On Metal Segregation of Bimetallic Nanocatalysts Prepared by a One-Pot Method in Microemulsions
Abstract
:1. Introduction
1.1. Bimetallic Nanoparticles as Catalysts
1.2. Metal Arrangement in Bimetallic Nanoparticles
1.3. Synthesis of Bimetallic Nanoparticles in Microemulsions
1.4. Scope
2. Bimetallic Nanoparticles Obtained from Microemulsions Using a One Pot Method. Experimental Observations
3. Factors Concerning Metal Separation in Bimetallic Nanoparticles
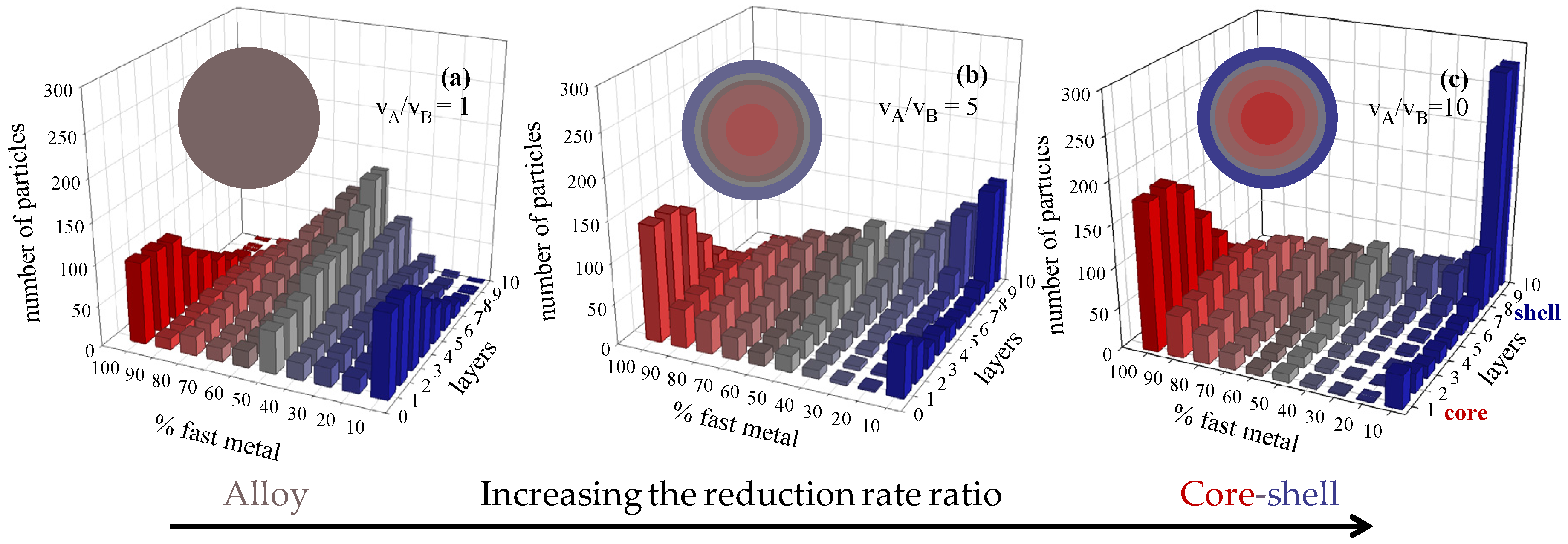
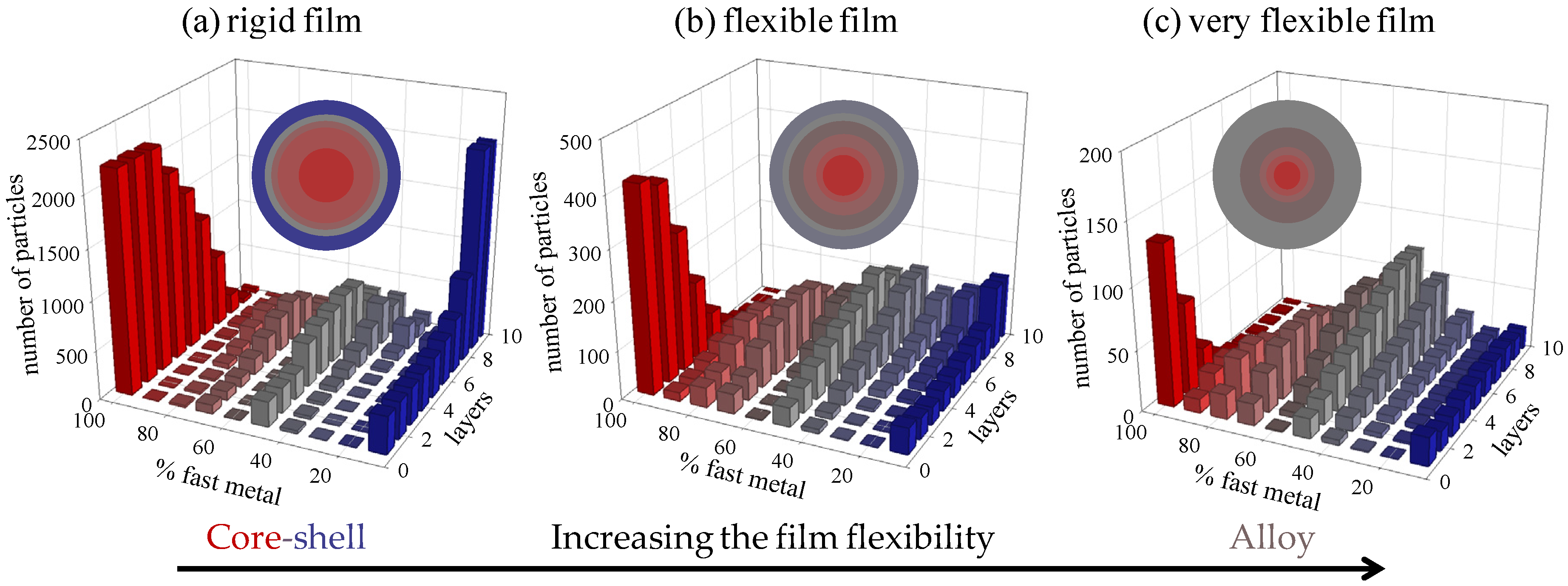
3.1. Keeping the Microemulsion Composition Fixed
3.2. Keeping the Pair of Metals Fixed
- Platinum salt H2[PtCl6]: This precursor was proved to be reduced from Pt4+ to Pt via Pt2+ [102] by means of the reactions: PtCl62− + 2e− → PtCl42− + 2Cl− (ε0 = 0.726 V [103]) and PtCl42− + 2e− → Pt + 2Cl− (ε0 = 0.758 V [103]). From these data, standard electrode potential of the couple PtCl62−/Pt (PtCl62− + 4e− → Pt + 6Cl−) was calculated to be ε0 = 0.742 V [104]
- Platinum salt PtCl2: The standard electrode reduction is: Pt2+ + 2e− → Pt (ε0 = 1.188 V [103])
- Paladium salt H2[PtCl4]: PdCl42− + 2e− → Pd + 2Cl− (ε0 = 0.591 V [105])
- Paladium salt PdCl2: Pd2+ + 2e− → Pd (ε0 = 0.915 V [103]).
3.3. Changing Concentration
4. Conclusions
- A bimetallic nanocatalyst can be obtained as a nanoalloy or as a core-shell depending on the combination of metal precursors, microemulsion composition, and metal concentration;
- A minimum difference is needed between the reduction standard potentials of the two metals of 0.20 V to obtain a core-shell structure. For values of ∆ε0 smaller than 0.20 V the obtaining of alloy cannot be avoided, neither by changing the microemulsion nor by increasing the metal concentration;
- As a rule, the higher the film flexibility around the micelles, the greater the degree of mixing in the nanocatalyst; and
- A minimum concentration of metal precursors is required to obtain a core-shell structure. This minimum concentration depends on the microemulsion flexibility and on the difference in reduction rates.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, W.; Vara, M.; Luo, M.; Huang, H.; Ruditskiy, A.; Park, J.; Bao, S.; Liu, J.; Howe, J.; Chi, M.; et al. Pd@Pt core-shell concave decahedra: A class of catalysts for the oxygen reduction reaction with enhanced activity and durability. J. Am. Chem. Soc. 2015, 137, 15036–15042. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ma, R.; Gao, N.; Garai, M.; Xu, Q.-H. Plasmon coupling-enhanced two-photon photoluminescence of Au@Ag core-shell nanoparticles and applications in the nuclease assay. Nanoscale Res. Lett. 2015, 7, 10233–10239. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Tuaev, X.; Rudi, S.; Petkov, V.; Hoell, A.; Strasser, P. In situ study of atomic structure transformations of Pt-Ni nanoparticle catalysts during electrochemical potential cycling. ACS Nano 2013, 7, 5666–5674. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Lu, Z.-H.; Jiang, H.-L.; Akita, T.; Xu, Q. Synergistic catalysis of metal-organic framework-immobilized Au-Pd nanoparticles in dehydrogenation of formic acid for chemical hydrogen storage. J. Am. Chem. Soc. 2011, 133, 11822–11825. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wang, L.; Su, R.; Qi, W.; Wang, M.; Yu, Y.; He, Z. Synthesis of silver nanoparticles within cross-linked lysozyme crystals as recyclable catalysts for 4-nitrophenol reduction. Catal. Sci. Technol. 2013, 3, 1910–1914. [Google Scholar] [CrossRef]
- Panacek, A.; Prucek, R.; Hrbac, J.; Nevecna, T.; Steffkova, J.; Zboril, R.; Kvitek, L. Polyacrylate-assisted size control of silver nanoparticles and their catalytic activity. Chem. Mat. 2014, 26, 1332–1339. [Google Scholar] [CrossRef]
