Eco-Friendly Physical Activation Methods for Suzuki–Miyaura Reactions
Abstract
:1. Introduction
- 1. Introduction
- Some Insights into the Suzuki–Miyaura reaction
- 2. Microwave-Assisted SMC Reactions
- 2.1. MW Irradiation in Homogeneous Catalyzed SMC
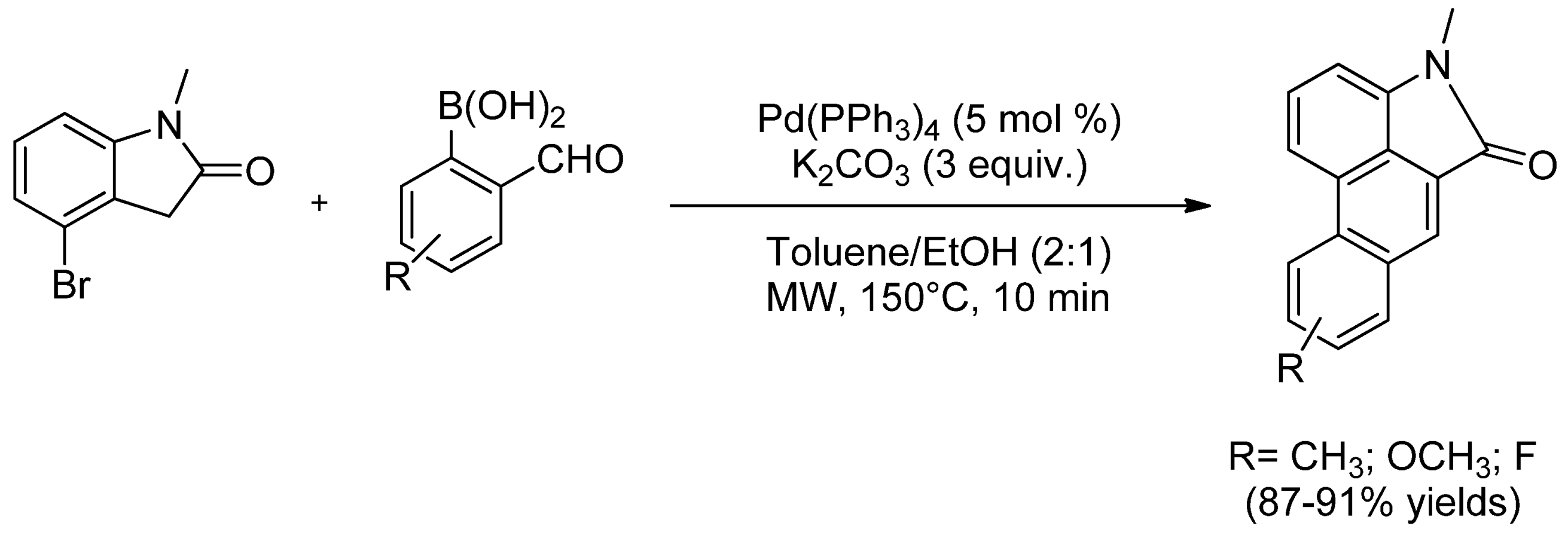
- 2.1.1. SMC from Arene to Heterocycle Decoration
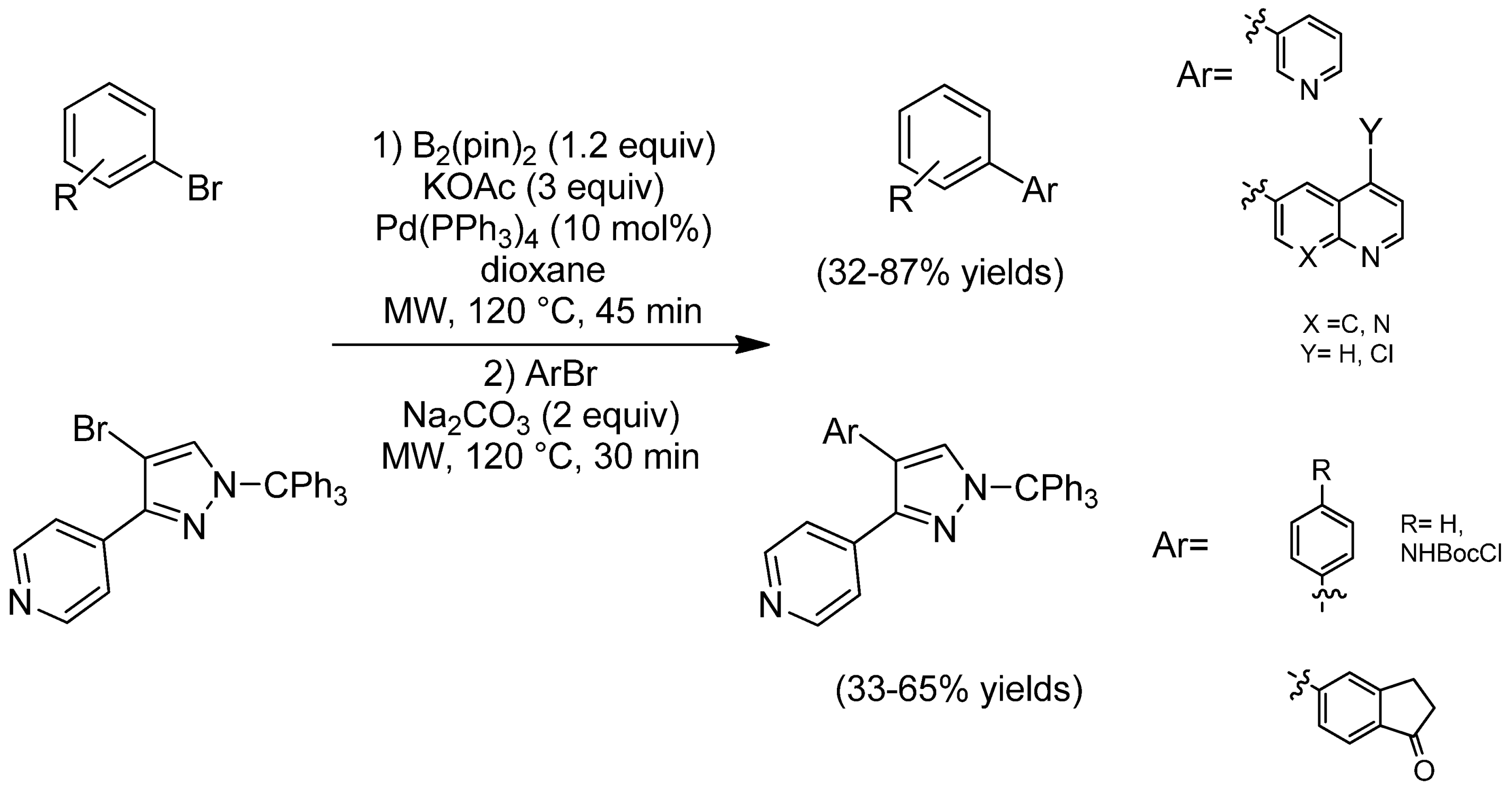
- 2.1.2. Organotrifluoroborates in MW-Assisted SMC
- 2.1.3. MW-Assisted One-Pot Protocols
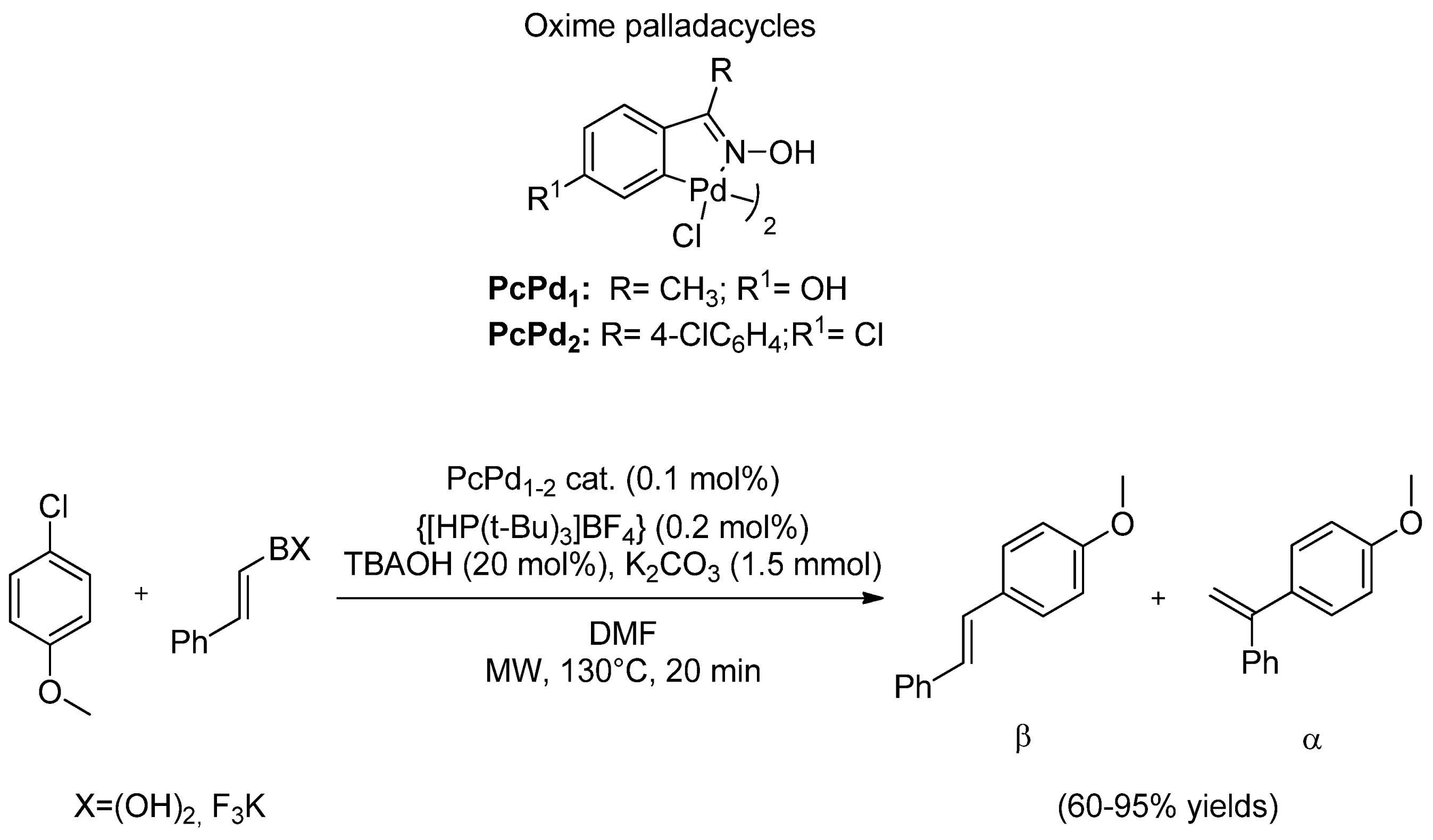
- 2.1.4. Ligands in MW-Assisted SMC
- 2.1.5. MW Promoted Ni Catalyzed SMC
- 2.2. Moving from Homogeneous to Heterogeneous Catalysis in MW Promoted SMC
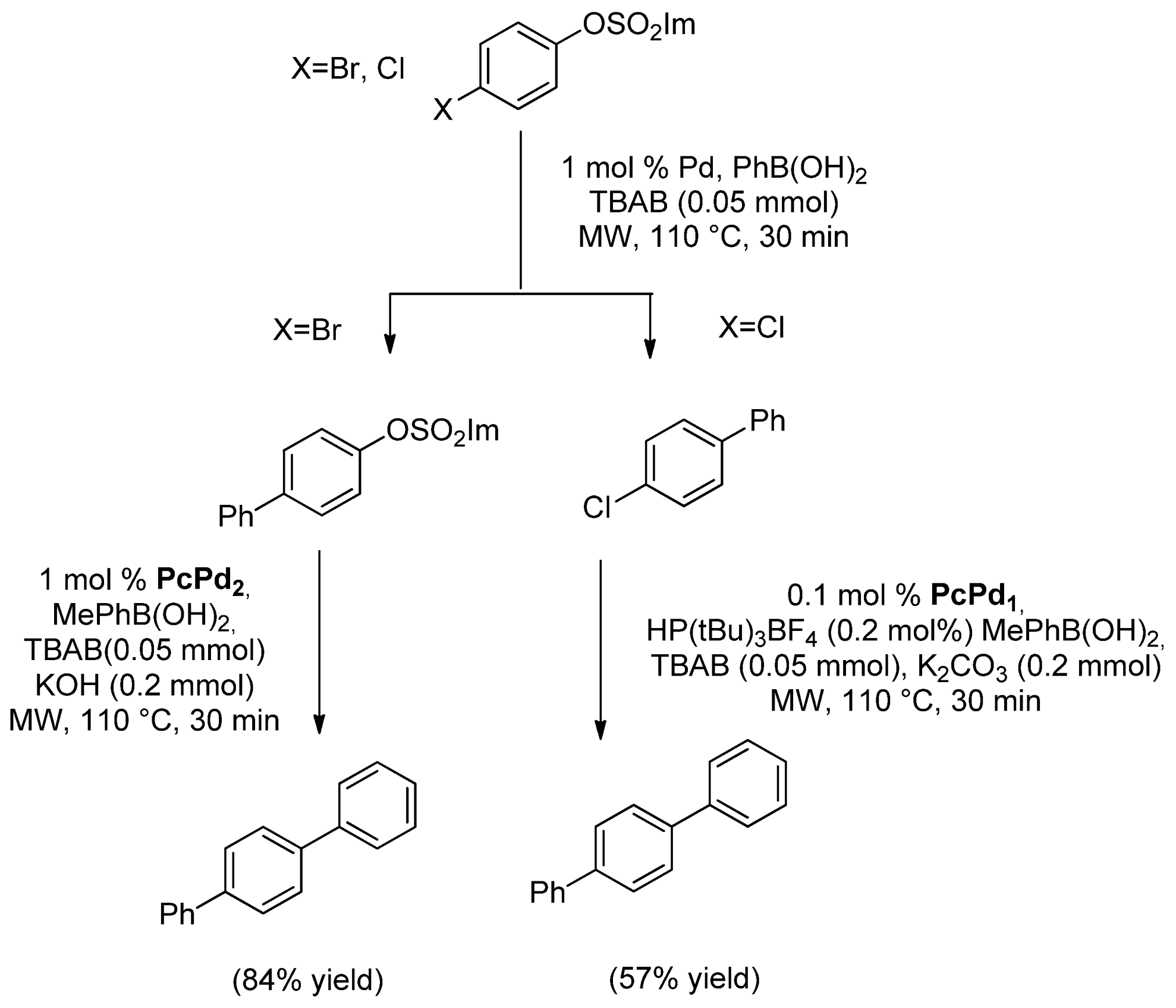
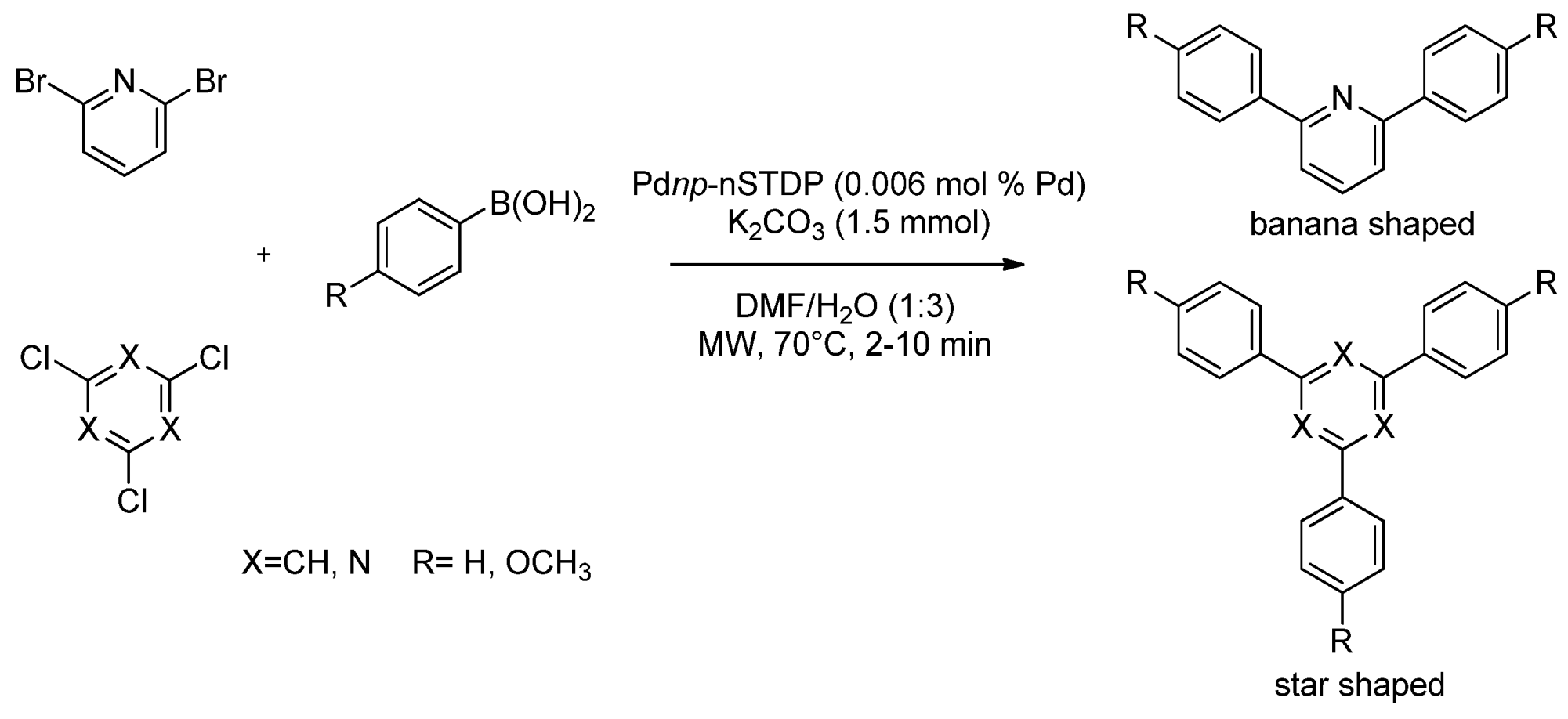
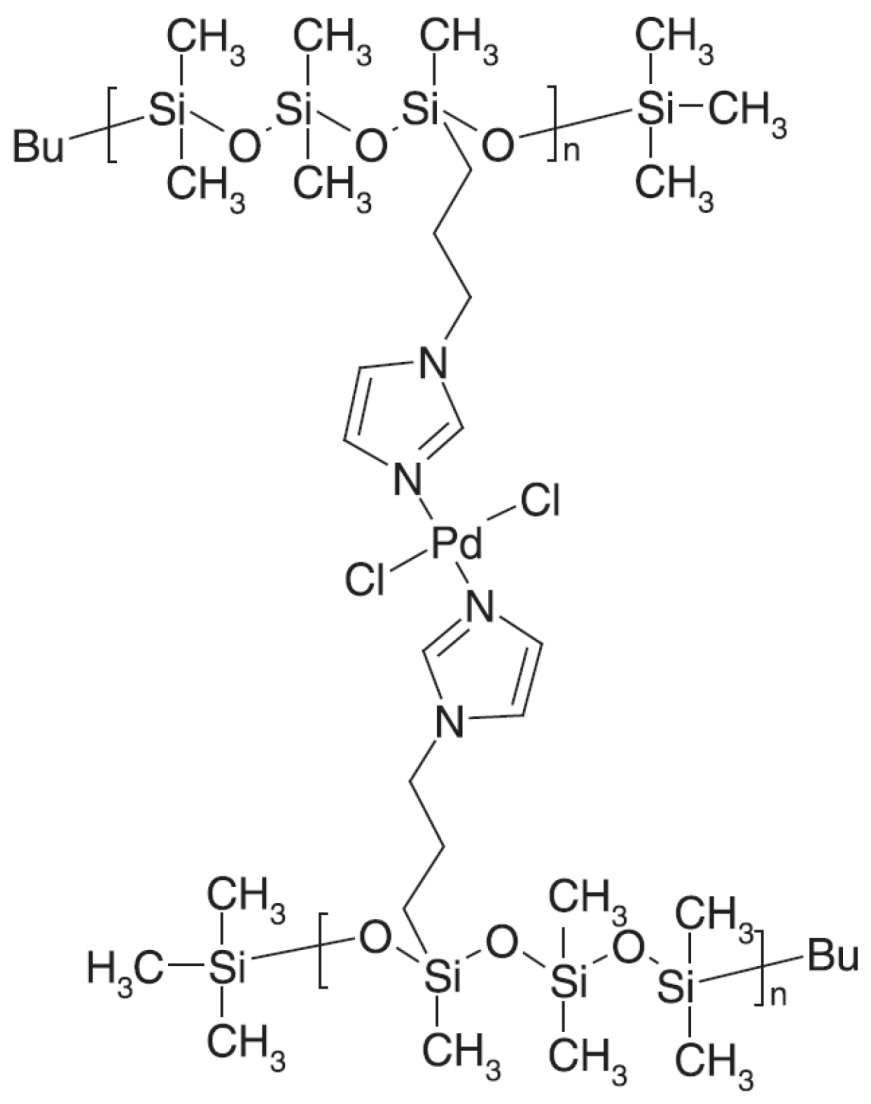
- 2.3. Solid Supported Pd Catalyzed MW Promoted SMC
- Use of MWs for the Synthesis of the Catalysts for the SMC Reaction
- 3. Ultrasound-Assisted SMC Reactions
- 3.1. Ultrasound-Assisted SMC Reactions
- 3.2. Ultrasound Assisted Heterogeneous Preparation of Catalysts Suitable for SMC Reactions
- 3.3. Ultrasound Assisted Heterogeneous Catalyst Preparation for MW Promoted SMC Reactions
- 4. Mechanochemical Activation of SMC
- 5. When the SMC Reaction is Driven by Light
- 6. Conclusions and Future Perspectives
Some Insights into the Suzuki–Miyaura Reaction
2. Microwave-Assisted SMC Reactions
2.1. MW Irradiation in Homogeneous Catalyzed SMC
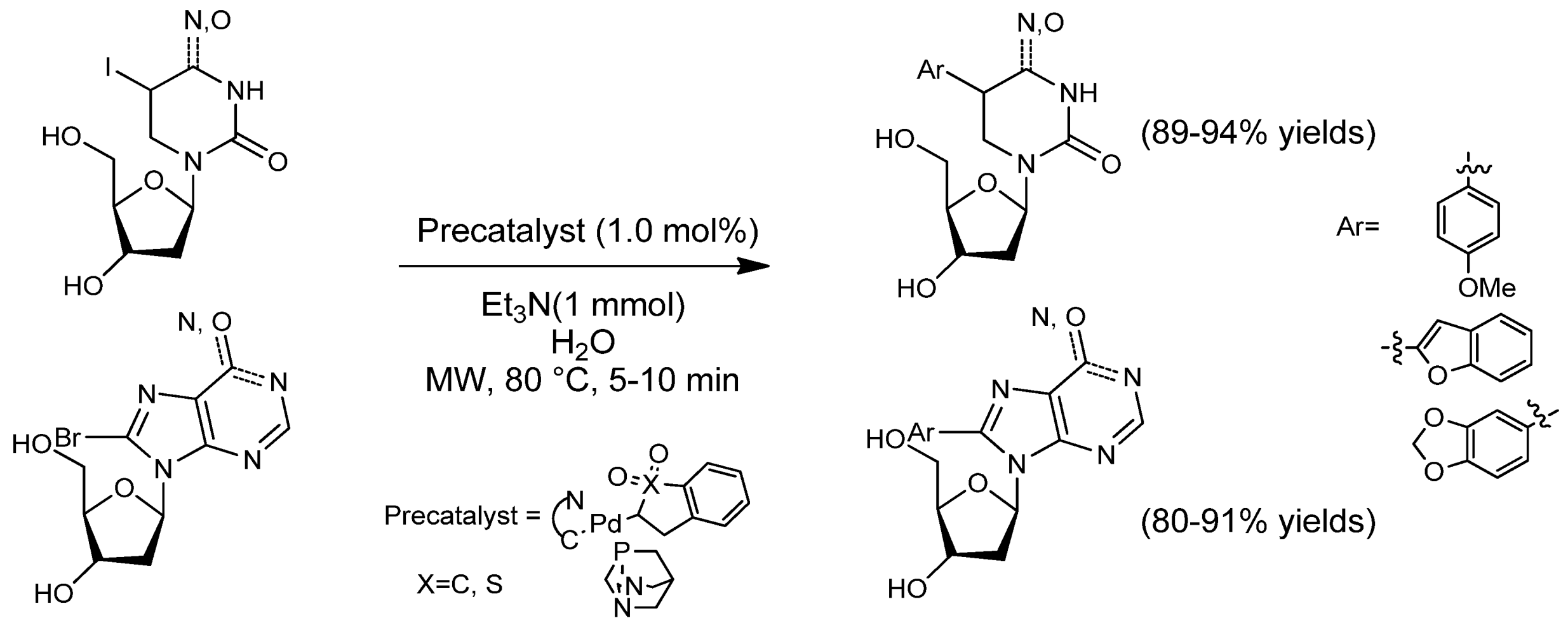
2.1.1. SMC from Arenes to Heterocycles
2.1.2. Organotrifluoroborates in MW-Assisted SMC
2.1.3. MW-Assisted One-Pot Protocols
2.1.4. Ligands in MW-Assisted SMC
2.1.5. MW Promoted Ni Catalyzed SMC
2.2. Moving from Homogeneous to Heterogeneous Catalysis in MW Promoted SMC
2.3. Solid Supported Pd Catalyzed MW Promoted SMC
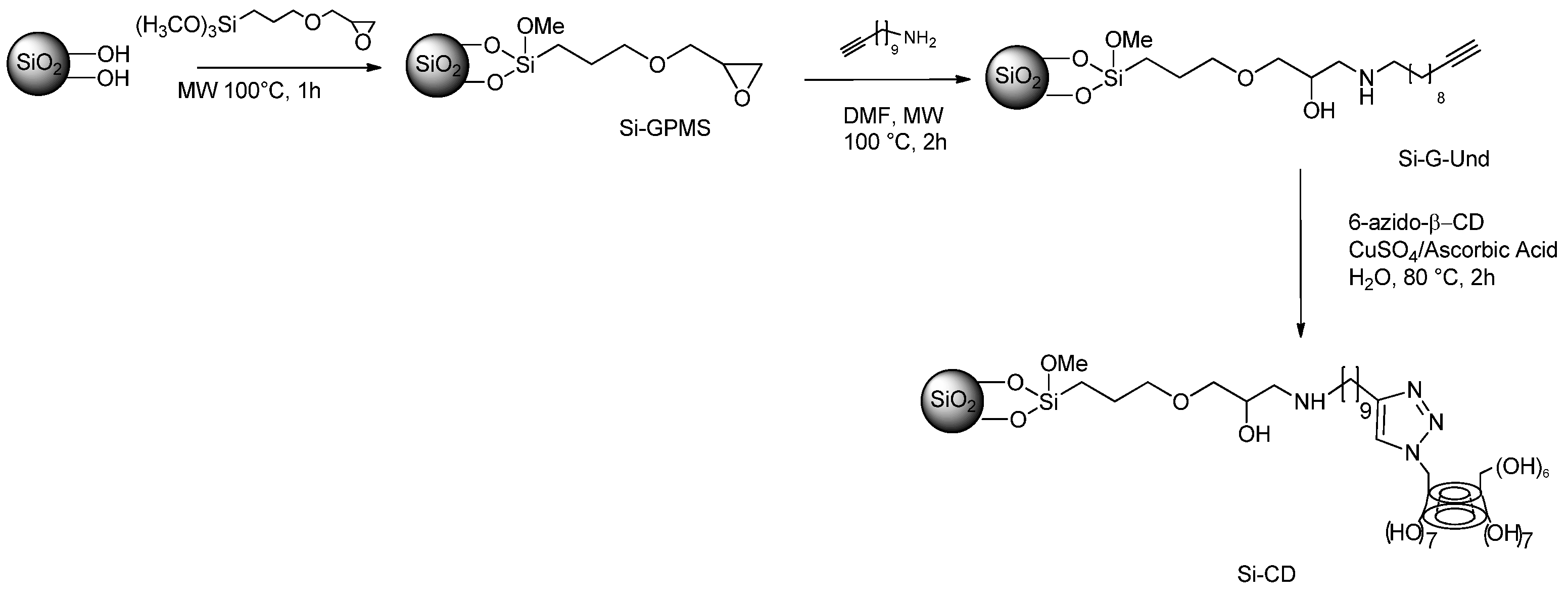
Use of MWs for the Synthesis of the Catalysts for the SMC Reaction
3. Ultrasound-Assisted SMC Reactions
3.1. Ultrasound-Assisted SMC Reactions
3.2. Ultrasound Assisted Heterogeneous Preparation of Catalysts Suitable for SMC Reactions
3.3. Ultrasound Assisted Heterogeneous Catalyst Preparation for MW Promoted SMC Reactions
4. Mechanochemical Activation of SMC
5. When the SMC Reaction Is Driven by Light
6. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the nature of the active species in palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura couplings—Homogeneous or heterogeneous catalysis, A critical review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Kappe, O.K.; Pieber, B.; Dallinger, D. Microwave effects in organic synthesis: Myth or reality? Angew. Chem. Int. Ed. 2013, 52, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Kreuzkupplungen von Organoboranen: Ein einfacher Weg zum Aufbau von C-C-Bindungen (Nobel-Aufsatz). Angew. Chem. 2011, 123, 6854–6869. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds. Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl–Aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Han, F.S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-coupling reactions via organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Abela, A.R.; Boskovic, Z.V.; Nishikata, T.; Duplais, C.; Krasovskiy, A. “Greening up” cross-coupling chemistry. Top. Catal. 2010, 53, 985–990. [Google Scholar] [CrossRef]
- Paul, S.; Islam, M.M.; Islam, S.M. Suzuki–Miyaura reaction by heterogeneously supported Pd in water: Recent studies. RSC Adv. 2015, 5, 42193–42221. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. Suzuki–Miyaura cross-coupling coupling reactions with low catalyst loading: A green and sustainable protocol in pure water. ChemSusChem 2010, 3, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Ward, T.R. Recent advances in the palladium catalyzed Suzuki–Miyaura cross-coupling reaction in water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef]
- Cravotto, G.; Garella, D.; Tagliapietra, S.; Stolle, A.; Schuessler, S.; Leonhardt, S.E.S.; Ondruschka, B. Suzuki cross-couplings of (hetero)aryl chlorides in the solid-state. New J. Chem. 2012, 36, 1304–1307. [Google Scholar] [CrossRef]
- Larhed, M.; Moberg, C.; Hallberg, A. Microwave-accelerated homogeneous catalysis in organic chemistry. Acc. Chem. Res. 2002, 35, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.P.; Van der Eycken, E.V. Microwave-assisted C–C bond forming cross-coupling reactions: An overview. Chem. Soc. Rev. 2011, 40, 4925–4936. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, J.X. Environmentally friendly Suzuki aryl–aryl cross-coupling reaction. Curr. Org. Chem. 2005, 9, 535–553. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Meier, M.A.R.; Schubert, U.S. The introduction of high-throughput experimentation methods for Suzuki–Miyaura coupling reactions in university education. J. Chem. Educ. 2005, 82, 1693–1696. [Google Scholar] [CrossRef]
- Costa, N.E.; Pelotte, A.L.; Simard, J.M.; Syvinski, C.A.; Deveau, A.M. Discovering green, aqueous Suzuki coupling reactions: Synthesis of ethyl (4-phenylphenyl)acetate, a biaryl with anti-arthritic potential. J. Chem. Educ. 2012, 89, 1064–1067. [Google Scholar] [CrossRef]
- Hill, N.J.; Bowman, M.D.; Esselman, B.J.; Byron, S.D.; Kreitinger, J.; Leadbeater, N.E. Ligand-free Suzuki–Miyaura coupling reactions using an inexpensive aqueous palladium source: A synthetic and computational exercise for the undergraduate organic chemistry laboratory. J. Chem. Educ. 2014, 91, 1054–1057. [Google Scholar] [CrossRef]
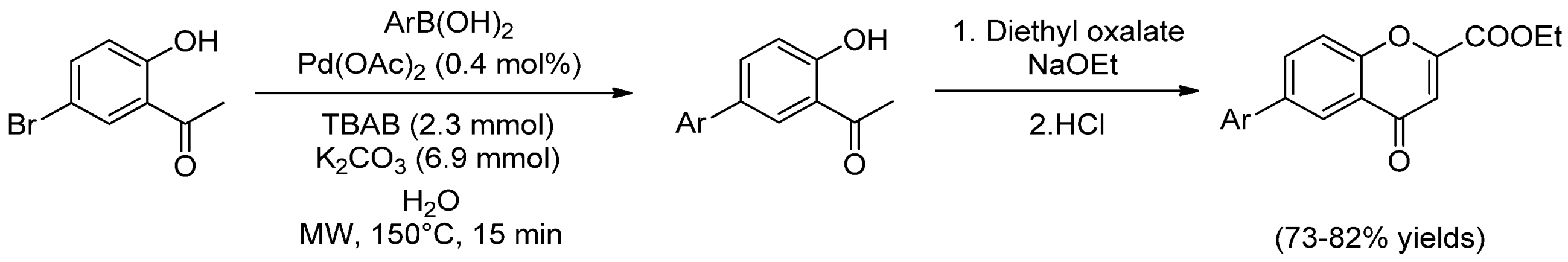
- Soares, P.; Fernandes, C.; Chavarria, D.; Borges, F. Microwave-assisted synthesis of 5-phenyl-2-hydroxyacetophenone derivatives by a green Suzuki coupling reaction. J. Chem. Educ. 2015, 92, 575–578. [Google Scholar] [CrossRef]
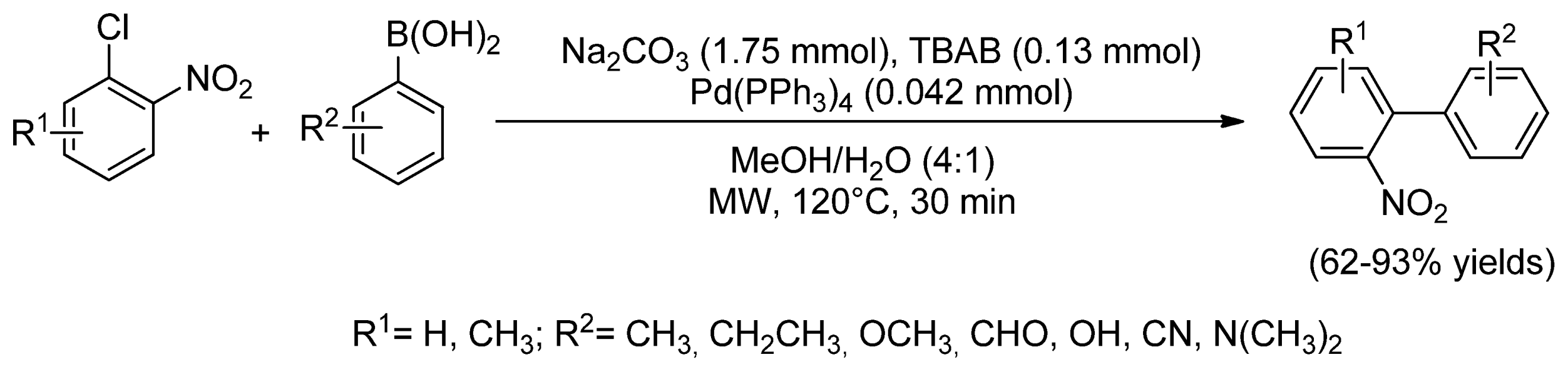
- Elumalai, V.; Sandtorv, A.H.; Bjorsvik, H.-R. A highly efficient Pd(PPh3)4-catalyzed suzuki cross-coupling method for the preparation of 2-nitrobiphenyls from 1-chloro-2-nitrobenzenes and phenylboronic acids. Eur. J. Org. Chem. 2016, 2016, 1344–1354. [Google Scholar] [CrossRef]
- Liu, J.; Robins, M.J. Fluoro, alkylsulfanyl, and alkylsulfonyl leaving groups in Suzuki cross-coupling reactions of purine 2′-deoxynucleosides and nucleosides. Org. Lett. 2005, 7, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.J.; Schade, M.A.; Wirth, S.; Knochel, P. Cobalt(II)-catalyzed cross-coupling between polyfunctional arylcopper reagents and aryl fluorides or tosylates. Org. Lett. 2006, 8, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Alfonso, B.J.; Love, J.A. Platinum(II)-catalyzed cross-coupling of polyfluoroaryl imines. Org. Lett. 2007, 9, 5629–5631. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kong, F.; Kanno, K.-I.; He, J.; Nakajima, K.; Takahashi, T. Early transition metal-catalyzed cross-coupling reaction of aryl fluorides with a phenethyl Grignard reagent accompanied by rearrangement of the phenethyl group. Organometallics 2006, 25, 2045–2048. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Jimenez-Sanchidrian, C.; Mora, M. Suzuki cross-coupling reaction of fluorobenzene with heterogeneous Palladium catalysts. J. Fluorine Chem. 2006, 127, 443–445. [Google Scholar] [CrossRef]
