Preparation of Salen–Metal Complexes (Metal = Co or Ni) Intercalated ZnCr-LDHs and Their Photocatalytic Degradation of Rhodamine B
Abstract
:1. Introduction
2. Results and Discussion
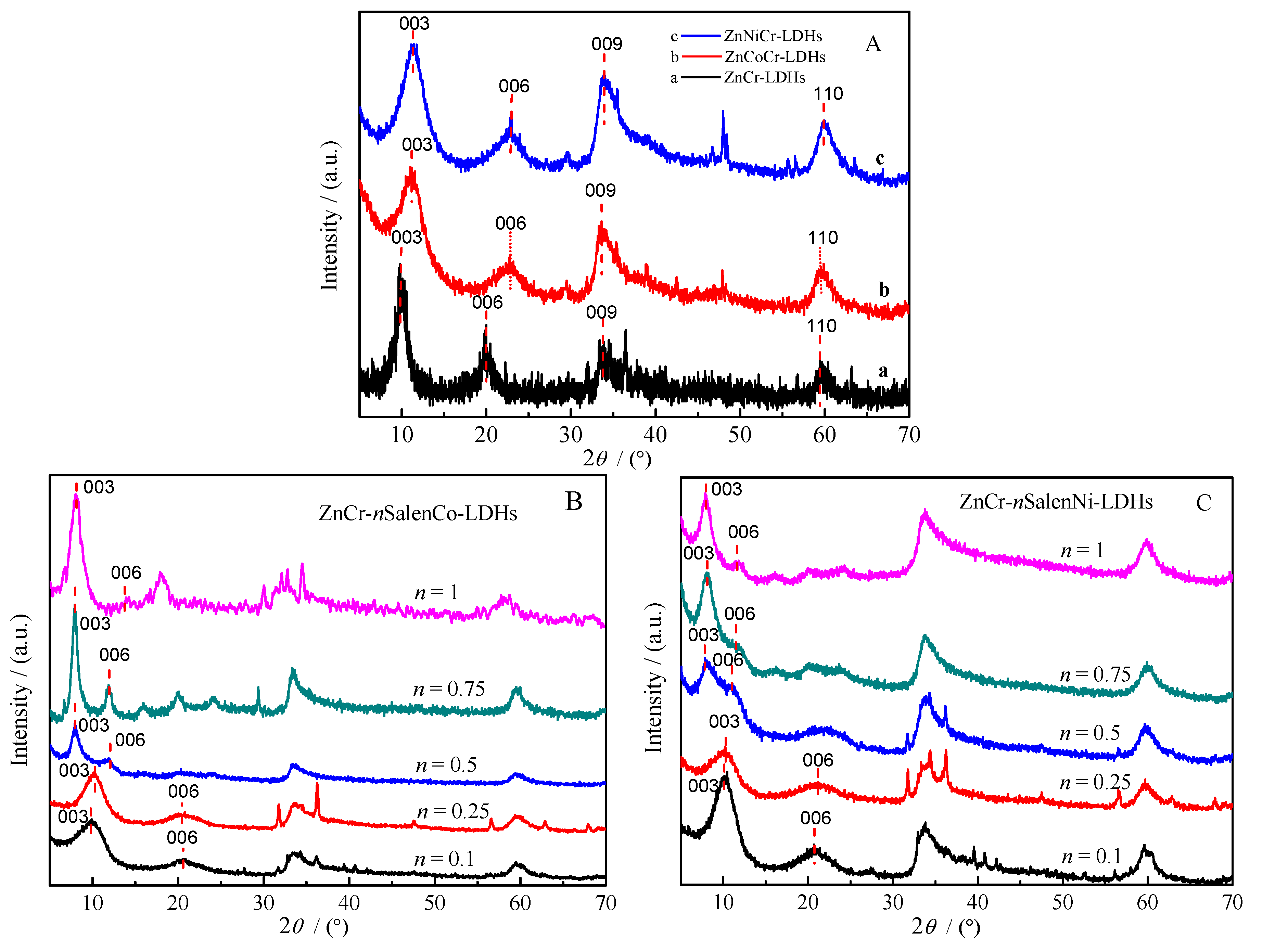
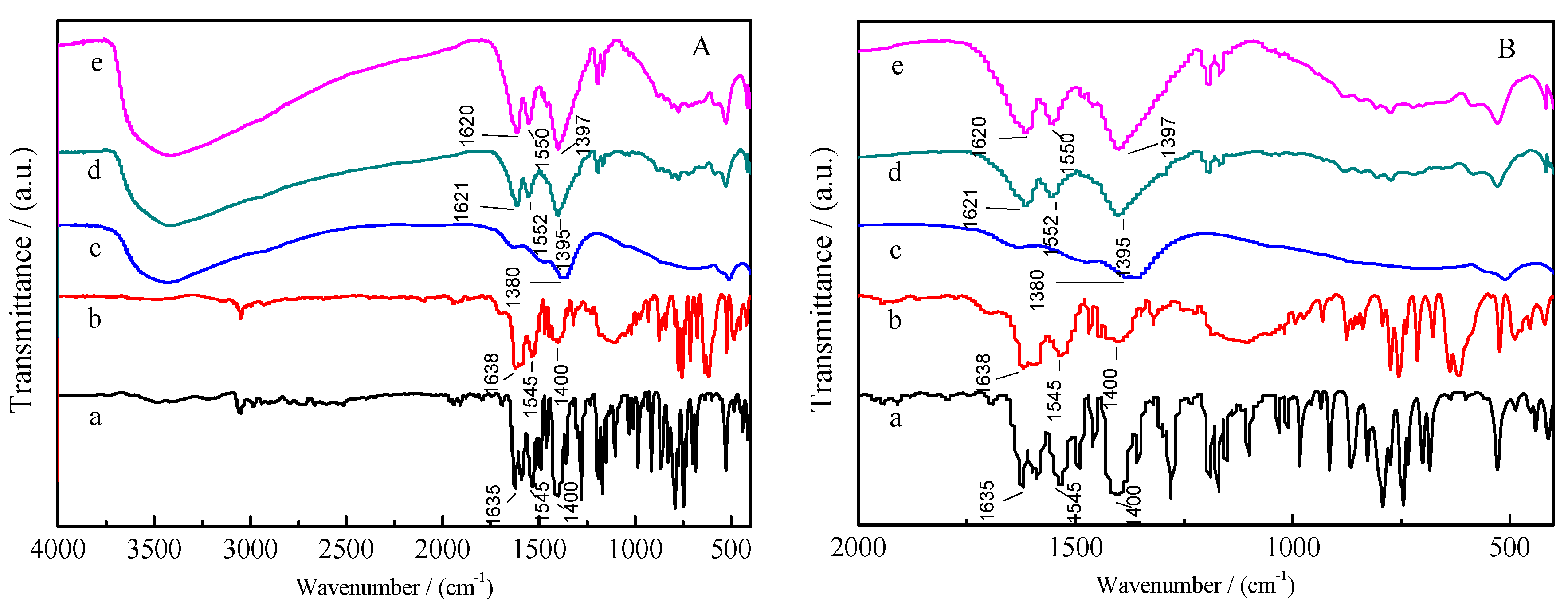
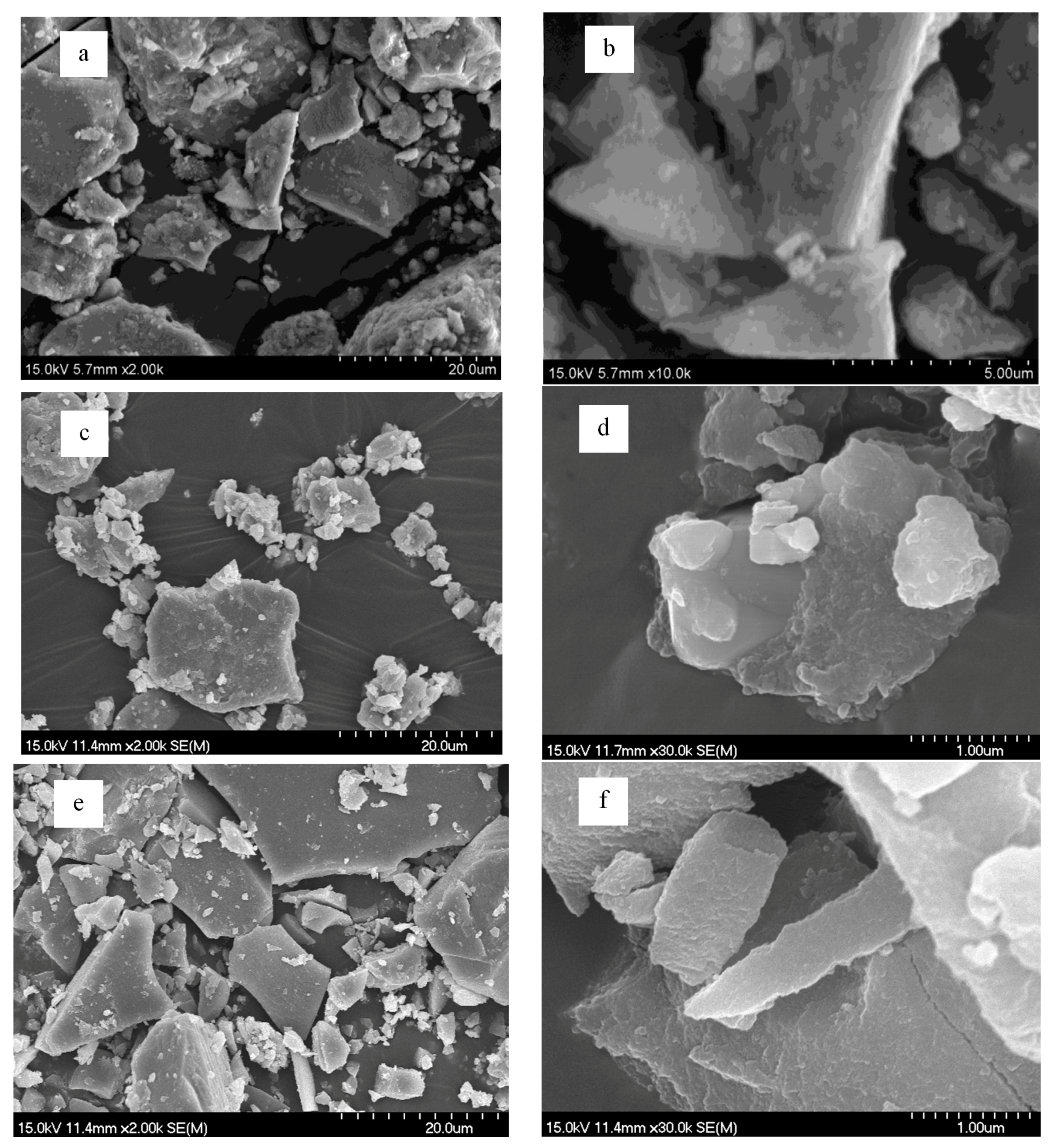
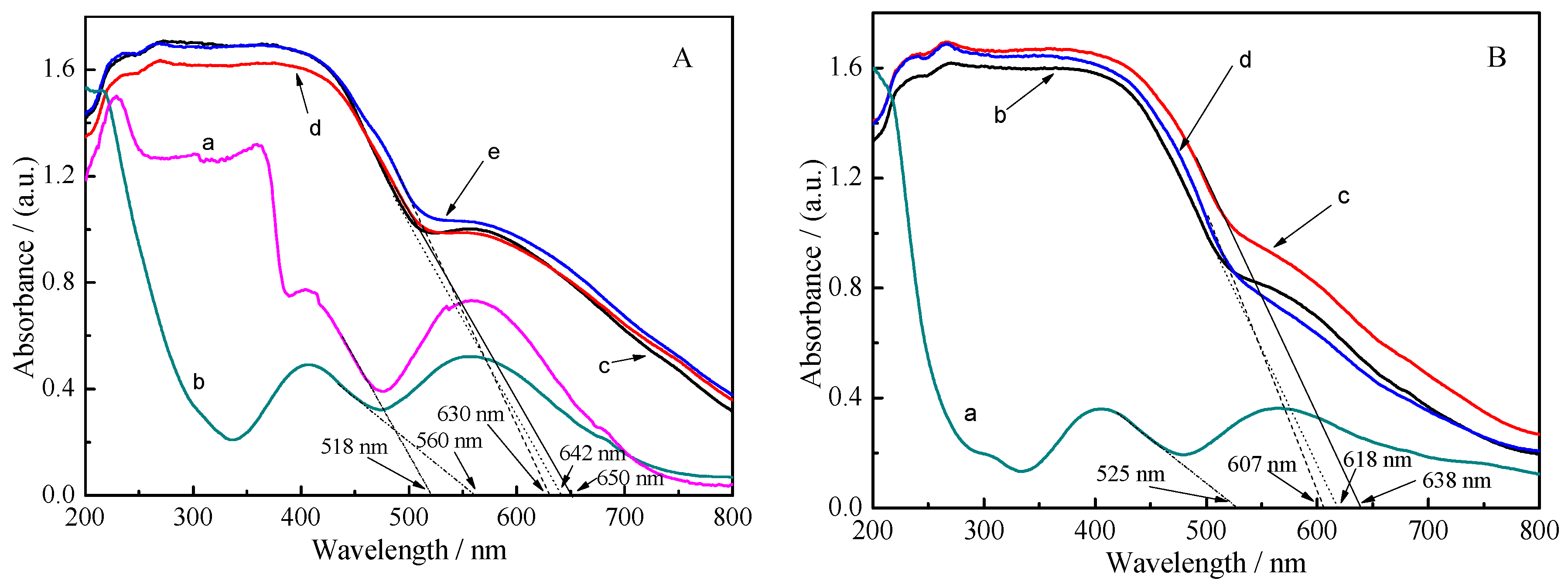
2.1. Characterization of Materials
2.2. Photocatalytic Activity
2.3. Recycling of Catalytic Materials
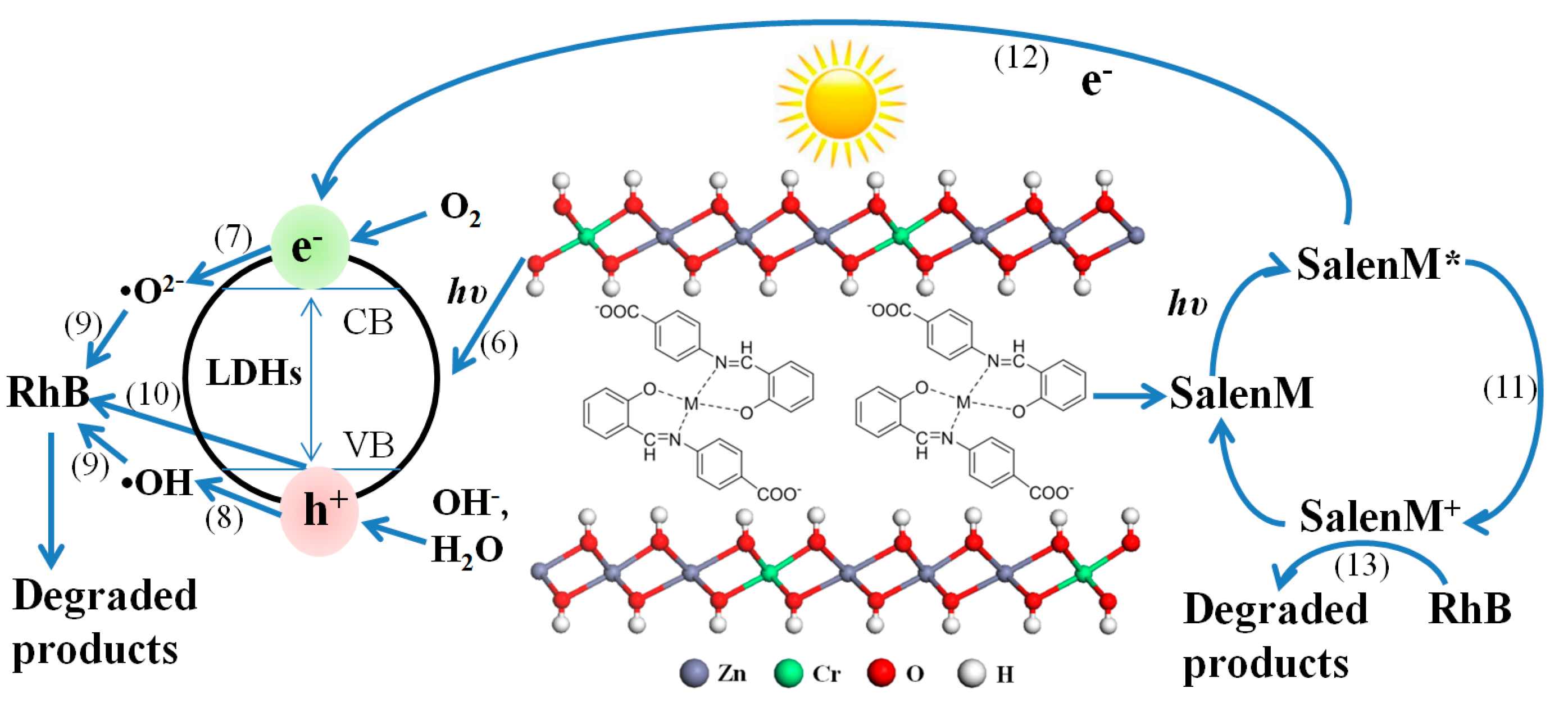
2.4. Mechanism of Photocatalytic Degradation
3. Materials and Methods
3.1. Synthesis of SalenM
3.2. Synthesis of LDHs
3.3. Characterizations
3.4. Photocatalytic Degradation Performance Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.C.; Cheng J., H; Chen, Y.C. Indium-based metal-organic framework/graphite oxide composite as an efficient adsorbent in the adsorption of Rhodamine B from aqueous solution. J. Alloy. Compd. 2016, 687, 804–812. [Google Scholar] [CrossRef]
- Dong, S.Y.; Feng, J.L.; Fan, M.H.; Pi, Y.Q.; Hu, L.M.; Han, X.; Liu, M.L.; Sun, J.Y.; Sun, J.H. Recent developments in heterogeneous photocatalytic water treatment using visible light responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Alessia, S.; Antonio, G.C.; Melissa, A.D.; Stefan, M.; Massimo, P.; Anna, S.; Roberto, T.; Antonio, V. Nature and reactivity of layered double hydroxides formed by coprecipitating Mg, Al and As(V): Effect of arsenic concentration, pH, and aging. J. Hazard. Mater. 2015, 300, 504–512. [Google Scholar]
- Pan, G.X.; Xia, X.H.; Luo, J.S.; Cao, F.; Yang, Z.H.; Fan, H.J. Preparation of CoAl layered double hydroxide nanoflake arrays and their high supercapacitance performance. Appl. Clay Sci. 2014, 102, 28–32. [Google Scholar]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, Guido. Exploring, Tuning, and Exploiting the Basicity of Hydrotalcites for Applications in Heterogeneous Catalysis. Chemistry 2009, 15, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Urdă, A.; Popescu, I.; Cacciaguerra, T.; Tanchoux, N.; Tichit, D.; Marcu, I. Total oxidation of methane over rare earth cation-containing mixed oxides derived from LDH precursors. Appl. Catal. A Gen. 2013, 464–465, 20–27. [Google Scholar]
