Characterization of MoVTeNbOx Catalysts during Oxidation Reactions Using In Situ/Operando Techniques: A Review
Abstract
:1. Introduction
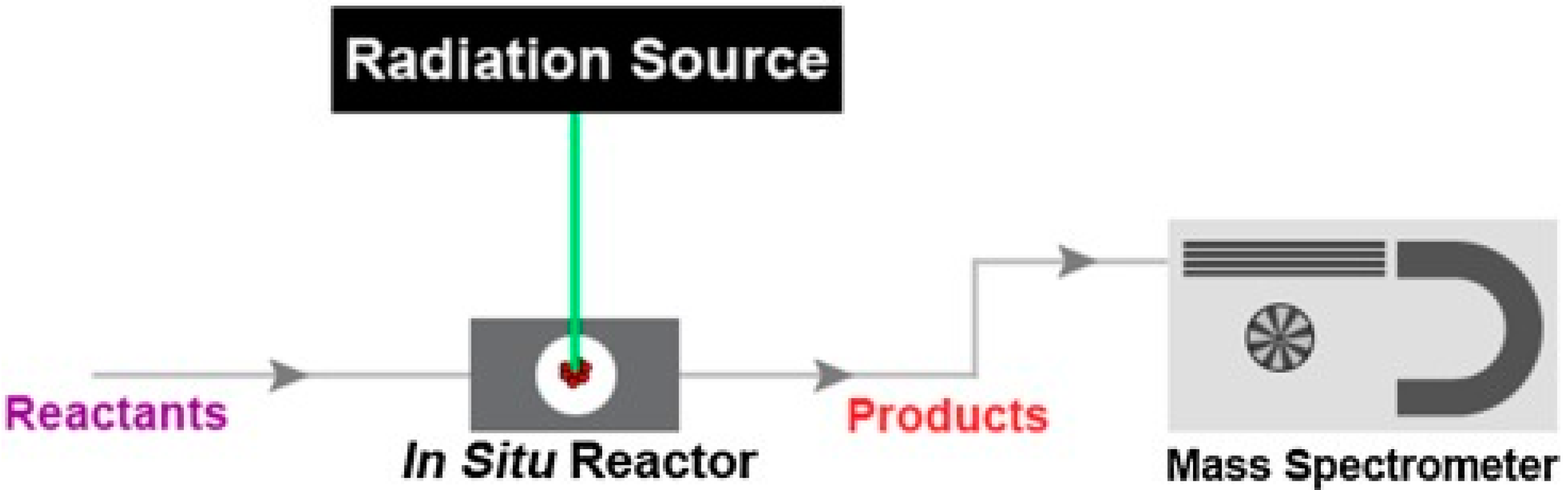
2. Operando Techniques
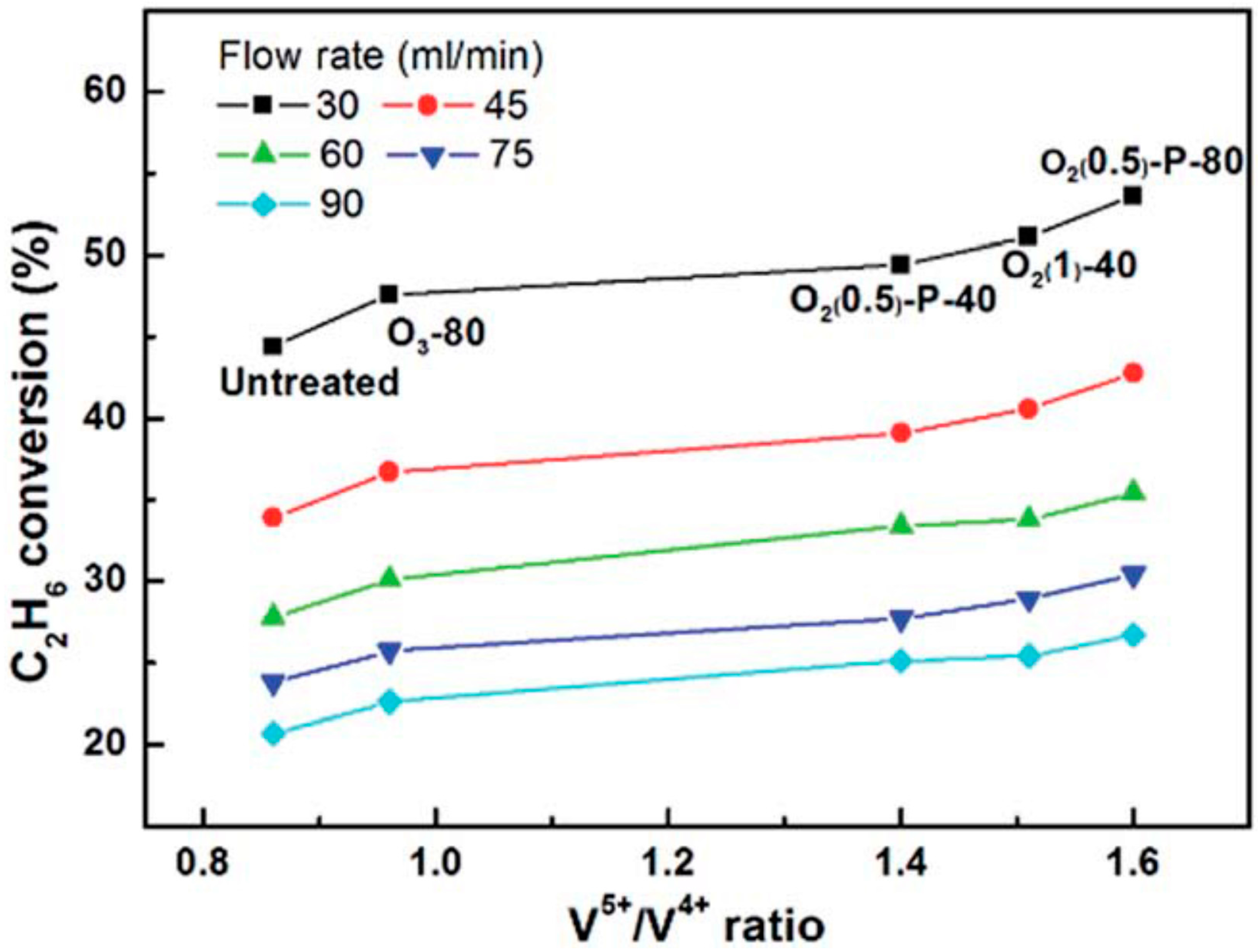
3. Oxidative Dehydrogenation (ODH) of Light Alkanes (Ethane and Propane)
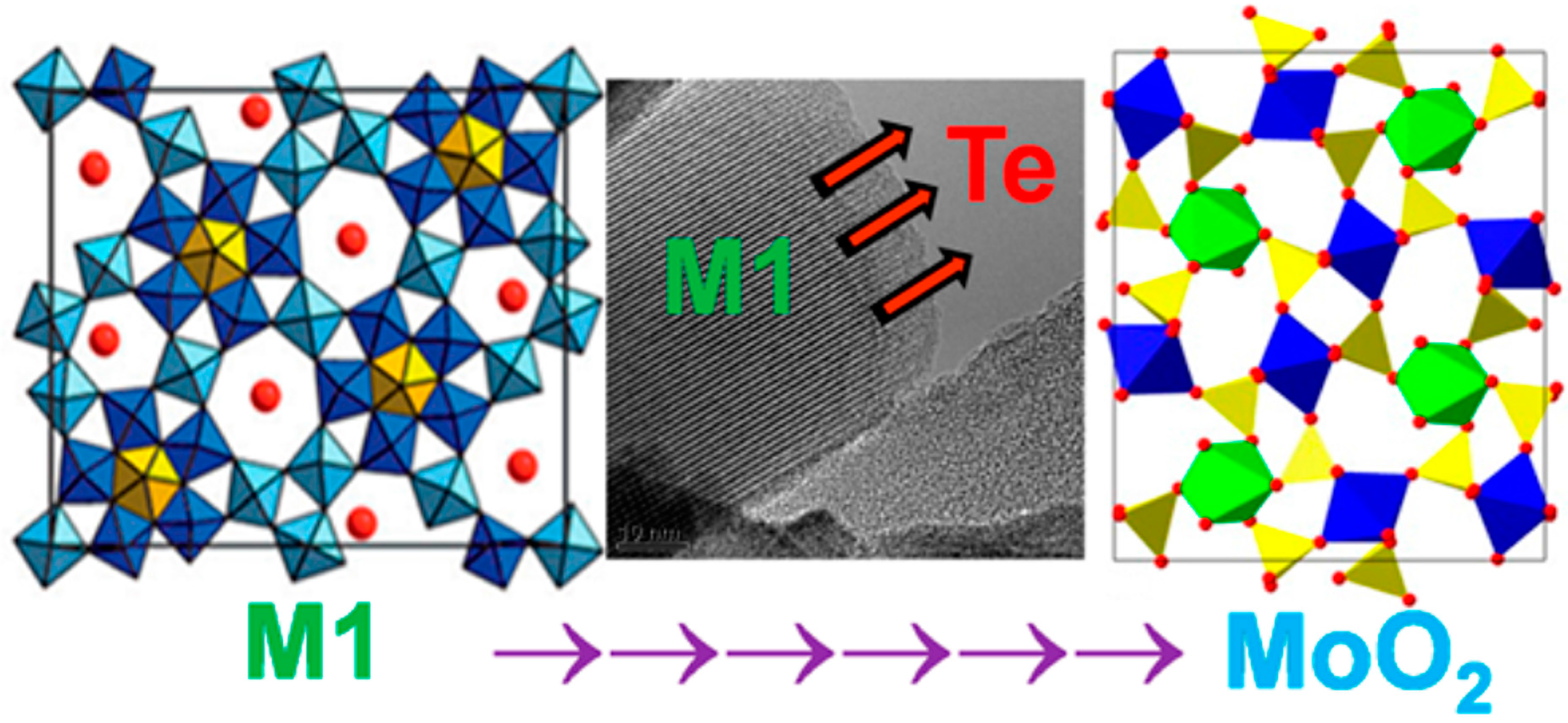
4. Propane Ammoxidation to Acrylonitrile
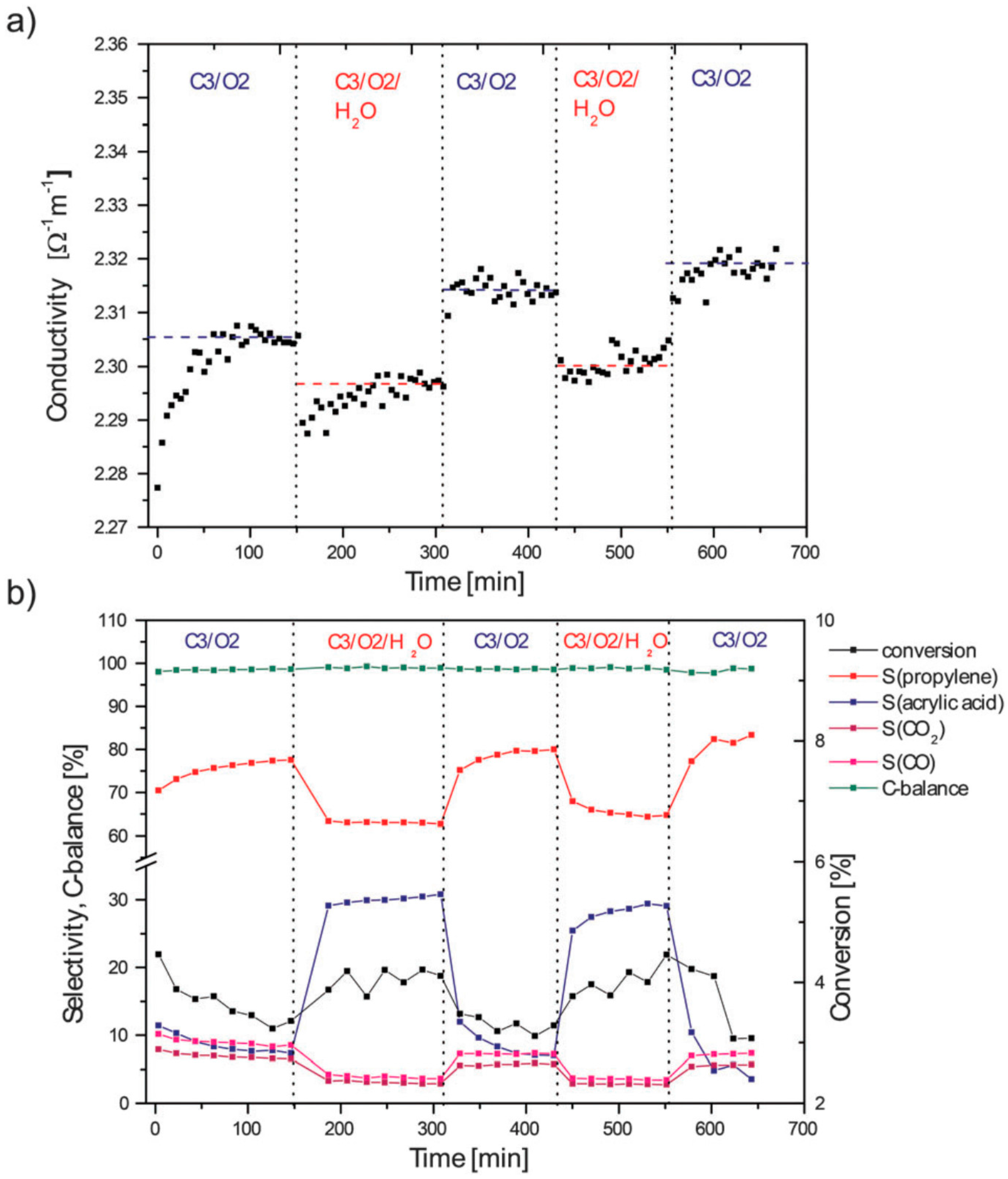
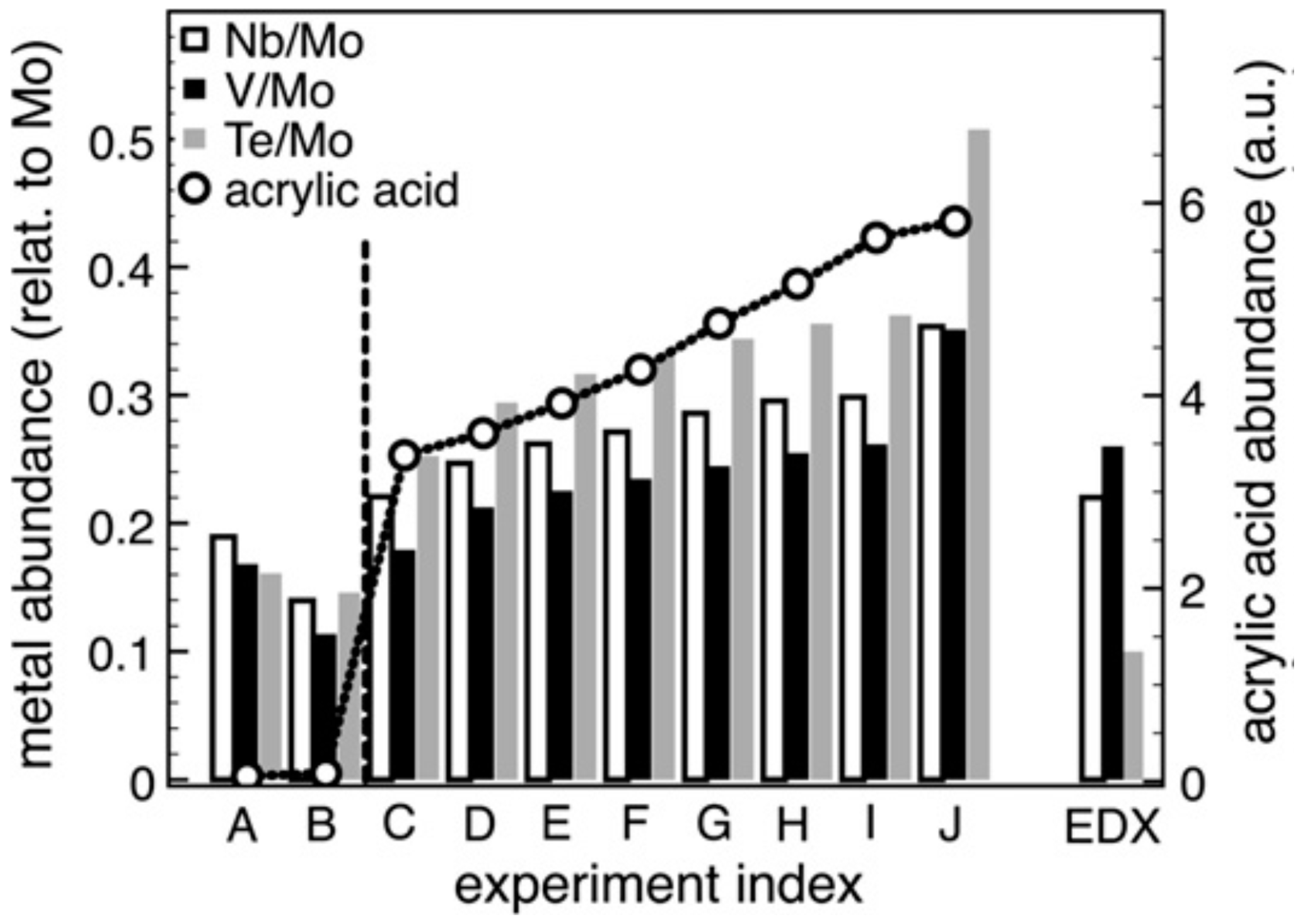
5. Selective Oxidation of Propane to Acrylic Acid
6. Discussion
7. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Faramawy, S.; Zaki, T.; Sakr, A.A.-E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Vedrine, J.C. Heterogeneous partial (amm) oxidation and oxidative dehydrogenation catalysis on mixed metal oxides. Catalysts 2016, 6, 22. [Google Scholar] [CrossRef]
- Gartner, C.A.; van Veen, A.C.; Lercher, J.A. Oxidative dehydrogenation of ethane: Common principles and mechanistic aspects. ChemCatChem. 2013, 5, 3196–3217. [Google Scholar] [CrossRef]
- Shiju, N.R.; Guliants, V.V. Recent developments in catalysis using nanostructured materials. Appl. Catal. A Gen. 2009, 356, 1–17. [Google Scholar] [CrossRef]
- Hatano, M.; Kayo, A. Catalytic Conversion of Alkanes to Nitriles, and a Catalyst Therefor. U.S. Patent 5049692, 1991. [Google Scholar]
- Ushikubo, T.; Nakamura, H.; Koyasu, Y.; Wajiki, S. Method for Producing an Unsaturated Carboxylic Acid. European Patent 0608838, 1994. [Google Scholar]
- Grasselli, R.K.; Burrington, J.D.; Buttrey, D.J.; DeSanto, P.; Lugmair, C.G.; Volpe, A.F.; Weingand, T. Multifunctionality of active centers in (amm) oxidation catalysts: From Bi-Mo-Ox to Mo-V-Nb-(Te, Sb)-Ox. Top. Catal. 2003, 23, 5–22. [Google Scholar] [CrossRef]
- Ueda, W.; Vitry, D.; Katou, T. Structural organization of catalytic functions in Mo-based oxides for propane selective oxidation. Catal. Today 2004, 96, 235–240. [Google Scholar] [CrossRef]
- DeSanto, P.; Buttrey, D.J.; Grasselli, R.K.; Lugmair, C.G.; Volpe, A.F.; Toby, B.H.; Vogt, T. Structural aspects of the M1 and M2 phases in MoVNbTeO propane ammoxidation catalysts. Z. Für Krist. 2004, 219, 152–165. [Google Scholar] [CrossRef]
