Suzuki-Miyaura C-C Coupling Reactions Catalyzed by Supported Pd Nanoparticles for the Preparation of Fluorinated Biphenyl Derivatives
Abstract
:1. Introduction
2. Results and Discussion
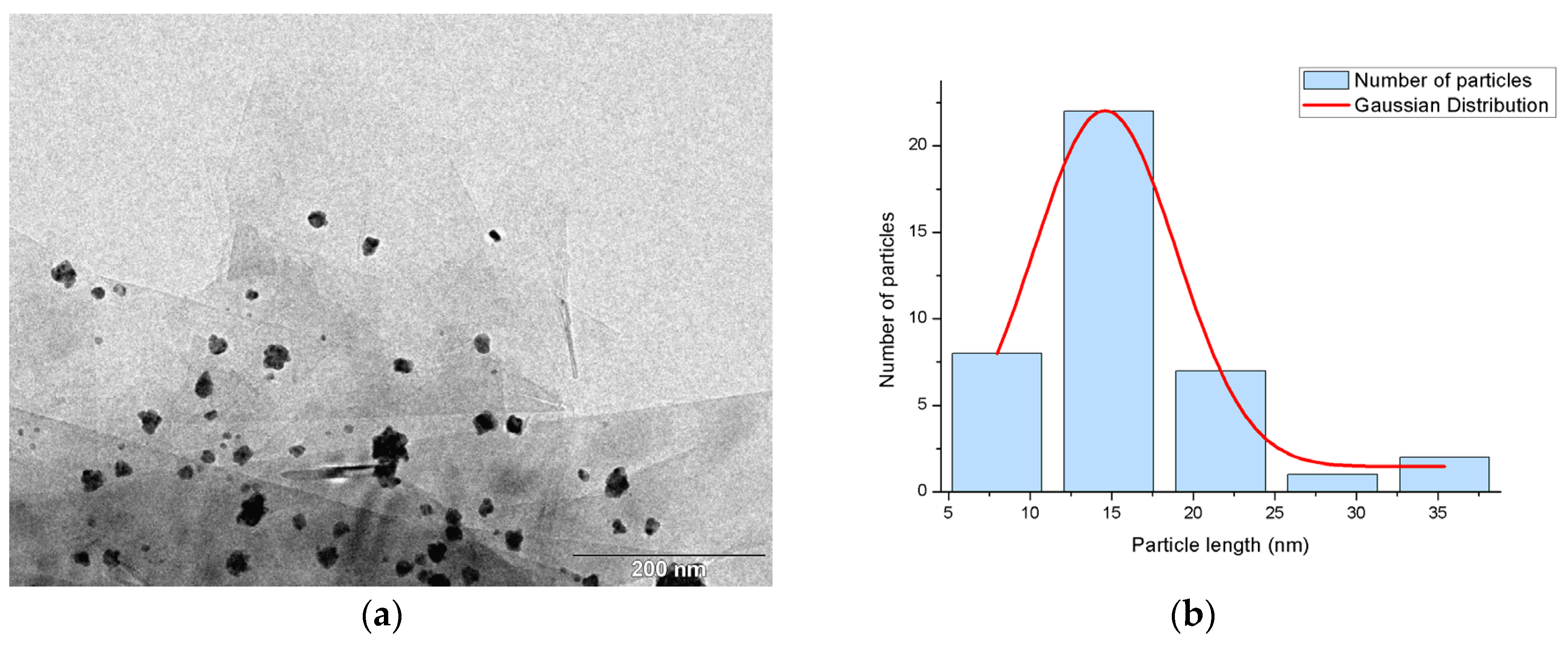
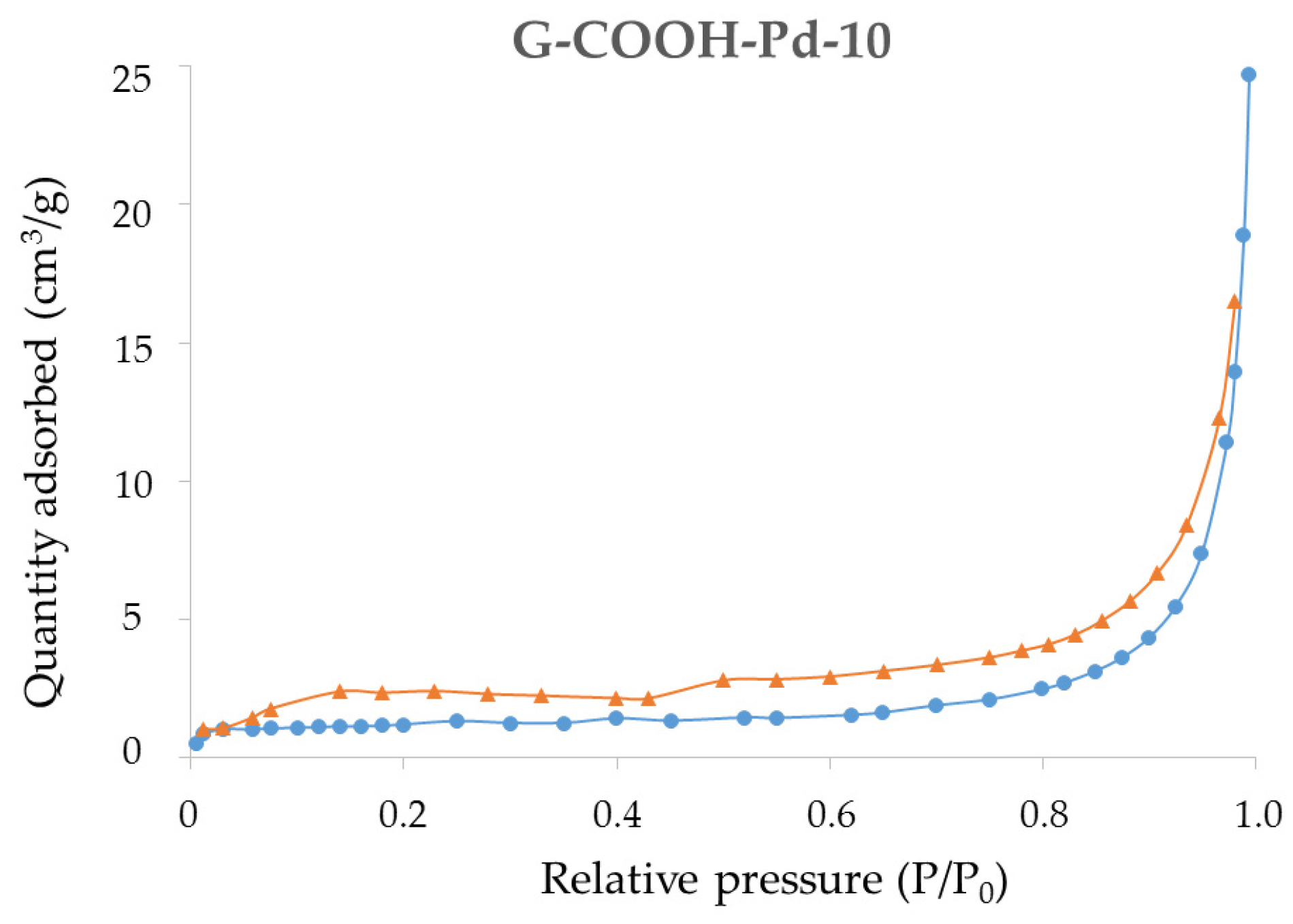
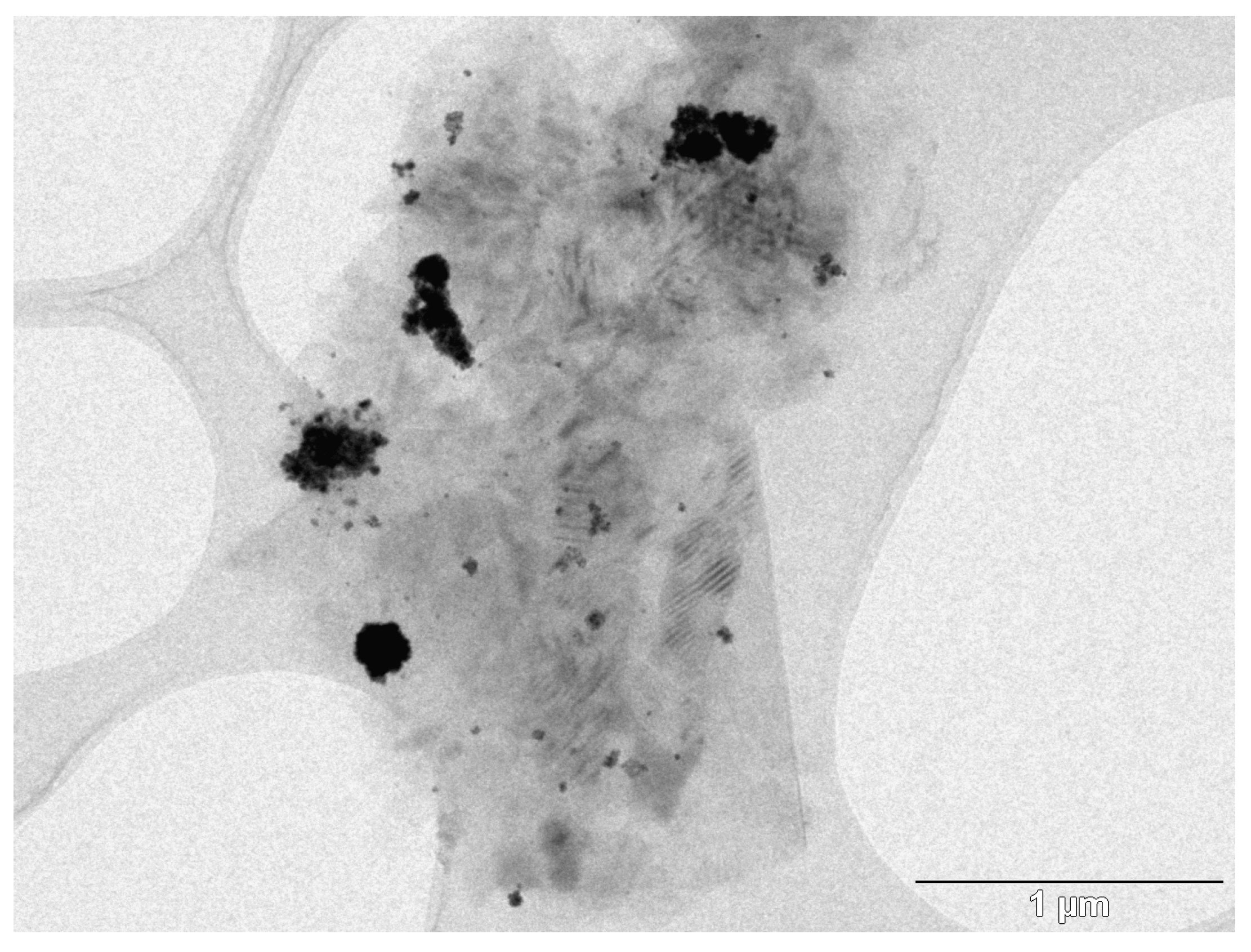
2.1. Synthesis and Characterization of the Supported PdNPs
2.2. Catalytic Study
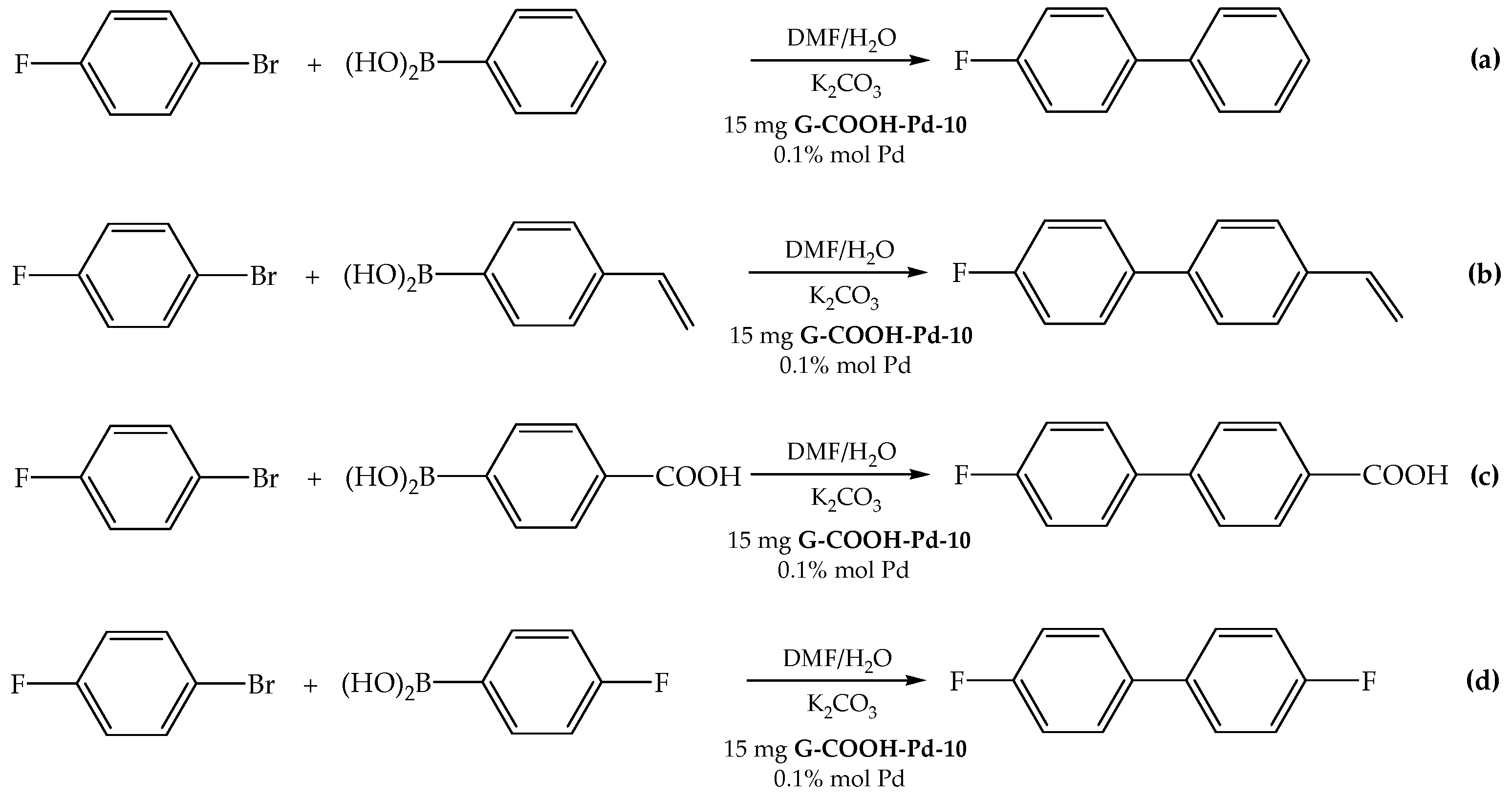
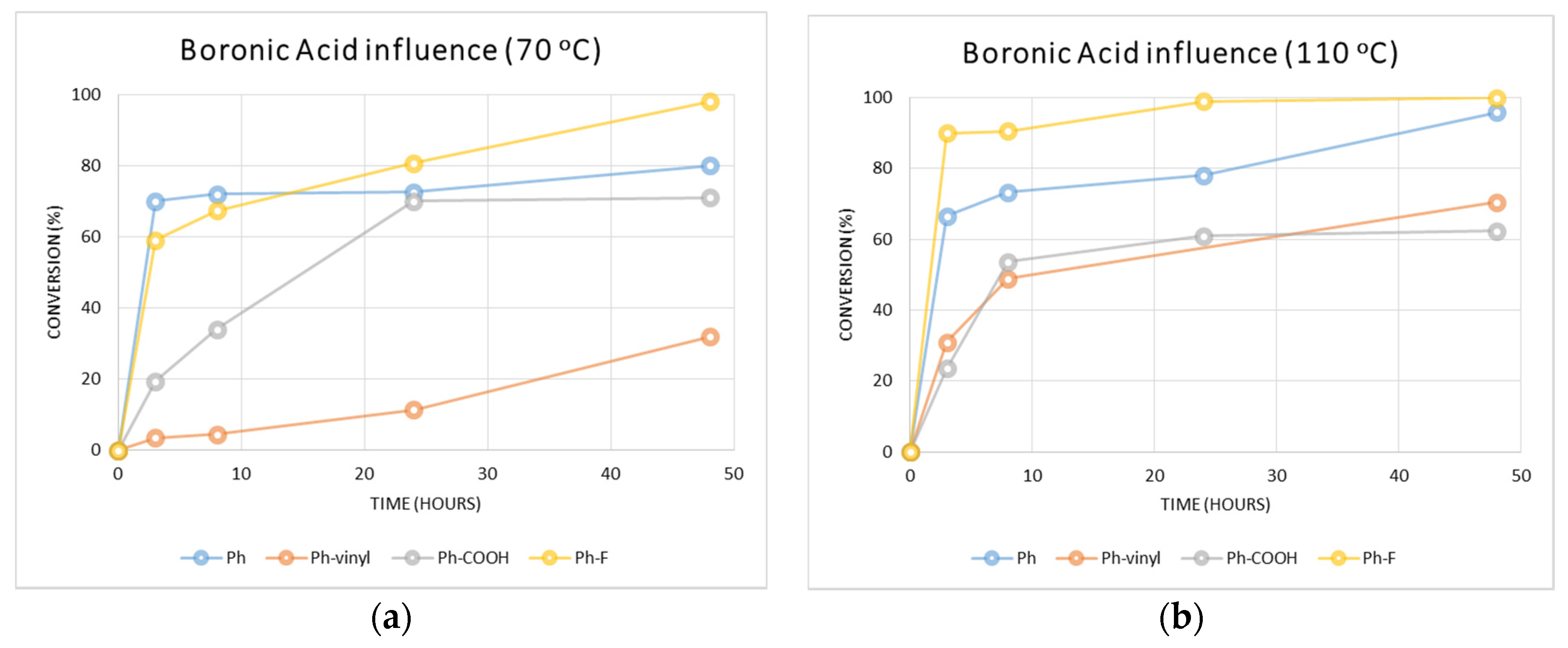
2.2.1. Determination of the Optimal Conditions and Influence of Different Boronic Acids
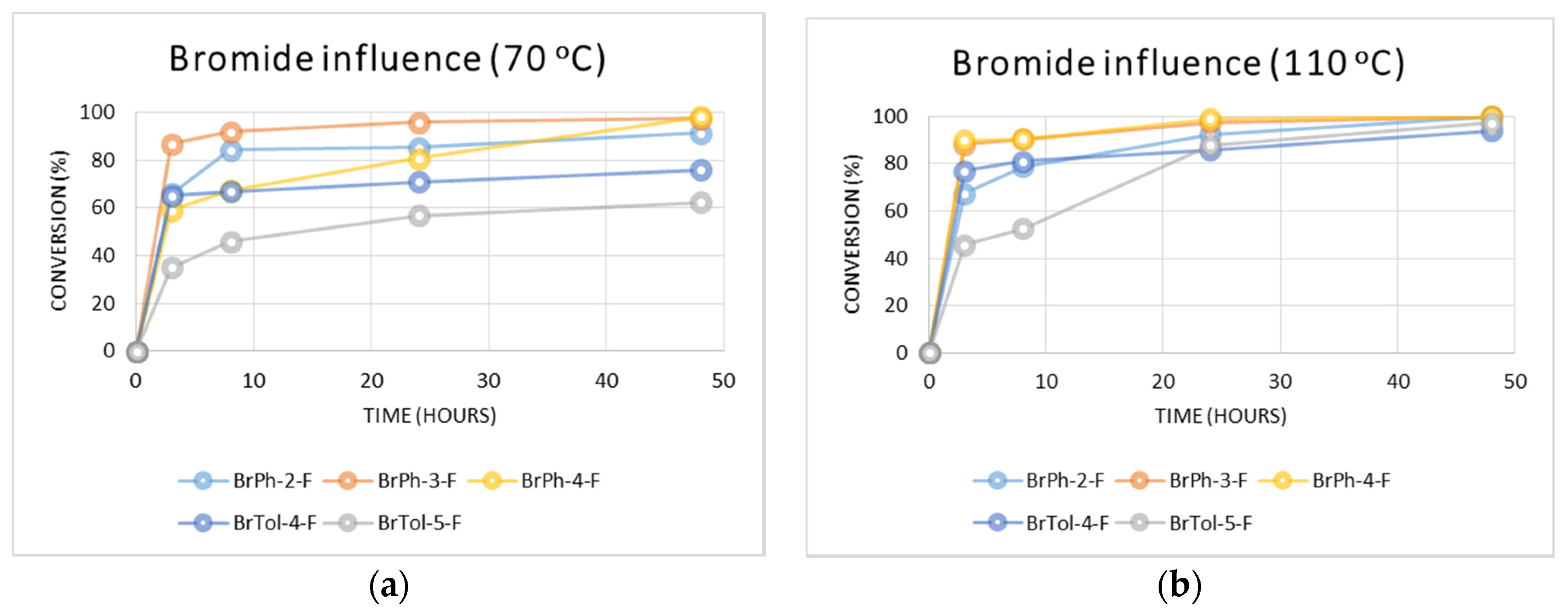
2.2.2. Influence of the Fluorinated Aryl Bromide
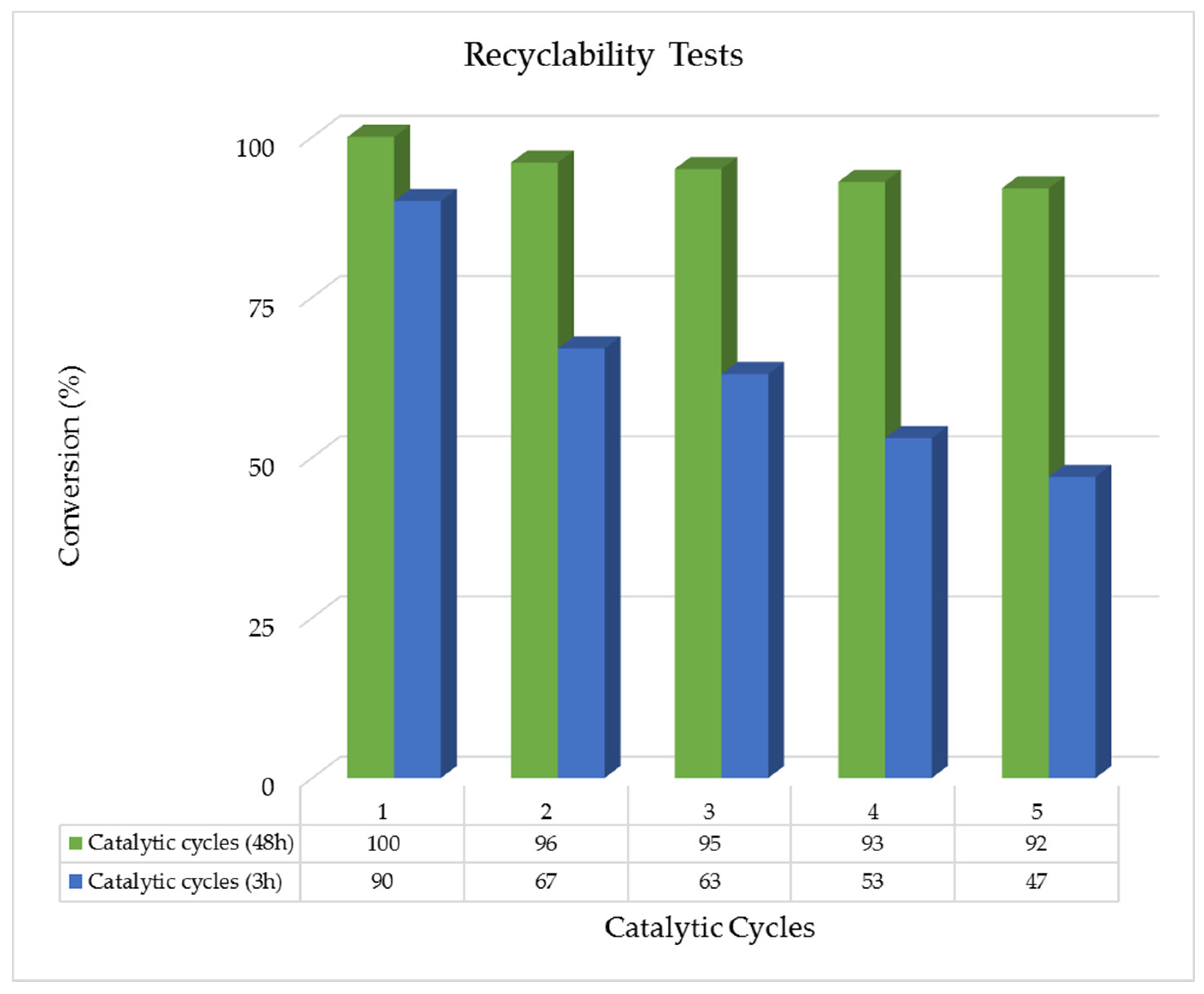
2.2.3. Recyclability Tests
3. Materials and Methods
3.1. General Conditions
3.2. General Remarks on the Characterization of the Materials
3.3. Preparation of the Hybrid Materials G-COOH-Pd-X
3.3.1. Preparation of the Palladium Precursor
3.3.2. Dehydration of G-COOH
3.3.3. Pd-Loading Study
3.4. Catalytic Study
3.4.1. Determination of the Optimal Conditions and Influence of the Boronic Acid
3.4.2. Quantification of the Conversion of 1-Bromo-4-Fluorobenzene
3.4.3. Isolation of the Coupling Products
3.4.4. Reactions of Different Fluorinated Aryl Bromides with 4-Fluorophenylboronic Acid
3.4.5. Studies of the Catalyst Recyclability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seechurn, C.C.C.J.