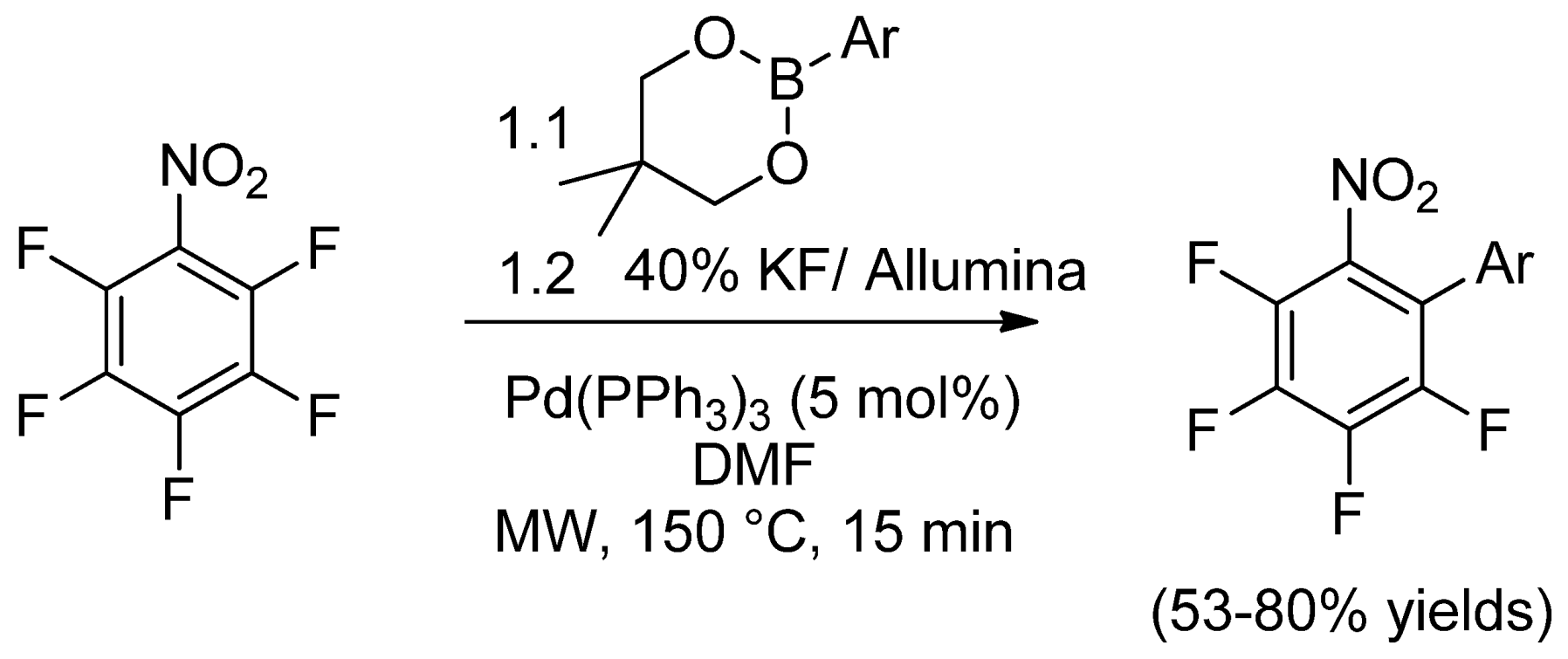
- Cargill, M.R.; Sandford, G.; Tadeusiak, A.J.; Yufit, D.S.; Howard, J.A.K.; Kilickiran, P.; Nelles, G. Palladium-catalyzed C–F activation of polyfluoronitrobenzene derivatives in Suzuki–Miyaura coupling reactions. J. Org. Chem. 2010, 75, 5860–5866. [Google Scholar]
- Louvis, A.D.; Silva, N.A.; Semaan, F.S.; Da Silva, F.D.; Saramago, G.; de Souza, L.C.; Ferreira, B.L.; Castro, H.C.; Salles, J.P.; Souza, A.L.; et al. Synthesis, characterization and biological activities of 3-aryl-1,4-naphthoquinones—Green palladium-catalysed suzuki cross coupling. New J. Chem. 2016, 40, 7643–7656. [Google Scholar] [CrossRef]
- Fernandes, C.; Soares, P.; Gaspar, A.; Martins, D.; Gomes, L.R.; Low, J.N.; Borges, F. Synthesis of 6-aryl/heteroaryl-4-oxo-4H-chromene-2-carboxylic ethyl ester derivatives. Tetrahedr. Lett. 2016, 57, 3006–3010. [Google Scholar] [CrossRef]
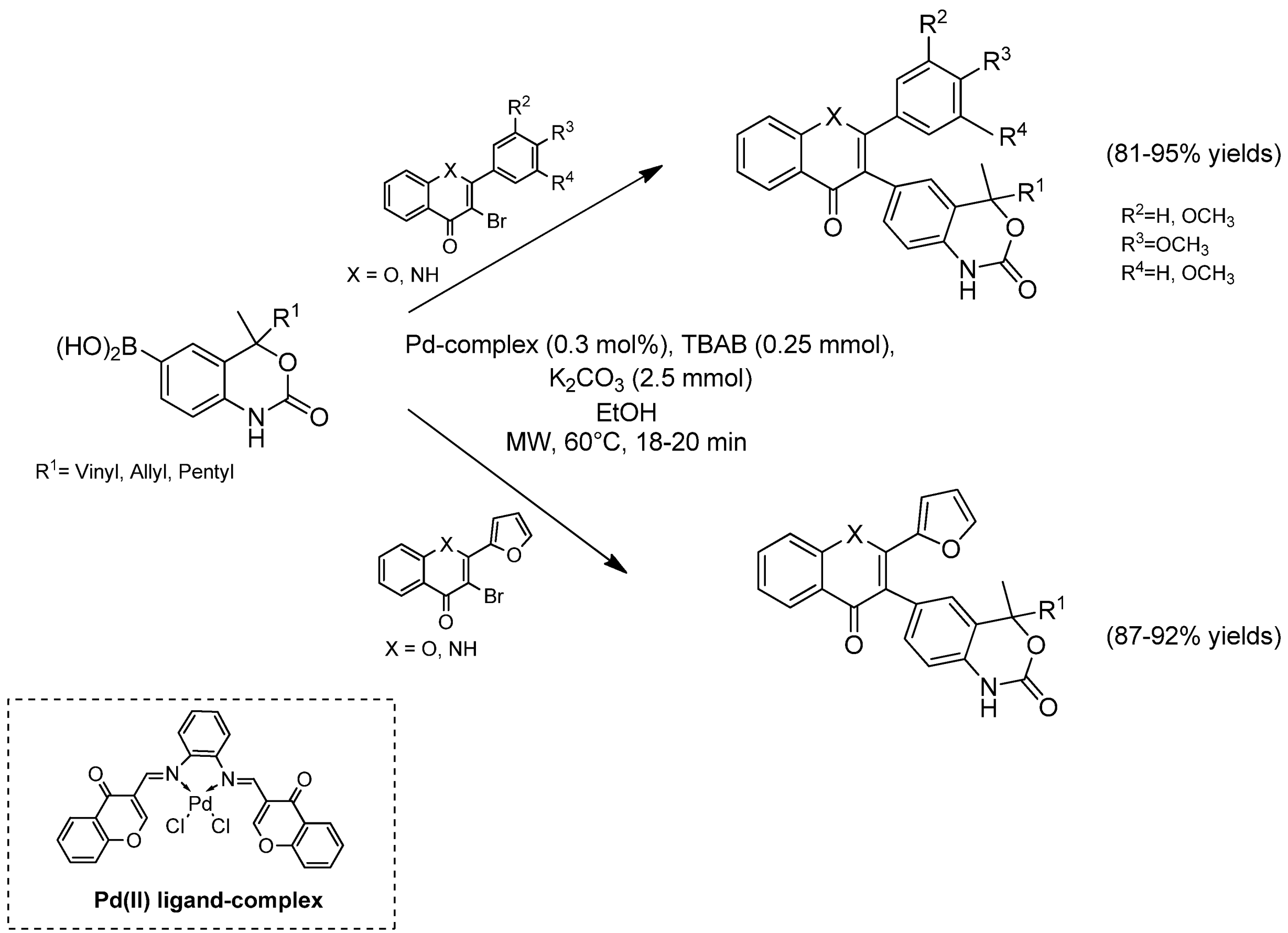
- Kumar, S.; Ahmed, N. A facile approach for the synthesis of novel 1-oxa- and 1-aza-flavonyl-4-methyl-1H-benzo[d][1,3]oxazin-2(4H)-ones by microwave enhanced Suzuki–Miyaura coupling using bidentate chromen-4-one-based Pd(II)–diimine complex as catalyst. RSC Adv. 2015, 5, 77075–77087. [Google Scholar] [CrossRef]
- Qu, R.Y.; Liu, Y.C.; Wu, Q.Y.; Chen, Q.; Yang, G.F. An efficinet method for the syntheses of funtionalized 6-bulkysubstituted salicilates under microwave irradiation. Tetrahedron 2015, 71, 8123–8130. [Google Scholar] [CrossRef]
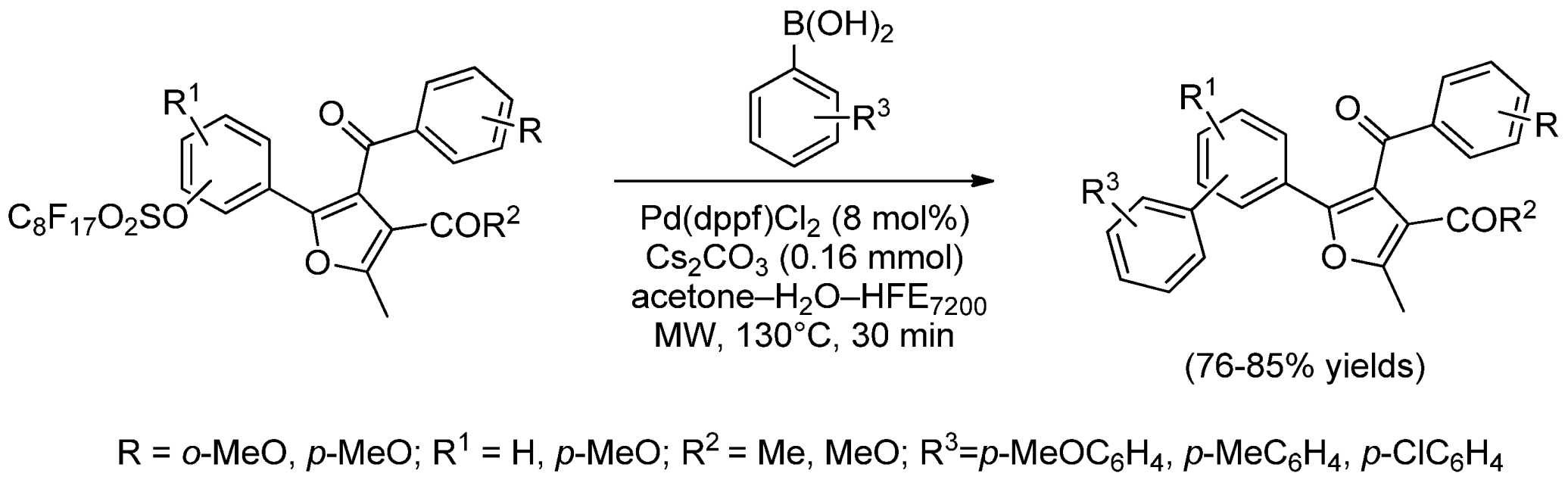
- Kadam, A.; Buckley, S.B.; Dinh, T.; Fitzgerald, R.; Zhang, W. Convertible fluorous linker assisted synthesis of tetrasubstituted furans. Synlett 2011, 11, 1608–1612. [Google Scholar]
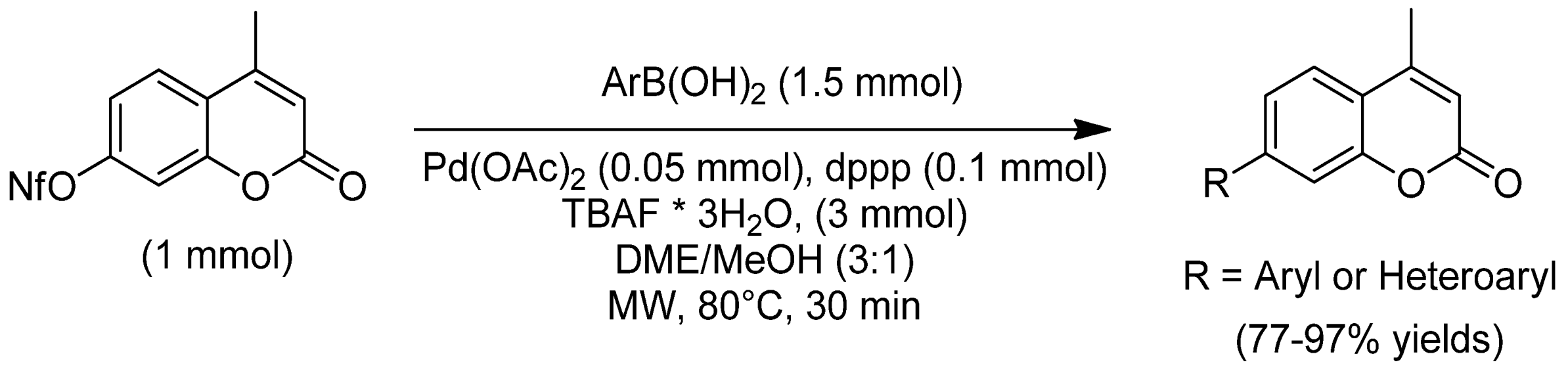
- Joy, M.N.; Bodke, Y.D.; Khader, K.K.A.; Sajith, A.M.; Venkatesh, T.; Kumar, A.K.A. Simultaneous exploration of TBAF·3H2O as a base as well as a solvating agent for the palladium catalyzed Suzuki cross-coupling of 4-methyl-7-nonafluorobutylsulfonyloxy coumarins under microwave irradiation. J. Fluorine Chem. 2016, 182, 109–120. [Google Scholar] [CrossRef]
- Vichier-Guerre, S.; Dugue, L.; Pochet, S. A convenient synthesis of 4(5)-(hetero)aryl-1H-imidazoles via microwave-assisted Suzuki–Miyaura cross-coupling reaction. Tetrahedron Lett. 2014, 55, 6347–6350. [Google Scholar] [CrossRef]
- Fuse, S.; Ohuchi, T.; Asawa, Y.; Sato, S.; Nakamura, H. Development of 1-aryl-3-furanyl/thienyl-imidazopyridine templates for inhibitors against hypoxia inducible factor (HIF)-1 transcriptional activity. Bioorg. Med. Chem. Lett. 2016, 26, 5887–5890. [Google Scholar] [CrossRef] [PubMed]
- Sandtorv, A.H.; Bjørsvika, H.-R. A three-way switchable process for Suzuki cross-coupling, hydrodehalogenation, or an assisted tandem hydrodehalogenation and Suzuki cross-coupling sequence. Adv. Synth. Catal. 2013, 355, 3231–3243. [Google Scholar] [CrossRef]
- Wang, S.; Guo, R.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Efficient synthesis of 3-aryl-1H-indazol-5-amine by Pd-catalyzed Suzuki–Miyaura cross-coupling reaction under microwave-assisted conditions. Tetrahedron Lett. 2015, 56, 3750–3753. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Ye, C.-J.; Chen, Q.; Yang, G.-F. Efficient synthesis of bulky 4-substituted-isatins via microwave-promoted Suzuki cross-coupling reaction. Tetrahedron Lett. 2013, 54, 949–955. [Google Scholar] [CrossRef]
- El Akkaoui, A.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumet, G. Direct arylation of imidazo[1,2-b]pyridazines: Microwave-assisted one-pot suzuki coupling/Pd-catalysed arylation. Eur. J. Org. Chem. 2010, 862–871. [Google Scholar] [CrossRef]
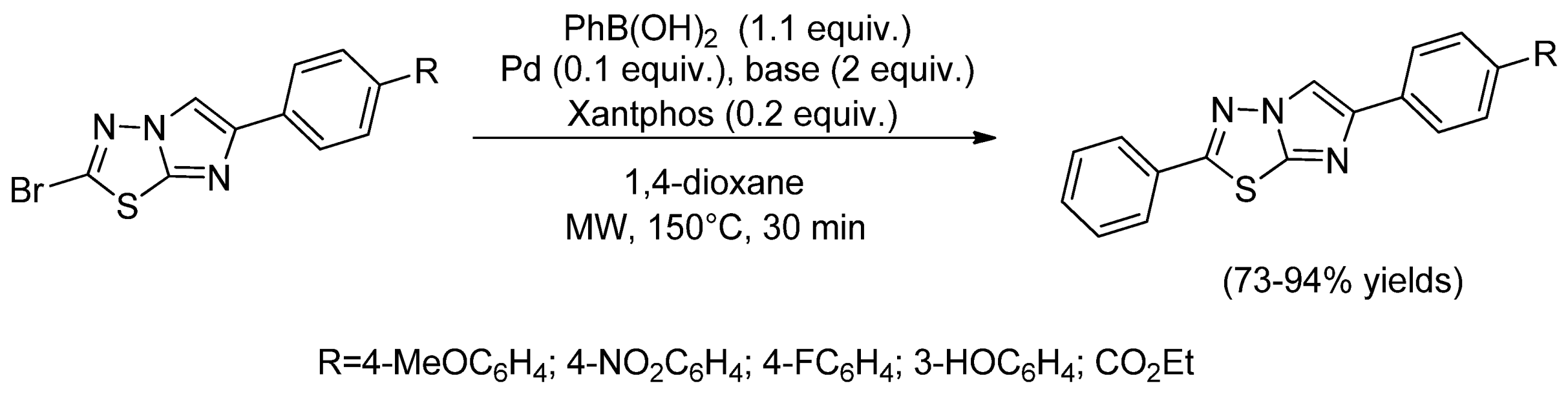
- Copin, C.; Henry, N.; Buron, F.; Routier, S. Synthesis of 2,6-disubstituted imidazo[2,1-b][1,3,4]thiadiazoles through cyclization and Suzuki–Miyaura cross-coupling reactions. Eur. J. Org. Chem. 2012, 2012, 3079–3083. [Google Scholar] [CrossRef]
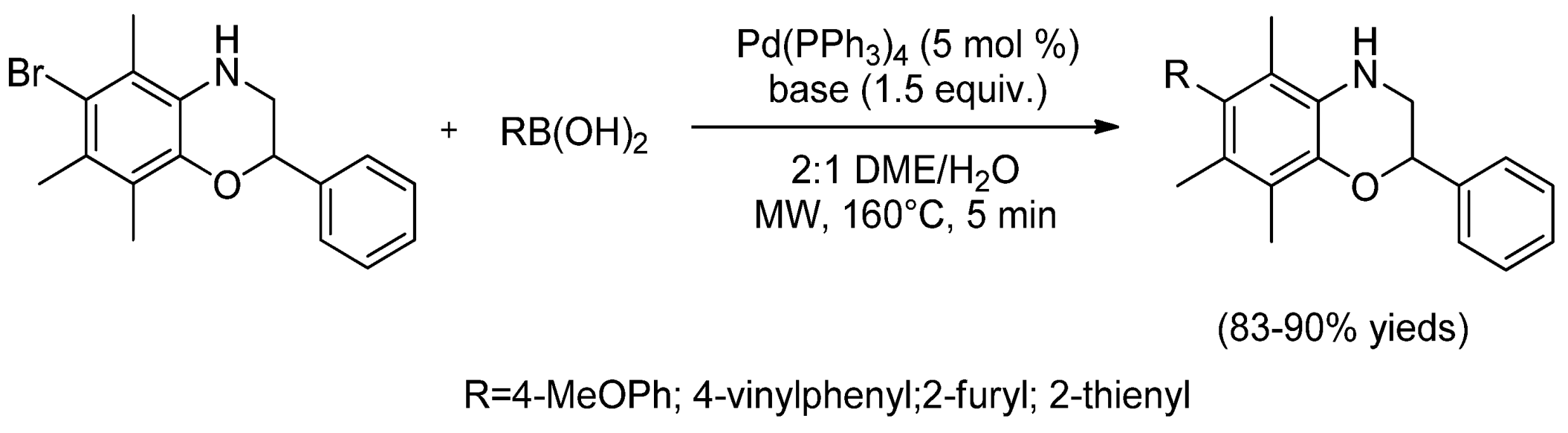
- Koini, E.N.; Avlonitis, N.; Martins-Duarte, E.S.; de Souza, W.; Vommaro, R.C.; Calogeropoulou, T. Divergent synthesis of 2,6-diaryl-substituted 5,7,8-trimethyl-1,4-benzoxazines via microwave-promoted palladium-catalyzed Suzuki–Miyaura cross coupling and biological evaluation. Tetrahedron 2012, 68, 10302–10309. [Google Scholar] [CrossRef]
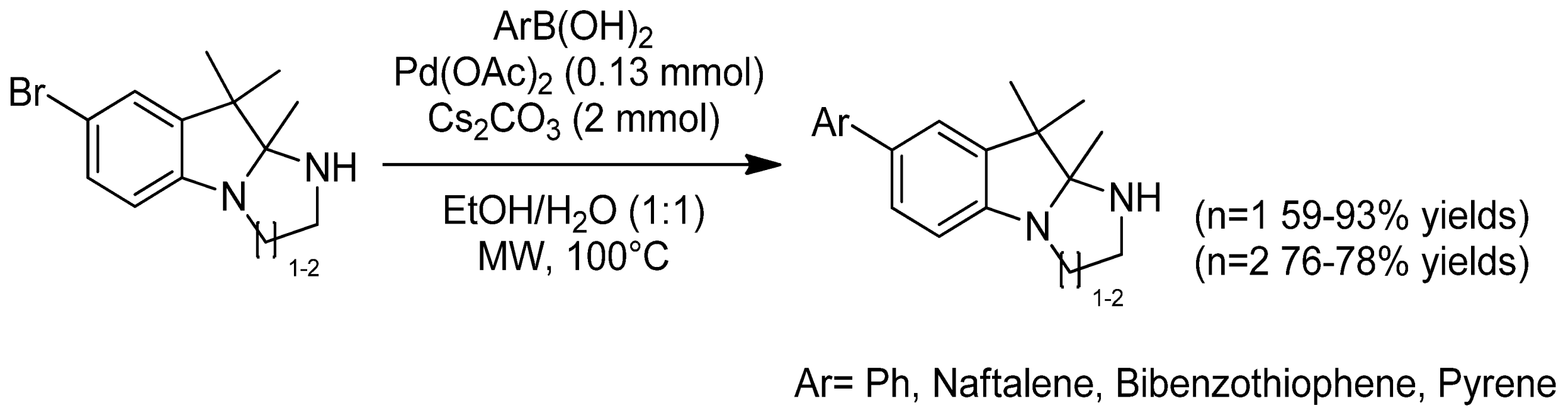
- Zukauskaite, Z.; Buinauskaite, V.; Solovjova, J.; Malinauskaite, L.; Kveselyte, A.; Bieliauskas, A.; Ragaite, G.; Sackus, A. Microwave-assisted synthesis of new fluorescent indoline-based building blocks by ligand free Suzuki–Miyaura cross-coupling reaction in aqueous media. Tetrahedron 2016, 72, 2955–2963. [Google Scholar] [CrossRef]
- Prieur, V.; Pujol, M.D.; Guillaumet, G. A strategy for the triarylation of pyrrolopyrimidines by using microwave-promoted cross-coupling reactions. Eur. J. Org. Chem. 2015, 2015, 6547–6556. [Google Scholar] [CrossRef]
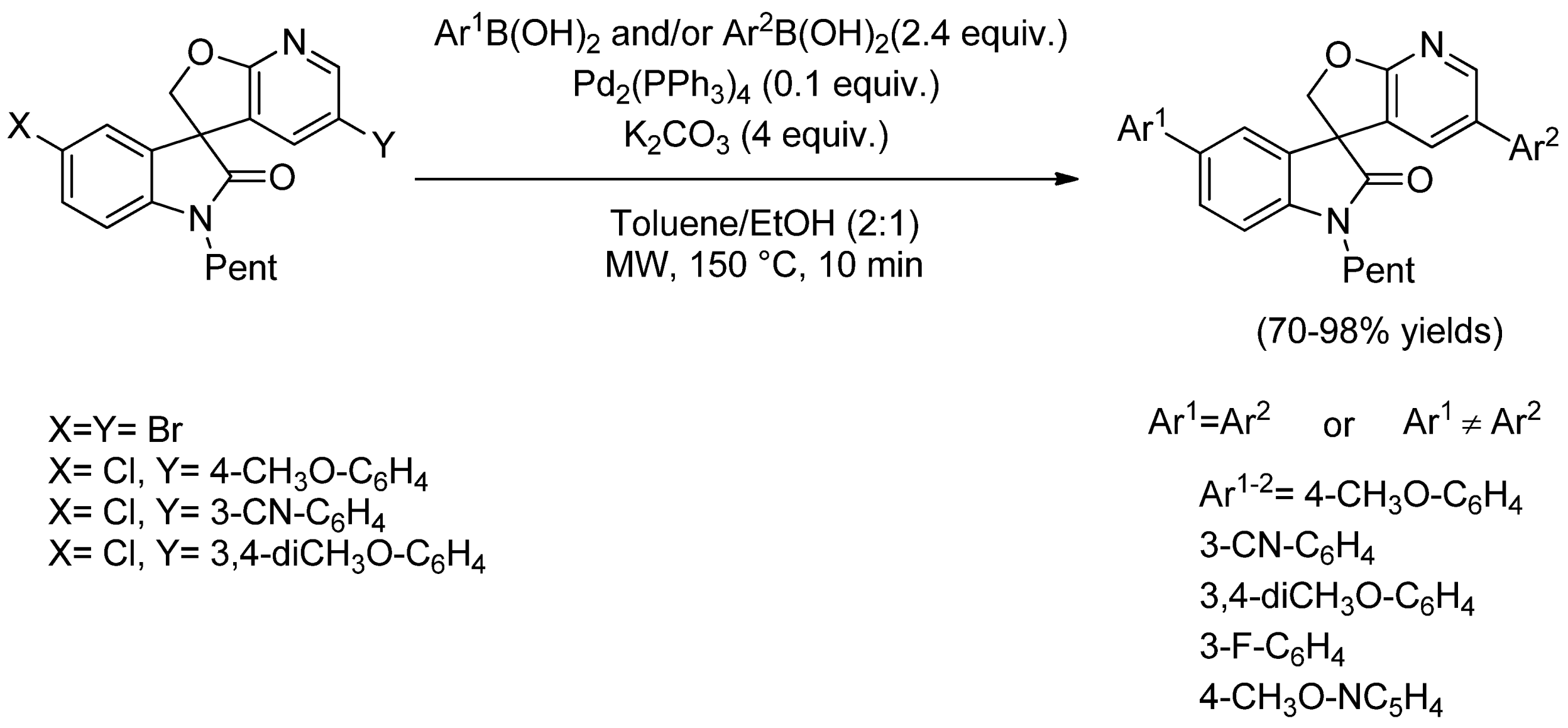
- El Bouakher, A.; Allouchi, H.; Abrunhosa-Thomas, I.; Troin, Y.; Guillaumet, G. Suzuki–Miyaura reactions of halospirooxindole derivatives. Eur. J. Org. Chem. 2015, 3450–3461. [Google Scholar] [CrossRef]
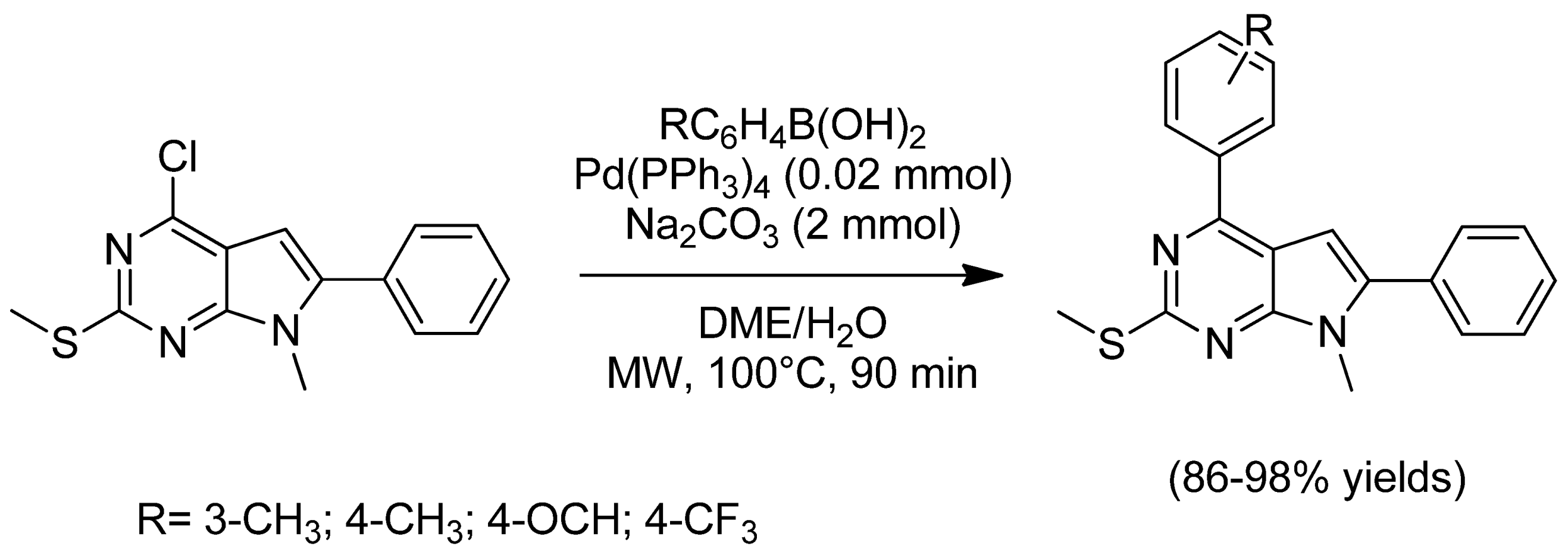
- Qu, G.-R.; Xin, P.-Y.; Niu, H.-Y.; Jin, X.; Guo, X.-T.; Yang, X.-N.; Guo, H.-M. Microwave promoted palladium-catalyzed Suzuki–Miyaura cross-coupling reactions of 6-chloropurines with sodium tetraarylborate in water. Tetrahedron 2011, 67, 9099–9103. [Google Scholar] [CrossRef]
- Kabri, Y.; Crozet, M.D.; Szabo, R.; Vanelle, P. Efficient and original microwave-assisted Suzuki–Miyaura cross-coupling reaction in the 4H-pyrido[1,2-a]pyrimidin-4-one series. Synthesis 2011, 19, 3115–3122. [Google Scholar] [CrossRef]
- Kabri, Y.; Crozet, M.D.; Primas, N.; Vanelle, P. One-pot chemoselective bis(Suzuki–Miyaura cross-coupling): Efficient access to 3,9-bis[(hetero)aryl]-4H-pyrido[1,2-a]pyrimidin-4-one derivatives under microwave irradiation. Eur. J. Org. Chem. 2012, 28, 5595–5604. [Google Scholar] [CrossRef]
- Kabri, Y.; Crozet, M.D.; Terme, T.; Vanelle, P. Efficient access to 2,6,8-trisubstituted 4-aminoquinazolines through microwave-assisted one-pot chemoselective tris-Suzuki–Miyaura or SNAr/bis-Suzuki–Miyaura reactions in water. Eur. J. Org. Chem. 2015. [Google Scholar] [CrossRef]