- Aouine, M.; Dubois, J.L.; Millet, J.M.M. Crystal chemistry and phase composition of the MoVTeNbO catalysts for the ammoxidation of propane. Chem. Commun. 2001, 1180–1181. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Buttrey, D.J.; DeSanto, P.; Burrington, J.D.; Lugmair, C.G.; Volpe, A.F.; Weingand, T. Active centers in Mo-V-Nb-Te-Ox (amm) oxidation catalysts. Catal. Today 2004, 91–92, 251–258. [Google Scholar] [CrossRef]
- Naraschewski, F.N.; Kumar, C.P.; Jentys, A.; Lercher, J.A. Phase formation and selective oxidation of propane over MoVTeNbOx catalysts with varying compositions. Appl. Catal. A Gen. 2011, 391, 63–69. [Google Scholar] [CrossRef]
- Baca, M.; Pigamo, A.; Dubois, J.L.; Millet, J.M.M. Fourier transform infrared spectroscopic study of surface acidity by pyridine adsorption on the M1 active phase of the MoVTe(Sb)NbO catalysts used in propane oxidation. Catal. Commun. 2005, 6, 215–220. [Google Scholar] [CrossRef]
- Concepcion, P.; Hernandez, S.; Lopez Nieto, J.M. On the nature of active sites in MoVTeO and MoVTeNbO catalysts: The influence of catalyst activation temperature. Appl. Catal. A Gen. 2011, 391, 92–101. [Google Scholar] [CrossRef]
- He, Q.; Woo, J.; Belianinov, A.; Guliants, V.V.; Borisevich, A.Y. Better catalysts through microscopy: Mesoscale M1/M2 intergrowth in molybdenum-vanadium based complex oxide catalysts for propane ammoxidation. ACS Nano 2015, 9, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Baca, M.; Aouine, M.; Dubois, J.L.; Millet, J.M.M. Synergetic effect between phases in MoVTe(Sb)NbO catalysts used for the oxidation of propane into acrylic acid. J. Catal. 2005, 233, 234–241. [Google Scholar] [CrossRef]
- Havecker, A.; Wrabetz, S.; Krohnert, J.; Csepei, L.; Naumann, R.; Alnoncourt, D.; Kolen’ko, Y.V.; Girgsdies, F.; Schlogl, R.; Trunschke, A. Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid. J. Catal. 2012, 285, 48–60. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Lugmair, C.G.; Volpe, A.F. Towards an understanding of the reaction pathways in propane ammoxidation based on the distribution of elements at the active centers of the M1 Phase of the MoV (Nb, Ta)TeO system. Top. Catal. 2011, 54, 595–604. [Google Scholar] [CrossRef]