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Maluenda, I.; Navarro, H. Recent Developments in the Suzuki-Miyaura Reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-Catalyzed Cross-Coupling Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent Applications of the Suzuki-Miyaura Cross-Coupling Reaction in Organic Synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Len, C.; Fihri, A. Silica-supported palladium: Sustainable catalysts for cross-coupling reactions. Coord. Chem. Rev. 2009, 253, 2599–2626. [Google Scholar] [CrossRef]
- Kann, N. Recent Applications of Polymer Supported Organometallic Catalysts in Organic Synthesis. Molecules 2010, 15, 6306–6331. [Google Scholar] [CrossRef] [PubMed]
- Bej, A.; Ghosh, K.; Sarkar, A.; Knight, D.W. Palladium nanoparticles in the catalysis of coupling reactions. RSC Adv. 2016, 6, 11446–11453. [Google Scholar] [CrossRef]
- Pérez-Lorenzo, M. Palladium Nanoparticles as Efficient Catalysts for Suzuki Cross-Coupling Reactions. J. Phys. Chem. Lett. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Parlett, C.M. A.; Bruce, D.W.; Hondow, N.S.; Newton, M.A.; Lee, A.F.; Wilson, K. Mesoporous Silicas as Versatile Supports to Tune the Palladium-Catalyzed Selective Aerobic Oxidation of Allylic Alcohols. ChemCatChem 2013, 5, 939–950. [Google Scholar] [CrossRef]
- Balbín, A.; Gaballo, F.; Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Kaluderovic, G.N.; Gómez-Ruiz, S. Dual application of Pd nanoparticles supported on mesoporous silica SBA-15 and MSU-2: Supported catalysts for C–C coupling reactions and cytotoxic agents against human cancer cell lines. RSC Adv. 2014, 4, 54775–54787. [Google Scholar] [CrossRef]
