Two-Dimensional Layered Double Hydroxide Derived from Vermiculite Waste Water Supported Highly Dispersed Ni Nanoparticles for CO Methanation
Abstract
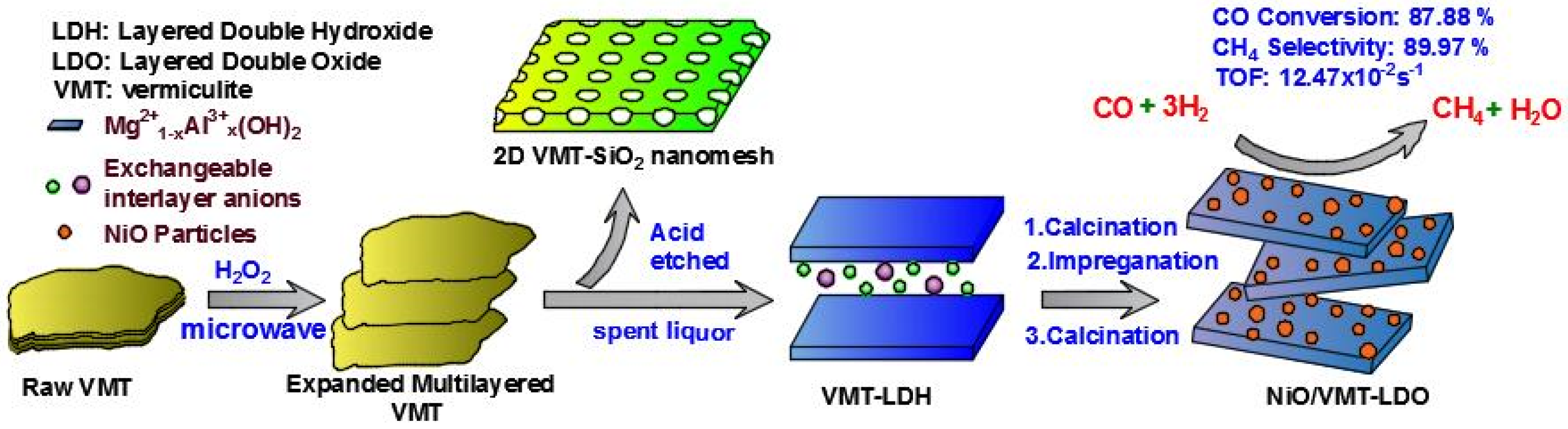
:1. Introduction
2. Results and Discussion
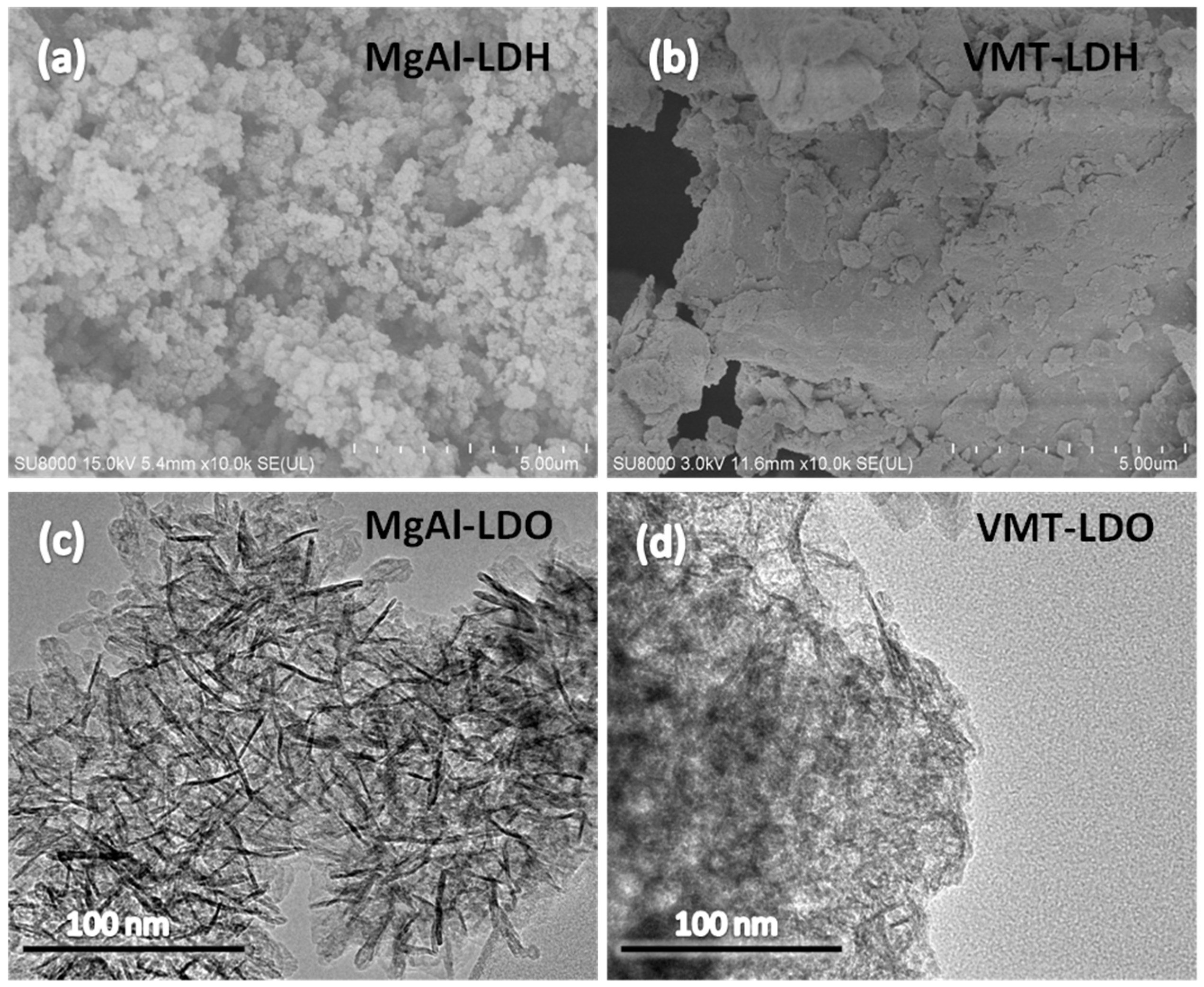
2.1. XRD, SEM, and TEM Characterization of LDHS and LDOS
2.2. XRD and TEM Characterization of Catalysts
2.3. TPR Characterization of Catalysts
2.4. Catalytic Performance of Catalysts
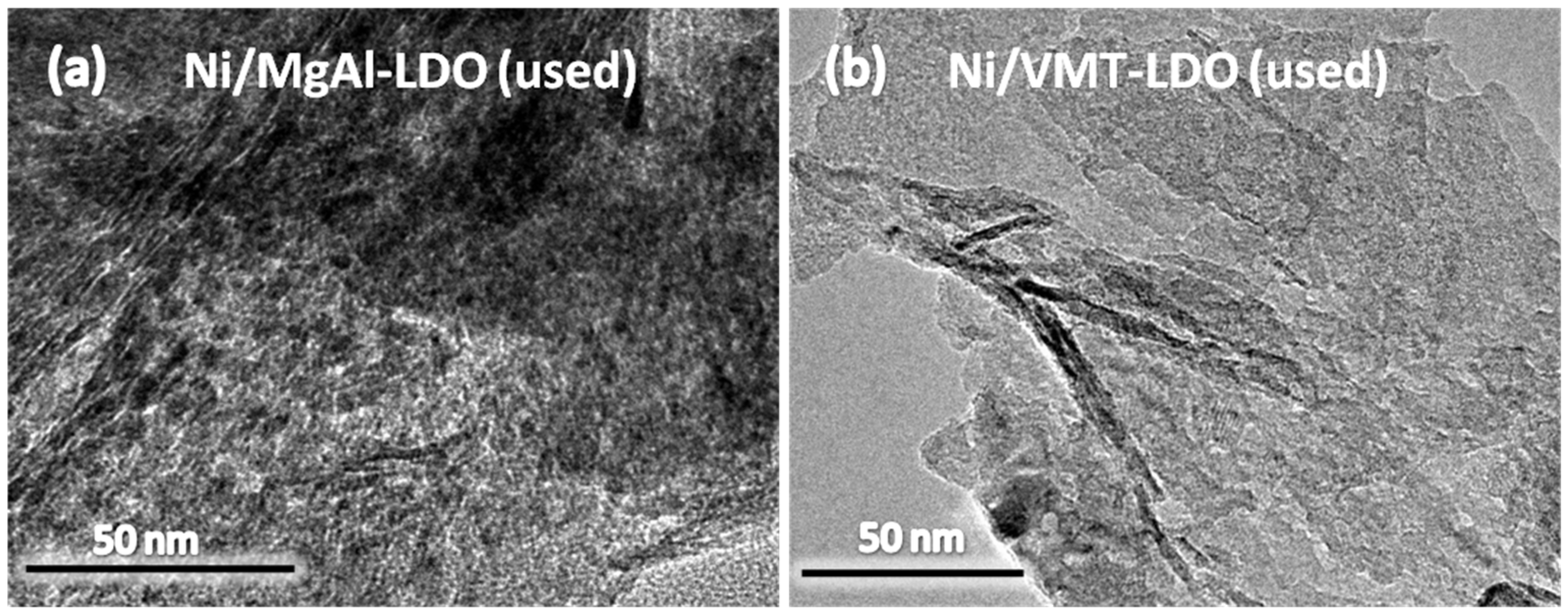
2.5. TEM and XRD Characterization of the Used Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization of Samples
3.3. Catalytic Performance Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Cheng, D.D.; Negreiros, F.R.; Aprà, E.; Fortunelli, A. Computational approaches to the chemical conversion of carbon dioxide. ChemSusChem 2013, 6, 944–965. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, P.; Senderens, J. New Synthesis of Methane. Comptes Rendus Hebdomadaires des Seances del Academie des Scrences 1902, 134, 514–516. [Google Scholar]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Production of synthetic natural gas (sng) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Seemann, M.C.; Schildhauer, T.J.; Biollaz, S.M.A. Fluidized bed methanation of wood-derived producer gas for the production of synthetic natural gas. Ind. Eng. Chem. Res. 2010, 49, 7034–7038. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced investigation of CO methanation over Ni/Al2O3 catalysts for synthetic natural gas production. Ind. Eng. Chem. Res. 2012, 51, 1254–1262. [Google Scholar] [CrossRef]
- Gabrovska, M.; Edreva-Kardjieva, R.; Crişan, D.; Tzvetkov, P.; Shopska, M.; Shtereva, I. Ni–Al layered double hydroxides as catalyst precursors for CO2 removal by methanation. React. Kinet. Mech. Catal. 2012, 105, 79–99. [Google Scholar] [CrossRef]