- Banares, M.A.; Guerrero-Perez, M.O.; Fierro, J.L.G.; Cortez, G.G. Raman spectroscopy during catalytic operations with on-line activity measurement (operando spectroscopy): A method for understanding the active centers of cations supported on porous materials. J. Mater. Chem. 2002, 12, 3337–3342. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. Determining the active site in a catalytic process: Operando spectroscopy is more than a buzzword. Phys. Chem. Chem. Phys. 2003, 5, 4351–4360. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Ford, M.E.; Gregory, D.; Hu, R.; Keturakis, C.J.; Lwin, S.; Tang, Y.; Yang, Z.; Zhu, M.; Banares, M.A.; Wachs, I.E. A decade + of operando spectroscopy studies. Catal. Today 2017, 283, 27–53. [Google Scholar] [CrossRef]
- Global Ethylene Demand to Grow 3.3% Annually. Available online: http://www.woodmac.com/analysis/11966725 (accessed on 4 August 2016).
- Chemical Economics Handbook: Propylene. Available online: https://www.ihs.com/products/propylene-chemical-economics-handbook.html (accessed on 4 August 2016).
- Arnold, S.C.; Gaffney, A.M.; Song, R.; Yeh, C.Y. Process for Producing Ethylene Via Oxidative Dehydrogenation (ODH) of Ethane. U.S. Patent 0256432, 2010. [Google Scholar]
- Che-Galicia, G.; Quintana-Solorzano, R.; Ruiz-Martinez, R.S.; Valente, J.S.; Castillo-Araiza, C.O. Kinetic modeling of the oxidative dehydrogenation of ethane to ethylene over a MoVTeNbO catalytic system. Chem. Eng. J. 2014, 252, 75–88. [Google Scholar] [CrossRef]
- Schlogl, R. Active sites for propane oxidation: Some generic considerations. Top. Catal. 2011, 54, 627–638. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Chu, B.; An, H.; Cheng, Y. Valence variation of phase-pure M1 MoVNbTe oxide by plasma treatment for improved catalytic performance in oxidative dehydrogenation of ethane. RSC Adv. 2015, 5, 91295–91301. [Google Scholar] [CrossRef]
- Chu, B.; An, H.; Nijhuis, T.A.; Schouten, J.C.; Cheng, Y. A self-redox pure-phase M1 MoVNbTeOx/CeO2 nanocomposite as a highly active catalyst for oxidative dehydrogenation of ethane. J. Catal. 2015, 329, 471–478. [Google Scholar] [CrossRef]
- Chu, B.; Truter, L.; Nijhuis, T.A.; Cheng, Y. Performance of phase-pure M1 MoVNbTeOx catalysts by hydrothermal synthesis with different post-treatments for the oxidative dehydrogenation of ethane. Appl. Catal. A Gen. 2015, 498, 99–106. [Google Scholar] [CrossRef]