- Kumar, A.P.; Kumar, B.P.; Kumar, A.B.V.K.; Huy, B.T.; Lee, Y.-I. Preparation of palladium nanoparticles on alumina surface by chemical co-precipitation method and catalytic applications. Appl. Surface Sci. 2013, 265, 500–509. [Google Scholar] [CrossRef]
- Hossain, A.M.S.; Balbín, A.; Erami, R.S.; Prashar, S.; Fajardo, M.; Gómez-Ruiz, S. Synthesis and study of the catalytic applications in C–C coupling reactions of hybrid nanosystems based on alumina and palladium nanoparticles. Inorg. Chim. Acta 2017, 455, 645–652. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Khder, A.E.R.S.; Abdelsayed, V.; El-Shall, M.S.; Gupton, B.F. Microwave-assisted synthesis of palladium nanoparticles supported on graphene: A highly active and recyclable catalyst for carbon–carbon cross-coupling reactions. J. Catal. 2011, 279, 1–11. [Google Scholar] [CrossRef]
- Gómez-Martínez, M.; Buxaderas, E.; Pastor, I.M.; Alonso, D.A. Palladium nanoparticles supported on graphene and reduced graphene oxide as efficient recyclable catalyst for the Suzuki–Miyaura reaction of potassium aryltrifluoroborates. J. Mol. Catal. A: Chem. 2015, 404–405, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Joshi, H.; Sharma, K.N.; Sharma, A.K.; Singh, A.K. Palladium–phosphorus/sulfur nanoparticles (NPs) decorated on graphene oxide: Synthesis using the same precursor for NPs and catalytic applications in Suzuki–Miyaura coupling. Nanoscale 2014, 6, 4588–4597. [Google Scholar] [CrossRef] [PubMed]
- Movahed, S.K.; Esmatpoursalmani, R.; Bazgir, A. N-Heterocyclic carbene palladium complex supported on ionic liquid-modified graphene oxide as an efficient and recyclable catalyst for Suzuki reaction. RSC Adv. 2014, 4, 14586–14591. [Google Scholar] [CrossRef]