- Sinfelt, J.H. Catalysis by alloys and bimetallic clusters. Acc. Chem. Res. 1977, 10, 15–20. [Google Scholar] [CrossRef]
- Sinfelt, J.H. Structure of bimetallic clusters. Acc. Chem. Res. 1987, 20, 134–139. [Google Scholar] [CrossRef]
- Tedsree, K.; Li, T.; Jones, S.; Chan, C.W.A.; Yu, K.M.K.; Bagot, P.A.J.; Marquis, E.A.; Smith, G.D.W.; Tsang, S.C.E. Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst. Nat. Nanotechnol. 2011, 6, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Bandarenka, A.S.; Varela, A.S.; Karamad, M.; Calle-Vallejo, F.; Bech, L.; Perez-Alonso, F.J.; Rossmeisl, J.; Stephens, I.E.L.; Chorkendorff, I. Design of an active site towards optimal electrocatalysis: Overlayers, surface alloys and near-surface alloys of Cu/Pt(111). Angew. Chem. Int. Ed. 2012, 51, 11845–11848. [Google Scholar] [CrossRef] [PubMed]
- Spanos, I.; Dideriksen, K.; Kirkensgaard, J.J.K.; Jelavic, S.; Arenz, M. Structural disordering of de-alloyed Pt bimetallic nanocatalysts: The effect on oxygen reduction reaction activity and stability. Phys. Chem. Chem. Phys. 2015, 17, 28044–28053. [Google Scholar] [CrossRef] [PubMed]
- König, R.Y.G.; Schwarze, M.; Schomäcker, R.; Stubenrauch, C. Catalytic Activity of Mono- and Bi-Metallic Nanoparticles Synthesized via Microemulsions. Catalysts 2014, 4, 256–275. [Google Scholar] [CrossRef]
- Singh, H.P.; Gupta, N.; Sharma, S.K.; Sharma, R.K. Synthesis of bimetallic Pt-Cu nanoparticles and their application in the reduction of rhodamine B. Colloids Surf. A 2013, 416, 43–50. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Santhanalakshmi, J.; Venkatesan, P. Mono and bimetallic nanoparticles of gold, silver and palladium-catalyzed NADH oxidation-coupled reduction of Eosin-Y. J. Nanoparticle Res. 2011, 13, 479–490. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Abazari, R.; Balalaie, S. Preparation of monometallic (Pd, Ag) and bimetallic (Pd/Ag, Pd/Ni, Pd/Cu) nanoparticles via reversed micelles and their use in the Heck reaction. Tetrahedron 2012, 68, 3001–3011. [Google Scholar] [CrossRef]
- Notar Francesco, I.; Fontaine-Vive, F.; Antoniotti, S. Synergy in the catalytic activity of bimetallic nanoparticles and new synthetic methods for the preparation of fine chemicals. Chem. Cat. Chem. 2014, 6, 2784–2791. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Hernández-Fernández, P.; Rojas, S.; Ocón, P.; Gómez de la Fuente, J.L.; San Fabián, J.; Sanza, J.; Peña, M.A.; García-García, F.J.; Terreros, P.; Fierro, J.L.G. Influence of the preparation route of bimetallic Pt-Au nanoparticle electrocatalyst for the oxygen reduction reaction. J. Phys. Chem. B 2007, 111, 2913–2923. [Google Scholar] [CrossRef]
- Boutonnet, M.; Lögdberg, S.; Svensson, E.E. Recent developments in the aplication of nanoparticles prepared from w/o microemulsions in heterogeneous catalysis. Curr. Opin. Colloid Interface Sci. 2008, 13, 270–286. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Plasmonic Au@Pd nanoparticles supported on a basic metal-organic framework: Synergic boosting of H2 production from formic acid. ACS Energy Lett. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Wang, A.-Q.; Chang, C.-M.; Mou, C.-Y. Evolution of catalytic activity of Au-Ag bimetallic nanoparticles on mesoporous support for CO oxidation. J. Phys. Chem. B 2005, 109, 18860–18867. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem. Rev. 2013, 113, 2139–2181. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.M.; Pawelec, B.; Trejo, J.M.; Mariscal, R.; Fierro, J.L.G. Hydrogenation of aromatics on sulfur-resistant PtPd bimetallic catalysts. J. Catal. 2000, 189, 184–194. [Google Scholar] [CrossRef]