- Mirodatos, C.; Praliaud, H.; Primet, M. Deactivation of nickel-based catalysts during CO methanation and disproportionation. J. Catal. 1987, 107, 275–287. [Google Scholar] [CrossRef]
- Struis, R.P.W.J.; Schildhauer, T.J.; Czekaj, I.; Janousch, M.; Biollaz, S.M.A.; Ludwig, C. Sulphur poisoning of Ni catalysts in the sng production from biomass: A TPO/XPS/XAS study. Appl. Catal. A Gen. 2009, 362, 121–128. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni–Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Hong, U.G.; Seo, J.G.; Ji, C.J.; Dong, J.K.; Lim, H.; Byun, C.; Song, I.K. Methane production from carbon monoxide and hydrogen over nickel–alumina xerogel catalyst: Effect of nickel content. J. Ind. Eng. Chem. 2011, 17, 154–157. [Google Scholar] [CrossRef]
- Fan, M.T.; Miao, K.P.; Lin, J.D.; Zhang, H.B.; Liao, D.W. Mg-Al oxide supported Ni catalysts with enhanced stability for efficient synthetic natural gas from syngas. Appl. Surf. Sci. 2014, 307, 682–688. [Google Scholar] [CrossRef]
- Bradford, M.C.; Vannice, M.A. Catalytic reforming of methane with carbon-dioxide over nickel-catalysts I. Catalyst characterization and activity. Appl. Catal. A Gen. 1996, 142, 73–96. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichikuni, N.; Sato, S.; Nozaki, F. Ni/MgO catalyst prepared using citric acid for hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 158, 185–199. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, C.; Du, S.; Yang, J.; Yu, B.; Luo, J.; Yin, W.; Li, E.; Dong, S.; Ye, P. Contacts between two- and three-dimensional materials: Ohmic, schottky, and p-n heterojunctions. ACS Nano 2016, 10, 4895–4919. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; He, Y.; Liu, Y.; Du, Y.; Li, D. Cheminform abstract: Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: General functionality and promising application prospects. Cheminform 2015, 46, 5291–5319. [Google Scholar] [CrossRef]