- Yamamoto, S.-I.; Kinoshita, H.; Hashimoto, H.; Nishina, Y. Facile preparation of Pd nanoparticles supported on single-layer graphene oxide and application for the Suzuki–Miyaura cross-coupling reaction. Nanoscale 2014, 6, 6501–6505. [Google Scholar] [CrossRef] [PubMed]
- Rumi, L.; Scheuermann, G.M.; Mülhaupt, R.; Bannwarth, W. Palladium Nanoparticles on Graphite Oxide as Catalyst for Suzuki-Miyaura, Mizoroki-Heck, and Sonogashira Reactions. Helv. Chim. Acta 2011, 94, 966–976. [Google Scholar] [CrossRef]
- Lin, J.; Mei, T.; Lv, M.; Zhang, C.; Zhao, Z.; Wang, X. Size-controlled PdO/graphene oxides and their reduction products with high catalytic activity. RSC Adv. 2014, 4, 29563–29570. [Google Scholar] [CrossRef]
- Hattori, T.; Tsubone, A.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Palladium on Carbon-Catalyzed Suzuki-Miyaura Coupling Reaction Using an Efficient and Continuous Flow System. Catalysts 2015, 5, 18–25. [Google Scholar] [CrossRef]
- Groult, H.; Leroux, F.; Tressaud, A. Modern synthesis Processes and Reactivity of Fluorinated Compounds; Elsevier: London, UK, 2017. [Google Scholar]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.D. Drug metabolism in microorganisms. Biotechnol. Lett. 2015, 37, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weberd, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Coates, D.; Greenfield, S.; Smith, G.; Chambers, M.K.; Kurmeier, H.-A.; Dorsch, D. Fluorinated biphenyl derivatives. Merck, Darmstadt, Germany. WO1990015115, 1990. [Google Scholar]
