Highly Dispersed PdNPs/α-Al2O3 Catalyst for the Selective Hydrogenation of Acetylene Prepared with Monodispersed Pd Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
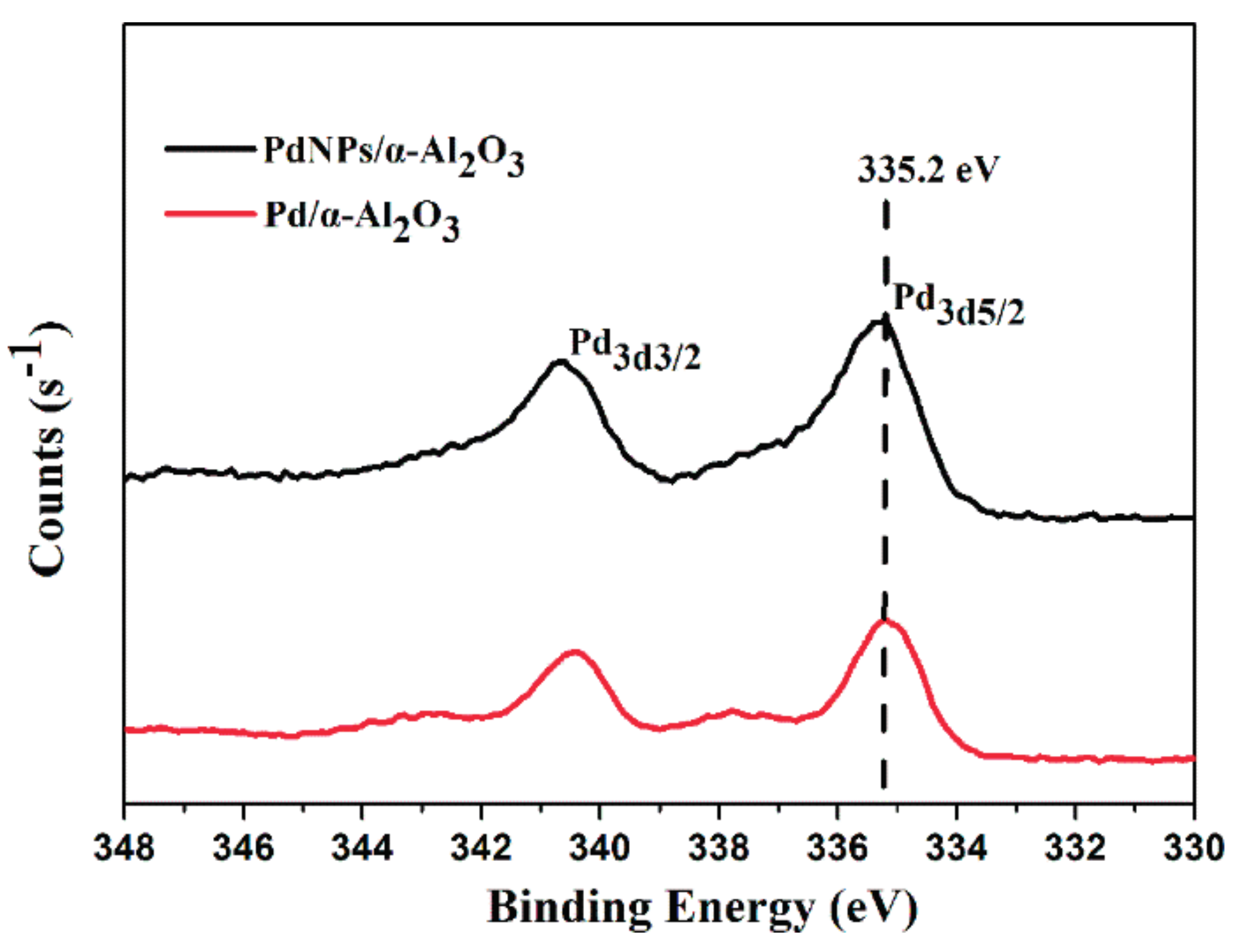
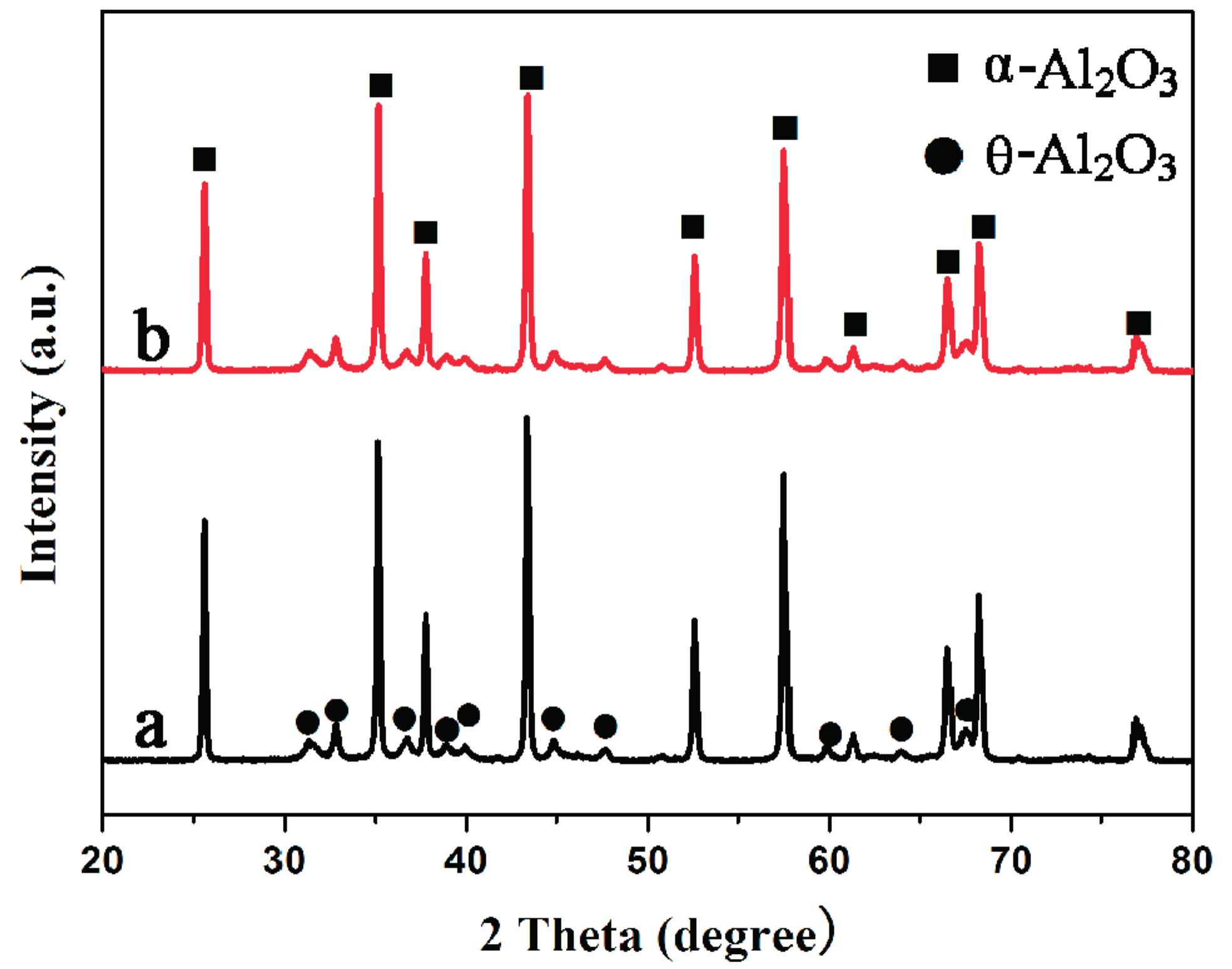
2.1. Catalyst Characterization
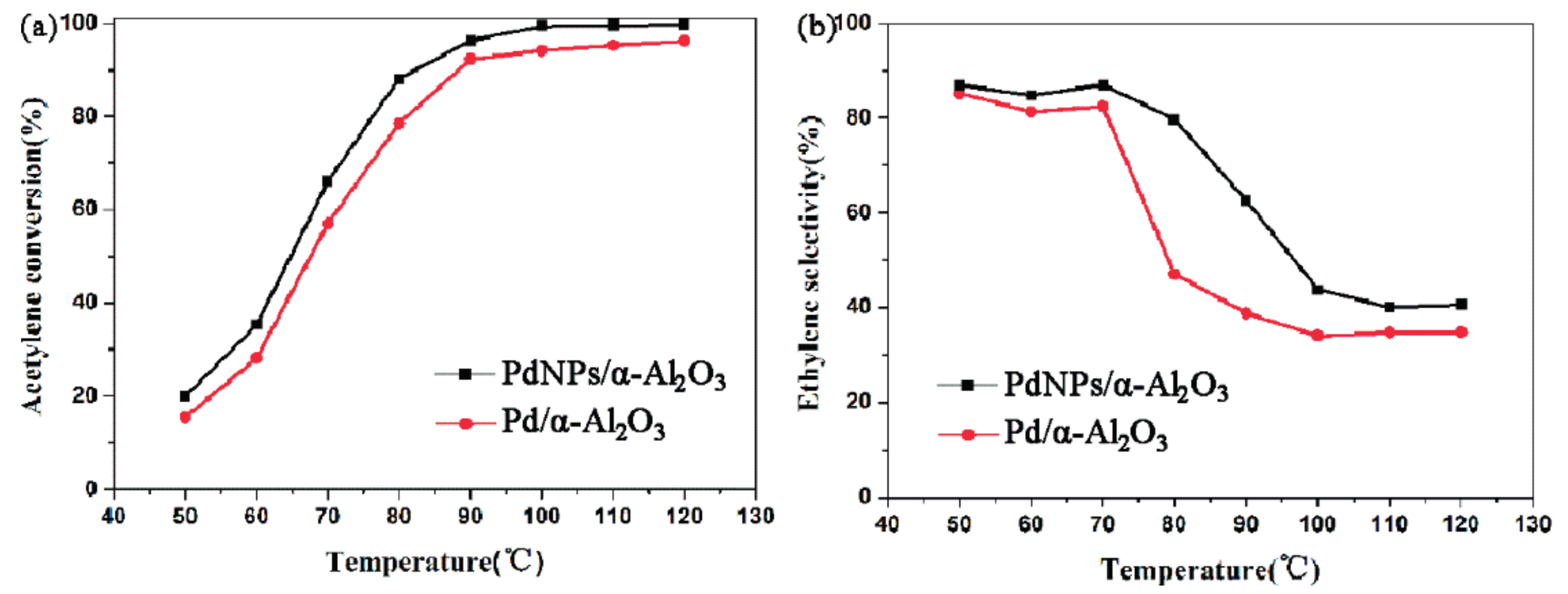
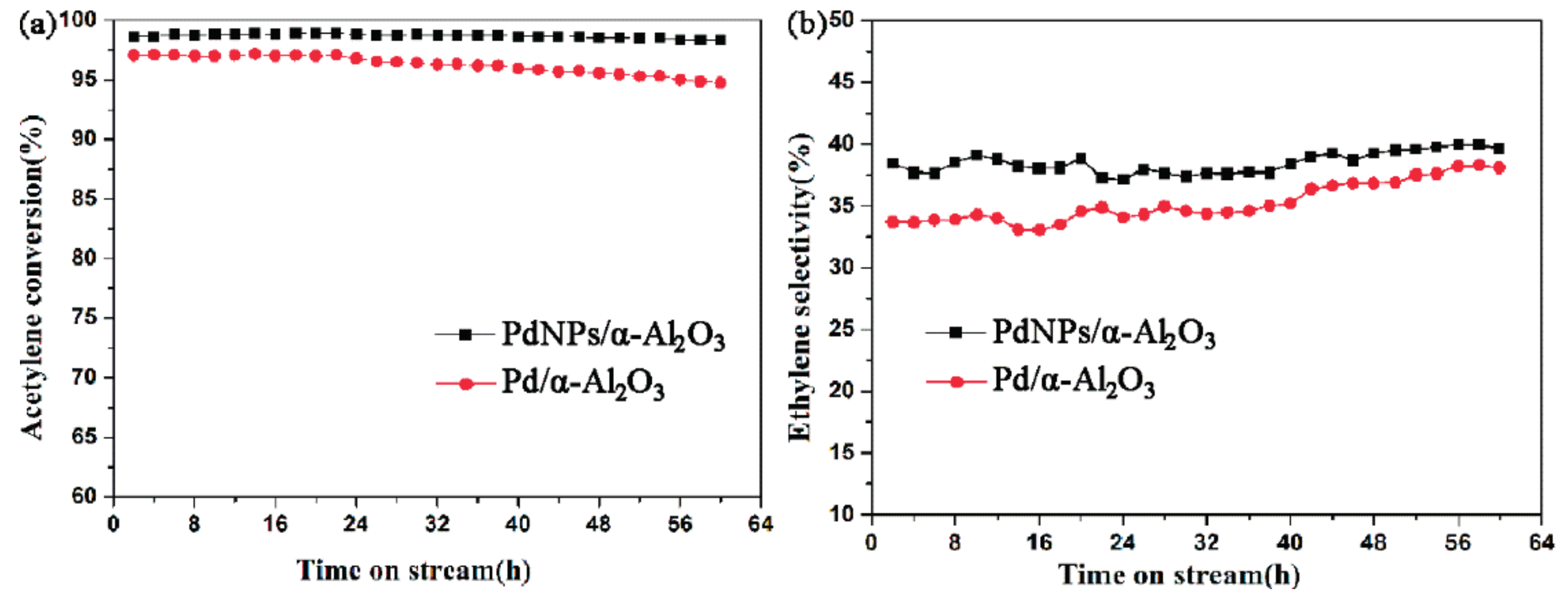
2.2. Catalytic Performance
3. Materials and Methods
3.1. Synthesis
3.2. Characterizations
3.3. Catalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Borodziński, A.; Bond, G.C. Selective hydrogenation of ethyne in ethane-rich streams on palladium catalysts, Part 1: Effect of changes to the catalyst during reaction. Catal. Rev. 2006, 48, 91–144. [Google Scholar] [CrossRef]
- Borodziński, A.; Bond, G.C. Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts, Part 2: Steady-state kinetics and effects of palladium particle size, carbon monoxide, and promoters. Catal. Rev. 2008, 50, 379–469. [Google Scholar] [CrossRef]
- Árpád, M.; Sárkány, A.; Varga, M. Hydrogenation of carbon-carbon multiple bonds: Chemo-, regio- and stereo-selectivity. J. Mol. Catal. A: Chem. 2001, 173, 185–221. [Google Scholar]
- Yang, B.; Burch, R.; Hardacre, C.; Hu, P.; Hughes, P. Importance of surface carbide formation on the activity and selectivity of Pd surfaces in the selective hydrogenation of acetylene. Surf. Sci. 2016, 646, 45–49. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, J.; Wu, B.; Dai, W.; Chen, Z.; Ma, M. An alumina-coated, egg-shell Pd/α-Al2O3@SiC catalyst with enhanced ethylene selectivity in the selective hydrogenation of acetylene. RSC Adv. 2016, 6, 57174–57182. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Sahebdelfar, S. Pd-Ag/Al2O3 catalyst: Stages of deactivation in tail-end acetylene selective hydrogenation. Appl. Catal. A: Gen. 2016, 525, 197–203. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Sahebdelfar, S.; Fard, M.R.; Fadaeerayeni, S.; Bigdeli, P. Pd-Ag/α-Al2O3 Catalyst Deactivation in Acetylene Selective Hydrogenation Process. Chem. Eng. Technol. 2016, 39, 301–310. [Google Scholar] [CrossRef]
- He, Y.; Liang, L.; Liu, Y.; Feng, J.; Ma, C.; Li, D. Partial hydrogenation of acetylene using highly stable dispersed bimetallic Pd–Ga/MgO–Al2O3 catalyst. J. Catal. 2014, 309, 166–173. [Google Scholar] [CrossRef]
- McCue, A.J.; McRitchie, C.J.; Shepherd, A.M.; Anderson, J.A. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation. J. Catal. 2014, 319, 127–135. [Google Scholar] [CrossRef]
- Zhu, B.; Jang, B.W.L. Insights into surface properties of non-thermal RF plasmas treated Pd/TiO2 in acetylene hydrogenation. J. Mol. Catal. A: Chem. 2014, 395, 137–144. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Lee, A.F.; Isaacs, M.A.; Li, L.; Pan, X.; Yang, X.; Wang, X.; Tai, Z.; et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal. 2015, 5, 3717–3725. [Google Scholar] [CrossRef]