- Bian, L.; Wang, W.; Xia, R.; Li, Z. Ni-based catalyst derived from Ni/Al hydrotalcite-like compounds by the urea hydrolysis method for CO methanation. RSC Adv. 2015, 6, 677–686. [Google Scholar] [CrossRef]
- Hwang, S.; Hong, U.G.; Lee, J.; Seo, J.G.; Baik, J.H.; Dong, J.K.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous Ni–Fe–Al2O3 catalysts prepared by a coprecipitation method: Effect of precipitation agent. J. Ind. Eng. Chem. 2013, 19, 2016–2021. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Cheminform abstract: Supported gold nanoparticles as a reusable catalyst for synthesis of lactones from diols using molecular oxygen as an oxidant under mild conditions. Green Chem. 2009, 11, 793–797. [Google Scholar] [CrossRef]
- Liu, P.; Li, P.C.; Hensen, P.E.J.M. Efficient tandem synthesis of methyl esters and imines by using versatile hydrotalcite-supported gold nanoparticles. Chemistry 2012, 18, 12122–12129. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bian, L.; Zhu, Q.; Wang, W. Ni-based catalyst derived from Ni/Mg/Al hydrotalcite-like compounds and its activity in the methanation of carbon monoxide. Kinet. Catal. 2014, 55, 217–223. [Google Scholar] [CrossRef]
- Buelens, L.C.; Galvita, V.; Poelman, H.; Detavernier, C.; Marin, G.B. Super-dry reforming of methane intensifies CO2 utilization via le chatelier’s principle. Science 2016, 354, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Huang, X.; Li, P.; Zhang, Y.; Zhu, M.; Guo, X.; Dai, B.; Wang, Q.; Yu, F. Two-dimensional porous silica nanomesh from expanded multilayered vermiculite via mixed acid leaching. Nanosci. Nanotechnol. Lett. 2016, 8, 1028–1032. [Google Scholar] [CrossRef]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, W.; Chu, W. Effect of ca modification on the catalytic performance of Ni/AC for comethanation. Integr. Ferroelectr. 