- Fan, J.; Wu, X.; Wu, X.; Liang, Q.; Ran, R.; Weng, D. Thermal ageing of Pt on low-surface-area CeO2–ZrO2–La2O3 mixed oxides: Effect on the OSC performance. Appl. Catal. B Environ. 2008, 81, 38–48. [Google Scholar] [CrossRef]
- Ishchenko, E.V.; Kardash, T.Y.; Gulyaev, R.V.; Ishchenko, A.V.; Sobolev, V.I.; Bondareva, V.M. Effect of K and Bi doping on the M1 phase in MoVTeNbO catalysts for ethane oxidative conversion to ethylene. Appl. Catal. A Gen. 2016, 514, 1–13. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Deniau, B.; Delichere, P.; Millet, J.M.M. Influence of the content and distribution of vanadium in the M1 phase of the MoVTe(Sb)NbO catalysts on their catalytic properties in light alkanes oxidation. Top. Catal. 2014, 57, 1152–1162. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Aouine, M.; Millet, J.M.M. Optimizing the efficiency of MoVTeNbO catalysts for ethane oxidative dehydrogenation to ethylene. Catal. Commun. 2012, 21, 22–26. [Google Scholar] [CrossRef]
- Deniau, B.; Nguyen, T.T.; Delichere, P.; Safonova, O.; Millet, J.M. Redox state dynamics at the surface of MoVTe(Sb)NbO M1 phase in selective oxidation of light alkanes. Top. Catal. 2013, 56, 1952–1962. [Google Scholar] [CrossRef]
- Solsona, B.; Vazquez, M.I.; Ivars, F.; Dejoz, A.; Concepcion, P.; Lopez, J.M. Selective oxidation of propane and ethane on diluted Mo-V-Nb-Te mixed-oxide catalysts. J. Catal. 2007, 252, 271–280. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, L.; Weng, W.; Wan, H. Preparation of MoVTe (Sb) Nb mixed oxide catalysts using a slurry method for selective oxidative dehydrogenation of ethane. J. Mol. Catal. A Chem. 2005, 240, 191–196. [Google Scholar] [CrossRef]
- Botella, P.; Garcia-Gonzalez, E.; Dejoz, A.; Lopez Nieto, J.M.; Vazquez, M.I.; Gonzalez-Calbet, J. Selective oxidative dehydrogenation of ethane on MoVTeNbO mixed metal oxide catalysts. J. Catal. 2004, 225, 428–438. [Google Scholar] [CrossRef]
- Valente, J.S.; Armendariz-Herrera, H.; Quintana-Solorzano, R.; del Angel, P.; Nava, N.; Masso, A.; Lopez Nieto, J.M. Chemical, structural, and morphological changes of a MoVTeNb catalyst during oxidative dehydrogenation of ethane. ACS Catal. 2014, 4, 1292–1301. [Google Scholar] [CrossRef]
