallic Monolith. Available online: http://wisdom-sh2015.en.made-in-china.com/product/NKZEBLPYZnRy/China-High-Machine-Strength-Metallic-Monolith.html (accessed on 9 September 2016).
- Oberlander, K. Applied Industrial Catalysis; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Wefers, K. Alumina Chemicals: Science and Technology Handbook; The American Ceramic Society Incorporated: Westerville, OH, USA, 1990. [Google Scholar]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hart, R.D.; Winter, K.; Studer, A.J.; Hunter, B.A.; Hanna, J.V. Boehmite derived γ-alumina system. 1. Structural evolution with temperature, with the identification and structural determination of a new transition phase, γ′-alumina. Chem. Mater. 2003, 16, 220–236. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of spinel. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Ionescu, A.; Allouche, A.; Aycard, J.P.; Rajzmann, M.; Hutschka, F. Study of γ-alumina surface reactivity: Adsorption of water and hydrogen sulfide on octahedral aluminum sites. J. Phys. Chem. B 2002, 106, 9359–9366. [Google Scholar] [CrossRef]
- Villegas, L.; Masset, F.; Guilhaume, N. Wet impregnation of alumina-washcoated monoliths: Effect of the drying procedure on Ni distribution and on autothermal reforming activity. Appl. Catal. A 2007, 320, 43–55. [Google Scholar] [CrossRef]
- Su, J.; Liu, Q.; Liu, Z.; Huang, Z. Honeycomb CuO/Al2O3/cordierite catalyst for selective catalytic reduction of NO by NH3-effect of Al2O3 coating. Ind. Eng. Chem. Res. 2008, 47, 4295–4301. [Google Scholar] [CrossRef]
- Fauchadour, D.; Kolenda, F.; Rouleau, L.; Barré, L.; Normand, L. Peptization mechanisms of boehmite used as precursors for catalysts. In Studies in Surface Science and Catalysis; Gaigneaux, E., De Vos, D.E., Grange, P., Jacobs, P.A., Martens, J.A., Ruiz, P., Poncelet, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 143, pp. 453–461. [Google Scholar]
- Agrafiotis, C.; Tsetsekou, A.; Ekonomakou, A. The effect of particle size on the adhesion properties of oxide washcoats on cordierite honeycombs. J. Mater. Sci. Lett. 1999, 18, 1421–1424. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A. The effect of processing parameters on the properties of γ-alumina washcoats deposited on ceramic honeycombs. J. Mater. Sci. 2000, 35, 951–960. [Google Scholar] [CrossRef]
- Jiang, P.; Lu, G.; Guo, Y.; Guo, Y.; Zhang, S.; Wang, X. Preparation and properties of a γ-Al2O3 washcoat deposited on a ceramic honeycomb. Surf. Coat. Technol. 2005, 190, 314–320. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tsetsekou, A. The effect of powder characteristics on washcoat quality. Part I: Alumina washcoats. J. Eur. Ceram. Soc. 2000, 20, 815–824. [Google Scholar] [CrossRef]
- Beauseigneur, P.A.; Lachman, I.M.; Patil, M.D.; Swaroop, S.H. Pore Impregnated Catalyst Device. U.S. Patent 5,334,570, 2 August 1994. [Google Scholar]
- Larsson, L.B.; Löwendahl, L.O.; Otterstedt, J.-E. The effect of the chemical nature of the wash-coat on the catalytic performance of CO oxidation catalysts of monolith type. In Studies in Surface Science and Catalysis; Crucq, A., Frennet, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 30, pp. 333–344. [Google Scholar]
- Morgado, E., Jr.; Lam, Y.L.; Menezes, S.M.C.; Nazar, L.F. Characterization of peptized boehmite systems: An 27Al nuclear magnetic resonance study. J. Colloid Interface Sci. 1995, 176, 432–441. [Google Scholar] [CrossRef]
- Özdemir, S.; Önsan, Z.I.; Yildirım, R. Selective CO oxidation over monolithic Au-MgO/Al2O3 catalysts. J. Chem. Technol. Biotechnol. 2012, 87, 58–64. [Google Scholar] [CrossRef]
- Barbero, B.P.; Costa-Almeida, L.; Sanz, O.; Morales, M.R.; Cadus, L.E.; Montes, M. Washcoating of metallic monoliths with a MnCu catalyst for catalytic combustion of volatile organic compounds. Chem. Eng. J. 2008, 139, 430–435. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Schmidt, H. Chemistry of material preparation by the sol-gel process. J. Non-Cryst. Solids 1988, 100, 51–64. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Kvande, I.; Chen, D.; Rønning, M. Carbon nanofibers uniformly grown on γ-alumina washcoated cordierite monoliths. Adv. Mater. 2006, 18, 1589–1592. [Google Scholar] [CrossRef]
- Blachou, V.; Goula, D.; Phillippopulos, C. Wet milling of alumina and preparation of slurries for monolithic structures impregnation. Ind. Eng. Chem. Res. 1922, 31, 364–369. [Google Scholar] [CrossRef]
- Adegbite, S.A. Particle characterisation and grinding behaviour of gamma-alumina slurries prepared in a stirred media mill. J. Mater. Sci. Res. 2013, 2, 135–147. [Google Scholar] [CrossRef]
- González-Velasco, J.R.; Gutiérrez-Ortiz, M.A.; Marc, J.L.; Botas, J.A.; González-Marcos, M.P.; Blanchard, G. Pt/Ce0.68Zr0.32O2 washcoated monoliths for automotive emission control. Ind. Eng. Chem. Res. 2002, 42, 311–317. [Google Scholar] [CrossRef]
- Mogalicherla, A.K.; Kunzru, D. The effect of prewetting on the loading of γ-alumina washcoated cordierite monolith. Int. J. Appl. Ceram. Technol. 2011, 8, 430–436. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Maury, F. Al2O3 coatings on stainless steel from Al metal-organic chemical vapor deposition and thermal treatments. Surf. Coat. Technol. 2000, 125, 419–423. [Google Scholar] [CrossRef]
- Ferrandon, M.; Berg, M.; Björnbom, E. Thermal stability of metal-supported catalysts for reduction of cold-start emissions in a wood-fired domestic boiler. Catal. Today 1999, 53, 647–659. [Google Scholar] [CrossRef]