- Hermans, S.; Raja, R.; Thomas, J.M.; Johnson, B.F.G.; Sankar, G.; Gleeson, D. Solvent-free, low-temperature, selective hydrogenation of polyenes using a bimetallic nanoparticle Ru-Sn catalyst. Angew. Chem. Int. Ed. 2001, 40, 1211–1215. [Google Scholar] [CrossRef]
- Lu, P.; Teranishi, T.; Asakura, K.; Miyake, M.; Toshima, N. Polymer-protected Ni/Pd bimetallic nano-clusters: Preparation, characterization and catalysis for hydrogenation of nitrobenzene. J. Phys. Chem. B 1999, 103, 9673–9682. [Google Scholar] [CrossRef]
- Cheney, B.A.; Lauterbach, J.A.; Chen, J.G. Reverse micelle synthesis and characterization of supported Pt/Ni bimetallic catalysts on γ-Al2O3. App. Catal. A 2011, 394, 41–47. [Google Scholar] [CrossRef]
- Parera, J.M.; Beltramini, J.N. Stability of bimetallic reforming catalysts. J. Catal. 1988, 112, 357–365. [Google Scholar] [CrossRef]
- Skoplyak, O.; Menning, C.A.; Barteau, M.A.; Chen, J.G. Reforming of oxygenates for H2 production on 3d/Pt(111) bimetallic surfaces. Top. Catal. 2008, 51, 49–59. [Google Scholar] [CrossRef]
- Peng, X.; Pan, Q.; Rempel, G.L. Bimetallic dendrimer-encapsulated nanoparticles as catalysts: A review of the research advances. Chem. Soc. Rev. 2008, 37, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.; Naota, T.; Hirai, N. Aerobic oxidation of alcohols with ruthenium-cobalt bimetallic catalyst in the presence of aldehydes. J. Org. Chem. 1993, 58, 7318–7319. [Google Scholar] [CrossRef]
- Araña, J.; Ramírez de la Piscina, P.; Llorca, J.; Sales, J.; Homs, N.; Fierro, J.L.G. Bimetallic silica-supported catalysts based on Ni-Sn, Pd-Sn, and Pt-Sn as materials in the CO oxidation reaction. Chem. Mater. 1998, 10, 1333–1342. [Google Scholar] [CrossRef]
- Dhakad, M.; Fino, D.; Rayalu, S.; Kumar, R.; Watanabe, A.; Haneda, H.; Devotta, S.; Mitsuhashi, T.; Labhsetwar, N. Zirconia supported Ru-Co bimetallic catalysts for diesel soot oxidation. Top. Catal. 2007, 42, 273–276. [Google Scholar] [CrossRef]
- Zhao, L.; Thomas, J.P.; Heinig, N.F.; Abd-Ellah, M.; Wang, X.; Leung, K.T. Au-Pt alloy nanocatalysts for electro-oxidation of methanol and their application for fast-response non-enzymatic alcohol sensing. J. Mater. Chem. C 2014, 2, 2707–2714. [Google Scholar] [CrossRef]
- Shao, M.; Peles, A.; Shoemaker, K.; Gummalla, M.; Njoki, P.N.; Luo, J.; Zhong, C.-J. Enhanced oxygen reduction activity of platinum monolayer on gold nanoparticles. J. Phys. Chem. Lett. 2011, 2, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, Y.; Xin, H.L.; Hovden, R.; Ercius, P.; Mundy, J.A.; Chen, H.; Richard, J.H.; Muller, D.A.; DiSalvo, F.J.; et al. Tuning oxygen reduction reaction activity via controllable dealloying: A model study of ordered Cu3Pt/C intermetallic nanocatalysts. Nano Lett. 2012, 12, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Strasser, P. Electrocatalysis on bimetallic surfaces: Modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J. Am. Chem. Soc. 2007, 129, 12624–12625. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core-shell compositional fine structures of dealloyed PtxNi1-x nanoparticles and their impact on oxygen reduction catalysis. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis goes bio: From carbohydrates and sugar alcohols to platform chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Rao, L.; Ito, T.; Fukuoka, A. Ensemble and ligand effects in selective alkane hydrogenolysis catalyzed on well characterized rhodium-iridium and rhodium-iron bimetallic clusters inside NaY zeolite. Faraday Discuss. Chem. Soc. Rev. 1989, 87, 321–336. [Google Scholar]