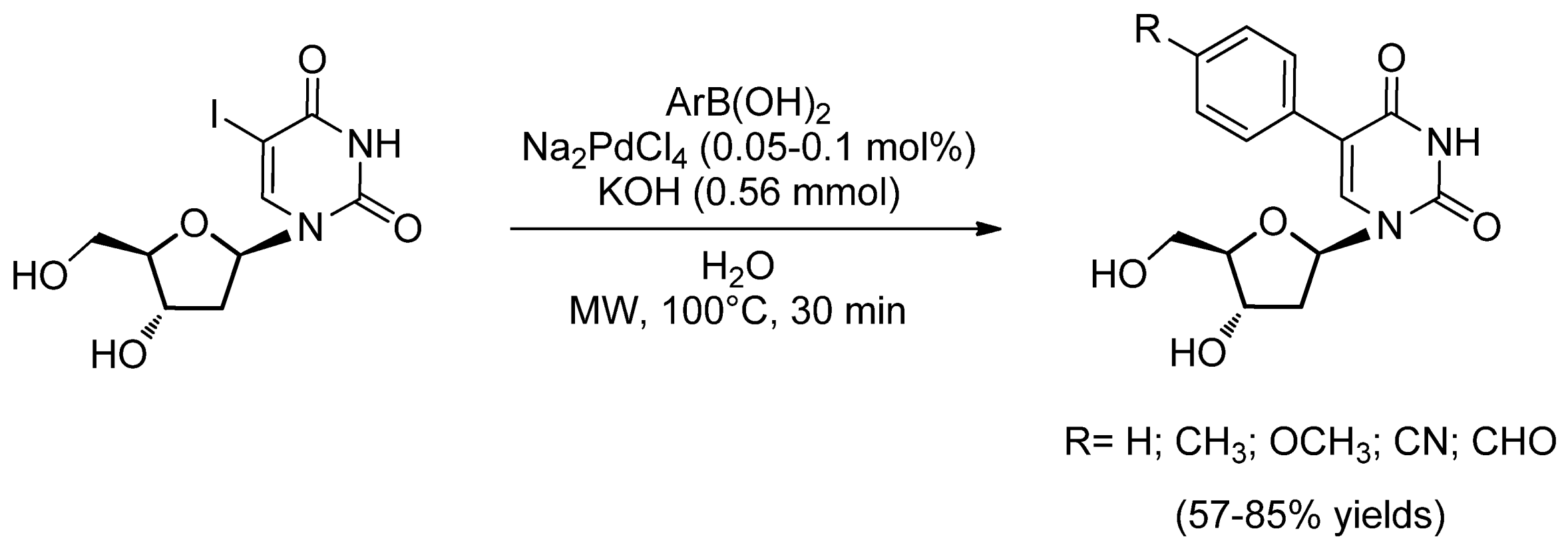
- Gallagher-Duval, S.; Hervé, G.; Sartori, G.; Enderlin, G.; Len, C. Improved microwave-assisted ligand-free Suzuki–Miyaura cross-coupling of 5-iodo-2′-deoxyuridine in pure water. New J. Chem. 2013, 37, 1989–1995. [Google Scholar] [CrossRef]
- Darses, S.; Genet, J.-P. Potassium organotrifluoroborates: New perspectives in organic synthesis. Chem. Rev. 2008, 108, 288–325. [Google Scholar] [CrossRef] [PubMed]
- Gary, A. Molander organotrifluoroborates: Another branch of the mighty oak. J. Org. Chem. 2015, 80, 7837–7848. [Google Scholar]
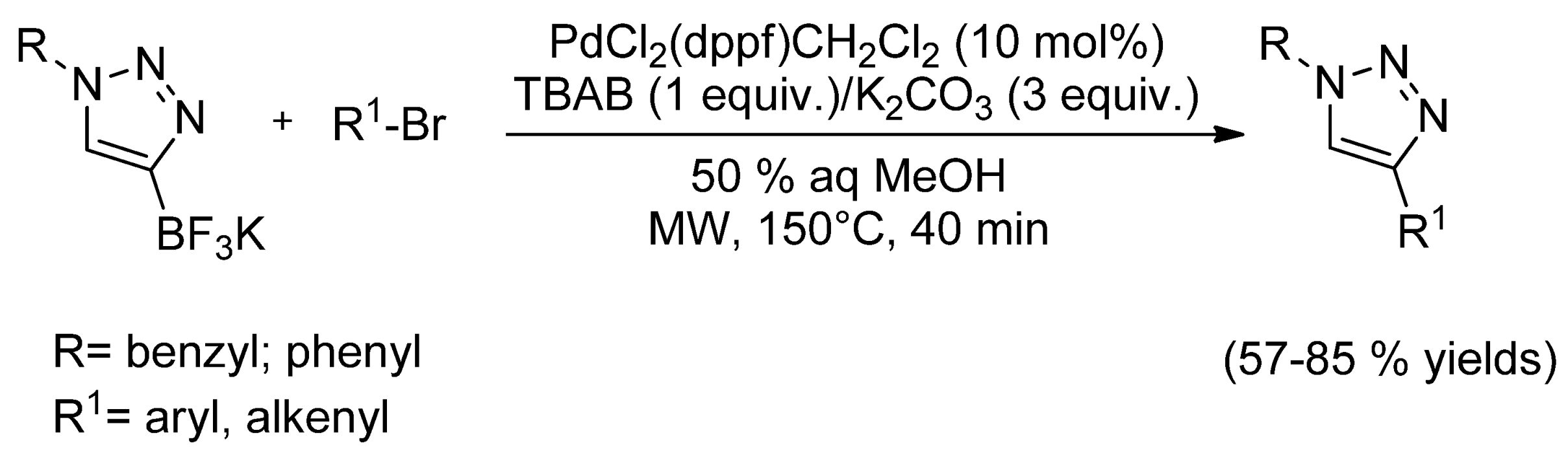
- Kim, T.; Song, J.H.; Jeong, K.H.; Lee, S.; Ham, J. Potassium (1-organo-1H-1,2,3-triazol-4-yl)trifluoroborates from ethynyltrifluoroborate through a regioselective one-pot Cu-catalyzed azide–alkyne cycloaddition reaction. Eur. J. Org. Chem. 2013. [Google Scholar] [CrossRef]
- Jain, P.; Yi, S.; Flaherty, P.T. Suzuki–Miyaura cross-coupling of potassium organoborates with 6-sulfonate benzimidazoles using microwave irradiation. J. Heterocycl. Chem. 2013, 50, E166–E173. [Google Scholar] [CrossRef]
- Henderson, L.; Knight, D.W.; Rutkowski, P.; Williams, A.C. Optimised conditions for styrene syntheses using Suzuki–Miyaura couplings and catalyst-ligand-base pre-mixes. Tetrahedron Lett. 2012, 53, 4654–4656. [Google Scholar] [CrossRef]
- Brooker, M.D.; Cooper, S.M., Jr.; Hodges, D.R.; Carter, R.R.; Wyatt, J.K. Studies of microwave-enhanced Suzuki–Miyaura vinylation of electron-rich sterically hindered substrates utilizing potassium vinyltrifluoroborate. Tetrahedron Lett. 2010, 51, 6748–6752. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Y.; Yang, J.-F.; Song, K.; Chen, Q.; Zhou, S.-L.; Hao, G.-F.; Yang, G.-F. One-pot approach to N-quinolyl 3′/4′-biaryl carboxamides by microwave-assisted Suzuki–Miyaura coupling and N-boc deprotection. J. Org. Chem. 2016, 81, 9647–9657. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Y.; Yang, J.-F.; Chen, Q.; Cao, R.-J.; Huang, W.; Hao, G.-F.; Yang, G.-F. An efficient one-pot access to N-(pyridin-2-ylmethyl) substituent biphenyl-4-sulfonamides through water-promoted, palladium-catalyzed, microwave-assisted reactions. RSC Adv. 2015, 5, 75182–75186. [Google Scholar] [CrossRef]
- Grob, J.E.; Nunez, J.; Dechantsreiter, M.A.; Hamann, L.G. One-pot reductive amination and Suzuki–Miyaura cross-coupling of formyl aryl and heteroaryl mida boronates in array format. J. Org. Chem. 2011, 76, 4930–4940. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-Y.; Kim, B.T.; Heo, J.-N. Direct one-pot synthesis of naphthoxindoles from 4-bromooxindoles by Suzuki–Miyaura coupling and aldol condensation reactions. Eur. J. Org. Chem. 2014, 1, 164–170. [Google Scholar] [CrossRef]
- Hooper, A.; Zambon, A.; Springer, C.J. A novel protocol for the one-pot borylation/suzuki reaction provides easy access to hinge-binding groups for kinase inhibitors. Org. Biomol. Chem. 2016, 14, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Cívicos, J.F.; Alonso, D.A.; Nájera, C. Oxime palladacycle-catalyzed Suzuki–Miyaura alkenylation of aryl, heteroaryl, benzyl, and allyl chlorides under microwave irradiation conditions. Adv. Synth. Catal. 2011, 353, 1683–1687. [Google Scholar] [CrossRef]
- Cívicos, J.F.; Alonso, D.A.; Najera, C. Oxime-palladacycle-catalyzed Suzuki–Miyaura arylation and alkenylation of aryl imidazolesulfonates under aqueous and phosphane-free conditions. Eur. J. Org. Chem. 2012, 2012, 3670–3676. [Google Scholar] [CrossRef]
- Susanto, W.; Chu, C.-Y.; Ang, W.J.; Chou, T.-C.; Lo, L.-C.; Lam, Y. Development of a fluorous, oxime-based palladacycle for microwave-promoted carbon–carbon coupling reactions in aqueous media. Green Chem. 2012, 14, 77–80. [Google Scholar] [CrossRef]
- Dawood, K.M.; Elamin, M.B.; Farag, A.M. Microwave-assisted synthesis of 2-acetyl-5-arylthiophenes and 4-(5-arylthiophen-2-yl)thiazoles via Suzuki coupling in water. ARKIVOC 2015, 7, 50–62. [Google Scholar]
- Hanhan, M.E.; Martínez-Máñez, R.; Ros-Lis, J.V. Highly effective activation of aryl chlorides for Suzuki coupling in aqueous media using a ferrocene-based Pd(II)–diimine catalyst. Tetrahedron Lett. 2012, 53, 2388–2391. [Google Scholar] [CrossRef]
- Balam-Villarreal, J.A.; Sandoval-Chavez, C.I.; Ortega-Jimenez, F.; Toscano, R.A.; Carreon-Castro, M.P.; Lopez-Cortes, J.G.; Ortega-Alfaro, M.C. Infrared irradiation or microwave assisted cross-coupling reactions using sulfur-containing ferrocenyl-palladacycles. J. Organomet. Chem. 2016, 818, 7–14. [Google Scholar] [CrossRef]
- Yılmaz, Ü.; Şireci, N.; Deniz, S.; Küçükbay, H. Synthesis and microwave-assisted catalytic activity of novel bis-benzimidazole salts bearing furfuryl and thenylmoieties in Heck and Suzuki cross-coupling reactions. Appl. Organomet. Chem. 2010, 24, 414–420. [Google Scholar]
- Yılmaz, Ü.; Küçükbay, H.; Şireci, N.; Akkurt, M.; Günald, S.; Durmaz, R.; Tahir, M.N. Synthesis, microwave-promoted catalytic activity in Suzuki–Miyaura cross-coupling reactions and antimicrobial properties of novel benzimidazole salts bearing trimethylsilyl group. Appl. Organomet. Chem. 2011, 25, 366–373. [Google Scholar] [CrossRef]
- Silarska, E.; Trzeciak, A.M.; Pernak, J.; Skrzypczak, A. [IL]2[PdCl4] complexes (IL = imidazolium cation) as efficient catalysts for Suzuki–Miyaura cross-coupling of aryl bromides and aryl chlorides. Appl. Catal. A 2013, 466, 216–223. [Google Scholar] [CrossRef]
- Hanhan, M.E.; Senemoglu, Y. Microwave-assisted aqueous Suzuki coupling reactions catalyzed by ionic palladium(II) complexes. Transit. Met. Chem. 2012, 37, 109–116. [Google Scholar] [CrossRef]
- Shen, L.; Huang, S.; Nie, Y.; Lei, F. An efficient microwave-assisted Suzuki reaction using a new pyridine-pyrazole/Pd(II) species as catalyst in aqueous media. Molecules 2013, 18, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Kumaravel, M.; Mague, J.T.; Balakrishna, M.S. Bisamino(diphosphonite) with dangling olefin functionalities: Synthesis, metal chemistry and catalytic utility of RhI and PdII complexes in hydroformylation and Suzuki–Miyaura reactions. Dalton Trans. 2014, 43, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Basauri-Molina, M.; Hernández-Ortega, S.; Morales-Morales, D. Microwave-assisted C–C and C–S couplings catalysed by organometallic Pd-SCS or coordination Ni-SNS pincer complexes. Eur. J. Inorg. Chem. 2014, 27, 4619–4625. [Google Scholar] [CrossRef]
- Gayakhe, V.; Ardhapure, A.; Kapdi, A.R.; Sanghvi, Y.S.; Serrano, J.L.; Garcia, L.; Perez, J.; Garcia, J.; Sanchez, G.; Fischer, C.; et al. Water-soluble Pd-imidate complexes: Broadly applicable catalysts for the synthesis of chemically modified nucleosides via Pd-catalyzed cross-coupling. J. Org. Chem. 2016, 81, 2713–2729. [Google Scholar] [CrossRef] [PubMed]