- Valentini, M.; Groppi, G.; Cristiani, C.; Levi, M.; Tronconi, E.; Forzatti, P. The deposition of γ-Al2O3 layers on ceramic and metallic supports for the preparation of structured catalysts. Catal. Today 2001, 69, 307–314. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Yue, P.L.; Lu, G.Q.; Greenfield, P.F. Synthesis of anatase TiO2 supported on porous solids by chemical vapor deposition. Catal. Today 2001, 68, 173–182. [Google Scholar] [CrossRef]
- Burgos, N.; Paulis, M.; Montes, M. Preparation of Al2O3/Al monoliths by anodisation of aluminium as structured catalytic supports. J. Mater. Chem. 2003, 13, 1458–1467. [Google Scholar] [CrossRef]
- Sanz, O.; Martínez T, L.M.; Echave, F.J.; Domínguez, M.I.; Centeno, M.A.; Odriozola, J.A.; Montes, M. Aluminium anodisation for Au-CeO2/Al2O3-Al monoliths preparation. Chem. Eng. J. 2009, 151, 324–332. [Google Scholar]
- Jia, J.; Zhou, J.; Zhang, J.; Yuan, Z.; Wang, S. The influence of preparative parameters on the adhesion of alumina washcoats deposited on metallic supports. Appl. Surf. Sci. 2007, 253, 9099–9104. [Google Scholar] [CrossRef]
- Zwinkels, M.F.M.; Järs, S.G.; Govind Menon, P. Preparation of combustion catalysts by wash coating alumina whiskers-covered metal monoliths using a sol-gel method. In Studies in Surface Science and Catalysis; Poncelet, G., Martens, J., Delmon, B., Jacobs, P.A., Grange, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 91, pp. 85–94. [Google Scholar]
- Bröcker, F.J.; Schwab, E. Impregnating Process for the Application of Active Composition to Structured Supports or Monoliths. U.S. Patent 6,436,873, 20 August 2002. [Google Scholar]
- Euzen, P.; Tocque, E.; Rebours, S.; Mabilon, G. Combustion Catalyst and Combustion Process Using Such a Catalyst. U.S. Patent 6,284,210, 4 September 2001. [Google Scholar]
- Guibard, I.; Durand, D.; Mabilon, G.; des Courtils, N. Catalyst for Treatment of Exhaust Gases from an Internal Combustion Engine. U.S. Patent 5,643,543, 1 July 1997. [Google Scholar]
- Shimrock, T.; Taylor, R.D.; Collins, J.M., Jr. Method of Impregnating Ceramic Monolithic Structures with Predetermined Amounts of Catalyst. U.S. Patent 4,550,034, 25 October 1985. [Google Scholar]
- Xiaodong, M.; Hongwen, S.; Quan, S.; Hongwen, G.; Bo, F.; Shuo, Z. Hierarchically porous Fe2O3/CuO composite monoliths: Synthesis and characterization. J. Nat. Gas Chem. 2010, 19, 589–592. [Google Scholar]
- Thimmaraju, N.; Pratap, S.R.; Senthilkumar, M.; Shamshuddin, S.Z.M. Honeycomb monolith coated with Mo(VI)/ZrO2 as a versatile catalyst system for liquid phase transesterificaiton. J. Korean Chem. Soc. 2012, 56, 563–570. [Google Scholar] [CrossRef]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Monolithic catalysts—Non-uniform active phase distribution by impregnation. Appl. Catal. A 2001, 213, 179–187. [Google Scholar] [CrossRef]
- Berčič, G. Through control of wetting and drying conditions of monolithic supports toward a uniform catalyst distribution. Dry. Technol. 2015, 33, 72–82. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.T.; Du, L. Development of nano-NiO/Al2O3 catalyst to be used for tar removal in biomass gasification. Environ. Sci. Technol. 2008, 42, 6224–6229. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Pirone, R.; Russo, G.; Turco, M. Methane combustion on perovskites-based structured catalysts. Catal. Today 2000, 59, 19–31. [Google Scholar] [CrossRef]
- Carnö, J.; Ferrandon, M.; Björnbom, E.; Järås, S. Mixed manganese oxide/platinum catalysts for total oxidation of model gas from wood boilers. Appl. Catal. A 1997, 155, 265–281. [Google Scholar] [CrossRef]
- Barrio, I.; Legórburu, I.; Montes, M.; Domínguez, M.; Centeno, M.; Odriozola, J. New redox deposition-precipitation method for preparation of supported manganese oxide catalysts. Catal. Lett. 2005, 101, 151–157. [Google Scholar] [CrossRef]
- Ouzzine, M.; Cifredo, G.A.; Gatica, J.M.; Harti, S.; Chafik, T.; Vidal, H. Original carbon-based honeycomb monoliths as support of Cu or Mn catalysts for low-temperature SCR of NO: Effects of preparation variables. Appl. Catal. A 2008, 342, 150–158. [Google Scholar] [CrossRef]
- Pinna, F. Supported metal catalysts preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Ran, R.; Xiong, G.; Yang, W. An in-situ modified sol-gel process for monolith catalyst preparation used in the partial oxidation of methane. J. Mater. Chem. 2002, 12, 1854–1859. [Google Scholar] [CrossRef]
- Sharma, S.; Hegde, M.S. Single step direct coating of 3-way catalysts on cordierite monolith by solution combustion method: High catalytic activity of Ce0.98Pd0.02O2-δ. Catal. Lett. 2006, 112, 69–75. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Stöcker, M. Gas phase catalysis by zeolites. Microporous Mesoporous Mater. 2005, 82, 257–292. [Google Scholar] [CrossRef]
- Antia, J.E.; Israni, K.; Govind, R. n-Hexane cracking on binderless zeolite HZSM-5 coated monolithic reactors. Appl. Catal. A 1997, 159, 89–99. [Google Scholar] [CrossRef]
- Jansen, J.C.; Koegler, J.H.; van Bekkum, H.; Calis, H.P.A.; van den Bleek, C.M.; Kapteijn, F.; Moulijn, J.A.; Geus, E.R.; van der Puil, N. Zeolitic coatings and their potential use in catalysis. Microporous Mesoporous Mater. 1998, 21, 213–226. [Google Scholar] [CrossRef]
- Suppiah, S. Supported High Silica Zeolites. U.S. Patent 5,157,005, 20 October 1992. [Google Scholar]
- Abe, F.; Noda, K. Heater and Catalytic Converter. U.S. Patent 5,296,198, 22 March 1994. [Google Scholar]
- Dessau, R.M.; Grasselli, R.K.; Lago, R.M.; Tsikoyiannis, J.G. Processes for Converting Feedstock Organic Compounds. U.S. Patent 5,316,661, 31 May 1994. [Google Scholar]