- Zhou, S.; Johnson, M.; Veinot, J.G.C. Iron/iron oxide nanoparticles: A versatile support for catalytic metals and their application in Suzuki-Miyaura cross-coupling reactions. Chem. Commun. 2010, 46, 2411–2413. [Google Scholar] [CrossRef]
- Hudson, R.; Li, C.-J.; Moores, A. Magnetic copper-iron nanoparticles as simple heterogeneous catalysts for the azide-alkyne click reaction in water. Green Chem. 2012, 14, 622–624. [Google Scholar] [CrossRef]
- Rai, R.K.; Tyagi, D.; Gupta, K.; Singh, S.K. Activated nanostructured bimetallic catalysts for C-C coupling reactions: Recent progress. Catal. Sci. Technol. 2016, 6, 3341–3361. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef] [PubMed]
- Bracey, C.L.; Ellis, P.R.; Hutchings, G.J. Application of copper-gold alloys in catalysis: Current status and future perspectives. Chem. Soc. Rev. 2009, 38, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles—Novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Bönnemann, H.; Richards, R.M. Nanoscopic metal particles—Synthetic methods and potential applications. Eur. J. Inorg. Chem. 2001, 2001, 2455–2480. [Google Scholar] [CrossRef]
- Muñoz-Flores, B.M.; Kharisov, B.I.; Jiménez-Pérez, V.M.; Elizondo Martínez, P.; López, S.T. Recent advances in the synthesis and main applications of metallic nanoalloys. Ind. Eng. Chem. Res. 2011, 50, 7705–7721. [Google Scholar] [CrossRef]
- Suntivich, J.; Xu, Z.; Carlton, C.E.; Kim, J.; Han, B.; Lee, S.W.; Bonnet, N.; Marzari, N.; Allard, L.F.; Gasteiger, H.A.; et al. Surface composition tuning of Au-Pt bimetallic nanoparticles for enhanced carbon monoxide and methanol electro-oxidation. J. Am. Chem. Soc. 2013, 135, 7985–7991. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.J.; Sarma, L.S.; Chen, J.M.; Chen, C.H.; Shih, S.C.; Wang, G.R.; Liu, D.G.; Lee, J.F.; Tang, M.T. Structural models and atomic distribution of bimetallic nanoparticles as investigated by X-ray Absorption spectroscopy. J. Am. Chem. Soc. 2005, 127, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Somorjai, G.A.; Park, J.Y. Molecular factors of catalytic selectivity. Angew. Chem. Int. Ed. 2008, 47, 9212–9228. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Fisher, A.; Xu, Z.J. Surface segregation in bimetallic nanoparticles: A critical issue in electrocatalyst engineering. Small 2015, 11, 3221–3246. [Google Scholar] [CrossRef] [PubMed]
- Hartl, K.; Mayrhofer, K.J.J.; López, M.; Goia, D.; Arenz, M. AuPt core-shell nanocatalysts with bulk Pt activity. Electrochem. Commun. 2010, 12, 1487–1489. [Google Scholar] [CrossRef]
- Zhang, G.-R.; Zhao, D.; Feng, Y.-Y.; Zhang, B.; Su, D.S.; Liu, G.; Xu, B.-Q. Catalytic Pt-on-Au nanostructures: Why Pt becomes more active on smaller Au particles. ACS Nano 2012, 6, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Gao, G.; Huang, P.; Wang, K.; Wang, X.; Luo, T.; Zhang, C. Optical properties and catalytic activity of bimetallic gold-silver nanoparticles. Nano Biomed. Eng. 2010, 2, 258–267. [Google Scholar] [CrossRef]
- Chen, G.; Desinan, S.; Nechache, R.; Rosei, R.; Rosei, F.; Ma, D. Bifunctional catalytic/magnetic Ni@Ru core-shell nanoparticles. Chem. Commun. 2011, 47, 6308–6310. [Google Scholar] [CrossRef] [PubMed]
- Magno, L.M.; Sigle, W.; Aken, P.A.v.; Angelescu, D.G.; Stubenrauch, C. Microemulsions as reaction media for the synthesis of bimetallic nanoparticles: Size and composition of particles. Chem. Mater. 2010, 22, 6263–6271. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J. Am. Chem. Soc. 2007, 129, 8698. [Google Scholar] [CrossRef] [PubMed]