- Safonova, O.V.; Deniau, B.; Millet, J.M. Mechanism of the oxidation-reduction of the MoVSbNbO catalyst: In operando X-ray absorption spectroscopy and electrical conductivity measurements. J. Phys. Chem. B 2006, 110, 23962–23967. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Xu, P.; Hartmann, D.; Zhu, Y.; Browning, N.D.; Sanchez-Sanchez, M.; Lercher, J.A. Atomic-scale determination of active facets on the MoVTeNb oxide M1 phase and their intrinsic catalytic activity for ethane oxidative dehydrogenation. Angew. Chem. Int. Ed. 2016, 55, 8873–8877. [Google Scholar] [CrossRef] [PubMed]
- Aouine, M.; Epicier, T.; Millet, J.M. In situ environmental STEM study of the MoVTe oxide M1 phase catalysts for ethane oxidative dehydrogenation. ACS Catal. 2016, 6, 4775–4781. [Google Scholar] [CrossRef]
- Handbook of Heterogeneous Catalysis; Ertl, G.; Knozinger, H.; Weitkamp, J. (Eds.) Wiley/VHC: New York, NY, USA, 1997; pp. 2302–2326. [Google Scholar]
- Grasselli, R.K. Advances and future trends in selective oxidation and ammoxidation catalysis. Catal. Today 1999, 49, 141–153. [Google Scholar] [CrossRef]
- Holmberg, J.; Grasselli, R.K.; Andersson, A. Catalytic behavior of M1, M2, and M1/M2 physical mixtures of the Mo-V-Nb-Te-oxide system in propane and propene ammoxidation. Appl. Catal. A Gen. 2004, 270, 121–134. [Google Scholar] [CrossRef]
- Guttman, A.T.; Grasselli, R.K.; Bradzil, J.F. Ammoxidation of Paraffins and Catalysts Therefor. U.S. Patent 4746641, 1988. [Google Scholar]
- Nilsson, J.; Landa-Canovas, A.R.; Hansen, S.; Andersson, A. An investigation of the Al-Sb-V-W-Oxide system for propane ammoxidation. J. Catal. 1999, 186, 442–457. [Google Scholar] [CrossRef]
- Zenkovets, G.A.; Kryukova, G.N.; Tsybulya, S.V.; Anufrienko, V.F.; Larina, T.V.; Burgina, E.B. The structure of oxide Ga-Sb-Ni-P-W-O/SiO2 catalyst and its catalytic properties in propane ammoxidation. Kinet. Catal. 2002, 43, 384–390. [Google Scholar] [CrossRef]
- Ushikubo, T.; Oshima, K.; Kayo, A.; Umezawa, T.; Kiyono, K.; Sawaki, I. Process for Producing Nitriles. European Patent 529853, 1992. [Google Scholar]
- Komada, S.; Hinago, H.; Kaneta, M.; Watanabe, M. Process Using Niobium-Containing Aqueous Solution in Producing Niobium-Containing Oxide Catalyst. European Patent 0859809, 1998. [Google Scholar]