- Hampton, A.S.; Mikulski, L.; Palmer-Brown, W.; Murphy, C.D.; Sandford, G. Evaluation of fluorinated biphenyl ether pro-drug scaffolds employing the chemical-microbial approach. Bioorg. Med. Chem. Lett. 2016, 26, 2255–2258. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Huang, Y.; Chen, X.; Lin, X.; Bai, Z.; Huang, K.-W.; Yuan, Y.; Weng, Z. Preparation of fluorinated biaryls through direct palladium-catalyzed coupling of polyfluoroarenes with aryltrifluoroborates. J. Fluorine Chem. 2013, 151, 50–57. [Google Scholar] [CrossRef]
- Wei, Y.; Su, W. Pd(OAc)2-Catalyzed Oxidative C-H/C-H Cross-Coupling of Electron-Deficient Polyfluoroarenes with Simple Arenes. J. Am. Chem. Soc. 2010, 132, 16377–16379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, J.; Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Palladium-Catalyzed Cross-Coupling of Polyfluoroarenes with Simple Arenes. Org. Lett. 2011, 13, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liu, C.; Jin, Z. Green synthesis of fluorinated biaryl derivatives via thermoregulated ligand/palladium-catalyzed Suzuki reaction. J. Organomet. Chem. 2011, 696, 2641–2647. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Synthesis of 4-Substituted Styrene Compounds via Palladium-Catalyzed Suzuki Coupling Reaction Using Free Phosphine Ligand in Air. Synth. Commun. 2010, 40, 196–205. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Ma, Q.-M.; Lia, R.-H.; Shao, L.