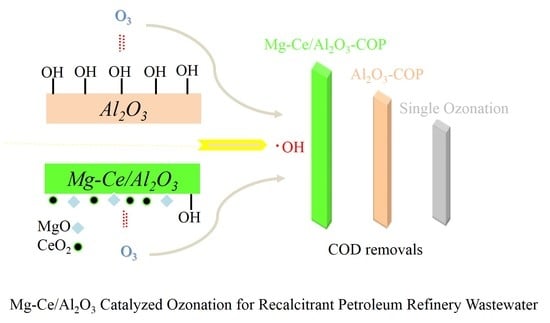
Comparison of Efficiencies and Mechanisms of Catalytic Ozonation of Recalcitrant Petroleum Refinery Wastewater by Ce, Mg, and Ce-Mg Oxides Loaded Al2O3
Abstract
:1. Introduction
2. Results and Discussion
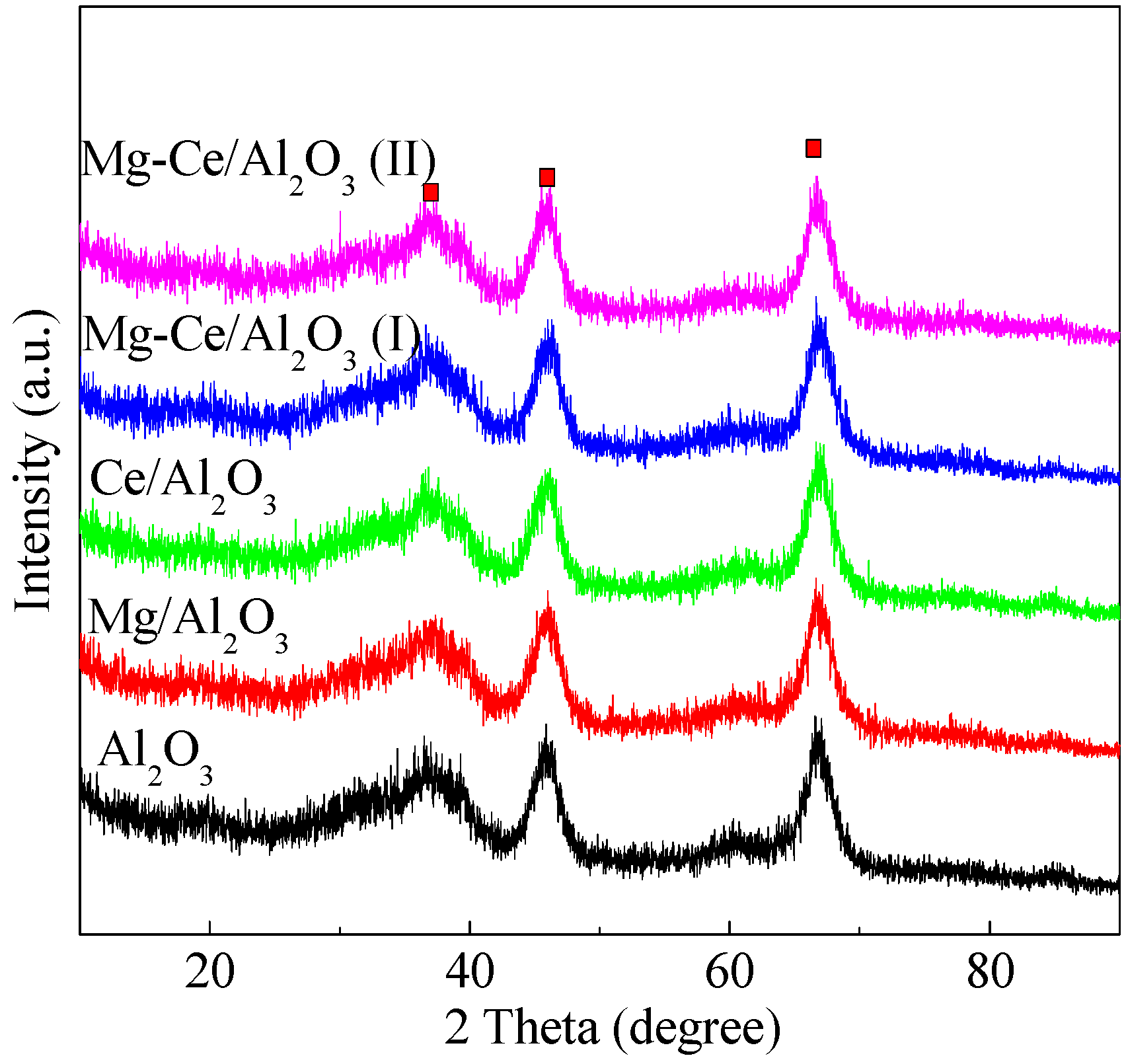
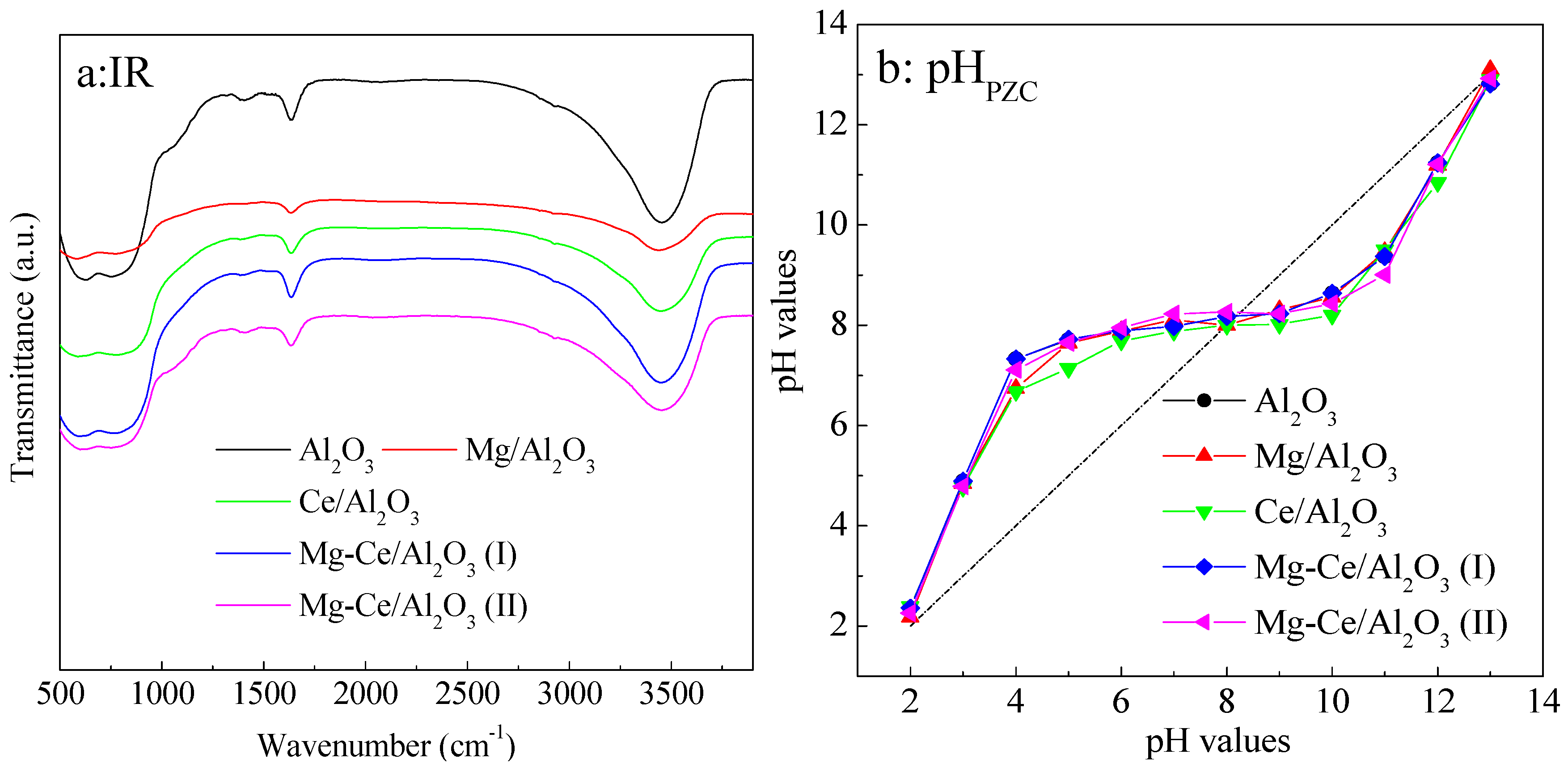
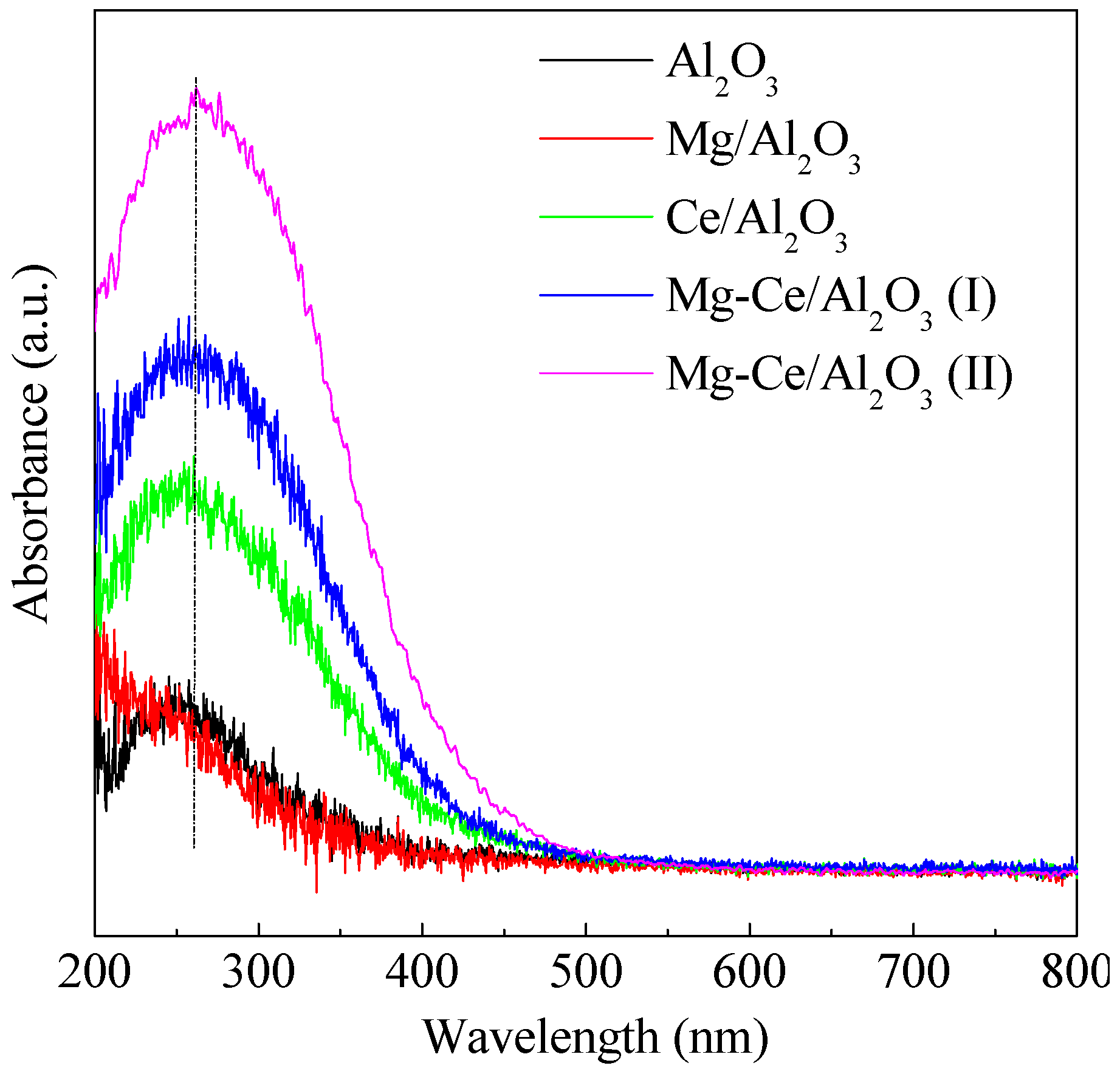
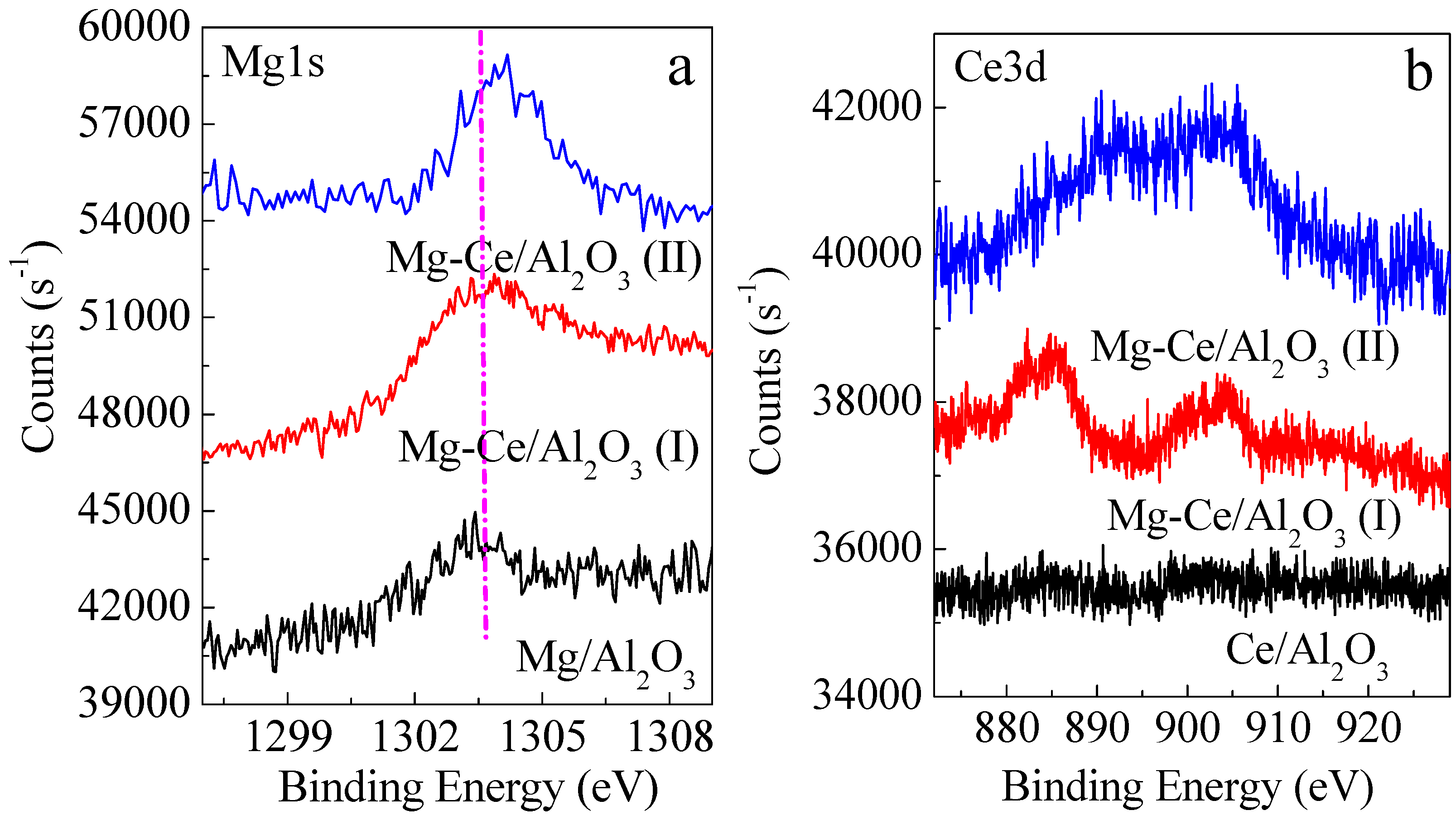
2.1. Characteristics of Catalysts
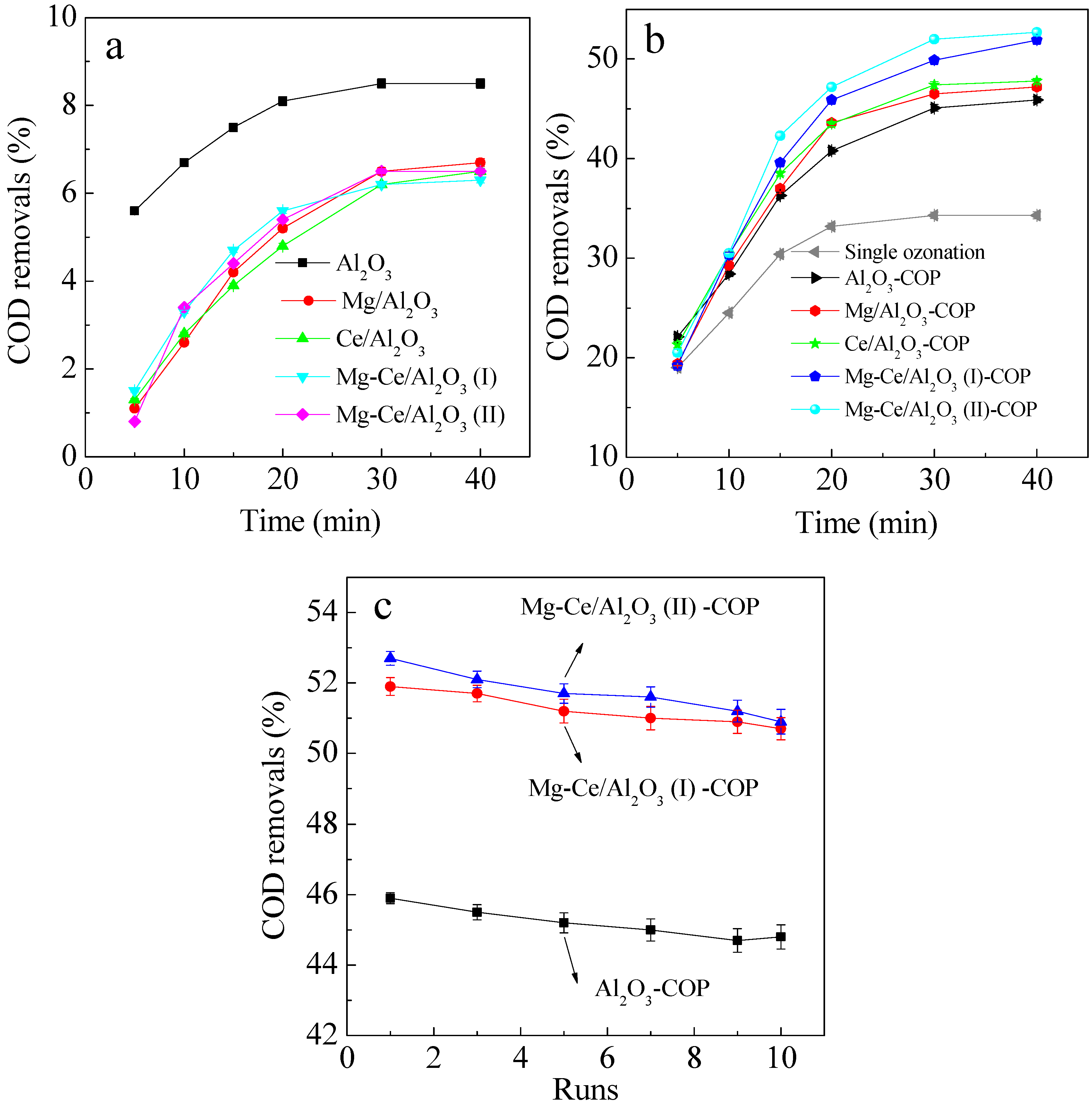
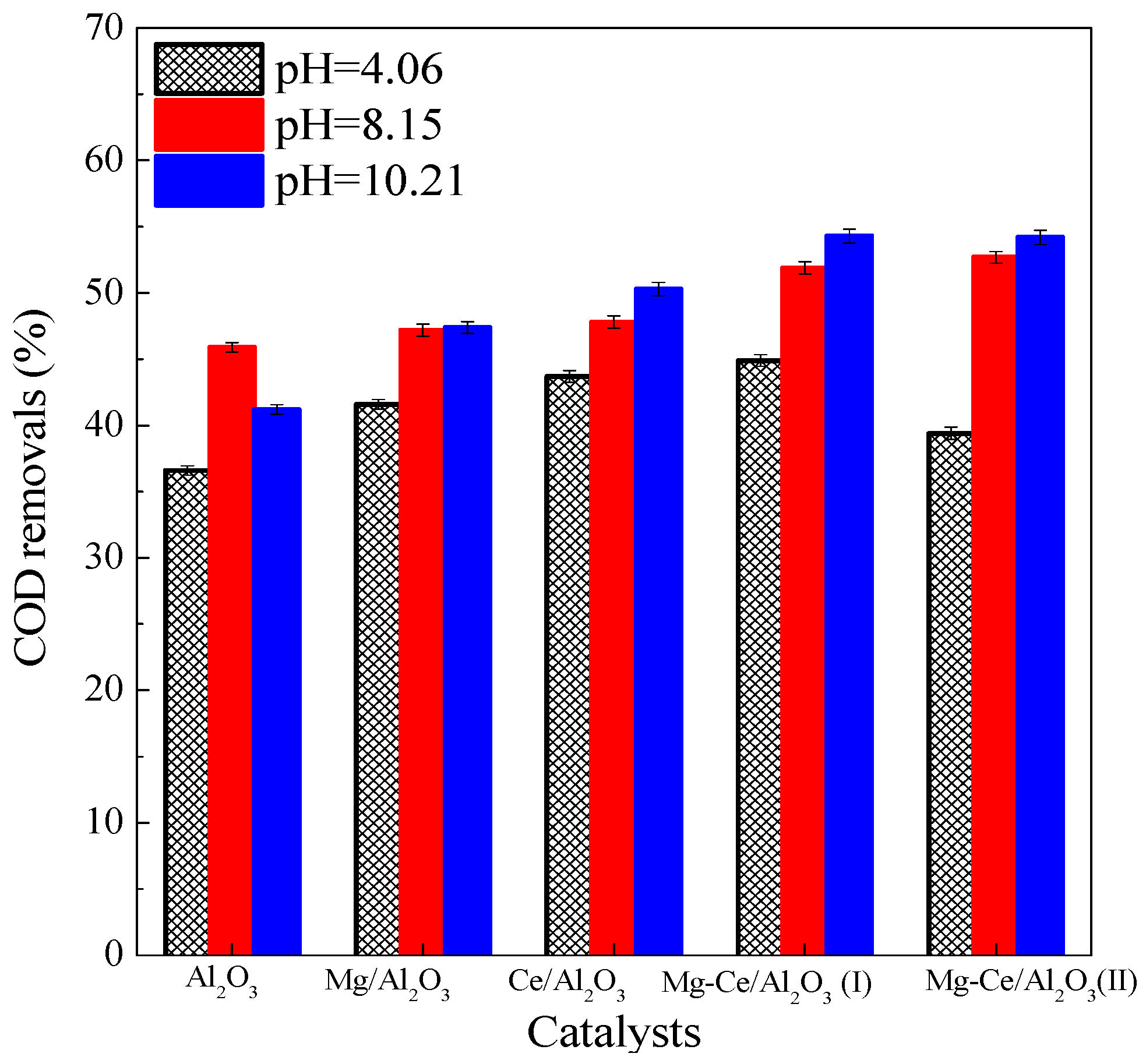
2.2. Efficiencies of Catalysts
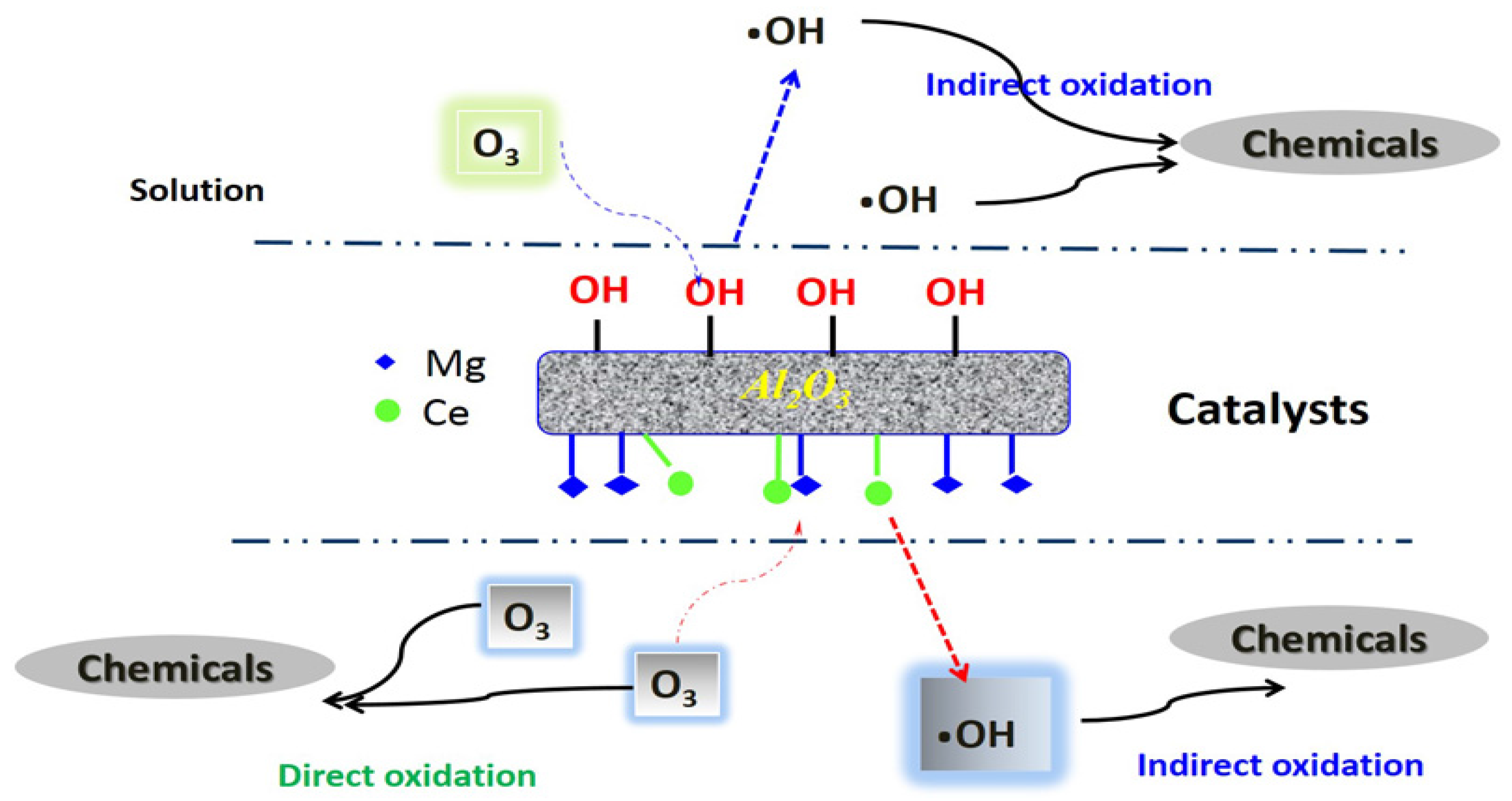
2.3. Mechanisms of Catalytic Ozonation
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization of Catalysts
3.3. Ozonation of RPRW
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Al Zarooni, M.; Elshorbagy, W. Characterization and assessment of Al Ruwais refinery wastewater. J. Hazard. Mater. 2006, 136, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhan, Y.; Chen, C.M.; Zhao, L.; Guo, S. The influence of microbial synergistic and antagonistic effects on the performance of refinery wastewater microbial fuel cells. J. Power Sources 2014, 251, 229–236. [Google Scholar] [CrossRef]
- EI-Naas, M.H.; Al-Zuhair, S.; Alhaija, M.A. Reduction of COD in refinery wastewater through adsorption on date-pit activate carbon. J. Hazard. Mater. 2009, 173, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, L.; Chen, Y.; Cheng, Y.; Liu, Y.; Zha, X. Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways. Water Res. 2016, 92, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, D.; Roy, R.A. Advances in catalytic oxidation of organic pollutants—Prospects for thorough mineralization by natural clay catalysts. Appl. Catal. B 2015, 174–175, 277–292. [Google Scholar] [CrossRef]