- Grasselli, R.K.; Lago, R.M.; Socha, R.F.; Tsikoyiannis, J.G. NOx Abatement Process. U.S. Patent 5,374,410, 20 December 1994. [Google Scholar]
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Ulla, M.A.; Miró, E.E. Zeolite washcoating onto cordierite honeycomb reactors for environmental applications. Chem. Eng. J. 2005, 106, 25–33. [Google Scholar] [CrossRef]
- Beers, A.E.W.; Nijhuis, T.A.; Aalders, N.; Kapteijn, F.; Moulijn, J.A. BEA coating of structured supports—Performance in acylation. Appl. Catal. A 2003, 243, 237–250. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Ulla, M.A.; Miró, E.E. The effect of different slurry compositions and solvents upon the properties of ZSM5-washcoated cordierite honeycombs for the SCR of NOx with methane. Catal. Today 2005, 107–108, 86–93. [Google Scholar] [CrossRef]
- Mitra, B.; Kunzru, D. Washcoating of different zeolites on cordierite monoliths. J. Am. Cer. Soc. 2008, 91, 64–70. [Google Scholar] [CrossRef]
- Beers, A.E.W.; Nijhuis, T.A.; Kapteijn, F.; Moulijn, J.A. Zeolite coated structures for the acylation of aromatics. Microporous Mesoporous Mater. 2001, 48, 279–284. [Google Scholar] [CrossRef]
- Lisi, L.; Pirone, R.; Russo, G.; Stanzione, V. Cu-ZSM5 based monolith reactors for NO decomposition. Chem. Eng. J. 2009, 154, 341–347. [Google Scholar] [CrossRef]
- Boix, A.V.; Zamaro, J.M.; Lombardo, E.A.; Miró, E.E. The beneficial effect of silica on the activity and thermal stability of PtCoFerrierite-washcoated cordierite monoliths for the SCR of NOx with CH4. Appl. Catal. B 2003, 46, 121–132. [Google Scholar] [CrossRef]
- Wang, T.; Yang, S.; Sun, K.; Fang, X. Preparation of Pt/beta zeolite–Al2O3/cordierite monolith for automobile exhaust purification. Ceram. Int. 2011, 37, 621–626. [Google Scholar] [CrossRef]
- Boix, A.V.; Miró, E.E.; Lombardo, E.A.; Mariscal, R.; Fierro, J.L.G. Binder effect upon the catalytic behavior of PtCoZSM5 washcoated on cordierite monoliths. Appl. Catal. A 2004, 276, 197–205. [Google Scholar] [CrossRef]
- Buciuman, F.-C.; Kraushaar-Czarnetzki, B. Preparation and characterization of ceramic foam supported nanocrystalline zeolite catalysts. Catal. Today 2001, 69, 337–342. [Google Scholar] [CrossRef]
- Landi, G.; Lisi, L.; Pirone, R.; Tortorelli, M.; Russo, G. NO decomposition over La-doped Cu-ZSM5 monolith under adsorption-reaction conditions. Appl. Catal. A 2013, 464–465, 61–67. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Miró, E.E. Novel binderless zeolite-coated monolith reactor for environmental applications. Chem. Eng. J. 2010, 165, 701–708. [Google Scholar] [CrossRef]
- Lachman, I.M.; Patil, M.D. Method of Crystallizing a Zeolite on the Surface of a Monolithic Ceramic Substrate. U.S. Patent 4,800,187, 24 January 1989. [Google Scholar]
- Okada, K.; Kameshima, Y.; Madhusoodana, C.D.; Das, R.N. Preparation of zeolite-coated cordierite honeycombs prepared by an in situ crystallization method. Sci. Technol. Adv. Mater. 2004, 5, 479–484. [Google Scholar] [CrossRef]
- Seijger, G.B.F.; Oudshoorn, O.L.; van Kooten, W.E.J.; Jansen, J.C.; van Bekkum, H.; van den Bleek, C.M.; Calis, H.P.A. In situ synthesis of binderless ZSM-5 zeolitic coatings on ceramic foam supports. Microporous Mesoporous Mater. 2000, 39, 195–204. [Google Scholar] [CrossRef]
- Seijger, G.B.F.; van den Berg, A.; Riva, R.; Krishna, K.; Calis, H.P.A.; van Bekkum, H.; van den Bleek, C.M. In situ preparation of ferrierite coatings on cordierite honeycomb supports. Appl. Catal. A 2002, 236, 187–203. [Google Scholar] [CrossRef]
- Seijger, G.B.F.; Palmaro, S.G.; Krishna, K.; van Bekkum, H.; van den Bleek, C.M.; Calis, H.P.A. In situ preparation of ferrierite coatings on structured metal supports. Microporous Mesoporous Mater. 2002, 56, 33–45. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Ulla, M.A.; Miró, E.E. Growth of mordenite on monoliths by secondary synthesis: Effects of the substrate on the coating structure and catalytic activity. Appl. Catal. A 2006, 314, 101–113. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Ulla, M.A.; Miró, E.E. ZSM5 growth on a FeCrAl steel support. Coating characteristics upon the catalytic behavior in the NOx SCR. Microporous Mesoporous Mater. 2008, 115, 113–122. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P.; Schäfer, R. Zeolite membranes–state of their development and perspective. Microporous Mesoporous Mater. 2000, 38, 3–24. [Google Scholar] [CrossRef]
- Zamaro, J.M.; Ulla, M.A.; Miró, E.E. Improvement in the catalytic performance of In-mordenite through preferential growth on metallic monoliths. Appl. Catal. A 2006, 308, 161–171. [Google Scholar] [CrossRef]
- Ulla, M.A.; Mallada, R.; Coronas, J.; Gutierrez, L.; Miró, E.; Santamarı́a, J. Synthesis and characterization of ZSM-5 coatings onto cordierite honeycomb supports. Appl. Catal. A 2003, 253, 257–269. [Google Scholar] [CrossRef]
- Eleta, A.; Navarro, P.; Costa, L.; Montes, M. Deposition of zeolitic coatings onto Fecralloy microchannels: Washcoating vs. in situ growing. Microporous Mesoporous Mater. 2009, 123, 113–122. [Google Scholar] [CrossRef]
- Öhrman, O.; Hedlund, J.; Sterte, J. Synthesis and evaluation of ZSM-5 films on cordierite monoliths. Appl. Catal. A 2004, 270, 193–199. [Google Scholar] [CrossRef]
- Li, L.; Xue, B.; Chen, J.; Guan, N.; Zhang, F.; Liu, D.; Feng, H. Direct synthesis of zeolite coatings on cordierite supports by in situ hydrothermal method. Appl. Catal. A 2005, 292, 312–321. [Google Scholar] [CrossRef]
- Basaldella, E.I.; Kikot, A.; Bengoa, J.F.; Tara, J.C. ZSM-5 zeolite films on cordierite modules. Effect of dilution on the synthesis medium. Mater. Lett. 2002, 52, 350–354. [Google Scholar] [CrossRef]