- Zubris, M.; King, R.B.; Garmestani, H.; Tannenbaum, R. FeCo nanoalloy formation by decomposition of their carbonyl precursors. J. Mater. Chem. 2005, 15, 1277–1285. [Google Scholar] [CrossRef]
- Kolb, U.; Quaiser, S.A.; Winter, M.; Reetz, M.T. Investigation of tetraalkylammonium bromide stabilized palladium/platinum bimetallic clusters using extended X-ray absorption fine structure spectroscopy. Chem. Mater. 1996, 8, 1889–1894. [Google Scholar] [CrossRef]
- Juškėnas, R.; Mockus, Z.; Kanapeckaitė, S.; Stalnionis, G.; Survila, A. XRD studies of the phase composition of the electrodeposited copper-rich Cu–Sn alloys. Electrochim. Acta 2006, 52, 928–935. [Google Scholar]
- Abdelsayed, V.; Aljarash, A.; El-Shall, M.S.; Othman, Z.A.; Alghamdi, A.H. Microwave synthesis of bimetallic nanoalloys and CO oxidation on ceria-supported nanoalloys. Chem. Mater. 2009, 21, 2825–2834. [Google Scholar] [CrossRef]
- Gerbec, J.A.; Magana, D.; Washington, A.; Strouse, G.F. Microwave-enhanced reaction rates for nanoparticle synthesis. J. Am. Chem. Soc. 2005, 127, 15791–15800. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, C. Preparation of Cu-Ni alloy nanocrystallites in water-in-oil microemulsions. J. Colloid Interface Sci. 2006, 293, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ma, D.; Bao, X. Emulsion-assisted synthesis of monodisperse binary metal nanoparticles. Chem. Commun. 2010, 46, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Parapat, R.Y.; Parwoto, V.; Schwarze, M.; Zhang, B.; Su, D.-S.; Schomäcker, R. A new method to synthesize very active and stable supported metal Pt catalysts: Thermo-destabilization of microemulsions. J. Mater. Chem. 2012, 22, 11605–11614. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.G.; Zhou, S. Size effect of Au seeds on structure of Au-Pt bimetallic nanoparticles. Mater. Lett. 2011, 65, 2649–2651. [Google Scholar] [CrossRef]
- Fletcher, P.D.I.; Howe, A.M.; Robinson, B.H. The kinetics of solubilisate exchange between water droplets of a water–in-oil microemulsion. J. Chem. Soc. Faraday Trans. 1987, 83, 985–1006. [Google Scholar] [CrossRef]
- Tojo, C.; de Dios, M.; López-Quintela, M.A. On the structure of bimetallic nanoparticles synthesized in microemulsions. J. Phys. Chem. C 2009, 113, 19145–19154. [Google Scholar] [CrossRef]
- López-Quintela, M.A. Synthesis of nanomaterials in microemulsions: Formation mechanisms and growth control. Curr. Opin. Colloid Interface. Sci. 2003, 8, 137–144. [Google Scholar] [CrossRef]
- Ban, I.; Drofenik, M.; Makovec, D. The synthesis of iron-nickel alloy nanoparticles using a reverse micelle technique. J. Magn. Magn. Mater. 2006, 307, 250–256. [Google Scholar] [CrossRef]
- Buceta, D.; Tojo, C.; Vukmirovik, M.; Deepak, F.L.; López-Quintela, M.A. Controlling bimetallic nanostructures by the microemulsion method with sub-nanometer resolution using a prediction model. Langmuir 2015, 31, 7435–7439. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Ramanujachary, K.V.; Lofland, S.E.; Furiato, A.; Gupta, G.; Shivaprasad, S.M.; Ganguli, A.K. Bimetallic Cu-Ni nanoparticles of varying composition (CuNi3, CuNi, Cu3Ni). Colloids Surf. A 2008, 331, 206–212. [Google Scholar] [CrossRef]
- Wu, M.; Lai, L. Synthesis of Pt/Ag bimetallic nanoparticles in water-in-oil microemulsions. Colloids Surf. A 2004, 244, 149–157. [Google Scholar] [CrossRef]
- Wei, G.; Dai, W.; Qian, L.; Cao, W.; Zhang, J. Reverse microemulsions synthesis and characterization of Pd-Ag bimetallic alloy catalysts supported on Al2O3 for acetylene hydrogenation. China Pet. Process. Petrochem. Technol. 2012, 14, 59–67. [Google Scholar]