- Ma, J.; Sui, M.; Zhang, T.; Guan, C. Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene. Water Res. 2005, 39, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ye, W.; Zhang, Q.; Sun, F.; Lu, P.; Li, X. Catalytic ozonation of dimethyl phthalate over cerium supported on activated carbon. J. Hazard. Mater. 2009, 170, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Xu, B.; Chen, Z.; Ma, J.; Sun, D.; Zhang, L. Influence of aluminum oxides surface properties on catalyzed ozonation of 2,4,6-trichloroanisole. Sep. Purif. Technol. 2009, 66, 405–410. [Google Scholar] [CrossRef]
- Vittenet, J.; Aboussaoud, W.; Mendret, J.; Pic, J.; Debellefontaine, H.; Lesagee, N.; Faucher, K.; Manero, M.H.; Thibault-Starzyk, F.; Leclerc, H.; et al. Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl. Catal. A 2015, 504, 519–532. [Google Scholar] [CrossRef]
- Chen, C.; Wei, L.; Guo, X.; Guo, S.; Yan, G. Investigation of heavy oil refinery wastewater treatment by integrated ozone and activated carbon supported manganese oxides. Fuel Process. Technol. 2014, 124, 165–173. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Guo, X.; Guo, S.; Yan, G. Advanced ozone treatment of heavy oil refining wastewater by activated carbon supported iron oxide. J. Ind. Eng. Chem. 2014, 20, 2782–2791. [Google Scholar] [CrossRef]