- Brown, S.M.; Woltermann, G.M. Conversion of Nitrogen Oxides. U.S. Patent 4,157,375, 5 June 1979. [Google Scholar]
- Lester, G.R.; Brennan, J.F. Pollution Control Catalyst. U.S. Patent 4,663,300, 5 May 1987. [Google Scholar]
- Li, Y.Y.; Perera, S.P.; Crittenden, B.D. Zeolite monoliths for air separation Part 1: Manufacture and characterization. Chem. Eng. Res. Des. 1998, 76, 921–930. [Google Scholar] [CrossRef]
- Aranzabal, A.; Iturbe, D.; Romero-Sáez, M.; González-Marcos, M.P.; González-Velasco, J.R.; González-Marcos, J.A. Optimization of process parameters on the extrusion of honeycomb shaped monolith of H-ZSM-5 zeolite. Chem. Eng. J. 2010, 162, 415–423. [Google Scholar] [CrossRef]
- Li, Y.Y.; Perera, S.P.; Crittenden, B.D.; Bridgwater, J. The effect of the binder on the manufacture of a 5A zeolite monolith. Powder Technol. 2001, 116, 85–96. [Google Scholar] [CrossRef]
- Grande, C.A.; Cavenati, S.; Barcia, P.; Hammer, J.; Fritz, H.G.; Rodrigues, A.E. Adsorption of propane and propylene in zeolite 4A honeycomb monolith. Chem. Eng. Sci. 2006, 61, 3053–3067. [Google Scholar] [CrossRef]
- Shams, K.; Mirmohammadi, S.J. Preparation of 5A zeolite monolith granular extrudates using kaolin: Investigation of the effect of binder on sieving/adsorption properties using a mixture of linear and branched paraffin hydrocarbons. Microporous Mesoporous Mater. 2007, 106, 268–277. [Google Scholar] [CrossRef]
- Akhtar, F.; Vasiliev, P.O.; Bergström, L. Hierarchically porous ceramics from diatomite powders by pulsed current processing. J. Am. Ceram. Soc. 2009, 92, 338–343. [Google Scholar] [CrossRef]
- Vasiliev, P.; Akhtar, F.; Grins, J.; Mouzon, J.; Andersson, C.; Hedlund, J.; Bergström, L. Strong hierarchically porous monoliths by pulsed current processing of zeolite powder assemblies. ACS Appl. Mater. Interfaces 2010, 2, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, P.O.; Ojuva, A.; Grins, J.; Bergström, L. The effect of temperature on the pulsed current processing behaviour and structural characteristics of porous ZSM-5 and zeolite Y monoliths. J. Eur. Ceram. Soc. 2010, 30, 2977–2983. [Google Scholar] [CrossRef]
- Besser, B.; Tajiri, H.A.; Mikolajczyk, G.; Möllmer, J.; Schumacher, T.C.; Odenbach, S.; Gläser, R.; Kroll, S.; Rezwan, K. Hierarchical porous zeolite structures for pressure swing adsorption applications. ACS Appl. Mater. Interfaces 2016, 8, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.A.; Basaldella, E.; Rodrigues, A.E. Crystal size effect in vacuum pressure-swing adsorption for propane/propylene separation. Ind. Eng. Chem. Res. 2004, 43, 7557–7565. [Google Scholar] [CrossRef]
- Kopaygorodsky, E.M.; Guliants, V.V.; Krantz, W.B. Predictive dynamic model of single-stage ultra-rapid pressure swing adsorption. AIChE J. 2004, 50, 953–962. [Google Scholar] [CrossRef]
- Rodríguez-reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Frackowiak, E.; Jurewicz, K.; Friebe, M.; Parmentier, J.; Béguin, F. Electrochemical energy storage in ordered porous carbon materials. Carbon 2005, 43, 1293–1302. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Alcañiz-Monge, J.; de la Casa-Lillo, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Advances in the study of methane storage in porous carbonaceous materials. Fuel 2002, 81, 1777–1803. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; De La Casa-Lillo, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Methane storage in activated carbon fibres. Carbon 1997, 35, 291–297. [Google Scholar] [CrossRef]
- Gadkaree, K.P. Carbon honeycomb structures for adsorption applications. Carbon 1998, 36, 981–989. [Google Scholar] [CrossRef]
- Valdés-Solı́s, T.; Marbán, G.; Fuertes, A.B. Preparation of microporous carbon–ceramic cellular monoliths. Microporous Mesoporous Mater. 2001, 43, 113–126. [Google Scholar] [CrossRef]
- Garcı́a-Bordejé, E.; Calvillo, L.; Lázaro, M.J.; Moliner, R. Vanadium supported on carbon-coated monoliths for the SCR of NO at low temperature: Effect of pore structure. Appl. Catal. B 2004, 50, 235–242. [Google Scholar] [CrossRef]
- Valdés-Solı́s, T.; Linders, M.J.G.; Kapteijn, F.; Marbán, G.; Fuertes, A.B. Adsorption and breakthrough performance of carbon-coated ceramic monoliths at low concentration of n-butane. Chem. Eng. Sci. 2004, 59, 2791–2800. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Kapteijn, F.; Moulijn, J.A.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Moreno-Castilla, C. Pd and Pt catalysts supported on carbon-coated monoliths for low-temperature combustion of xylenes. Carbon 2006, 44, 2463–2468. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pérez-Cadenas, A.F.; Kapteijn, F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J.; Moulijn, J.A. Palladium and platinum catalysts supported on carbon nanofiber coated monoliths for low-temperature combustion of BTX. Appl. Catal. B 2009, 89, 411–419. [Google Scholar] [CrossRef]
- Garcı́a-Bordejé, E.; Kapteijn, F.; Moulijn, J.A. Preparation and characterisation aspects of carbon-coated monoliths. Catal. Today 2001, 69, 357–363. [Google Scholar] [CrossRef]
- Vergunst, T.; Linders, M.J.G.; Kapteijn, F.; Moulijn, J.A. Carbon-based monollithic structures. Catal. Rev. 2001, 43, 291–314. [Google Scholar] [CrossRef]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Preparation of carbon-coated monolithic supports. Carbon 2002, 40, 1891–1902. [Google Scholar] [CrossRef]
- Garcı́a-Bordejé, E.; Kapteijn, F.; Moulijn, J.A. Preparation and characterisation of carbon-coated monoliths for catalyst supports. Carbon 2002, 40, 1079–1088. [Google Scholar] [CrossRef]