- Zhang, Y.; Diao, W.; Williams, C.T.; Monnier, J.R. Selective hydrogenation of acetylene in excess ethylene using Ag-and Au–Pd/SiO2 bimetallic catalysts prepared by electroless deposition. Appl. Catal. A: Gen. 2014, 469, 419–426. [Google Scholar] [CrossRef]
- Menezes, W.G.; Altmann, L.; Zielasek, V.; Thiel, K.; Bäumer, M. Bimetallic Co–Pd catalysts: Study of preparation methods and their influence on the selective hydrogenation of acetylene. J. Catal. 2013, 300, 125–135. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.Y.; Jang, B.W.L.; Zhu, A.M. Radio-frequency H2 plasma treatment of AuPd/TiO2 catalyst for selective hydrogenation of acetylene in excess ethylene. Catal. Today 2015, 256, 161–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Diao, W.; Monnier, J.R.; Williams, C.T. Pd–Ag/SiO2 bimetallic catalysts prepared by galvanic displacement for selective hydrogenation of acetylene in excess ethylene. Catal. Sci. Technol. 2015, 5, 4123–4132. [Google Scholar] [CrossRef]
- Kuhn, M.; Lucas, M.; Claus, P. Long-Time Stability vs. Deactivation of Pd–Ag/Al2O3 Egg-Shell Catalysts in Selective Hydrogenation of Acetylene. Ind. Eng. Chem. Res. 2015, 54, 6683–6691. [Google Scholar] [CrossRef]
- Komeili, S.; Ravanchi, M.T.; Taeb, A. The influence of alumina phases on the performance of the Pd–Ag/Al2O3 catalyst in tail-end selective hydrogenation of acetylene. Appl. Catal. A: Gen. 2015, 502, 287–296. [Google Scholar] [CrossRef]