- Solla-Gullón, J.; Vidal-Iglesias, F.J.; Montiel, V.; Aldaz, A. Electrochemical characterization of platinum-ruthenium nanoparticles prepared by water-in-oil microemulsion. Electrochim. Acta 2004, 49, 5079–5088. [Google Scholar] [CrossRef]
- Rojas, S.; García-García, F.J.; Jaeras, S.; Martínez-Huerta, M.V.; García Fierro, J.L.; Boutonnet, M. Preparation of carbon supported Pt and PtRu nanoparticles from microemulsion. Appl. Catal. A 2005, 285, 24–35. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, J.Y.; Han, M.; Chen, W.; Gan, L.M. Synthesis and characterization of PtRu/C catalysts from microemulsions and emulsions. J. Mater. Chem. 2002, 12, 2453–2458. [Google Scholar] [CrossRef]
- Kim, T.; Koboyashi, K.; Nagai, M. Preparation and characterization of platinum-ruthenium bimetallic nanoparticles using reverse microemulsions for fuel cell catalyst. J. Oleo Sci. 2007, 56, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chan, K.Y. Water-in-oil microemulsion synthesis of platinum-ruthenium nanoparticles, their characterization and electrocatalytic properties. Chem. Mater. 2003, 15, 451–459. [Google Scholar] [CrossRef]
- Wu, M.; Chen, D.; Huang, T. Preparation of Pd/Pt bimetallic nanoparticles in water/AOT/isooctane microemulsions. J. Colloids Interface Sci. 2001, 243, 102–108. [Google Scholar] [CrossRef]
- Touroude, R.; Girard, P.; Maire, G.; Kizling, J.; Boutonnet, M.; Stenius, P. Preparation of colloidal platinum/palladium alloy particles from non-ionic microemulsions: Characterization and catalytic behaviour. Colloids Surf. A 1992, 67, 9–19. [Google Scholar] [CrossRef]
- Yashima, M.; Falk, L.K.L.; Palmqvist, A.E.C.; Holmberg, K. Structure and catalytic properties of nanosized alumina supported platinum and palladium particles synthesized by reaction in microemulsion. J. Colloids Interface Sci. 2003, 268, 348–356. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C. Formation and characterization of Au-Ag bimetallic nanoparticles in water-in-oil microemulsions. J. Mater. Chem. 2002, 12, 1557–1562. [Google Scholar] [CrossRef]
- Cheng, J.; Bordes, R.; Olsson, E.; Holmberg, K. One-pot synthesis of porous gold nanoparticles by preparation of Ag/Au nanoparticles followed by dealloying. Colloids Surf. A 2013, 436, 823–829. [Google Scholar] [CrossRef]
- Pal, A.; Shah, S.; Devi, S. Preparation of silver, gold and silver-gold bimetallic nanoparticles in w/o microemulsion containing Triton X-100. Colloids Surf. A 2007, 302, 483–487. [Google Scholar] [CrossRef]
- Wu, M.; Chen, D.; Huang, T. Preparation of Au/Pt bimetallic nanoparticles in water-in-oil microemulsions. Chem. Mater. 2001, 13, 599–606. [Google Scholar] [CrossRef]
- Habrioux, A.; Vogel, W.; Guinel, M.; Guetaz, L.; Servat, K.; Kokoh, B.; Alonso-Vante, N. Structural and electrochemical studies of Au-Pt nanoalloys. Phys. Chem. Chem. Phys. 2009, 11, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Pal, A. Gold–platinum alloy nanoparticles through water-in-oil microemulsion. J. Nanostruct. Chem. 2015, 5, 65–69. [Google Scholar] [CrossRef]
- Wu, M.; Chen, D.; Huang, T. Synthesis of Au/Pd bimetallic nanoparticles in reverse micelles. Langmuir 2001, 17, 3877–3883. [Google Scholar] [CrossRef]
- Simoes, M.; Baranton, S.; Coutanceau, C. Electrooxidation of sodium borohydride at Pd, Au, and PdxAu1-x carbon-supported nanocatalysts. J. Phys. Chem. C 2009, 113, 13369–13376. [Google Scholar] [CrossRef]
- Li, T.; Zhou, H.; Huang, J.; Yin, J.; Chen, Z.; Liu, D.; Zhang, N.; Kuang, Y. Facile preparation of Pd-Au bimetallic nanoparticles via in-situ self-assembly in reverse microemulsion and their electrocatalytic properties. Colloids Surf. A 2014, 463, 55–62. [Google Scholar] [CrossRef]