-X. Palladium-catalyzed Suzuki–Miyaura coupling of aryl sulfamates with arylboronic acids. Org. Biomol. Chem. 2013, 11, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Chen, G.-Q.; Shao, L.-X. N‑Heterocyclic Carbene−Palladium(II)−1-Methylimidazole Complex-Catalyzed Suzuki−Miyaura Coupling of Aryl Sulfonates with Arylboronic Acids. J. Org. Chem. 2012, 77, 6608–6614. [Google Scholar] [CrossRef] [PubMed]
- Kurscheid, B.; Belkoura, L.; Hoge, B. Air-Stable and Catalytically Active Phosphinous Acid Transition-Metal Complexes. Organometallics 2012, 31, 1329–1334. [Google Scholar] [CrossRef]
- Lázaro-Navas, S.; Prashar, S.; Fajardo, M.; Gómez-Ruiz, S. Visible light-driven photocatalytic degradation of the organic pollutant methylene blue with hybrid palladium–fluorine-doped titanium oxide nanoparticles. J. Nanopart. Res. 2015, 17, 94. [Google Scholar] [CrossRef]
- Rico-Oller, B.; Boudjemaa, A.; Bahruji, H.; Kebir, M.; Prashar, S.; Bachari, K.; Fajardo, M.; Gómez-Ruiz, S. Photodegradation of organic pollutants in water and green hydrogen production via methanol photoreforming of doped titanium oxide nanoparticles. Sci. Total Environ. 2016, 563–564, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lennox, A.J. J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Trilla, M.; Borja, G.; Pleixats, R.; Man, M.W.C.; Bied, C.; Moreau, J.J.E. Recoverable Palladium Catalysts for Suzuki–Miyaura Cross-Coupling Reactions Based on Organic-Inorganic Hybrid Silica Materials Containing Imidazolium and Dihydroimidazolium Salts. Adv. Synth. Catal. 2008, 350, 2566–2574. [Google Scholar] [CrossRef]