2016, 172, 40–48. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.; Hong, U.G.; Ji, C.J.; Dong, J.K.; Lim, H.; Byun, C.; Song, I.K. Hydrogenation of carbon monoxide to methane over mesoporous nickel-m-alumina (Fe, Ni, CO, Ce, and La) xerogel catalysts. J. Ind. Eng. Chem. 2012, 18, 243–248. [Google Scholar] [CrossRef]
- Hwang, S.; Hong, U.G.; Lee, J.; Baik, J.H.; Dong, J.K.; Lim, H.; Song, I.K. Methanation of carbon dioxide over mesoporous Nickel–M–Alumina (M = Fe, Zr, Ni, Y, and Mg) xerogel catalysts: Effect of second metal. Catal. Lett. 2012, 142, 860–868. [Google Scholar] [CrossRef]
- Fornasari, G.; Gazzano, M.; Matteuzzi, D.; Trifirò, F.; Vaccari, A. Structure and reactivity of high-surface-area Ni/Mg/Al mixed oxides. Appl. Clay Sci. 1995, 10, 69–82. [Google Scholar] [CrossRef]
- Olsbye, U.; Akporiaye, D.; Rytter, E.; Rønnekleiv, M.; Tangstad, E. On the stability of mixed M2+/M3+ oxides. Appl. Catal. A Gen. 2002, 224, 39–49. [Google Scholar] [CrossRef]
- He, L.; Lin, Q.; Liu, Y.; Huang, Y. Unique catalysis of ni-al hydrotalcite derived catalyst in CO2 methanation: Cooperative effect between ni nanoparticles and a basic support. J. Energy Chem. 2014, 23, 587–592. [Google Scholar] [CrossRef]
- Kustov, A.L.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Nørskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni–Fe catalysts: From computational studies towards catalyst optimization. Appl. Catal. A Gen. 2007, 320, 98–104. [Google Scholar] [CrossRef]




| Samples | SBET (m2·g−1) a | Pore Volume (cm3·g−1) b | Pore Size (nm) b |
|---|---|---|---|
| MgAl-LDO | 177.3 | 0.76 | 14.90 |
