Novel Synthesis of Plasmonic Ag/AgCl@TiO2 Continues Fibers with Enhanced Broadband Photocatalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Textural Properties
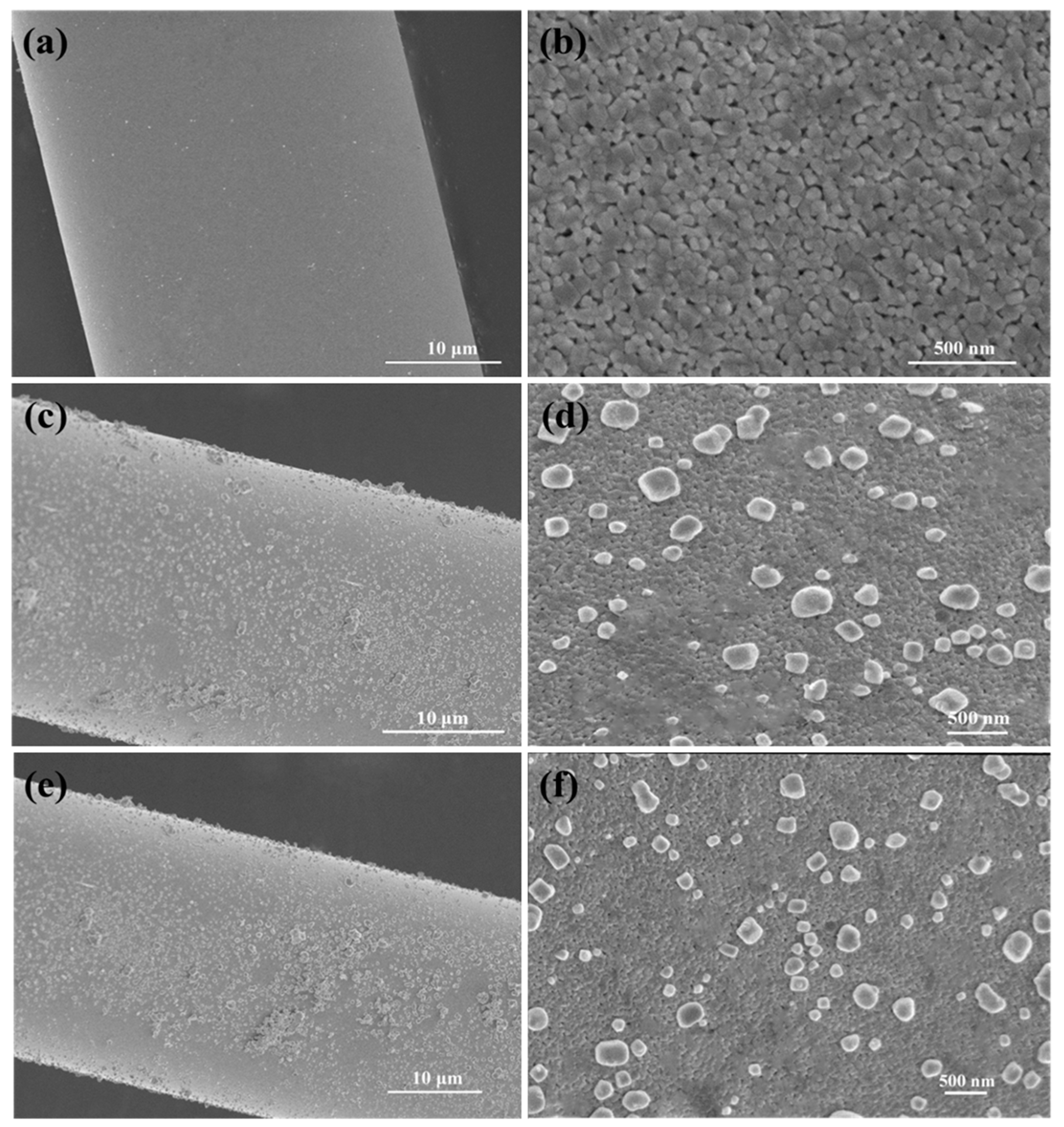
2.1.1. SEM
2.1.2. Textural Properties
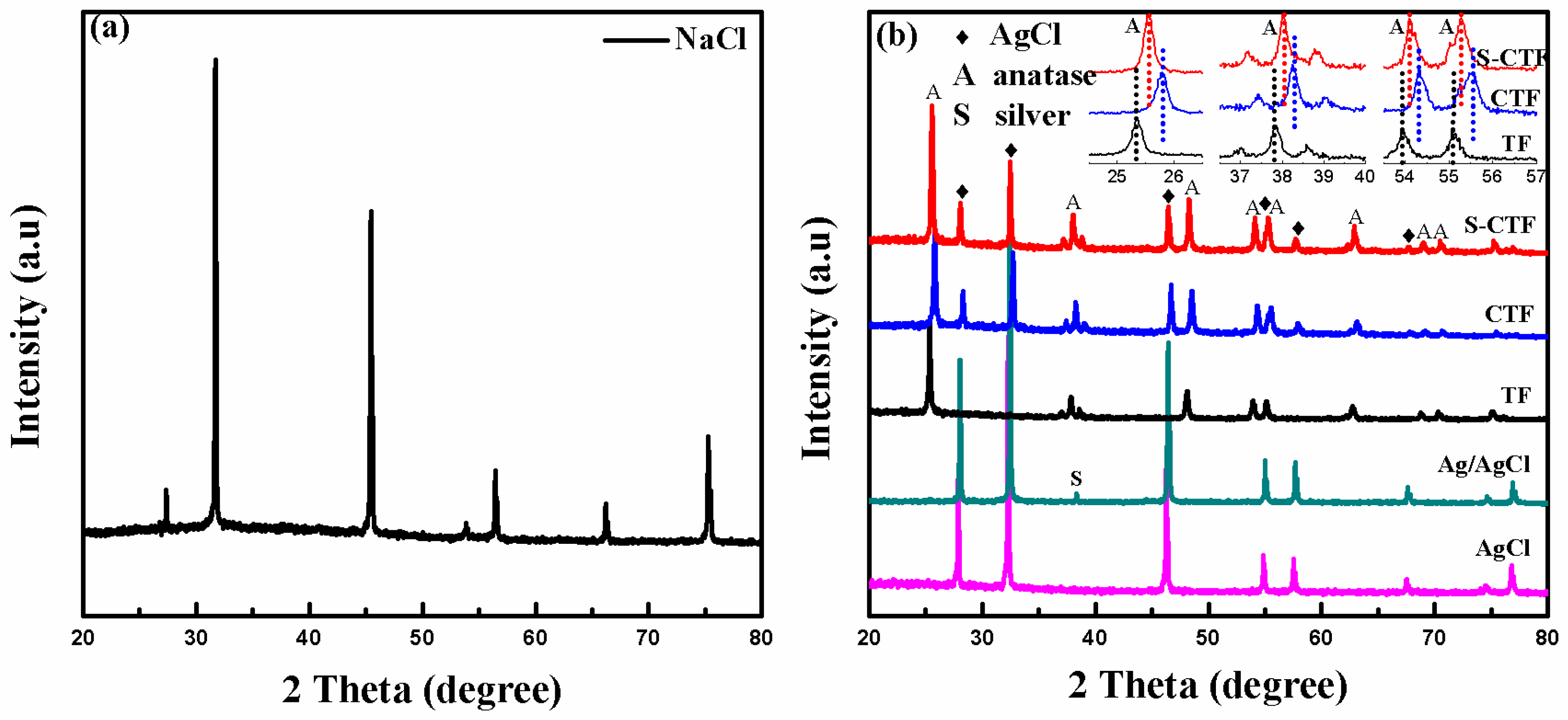
2.2. XRD
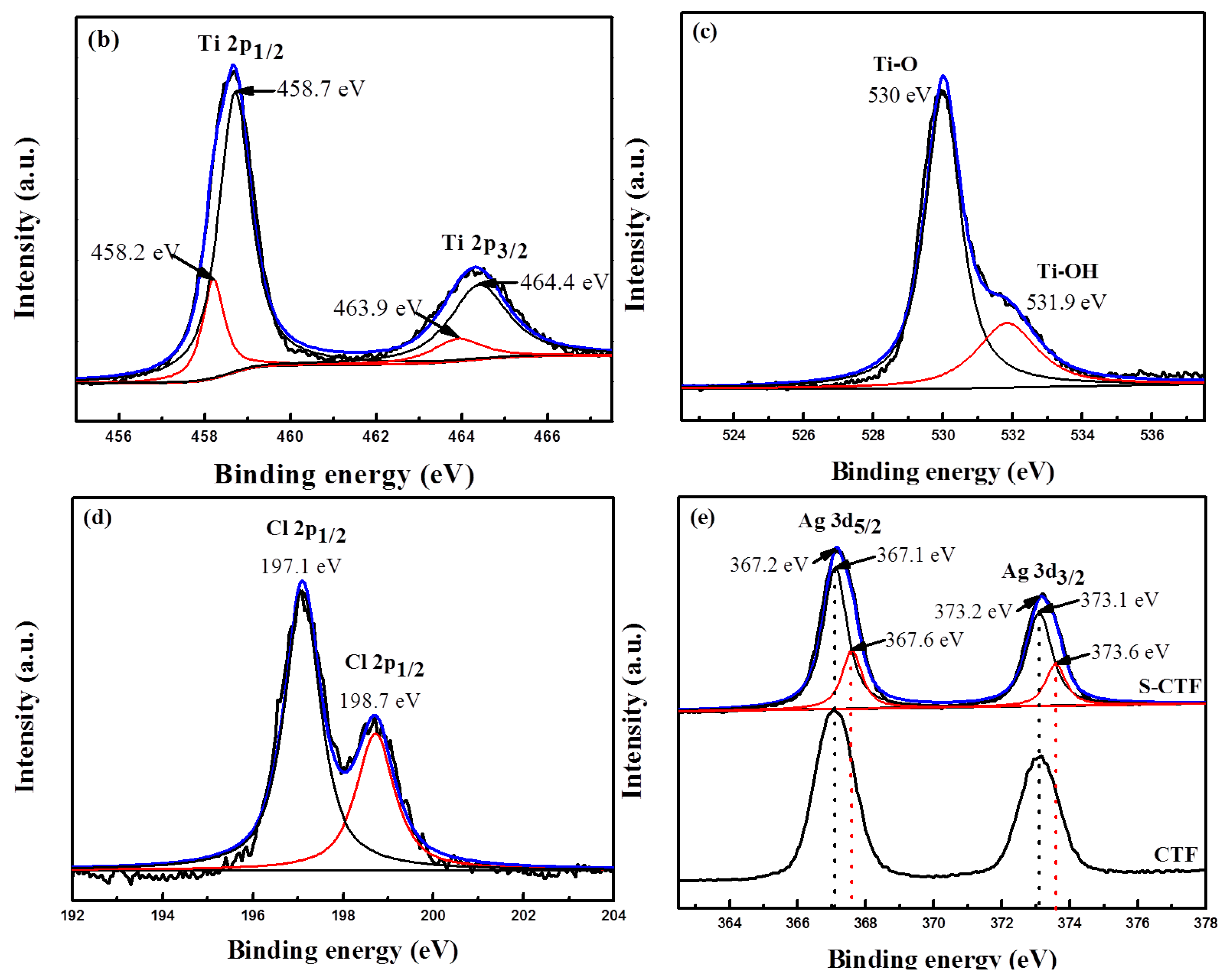
2.3. XPS
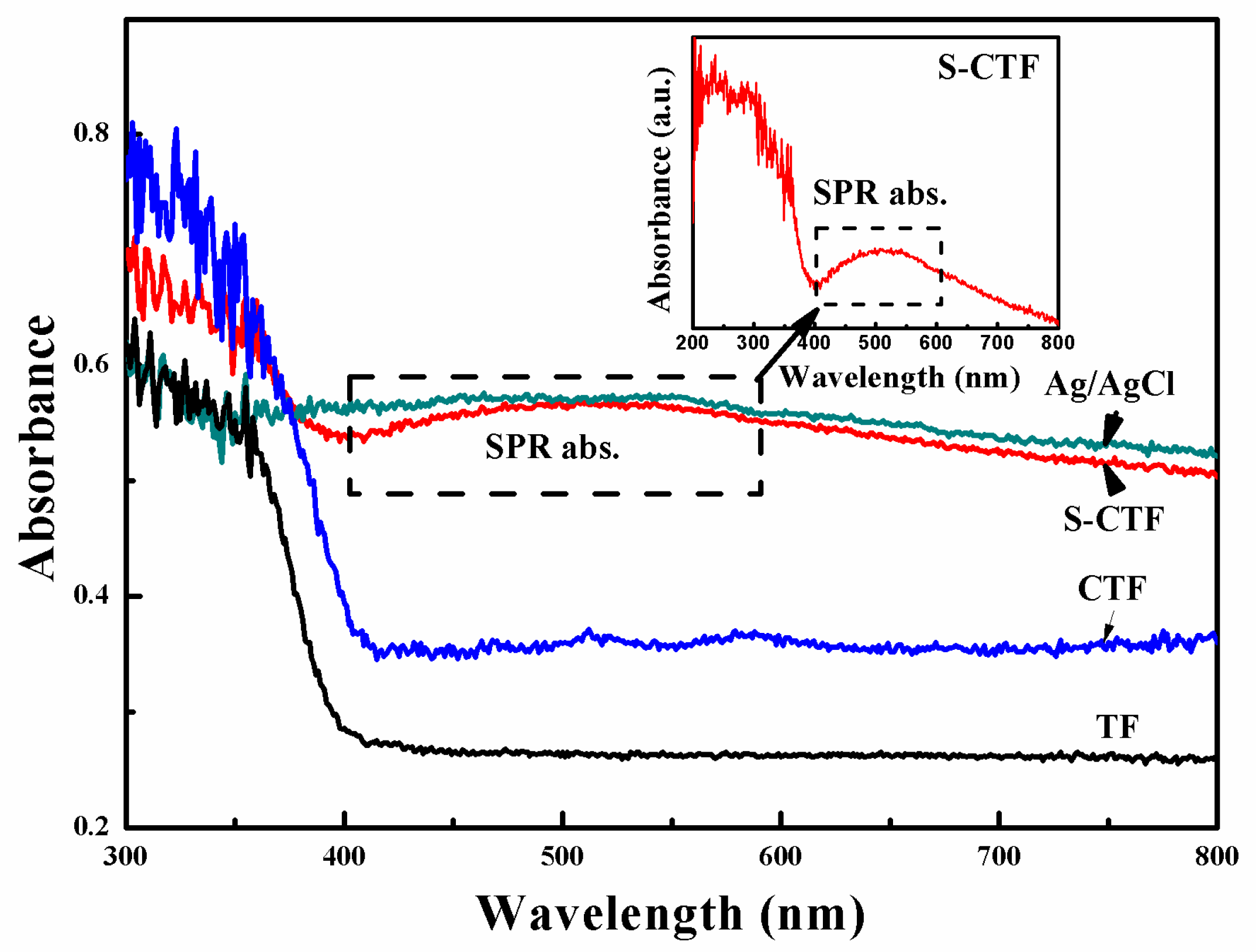
2.4. UV-Vis DRS
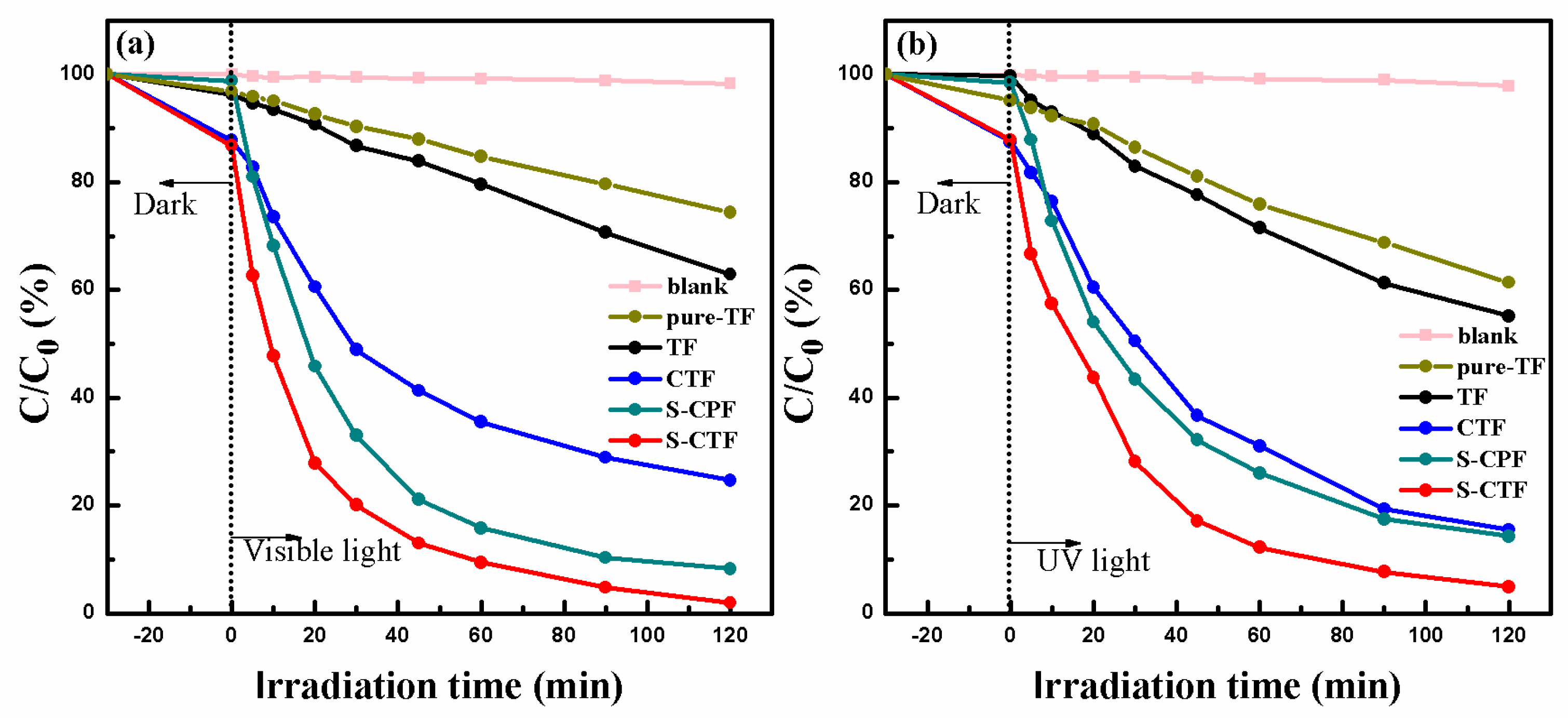
2.5. Photocatalytic Activity
2.6. Photocatalytic Mechanism
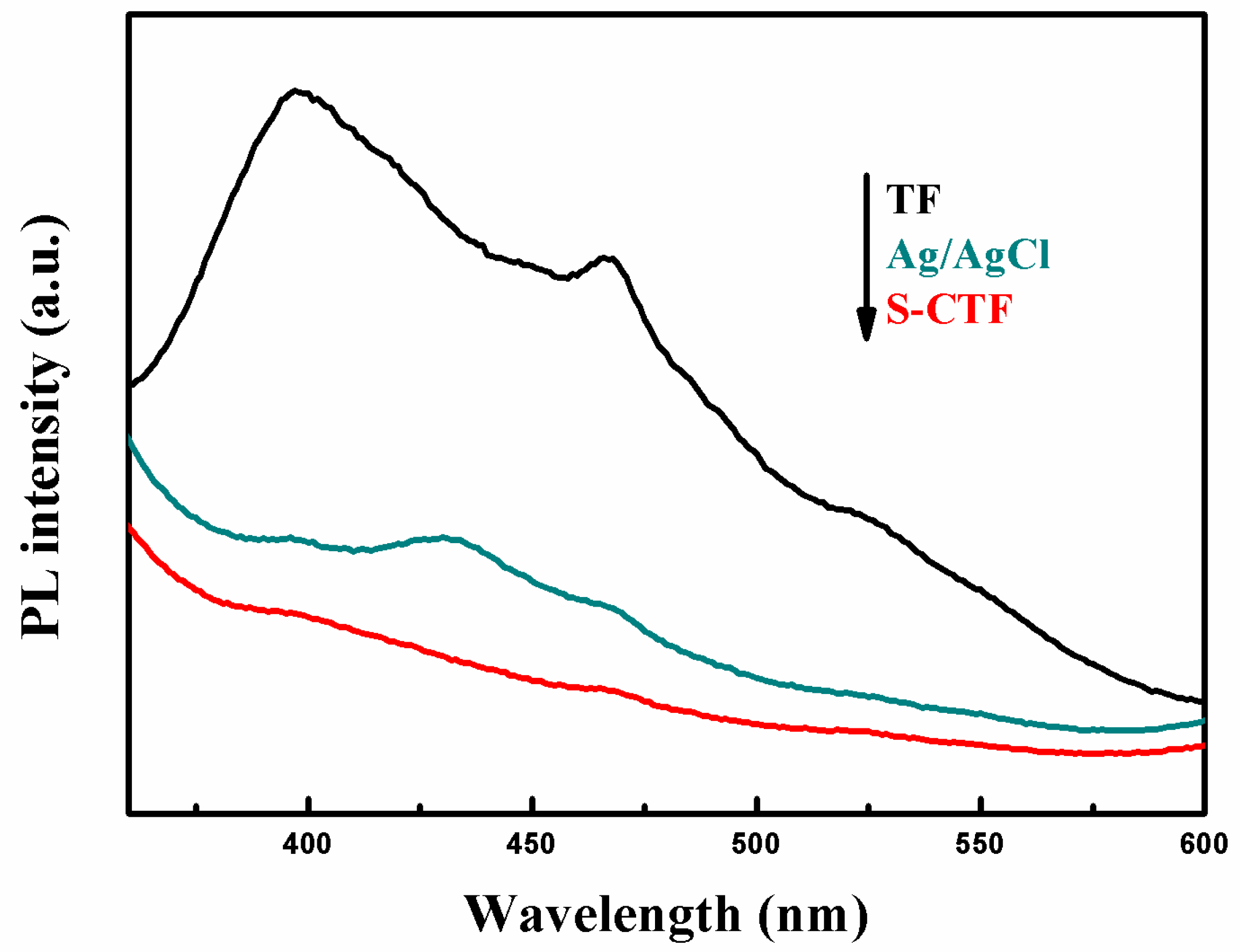
2.6.1. Photoluminescence (PL)
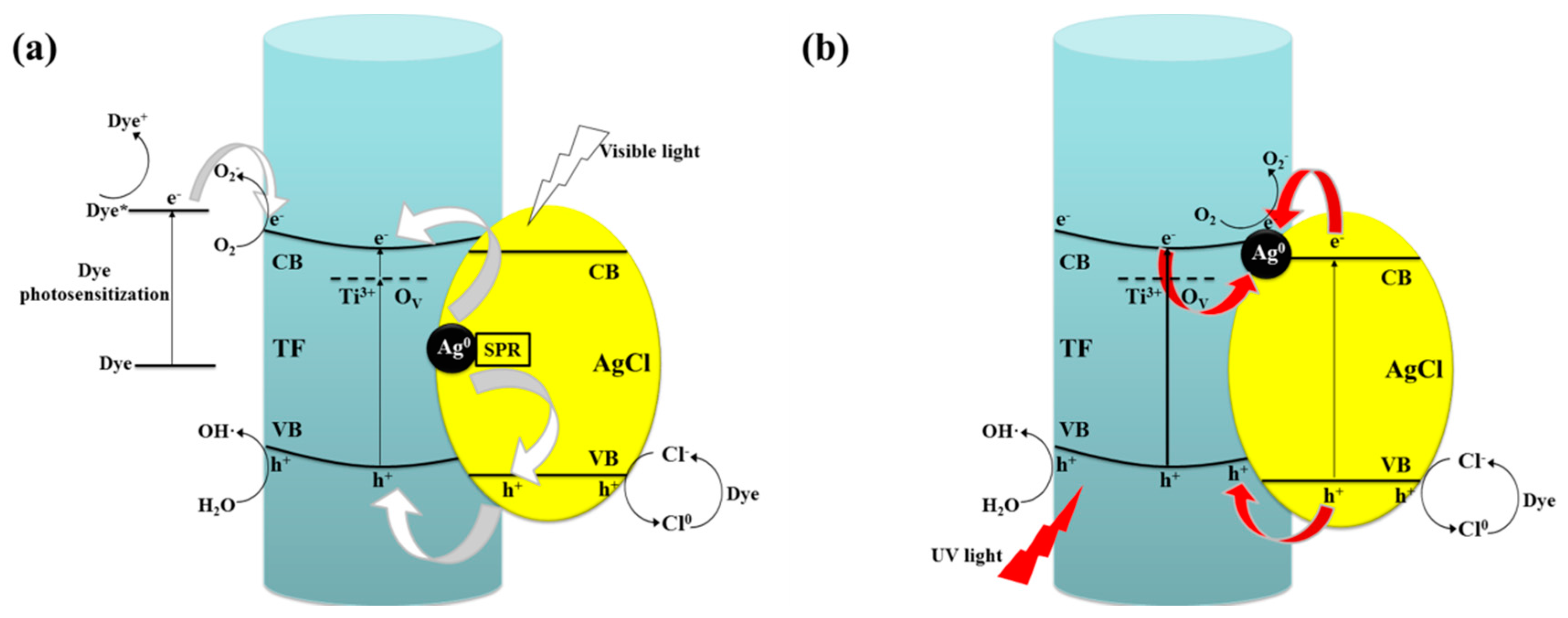
2.6.2. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Catalysts
3.3. Characterization of Catalysts
3.4. Photoactivity Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yin, L.-C.; Wang, J.; Niu, P.; Zhen, C.; Xie, Y.; Cheng, H.-M. A red anatase TiO2 photocatalyst for solar energy conversion. Energy Environ. Sci. 2012, 5, 9603–9610. [Google Scholar] [CrossRef]
- Kudo, A. Development of photocatalyst materials for water splitting with the aim at photon energy conversion. J. Ceram. Soc. Jpn. 2001, 109, S81–S88. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Lin, Y.; Zhang, Y.; Wei, Y. Simple fabrication and photocatalytic activity of S-doped TiO2 under low power led visible light irradiation. Ceram. Int. 2009, 35, 3061–3065. [Google Scholar] [CrossRef]