- Weihua, W.; Xuelin, T.; Kai, C.; Gengyu, C. Synthesis and characterization of Pt-Cu bimetallic alloy nanoparticles by reverse micelles method. Colloids Surf. A 2006, 273, 35. [Google Scholar] [CrossRef]
- Zhang, X.; Chan, K.Y. Microemulsion synthesis and electrocatalytic properties of Platinum-Cobalt nanoparticles. J. Mater. Chem. 2002, 12, 1203–1206. [Google Scholar] [CrossRef]
- Xia, L.; Hu, X.; Kang, X.; Zhao, H.; Sun, M.; Cihen, X. A one-step facile synthesis of Ag-Ni core-shell nanoparticles in water-in-oil microemulsions. Colloids Surf. A 2010, 367, 96–101. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Abazari, R. Formation of dispersed palladium-nickel bimetallic nanoparticles in microemulsions: Synthesis, characterization, and their use as efficient heterogeneous recyclable catalysts for the amination reactions of aryl chlorides under mild conditions. RSC Adv. 2014, 4, 55815–55826. [Google Scholar] [CrossRef]
- Tojo, C.; Vila-Romeu, N. Kinetic study on the formation of bimetallic core-shell nanoparticles via microemulsions. Materials 2014, 7, 7513–7532. [Google Scholar] [CrossRef]
- López-Quintela, M.A.; Tojo, C.; Blanco, M.C.; García Río, L.; Leis, J.R. Microemulsion dynamics and reactions in microemulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 264–279. [Google Scholar] [CrossRef]
- Hwang, B.-J.; Tsai, Y.-W.; Sarma, L.S.; Tseng, Y.-L.; Liu, D.-G.; Lee, J.-F. Genesis of bimetallic Pt-Cu clusters in reverse micelles investigated by in situ X-ray absorption spectroscopy. J. Phys. Chem. B 2004, 108, 20427–20434. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical methods. In Fundamentals and Applications, 2nd ed.; John Wiley and Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Li, Y.; Jiang, Y.; Chen, M.; Liao, H.; Huang, R.; Zhou, Z.; Tian, N.; Chen, S.; Sun, S. Electrochemically shape-controlled synthesis of trapezohedral platinum nanocrystals with high electrocatalytic activity. Chem. Commun. 2012, 48, 9531–9533. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Eichelsdoerfer, D.J.; Rasin, B.; Zhou, Y.; Brown, K.A.; Liao, X.; Mirkin, C.A. Delineating the pathways for the site-directed synthesis of individual nanoparticles on surfaces. Proc. Natl. Acad. Sci. USA 2013, 110, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hwang, B.; Wang, G.; Subramanya Sarma, L.; Tang, M.; Liu, D.; Lee, J. Nucleation and Growth Mechanism of Pd/Pt Bimetallic Clusters in Sodium Bis(2-ethylhexyl) sulfosuccinate (AOT) Reverse Micelles as Studied by in Situ X-ray Absorption Spectroscopy. J. Phys. Chem. B 2005, 109, 21566–21575. [Google Scholar] [CrossRef] [PubMed]
- Tojo, C.; Buceta, D.; López-Quintela, M.A. Understanding the Metal Distribution in Core-Shell Nanoparticles Prepared in Micellar Media. Nanoscale Res. Lett. 2015, 10, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Tojo, C.; Buceta, D.; López-Quintela, M.A. A kinetic study on simultaneous reduction of metals within reverse micelles. 2017. to be published. [Google Scholar]

| N° | Metals | ∆ε0/V | Structure | Microemulsion; Reduction Agent; Metal Precursor | Film Flex | c/M | Size/nm | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ag-Pt | 0.06 | alloy | water/AOT/isooctane; N2H5OH; Ag+, PtCl62− | rigid | 0.1 | 4.2 | [77] |
| 2 | Pd-Ag | 0.12 | alloy | water/Brij30/n-octane; N2H4; Ag+, Pd2+ | rigid | - | 2 | [78] |
| 3 | alloy | water/AOT/isooctane; N2H4; Ag+, Pd2+ | rigid | - | - | [19] | ||
| 4 | Pt-Ru | 0.14 | alloy | water/Brij-30/n-heptane; NaBH4; PtCl62−, Ru3+ | rigid | 0.1 | 3.7 | [79] |
| 5 | alloy | water/Berol 050/isooctane; N2H5OH; PtCl62−, Ru3+ | flex | - | - | [80] | ||