| NiO/MgAl-LDO | 52.5 | 0.15 | 3.91 |
| Ni/MgAl-LDO (used) | 45.0 | 0.22 | 24.98 |
| VMT-LDO | 72.4 | 0.16 | 7.36 |
| NiO/VMT-LDO | 53.2 | 0.22 | 5.63 |
| Ni/VMT-LDO (used) | 46.2 | 0.20 | 3.91 |
| Catalysts | Optical Temperature (°C) | Pressure (MPa) | Space Velocity (mL/g/h) | CO/CO2 Conversion (%) | CH4 Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|
| 40 wt % NiMgAl-LDO | 250 | 0.1 | 40,000 | 98.4 (CO2 + CO) | 100 | [12] |
| 78 wt % NiAl-LDO | 350 | 0.1 | 75,000 | 82.5 (CO2) | 99.5 | [30] |
| NiAl-LDO(NiAl4) | 400 | 0.1 | 300,000 | 100 (CO) | 92 | [17] |
| 11 wt % NiMgAl-LDO | 400 | - | 15,000 h−1 | 99.9 (CO) | 73.6 | [21] |
| 10 wt % Ni/MgAl-LDO | 400 | 1.5 | 20,000 | 79.5 (CO) | 93.9 | this work |
| 10 wt % Ni/VMT-LDO | 400 | 1.5 | 20,000 | 87.9 (CO) | 90.0 | this work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhu, M.; Tian, Z.; Han, Y.; Zhang, Y.; Zhou, T.; Kang, L.; Dan, J.; Guo, X.; Yu, F.; et al. Two-Dimensional Layered Double Hydroxide Derived from Vermiculite Waste Water Supported Highly Dispersed Ni Nanoparticles for CO Methanation. Catalysts 2017, 7, 79. https://doi.org/10.3390/catal7030079
Li P, Zhu M, Tian Z, Han Y, Zhang Y, Zhou T, Kang L, Dan J, Guo X, Yu F, et al. Two-Dimensional Layered Double Hydroxide Derived from Vermiculite Waste Water Supported Highly Dispersed Ni Nanoparticles for CO Methanation. Catalysts. 2017; 7(3):79. https://doi.org/10.3390/catal7030079
Chicago/Turabian StyleLi, Panpan, Mingyuan Zhu, Zhiqun Tian, Yang Han, Yu Zhang, Tuantuan Zhou, Lihua Kang, Jianming Dan, Xuhong Guo, Feng Yu, and et al. 2017. "Two-Dimensional Layered Double Hydroxide Derived from Vermiculite Waste Water Supported Highly Dispersed Ni Nanoparticles for CO Methanation" Catalysts 7, no. 3: 79. https://doi.org/10.3390/catal7030079