- Kameda, T.; Uchiyama, T.; Yoshioka, T. Equilibrium and kinetics studies on the adsorption of substituted phenols by a Cu-Al layered double hydroxide intercalated with 1-naphthol-3,8-disulfonate. J. Alloy. Compd. 2016, 670, 322–328. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, S.G.; Chen, H.Z.; He, H.; Sun, C. Adsorption behavior and mechanism of reactive brilliant red X-3B in aqueous solution over three kinds of hydrotalcite-like LDHs. Appl. Surf. Sci. 2014, 301, 329–337. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Beak, M.; Huy, B.T.; Lee, Y. Adsorption and photodegradation kinetics of herbicide 2,4,5-trichlorophenoxyacetic acid with MgFeTi layered double hydroxides. Chemosphere 2016, 146, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.M.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Yan, Q.J.; Zhang, Z.; Zhang, Y.L.; Umar, A.; Guo, Z.H.; Hare, D.O.; Wang, Q. Hierarchical Fe3O4 Core–Shell Layered Double Hydroxide Composites as Magnetic Adsorbents for Anionic Dye Removal from Wastewater. Eur. J. Inorg. Chem. 2015, 25, 4182–4191. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.Q.; Qian, G.R.; Wu, D.S.; Frost, R.L. Removal of methyl orange from aqueous solutions through adsorption by calcium aluminate hydrates. J. Colloid Interf. Sci. 2014, 426, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Lo, W.H.; Chan, G.Y. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006, 129, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Habibi-Yangjeh, A.; Akhundi, A. Novel ternary g-C3N4/Fe3O4/Ag2CrO4 nanocomposites: magnetically separable and visible-light-driven photocatalysts for degradation of water pollutants. J. Mol. Catal. A Chem. 2016, 415, 122–130. [Google Scholar] [CrossRef]