| Material | Theoretical Pd Quantity (wt %) | Experimental Pd Quantity (wt %) | Incorporation Rate (%) |
|---|---|---|---|
| G-COOH-Pd-5 | 5 | 3.06 | 61.2 |
| G-COOH-Pd-10 | 10 | 7.93 | 79.3 |
| G-COOH-Pd-15 | 15 | 11.20 | 74.6 |
| Time (h) | T (°C) | Bromide Conversion (%) | TON a | TOF b (h−1) | Reaction |
|---|---|---|---|---|---|
| 3 | 70 | 70 | 157 | 52 |  |
| 8 | 72 | 161 | 20 | ||
| 24 | 73 | 162 | 7 | ||
| 48 | 80 | 179 | 4 | ||
| 3 | 110 | 67 | 149 | 50 | |
| 8 | 73 | 164 | 21 | ||
| 24 | 78 | 175 | 7 | ||
| 48 | 96 | 215 | 5 | ||
| 3 | 70 | 3 | 8 | 3 |  |
| 8 | 5 | 10 | 1 | ||
| 24 | 11 | 26 | 1 | ||
| 48 | 32 | 71 | 2 | ||
| 3 | 110 | 31 | 69 | 23 | |
| 8 | 49 | 109 | 14 | ||
| 24 | 1 | ||||
| 48 | 71 | 158 | 3 | ||
| 3 | 70 | 19 | 43 | 15 |  |
| 8 | 34 | 76 | 10 | ||
| 24 | 70 | 157 | 7 | ||
| 48 | 71 | 159 | 3 | ||
| 3 | 110 | 24 | 53 | 18 | |
| 8 | 54 | 120 | 15 | ||
| 24 | 61 | 136 | 6 | ||
| 48 | 62 | 139 | 3 | ||
| 3 | 70 | 59 | 132 | 44 |  |
| 8 | 67 | 151 | 19 | ||
| 24 | 81 | 181 | 8 | ||
| 48 | 98 | 219 | 5 | ||
| 3 | 110 | 90 | 201 | 67 | |
| 8 | 91 | 202 | 25 | ||
| 24 | 99 | 221 | 9 | ||
| 48 | 100 | 223 | 5 |
| Time (h) | T (°C) | Bromide Conversion (%) | TON | TOF (h−1) | Reaction |
|---|---|---|---|---|---|
| 3 | 70 | 66 | 147 | 49 |  |
| 8 | 84 | 189 | 24 | ||
| 24 | 85 | 191 | 8 | ||
| 48 | 91 | 204 | 4 | ||
| 3 | 110 | 67 | 151 | 50 | |
| 8 | 79 | 177 | 22 | ||
| 24 | 92 | 207 | 9 | ||
| 48 | 100 | 224 | 5 | ||
| 3 | 70 | 87 | 195 | 65 |  |
| 8 | 92 | 206 | 26 | ||
| 24 | 96 | 215 | 9 | ||