- Chen, C.; Cai, W.; Long, M.; Zhou, B.; Wu, Y.; Wu, D.; Feng, Y. Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Yin, Z.; Zhang, Q.; He, S.; Hu, X.; Miao, X. Synthesis of flower-like monoclinic BiVO4/surface rough TiO2 ceramic fiber with heterostructures and its photocatalytic property. Ceram. Int. 2016, 42, 1791–1800. [Google Scholar] [CrossRef]
- Yang, Y.; Wen, J.; Wei, J.; Xiong, R.; Shi, J.; Pan, C. Polypyrrole-decorated Ag-TiO2 nanofibers exhibiting enhanced photocatalytic activity under visible-light illumination. ACS Appl. Mater. Interfaces 2013, 5, 6201–6207. [Google Scholar] [CrossRef] [PubMed]
- Li, F.B.; Li, X.Z. Photocatalytic properties of gold/gold ion-modified titanium dioxide for wastewater treatment. Appl. Catal. A Gen. 2002, 228, 15–27. [Google Scholar] [CrossRef]
- Zhang, Q.; Thrithamarassery Gangadharan, D.; Liu, Y.; Xu, Z.; Chaker, M.; Ma, D. Recent advancements in plasmon-enhanced visible light-driven water splitting. J. Materiomics 2017, 3, 33–50. [Google Scholar] [CrossRef]
- Kumar, R.; El-Shishtawy, M.R.; Barakat, A.M. Synthesis and characterization of Ag-Ag2O/TiO2@polypyrrole heterojunction for enhanced photocatalytic degradation of methylene blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.-H. Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, N.; Goto, N.; Ohkita, H.; Mizushima, T. Silver bromide as a photocatalyst for hydrogen generation from CH3OH/H2O solution. J. Phys. Chem. B 1999, 103, 5917–5919. [Google Scholar] [CrossRef]
- Yu, J.; Dai, G.; Huang, B. Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanotube arrays. J. Phys. Chem. C 2009, 113, 16394–16401. [Google Scholar] [CrossRef]