- Riyapan, S.; Zhang, Y.; Wongkaew, A.; Pongthawornsakun, B.; Monnier, J.R.; Panpranot, J. Preparation of improved Ag–Pd/TiO2 catalysts using the combined strong electrostatic adsorption and electroless deposition methods for the selective hydrogenation of acetylene. Catal. Sci. Technol. 2016, 6, 5608–5617. [Google Scholar] [CrossRef]
- Jin, Q.; He, Y.; Miao, M.; Guan, C.; Du, Y.; Feng, J.; Li, D. Highly selective and stable PdNi catalyst derived from layered double hydroxides for partial hydrogenation of acetylene. Appl. Catal. A: Gen. 2015, 500, 3–11. [Google Scholar] [CrossRef]
- Liu, J.H.; Meng, L.D.; Lv, C.Q.; Wang, G.C. Selective hydrogenation of acetylene over TiO2-supported PdAg cluster: Carbon species effect. RSC Adv. 2016, 6, 14593–14601. [Google Scholar] [CrossRef]
- Krajčí, M.; Hafner, J. Selective semi-hydrogenation of acetylene: Atomistic scenario for reactions on the polar threefold surfaces of GaPd. J. Catal. 2014, 312, 232–248. [Google Scholar] [CrossRef]
- Han, Y.; Peng, D.; Xu, Z.; Wan, H.; Zheng, S.; Zhu, D. TiO2 supported Pd@Ag as highly selective catalysts for hydrogenation of acetylene in excess ethylene. Chem. Commun. 2013, 49, 8350–8352. [Google Scholar] [CrossRef] [PubMed]
- Yarulin, A.E.; Crespo-Quesada, R.M.; Egorova, E.V.; Kiwi-Minsker, L.L. Structure sensitivity of selective acetylene hydrogenation over the catalysts with shape-controlled palladium nanoparticles. Kinet. Catal. 2012, 53, 253–261. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Shin, K.Y.; Lee, J.S.; Hackett, M.J.; Jun, S.W.; Oh, M.H.; Jang, J.; Hyeon, T. Magnetically recyclable core–shell nanocatalysts for efficient heterogeneous oxidation of alcohols. J. Mater. Chem. A 2014, 2, 7593–7599. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Andanson, J.M.; Yarulin, A.; Lim, B.; Xia, Y.; Kiwi-Minsker, L. UV–ozone cleaning of supported poly (vinylpyrrolidone)-stabilized palladium nanocubes: Effect of stabilizer removal on morphology and catalytic behavior. Langmuir 2011, 27, 7909–7916. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Feng, J.T.; Du, Y.Y.; Li, D.Q. Controllable synthesis and acetylene hydrogenation performance of supported Pd nanowire and cuboctahedron catalysts. ACS Catal. 2012, 2, 1703–1710. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, C.; Lee, J.H.; Kim, J.; Lee, H.; Moon, S.H. Performance of shape-controlled Pd nanoparticles in the selective hydrogenation of acetylene. J. Catal. 2013, 306, 146–154. [Google Scholar] [CrossRef]
- Yu, H.; Mao, Z.; Dai, W.; Peng, J.; Zhai, M.; Wei, G. Highly selective Pd/Al2O3 catalyst for hydrogenation of methylacetylene and propadiene in propylene stream prepared by γ-radiation. Appl. Catal. A: Gen. 2012, 445, 246–251. [Google Scholar] [CrossRef]
- Lin, X.; Xu, D.; Xi, Y.; Zhao, R.; Zhao, L.; Song, M.; Zhai, H.; Che, G.; Chang, L. Construction of leaf-like g-C3N4/Ag/BiVO4 nanoheterostructures with enhanced photocatalysis performance under visible-light irradiation. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 513, 117–124. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Shin, E.W.; Kim, W.J.; Park, J.D.; Moon, S.H. Selective hydrogenation of acetylene on TiO2-added Pd catalysts. J. Catal. 2002, 208, 310–320. [Google Scholar] [CrossRef]