| 6 | alloy | water/NP5-NP9/cyclohexane; NaBH4; PtCl62−, Ru3+ | flex | 0.025 | 4–9 | [81] | ||
| 7 | alloy | water/igepal CA-630/isooctane/2-propanol; NaBH4; PtCl62−, Ru3+ | flex | 0.004 | 2–4 | [82] | ||
| 8 | alloy | water/TritonX-100/propanol/cyclohexane; NaBH4; PtCl62−, Ru3+ | flex | 0.002 | 2.5–4.5 | [83] | ||
| 9 | Pt-Pd | 0.15 | alloy | water/AOT/isooctane; N2H5OH; PdCl42−, PtCl62− | rigid | 0.1 | 9.8 | [84] |
| 10 | alloy | water/C12E5/hexadecane; N2H5OH; Pd2+, PtCl62− | flex | - | - | [85] | ||
| 11 | alloy | water/Brij-L4/cyclohexane; N2H5OH; Pd2+, Pt2+ | rigid | - | - | [86] | ||
| 12 | Au-Ag | 0.20 | Au core-enriched in Ag shell | water/AOT/isooctane; N2H5OH; Ag+, AuCl4− | rigid | 0.1 | 5.1 | [87] |
| 13 | alloy | water/C11E3-C11E5/cyclohexane; NaBH4; Ag+, AuCl4− | flex | 0.05 | 6.7 | [88] | ||
| 14 | alloy | water/TritonX-100/cyclohexane NaBH4; Ag+, AuCl4− | flex | 0.05 | 23 | [89] | ||
| 15 | Fe-Ni | 0.20 | alloy | water/CTAB/isooctane/n-butanol; NaBH4; Fe2+, Ni2+ | very flex | 0.4/0.1 | 4–12 | [74] |
| 16 | Au-Pt | 0.26 | core-shell | water/AOT/isooctane; N2H5OH; AuCl4−, PtCl62− | rigid | 0.5 | 3.8 | [90] |
| 17 | alloy/Pt enriched surface | water/Brij 30/n-heptane; NaBH4; AuCl4−, PtCl62− | rigid | - | - | [91] | ||
| 18 | core-shell | water/tergitol/isooctane; N2H5OH; AuCl4−, PtCl62− | flex | 0.08–0.4 | - | [75] | ||
| 19 | alloy | water/tergitol 15-S-5/isooctane; N2H5OH; AuCl4−, PtCl62− | flex | - | - | [22] | ||
| 20 | alloy | water/TritonX-100/cyclohexane/1-hexanol; NaBH4; AuCl4−, PtCl62− | flex | 2.7 × 10−4 | 2.5 | [92] | ||
| 21 | Au-Pd | 0.39 | core-shell | water/AOT/isooctane; N2H5OH; AuCl4−, PdCl42− | rigid | 0.5 | 2.8 | [93] |
| 22 | enriched in Au core/enriched in Pd shell | water/Brij-30/n-heptane; NaBH4; AuCl4−, PdCl42− | rigid | - | 5.0 | [94] | ||
| 23 | alloy | water/TritonX-100/n-hexane/n-hexanol; N2H5OH; AuCl4−, PdCl64− | flex | 0.005/0.006 | 5.1 | [95] | ||
| 24 | Pt-Cu | 0.40 | alloy | water/AOT/hexane; N2H5OH; Cu2+, PtCl62− | rigid | 0.058/0.015 | 3 | [16] |
| 25 | PtCu3 alloy | water/CTAB/isooctane/n-butanol; N2H5OH; Cu2+, PtCl62− | very flex | 0.02 | 1.6 | [96] | ||
| 26 | Pt-Bi | 0.43 | PtBi2 intermetall | water/Brij-30/n-octane/1-octanol; NaBH4; Bi3+, PtCl62− | rigid | 0.013 | 4.5 | [60] |
| 27 | Cu-Ni | 0.59 | alloy | water/SDS/n-butanol/n-heptane; N2H5OH; Cu2+, Ni2+ | very flex | - | 4.6–9.3 | [67] |
| 28 | alloy | water/CTAB/isooctane/1-butanol; N2H5OH; Cu2+, Ni2+ | very flex | 0.1 | 7 | [76] | ||
| 29 | Pt-Pb | 0.87 | intermetal (Pt/Pb3, Pt/Pb) | water/Brij-30/n-octane/1-octanol; NaBH4; Pb2+, PtCl62− | rigid | 0.013 | 4.0 | [60] |
| 30 | Pt-Co | 1.02 | alloy | water/TritonX-100/cyclohexane/propanol; NaBH4; PtCl62−, Co2+ | flex | 0.04/0.120 | 3–4 | [97] |
| 31 | Ag-Ni | 1.04 | core-shell | water/OP-4,OP-7/n-heptane; NaBH4; Ag+, Ni2+ | flex | - | 50–100 | [98] |
| 32 | Pd-Ni | 1.15 | alloy | water/AOT/isooctane; N2H5OH; Pd2+, Ni2+ | rigid | 0.0017 | 6–20 | [99] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tojo, C.; Buceta, D.; López-Quintela, M.A. On Metal Segregation of Bimetallic Nanocatalysts Prepared by a One-Pot Method in Microemulsions. Catalysts 2017, 7, 68. https://doi.org/10.3390/catal7020068
Tojo C, Buceta D, López-Quintela MA. On Metal Segregation of Bimetallic Nanocatalysts Prepared by a One-Pot Method in Microemulsions. Catalysts. 2017; 7(2):68. https://doi.org/10.3390/catal7020068
Chicago/Turabian StyleTojo, Concha, David Buceta, and Manuel Arturo López-Quintela. 2017. "On Metal Segregation of Bimetallic Nanocatalysts Prepared by a One-Pot Method in Microemulsions" Catalysts 7, no. 2: 68. https://doi.org/10.3390/catal7020068