- Moussavi, G.; Aghapour, A.A.; Yaghmaeian, K. The degradation and mineralization of catechol using ozonation catalyzed with MgO/GAC composite in a fluidized bed reactor. Chem. Eng. J. 2014, 249, 302–310. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, J.; Chen, J.; Chen, J. Ozonation catalyzed by cerium supported on activated carbon for the degradation of typical pharmaceutical wastewater. Sep. Purif. Technol. 2014, 127, 112–120. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, H.; Hou, B.; Jia, S.; Zhao, Q. Heterogeneous catalytic ozonation of biologically pretreated Lurgi coal gasification wastewater using sewage sludge based activated carbon supported manganese and ferric oxides as catalysts. Bioresource Technol. 2014, 166, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sa´nchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manage. 2007, 85, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Pocostales, P.; Álvarez, P.; Beltrán, F.J. Catalytic ozonation promoted by alumina-based catalysts for the removal of some pharmaceutical compounds from water. Chem. Eng. J. 2011, 168, 1289–1295. [Google Scholar] [CrossRef]
- Roshani, B.; McMaster, I.; Rezaei, E.; Soltan, J. Catalytic ozonation of benzotriazole over alumina supported transition metal oxide catalysts in water. Sep. Purif. Technol. 2014, 135, 158–164. [Google Scholar] [CrossRef]
- Yang, L.; Hu, C.; Nie, Y.; Qu, J. Catalytic ozonation of selected pharmaceuticals over mesoporous alumina-supported manganese oxide. Environ. Sci. Technol. 2009, 43, 2525–2529. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, N.; Hu, C. Enhanced inhibition of bromate formation in catalytic ozonation of organic pollutants over Fe-Al LDH/Al2O3. Sep. Purif. Technol. 2015, 151, 256–261. [Google Scholar] [CrossRef]