- Xie, C.; Yang, S.H.; Shi, J.W.; Niu, C.M. Highly Crystallized C-Doped Mesoporous Anatase TiO2 with Visible Light Photocatalytic Activity. Catalysts 2016, 6, 117. [Google Scholar] [CrossRef]
- Liu, F.F.; Ruxangul, J.; Wang, Y.J.; Wang, M.C.; Yang, L.; Abdiryim, T. Photodegradation of methylene blue by photocatalyst of D-A-D type polymer/functionalized multi-walled carbon nanotubes composite under visible-light irradiation. Chemosphere 2017, 168, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.M.; Xue, J.L. Synthesis of CuMgAl layered double hydroxides for efficient photocatalysis of Rhodamine B. Chem. J. Chin. Univ. 2013, 3, 503–508. [Google Scholar]
- Jayaseeli, A.M.I.; Ramdass, A.; Rajagopal, S. Selective H2O2 oxidation of organic sulfides to sulfoxides catalyzed by cobalt(III)–salen ion. Polyhedron 2015, 100, 59–66. [Google Scholar] [CrossRef]
- Matsunaga, S.; Shibasaki, M. Recent advances in cooperative bimetallic asymmetric catalysis: dinuclear Schiff base complexes. Chem. Commun. 2014, 50, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Higashi, K.; Watanabe, M.; Kakigi, R.; Ida, S.; Ishihara, T. Effect of porphyrin molecular structure on water splitting activity of a KTaO photocatalyst. Catalysts 2016, 6, 42. [Google Scholar] [CrossRef]
- Song, Q.; Ma W, .H.; Jia, M.K.; Johnson, D.; Huang, Y.P. Degradation of organic pollutants in waters by a water-insoluble iron(III) Schiff base complex. Appl. Catal. A-Gen. 2015, 505, 70–76. [Google Scholar] [CrossRef]
- Wang, X.L.; Shang, C.X.; Wu, G.D.; Liu, X.F.; Liu, H. Base-Free Selective Oxidation of Glycerol over LDH Hosted Transition Metal Complexes Using 3% H2O2 as Oxidant. Catalysts 2016, 6, 101. [Google Scholar] [CrossRef]
- Xia, S.J.; Liu, F.X.; Ni, Z.M.; Shi, W.; Xue, J.L.; Qian, P.P. Ti-based layered double hydroxides: Efficient photocatalysts for azo dyes degradation under visible light. Appl. Catal. B-Environ. 2014, 144, 570–579. [Google Scholar] [CrossRef]