- Pachulski, A.; Schödel, R.; Claus, P. Performance and regeneration studies of Pd-Ag/Al2O3 catalysts for the selective hydrogenation of acetylene. Appl. Catal. A: Gen. 2011, 400, 14–24. [Google Scholar] [CrossRef]



| Sample | The Design Value of Pd-Loading (wt %) | The Real Value of Pd-Loading before Reaction (wt %) | The Real Value of Pd-Loading after 60 h on Stream Reaction (wt %) |
|---|---|---|---|
| PdNPs/α-Al2O3 | 0.030 | 0.028 | 0.028 |
| Pd/α-Al2O3 | 0.030 | 0.029 | 0.029 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, Y.; Wang, Y.; Cao, J.; Kang, P.; Tang, Q.; Ma, M. Highly Dispersed PdNPs/α-Al2O3 Catalyst for the Selective Hydrogenation of Acetylene Prepared with Monodispersed Pd Nanoparticles. Catalysts 2017, 7, 128. https://doi.org/10.3390/catal7050128
Zhang H, Wang Y, Wang Y, Cao J, Kang P, Tang Q, Ma M. Highly Dispersed PdNPs/α-Al2O3 Catalyst for the Selective Hydrogenation of Acetylene Prepared with Monodispersed Pd Nanoparticles. Catalysts. 2017; 7(5):128. https://doi.org/10.3390/catal7050128
Chicago/Turabian StyleZhang, Huoli, Youchao Wang, Yan Wang, Jianliang Cao, Peng Kang, Qingjie Tang, and Mingjie Ma. 2017. "Highly Dispersed PdNPs/α-Al2O3 Catalyst for the Selective Hydrogenation of Acetylene Prepared with Monodispersed Pd Nanoparticles" Catalysts 7, no. 5: 128. https://doi.org/10.3390/catal7050128