- Guo, J.-F.; Ma, B.; Yin, A.; Fan, K.; Dai, W.-L. Highly stable and efficient Ag/AgCl@TiO2 photocatalyst: Preparation, characterization, and application in the treatment of aqueous hazardous pollutants. J. Hazard. Mater. 2012, 211–212, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lan, Y.; Qu, J.; Hu, X.; Wang, A. Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Martins, R. Photocatalytic TiO2 nanorod spheres and arrays compatible with flexible applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, Y.; Zhang, M.; Murugananthan, M.; Yoshihara, S. Photoelectrocatalytic degradation of microcystin-LR using Ag/AgCl/TiO2 nanotube arrays electrode under visible light irradiation. Chem. Eng. J. 2013, 231, 455–463. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Li Puma, G.; Wang, C.; Wang, P.; Zhang, W.; Wang, Q. Ag/AgCl@helical chiral TiO2 nanofibers as a visible-light driven plasmon photocatalyst. Chem. Commun. 2013, 49, 10367–10369. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of highly efficient Ag@AgCl plasmonic photocatalysts with various structures. Chem. Eur. J. 2010, 16, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Guo, J.; Dai, W.-L.; Fan, K. Ag-AgCl/WO3 hollow sphere with flower-like structure and superior visible photocatalytic activity. Appl. Catal. B Environ. 2012, 123–124, 193–199. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Ceccato, R.; Palmisano, L.; Parrino, F. Highly active photocatalytic TiO2 powders obtained by thermohydrolysis of TiCl4 in water. J. Phys. Chem. C 2009, 113, 15166–15174. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Gao, C.; Cao, L. The synthesis of nanosized TiO2 powder using a sol-gel method with TiCl4 as a precursor. J. Mater. Sci. 2000, 35, 4049–4054. [Google Scholar] [CrossRef]
- Xu, H.; Gao, L.; Guo, J. Hydrothermal synthesis of tetragonal barium titanate from barium chloride and titanium tetrachloride under moderate conditions. J. Am. Ceram. Soc. 2002, 85, 727–729. [Google Scholar] [CrossRef]
- Bao, N.; Yin, G.-B.; Wei, Z.-T.; Li, Y.; Ma, Z.-H. Preparation of TiO2 continuous fibers with oxygen vacancies and photocatalytic activity. Integr. Ferroelectr. 2011, 127, 97–105. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, Y.; Yu, J. Preparation and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanocomposite thin films. J. Photochem. Photobiol. A Chem. 2011, 223, 82–87. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, X.; McKiernan, M.; Lee, E.P.; Sun, Y.; Xia, Y. Hierarchical nanostructures of K-birnessite nanoplates on anatase nanofibers and their application for decoloration of dye solution. J. Mater. Chem. 2010, 20, 3157–3162. [Google Scholar] [CrossRef]
- Zhuang, X.; Wan, Y.; Feng, C.; Shen, Y.; Zhao, D. Highly efficient adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons. Chem. Mater. 2009, 21, 706–716. [Google Scholar] [CrossRef]