- Glorius, F. Asymmetric cross-coupling of non-activated secondary alkyl halides. Angew. Chem. Int. Ed. 2008, 47, 8347–8349. [Google Scholar] [CrossRef] [PubMed]
- Glasson, C.R.K.; Meehan, G.V.; Motti, C.A.; Clegg, J.K.; Turner, P.; Jensen, P.; Lindoy, L.F. New nickel(II) and iron(II) helicates and tetrahedra derived from expanded quaterpyridines. Dalton Trans. 2011, 40, 10481–10490. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.M.; Loechtefeld, R.; Menssen, R.; Bierer, D.E.; Riedl, B.; Baker, R.T. Synthesis of bromodifluoromethyl(arylsulfonyl) compounds and microwave-assisted nickel catalyzed cross coupling with arylboronic acids. Tetrahedron Lett. 2016, 57, 5464–5468. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Pilger, C.; Kappe, C.O. Rapid nickel-catalyzed Suzuki–Miyaura cross-couplings of aryl carbamates and sulfamates utilizing microwave heating. J. Org. Chem. 2011, 76, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- De Luna Martins, D.; Aguiar, L.C.S.; Antunes, O.A.C. Microwave promoted Suzuki reactions between aroyl chlorides and boronic acids catalyzed by heterogeneous and homogeneous phosphine-free palladium catalysts. J. Organomet. Chem. 2011, 696, 2845–2849. [Google Scholar] [CrossRef]
- Feng, G.; Liu, F.; Lin, C.; Li, W.; Wang, S.; Qi, C. Crystalline mesoporous γ-Al2O3 supported palladium: Novel and efficient catalyst for Suzuki–Miyaura reaction under controlled microwave heating. Catal. Commun. 2013, 37, 27–31. [Google Scholar] [CrossRef]
- Smith, S.E.; Siamaki, A.R.; Gupton, B.F.; Carpenter, E.E. CuPd nanoparticles as a catalyst in carbon–carbon cross-coupling reactions by a facile oleylamine synthesis. RSC Adv. 2016, 6, 91541–91545. [Google Scholar] [CrossRef]
- Molnár, Á. Efficient, selective, and recyclable palladium catalysts in carbon–carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, M.; Lee, W.Y.; Hyeon, T. Fabrication of hollow palladium spheres and their successful application to the recyclable heterogeneous catalyst for Suzuki coupling reactions. J. Am. Chem. Soc. 2002, 124, 7642–7643. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, G.M.; Rumi, L.; Steurer, P.; Bannwarth, W.; Mulhaupt, R. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 2009, 131, 8262–8270. [Google Scholar] [CrossRef] [PubMed]
- Kotadia, D.A.; Patel, U.H.; Gandhi, S.; Soni, S.S. Pd doped SiO2 nanoparticles: An efficient recyclable catalyst for Suzuki, Heck and Sonogashira reactions. RSC Adv. 2014, 4, 32826–32833. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pandarus, V.; Gingras, G.; Beland, F.; Demma Cara, P.; Pagliaro, M. Heterogeneously catalyzed Suzuki–Miyaura conversion of broad scope. RSC Adv. 2012, 2, 10798–10804. [Google Scholar] [CrossRef]
- Verho, O.; Nagendiran, A.; Johnston, E.V.; Tai, C.; Baeckvall, J.-E. Nanopalladium on amino-functionalized mesocellular foam. An efficient catalyst for Suzuki reactions and transfer hydrogenations. ChemCatChem 2013, 5, 612–618. [Google Scholar] [CrossRef]
- Parvulescu, A.N.; Van der Eycken, E.; Jacobs, P.A.; De Vos, D.E. Microwave-promoted racemization and dynamic kinetic resolution of chiral amines over Pd on alkaline earth supports and lipases. J. Catal. 2008, 255, 206–212. [Google Scholar] [CrossRef]
- Chang, W.; Chae, G.H.; Jang, S.R.; Shin, J.; Ahn, B.J. An efficient microwave-assisted Suzuki reaction using Pd/MCM-41 and Pd/SBA-15 as catalysts in solvent-free condition. J. Ind. Eng. Chem. 2012, 18, 581–585. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, H.; Liu, T.; Cao, R. Monodisperse noble metal nanoparticles stabilized in SBA-15: Synthesis, characterization and application in microwave-assisted Suzuki–Miyaura coupling reaction. J. Catal. 2010, 270, 268–274. [Google Scholar] [CrossRef]
- Luo, M.; Dai, C.; Han, Q.; Fan, G.; Song, G. Preparation and characterization of palladium immobilized on silica-coated Fe3O4 and its catalytic performance for Suzuki reaction under microwave irradiation. Surf. Interface Anal. 2016, 48, 1066–1071. [Google Scholar] [CrossRef]
- Nehlig, E.; Waggeh, B.; Millot, N.; Lalatonne, Y.; Motte, L.; Guenin, E. Immobilized Pd on magnetic nanoparticles bearing proline as a highly efficient and retrievable Suzuki–Miyaura catalyst in aqueous media. Dalton Trans. 2015, 44, 501–505. [Google Scholar] [CrossRef] [PubMed]
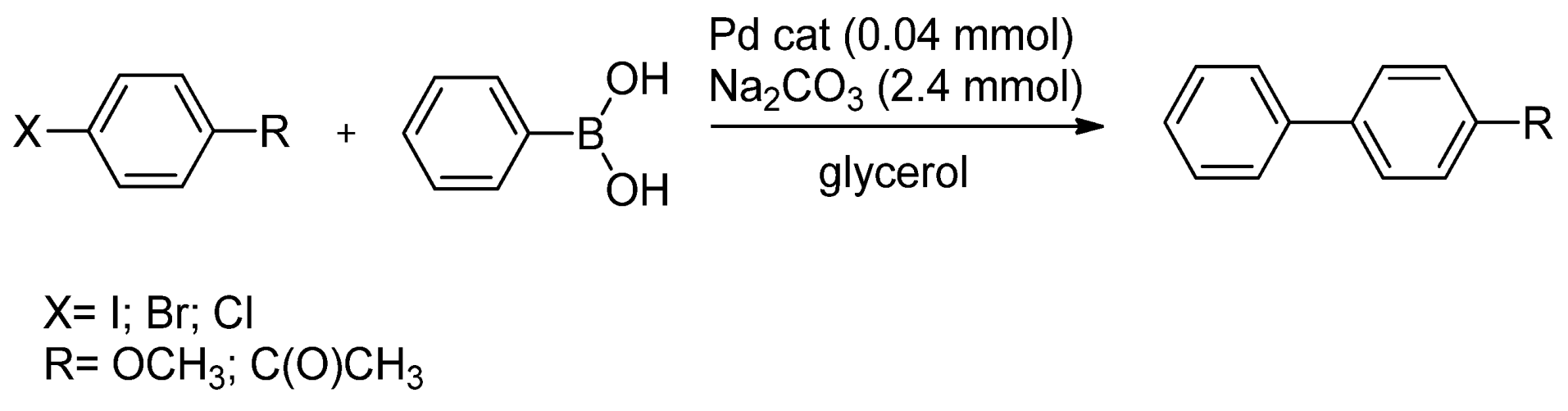
- Cravotto, G.; Orio, L.; Calcio Gaudino, E.; Martina, K.; Tavor, D.; Wolfson, A. Efficient synthetic protocols in glycerol under heterogeneous catalysis. ChemSusChem 2011, 4, 1130–1134. [Google Scholar] [CrossRef]
- Martina, K.; Leonhardt, S.E.; Ondruschka, B.; Curini, M.; Binello, A.; Cravotto, G. In situ cross-linked chitosan Cu(I) or Pd(II) complexes as a versatile, eco-friendly recyclable solid catalyst. J. Mol. Catal. A 2011, 334, 60–64. [Google Scholar] [CrossRef]
- Leonhardt, S.E.S.; Stolle, A.; Ondruschka, B.; Cravotto, G.; De Leo, C.; Jandt, K.D.; Keller, T.F. Chitosan as a support for heterogeneous Pd catalysts in liquid phase catalysis. Appl. Catal. A 2010, 379, 30–37. [Google Scholar] [CrossRef]
- Baran, T.; Sargin, I.; Kaya, M.; Mentes, A. Green heterogeneous Pd(II) catalyst produced from chitosan-cellulose micro beads for green synthesis of biaryls. Carbohydr. Polym. 2016, 152, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Calcio Gaudino, E.; Tagliapietra, S.; Carnaroglio, D.; Procopio, A. A green approach to heterogeneous catalysis using ligand-free, metal-loaded cross-linked cyclodextrins. Green Proc. Synth. 2012, 1, 269–273. [Google Scholar] [CrossRef]
- Martina, K.; Baricco, F.; Caporaso, M.; Berlier, G.; Cravotto, G. Cyclodextrin-grafted silica-supported Pd nanoparticles: An efficient and versatile catalyst for ligand-free C–C coupling and hydrogenation. ChemCatChem 2016, 8, 1176–1184. [Google Scholar] [CrossRef]
- Isfahani, A.L.; Mohammadpoor-Baltork, I.; Mirkhani, V.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Kia, R. Palladium nanoparticles immobilized on nano-silica triazine dendritic polymer (Pdnp-nSTDP): An efficient and reusable catalyst for Suzuki–Miyaura cross-coupling and Heck reactions. Adv. Synth. Catal. 2013, 355, 957–972. [Google Scholar] [CrossRef]
- Borkowski, T.; Zawartka, W.; Pospiech, P.; Mizerska, U.; Trzeciak, A.M.; Cypryk, M.; Tylus, W. Reusable functionalized polysiloxane-supported palladium catalyst for Suzuki–Miyaura cross-coupling. J. Catal. 2011, 282, 270–277. [Google Scholar] [CrossRef]
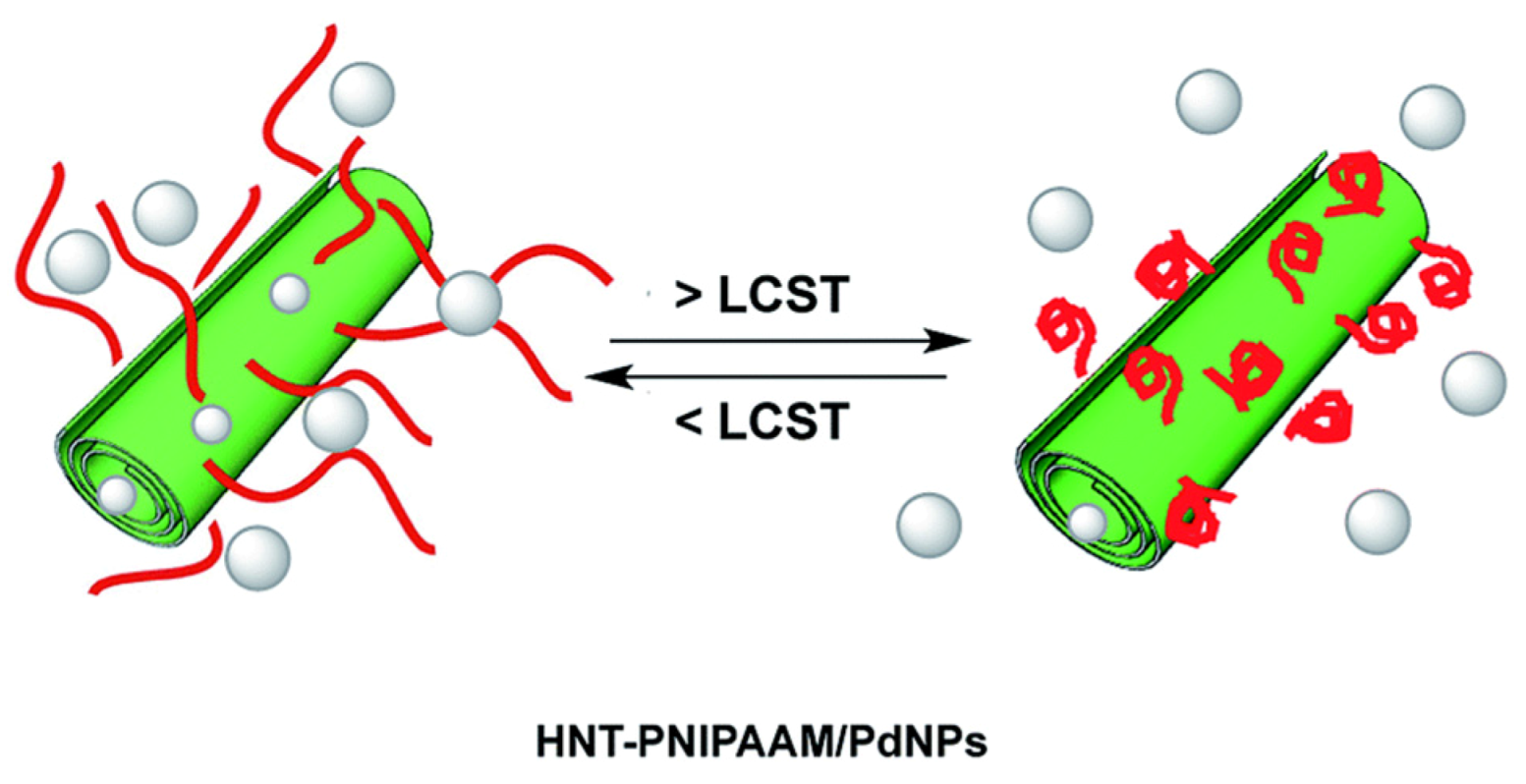