- Chen, K.C.; Wang, Y.H. The effects of Fe-Mn oxide and TiO2/α-Al2O3 on the formation of disinfection by-products in catalytic ozonation. Chem. Eng. J. 2014, 253, 84–92. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Bradu, C.; Udrea, I.; Mihalache, N.; Ruta, F. Degradation of oxalic acid from aqueous solutions by ozonation in presence of Ni/Al2O3 catalysts. Catal. Commun. 2008, 9, 2386–2391. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.; Wang, C.; Yang, S.; Zhu, W. Catalytic ozonation of dimethyl phthalate with RuO2/Al2O3 catalysts prepared by microwave irradiation. Catal. Commun. 2013, 41, 1–5. [Google Scholar] [CrossRef]
- Chen, C.; Yoza, B.A.; Wang, Y.; Wang, P.; Li, Q.X.; Guo, S.; Yan, G. Catalytic ozonation of petroleum refinery wastewater utilizing Mn-Fe-Cu/Al2O3 catalyst. Environ. Sci. Pollut. Res. 2015, 22, 5552–5562. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Shi, R.; Zhang, H.; Ma, C. Catalytic performance of Fe3O4-CoO/Al2O3 catalyst in ozonation of 2-(2, 4-dichlorophenoxy) propionic acid, nitrobenzene and oxalic acid in water. J. Environ. Sci. 2010, 22, 1623–1628. [Google Scholar] [CrossRef]