- Dawson, E.A.; Barnes, P.A.; Chinn, M.J. Preparation and characterisation of carbon-coated ceramic foams for organic vapour adsorption. Carbon 2006, 44, 1189–1197. [Google Scholar] [CrossRef]
- De Jong, K.P.; Geus, J.W. Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Jarrah, N.A.; van Ommen, J.G.; Lefferts, L. Mechanistic aspects of the formation of carbon-nanofibers on the surface of Ni foam: A new microstructured catalyst support. J. Catal. 2006, 239, 460–469. [Google Scholar] [CrossRef]
- Jarrah, N.; van Ommen, J.G.; Lefferts, L. Development of monolith with a carbon-nanofiber-washcoat as a structured catalyst support in liquid phase. Catal. Today 2003, 79–80, 29–33. [Google Scholar] [CrossRef]
- Jarrah, N.A.; van Ommen, J.G.; Lefferts, L. Growing a carbon nano-fiber layer on a monolith support; effect of nickel loading and growth conditions. J. Mater. Chem. 2004, 14, 1590–1597. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Kvande, I.; Chen, D.; Rønning, M. Synthesis of composite materials of carbon nanofibres and ceramic monoliths with uniform and tuneable nanofibre layer thickness. Carbon 2007, 45, 1828–1838. [Google Scholar] [CrossRef]
- DeLiso, E.M.; Lachman, I.M.; Patil, M.D.; Zaun, K.E. Activated Carbon Structures. U.S. Patent 5,356,852, 18 October 1994. [Google Scholar]
- Kyotani, T. Control of pore structure in carbon. Carbon 2000, 38, 269–286. [Google Scholar] [CrossRef]
- Taguchi, A.; Smått, J.H.; Lindén, M. Carbon monoliths possessing a hierarchical, fully interconnected porosity. Adv. Mater. 2003, 15, 1209–1211. [Google Scholar] [CrossRef]
- Alvarez, S.; Esquena, J.; Solans, C.; Fuertes, A.B. Meso/Macroporous carbon monoliths from polymeric foams. Adv. Eng. Mater. 2004, 6, 897–899. [Google Scholar] [CrossRef]
- Lu, A.-H.; Smått, J.-H.; Backlund, S.; Lindén, M. Easy and flexible preparation of nanocasted carbon monoliths exhibiting a multimodal hierarchical porosity. Microporous Mesoporous Mater. 2004, 72, 59–65. [Google Scholar] [CrossRef]
- Lu, A.H.; Smått, J.H.; Lindén, M. Combined surface and volume templating of highly porous nanocast carbon monoliths. Adv. Funct. Mater. 2005, 15, 865–871. [Google Scholar] [CrossRef]
- Shi, Z.-G.; Feng, Y.-Q.; Xu, L.; Da, S.-L.; Zhang, M. Synthesis of a carbon monolith with trimodal pores. Carbon 2003, 41, 2677–2679. [Google Scholar] [CrossRef]
- Álvarez, S.; Fuertes, A.B. Synthesis of macro/mesoporous silica and carbon monoliths by using a commercial polyurethane foam as sacrificial template. Mater. Lett. 2007, 61, 2378–2381. [Google Scholar] [CrossRef]
- Klepel, O.; Strauβ, H.; Garsuch, A.; Böhme, K. Several ways to produce porous carbon monoliths by template assisted routes. Mater. Lett. 2007, 61, 2037–2039. [Google Scholar] [CrossRef]
- Vinu, A.; Srinivasu, P.; Takahashi, M.; Mori, T.; Balasubramanian, V.V.; Ariga, K. Controlling the textural parameters of mesoporous carbon materials. Microporous Mesoporous Mater. 2007, 100, 20–26. [Google Scholar] [CrossRef]
- Yu, L.; Brun, N.; Sakaushi, K.; Eckert, J.; Titirici, M.M. Hydrothermal nanocasting: Synthesis of hierarchically porous carbon monoliths and their application in lithium–sulfur batteries. Carbon 2013, 61, 245–253. [Google Scholar] [CrossRef]
- Lu, A.-H.; Li, W.-C.; Schmidt, W.; Schüth, F. Fabrication of hierarchically structured carbon monoliths via self-binding and salt templating. Microporous Mesoporous Mater. 2006, 95, 187–192. [Google Scholar] [CrossRef]
- Tomasic, V. Application of the monoliths in DeNOx catalysis. Catal. Today 2007, 119, 106–113. [Google Scholar] [CrossRef]
- Lee, J.H.; Trimm, D.L. Catalytic combustion of methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.K.; Blok, K. Steam cracking and methane to olefins: Energy use, CO2 emissions and production costs. Energy 2008, 33, 817–833. [Google Scholar] [CrossRef]
- Govender, N.; Friedrich, H.B.; van Vuuren, M.J. Controlling factors in the selective conversion of n-butane over promoted VPO catalysts at low temperature. Catal. Today 2004, 97, 315–324. [Google Scholar] [CrossRef]
- Pillay, B.; Mathebula, M.R.; Friedrich, H.B. The oxidative dehydrogenation of n-hexane over Ni–Mo–O catalysts. Appl. Catal. A 2009, 361, 57–64. [Google Scholar] [CrossRef]
- Elkhalifa, E.A.; Friedrich, H.B. Oxidative dehydrogenation of n-octane using vanadium-magnesium oxide catalysts with different vanadium loadings. Appl. Catal. A 2010, 373, 122–131. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.; Green, M.L.H. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Huff, M.; Schmidt, L.D. Ethylene formation by oxidative dehydrogenation of ethane over monoliths at very short contact times. J. Phys. Chem. 1993, 97, 11815–11822. [Google Scholar] [CrossRef]
- Huff, M.; Schmidt, L.D. Production of olefins by oxidative dehydrogenation of propane and butane over monoliths at short contact times. J. Catal. 1994, 149, 127–141. [Google Scholar] [CrossRef]
- Huff, M.; Schmidt, L.D. Oxidative dehydrogenation of isobutane over monoliths at short contact times. J. Catal. 1995, 155, 82–94. [Google Scholar] [CrossRef]
- Huff, M.; Torniainen, P.M.; Schmidt, L.D. Partial oxidation of alkanes over noble metal coated monoliths. Catal. Today 1994, 21, 113–128. [Google Scholar] [CrossRef]
- Liebmann, L.S.; Schmidt, L.D. Oxidative dehydrogenation of isobutane at short contact times. Appl. Catal. A 1999, 179, 93–106. [Google Scholar] [CrossRef]
- Beretta, A.; Piovesan, L.; Forzatti, P. An investigation on the role of a Pt/Al2O3 catalyst in the oxidative dehydrogenation of propane in annular reactor. J. Catal. 1999, 184, 455–468. [Google Scholar] [CrossRef]