| 48 | 97 | 218 | 5 | ||
| 3 | 110 | 88 | 197 | 66 | |
| 8 | 90 | 202 | 25 | ||
| 24 | 98 | 218 | 9 | ||
| 48 | 100 | 224 | 5 | ||
| 3 | 70 | 35 | 78 | 26 |  |
| 8 | 46 | 102 | 13 | ||
| 24 | 57 | 127 | 5 | ||
| 48 | 62 | 140 | 3 | ||
| 3 | 110 | 46 | 102 | 34 | |
| 8 | 53 | 117 | 15 | ||
| 24 | 88 | 197 | 8 | ||
| 48 | 97 | 217 | 5 | ||
| 3 | 70 | 65 | 149 | 50 |  |
| 8 | 67 | 151 | 19 | ||
| 24 | 71 | 159 | 6 | ||
| 48 | 76 | 169 | 3 | ||
| 3 | 110 | 77 | 172 | 57 | |
| 8 | 81 | 182 | 23 | ||
| 24 | 86 | 194 | 8 | ||
| 48 | 94 | 210 | 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erami, R.S.; Díaz-García, D.; Prashar, S.; Rodríguez-Diéguez, A.; Fajardo, M.; Amirnasr, M.; Gómez-Ruiz, S. Suzuki-Miyaura C-C Coupling Reactions Catalyzed by Supported Pd Nanoparticles for the Preparation of Fluorinated Biphenyl Derivatives. Catalysts 2017, 7, 76. https://doi.org/10.3390/catal7030076
Erami RS, Díaz-García D, Prashar S, Rodríguez-Diéguez A, Fajardo M, Amirnasr M, Gómez-Ruiz S. Suzuki-Miyaura C-C Coupling Reactions Catalyzed by Supported Pd Nanoparticles for the Preparation of Fluorinated Biphenyl Derivatives. Catalysts. 2017; 7(3):76. https://doi.org/10.3390/catal7030076
Chicago/Turabian StyleErami, Roghayeh Sadeghi, Diana Díaz-García, Sanjiv Prashar, Antonio Rodríguez-Diéguez, Mariano Fajardo, Mehdi Amirnasr, and Santiago Gómez-Ruiz. 2017. "Suzuki-Miyaura C-C Coupling Reactions Catalyzed by Supported Pd Nanoparticles for the Preparation of Fluorinated Biphenyl Derivatives" Catalysts 7, no. 3: 76. https://doi.org/10.3390/catal7030076