- Xu, B.; Qi, F.; Sun, D.; Chen, Z.; Robert, D. Cerium doped red mud catalytic ozonation for bezafibrate degradation in wastewater: Efficiency, intermediates, and toxicity. Chemosphere 2016, 146, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, J.; Yoza, B.A.; Li, Q.X.; Wang, G. A novel "wastes-treat-wastes" technology: role and potential of spent fluid catalytic cracking catalyst assisted ozonation of petrochemical wastewater. J. Environ. Manage. 2015, 152, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wang, J.; Yu, J.; Chen, J.; Chen, J. Catalytic ozonation for the degradation of acetylsalicylic acid in aqueous solution by magnetic CeO2 nanometer catalyst particles. Appl. Catal. B 2014, 144, 686–693. [Google Scholar] [CrossRef]
- Yan, H.; Lu, P.; Pan, Z.; Wang, X.; Zhang, Q.; Li, L. Ce/SBA-15 as a heterogeneous ozonation catalyst for efficient mineralization of dimethyl phthalate. J. Mol. Catal. A 2013, 377, 57–64. [Google Scholar] [CrossRef]
- Bing, J.; Wang, X.; Lan, B.; Liao, G.; Zhang, Q.; Li, L. Characterization and reactivity of cerium loaded MCM-41 for p-chlorobenzoic acid mineralization with ozone. Sep Purif. Technol. 2013, 118, 479–486. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yoza, B.A.; Chen, H.; Li, Q.X.; Guo, S. Manganese sand ore is an economical and effective catalyst for ozonation of organic contaminants in petrochemical wastewater. Water Air Soil Poll. 2015, 226, 182. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Pan, Z.; Ma, S.; Li, L. Synthesis of MnOx/SBA-15 for Norfloxacin degradation by catalytic ozonation. Sep. Purif. Technol. 2017, 173, 99–104. [Google Scholar] [CrossRef]
- Pines, D.S.; Reckhow, D.A. Solid phase catalytic ozonation process for the destruction of a model pollutant. Ozone: Sci. Eng. 2003, 25, 25–39. [Google Scholar] [CrossRef]