- Ahmed, N.; Shibata, Y.; Taniguchi, T.; Izumi, Y. Photocatalytic conversion of carbon dioxide into methanol using zinc–copper–M(III) (M = aluminum, gallium) layered double hydroxides. J. Catal. 2011, 279, 123–135. [Google Scholar] [CrossRef]
- Prince, J.; Tzompantzi, F.; Mendoza-Damián, G.; Hernández-Beltrán, F.; Valente, J.S. Photocatalytic degradation of phenol by semiconducting mixed oxides derived from Zn(Ga)Al layered double hydroxides. Appl. Catal. B-Environ. 2015, 163, 352–360. [Google Scholar] [CrossRef]
- Serdechnova, M.; Salak, A.N.; Barbosa, F.S.; Vieira D.E., L.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Interlayer intercalation and arrangement of 2-mercaptobenzothiazolate and 1,2,3-benzotriazolate anions in layered double hydroxides: In situ X-ray diffraction study. J. Solid State Chem. 2016, 233, 158–165. [Google Scholar] [CrossRef]
- Bu, R.; Chen, F.F.; Li, J.; Li, W.; Yang, F. Adsorption capability for anionic dyes on 2-hydroxyethylammonium acetate-intercalated layered double hydroxide. Colloid. Surface. A 2016, 511, 312–319. [Google Scholar] [CrossRef]
- Shakir, M.; Shahid, N.; Sami, N.; Azam, M.; Khan, A.U. Synthesis, spectroscopic characterization and comparative DNA binding studies of Schiff base complexes derived from L-leucine and glyoxal. Spectrochim. Acta A 2011, 82, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kovář, P.; Pospíšil, M.; Nocchetti, M.; Čapková, P.; Melánová, K. Molecular modeling of layered double hydroxide intercalated with benzoate, modeling and experiment. J. Mol. Model. 2007, 13, 937–942. [Google Scholar]
- Rad, F.A.; Rezvani., Z. Preparation of cubane-1,4-dicarboxylate–Zn–Al layered double hydroxide nanohybrid: Comparison of structural and optical properties between experimental and calculated results. RSC Adv. 2015, 5, 67384–67393. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wu, G.Q.; Evans, D.G. Synthesis and characterization of a layered double hydroxide containing an intercalated nickel(II) citrate complex. Mater. Chem. Phys. 2007, 104, 133–140. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.F.; Zhu, Y.; Zhang, F.Z. Experimental and theoretical investigation into the elimination of organic pollutants from solution by layered double hydroxides. Appl. Catal. B-Environ. 2013, 140–141, 241–248. [Google Scholar] [CrossRef]
- Emara, A.A.A.; Ali, A.M.; El-Asmy, A.F.; Ragab, E.M. Investigation of the oxygen affinity of manganese(II), cobalt(II) and nickel(II) complexes with some tetradentate Schiff bases. J. Saudi Chem. Soc. 2014, 18, 762–773. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhan, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, Y.; Kato, H.; Kobayashi, H.; Kudo, A. Photophysical Properties and Photocatalytic Activities of Bismuth Molybdates under Visible Light Irradiation. J. Phys. Chem. B 2006, 110, 17790–17797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Xiong, Z.G.; Li, L.; Burt, R.; Zhao, X.S. Uptake and degradation of Orange II by zinc aluminum layered double oxides. J. Colloid Interf. Sci. 2016, 469, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.J.; Zhang, L.Y.; Zhou, X.B.; Shao, M.M.; Pan, G.X.; Ni, Z.M. Fabrication of highly dispersed Ti/ZnO–Cr2O3 composite as highly efficient photocatalyst for naphthalene degradation. Appl. Catal. B-Environ. 2015, 176, 266–277. [Google Scholar] [CrossRef]
- Becker, J.; Raghupathi, K.R.; Pierre, J.S.; Zhao, D.; Koodali, R.T. Tuning of the crystallite and particle sizes of ZnO nanocrystalline materials in solvothermal synthesis and their photocatalytic activity for dye degradation. J. Phys. Chem. C 2011, 115, 13844–13850. [Google Scholar] [CrossRef]
- Chiou, C.H.; Wu, C.Y.; Juang, R.S. Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2 process. Chem. Eng. J. 2008, 139, 322–329. [Google Scholar] [CrossRef]