- Grasselli, R.K. Selectivity issues in (amm) oxidation catalysis. Catal. Today 2005, 99, 23–31. [Google Scholar] [CrossRef]
- Pyrz, W.D.; Blom, D.A.; Vogt, T.; Buttrey, D.J. Direct imaging of the MoVTeNbO M1 phase using an aberration-corrected high-resolution scanning transmission electron microscope. Angew. Chem. Int. Ed. 2008, 47, 2788–2791. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Sanghavi, U.; Vonderheide, A.; Guliants, V.V. A study of M1/M2 phase synergy in the MoVTe(Nb,Ta)O catalysts for propane ammoxidation to acrylonitrile. Appl. Catal. A Gen. 2016, 515, 179–189. [Google Scholar] [CrossRef]
- Korovchenko, P.; Shiju, N.R.; Dozier, A.K.; Graham, U.M.; Guerrero-Perez, M.O.; Guliants, V.V. M1 to M2 phase transformation and phase cooperation in bulk mixed metal Mo-V-M-O (M = Te, Nb) catalysts for selective ammoxidation of propane. Top. Catal. 2008, 50, 43–51. [Google Scholar] [CrossRef]
- Shiju, N.R.; Guliants, V.V.; Overbury, S.H.; Rondinone, A.J. Toward environmentally benign oxidations: Bulk mixed Mo-V-(Te-Nb)-O M1 phase catalysts for the selective ammoxidation of propane. ChemSusChem 2008, 1, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Guliants, V.V.; Brongersma, H.H.; Knoester, A.; Gaffney, A.M.; Han, S. Surface active sites present in the orthorhombic M1 phases: Low energy ion scattering study of methanol and allyl alcohol chemisorption over Mo-V-Te-Nb-O and Mo-V-O catalysts. Top. Catal. 2006, 38, 41–50. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Y.; Guliants, V.V. Propane ammoxidation over Mo–V–Te–Nb–O M1 phase investigated by DFT: Elementary steps of ammonia adsorption, activation and NH insertion into π-allyl intermediate. Top. Catal. 2014, 57, 1145–1151. [Google Scholar] [CrossRef]
- Yu, J.; Xu, Y.; Guliants, V.V. Propane ammoxidation over Mo-V-Te-Nb-O M1 phase: Density functional theory study of propane oxidative dehydrogenation steps. Catal. Today 2014, 238, 28–34. [Google Scholar] [CrossRef]
- DeSanto, P.; Buttrey, D.J.; Grasselli, R.K.; Pyrz, W.D.; Lugmair, C.G.; Volpe, A.F., Jr.; Vogt, T.; Toby, B.H. Comparison of MoVTaTeO and MoVNbTeO M1 crystal chemistry. Top. Catal. 2006, 38, 31–40. [Google Scholar] [CrossRef]