- Yang, L.; Hu, C.; Nie, Y.; Qu, J. Surface acidity and reactivity of β-FeOOH/Al2O3 for pharmaceuticals degradation with ozone: In situ ATR-FTIR studies. Appl. Catal. B 2010, 97, 340–346. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Lu, J.; Xiong, Y. Catalytic performance of MgO with different exposed crystal facets towards the ozonation of 4-chlorophenol. Appl. Catal. A 2015, 506, 118–125. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, F.J.; Montero-de-Espinosa, R. A TiO2/Al2O3 catalyst to improve the ozonation of oxalic acid in water. Appl. Catal. B 2004, 47, 101–109. [Google Scholar] [CrossRef]
- Xu, R.R.; Pang, W.Q.; Yu, J.H.; Huo, Q.S.; Chen, J.S. Chemistry Zeolites and Porous Materials, 1st ed.; Science Press: Beijing, China, 2004; pp. 145–149. (in Chinese) [Google Scholar]
- Rao, G.R.; Sahu, H.R. XRD and UV-Vis diffuse reflectance analysis of CeO2-ZrO2 solid solutions synthesized by combustion method. J. Chem. Sci. 2001, 113, 651–658. [Google Scholar]
- Mittal, V.K.; Bera, S.; Nithya, R.; Srinivasan, M.P.; Velmurugan, S.; Narasimhan, S.V. Solid state synthesis of Mg–Ni ferrite and characterization by XRD and XPS. J. Nucl. Mater. 2004, 335, 302–310. [Google Scholar] [CrossRef]
- Sheerin, E.; Reddy, G.K.; Smirniotis, P. Evaluation of Rh/CexTi1−xO2 catalysts for synthesis of oxygenates from syngas using XPS and TPR techniques. Catal. Today 2016, 263, 75–83. [Google Scholar] [CrossRef]