- Massaro, M.; Schembri, V.; Campisciano, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Noto, R.; Parisi, F.; Riela, S. Design of PNIPAAM covalently grafted on halloysite nanotubes as a support for metal-based catalysts. RSC Adv. 2016, 6, 55312–55318. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Cavallaro, G.; Colletti, C.G.; Milioto, S.; Noto, R.; Parisi, F.; Lazzara, G. Palladium supported on Halloysite-triazolium salts as catalyst for ligand free Suzuki cross-coupling in water under microwave irradiation. J. Mol. Catal. A 2015, 408, 12–19. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Lazzara, G.; Gruttadauria, M.; Milioto, S.; Noto, R. Green conditions for the Suzuki reaction using microwave irradiation and a new HNT-supported ionic liquid-like phase (HNT-SILLP) catalyst. Appl. Organomet. Chem. 2014, 28, 234–238. [Google Scholar] [CrossRef]
- Baruah, D.; Das, R.N.; Hazarika, S.; Konwar, D. Biogenic synthesis of cellulose supported Pd(0) nanoparticles using hearth wood extract of Artocarpus lakoocha Roxb—A green, efficient and versatile catalyst for Suzuki and Heck coupling in water under microwave heating. Catal. Commun. 2015, 72, 73–80. [Google Scholar] [CrossRef]
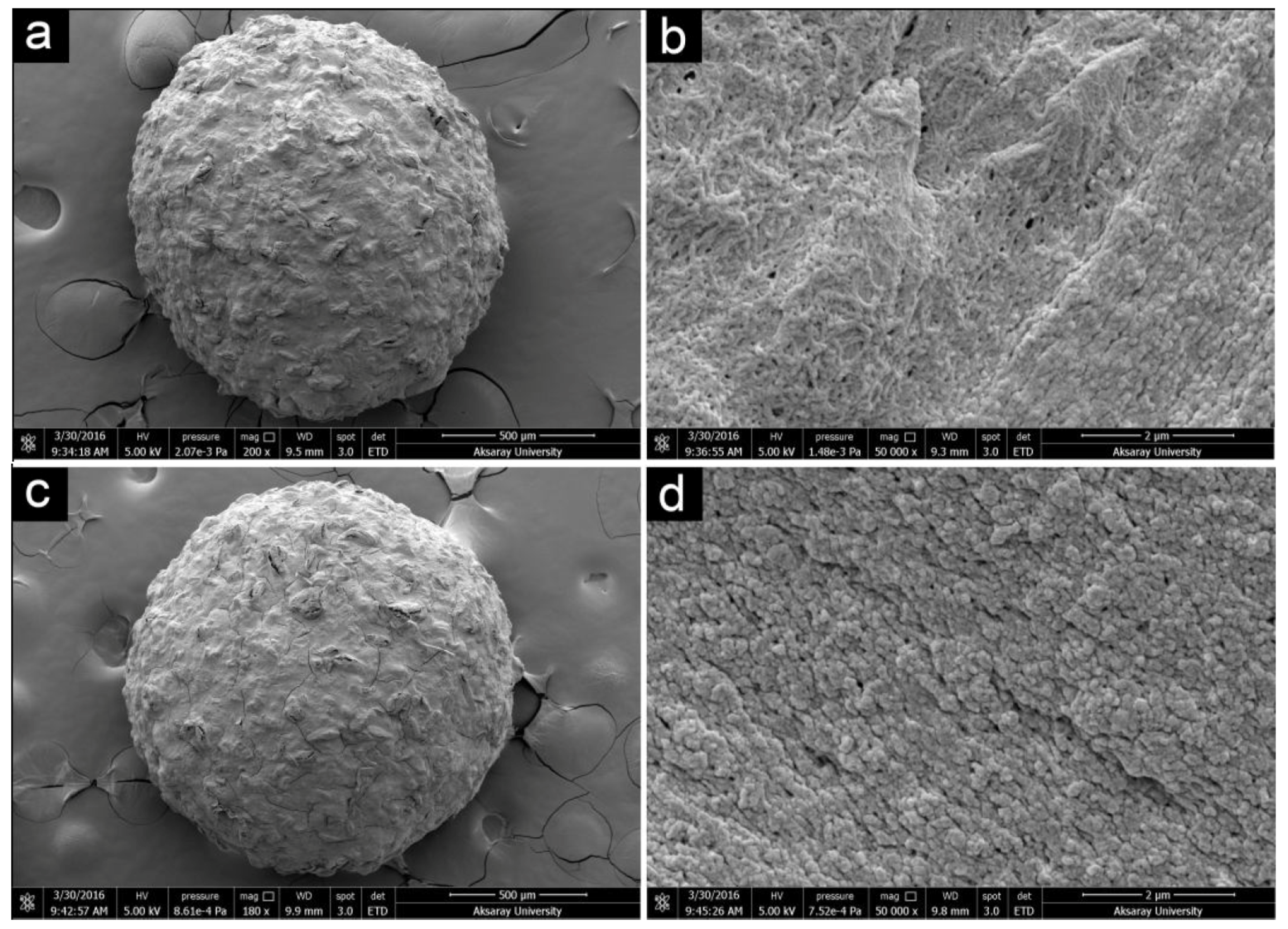
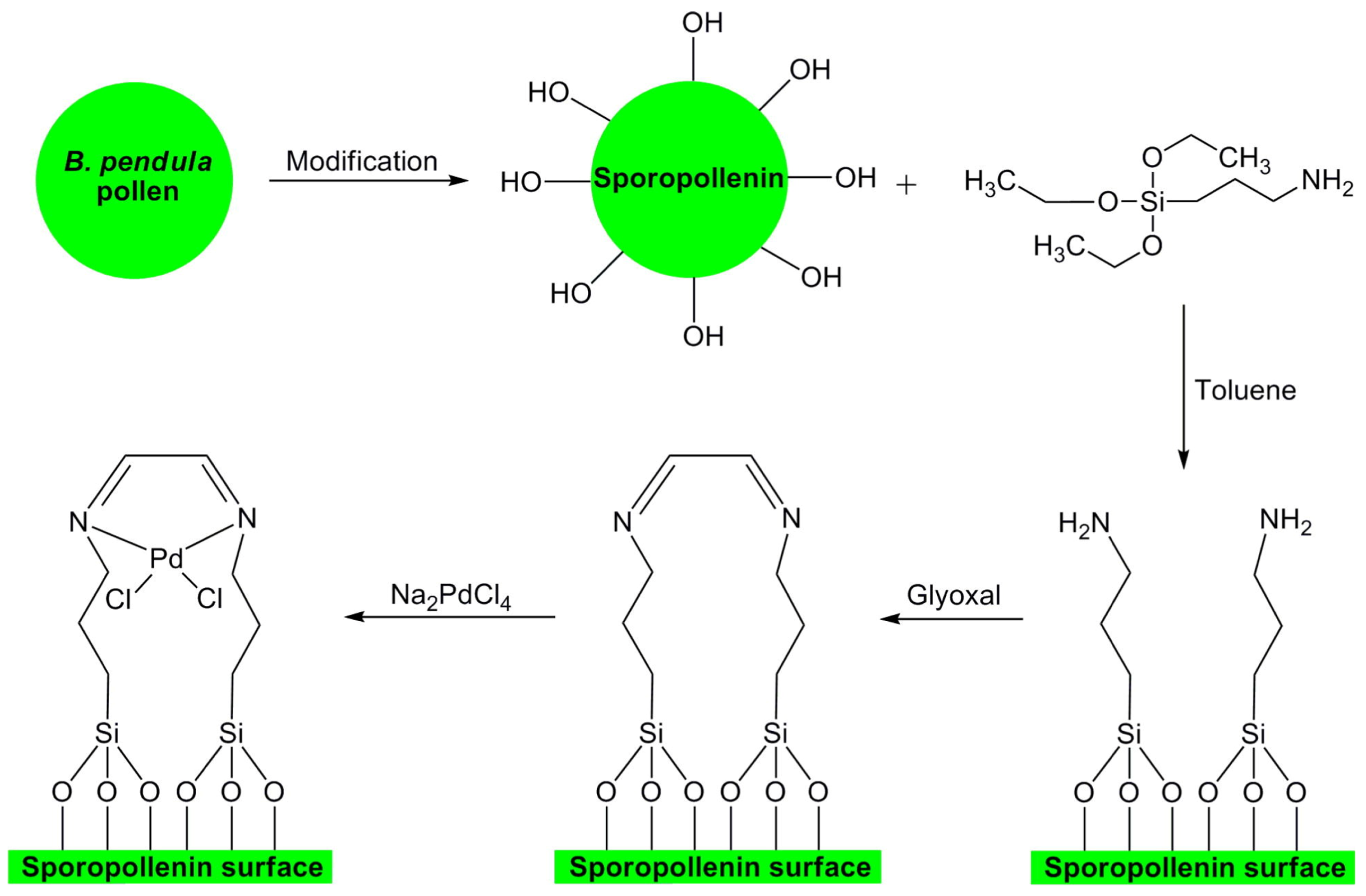
- Baran, T.; Sargin, I.; Kaya, M.; Mentes, A.; Ceter, T. Design and application of sporopollenin microcapsule supported palladium catalyst: Remarkably high turnover frequency and reusability in catalysis of biaryls. J. Coll. Interface Sci. 2017, 486, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Baran, T.; Sargin, I.; Mentes, A.; Kaya, M. Exceptionally high turnover frequencies recorded for a new chitosan-based palladium(II) catalyst. Appl. Catal. A 2016, 523, 12–20. [Google Scholar] [CrossRef]
- Baran, T.; Sargin, I.; Kaya, M.; Mentes, A. An environmental catalyst derived from biological waste materials for green synthesis of biaryls via Suzuki coupling reactions. J. Mol. Catal. A 2016, 420, 216–221. [Google Scholar] [CrossRef]
- Shah, D.; Kaur, H. Supported palladium nanoparticles: A general sustainable catalyst for microwave enhanced carbon-carbon coupling reactions. J. Mol. Catal. A 2016, 424, 171–180. [Google Scholar] [CrossRef]
- Kaur, H.; Shah, D.; Pal, U. Resin encapsulated palladium nanoparticles: An efficient and robust catalyst for microwave enhanced Suzuki–Miyaura coupling. Catal. Commun. 2011, 12, 1384–1388. [Google Scholar] [CrossRef]
- Djakovitch, L.; Dufaud, V.; Zaidi, R. Heterogeneous palladium catalysts applied to the synthesis of 2- and 2,3-functionalised indoles. Adv. Synth. Catal. 2006, 348, 715–724. [Google Scholar] [CrossRef]
- Djakovitch, L.; Koehler, K. Heck reaction catalyzed by Pd-modified zeolites. J. Am. Chem. Soc. 2001, 123, 5990–5999. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, F.; Campisciano, V.; Calabrese, C.; La Parola, V.; Liotta, L.F.; Aprile, C.; Gruttadauria, M. Supported C60-IL-PdNPs as extremely active nanocatalysts for C–C cross-coupling reactions. J. Mat. Chem. A 2016, 4, 17193–17206. [Google Scholar] [CrossRef]
- Guerra, R.R.G.; Martins, F.C.P.; Lima, C.G.S.; Gonçalves, R.H.; Leite, E.R.; Pereira-Filho, E.R.; Schwab, R.S. Factorial design evaluation of the Suzuki cross-coupling reaction using a magnetically recoverable palladium catalyst. Tetrahedron Lett. 2017, 58, 903–908. [Google Scholar] [CrossRef]
- Moussa, S.; Siamaki, A.R.; Gupton, B.F.; El-Shall, M.S. Pd-partially reduced graphene oxide catalysts (Pd/PRGO): Laser synthesis of Pd nanoparticles supported on PRGO nanosheets for carbon-carbon cross coupling reactions. ACS Catal. 2012, 2, 145–154. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Higashi, A.; Yamauchi, T.; Nakamura, T.; Yasuda, M.; Baba, A.; Wada, Y. In situ observation of nonequilibrium local heating as an origin of special effect of microwave on chemistry. J. Phys. Chem. C 2010, 114, 8965–8970. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Abe, M.; Serpone, N. On the generation of hot-spots by microwave electric and magnetic fields and their impact on a microwave-assisted reaction in the presence of metallic Pd nanoparticles on an activated carbon support. J. Phys. Chem. C 2011, 115, 23030–23035. [Google Scholar] [CrossRef]
- Gomez-Martinez, M.; Buxaderas, E.; Pastor, I.M.; Alonso, D.A. Palladium nanoparticles supported on graphene and reduced graphene oxide as efficient recyclable catalyst for the Suzuki–Miyaura reaction of potassium aryltrifluoroborates. J. Mol. Catal. A 2015. [Google Scholar] [CrossRef]
- Garcia-Suarez, E.J.; Lara, P.; Garcia, A.B.; Ojeda, M.; Luque, R.; Philippot, K. Efficient and recyclable carbon-supported Pd nanocatalysts for the Suzuki–Miyaura reaction in aqueous-based media: Microwave vs. conventional heating. Appl. Catal. A 2013, 468, 59–67. [Google Scholar] [CrossRef]
- Sanhes, D.; Raluy, E.; Rétory, S.; Saffon, N.; Teuma, E.; Gomez, M. Unexpected activation of carbon–bromide bond promoted by palladium nanoparticles in Suzuki C–C couplings. Dalton Trans. 2010, 39, 9719–9726. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Riemer, M.; Karras, M. 2,2′-biphenols via protecting group-free thermal or microwave-accelerated Suzuki–Miyaura coupling in water. J. Org. Chem. 2013, 78, 8680–8688. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Akimoto, M.; Tameno, T.; Ohki, Y.; Takahashi, N.; Hoshiya, N.; Shuto, S.; Arisawa, M. Suzuki–Miyaura cross-coupling reactions using a low-leaching and highly recyclable gold-supported palladium material and two types of microwave equipments. Green Chem. 2013, 15, 1142–1145. [Google Scholar] [CrossRef]
- Heinrich, F.; Kessler, M.T.; Dohmen, S.; Singh, M.; Prechtl, M.H.G.; Mathur, S. Molecular palladium precursors for Pd0 nanoparticle preparation by microwave irradiation: Synthesis, structural characterization and catalytic activity. Eur. J. Inorg. Chem. 2012, 2012, 6027–6033. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Abd El Rahman, S.K.; Abdelsayed, V.; El-Shall, M.S.; Gupton, B.F. Microwave-assisted synthesis of palladium nanoparticles supported on graphene: A highly active and recyclable catalyst for carbon-carbon cross-coupling reactions. J. Catal. 2011. [Google Scholar] [CrossRef]
- Elazab, H.A.; Siamaki, A.R.; Moussa, S.; Gupton, B.F.; El-Shall, M.S. Highly efficient and magnetically recyclable graphene-supported Pd/Fe3O4 nanoparticle catalysts for Suzuki and Heck cross-coupling reactions. Appl. Catal. A 2015, 491, 58–69. [Google Scholar] [CrossRef]
- Ni, Z.; Masel, R.I. Rapid production of metal-organic frameworks via microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Gao, W.; Zhang, L.; Liu, W.; Lu, J.; Wang, Z.; Deng, Y.J. Microwave-assisted synthesis of UIO-66 and its adsorption performance towards dyes. Cryst. Eng. Commun. 2014, 16, 7037–7042. [Google Scholar] [CrossRef]