- Beretta, A.; Forzatti, P.; Ranzi, E. Production of olefins via oxidative dehydrogenation of propane in autothermal conditions. J. Catal. 1999, 184, 469–478. [Google Scholar] [CrossRef]
- Beretta, A.; Ranzi, E.; Forzatti, P. Experimental and theoretical investigation on the roles of heterogeneous and homogeneous phases in the oxidative dehydrogenation of light paraffins in novel short contact time reactors. In Studies in Surface Science and Catalysis; Corma, A., Melo, F.V., Mendioroz, S., Fierro, J.L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 130, pp. 1913–1918. [Google Scholar]
- Huff, M.C.; Androulakis, I.P.; Sinfelt, J.H.; Reyes, S.C. The contribution of gas-phase reactions in the Pt-catalyzed conversion of ethane–oxygen mixtures. J. Catal. 2000, 191, 46–54. [Google Scholar] [CrossRef]
- Henning, A.D.; Schmidt, D.L. Oxidative dehydrogenation of ethane at short contact times: Species and temperature profiles within and after the catalyst. Chem. Eng. Sci. 2002, 57, 2615–2625. [Google Scholar] [CrossRef]
- Beretta, A.; Ranzi, E.; Forzatti, P. Oxidative dehydrogenation of light paraffins in novel short contact time reactors. Experimental and theoretical investigation. Chem. Eng. Sci. 2001, 56, 779–787. [Google Scholar] [CrossRef]
- Beretta, A.; Forzatti, P. Catalyst-assisted oxidative dehydrogenation of light paraffins in short contact time reactors. In Studies in Surface Science and Catalysis; Iglesia, E., Spivey, J.J., Fleisch, T.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 136, pp. 191–196. [Google Scholar]
- Beretta, A.; Ranzi, E.; Forzatti, P. Production of olefins via oxidative dehydrogenation of light paraffins at short contact times. Catal. Today 2001, 64, 103–111. [Google Scholar] [CrossRef]
- Dietz, A.G.; Carlsson, A.F.; Schmidt, L.D. Partial oxidation of C and C alkanes over monolith catalysts at short contact times. J. Catal. 1996, 176, 459–473. [Google Scholar] [CrossRef]
- O’Connor, R.P.; Schmidt, L.D. C6 oxygenates from n-hexane in a single-gauze reactor. Chem. Eng. Sci. 2000, 55, 5693–5703. [Google Scholar] [CrossRef]
- Hickman, D.A.; Schmidt, L.D. Synthesis gas formation by direct oxidation of methane over Pt monoliths. J. Catal. 1992, 138, 267–282. [Google Scholar] [CrossRef]
- Hickman, D.A.; Schmidt, L.D. Steps in CH4 oxidation on Pt and Rh surfaces: High-temperature reactor simulations. AIChE J. 1993, 39, 1164–1177. [Google Scholar] [CrossRef]
- Fathi, M.; Lødeng, R.; Nilsen, E.S.; Silberova, B.; Holmen, A. Short contact time oxidative dehydrogenation of propane. Catal. Today 2001, 64, 113–120. [Google Scholar] [CrossRef]
- Silberova, B.; Fathi, M.; Holmen, A. Oxidative dehydrogenation of ethane and propane at short contact time. Appl. Catal. A 2004, 276, 17–28. [Google Scholar] [CrossRef]
- Hickman, D.A.; Haupfear, E.A.; Schmidt, L.D. Synthesis gas formation by direct oxidation of methane over Rh monoliths. Catal. Lett. 1993, 17, 223–237. [Google Scholar] [CrossRef]
- Hickman, D.A.; Schmidt, L.D. Production of syngas by direct catalytic oxidation of methane. Science 1993, 259, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Torniainen, P.M.; Chu, X.; Schmidt, L.D. Comparison of monolith-supported metals for the direct oxidation of methane to syngas. J. Catal. 1994, 146, 1–10. [Google Scholar] [CrossRef]
- O’Connor, R.P.; Klein, E.J.; Schmidt, L.D. High yields of synthesis gas by millisecond partial oxidation of higher hydrocarbons. Catal. Lett. 2000, 70, 99–107. [Google Scholar] [CrossRef]
- Krummenacher, J.J.; West, K.N.; Schmidt, L.D. Catalytic partial oxidation of higher hydrocarbons at millisecond contact times: Decane, hexadecane, and diesel fuel. J. Catal. 2003, 215, 332–343. [Google Scholar] [CrossRef]
- Schmidt, D.L.; Klein, J.E.; Leclerc, A.C.; Krummenacher, J.J.; West, N.K. Syngas in millisecond reactors: Higher alkanes and fast lightoff. Chem. Eng. Sci. 2003, 58, 1037–1041. [Google Scholar] [CrossRef]
- Forzatti, P.; Groppi, G. Catalytic combustion for the production of energy. Catal. Today 1999, 54, 165–180. [Google Scholar] [CrossRef]
- Groppi, G.; Ibashi, W.; Tronconi, E.; Forzatti, P. Structured reactors for kinetic measurements under severe conditions in catalytic combustion over palladium supported systems. Catal. Today 2001, 69, 399–408. [Google Scholar] [CrossRef]
- Groppi, G.; Ibashi, W.; Tronconi, E.; Forzatti, P. Structured reactors for kinetic measurements in catalytic combustion. Chem. Eng. J. 2001, 82, 57–71. [Google Scholar] [CrossRef]
- Brambilla, A.; Frouzakis, C.E.; Mantzaras, J.; Bombach, R.; Boulouchos, K. Flame dynamics in lean premixed CO/H2 /air combustion in a mesoscale channel. Combust. Flame 2014, 161, 1268–1281. [Google Scholar] [CrossRef]
- Brambilla, A.; Schultze, M.; Frouzakis, C.E.; Mantzaras, J.; Bombach, R.; Boulouchos, K. An experimental and numerical investigation of premixed syngas combustion dynamics in mesoscale channels with controlled wall temperature profiles. Proc. Combust. Inst. 2015, 35, 3429–3437. [Google Scholar] [CrossRef]
- Karagiannidis, S.; Mantzaras, J. Numerical investigation on the hydrogen-assisted start-up of methane-fueled, catalytic microreactors. Flow Turbul. Combust. 2012, 89, 215–230. [Google Scholar] [CrossRef]
- Mantzaras, J.; Bombach, R.; Schaeren, R. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum at pressures up to 10 bar. Proc. Combust. Inst. 2009, 32, 1937–1945. [Google Scholar] [CrossRef]