- Bao, N.; Li, Y.; Wei, Z.; Yin, G.; Niu, J. Adsorption of dyes on hierarchical mesoporous TiO2 fibers and its enhanced photocatalytic properties. J. Phys. Chem. C 2011, 115, 5708–5719. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.; Shi, J.; Niu, C. Highly crystallized C-doped mesoporous anatase TiO2 with visible light photocatalytic activity. Catalysts 2016, 6, 117. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Xia, J.; Yin, S.; Luo, Z.; Liu, L.; Xu, L. One-pot synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl in ionic liquid. ACS Appl. Mater. Interfaces 2011, 3, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ghicov, A.; Yamamoto, M.; Schmuki, P. Lattice Widening in Niobium-Doped TiO2 Nanotubes: Efficient Ion Intercalation and Swift Electrochromic Contrast. Angew. Chem. Int. Ed. 2008, 47, 7934–7937. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Zhou, Y.; Ma, Y.; Yang, X.; Sheng, W.; Xing, M.; Zhang, J. Facile synthesis of the Ti3+ self-doped TiO2-graphene nanosheet composites with enhanced photocatalysis. Sci. Rep. 2015, 5, 8591. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-situ-reduced synthesis of Ti3+ self-doped TiO2/g-C3N4 heterojunctions with high photocatalytic performance under led light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Chen, Z.; Feng, C.; Li, W. Study of the promotion mechanism of the photocatalytic performance and stability of the Ag@AgCl/g-C3N4 composite under visible light. RSC Adv. 2014, 4, 38124–38132. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Ag/AgBr/WO3·H2O: Visible-light photocatalyst for bacteria destruction. Inorg. Chem. 2009, 48, 10697–10702. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, H.; Li, H.; Xia, J.; Liu, C.; Liu, L. Enhanced photocatalytic activity of new photocatalyst Ag/AgCl/ZnO. J. Alloys Compd. 2011, 509, 3286–3292. [Google Scholar] [CrossRef]
- Wang, Z.; Wen, B.; Hao, Q.; Liu, L.-M.; Zhou, C.; Mao, X.; Lang, X.; Yin, W.-J.; Dai, D.; Selloni, A.; et al. Localized excitation of Ti3+ ions in the photoabsorption and photocatalytic activity of reduced rutile TiO2. J. Am. Chem. Soc. 2015, 137, 9146–9152. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, X.; Wang, L.; Nie, Q.; Zhang, Y.; Yuan, Q.; Wu, W. Ag/AgCl modified self-doped TiO2 hollow sphere with enhanced visible light photocatalytic activity. J. Alloys Compd. 2016, 657, 44–52. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Zhu, C.; Zhai, Y.; Dong, S. Facile solvothermal synthesis of cube-like Ag@AgCl: A highly efficient visible light photocatalyst. Nanoscale 2011, 3, 2931–2935. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Guo, J.; Dai, W.-L.; Fan, K. Highly stable and efficient Ag/AgCl core-shell sphere: Controllable synthesis, characterization, and photocatalytic application. Appl. Catal. B Environ. 2013, 130, 257–263. [Google Scholar] [CrossRef]
- Sung-Suh, H.M.; Choi, J.R.; Hah, H.J.; Koo, S.M.; Bae, Y.C. Comparison of ag deposition effects on the photocatalytic activity of nanoparticulate TiO2 under visible and UV light irradiation. J. Photochem. Photobiol. A Chem. 2004, 163, 37–44. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. One-step synthesis of the nanostructured AgI/BiOI composites with highly enhanced visible-light photocatalytic performances. Langmuir 2010, 26, 6618–6624. [Google Scholar] [CrossRef] [PubMed]
- Klaysri, R.; Tubchareon, T.; Praserthdam, P. One-step synthesis of amine-functionalized TiO2 surface for photocatalytic decolorization under visible light irradiation. J. Ind. Eng. Chem. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, P.; Liu, M. Ag/Agbr/graphene oxide nanocomposite synthesized via oil/water and water/oil microemulsions: A comparison of sunlight energized plasmonic photocatalytic activity. Langmuir 2012, 28, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, R.; Wang, Y.; Chen, W. Plasmonic Ag@AgCl nanotubes fabricated from copper nanowires as high-performance visible light photocatalyst. ACS Appl. Mater. Interfaces 2014, 6, 14819–14826. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Li, Y.; Yu, X.-H.; Niu, J.-J.; Wu, G.-L.; Xu, X.-H. Removal of anionic azo dye from aqueous solution via an adsorption–photosensitized regeneration process on a TiO2 surface. Environ. Sci. Pollut. Res. 2013, 20, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two different roles of metallic Ag on Ag/AgX/BiOX (X = Cl, Br) visible light photocatalysts: Surface plasmon resonance and Z-scheme bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Hirakawa, T.; Kamat, P.V. Charge separation and catalytic activity of Ag@TiO2 core-shell composite clusters