- Dong, W.; Feng, C.; Zhang, L.; Shang, N.; Gao, S.; Wang, C.; Wang, Z. Pd@UiO-66: An efficient catalyst for Suzuki–Miyaura coupling reaction at mild condition. Catal. Lett. 2016, 146, 117–125. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Li, H. Water-medium organic reactions catalyzed by active and reusable Pd/Y heterobimetal-organic framework. ACS Catal. 2013, 3, 1526–1536. [Google Scholar] [CrossRef]
- Prasad, K.S.; Noh, H.-B.; Reddy, S.S.; Reddy, A.E.; Shim, Y.-B. Catalytic properties of Au and Pd nanoparticles decorated on Cu2O microcubes for aerobic benzyl alcohol oxidation and Suzuki–Miyaura coupling reactions in water. Appl. Catal. A 2014, 476, 72–77. [Google Scholar] [CrossRef]
- Veerakumar, P.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Hung, C.-T.; Tang, P.-H.; Wang, C.-B.; Liu, S.-B. Highly stable and active palladium nanoparticles supported on porous carbon for practical catalytic applications. J. Mater. Chem. A 2014, 2, 16015–16022. [Google Scholar] [CrossRef]
- Misch, L.M.; Birkel, A.; Figg, C.A.; Fors, B.P.; Hawker, C.J.; Stucky, G.D.; Seshadri, R. Rapid microwave-assisted sol-gel preparation of Pd-substituted LnFeO3 (Ln = Y, La): Phase formation and catalytic activity. Dalton Trans. 2014, 43, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Organotrifluoroborate hydrolysis: Boronic acid release mechanism and an acid–base paradox in cross-coupling. J. Am. Chem. Soc. 2012, 134, 7431–7441. [Google Scholar] [CrossRef] [PubMed]
- Shil, A.K.; Guha, N.R.; Sharma, D.; Das, P. A solid supported palladium(0) nano/microparticle catalyzed ultrasound induced continuous flow technique for large scale Suzuki reactions. RSC Adv. 2013, 3, 13671–13676. [Google Scholar] [CrossRef]
- Das, P.; Sharma, D.; Shil, A.K.; Kumari, A. Solid-supported Pd nano and microparticles: An efficient heterogeneous catalyst for ligand-free Suzuki–Miyaura cross coupling reaction. Tetrahedron Lett. 2011, 52, 1176–1178. [Google Scholar] [CrossRef]
- Azua, A.; Mata, J.A.; Heymes, P.; Peris, E.; Lamaty, F.; Martinez, J.; Colacino, E. Palladium N-heterocyclic carbene catalysts for the ultrasound-promoted Suzuki–Miyaura reaction in glycerol. Adv. Synth. Catal. 2013, 355, 1107–1116. [Google Scholar] [CrossRef]
- Chaudhary, A.R.; Bedekar, A.V. 1-(α-Aminobenzyl)-2-naphthol as phosphinefree ligand for Pd-catalyzed Suzuki and one-pot Wittig–Suzuki reaction. Appl. Organomet. Chem. 2012, 26, 430–437. [Google Scholar] [CrossRef]
- Chaudhary, A.R.; Bedekar, A.V. Application of 1-(α-aminobenzyl)-2-naphthols as air-stable ligands for Pd-catalyzed Mizoroki–Heck coupling reaction. Synth. Commun. 2012, 42, 1778–1785. [Google Scholar]
- Ghotbinejad, M.; Khosropour, A.R.; Mohammadpoor-Baltork, I.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Ultrasound-assisted C–C coupling reactions catalyzed by unique SPION-A-Pd(EDTA) as a robust nanocatalyst. RSC Adv. 2014, 4, 8590–8596. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Han, Z.; Zhu, J.; Lu, N.; Yang, Y.; Ma, D.; Chen, Y.; Huang, S. Pd embedded in porous carbon (Pd@CMK-3) as an active catalyst for Suzuki reactions: Accelerating mass transfer to enhance the reaction rate. Nano Res. 2014, 7, 1254–1262. [Google Scholar] [CrossRef]
- Suresh, N.; Prasanna, G.L.; Rao, M.V.B.; Pal, M. Ultrasound assisted synthesis of 6-flavonyl substituted 1,4-dihydro-benzo[d][1,3]oxazin-2-ones via Suzuki–Miyaura coupling under Pd/C catalysis. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Kulkarni, K.; Friend, J.; Yeo, L.; Perlmutter, P. An emerging reactor technology for chemical synthesis: Surface acoustic wave-assisted closed-vessel Suzuki coupling reactions. Ultrason. Sonochem. 2014, 21, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Senapati, K.K.; Roy, S.; Borgohain, C.; Phukan, P. Palladium nanoparticle supported on cobalt ferrite: An efficient magnetically separable catalyst for ligand free Suzuki coupling. J. Mol. Catal. A 2012, 352, 128–134. [Google Scholar] [CrossRef]
- Singh, A.S.; Patil, U.B.; Nagarkar, J.M. Palladium supported on zinc ferrite: A highly active, magnetically separable catalyst for ligand free Suzuki and Heck coupling. Catal. Commun. 2013, 35, 11–16. [Google Scholar] [CrossRef]
- Su, X.; Vinu, A.; Aldeyab, S.S.; Zhong, L. Highly uniform Pd nanoparticles supported on g-C3N4 for efficiently catalytic Suzuki–Miyaura reactions. Catal. Lett. 2015, 145, 1388–1395. [Google Scholar] [CrossRef]
- Li, J.; Bai, X. Ultrasonic synthesis of supported palladium nanoparticles for room-temperature Suzuki–Miyaura coupling. J. Mater. Sci. 2016, 51, 9108–9122. [Google Scholar] [CrossRef]
- Berry, D.E.; Carrie, P.; Fawkes, K.L.; Rebner, B.; Xing, Y. The mechanochemical reaction of palladium(II) chloride with a bidentate phosphine. J. Chem. Educ. 2010, 87, 533–534. [Google Scholar] [CrossRef]
- Ojeda, M.; Balu, A.M.; Barron, V.; Pineda, A.; Coleto, A.G.; Romero, A.A.; Luque, R. Solventless mechanochemical synthesis of magnetic functionalized catalytically active mesoporous SBA-15 nanocomposites. J. Mater. Chem. A 2014, 2, 387–393. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Lin, Y.; Woodberry, K.; Connell, J.W.; Gupton, B.F. Palladium nanoparticles supported on carbon nanotubes from solventless preparations: Versatile catalysts for ligand-free suzuki cross coupling reactions. J. Mater. Chem. A 2013, 1, 12909–12918. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Liu, M.; Xiao, Y.; Li, Z.; Chen, J.; Sun, Y.; Zhao, J.; Fang, S.; Jia, D.; et al. Homogeneous Pd nanoparticles produced in direct reactions: Green synthesis, formation mechanism and catalysis properties. J. Mater. Chem. A 2014, 2, 1369–1374. [Google Scholar] [CrossRef]
- Schneider, F.; Ondruschka, B. Mechanochemical solid-state Suzuki reactions using an in situ generated base. ChemSusChem 2008, 1, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Stolle, A.; Ondruschka, B.; Hopf, H. The Suzuki–Miyaura reaction under mechanochemical conditions. Org. Process Res. Dev. 2009, 13, 44–48. [Google Scholar] [CrossRef]
- Braga, D.; D’Addario, D.; Polito, M.; Grepioni, F. Mechanically induced expeditious and selective preparation of disubstituted pyridine/pyrimidine ferrocenyl complexes. Organometallics 2004, 23, 2810–2812. [Google Scholar] [CrossRef]
- Saha, P.; Naskar, S.; Paira, P.; Hazra, A.; Sahu, K.B.; Paira, R.; Banerjee, S.; Mondal, N.B. Basic alumina-supported highly effective Suzuki–Miyaura cross-coupling reaction under microwave irradiation: Application to fused tricyclic oxa-aza-quinolones. Green Chem. 2009, 11, 931–934. [Google Scholar] [CrossRef]