- DeSanto, P.; Buttrey, D.J.; Grasselli, R.K.; Lugmair, C.G.; Volpe, A.F.; Toby, B.H.; Vogt, T. Structural characterization of the orthorhombic phase M1 in MoVNbTeO propane ammoxidation catalyst. Top. Catal. 2003, 23, 23–38. [Google Scholar] [CrossRef]
- Shiju, N.R.; Liang, X.; Weimer, A.W.; Liang, C.; Dai, S.; Guliants, V.V. The role of surface basal planes of layered mixed metal oxides in selective transformation of lower alkanes: Propane ammoxidation over surface ab planes of Mo-V-Te-Nb-O M1 phase. J. Am. Chem. Soc. 2008, 130, 5850–5851. [Google Scholar] [CrossRef] [PubMed]
- Popova, G.Y.; Andrushkevich, T.V.; Chesalov, Y.A.; Plyasova, L.M.; Dovlitova, L.S.; Ischenko, E.V.; Aleshina, G.I.; Khramov, M.I. Formation of active phases in MoVTeNb oxide catalysts for ammoxidation of propane. Catal. Today. 2009, 144, 312–317. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Lu, G. Promotional effect of cerium on Mo–V–Te–Nb mixed oxide catalyst for ammoxidation of propane to acrylonitrile. Fuel Process. Technol. 2015, 130, 71–77. [Google Scholar] [CrossRef]
- Baca, M.; Millet, J.M. Bulk oxidation state of the different cationic elements in the MoVTe (Sb)NbO catalysts for oxidation or ammoxidation of propane. Appl. Catal. A Gen. 2005, 279, 67–77. [Google Scholar] [CrossRef]
- Shiju, N.R.; Rondinone, A.J.; Mullins, D.R.; Schwartz, V.; Overbury, S.H.; Guliants, V.V. XANES study of hydrothermal Mo-V-based mixed oxide M1-phase catalysts for the (amm) oxidation of propane. Chem. Mater. 2008, 20, 6611–6616. [Google Scholar] [CrossRef]
- Shiju, N.R.; Kale, R.R.; Iyer, S.S.; Guliants, V.V. 13C isotope labeling study of propane ammoxidation over M1 phase Mo-V-Te-Nb-O mixed oxide catalyst. J. Phys. Chem. C 2007, 111, 18001–18003. [Google Scholar] [CrossRef]
- Heine, C.; Havecker, M.; Trunschke, A.; Schlogl, R.; Eichelbaum, M. The impact of steam on the electronic structure of the selective propane oxidation catalyst MoVTeNb oxide (orthorhombic M1 phase). Phys. Chem. Chem. Phys. 2015, 17, 8983–8993. [Google Scholar] [CrossRef] [PubMed]
- Acrylic acid market worth 13.21 billion USD by 2020. Available online: http://www.marketsandmarkets.com/PressReleases/acrylic-acid.asp (accessed on 7 August 2016).