under UV-irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bao, N.; Wang, X.; Hu, X.; Miao, X.; Chaker, M.; Ma, D. Advanced fabrication of chemically bonded graphene/TiO2 continuous fibers with enhanced broadband photocatalytic properties and involved mechanisms exploration. Sci. Rep. 2016, 6, 38066. [Google Scholar] [CrossRef] [PubMed]
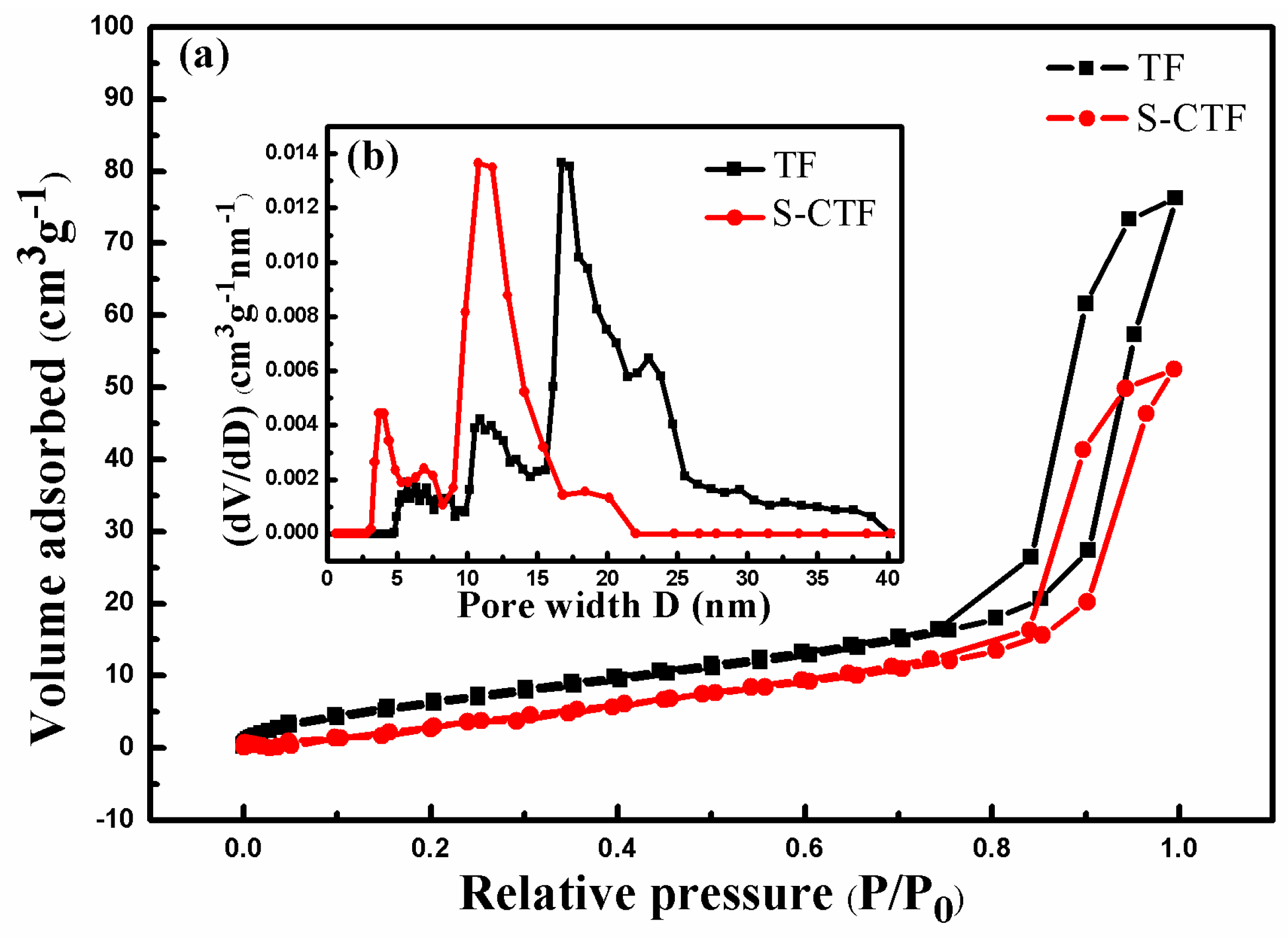

| Samples | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| TF | 27.9 | 0.111 | 16.7 |
| S-CTF | 30.6 | 0.074 | 10.8 |
| Temperature (°C) | Heating Rate (°C min−1) | Time (min) |
|---|---|---|
| 25–95 | 1.67 | 42 |
| 95–250 | 1.29 | 120 |
| 250–350 | 0.83 | 120 |
| 350–550 | 5.00 | 40 |
| 550–600 | 0.83 | 60 |
| 600–600 | - | 120 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, N.; Miao, X.; Hu, X.; Zhang, Q.; Jie, X.; Zheng, X. Novel Synthesis of Plasmonic Ag/AgCl@TiO2 Continues Fibers with Enhanced Broadband Photocatalytic Performance. Catalysts 2017, 7, 117. https://doi.org/10.3390/catal7040117
Bao N, Miao X, Hu X, Zhang Q, Jie X, Zheng X. Novel Synthesis of Plasmonic Ag/AgCl@TiO2 Continues Fibers with Enhanced Broadband Photocatalytic Performance. Catalysts. 2017; 7(4):117. https://doi.org/10.3390/catal7040117
Chicago/Turabian StyleBao, Nan, Xinhan Miao, Xinde Hu, Qingzhe Zhang, Xiuyan Jie, and Xiyue Zheng. 2017. "Novel Synthesis of Plasmonic Ag/AgCl@TiO2 Continues Fibers with Enhanced Broadband Photocatalytic Performance" Catalysts 7, no. 4: 117. https://doi.org/10.3390/catal7040117