- Bernhardt, F.; Trotzki, R.; Szuppa, T.; Stolle, A.; Ondruschka, B. Solvent-free and time-efficient Suzuki–Miyaura reaction in a ball mill: The solid reagent system KF-Al2O3 under inspection. Beilstein J. Org. Chem. 2010. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Li, Z.-H.; Yu, J.-B.; Su, W.-K. Liquid-assisted grinding accelerating: Suzuki–Miyaura reaction of aryl chlorides under high-speed ball-milling conditions. J. Org. Chem. 2016, 81, 10049–10055. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Sarina, S.; Bai, S.; Huang, Y.; Chen, C.; Jia, J.; Jaatinen, E.; Ayoko, G.A.; Bao, Z.; Zhu, H. Visible light enhanced oxidant free dehydrogenation of aromatic alcohols using Au–Pd alloy nanoparticle catalysts. Green Chem. 2014, 16, 331–341. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Mao, L. Recent progress in highly efficient Ag-based visible-light photocatalysts. RSC Adv. 2014, 4, 53649–53661. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Moriab, K.; Yamashita, H. Synthesis and characterization of a Pd/Ag bimetallic nanocatalyst on SBA-15 mesoporous silica as a plasmonic catalyst. J. Mater. Chem. A 2015, 3, 18889–18897. [Google Scholar] [CrossRef]
- Tanaka, A.; Fuku, K.; Nishi, T.; Hashimoto, K.; Kominami, H. Functionalization of Au/TiO2 plasmonic photocatalysts with Pd by formation of a core–shell structure for effective dechlorination of chlorobenzene under irradiation of visible light. J. Phys. Chem. C 2013, 117, 16983–16989. [Google Scholar] [CrossRef]
- Tanaka, A.; Nakanishi, K.; Hamada, R.; Hashimoto, K.; Kominami, H. Simultaneous and stoichiometric water oxidation and Cr(VI) reduction in aqueous suspensions of functionalized plasmonic photocatalyst Au/TiO2–Pt under irradiation of green light. ACS Catal. 2013, 3, 1886–1891. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Moriab, K.; Yamashita, H. Pd/Ag and Pd/Au bimetallic nanocatalysts on mesoporous silica for plasmon-mediated enhanced catalytic activity under visible light irradiation. J. Mater. Chem. A 2016, 4, 10142–10150. [Google Scholar] [CrossRef]
- Smith, J.G.; Faucheaux, J.A.; Jain, P.K. Plasmon resonances for solar energy harvesting: A mechanistic outlook. Nano Today 2015, 10, 67–80. [Google Scholar] [CrossRef]
- Del Fatti, N.; Voisin, C.; Achermann, M.; Tzortzakis, S.; Christofilos, D.; Vallée, F. Nonequilibrium electron dynamics in noble metals. Phys. Rev. B 2000, 61, 16956–16966. [Google Scholar] [CrossRef]
- Link, S.; Link, S.; El-Sayed, M.A.; El-Sayed, M. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Evanoff, D.D.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Fuku, K.; Nishi, T.; Hashimoto, K.; Kominami, H. Gold–titanium(IV) oxide plasmonic photocatalysts prepared by a colloid-photodeposition method: Correlation between physical properties and photocatalytic activities. J. Phys. Chem. C 2013, 117, 16983–16989. [Google Scholar] [CrossRef]
- Sarina, S.; Zhu, H.; Jaatinen, E.; Xiao, Q.; Liu, H.; Jia, J.; Chen, C.; Zhao, J. Enhancing catalytic performance of palladium in gold and palladium alloy nanoparticles for organic synthesis reactions through visible light irradiation at ambient temperatures. J. Am. Chem. Soc. 2013, 135, 5793–5801. [Google Scholar] [CrossRef] [PubMed]
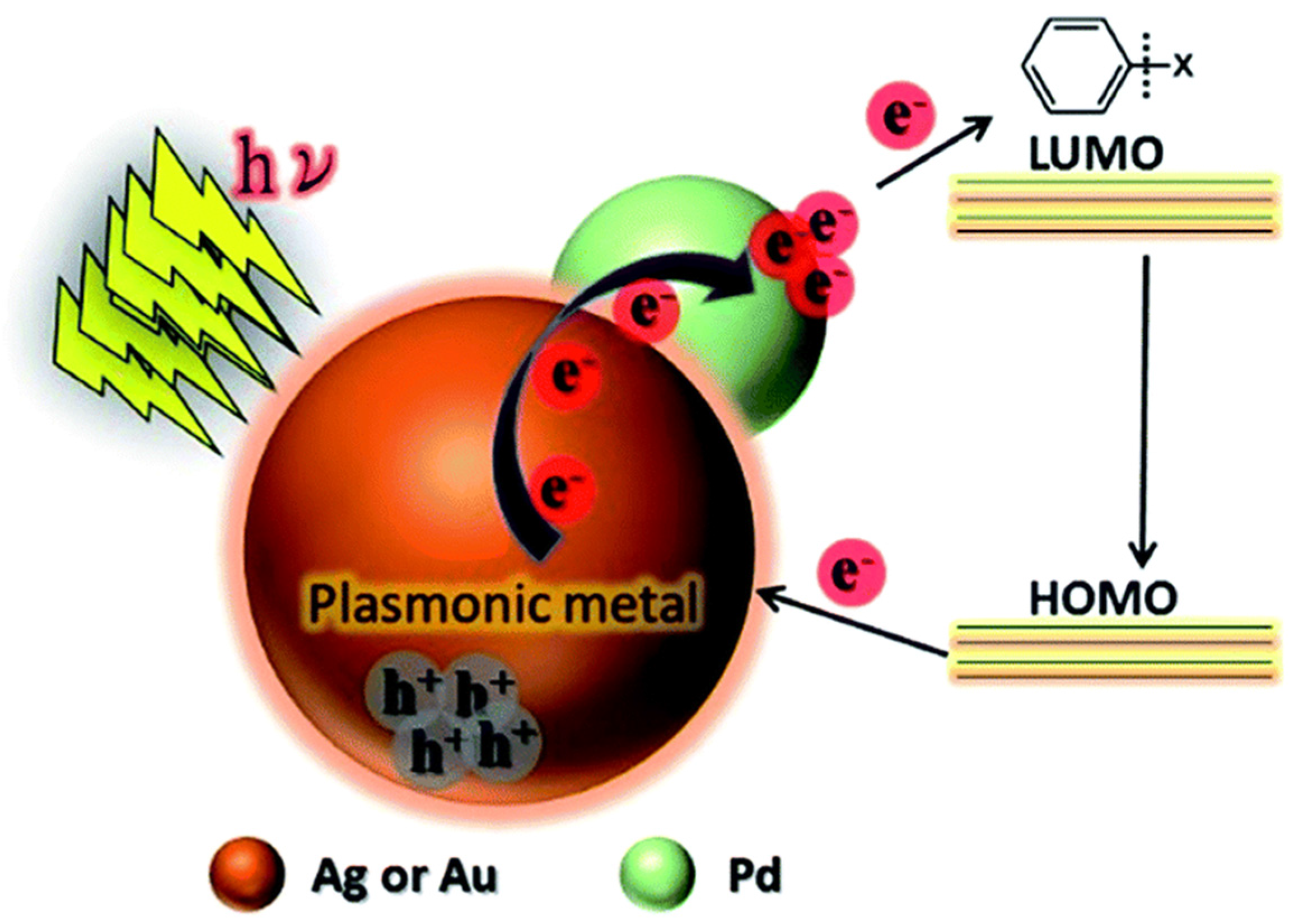
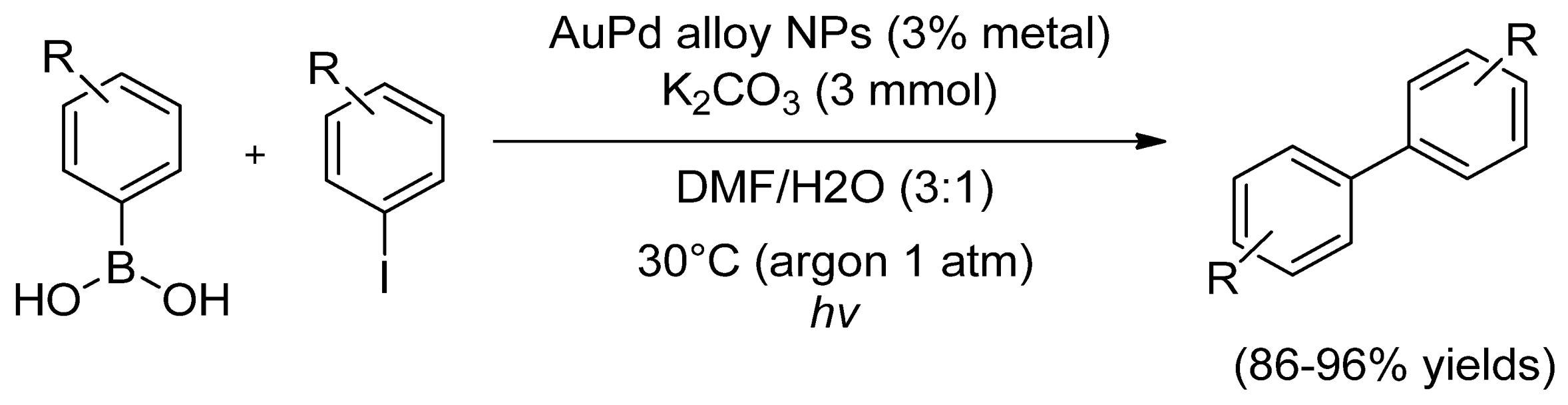
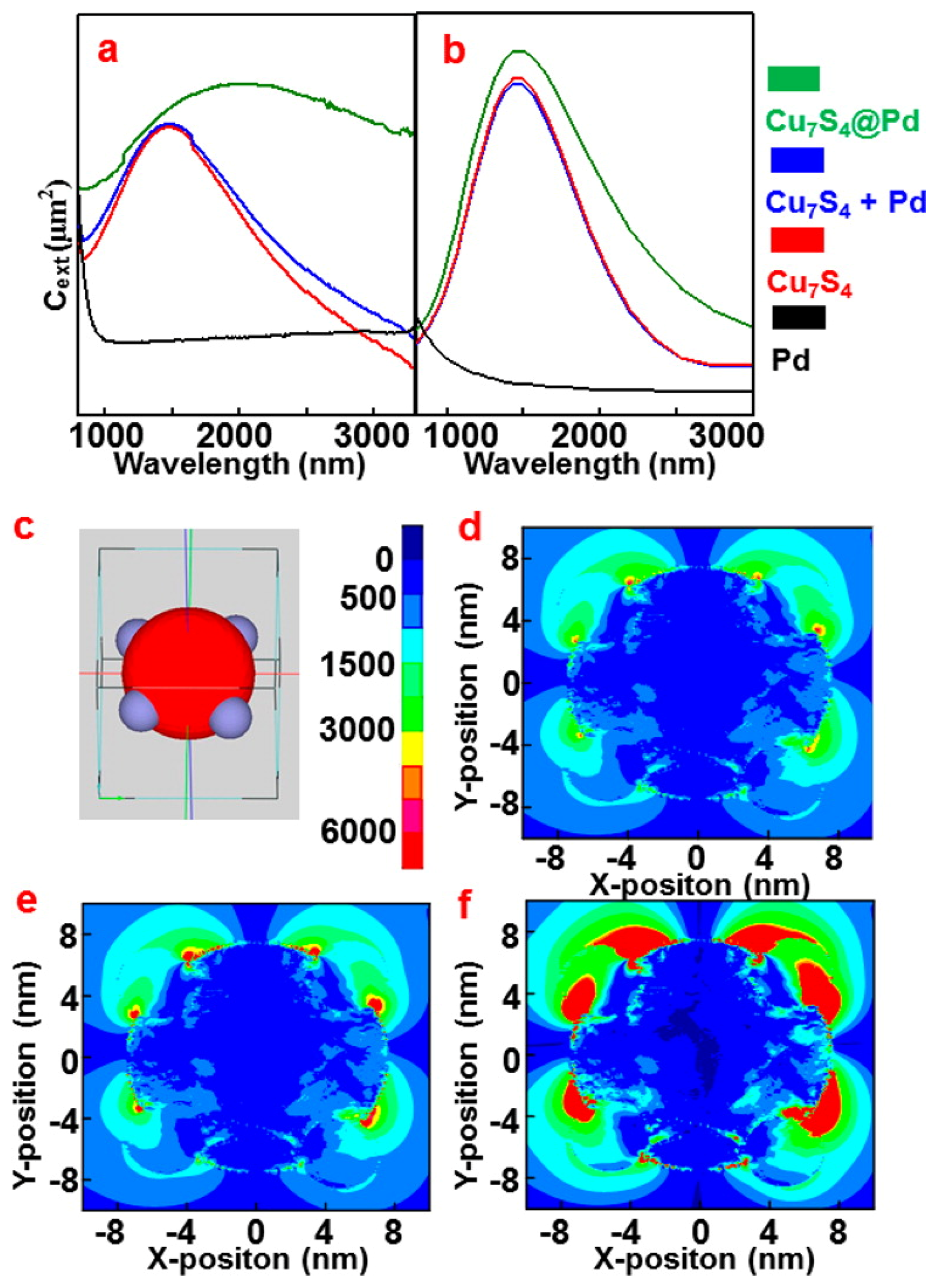
- Xiao, Q.; Sarina, S.; Jaatinen, E.; Jia, J.; Arnold, D.P.; Liu, H.; Zhu, H. Efficient photocatalytic Suzuki cross-coupling reactions on Au–Pd alloy nanoparticles under visible light irradiation. Green Chem. 2014, 16, 4272–4285. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Chen, H.; Jiang, R.; Sun, L.-D.; Li, Q.; Wang, J.; Yu, J.C.; Yan, C.-H. Plasmonic harvesting of light energy for Suzuki coupling reactions. J. Am. Chem. Soc. 2013, 135, 5588–5601. [Google Scholar] [CrossRef]
- Wang, F.; Sun, L.-D.; Feng, W.; Chen, H.J.; Yeung, M.H.; Wang, J.F.; Yan, C.-H. Heteroepitaxial growth of core–shell and core–multishell nanocrystals composed of palladium and gold. Small 2010, 6, 2566–2575. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.H.; Sun, L.-D.; Wu, H.S.; Ming, T.; Wang, J.F.; Yu, J.C.; Yan, C.-H. Heteroepitaxial growth of high-index-faceted palladium nanoshells and their catalytic performance. J. Am. Chem. Soc. 2011, 133, 1106–1111. [Google Scholar] [CrossRef]
- Hu, J.-W.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Sun, S.-G.; Tian, Z.-Q. Palladium-coated gold nanoparticles with a controlled shell thickness used as surface-enhanced Raman scattering substrate. J. Phys. Chem. C 2007, 111, 1105–1112. [Google Scholar] [CrossRef]
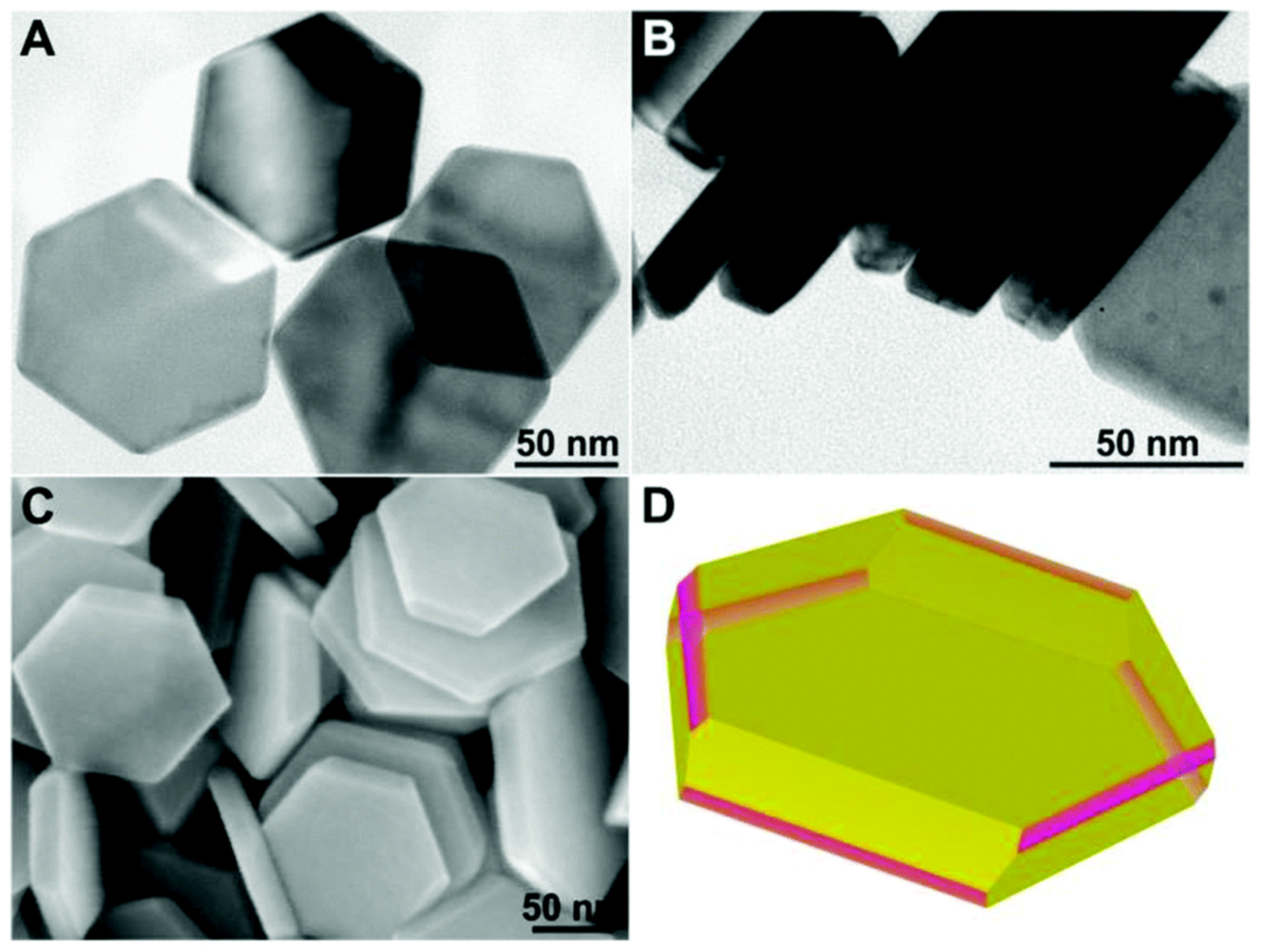
- Trinh, T.T.; Sato, R.; Sakamoto, M.; Fujiyoshi, Y.; Haruta, M.; Kurata, H.; Teranishi, T. Visible to near-infrared plasmon-enhanced catalytic activity of Pd hexagonal nanoplates for the Suzuki coupling reaction. Nanoscale 2015, 7, 12435–12444. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Baar, M.; Blechert, S.; Antonietti, M. Facilitating room-temperature Suzuki coupling reaction with light: Mott-Schottky photocatalyst for C–C-coupling. Sci. Rep. 2013, 3, 1743. [Google Scholar] [CrossRef]
- Möhlmann, L.; Baar, M.; Rieß, J.; Antonietti, M.; Wang, W.; Blecher, S. Carbon nitride-catalyzed photoredox C–C bond formation with N-aryltetrahydroisoquinolines. Adv. Synth. Catal. 2012, 354, 1909–1913. [Google Scholar] [CrossRef]
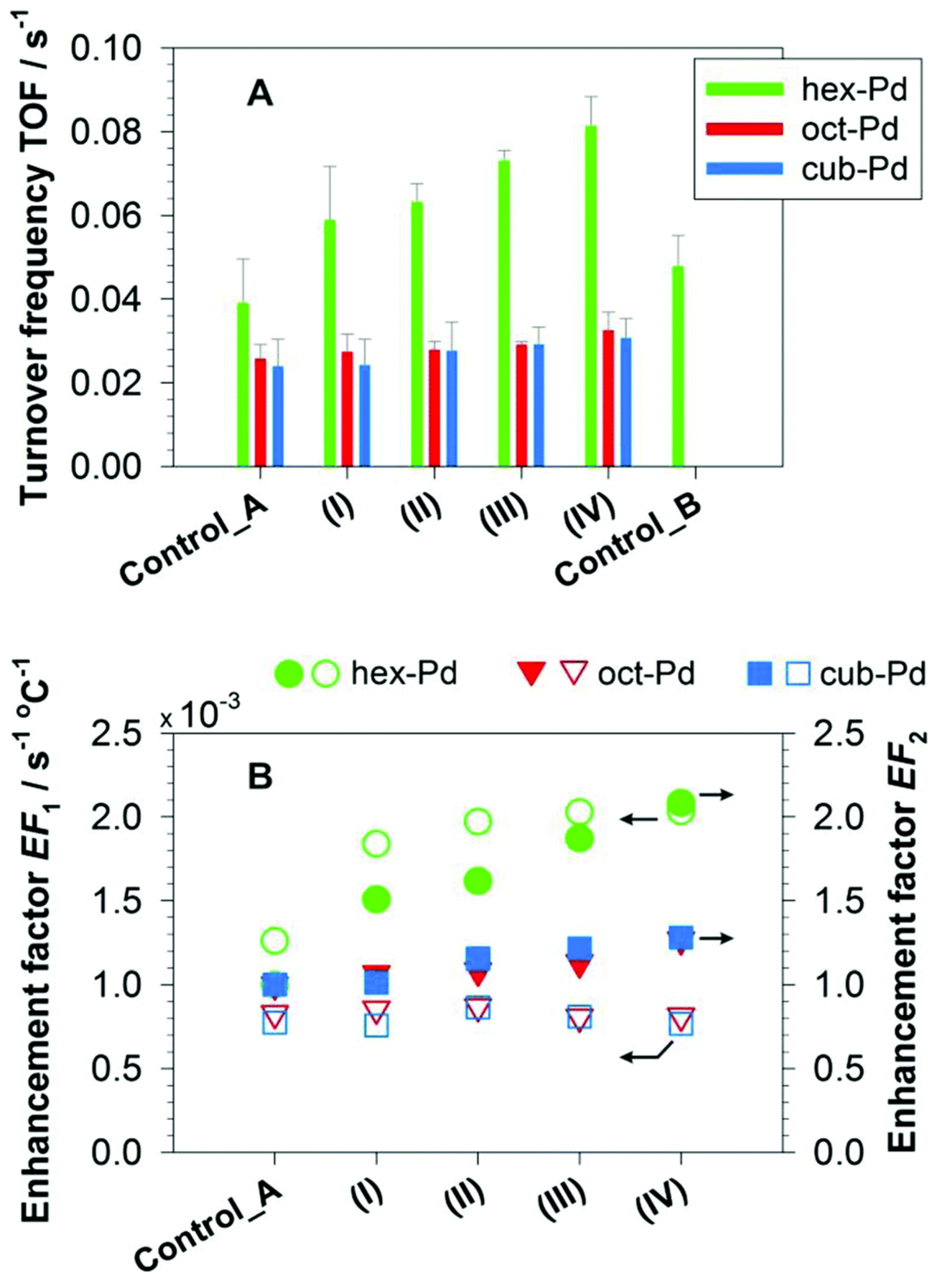
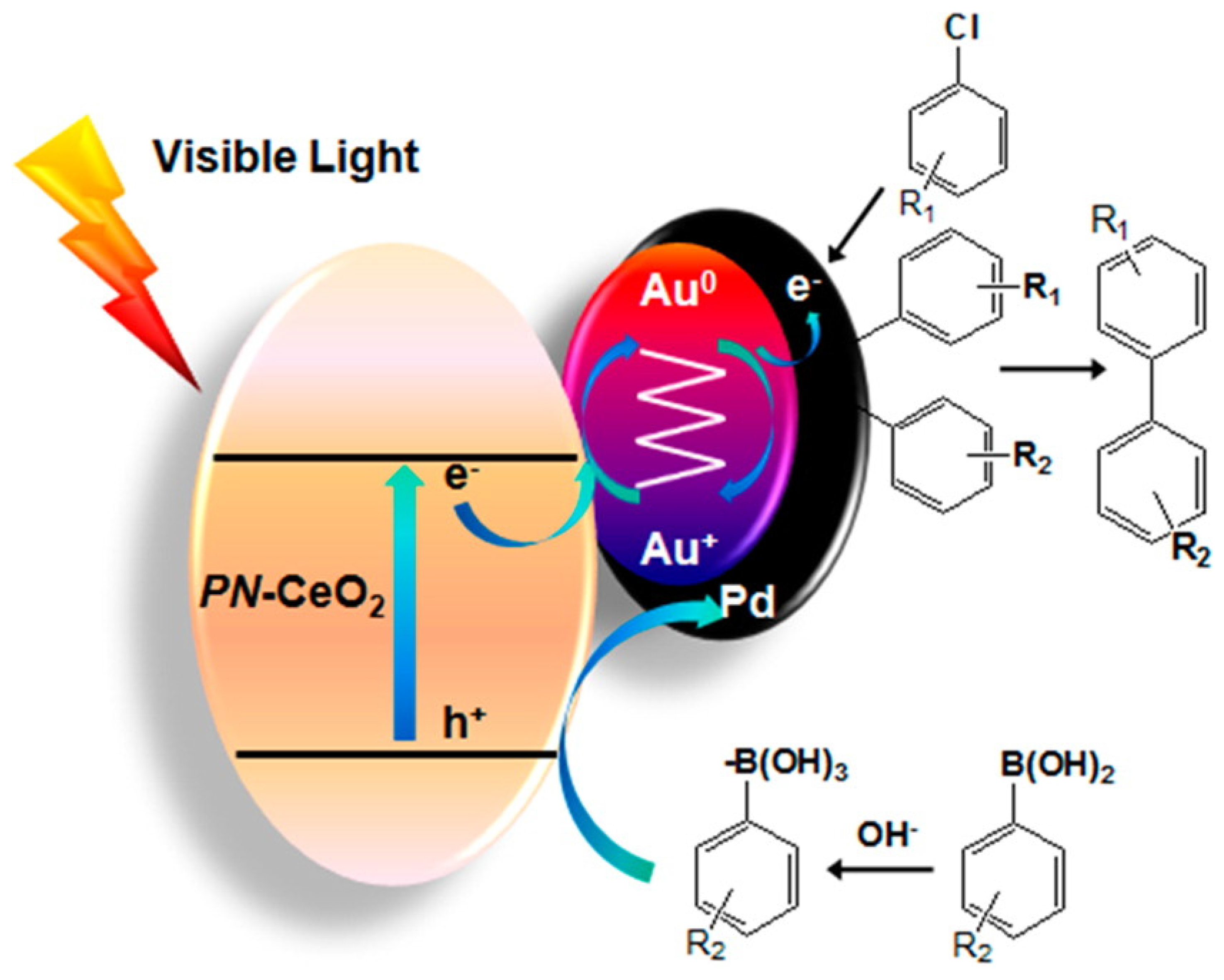
- Zhang, S.; Chang, C.; Huang, Z.; Ma, Y.; Gao, W.; Li, J.; Qu, Y. Visible-light-activated Suzuki–Miyaura coupling reactions of aryl chlorides over the multifunctional Pd/Au/porous nanorods of CeO2. ACS Catal. 2015, 5, 6481–6488. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Gao, W.; Qu, Y. Insights into the effects of surface properties of oxides on the catalytic activity of Pd for C–C coupling reactions. Nanoscale 2015, 7, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.; Liu, L.; Chen, L.; Xu, J.; Ma, J.; Fang, G.; Zhu, E.; Wu, H.; Zhao, L.; Wang, L.; Huang, Y. Near-infrared plasmonic-enhanced solar energy harvest for highly efficient photocatalytic reactions. Nano Lett. 2015, 15, 6295–6301. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
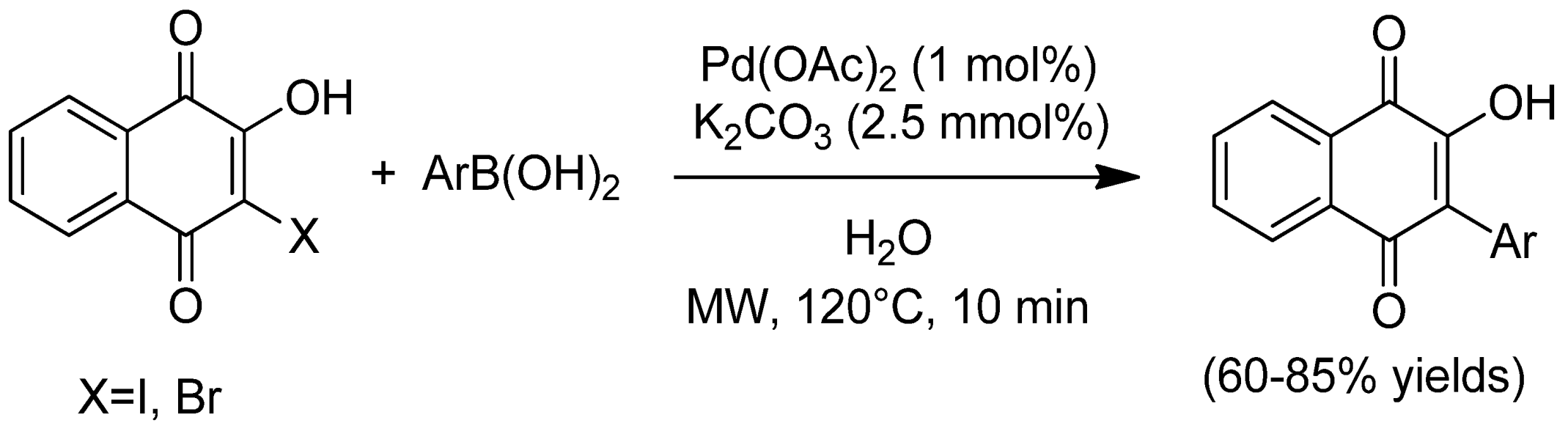
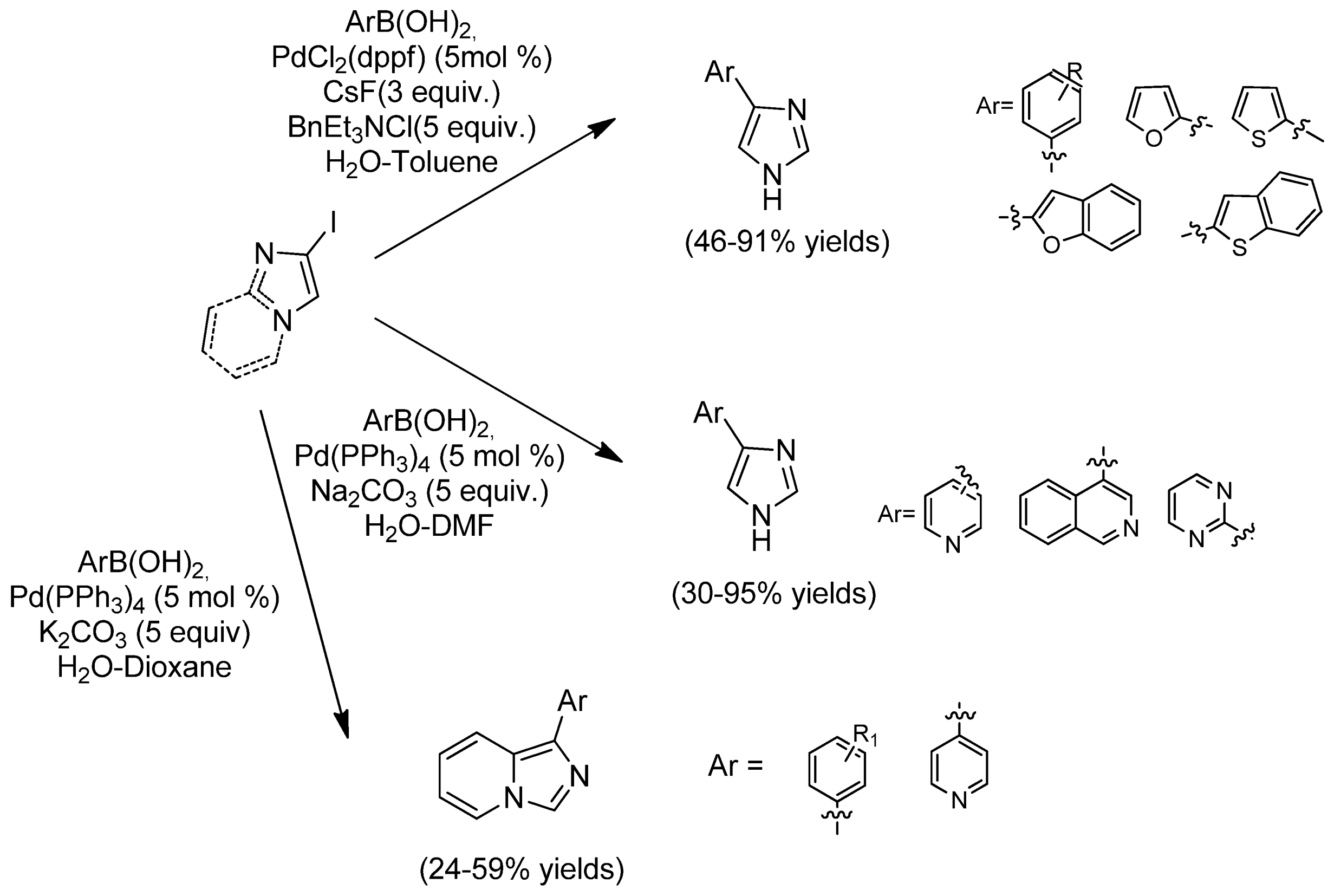
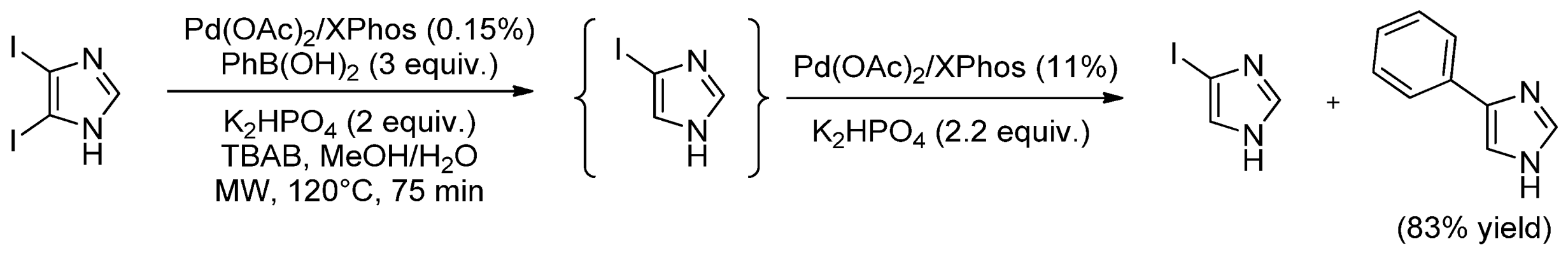

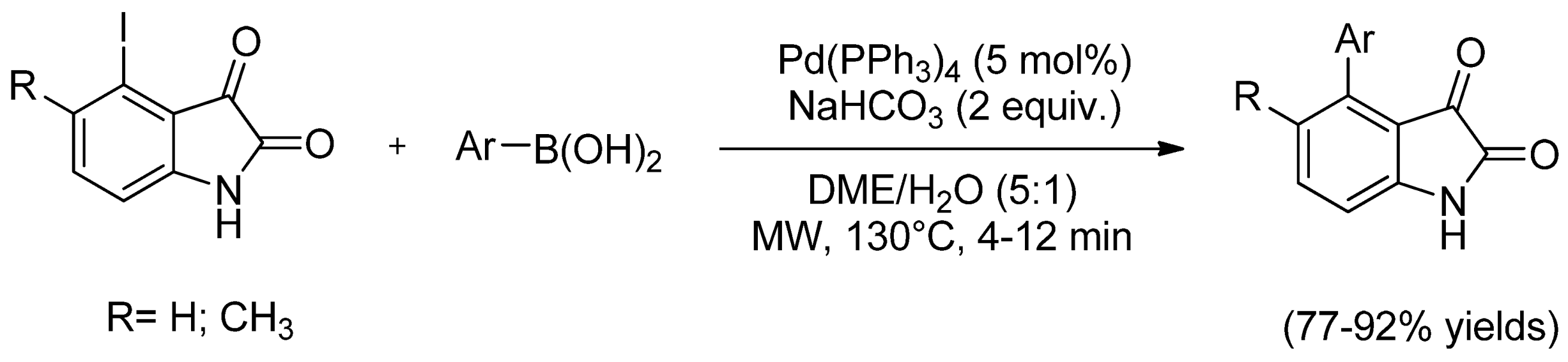
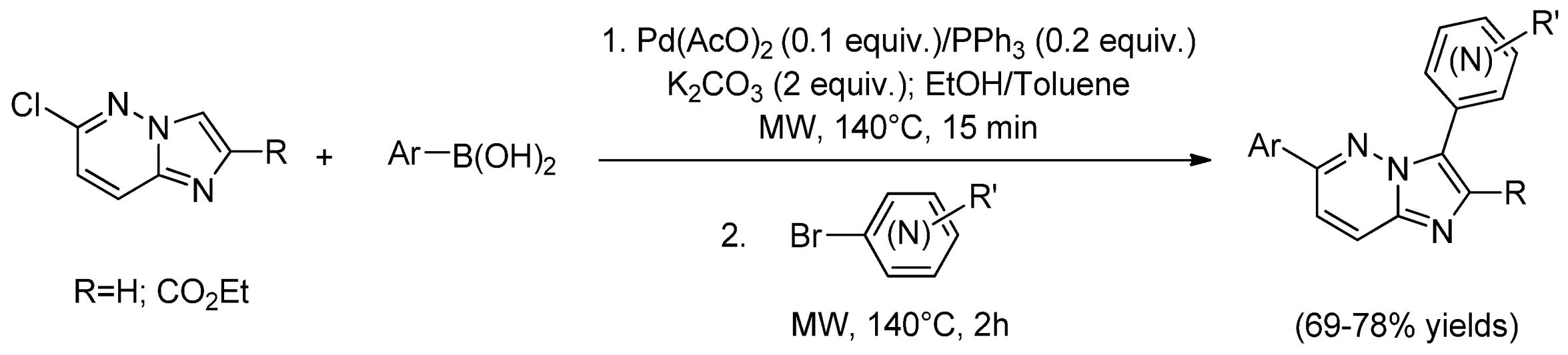
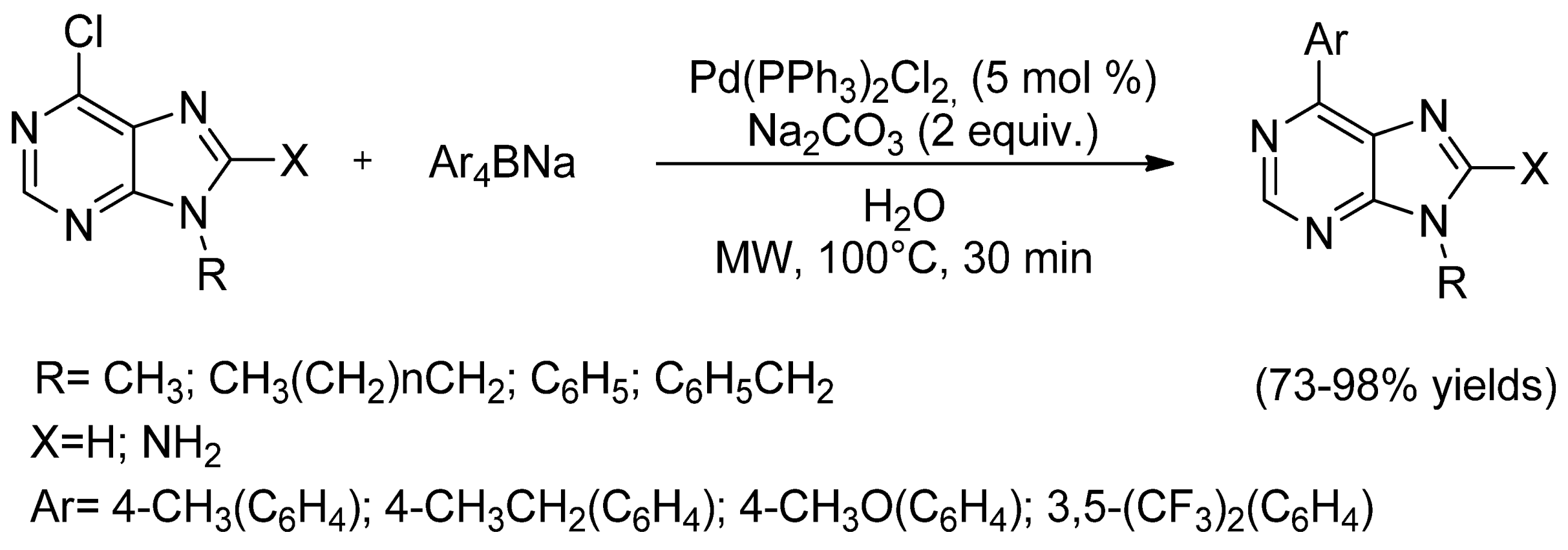


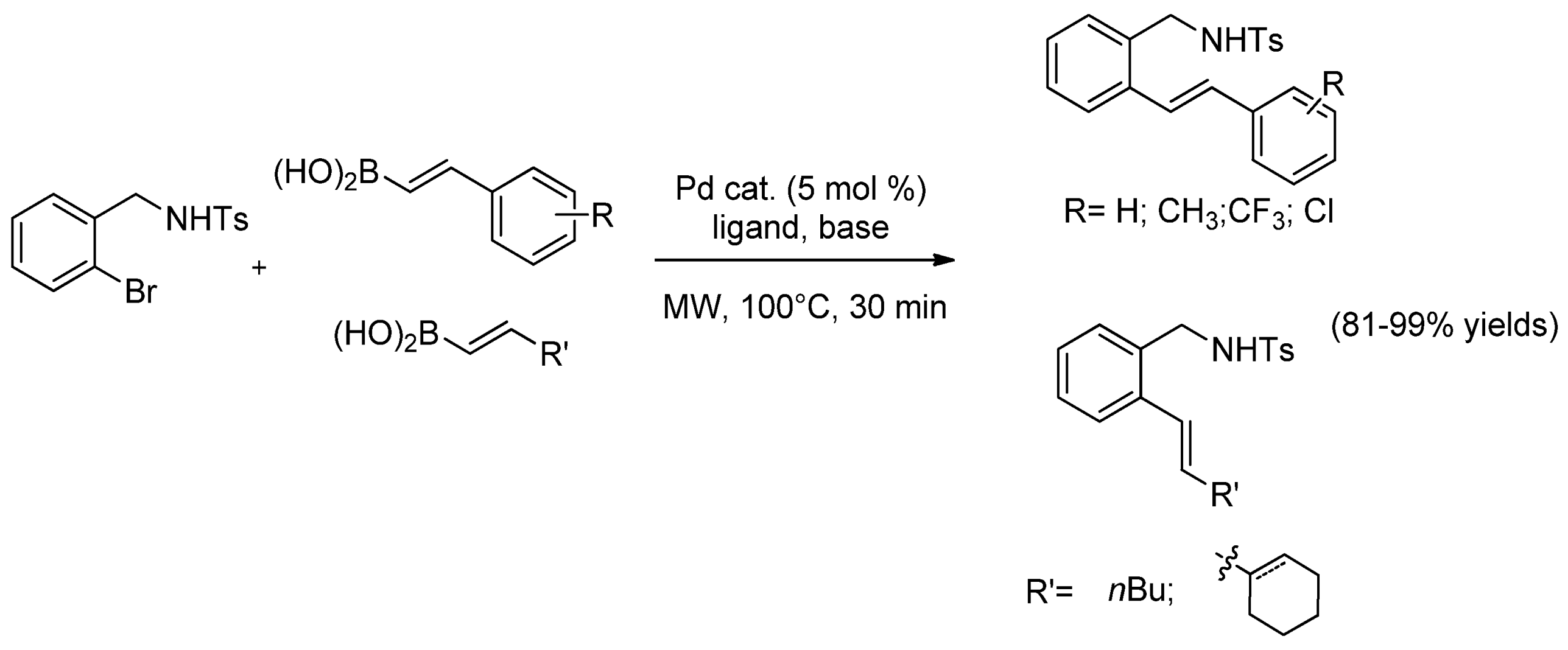
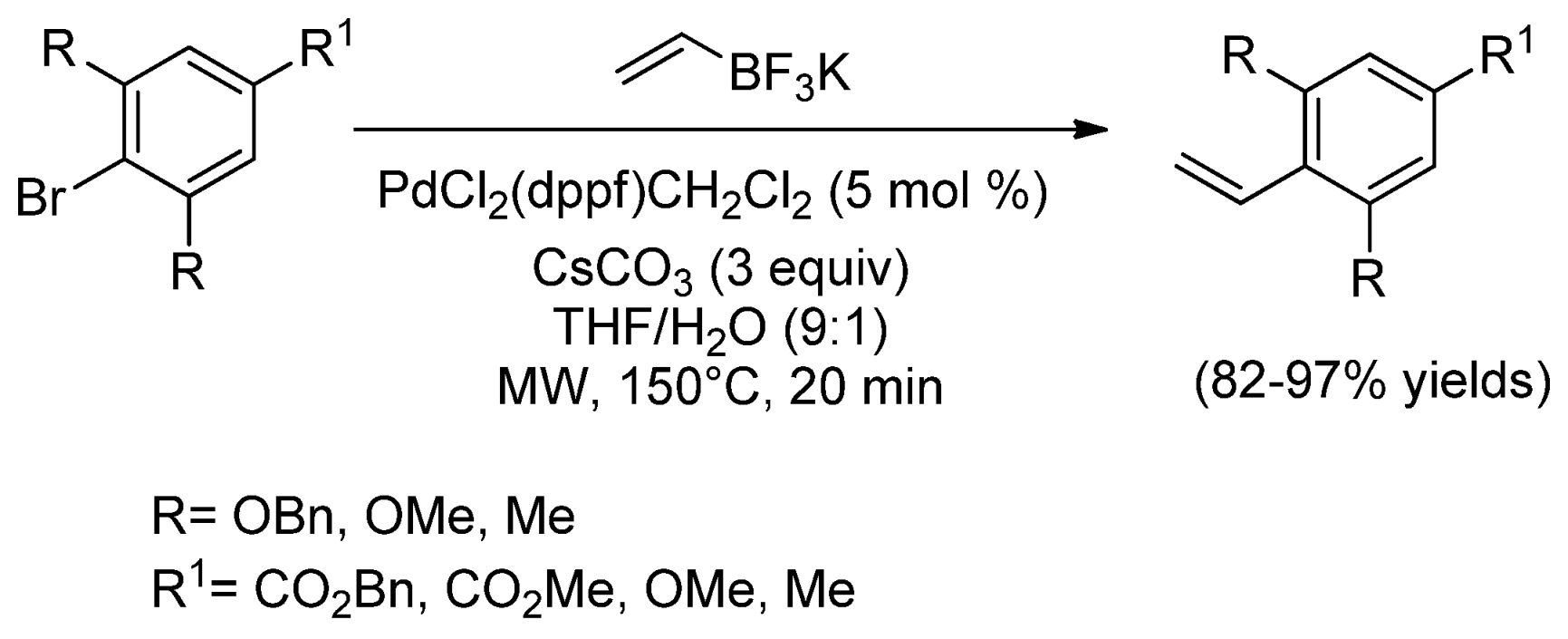
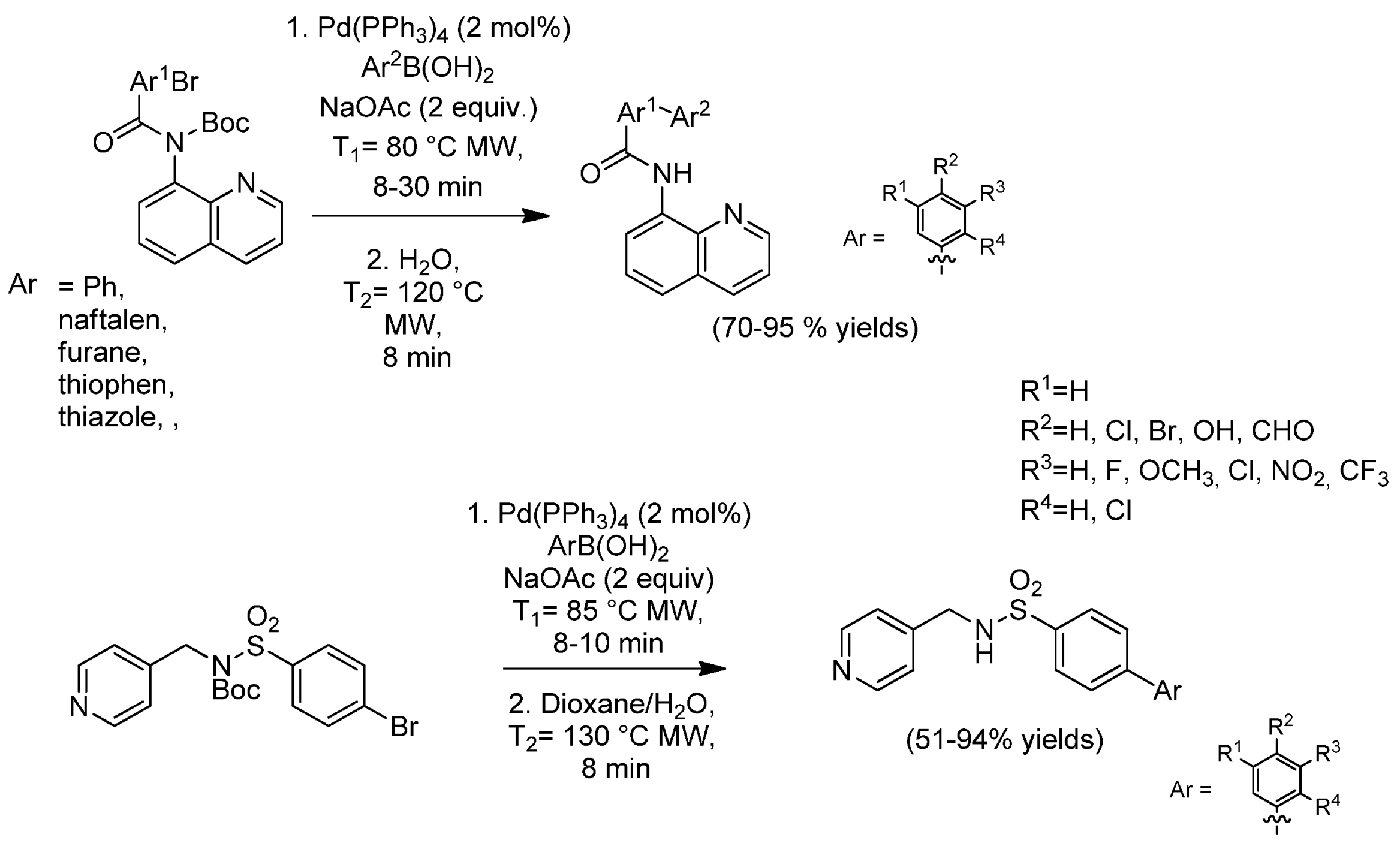
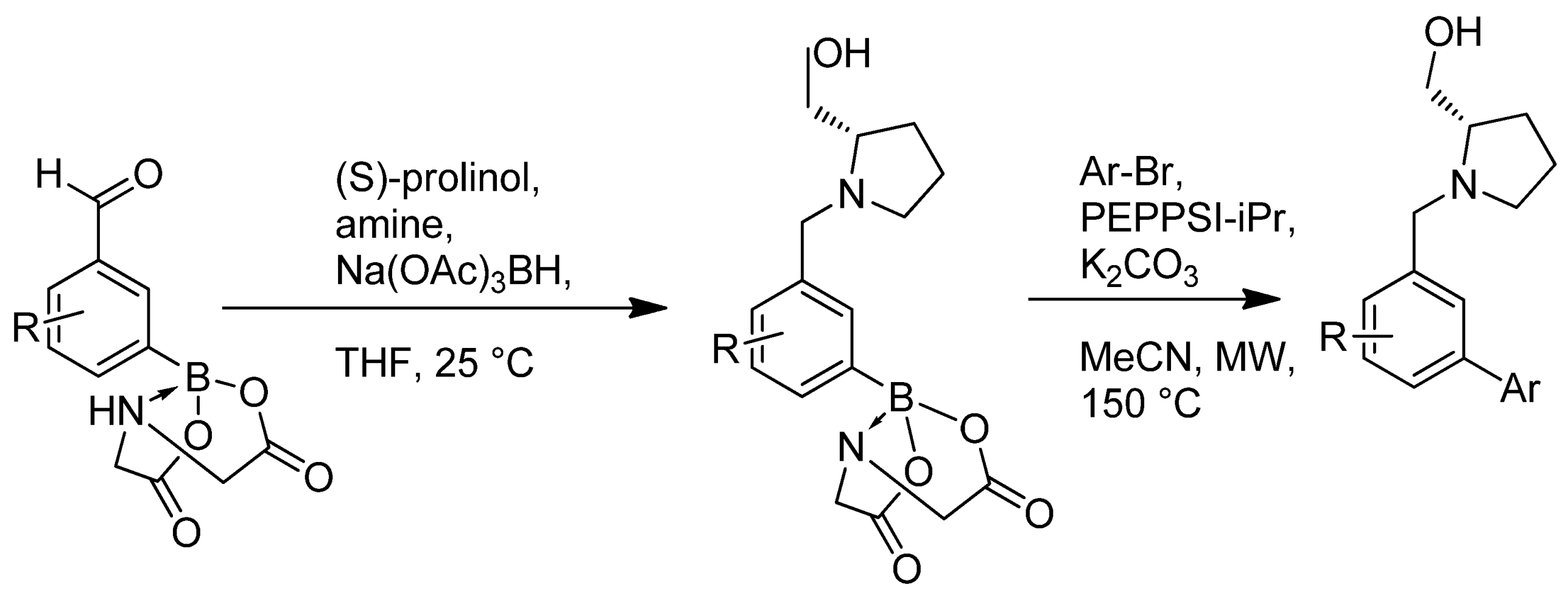

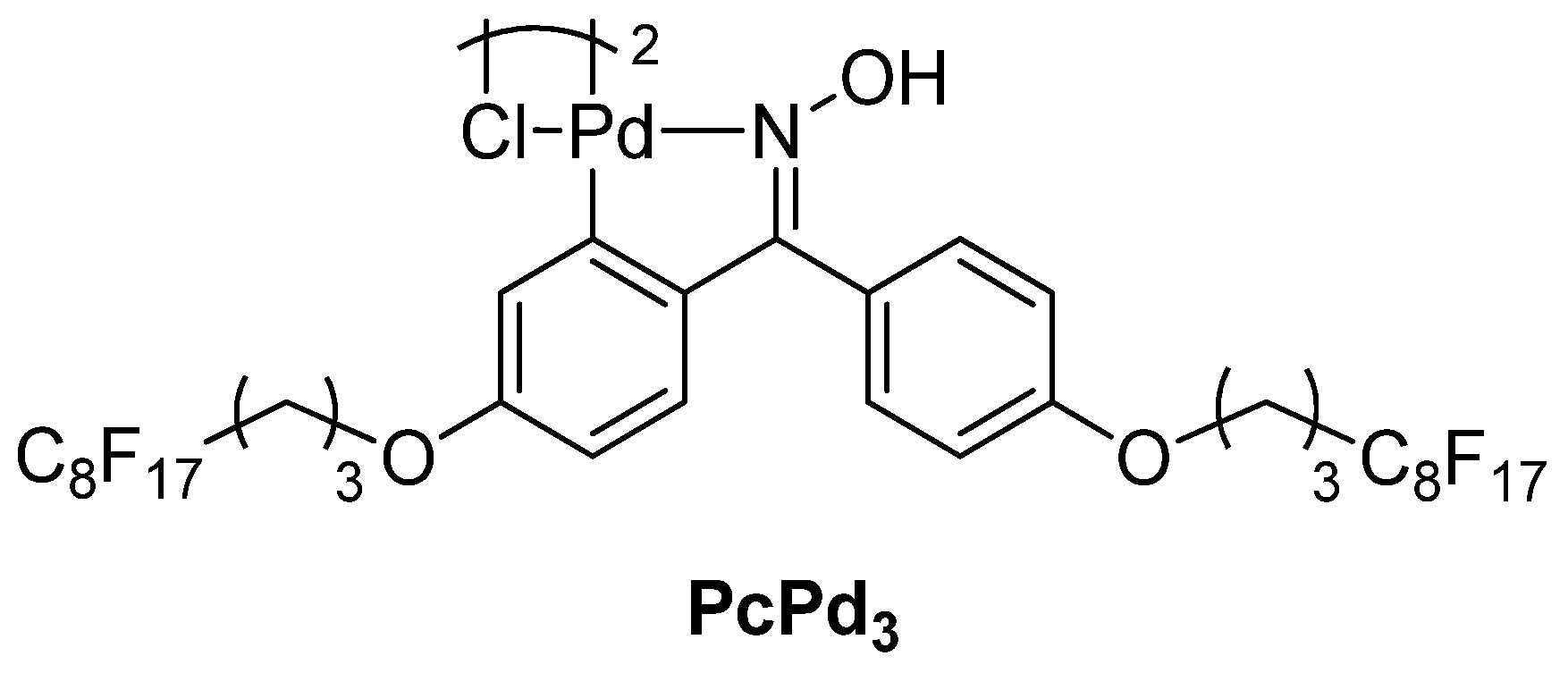
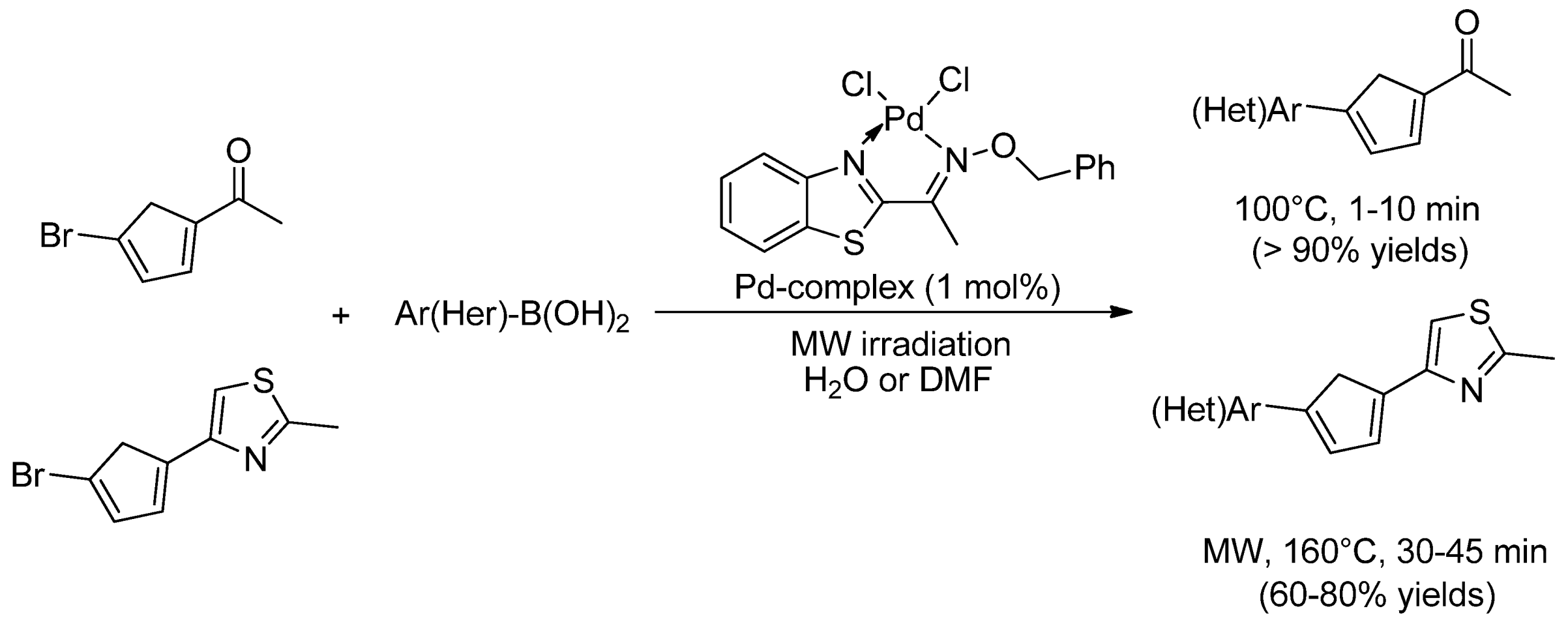
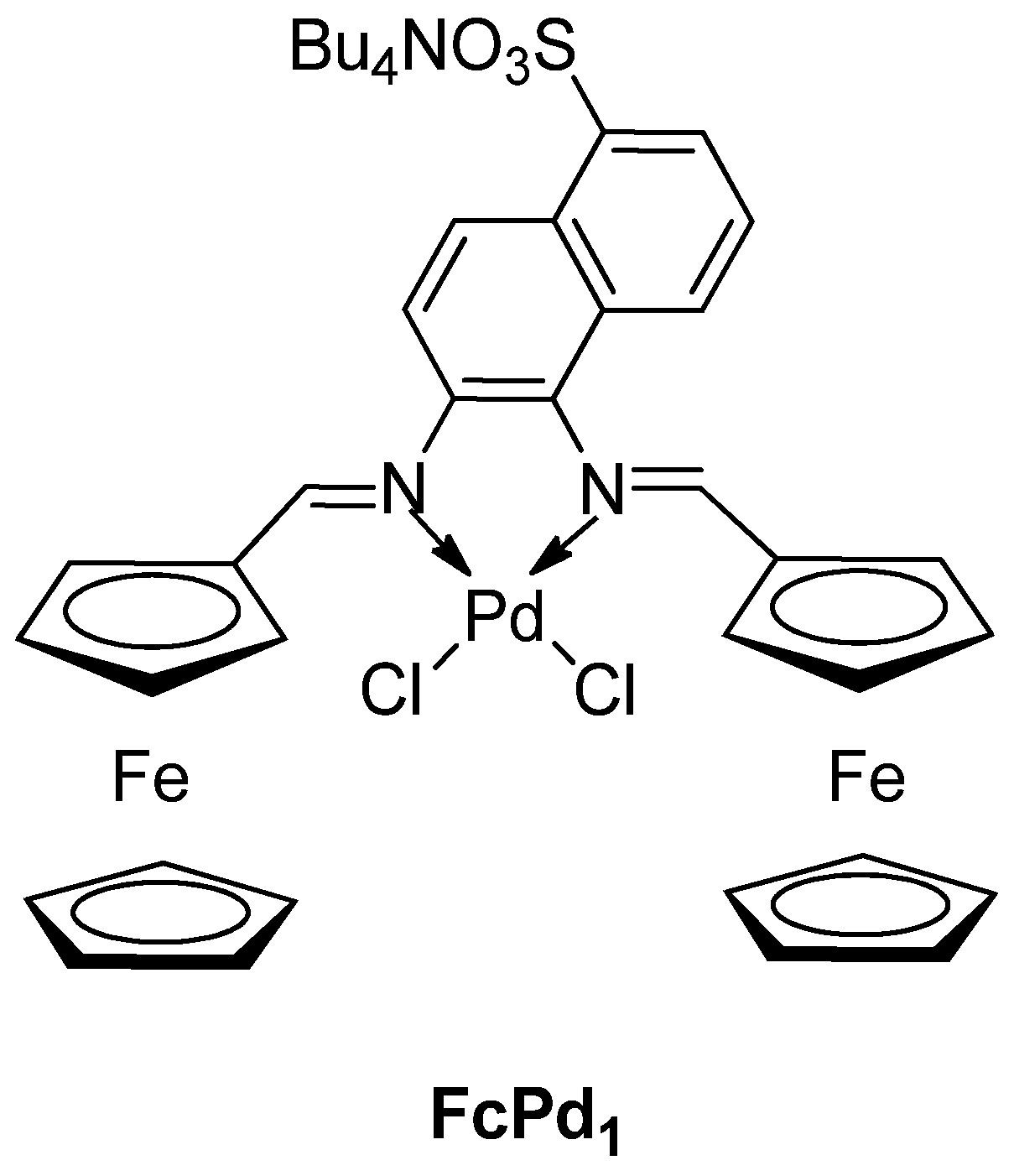
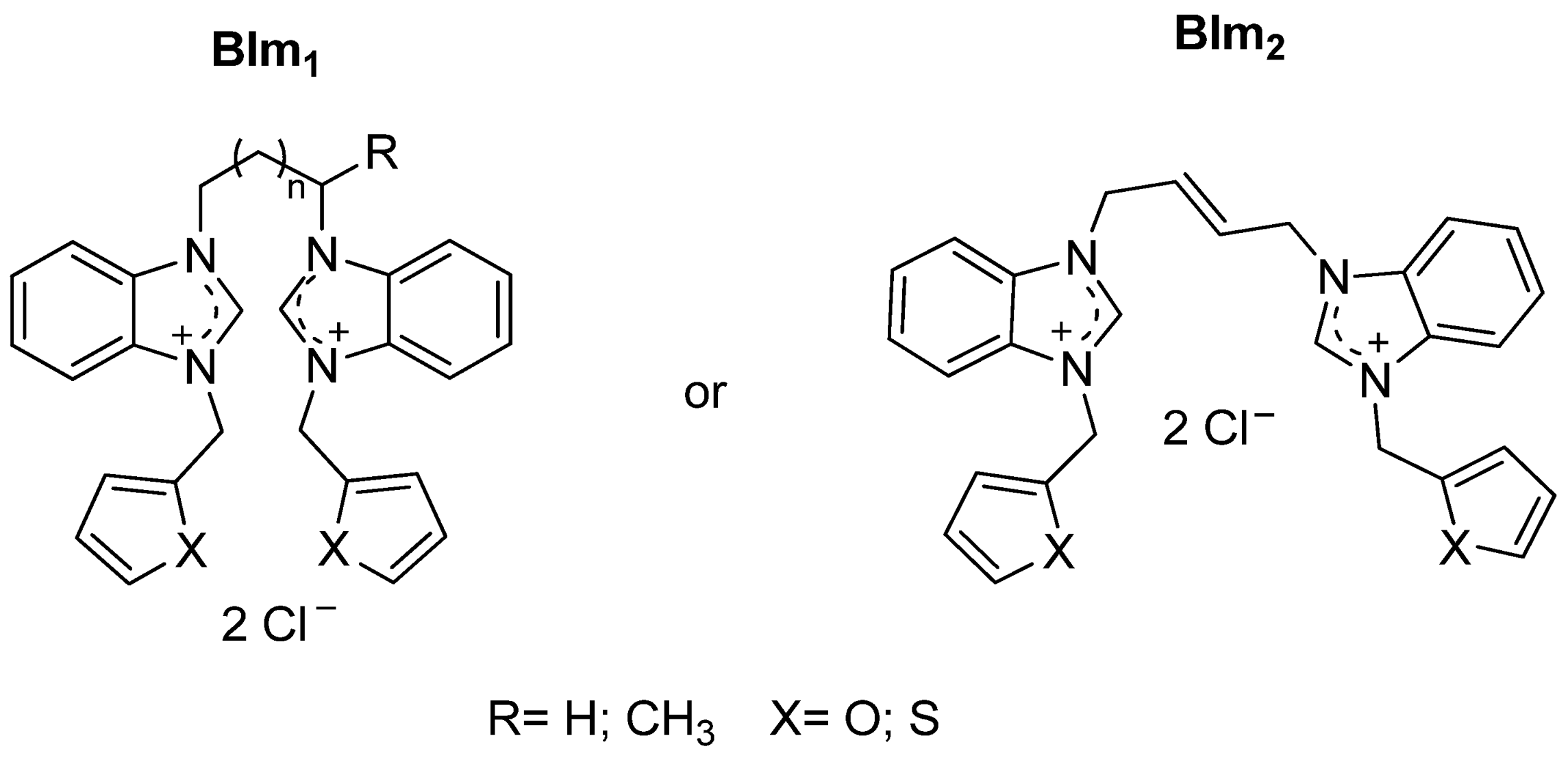