- Huang, Y.P.; Li, J.; MA, W.H.; Chen, M.M.; Zhao, J.; Yu, J.C. Efficient H2O2 oxidation of organic pollutants catalyzed by supported iron sulfophenylporphyrin under visible light irradiation. J. Phys. Chem. B 2004, 108, 7263–7270. [Google Scholar] [CrossRef]
- Li, J.; Ma, W.H.; Huang, Y.P.; Tao, X.; Zhao, J.C.; Xu, Y.M. Oxidative degradation of organic pollutants utilizing molecular oxygen and visible light over a supported catalyst of Fe(bpy)32+ in water. Appl. Catal. B-Environ. 2004, 48, 17–24. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Haq, E.U.; Licciulli, A. Nanocrystalline TiO2–diatomite composite catalysts: Effect of crystallization on the photocatalytic degradation of Rhodamine B. Appl. Catal. A Gen. 2014, 485, 157–162. [Google Scholar] [CrossRef]
- Madhusudan, P.; Zhang, J.; Cheng, B.; Yu, J.G. Fabrication of CdMoO4@CdS core–shell hollow superstructures as high performance visible-light driven photocatalysts. Phys. Chem. Chem. Phys. 2015, 17, 15339–15347. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, D.Z.; Wang, H.B.; Zeng, G.P.; Li, X.H.; Shao, Y. Role of active oxygen species in the liquid-phase photocatalytic degradation of RhB using BiVO4/TiO2 heterostructure under visible light irradiation. J. Mol. Catal. A Chem. 2015, 408, 172–178. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K.M. Zn–Cr layered double hydroxide: Visible light responsive photocatalyst for photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2012, 91, 73–80. [Google Scholar] [CrossRef]
- Fu, H.B.; Pan, C.S.; Yao, W.Q.; Zhu Yo., F. Visible-Light-Induced Degradation of Rhodamine B by Nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Rabbani, M.; Modirshahla, N.; Behnajady, M.A. Kinetic modeling of photocatalytic degradation of Acid Red 27 in UV/TiO2 process. J. Photoch. Photobio. A 2004, 168, 39–45. [Google Scholar] [CrossRef]