- Michelon, N.; Mantzaras, J.; Canu, P. Transient simulation of the combustion of fuel-lean hydrogen/air mixtures in platinum-coated channels. Combust. Theory Model. 2015, 19, 514–548. [Google Scholar] [CrossRef]
- Schultze, M.; Mantzaras, J.; Grygier, F.; Bombach, R. Hetero-/homogeneous combustion of syngas mixtures over platinum at fuel-rich stoichiometries and pressures up to 14 bar. Proc. Combust. Inst. 2015, 35, 2223–2231. [Google Scholar] [CrossRef]
- Sui, R.; Prasianakis, N.I.; Mantzaras, J.; Mallya, N.; Theile, J.; Lagrange, D.; Friess, M. An experimental and numerical investigation of the combustion and heat transfer characteristics of hydrogen-fueled catalytic microreactors. Chem. Eng. Sci. 2016, 141, 214–230. [Google Scholar] [CrossRef]
- Zheng, X.; Mantzaras, J. An analytical and numerical investigation of hetero-/homogeneous combustion with deficient reactants having larger than unity Lewis numbers. Combust. Flame 2014, 161, 1911–1922. [Google Scholar] [CrossRef]
- Zheng, X.; Mantzaras, J.; Bombach, R. Homogeneous combustion of fuel-lean syngas mixtures over platinum at elevated pressures and preheats. Combust. Flame 2013, 160, 155–169. [Google Scholar] [CrossRef]
- Dupont, V.; Moallemi, F.; Williams, A.; Zhang, S.H. Combustion of methane in catalytic honeycomb monolith burners. Int. J. Energy Res. 2000, 24, 1181–1201. [Google Scholar] [CrossRef]
- Euzen, P.; Le Gal, J.-H.; Rebours, B.; Martin, G. Deactivation of palladium catalyst in catalytic combustion of methane. Catal. Today 1999, 47, 19–27. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Banerjee, S.; Choudhary, V.R. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Zwinkels, M.F.M.; Haussner, O.; Govind Menon, P.; Järås, S.G. Preparation and characterization of LaCrO3 and Cr2O3 methane combustion catalysts supported on LaAl11O18- and Al2O3-coated monoliths. Catal. Today 1999, 47, 73–82. [Google Scholar] [CrossRef]
- Cimino, S.; Pirone, R.; Russo, G. Thermal stability of perovskite-based monolithic reactors in the catalytic combustion of methane. Ind. Eng. Chem. Res. 2001, 40, 80–85. [Google Scholar] [CrossRef]
- Deutschmann, O.; Maier, L.I.; Riedel, U.; Stroemman, A.H.; Dibble, R.W. Hydrogen assisted catalytic combustion of methane on platinum. Catal. Today 2000, 59, 141–150. [Google Scholar] [CrossRef]
- Cimino, S.; Di Benedetto, A.; Pirone, R.; Russo, G. CO, H2 or C3H8 assisted catalytic combustion of methane over supported LaMnO3 monoliths. Catal. Today 2003, 83, 33–43. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Di Sarli, V.; Landi, G.; Pirone, R. High-pressure methane combustion over a perovskyte catalyst. Ind. Eng. Chem. Res. 2012, 51, 7547–7558. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Landi, G.; Di Sarli, V.; Barbato, P.S.; Pirone, R.; Russo, G. Methane catalytic combustion under pressure. Catal. Today 2012, 197, 206–213. [Google Scholar] [CrossRef]
- Landi, G.; Di Benedetto, A.; Barbato, P.S.; Russo, G.; Di Sarli, V. Transient behavior of structured LaMnO3 catalyst during methane combustion at high pressure. Chem. Eng. Sci. 2014, 116, 350–358. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Benedetto, A.; Pirone, R.; Russo, G. High pressure kinetics of CH4, CO and H2 combustion over LaMnO3 catalyst. Appl. Catal. B 2013, 134–135, 110–122. [Google Scholar] [CrossRef]
- Di Sarli, V.; Barbato, P.S.; Di Benedetto, A.; Landi, G. Start-up behavior of a LaMnO3 partially coated monolithic combustor at high pressure. Catal. Today 2015, 242, 200–210. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Sarli, V.; Landi, G.; Di Benedetto, A. High pressure methane catalytic combustion over novel partially coated LaMnO3-based monoliths. Chem. Eng. J. 2015, 259, 381–390. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ayastuy, J.L.; Gamboa, N.K.; González-Marcos, M.P.; Gutiérrez-Ortiz, M.A. CuO/CeO2 washcoated ceramic monoliths for CO-PROx reaction. Chem. Eng. J. 2011, 171, 224–231. [Google Scholar] [CrossRef]
- Miguel-García, I.; Navlani-García, M.; García-Aguilar, J.; Berenguer-Murcia, Á.; Lozano-Castelló, D.; Cazorla-Amorós, D. Capillary microreactors based on hierarchical SiO2 monoliths incorporating noble metal nanoparticles for the preferential oxidation of CO. Chem. Eng. J. 2015, 275, 71–78. [Google Scholar] [CrossRef]
- Ouyang, X.; Bednarova, L.; Besser, R.S.; Ho, P. Preferential oxidation (PrOx) in a thin-film catalytic microreactor: Advantages and limitations. AIChE J. 2005, 51, 1758–1772. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Hungría, A.B.; Munuera, G.; Gamarra, D. Preferential oxidation of CO in rich H2 over CuO/CeO2: Details of selectivity and deactivation under the reactant stream. Appl. Catal. B 2006, 65, 207–216. [Google Scholar] [CrossRef]
- Korotkikh, O.; Farrauto, R. Selective catalytic oxidation of CO in H2: Fuel cell applications. Catal. Today 2000, 62, 249–254. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Liu, Y.; Ruettinger, W.; Ilinich, O.; Shore, L.; Giroux, T. Precious metal catalysts supported on ceramic and metal monolithic structures for the hydrogen economy. Catal. Rev. 2007, 49, 141–196. [Google Scholar] [CrossRef]
- Zhang, Q.; Shore, L.; Farrauto, R.J. Selective CO oxidation over a commercial PROx monolith catalyst for hydrogen fuel cell applications. Int. J. Hydrogen Energy 2012, 37, 10874–10880. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chen, Y.-Y.; Su, C.-C.; Hsu, C.-F. The cleanup of CO in hydrogen for PEMFC applications using Pt, Ru, Co, and Fe in PROx reaction. J. Power Sources 2007, 174, 294–301. [Google Scholar] [CrossRef]