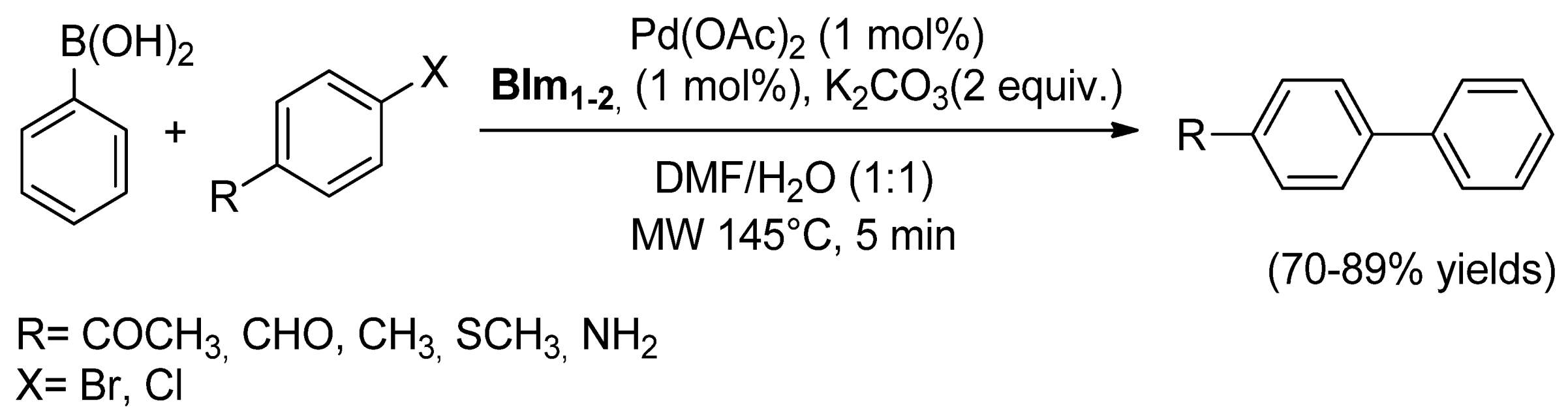
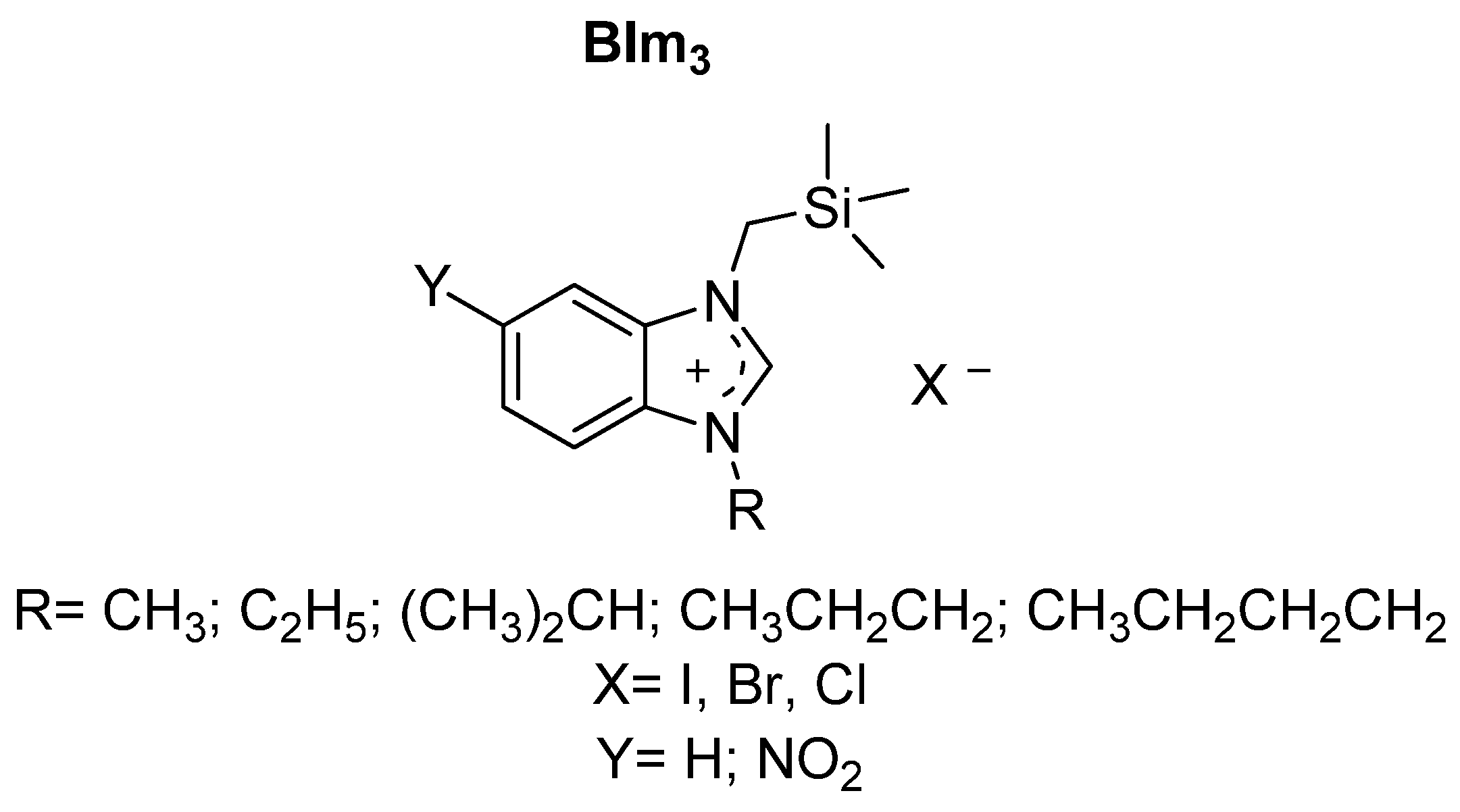
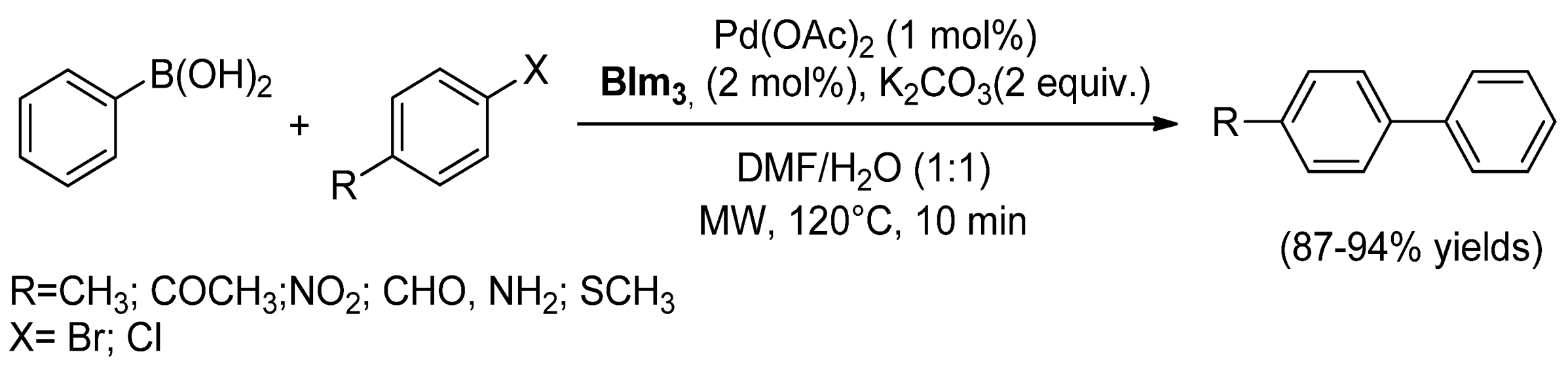
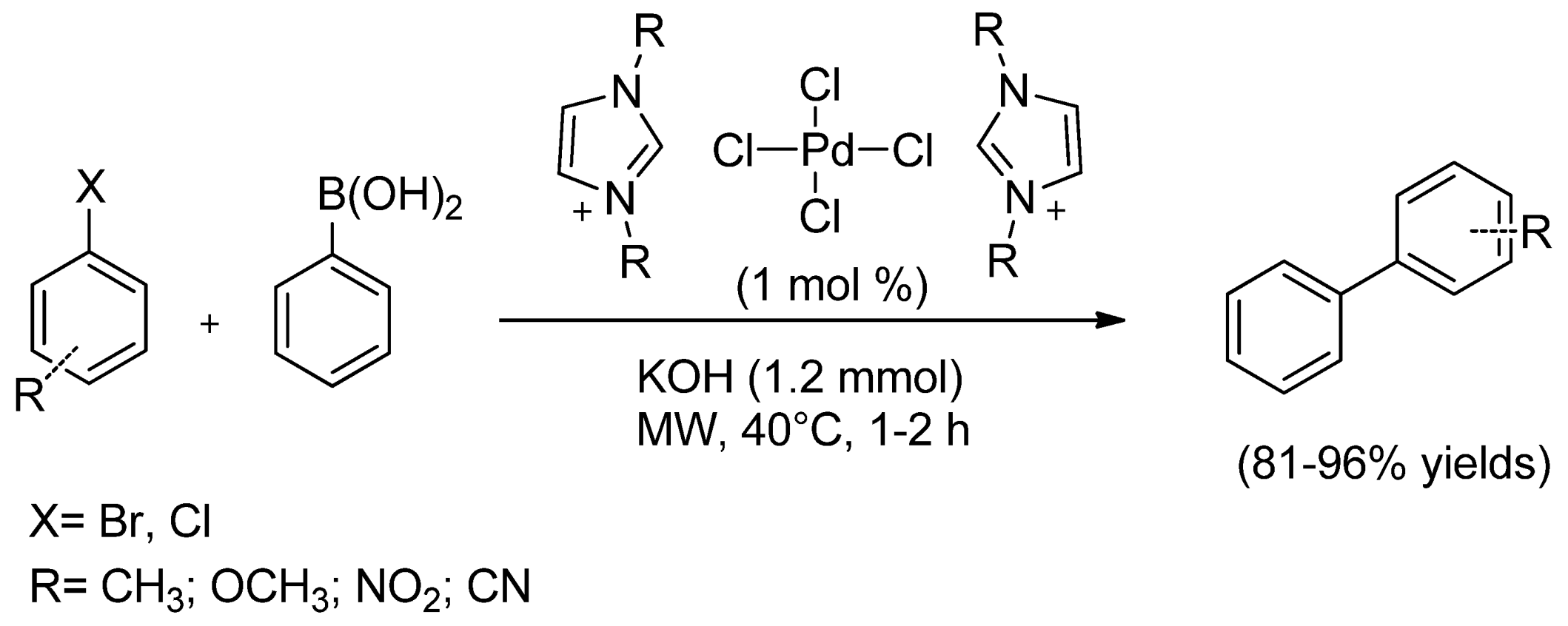
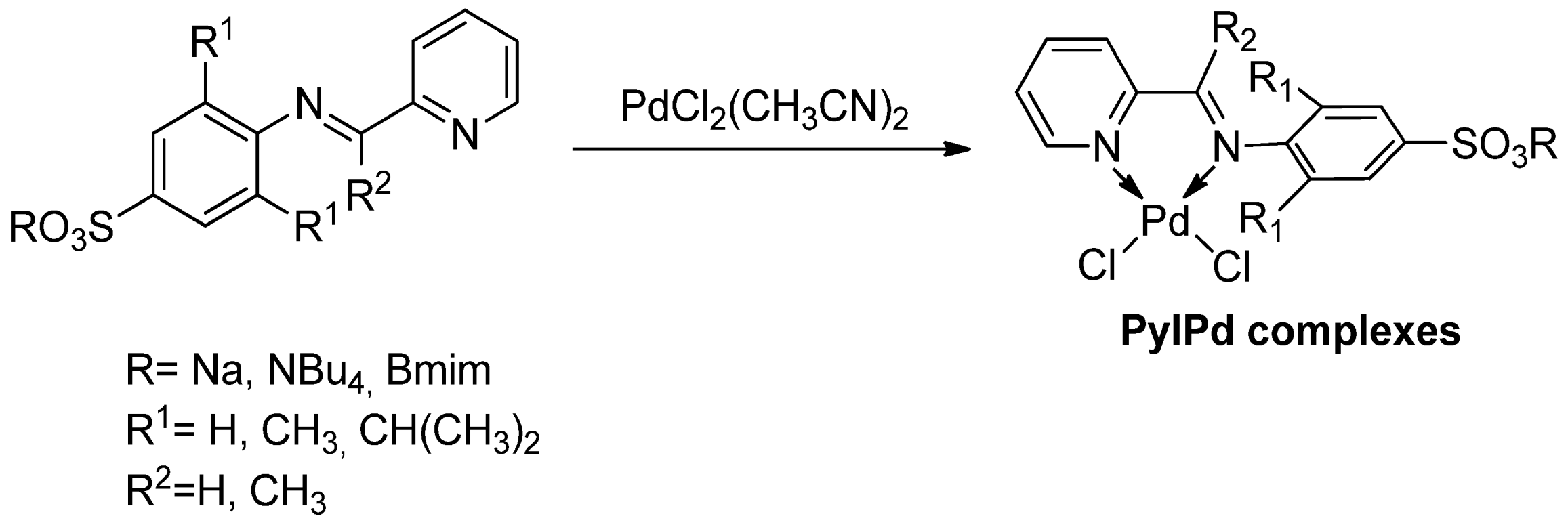
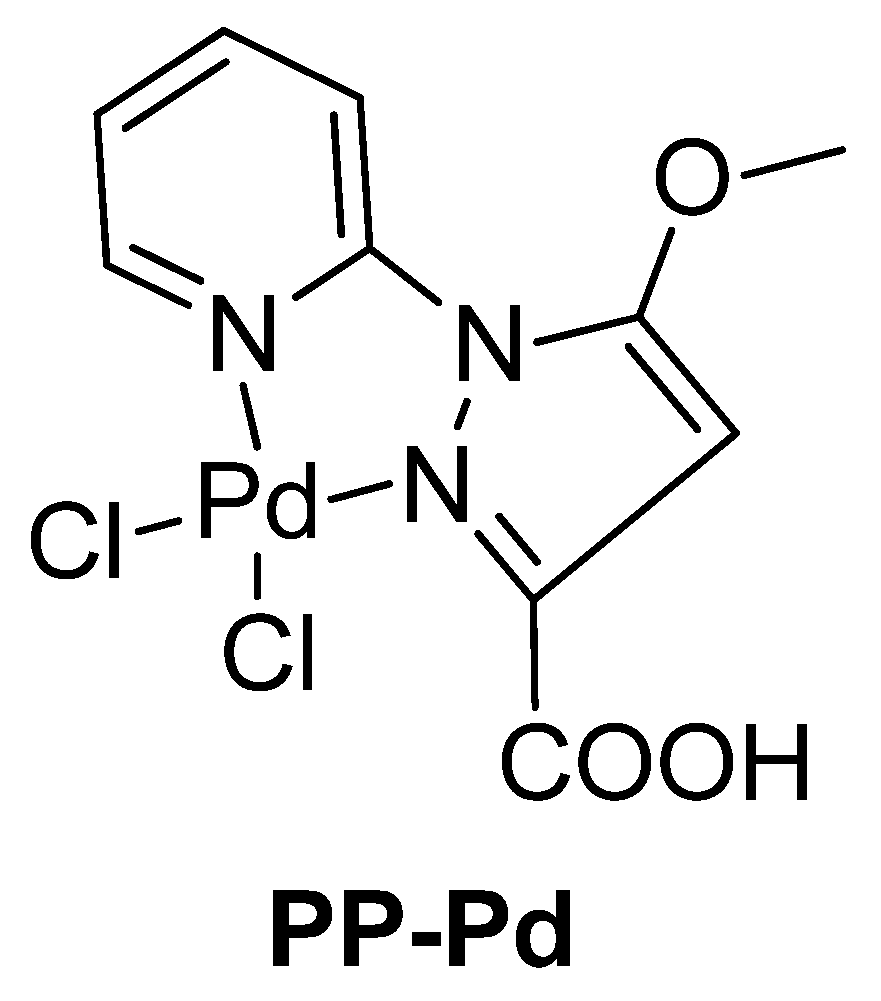
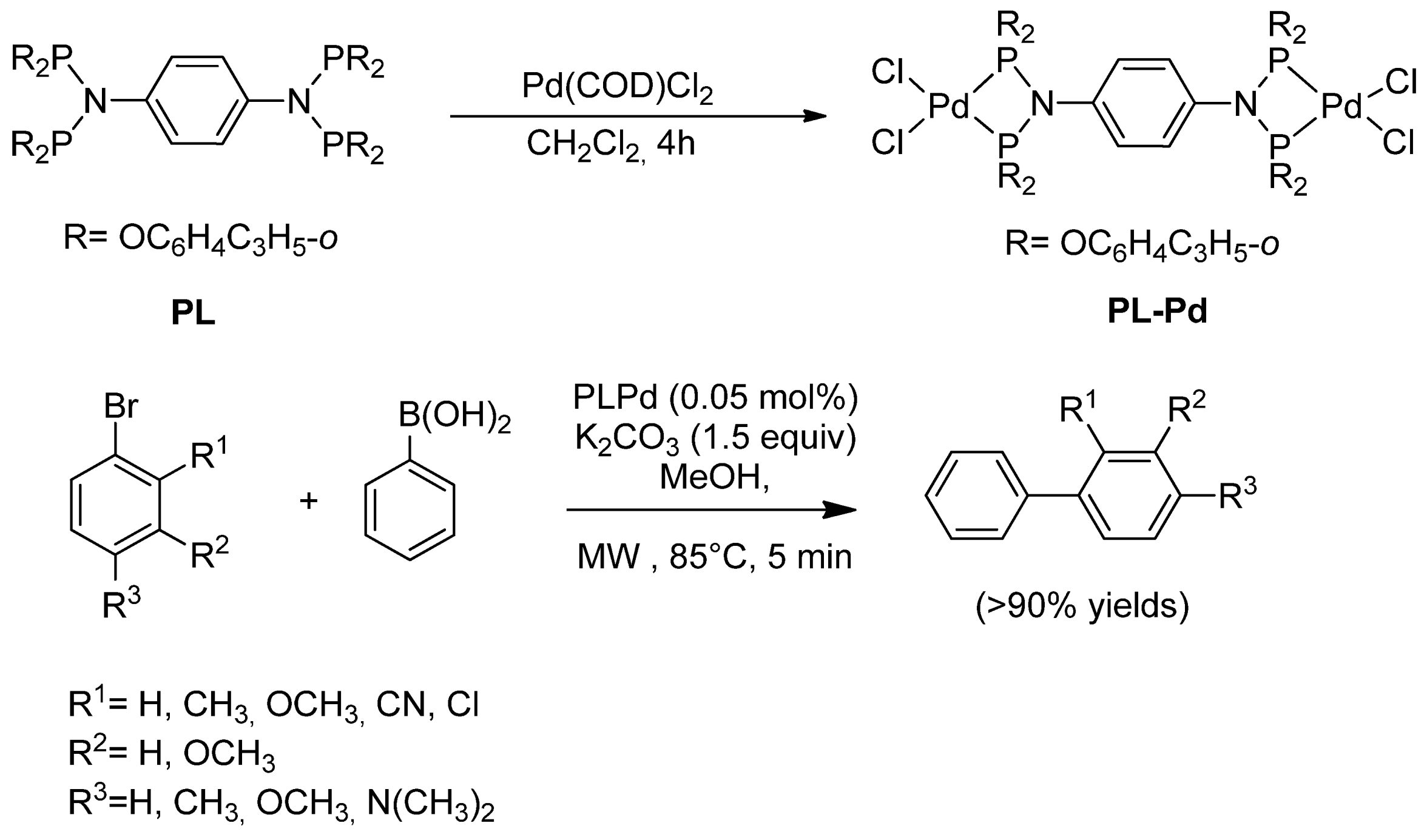
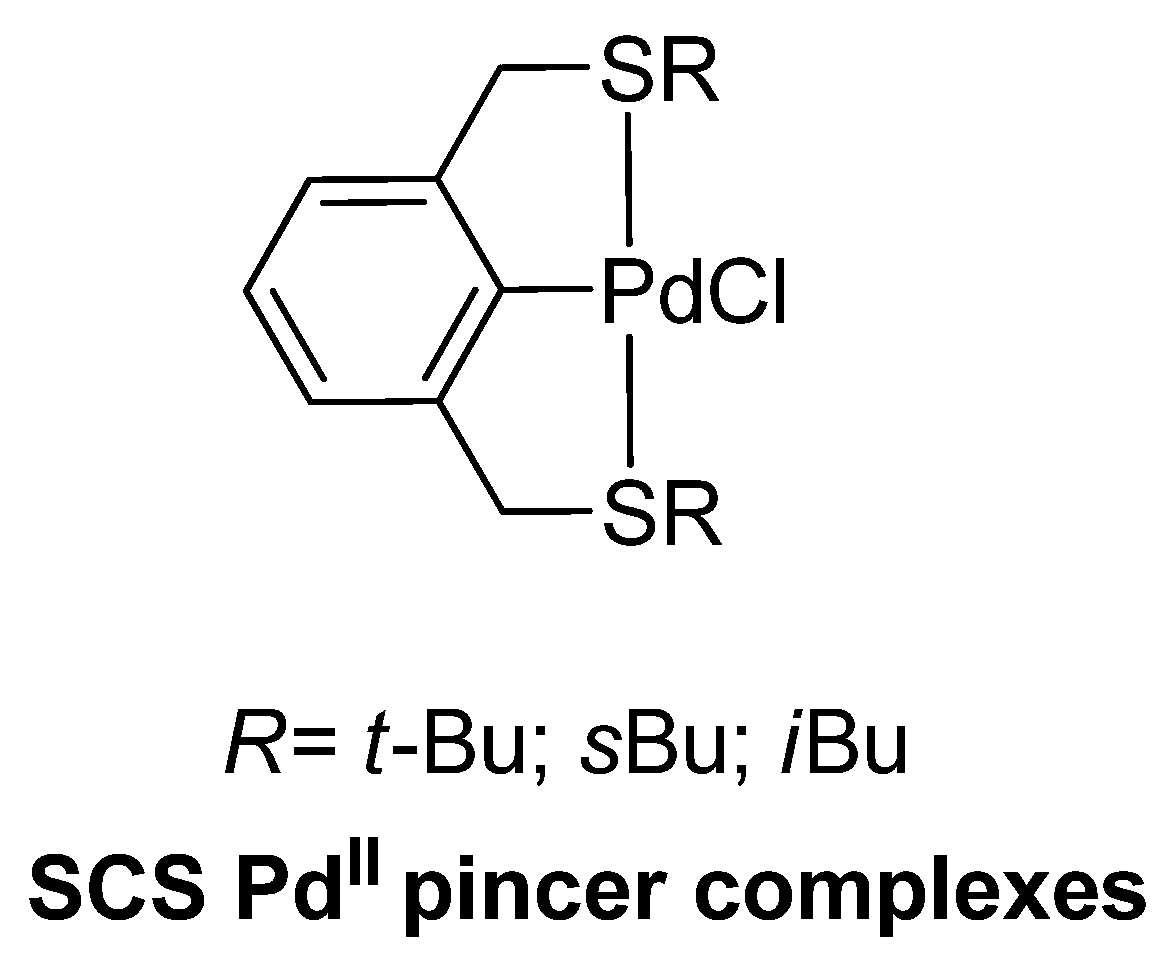
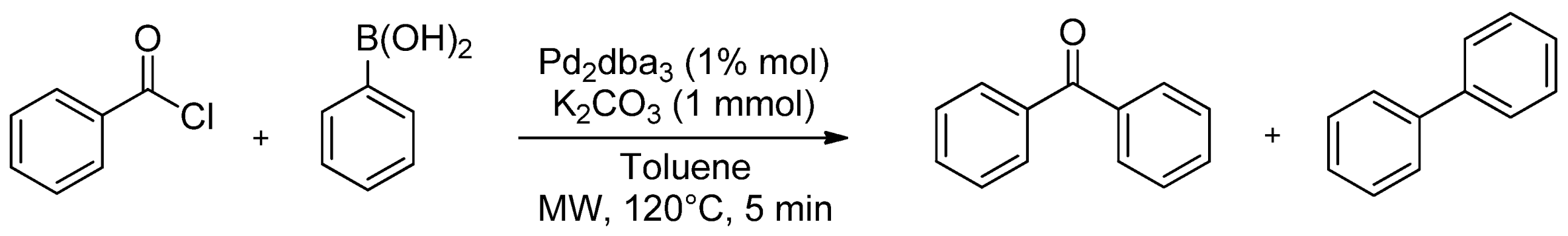

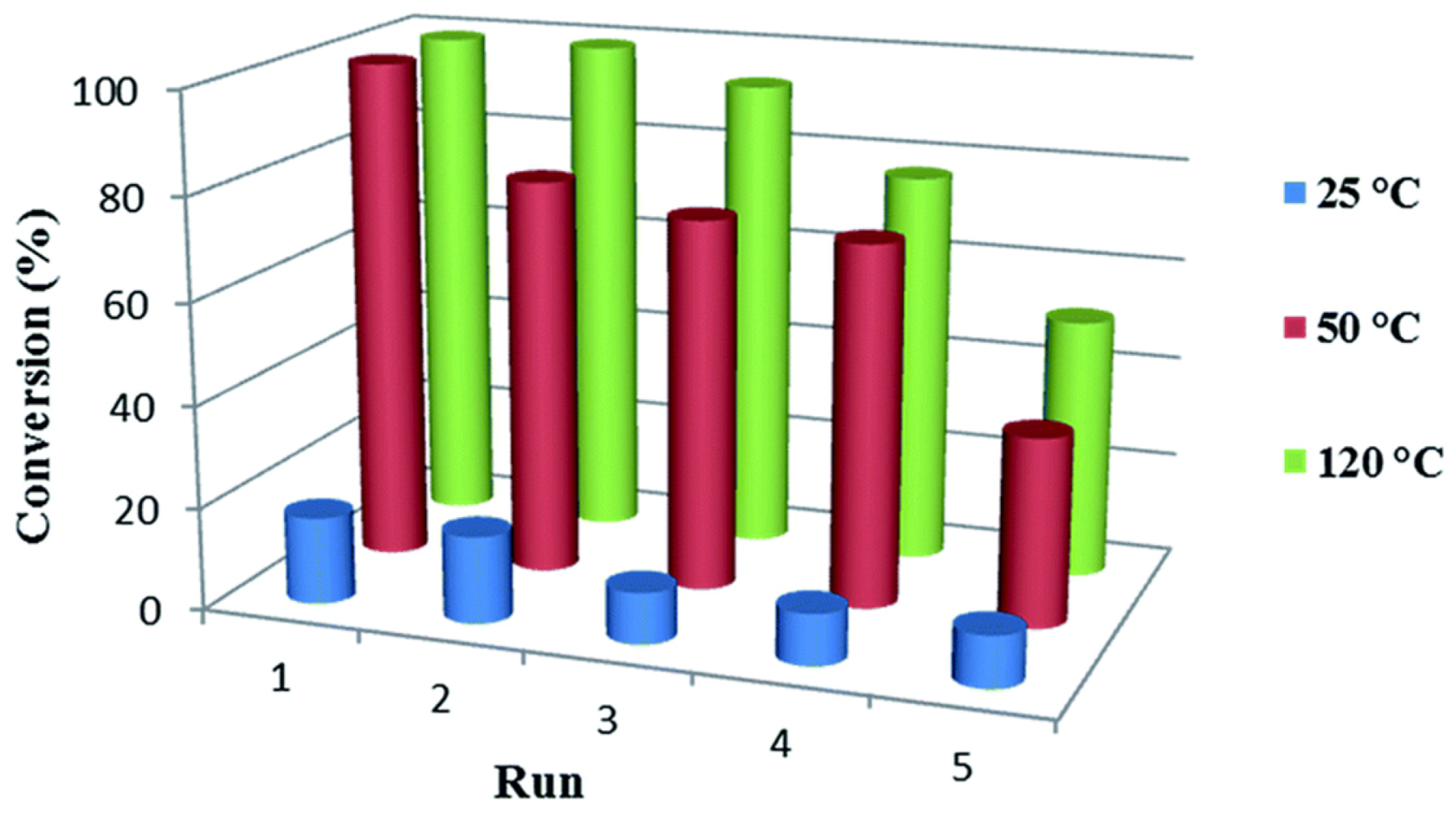
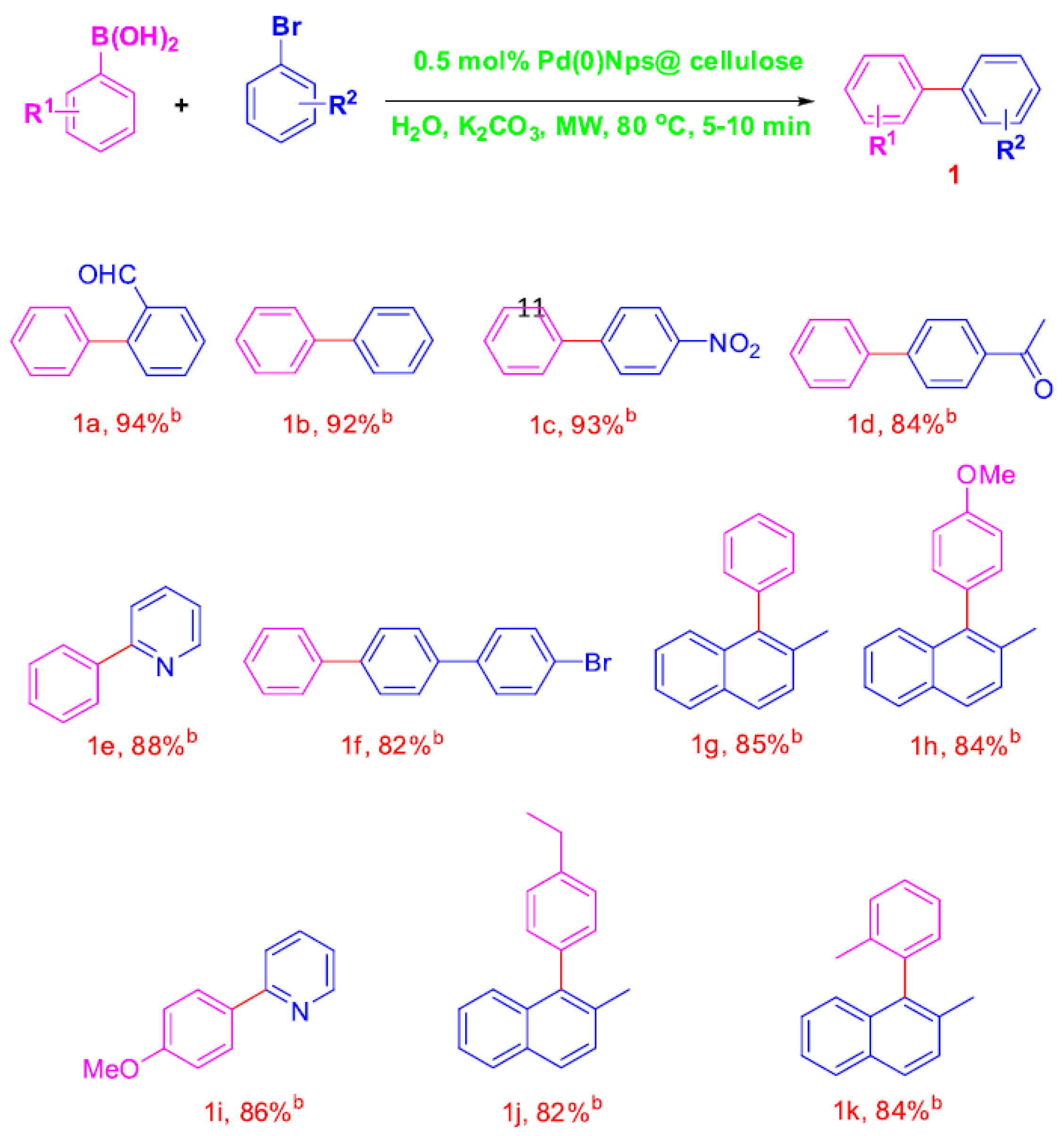
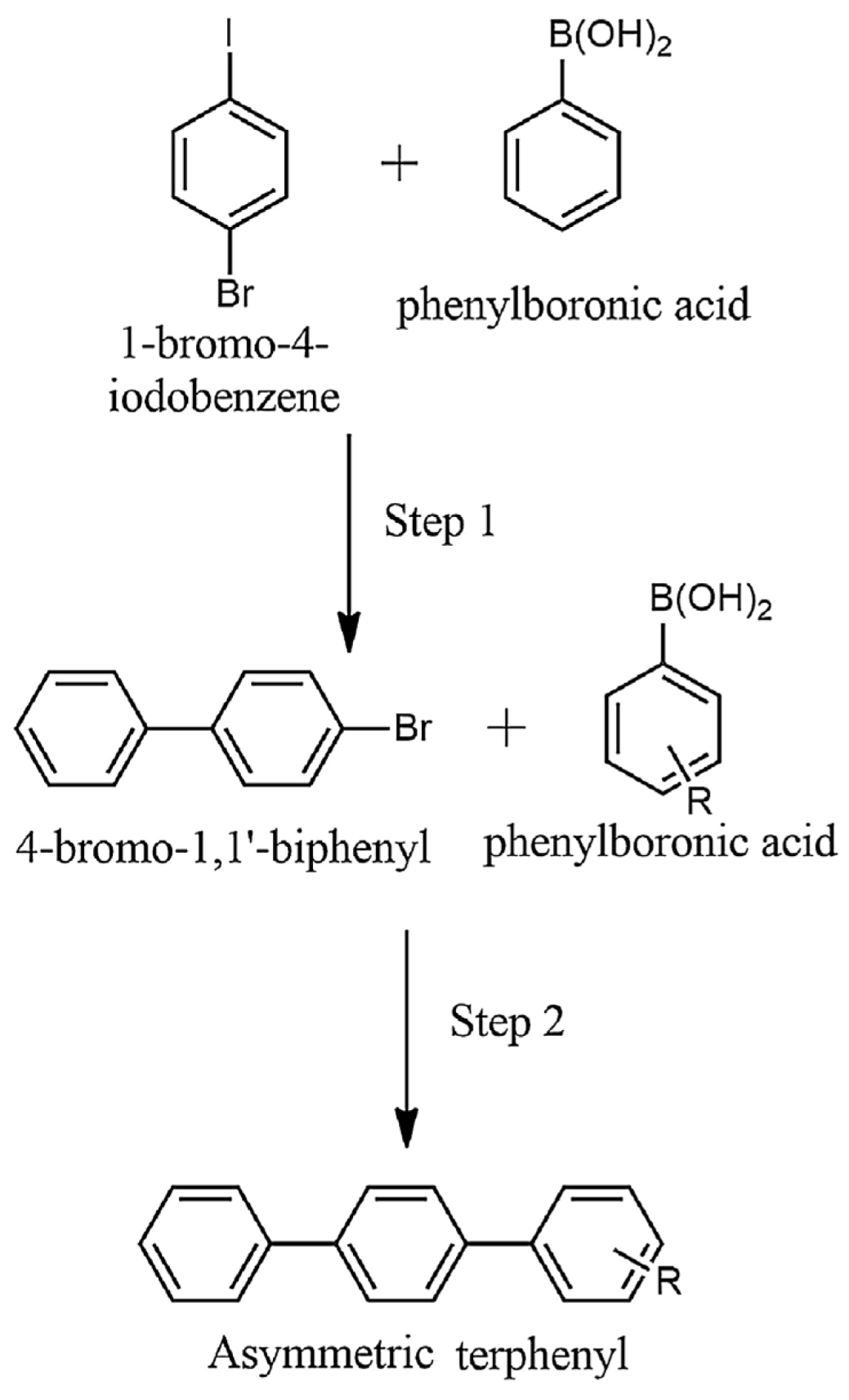
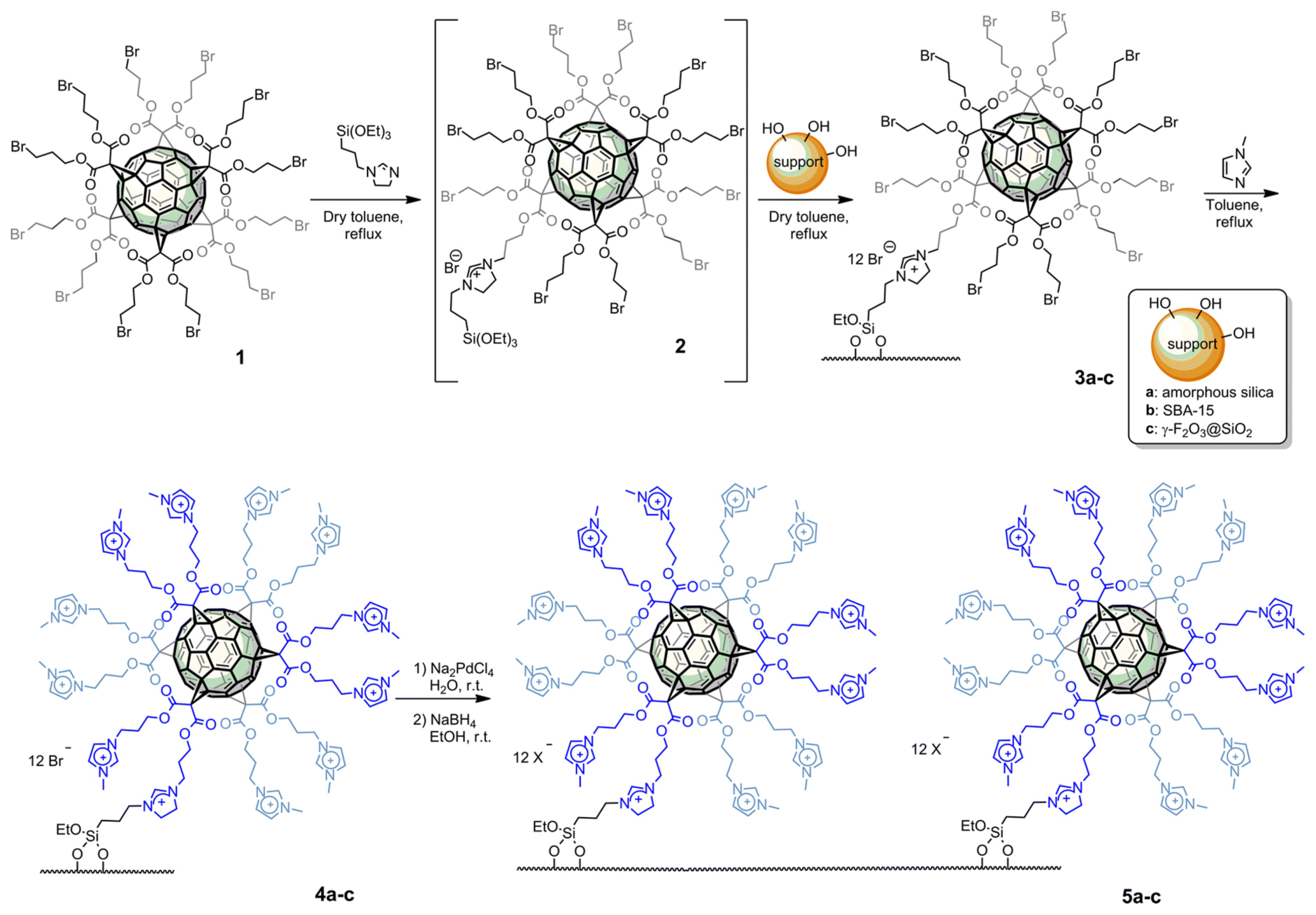
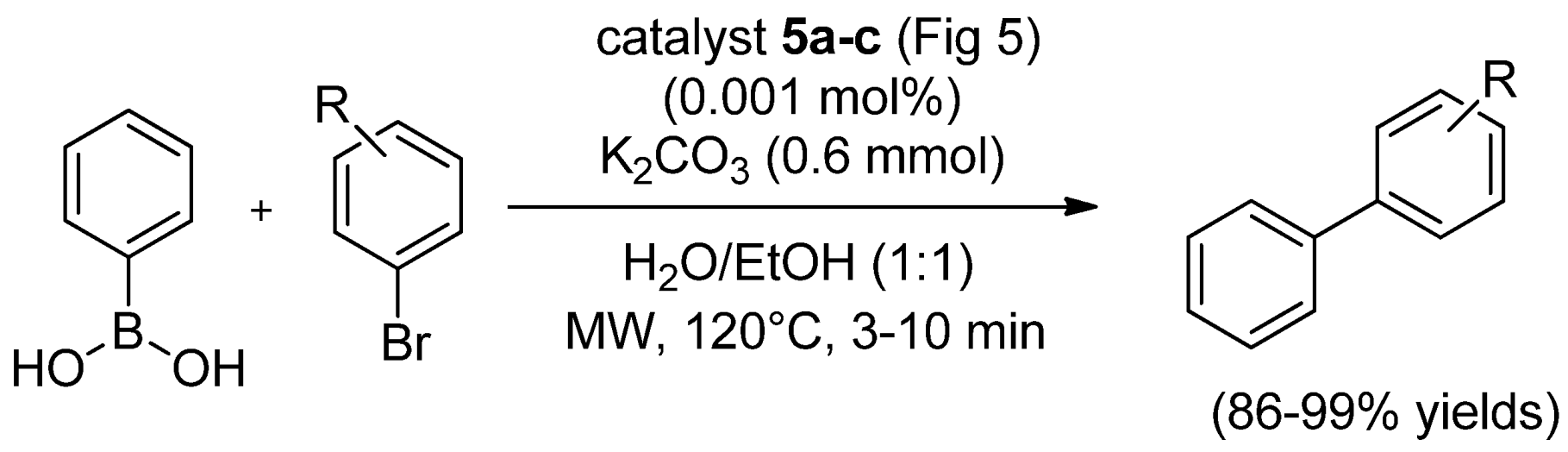
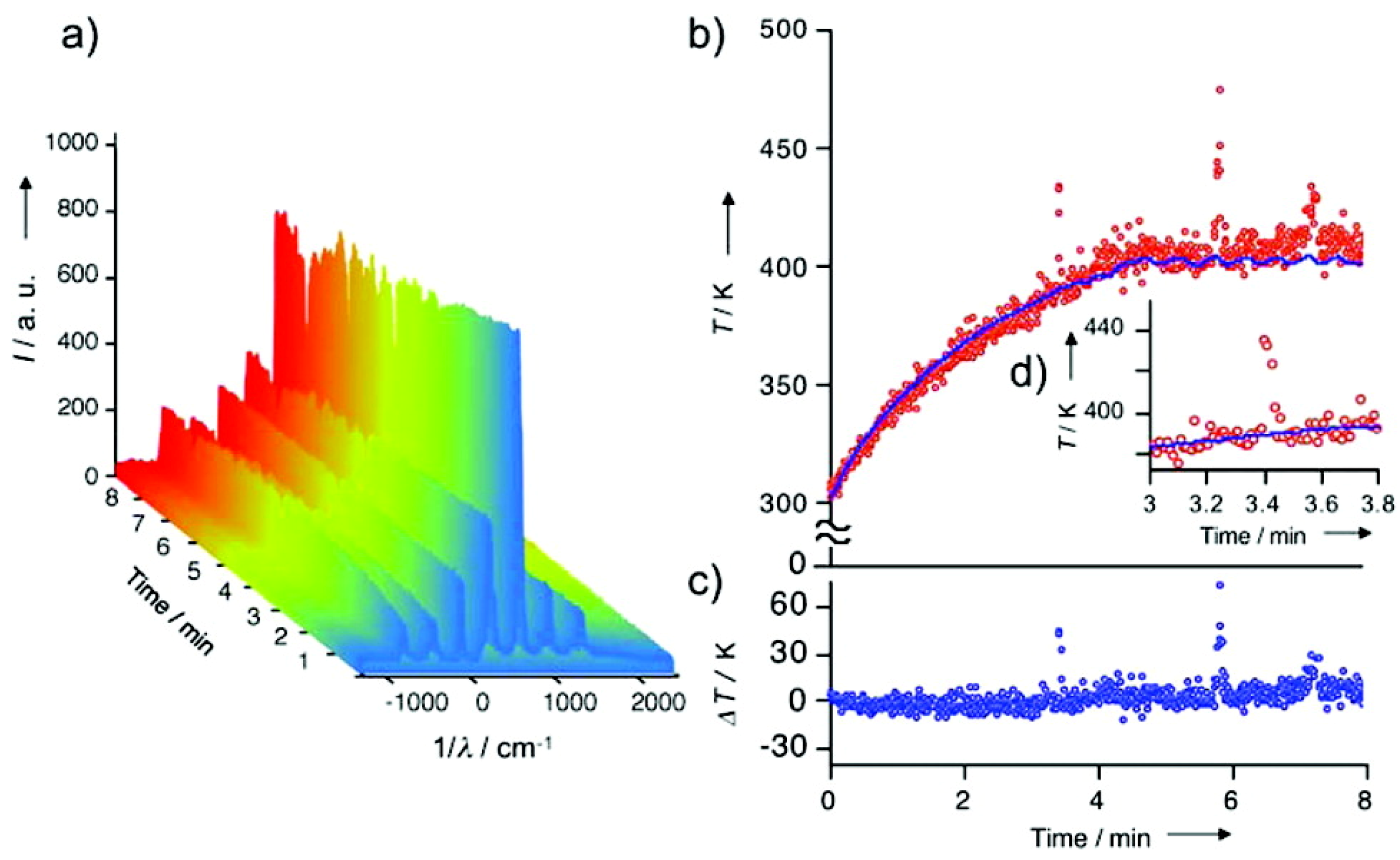
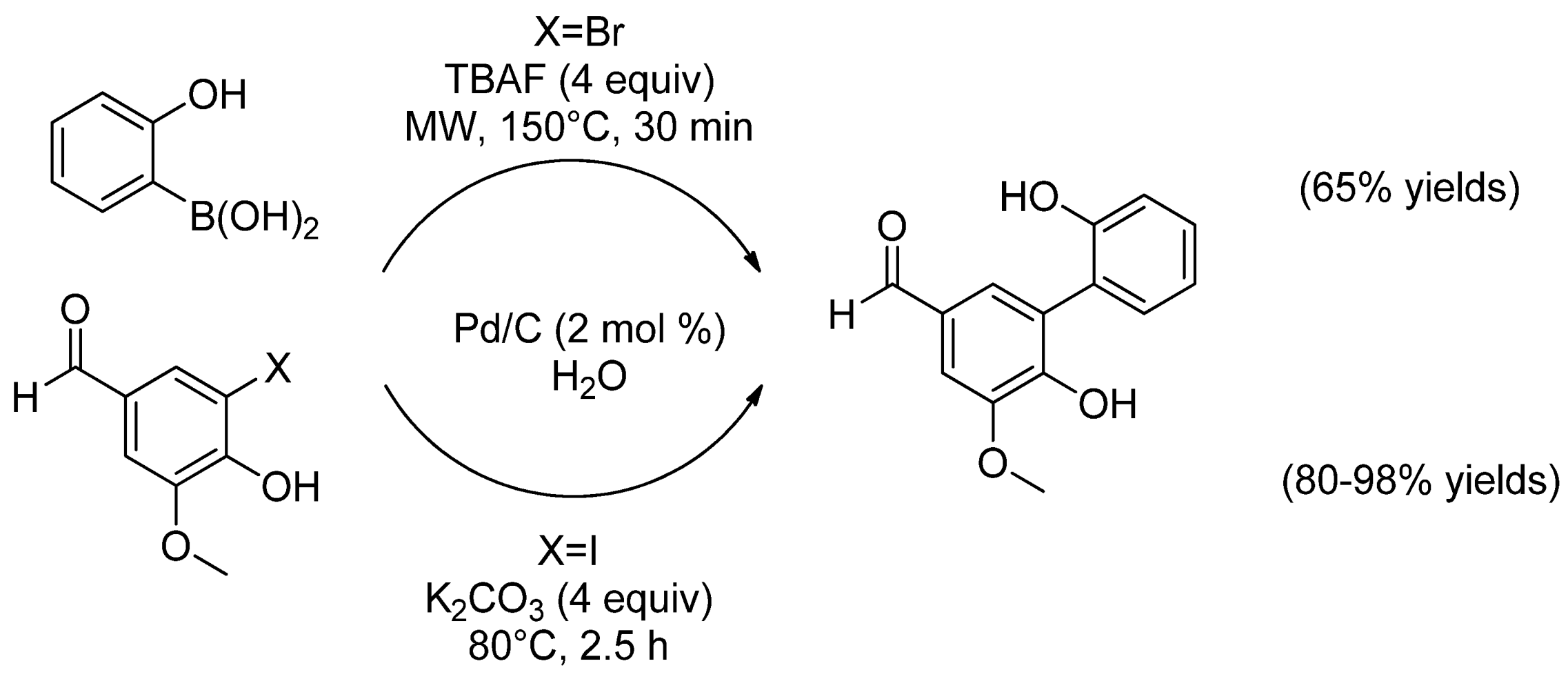
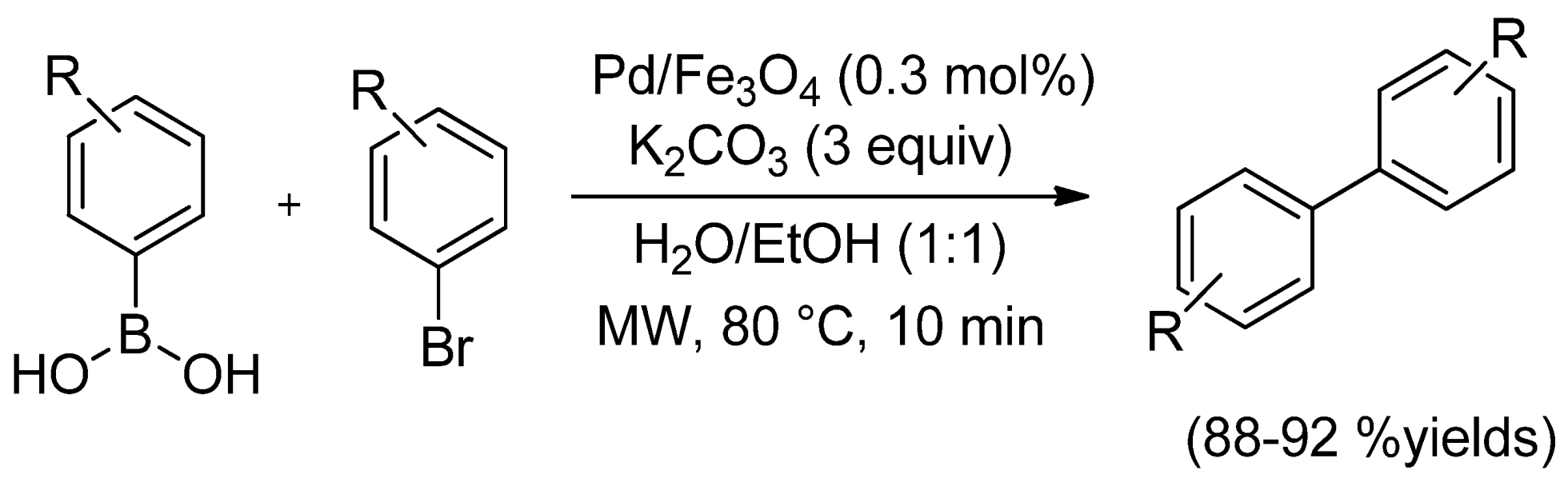
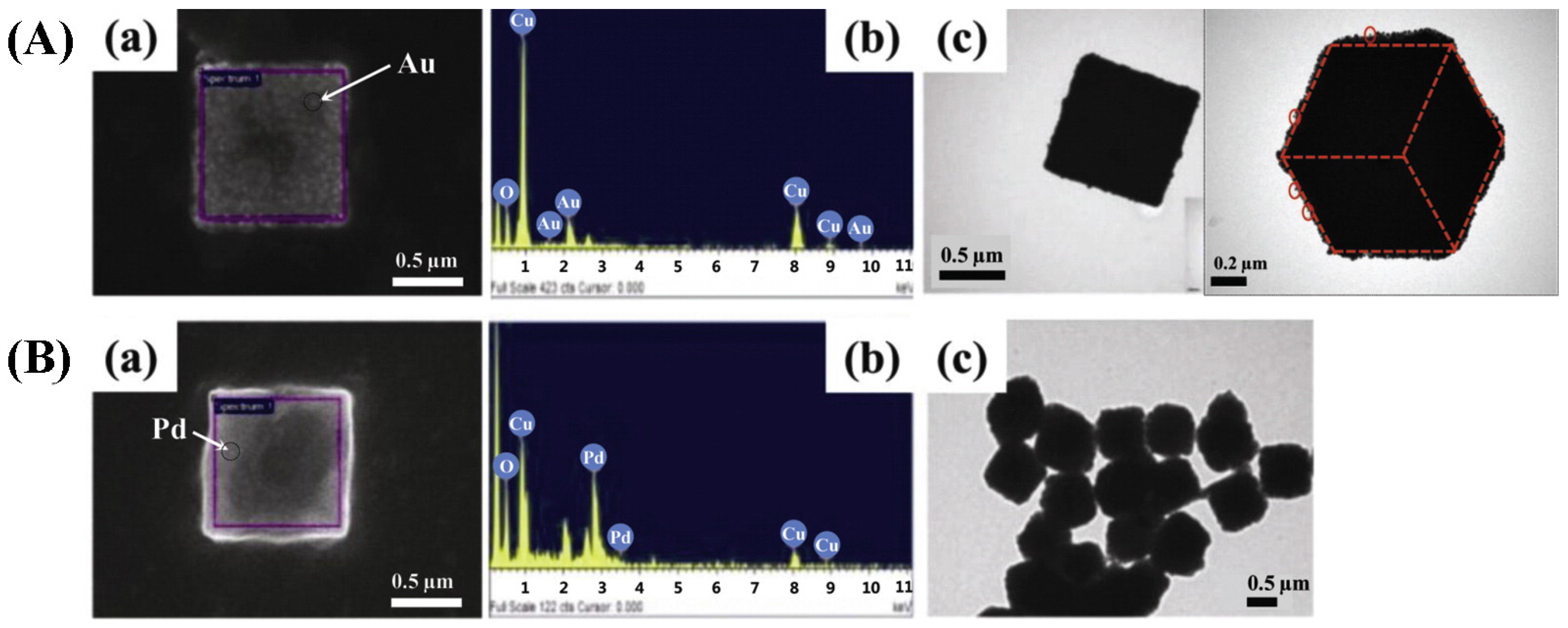

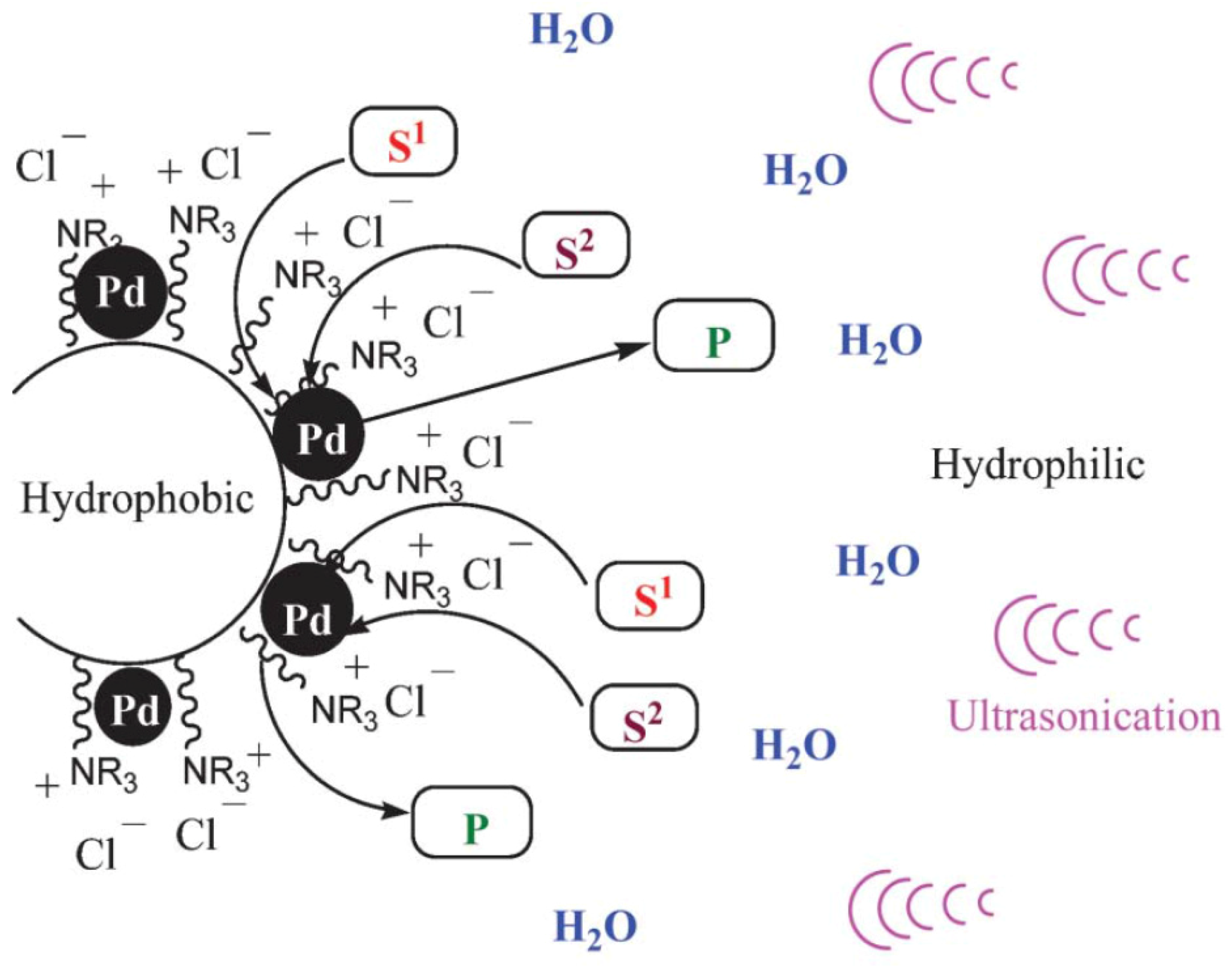
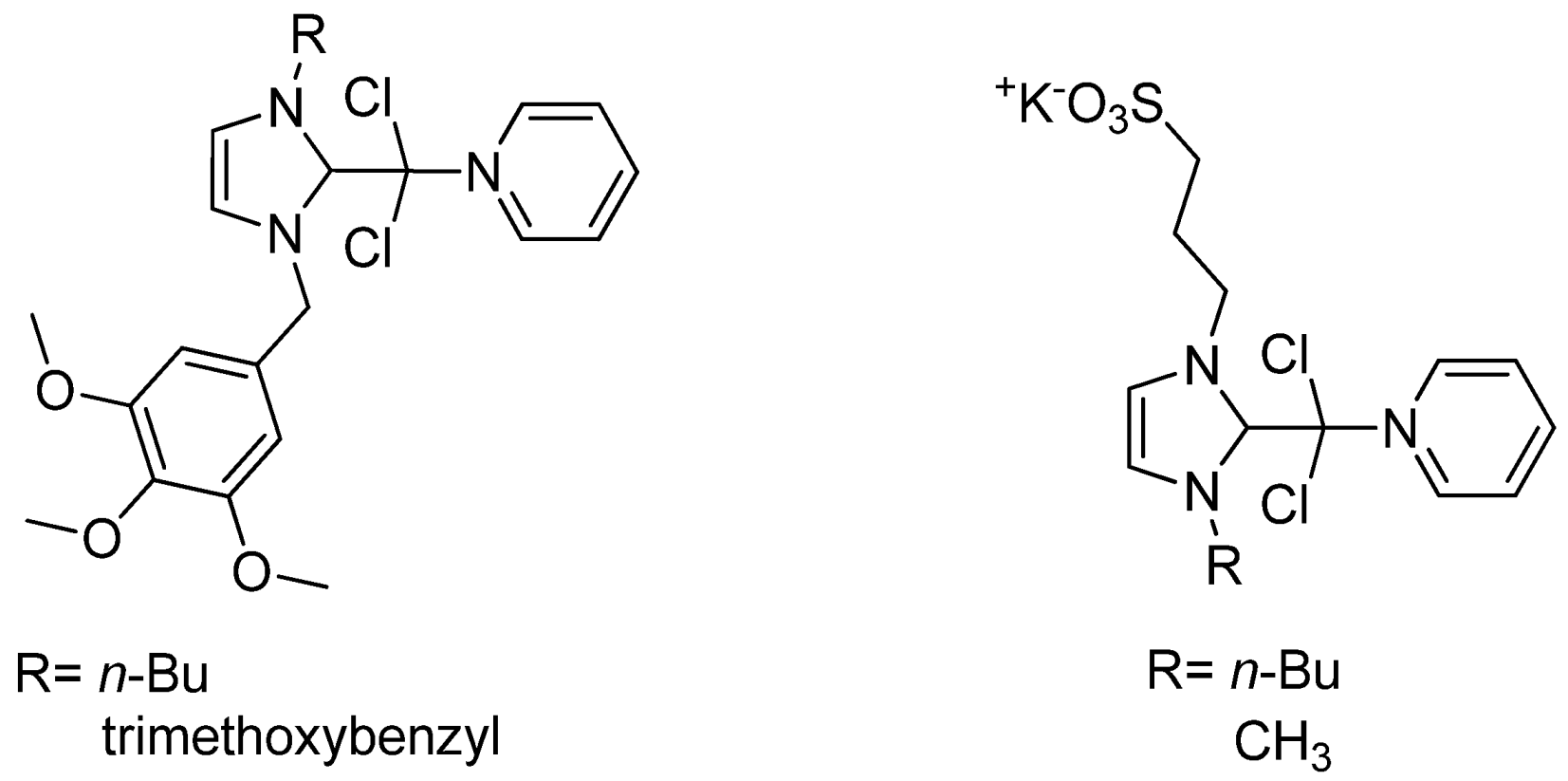
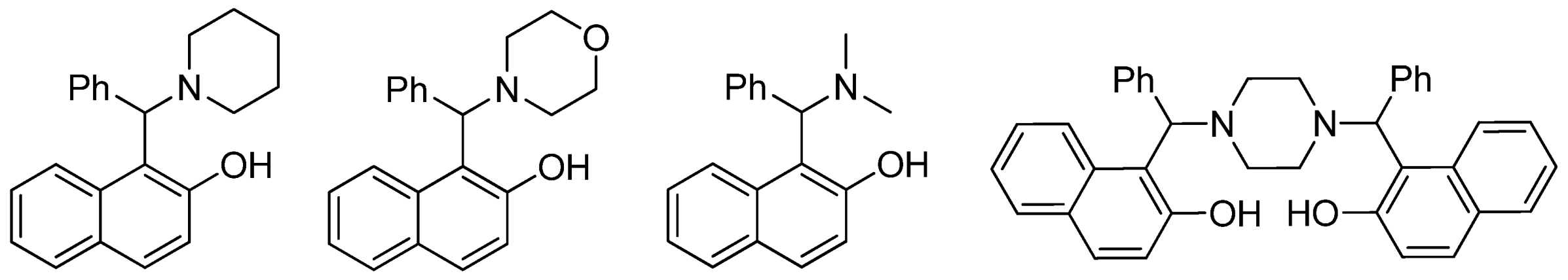
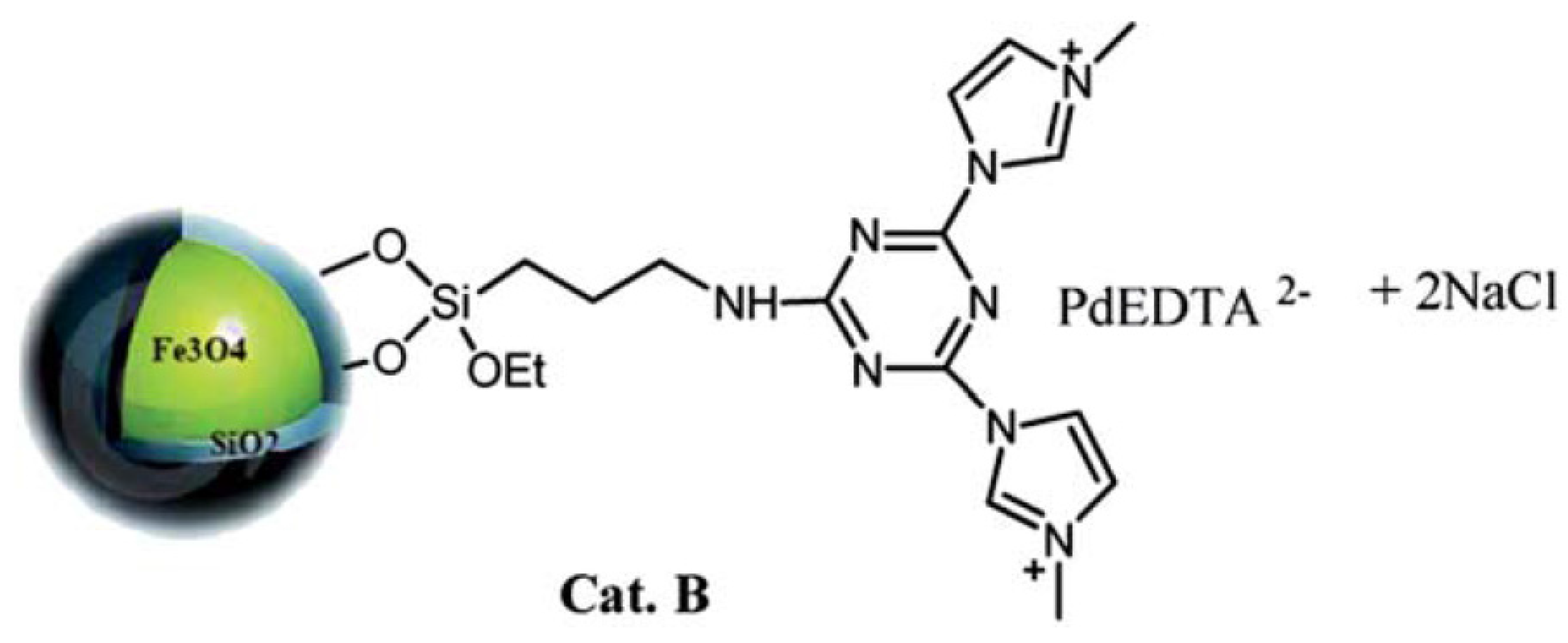
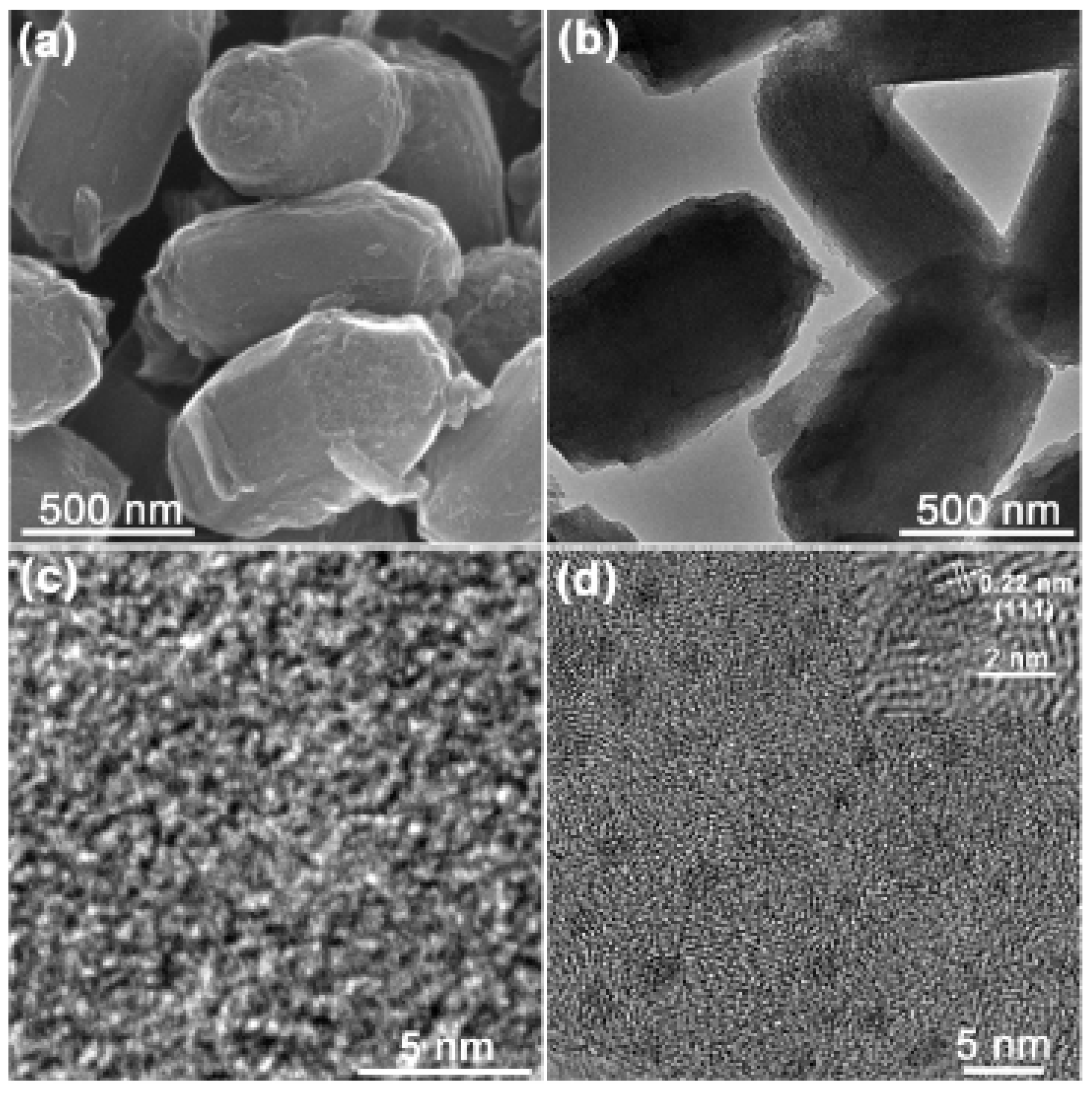
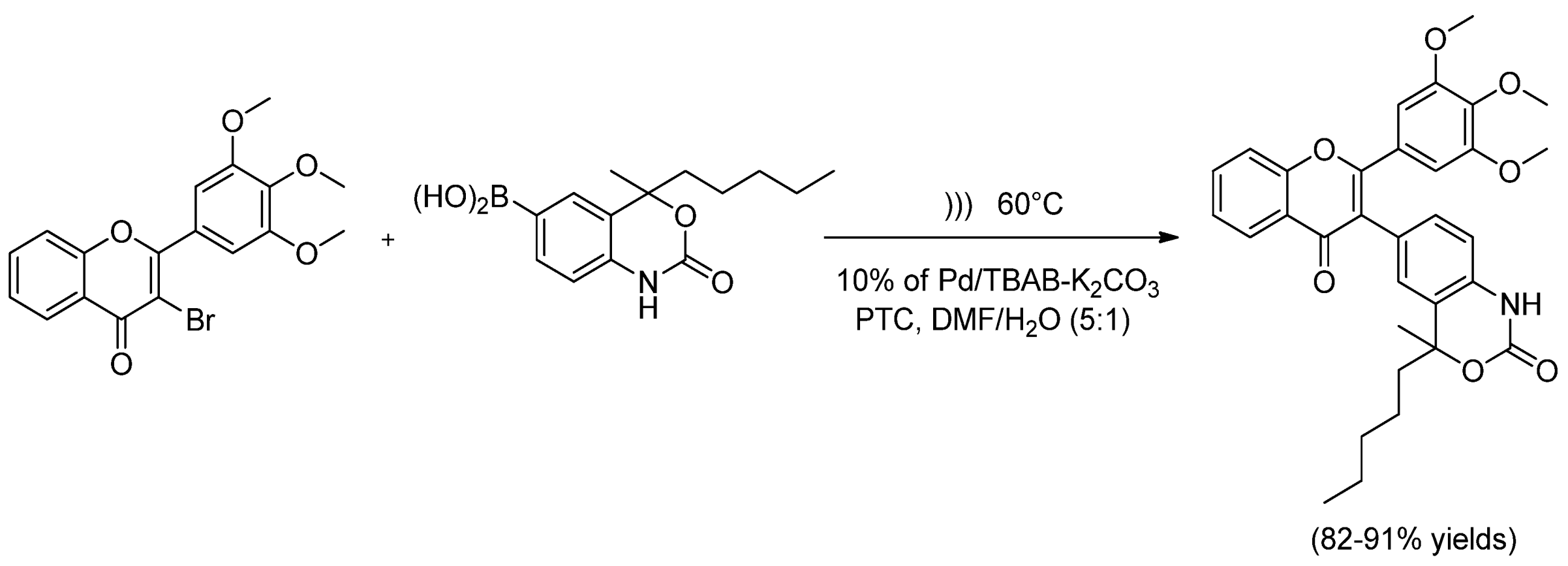
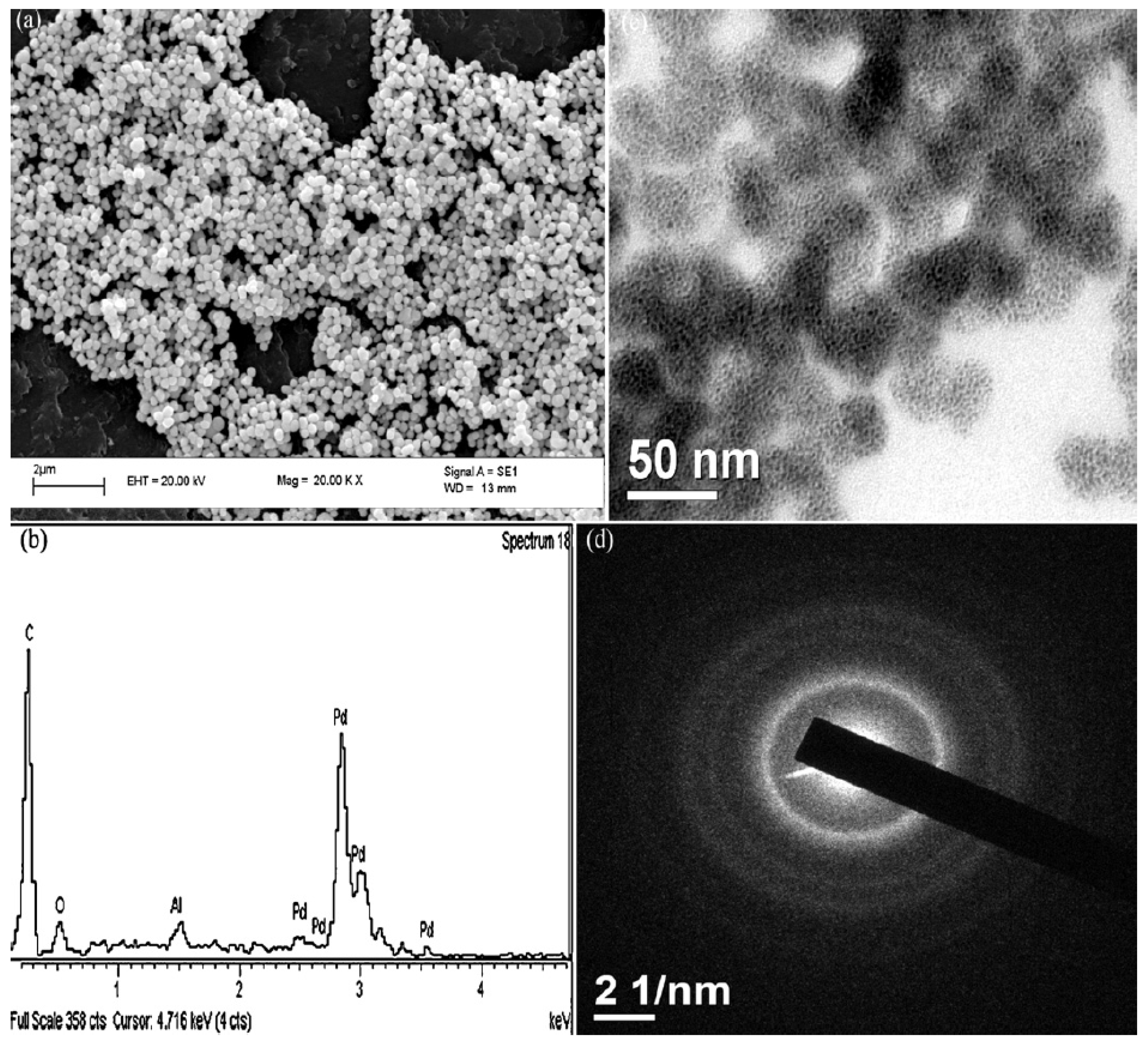
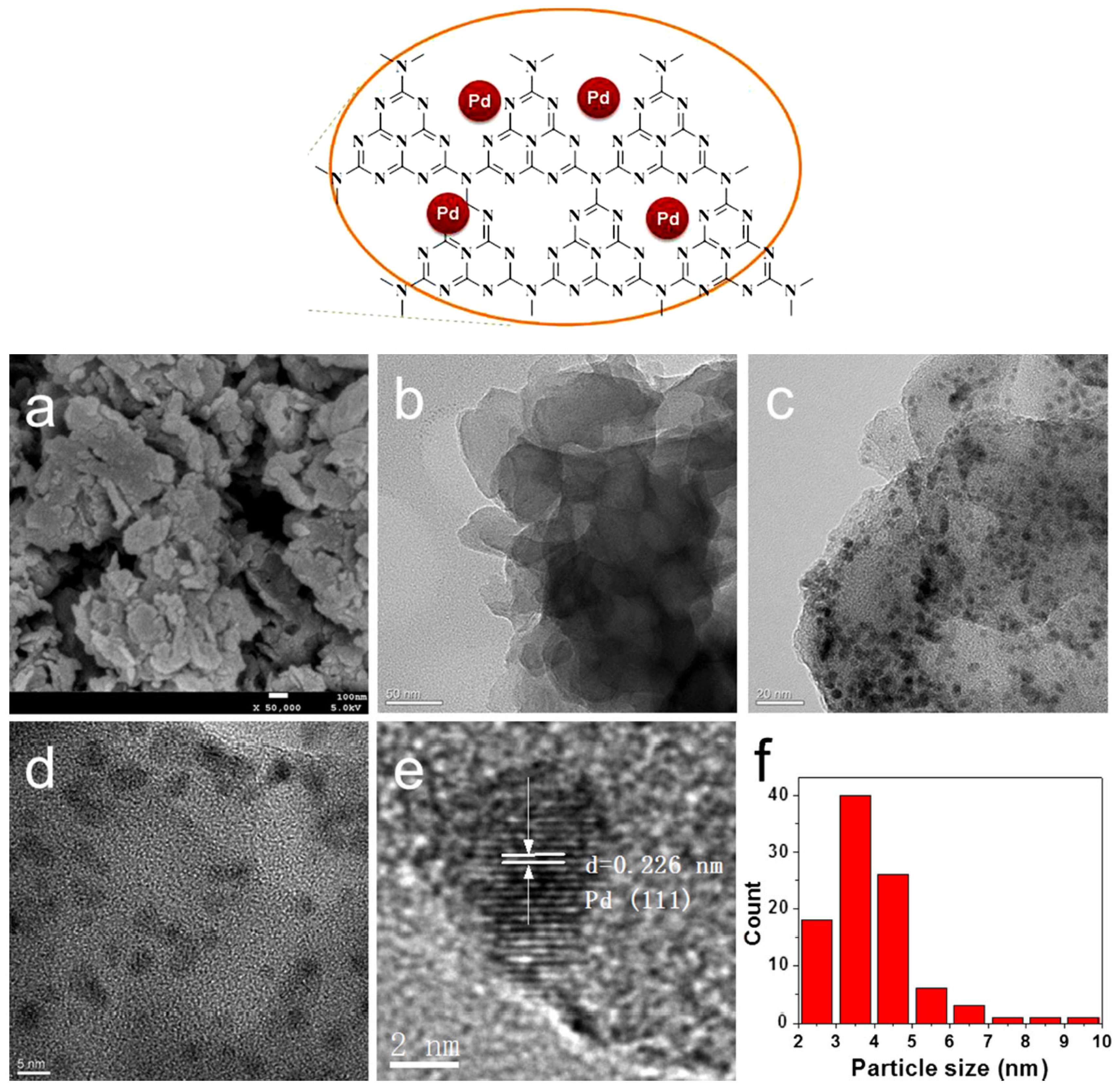
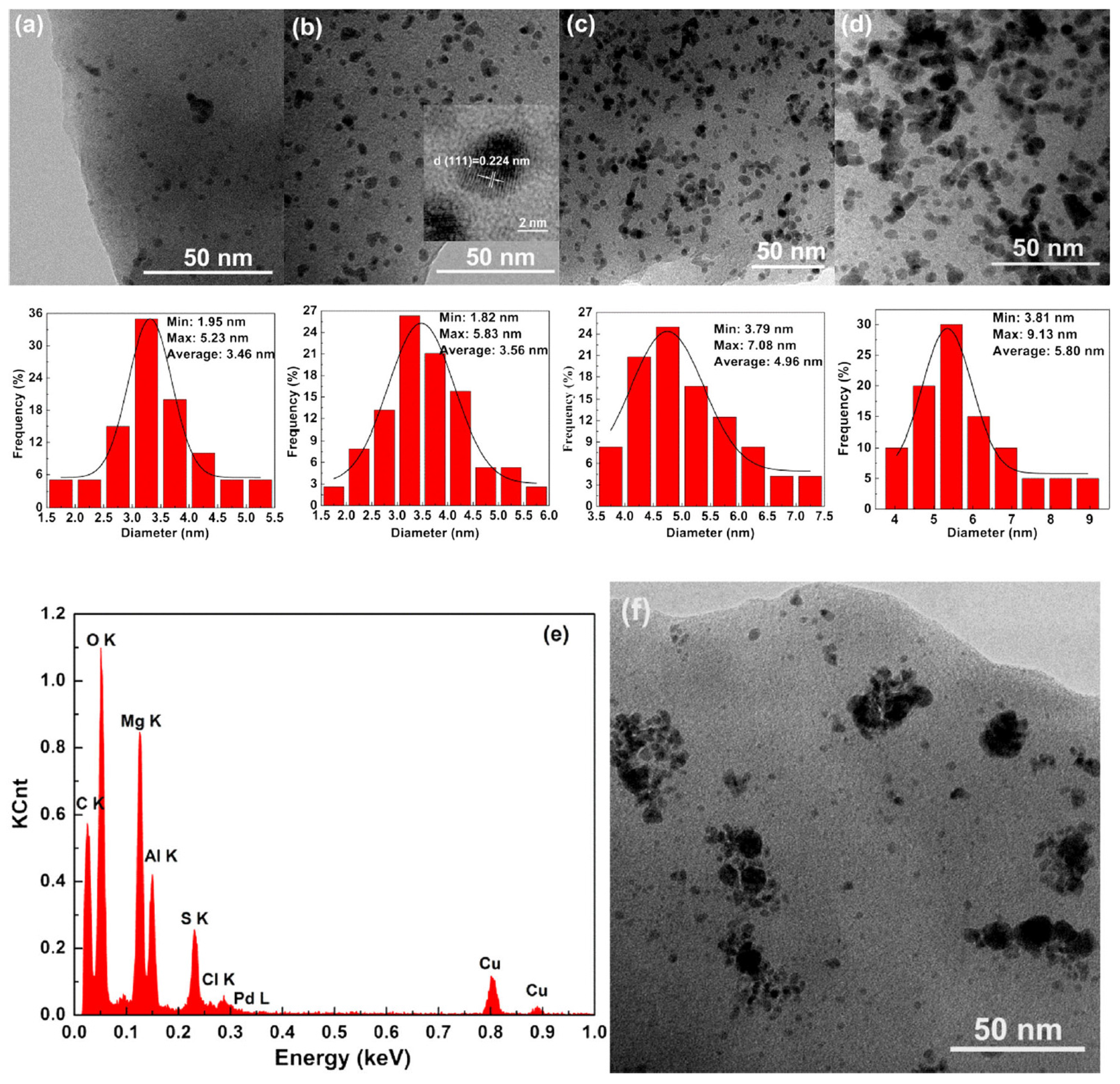

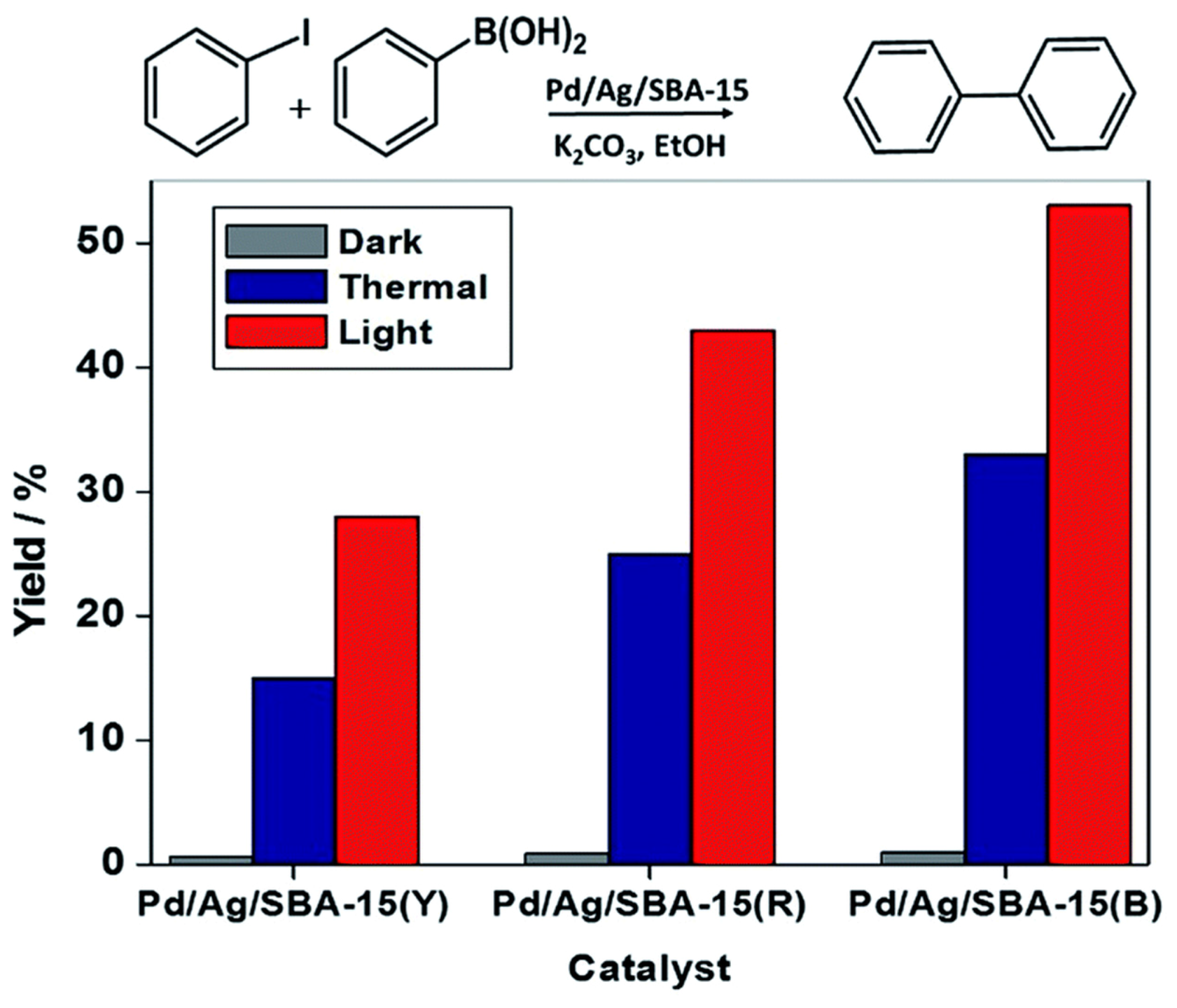
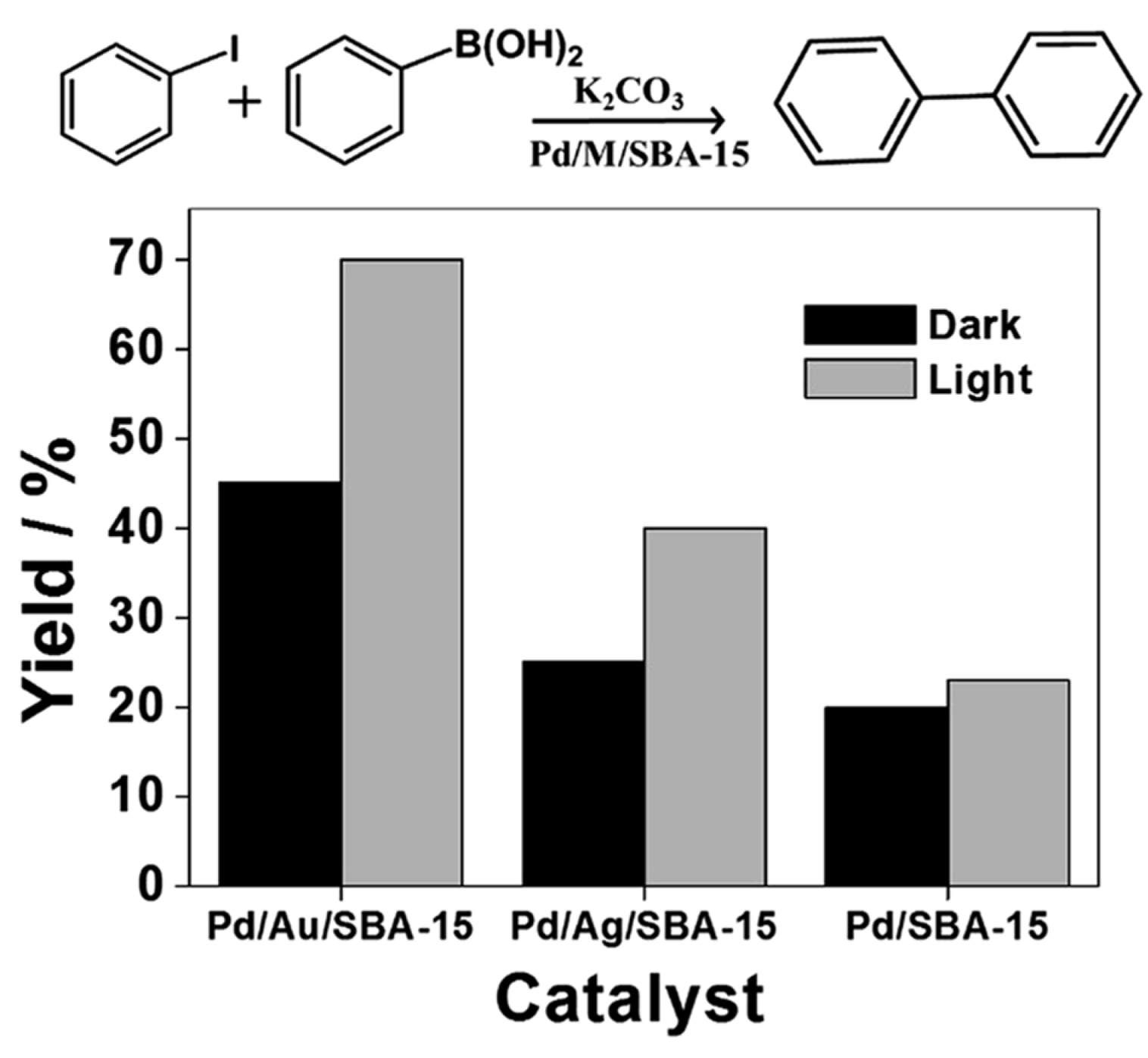


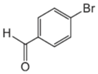
| Cat/Ligand/Base | Solvent | Yields (%) |
|---|---|---|
| Pd(PPh3)4–NaHCO3 | DMF | 0 |
| Pd(PPh3)4–NaOH | 1:1 DMF–H2O | 47 |
| Pd(dba)2–dtbpf–K3PO4 | 1:1 DMF–H2O | 43 |
| Pd(OAc)2–dtbpf–K3PO4 | 1:1 DMF–H2O | 63 |
| Pd(OAc)2–dtbpf–K3PO4 | 1:1 EtOH–H2O | 90 |
| Pd(OAc)2–dtbpf–K3PO4 | H2O | 72 |
| Pd(OAc)2–K2CO3 | H2O | 37 |
| Method 1 | T (min) | Yield (%) | ||
|---|---|---|---|---|
| Pd(OAc)2 | PdCl2 | Pd/C | ||
| OB 2 | 60 | 85 | 75 | 44 |
| US/OB | 60 | 99 | 98 | 86 |
| MW | 15 | 57 | 70 | 60 |
| MW | 60 | 74 | 92 | 67 |
| US/MW | 60 | 100 | 98 | 94 |
| Ar-X | Yield (%) 2 |
|---|---|
| 4-Bromoacetophenone | >99 |
| 3-Bromoacetophenone | 80 |
| 4-Bromobenzaldehyde | >99 |
| 3-Bromobenzaldehyde | >99 |
| 4-Bromoanisole | >99 |
| 3-Bromoanisole | 90 |
| 4-Bromotoluene | >99 |
| 2-Bromotoluene | >99 |
| 4-Bromoaniline | 78 |
| 2-Bromobenzonitrile | >99 |
| 3,5-Bis(trifluoromethyl)bromobenzene | >99 |
| 1-Bromo-2,4,6-triisopropylbenzene | <5 |
| 1-Bromo 4-nitrobenzene | >99 |
| 4-Iodoacetophenone | >99 |
| 4-Iodotoluene | >99 |
| Methyl 4-iodobenzoate | >99 |
| 2-Chlorobenzaldehyde | 14 |
| 4-Chlorobenzaldehyde | 33 |
| Entry | Catalyst | R | T (°C) | T (min) | Conv. 2 (%) | TON 3 | TOF 4 (h−1) |
|---|---|---|---|---|---|---|---|
| 1 | 5b | 4-COCH3 | 120 ∆ 5 | 10 | >99 | 100,000 | 600,000 |
| 2 | 5b | 4-COCH3 | 120 MW | 10 | >99 | 100,000 | 600,000 |
| 3 | 5b | 4-COCH3 | 120 ∆ | 3 | 88 | 88,000 | 1,760,000 |
| 4 | 5b | 4-COCH3 | 120 MW | 3 | >99 | 100,000 | 2,000,000 |
| 5 | 5a | 4-COCH3 | 120 MW | 3 | >99 | 100,000 | 2,000,000 |
| 6 | 5b | 3-OCH3 | 120 ∆ | 10 | 56 | 56,000 | 336,000 |
| 7 | 5b | 3-OCH3 | 120 MW | 3 | 73 | 73,000 | 1,460,000 |
| 8 6 | 5b | 4-COCH3 | 120 MW | 3 | 91 | 182,000 | 3,640,000 |
| Cycle | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Yield (%) | MW 1 | >99 | >99 | >99 | >99 | 91 | 95 | 97 | >99 |
| ∆ 1 | >99 | >99 | >99 | 90 | 83 | 58 | 44 | 40 | |
| Aryl-Halide | Boronic Acid | Isolated Yields |
|---|---|---|
 | 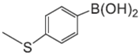 | 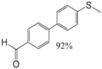 |
 | 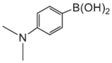 |  |
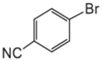 |  | 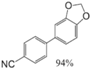 |
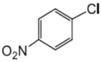 | 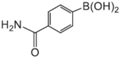 | 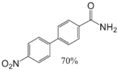 |
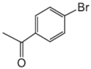 | 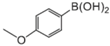 |  |
 | 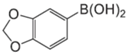 | 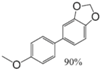 |
| Energy Source | Reaction Time (min) | Power (W) | Energy Density (kJ/mmol) | Catalyst Loading (mol %) | Yields (%) |
|---|---|---|---|---|---|
| SWA | 25 | 8 | 12 | 0.08 | 89 |
| MW | 17 | 5 | 5.1 | 0.8 | 72 |
| US | 10 | 120 | 144 | 0.9 | 85 |
| Catalyst | Loading Conditions | Technology | Pd Loading (wt %) | SMC Synthetic Protocol | Reference |
|---|---|---|---|---|---|
| Magnetically separable mesoporous SBA-15 nanocomposites | 1. SBA-15 silica support, Fe(NO3)3·9H2O 10 min at 350 rpm. 2. propionic acid at 85 °C for 3 h | Retsch PM-100 planetary ball mill 18 stainless steel balls (10 mm) | 19.2% | Bromobenzene, phenylboronic acid, Pd catalyst K2CO3, H2O, MW 150 °C, 20 min (59% yield) | [151] |
| Palladium nanoparticles supported on carbon nanotubes | MWCNT powder/Pd(OAc)2 (10:1) mechanical shaking for 30 min | Ball-mill mixer (SPEX CertiPrep 8000D), two ceramic balls (d ¼ 1.3 cm), 1060 lateral cycles per minute. | 9% | Bromobenzene, phenylboronic, Pd/MWCNT (Multiwall Carbon Nanotubes) (0.5 mol %) K2CO3, H2O–EtOH (1:1), MW 80 °C for 10 min (100% conv.) | [152] |
| Monodispersed Pd in ascorbic acid | Pd(NO3)2∙2H2O, and Ascorbic Acid (1.0568 g, 6 mmol) 10 min | Mortar | - | Phenyl iodide, phenylboronic acid, Na2CO3, TBAB, Pd (10 mol %), H2O, 80 °C, 24 h (Yield 97%) | [153] |
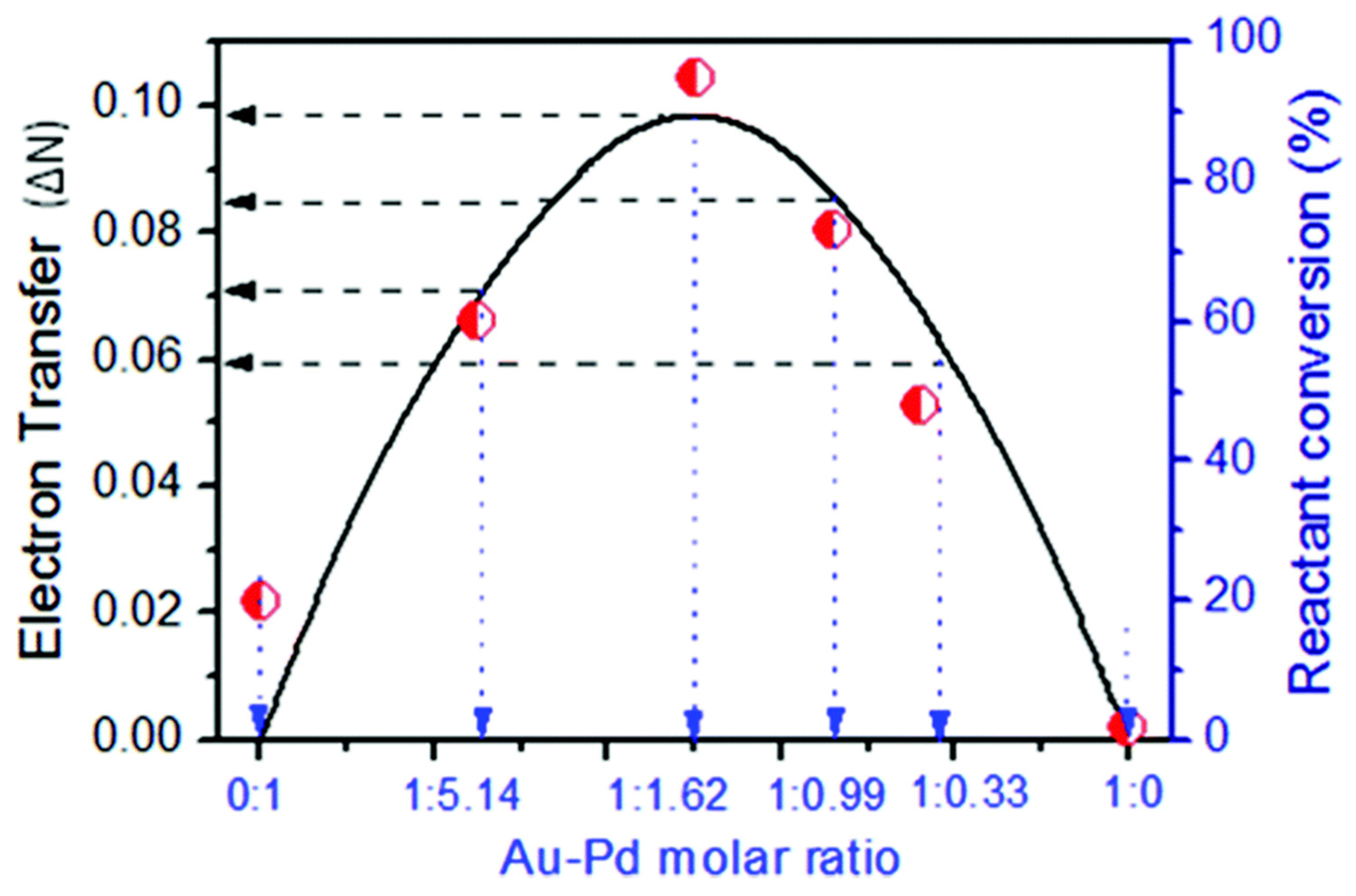
| R1 | R2 | Au/Pd 1 | Yield (%) | Selectivity (%) | TON | TOF (h−1) | Q.Y. 7 (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | ||||
| 3-CH3 | 4-H | 1:1.86 | 96 2 | 37 | 99 | 99 | 87 | 34 | 14.5 | 5.7 | 2.7 |
| 1:1.00 | 55 2 | 17 | 98 | 99 | 56 | 17 | 9.3 | 2.8 | 1.7 | ||
| 1:5.58 | 40 2 | 10 | 99 | 99 | 34 | 8 | 5.7 | 1.3 | 1.4 | ||
| 1:0.62 | 28 2 | 6 | 99 | 100 | 33 | 6 | 5.5 | 1.0 | 1.0 | ||
| 1:0 | 2 2 | 0 | 100 | - | 3 | 0 | 0.5 | 0 | 0.1 | ||
| 0:1 | 26 2 | 11 | 98 | 99 | 18 | 8 | 3.0 | 1.3 | 0.7 | ||
| 4-CH3 | 4-H | 1:1.86 | 94 2 | 46 | 98 | 97 | 95 | 46 | 15.8 | 7.7 | 2.2 |
| 2-CH3 | 4-H | 1:1.86 | 86 2 | 30 | 98 | 98 | 87 | 30 | 14.5 | 5.0 | 2.6 |
| 4-OCH3 | 4-H | 1:1.86 | 96 3 | 41 | 98 | 99 | 97 | 41 | 19.4 | 8.2 | 2.5 |
| 4-H | 4-OCH3 | 1:1.86 | 99 4 | 58 | 99 | 99 | 100 | 99 | 50.0 | 0.0 | 1.9 |
| 4-H | 4-CHO | 1:1.86 | 98 5 | 65 | 99 | 96 | 99 | 67 | 24.8 | 0.0 | 1.5 |
| 4-H | 4-N(CH3)2 | 1:1.86 | 80 6 | 55 | 68 | 60 | 81 | 56 | 3.0 | 0.0 | 0.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martina, K.; Manzoli, M.; Gaudino, E.C.; Cravotto, G. Eco-Friendly Physical Activation Methods for Suzuki–Miyaura Reactions. Catalysts 2017, 7, 98. https://doi.org/10.3390/catal7040098
Martina K, Manzoli M, Gaudino EC, Cravotto G. Eco-Friendly Physical Activation Methods for Suzuki–Miyaura Reactions. Catalysts. 2017; 7(4):98. https://doi.org/10.3390/catal7040098
Chicago/Turabian StyleMartina, Katia, Maela Manzoli, Emanuela Calcio Gaudino, and Giancarlo Cravotto. 2017. "Eco-Friendly Physical Activation Methods for Suzuki–Miyaura Reactions" Catalysts 7, no. 4: 98. https://doi.org/10.3390/catal7040098