- Yasui, K.; Isobe, T.; Nakajima, A. Preparation and photocatalytic activity of TiO2 powders from titanium citrate complex using two step hydrothermal treatments. Mater. Lett. 2010, 64, 2036–2039. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhai, S.Y.; Wang, M.H.; Ji, H.F.; He, L.H.; Ye, C.M.; Wang, C.B.; Fang, S.M.; Zhang, H.Z. Photocatalytic degradation of Rhodamine B by using a nanocomposite of cuprous oxide, three-dimensional reduced graphene oxide, and nanochitosan prepared via one-pot synthesis. J. Alloy. Compd. 2016, 659, 101–111. [Google Scholar] [CrossRef]
- Kiantazh, F.; Habibi-Yangjeh, A. Ag3VO4/ZnO nanocomposites with an n–n heterojunction as novel visible-light-driven photocatalysts with highly enhanced activity. Mat. Sci. Semicon. Proc. 2015, 39, 671–679. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- O'Regan, B.C.; López-Duarte, I.; Martínez-Díaz, M.V.; Forneli, A.; Albero, J.; Morandeira, A. Catalysis of recombination and its limitation on open circuit voltage for dye sensitized photovoltaic cells using phthalocyanine dyes. J. Am. Chem. Soc. 2008, 130, 2906–2907. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Deng, Z.G.; Li, X.P.; Zhang, J.L.; Zhao, J.C. Visible light detoxification by 2,9,16,23-tetracarboxyl phthalocyanine copper modified amorphous titania. Chem. Phys. Lett. 2005, 415, 85–88. [Google Scholar] [CrossRef]
- Zheng, J.J.; Jiao, Z.B. Modified Bi2WO6 with metal-organic frameworks for enhanced photocatalytic activity under visible light. J. Colloid Interf. Sci. 2017, 488, 234–239. [Google Scholar] [CrossRef] [PubMed]
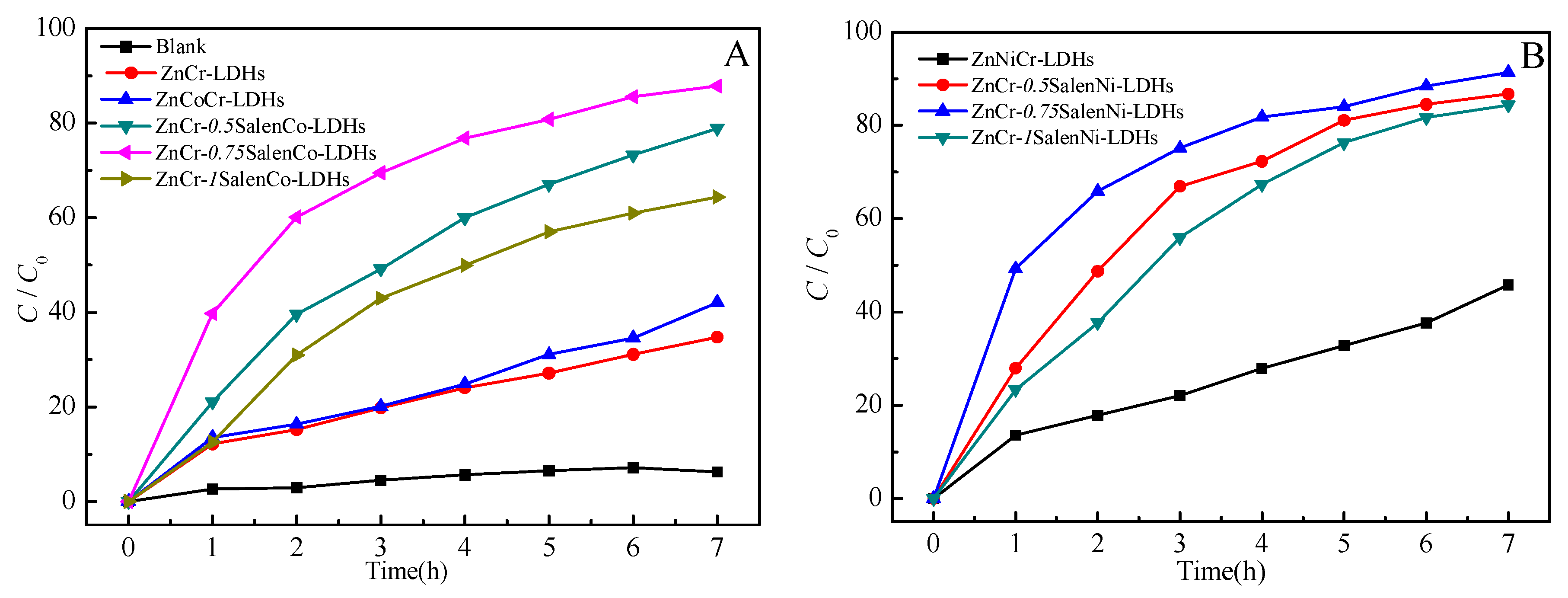

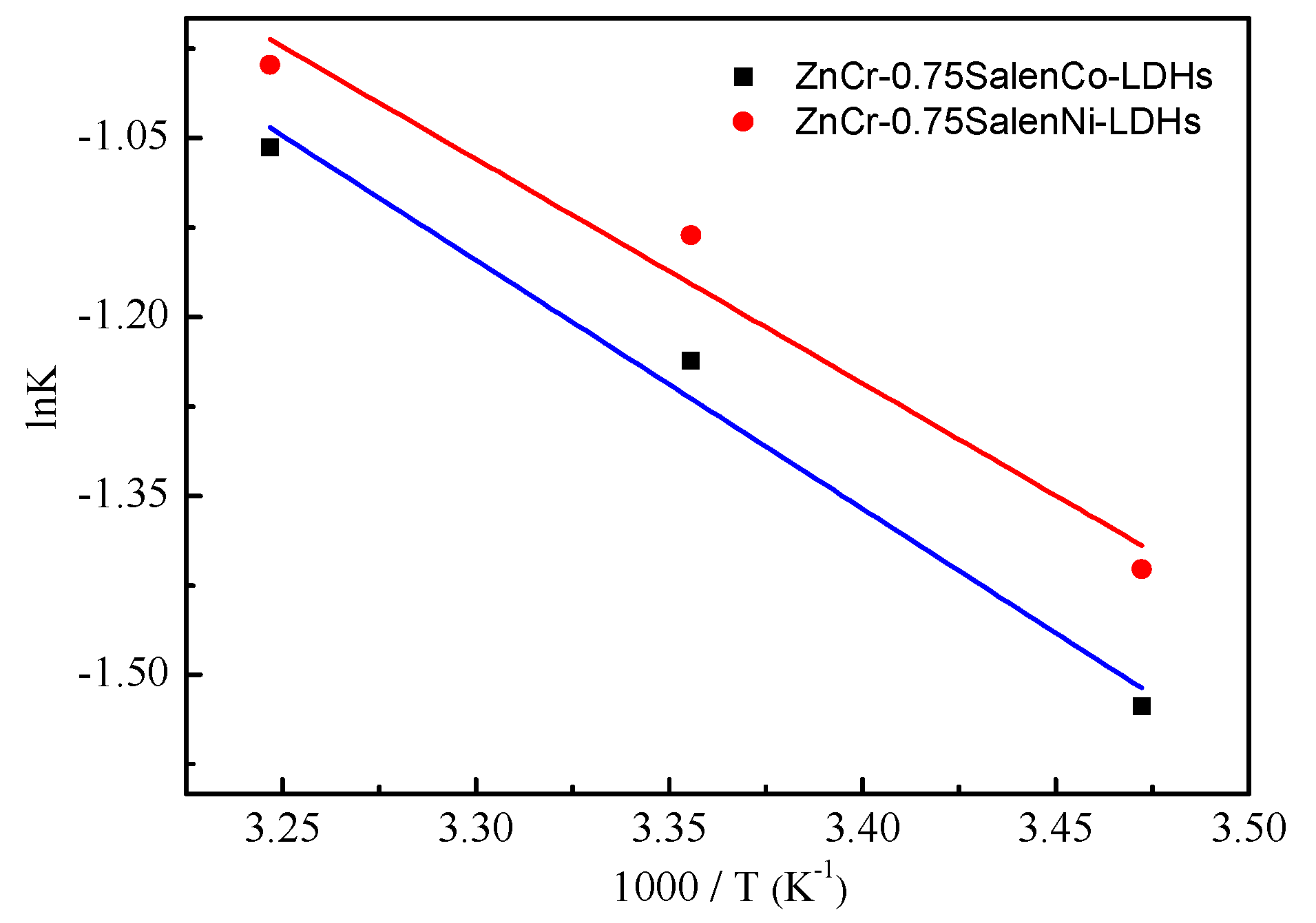
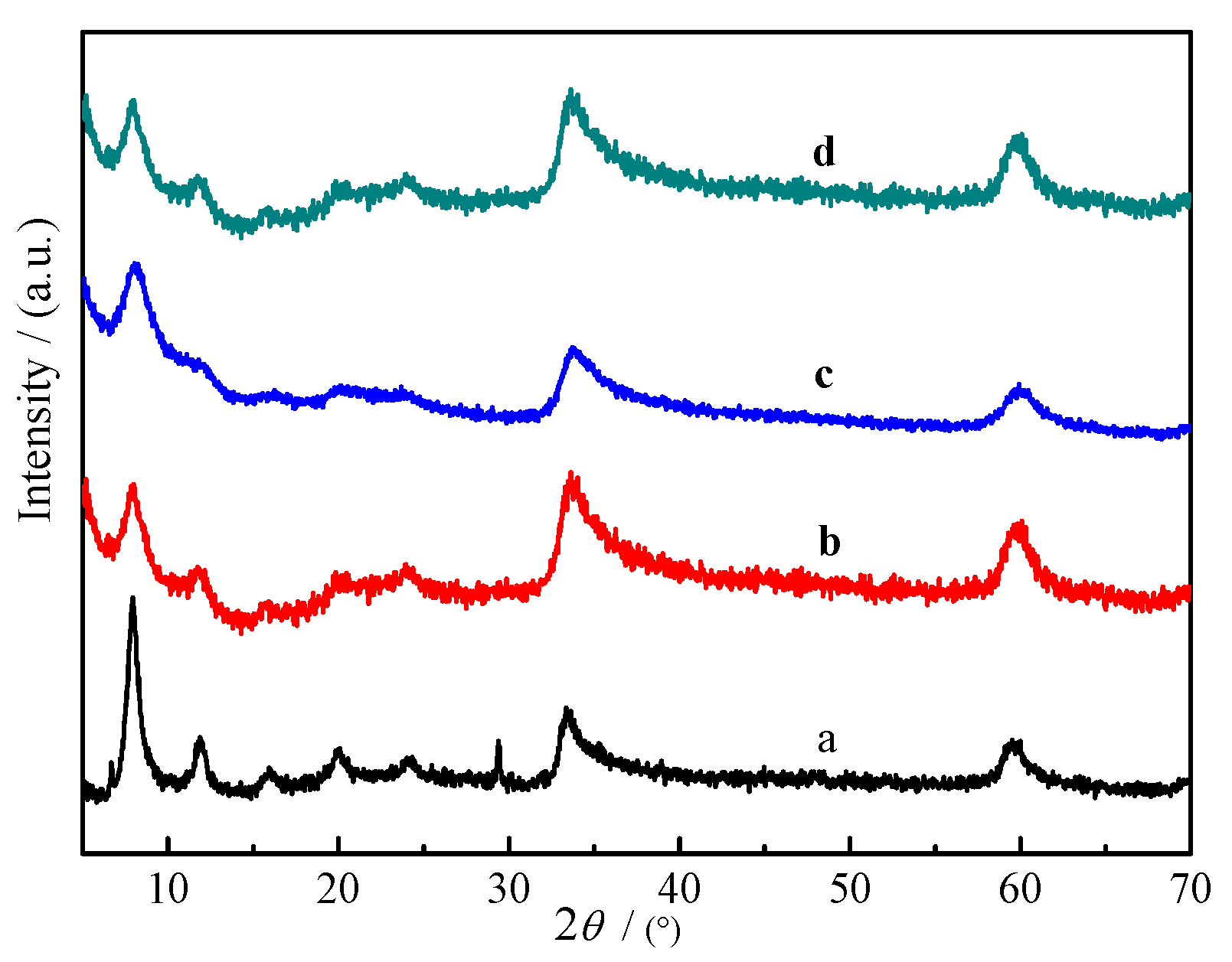
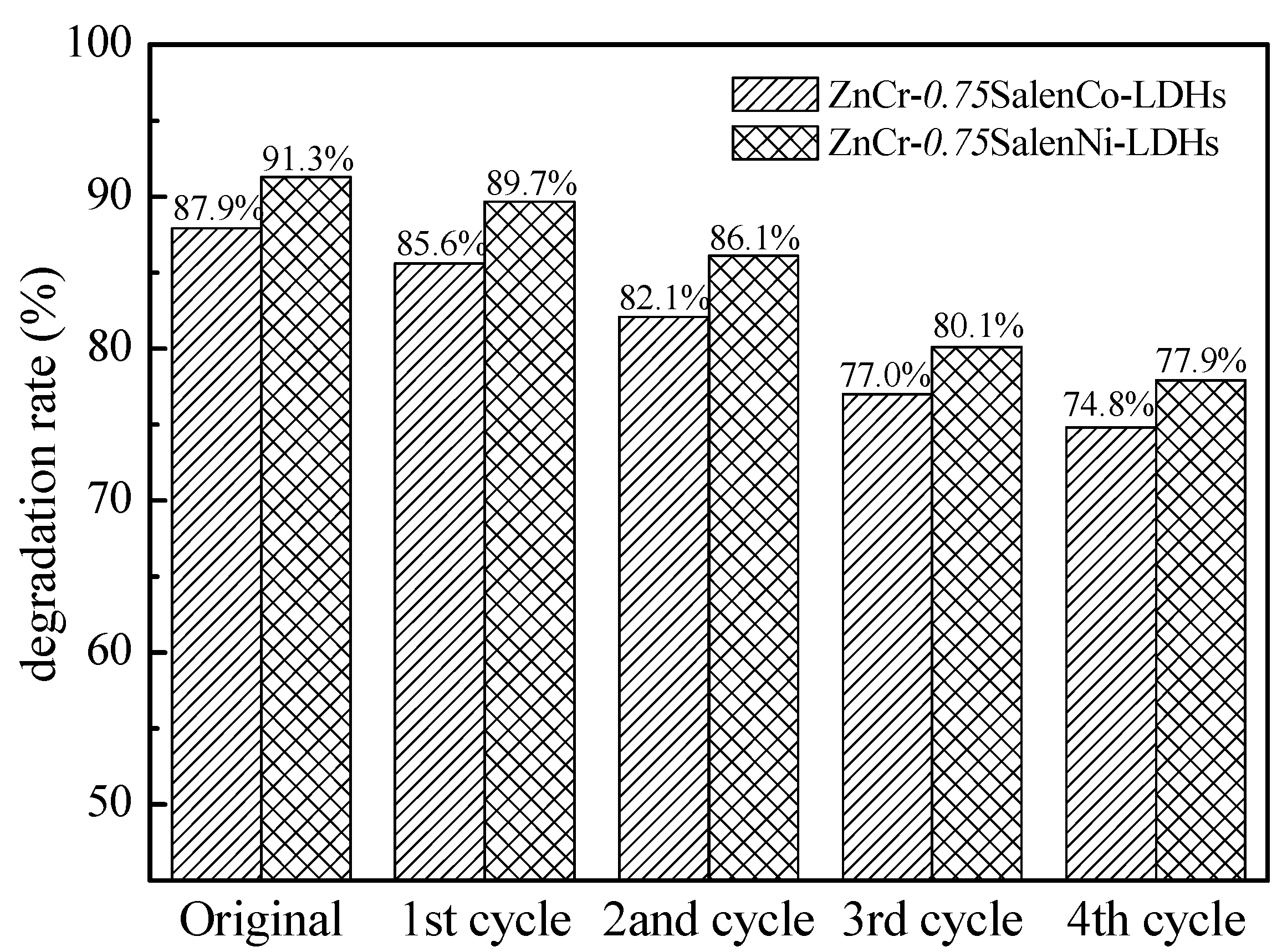

| Materials | Zn (ppm) | Co (ppm) | Ni (ppm) | Cr (ppm) | Experimental Mole Ratios (Zn:M:Cr) | Prospective Mole Ratios (Zn:M:Cr) |
|---|---|---|---|---|---|---|
| ZnCr-0.5SalenCo-LDHs | 21.86 | 3.13 | - | 5.52 | 3.15:0.501:1 | 3:0.50:1 |
| ZnCr-0.75SalenCo-LDHs | 15.08 | 3.24 | - | 3.81 | 3.15:0.753:1 | 3:0.75:1 |