- Lu, X.; Huangfu, X.; Ma, J. Removal of trace mercury(II) from aqueous solution by in situ formed Mn-Fe (hydr) oxides. J. Hazard. Mater. 2014, 280, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qu, H.; Liu, R.; Wu, R. Preparation and evaluation of a novel Fe-Mn binary oxide adsorbent for effective arsenite removal. Water Res. 2007, 41, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, J.; Yu, M. The microtopography of manganese dioxide formed in situ and its adsorptive properties for organic micropollutants. Solid State Sci. 2008, 10, 148–153. [Google Scholar] [CrossRef]
- Qi, F.; Xu, B.; Zhao, L.; Chen, Z.; Zhang, L.; Sun, D.; Ma, J. Comparison of the efficiency and mechanism of catalytic ozonation of 2,4,6-trichloroanisole by iron and manganese modified bauxite. Appl. Catal. B 2012, 121–122, 171–181. [Google Scholar] [CrossRef]
- Miyakoshi, A.; Ueno, A.; Ichikawa, M. XPS and TPD characterization of manganese-substituted iron-potassium oxide catalysts which are selective for dehydrogenation of ethylbenzene into styrene. Appl. Catal. A 2001, 219, 249–258. [Google Scholar] [CrossRef]
- Altenor, S.; Carene, B.; Emmanuel, E.; Lambert, J.; Ehrhardt, J.; Gaspard, S. Adsorption studies of methylene blue and phenol onto vetiver roots activated carbon prepared by chemical activation. J. Hazard. Mater. 2009, 165, 1029–1039. [Google Scholar] [CrossRef] [PubMed]

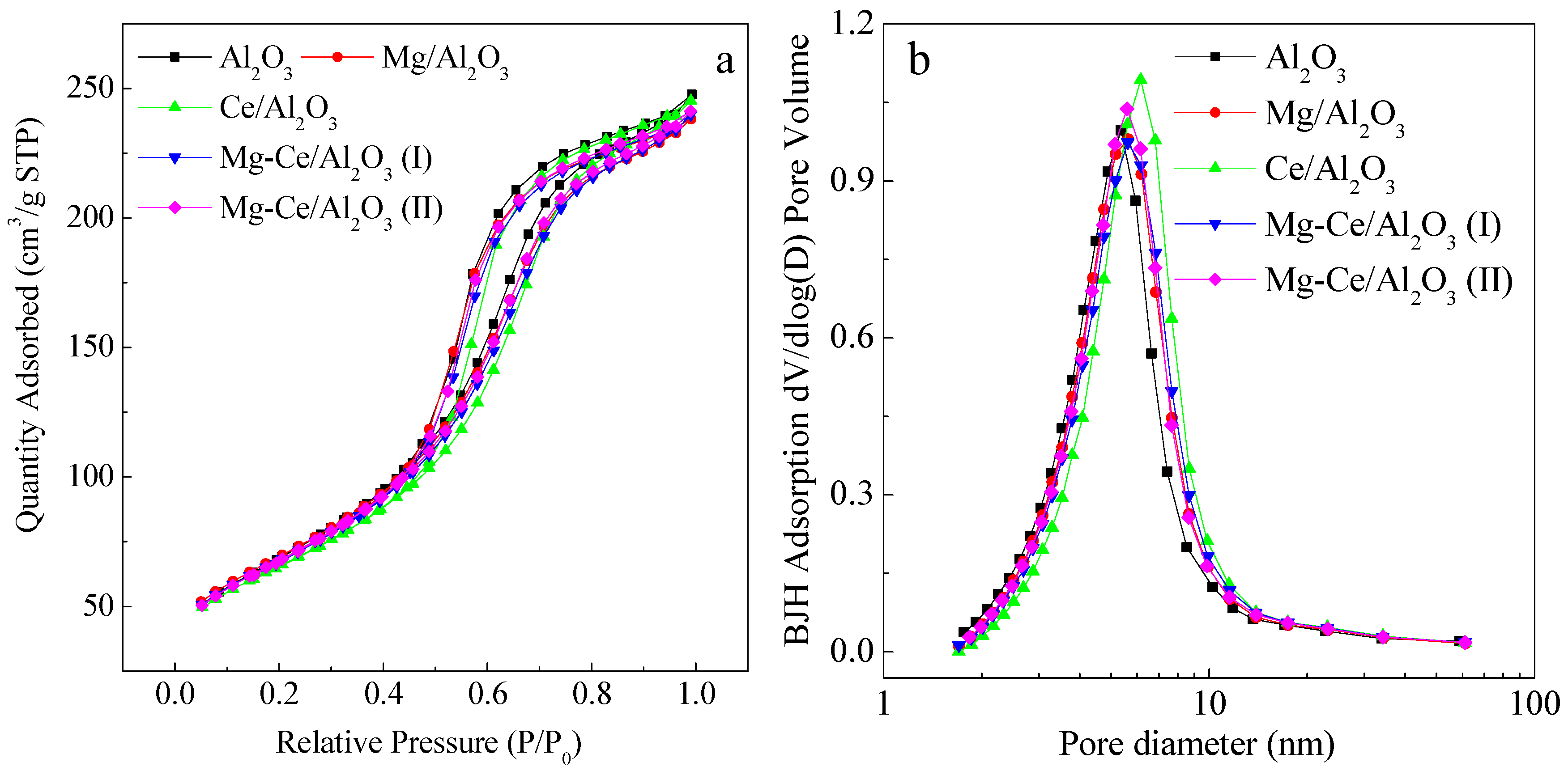

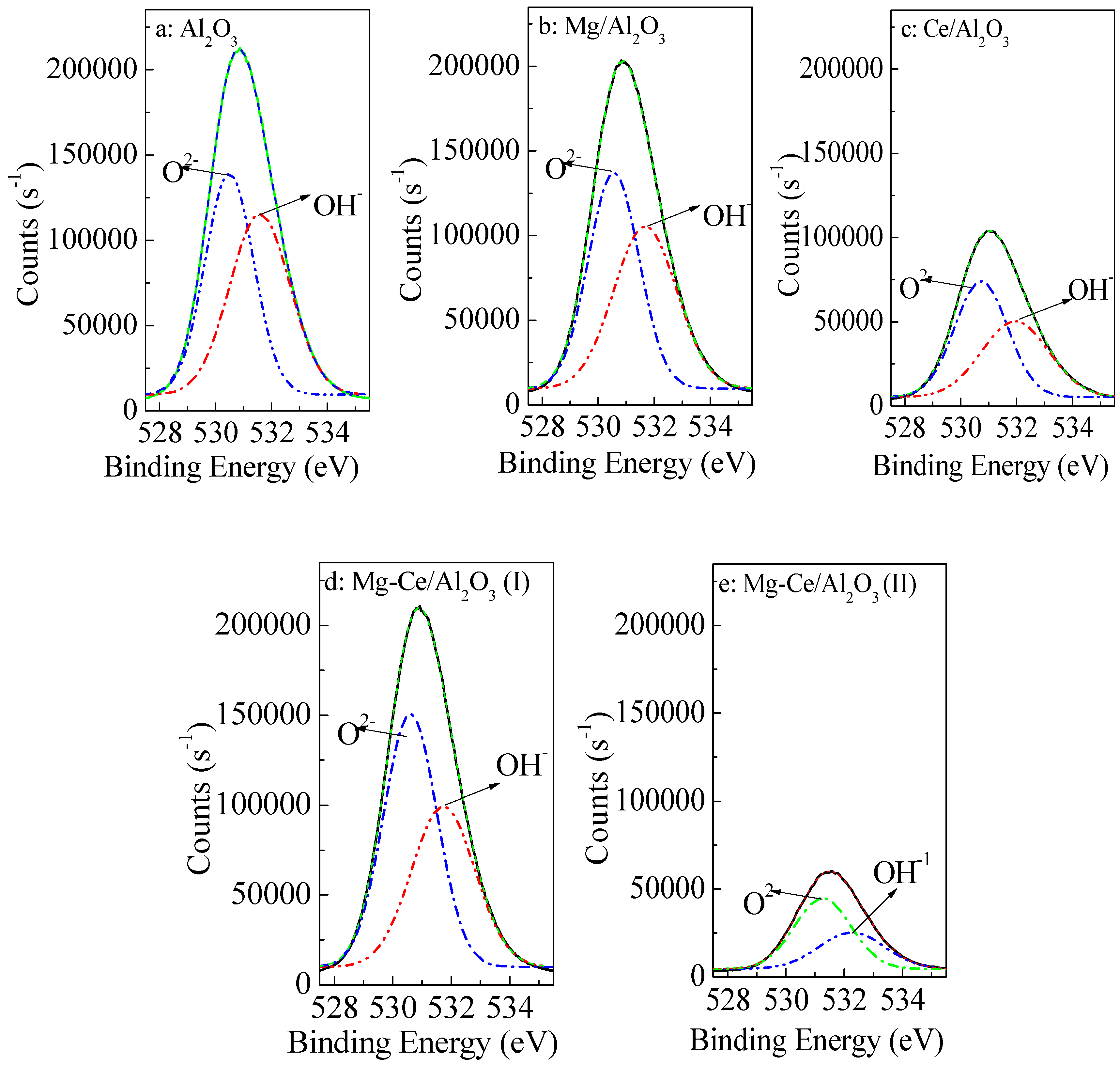
| Catalysts | Surface Areas and Pore Structures | Metal Oxide Contents (wt %) | |||
|---|---|---|---|---|---|
| SBET (m2/g) | VP (cm3/g) | Da (nm) | MgO | CeO2 | |
| Al2O3 | 250 | 0.36 | 6.1 | - | - |