- Maeda, N.; Matsushima, T.; Uchida, H.; Yamashita, H.; Watanabe, M. Performance of Pt-Fe/mordenite monolithic catalysts for preferential oxidation of carbon monoxide in a reformate gas for PEFCs. Appl. Catal. A 2008, 341, 93–97. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Tu, S.-T.; Yan, J.; Wang, Z. Pt–Co catalyst-coated channel plate reactor for preferential CO oxidation. Int. J. Hydrogen Energy 2011, 36, 3778–3788. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Papadopoulou, C.; Batista, J.; Hocevar, S.; Matralis, H.K. A comparative study of Pt/γ-Al2O3, Au/α-Fe2O3 and CuO–CeO2 catalysts for the selective oxidation of carbon monoxide in excess hydrogen. Catal. Today 2002, 75, 157–167. [Google Scholar] [CrossRef]
- Domínguez, M.I.; Sánchez, M.; Centeno, M.A.; Montes, M.; Odriozola, J.A. CO oxidation over gold-supported catalysts-coated ceramic foams prepared from stainless steel wastes. Appl. Catal. A 2006, 302, 96–103. [Google Scholar] [CrossRef]
- Centeno, M.A.; Paulis, M.; Montes, M.; Odriozola, J.A. Catalytic combustion of volatile organic compounds on Au/CeO2/Al2O3 and Au/Al2O3 catalysts. Appl. Catal. A 2002, 234, 65–78. [Google Scholar] [CrossRef]
- Martínez T, L.M.; Domínguez, M.I.; Sanabria, N.; Hernández, W.Y.; Moreno, S.; Molina, R.; Odriozola, J.A.; Centeno, M.A. Deposition of Al-Fe pillared bentonites and gold supported Al-Fe pillared bentonites on metallic monoliths for catalytic oxidation reactions. Appl. Catal. A 2009, 364, 166–173. [Google Scholar] [CrossRef]
- Milt, V.G.; Ivanova, S.; Sanz, O.; Domínguez, M.I.; Corrales, A.; Odriozola, J.A.; Centeno, M.A. Au/TiO2 supported on ferritic stainless steel monoliths as CO oxidation catalysts. Appl. Surf. Sci. 2013, 270, 169–177. [Google Scholar] [CrossRef]
- Martínez Tejada, L.M.; Domínguez, M.I.; Sanz, O.; Centeno, M.A.; Odriozola, J.A. Au/CeO2 metallic monolith catalysts: Influence of the metallic substrate. Gold Bull. 2013, 46, 221. [Google Scholar] [CrossRef]
- Laguna, O.H.; Domínguez, M.I.; Centeno, M.A.; Odriozola, J.A. Forced deactivation and postmortem characterization of a metallic microchannel reactor employed for the preferential oxidation of CO (PROx). Chem. Eng. J. 2016, 302, 650–662. [Google Scholar] [CrossRef]
- Snytnikov, P.V.; Potemkin, D.I.; Rebrov, E.V.; Sobyanin, V.A.; Hessel, V.; Schouten, J.C. Design, scale-out, and operation of a microchannel reactor with a Cu/CeO2−x catalytic coating for preferential CO oxidation. Chem. Eng. J. 2010, 160, 923–929. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Benedetto, A.; Lisi, L. Optimization of the preparation method of CuO/CeO2 structured catalytic monolith for CO preferential oxidation in H2-rich streams. Appl. Catal. B 2016, 181, 727–737. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Landi, G.; Lisi, L. CuO/CeO2 based monoliths for CO preferential oxidation in H2-rich streams. Chem. Eng. J. 2015, 279, 983–993. [Google Scholar] [CrossRef]
- Zeng, S.H.; Liu, Y.; Wang, Y.Q. CuO–CeO2/Al2O3 /FeCrAl monolithic catalysts prepared by sol-pyrolysis method for preferential oxidation of carbon monoxide. Catal. Lett. 2007, 117, 119–125. [Google Scholar] [CrossRef]
- Gu, C.; Miao, J.; Liu, Y.; Wang, Y. Meso-macroporous monolithic CuO–CeO2/γ-Al2O3 catalysts and their catalytic performance for preferential oxidation of CO. J. Mater. Sci. 2010, 45, 5660–5668. [Google Scholar] [CrossRef]
- Zeng, S.; Su, H.; Liu, Y.; Wang, Y.; Wang, D. CuO-CeO2/Al2O3/FeCrAl monolithic catalysts prepared by in situ combustion synthesis method for preferential oxidation of carbon monoxide. J. Rare Earths 2011, 29, 69–73. [Google Scholar] [CrossRef]
- Laguna, O.H.; González Castaño, M.; Centeno, M.A.; Odriozola, J.A. Microreactors technology for hydrogen purification: Effect of the catalytic layer thickness on CuOx/CeO2-coated microchannel reactors for the PROx reaction. Chem. Eng. J. 2015, 275, 45–52. [Google Scholar] [CrossRef]
- Tronconi, E.; Groppi, G. ‘High conductivity’ monolith catalysts for gas/solid exothermic reactions. Chem. Eng. Technol. 2002, 25, 743–750. [Google Scholar] [CrossRef]
- Tronconi, E.; Groppi, G.; Boger, T.; Heibel, A. Monolithic catalysts with ‘high conductivity’ honeycomb supports for gas/solid exothermic reactions: Characterization of the heat-transfer properties. Chem. Eng. Sci. 2004, 59, 4941–4949. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E.; Cortelli, C.; Leanza, R. Conductive monolithic catalysts: Development and industrial pilot tests for the oxidation of o-xylene to phthalic anhydride. Ind. Eng. Chem. Res. 2012, 51, 7590–7596. [Google Scholar] [CrossRef]
- Visconti, C.G.; Groppi, G.; Tronconi, E. Highly conductive “packed foams”: A new concept for the intensification of strongly endo- and exo-thermic catalytic processes in compact tubular reactors. Catal. Today 2016, 273, 178–186. [Google Scholar] [CrossRef]


| Pore Filling | Slurry Coating |
|---|---|
| sol-gel/colloidal suspensions | slurry |
| little decrease in open frontal area | larger decrease in open frontal area |
| limitation of macropore volume | high loading of washcoat possible |
| better suited for highly active catalyst | suitable even for catalyst with low activity |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govender, S.; Friedrich, H.B. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. https://doi.org/10.3390/catal7020062
Govender S, Friedrich HB. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts. 2017; 7(2):62. https://doi.org/10.3390/catal7020062
Chicago/Turabian StyleGovender, Sandeeran, and Holger B. Friedrich. 2017. "Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation" Catalysts 7, no. 2: 62. https://doi.org/10.3390/catal7020062