| ZnCr-1SalenCo-LDHs | 12.95 | 3.85 | - | 3.27 | 3.15:1.04:1 | 3:1:1 |
| ZnCr-0.5SalenNi-LDHs | 17.69 | - | 2.50 | 4.23 | 3.33:0.524:1 | 3:0.50:1 |
| ZnCr-0.75SalenNi-LDHs | 15.70 | - | 3.18 | 3.75 | 3.33:0.751:1 | 3:0.75:1 |
| ZnCr-1SalenNi-LDHs | 13.76 | - | 3.70 | 3.28 | 3.34:1.00:1 | 3:1:1 |
| Materials | 2θ003 (°) | d003 (nm) | Materials | 2θ003 (°) | d003 (nm) |
|---|---|---|---|---|---|
| ZnCr-LDHs | 9.99 | 0.89 | ZnCr-0.5SalenCo-LDHs | 7.99 | 1.11 |
| ZnCoCr-LDHs | 11.03 | 0.80 | ZnCr-0.75SalenCo-LDHs | 7.95 | 1.11 |
| ZnNiCr-LDHs | 11.18 | 0.79 | ZnCr-1SalenCo-LDHs | 8.01 | 1.10 |
| ZnCr-0.1SalenCo-LDHs | 10.00 | 0.88 | ZnCr-0.5SalenNi-LDHs | 7.91 | 1.12 |
| ZnCr-0.25SalenCo-LDHs | 10.11 | 0.87 | ZnCr-0.75SalenNi-LDHs | 7.99 | 1.11 |
| ZnCr-0.1SalenNi-LDHs | 10.42 | 0.85 | ZnCr-1SalenNi-LDHs | 7.95 | 1.11 |
| ZnCr-0.25SalenNi-LDHs | 10.41 | 0.85 |
| Catalytic Materials | Kapp (h−1) | r2 | t1/2 (h) |
|---|---|---|---|
| ZnCr-LDHs | 0.0560 | 0.9721 | 11.62 |
| ZnCoCr-LDHs | 0.0698 | 0.9710 | 9.61 |
| ZnNiCr-LDHs | 0.0784 | 0.9765 | 8.52 |
| ZnCr-0.75SalenCo-LDHs | 0.2902 | 0.9684 | 1.68 |
| ZnCr-0.75SalenNi-LDHs | 0.3225 | 0.9587 | 1.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Luo, W.; Xia, S.; Ni, Z. Preparation of Salen–Metal Complexes (Metal = Co or Ni) Intercalated ZnCr-LDHs and Their Photocatalytic Degradation of Rhodamine B. Catalysts 2017, 7, 143. https://doi.org/10.3390/catal7050143
Meng Y, Luo W, Xia S, Ni Z. Preparation of Salen–Metal Complexes (Metal = Co or Ni) Intercalated ZnCr-LDHs and Their Photocatalytic Degradation of Rhodamine B. Catalysts. 2017; 7(5):143. https://doi.org/10.3390/catal7050143
Chicago/Turabian StyleMeng, Yue, Wei Luo, Shengjie Xia, and Zheming Ni. 2017. "Preparation of Salen–Metal Complexes (Metal = Co or Ni) Intercalated ZnCr-LDHs and Their Photocatalytic Degradation of Rhodamine B" Catalysts 7, no. 5: 143. https://doi.org/10.3390/catal7050143