| Mg/Al2O3 | 250 | 0.38 | 5.9 | 0.30 | - |
| Ce/Al2O3 | 246 | 0.38 | 6.1 | - | 0.31 |
| Mg-Ce/Al2O3 (I) | 245 | 0.38 | 6.0 | 0.31 | 0.33 |
| Mg-Ce/Al2O3 (II) | 246 | 0.37 | 6.1 | 0.92 | 0.90 |
| Items | Mg/Al2O3 | Ce/Al2O3 | Mg-Ce/Al2O3 (I) | Mg-Ce/Al2O3 (II) | |
|---|---|---|---|---|---|
| Surface molar ratios | Mg1s/Al2p | 0.009 | - | 0.010 | 0.0099 |
| Ce3d/Al2p | - | 0.0012 | 0.002 | 0.0079 | |
| Ce3d/Mg1s | - | - | 0.2 | 0.8 | |
| Mg1s + Ce3d/Al2p | 0.009 | 0.0012 | 0.012 | 0.0198 | |
| Surface contents (wt %) | MgO | 0.71 | - | 0.78 | 0.75 |
| CeO2 | - | 0.40 | 0.67 | 2.57 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Chen, Y.; Yoza, B.A.; Du, Y.; Wang, Y.; Li, Q.X.; Yi, L.; Guo, S.; Wang, Q. Comparison of Efficiencies and Mechanisms of Catalytic Ozonation of Recalcitrant Petroleum Refinery Wastewater by Ce, Mg, and Ce-Mg Oxides Loaded Al2O3. Catalysts 2017, 7, 72. https://doi.org/10.3390/catal7030072
Chen C, Chen Y, Yoza BA, Du Y, Wang Y, Li QX, Yi L, Guo S, Wang Q. Comparison of Efficiencies and Mechanisms of Catalytic Ozonation of Recalcitrant Petroleum Refinery Wastewater by Ce, Mg, and Ce-Mg Oxides Loaded Al2O3. Catalysts. 2017; 7(3):72. https://doi.org/10.3390/catal7030072
Chicago/Turabian StyleChen, Chunmao, Yu Chen, Brandon A. Yoza, Yuhao Du, Yuxian Wang, Qing X. Li, Lanping Yi, Shaohui Guo, and Qinghong Wang. 2017. "Comparison of Efficiencies and Mechanisms of Catalytic Ozonation of Recalcitrant Petroleum Refinery Wastewater by Ce, Mg, and Ce-Mg Oxides Loaded Al2O3" Catalysts 7, no. 3: 72. https://doi.org/10.3390/catal7030072