- Cavani, F. Catalytic selective oxidation: The forefront in the challenge for a more sustainable chemical industry. Catal. Today 2010, 157, 8–15. [Google Scholar] [CrossRef]
- Novakova, E.K.; Vedrine, V.C. Metal Oxides, Chemistry and Applications; Fierro, J.L.G., Ed.; CRC Press: New York, NY, USA, 2006; pp. 414–416. [Google Scholar]
- Centi, G.; Cavani, F.; Trifiro, F. Selective Oxidation by Heterogeneous Catalysis; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; pp. 363–364. [Google Scholar]
- Lin, M.M. Selective oxidation of propane to acrylic acid with molecular oxygen. Appl. Catal. A 2001, 207, 1–16. [Google Scholar] [CrossRef]
- Ushikubo, T.; Nakamura, H.; Koyasu, Y.; Wajiki, S. Method for Producing an Unsaturated Carboxylic Acid. U.S. Patent 5380933, 1995. [Google Scholar]
- Deniau, B.; Millet, J.M.M.; Loridant, S.; Christin, N.; Dubois, J.L. Effect of several cationic substitutions in the M1 active phase of the MoVTeNbO catalysts used for the oxidation of propane to acrylic acid. J. Catal. 2008, 260, 30–36. [Google Scholar] [CrossRef]
- Ivars, F.; Solsona, B.; Botella, P.; Soriano, M.D.; Lopez Nieto, J.M. Selective oxidation of propane over alkali-doped Mo-V-Sb-O catalysts. Catal. Today 2009, 141, 294–299. [Google Scholar] [CrossRef]
- Sanfiz, A.C.; Hansen, T.W.; Teschner, D.; Schnorch, P.; Girgsdies, F.; Trunschke, A.; Schlogl, R.; Looi, M.H.; Hamid, S.B.A. Dynamics of the MoVTeNb oxide M1 phase in propane oxidation. J. Phys. Chem. C 2010, 114, 1912–1921. [Google Scholar] [CrossRef]
- D’Alnoncourt, R.N.; Csepei, L.; Havecker, M.; Girgsdies, F.; Schuster, M.E.; Schlogl, R.; Trunschke, A. The reaction network in propane oxidation over phase-pure MoVTeNb M1 oxide catalysts. J. Catal. 2014, 311, 369–385. [Google Scholar] [CrossRef]
- Blom, D.; Vogt, T.; Allard, L.; Buttrey, D. Observation of sublattice disordering of the catalytic sites in a complex Mo-V-Nb-Te-O oxidation catalyst using high temperature STEM imaging. Top. Catal. 2014, 57, 1138–1144. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, B.; Zheng, W.; Su, D.S. Electron-irradiation-stimulated atomic-scale structural dynamics of the pentagonal channel in a complex MoVTeNbOx catalyst. ChemCatChem 2015, 7, 3651–3654. [Google Scholar] [CrossRef]
- Ueda, W.; Vitry, D.; Katou, T. Crystalline Mo-V-O based complex oxides as selective oxidation catalysts of propane. Catal. Today 2005, 99, 43–49. [Google Scholar] [CrossRef]
- Sanfiz, A.C.; Hansen, T.W.; Sakthivel, A.; Trunschke, A.; Schologl, R.; Knoester, A.; Brongersma, H.H.; Looi, M.H.; Hamid, S.B.A. How important is the (001) plane of M1 for selective oxidation of propane to acrylic acid? J. Catal. 2008, 258, 35–43. [Google Scholar]
- Schlogl, R. Selective oxidation: From a still immature technology to the roots of catalysis science. Top. Catal. 2016, 59, 1461–1476. [Google Scholar] [CrossRef]
- Grasselli, R.K. Fundamental principles of selective heterogeneous oxidation catalysis. Top. Catal. 2002, 21, 79–88. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Buttrey, D.J.; Burrington, J.D.; Andersson, A.; Holmberg, J.; Ueda, W.; Kubo, J.; Lugmair, C.G.; Volpe, A.F., Jr. Active centers, catalytic behavior, symbiosis and redox properties of MoV(Nb,Ta)TeO ammoxidation catalysts. Top. Catal. 2006, 38, 7–16. [Google Scholar] [CrossRef]
- Taylor, H.S. A theory of the catalytic surface. Proc. R. Soc. London Ser. A 1925, 108, 105–111. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lwin, S.; Diao, W.; Baroi, C.; Gaffney, A.M.; Fushimi, R.R. Characterization of MoVTeNbOx Catalysts during Oxidation Reactions Using In Situ/Operando Techniques: A Review. Catalysts 2017, 7, 109. https://doi.org/10.3390/catal7040109
Lwin S, Diao W, Baroi C, Gaffney AM, Fushimi RR. Characterization of MoVTeNbOx Catalysts during Oxidation Reactions Using In Situ/Operando Techniques: A Review. Catalysts. 2017; 7(4):109. https://doi.org/10.3390/catal7040109
Chicago/Turabian StyleLwin, Soe, Weijian Diao, Chinmoy Baroi, Anne M. Gaffney, and Rebecca R. Fushimi. 2017. "Characterization of MoVTeNbOx Catalysts during Oxidation Reactions Using In Situ/Operando Techniques: A Review" Catalysts 7, no. 4: 109. https://doi.org/10.3390/catal7040109





