Cellulose with a High Fractal Dimension Is Easily Hydrolysable under Acid Catalysis
Abstract
:1. Introduction
2. Results and Discussion
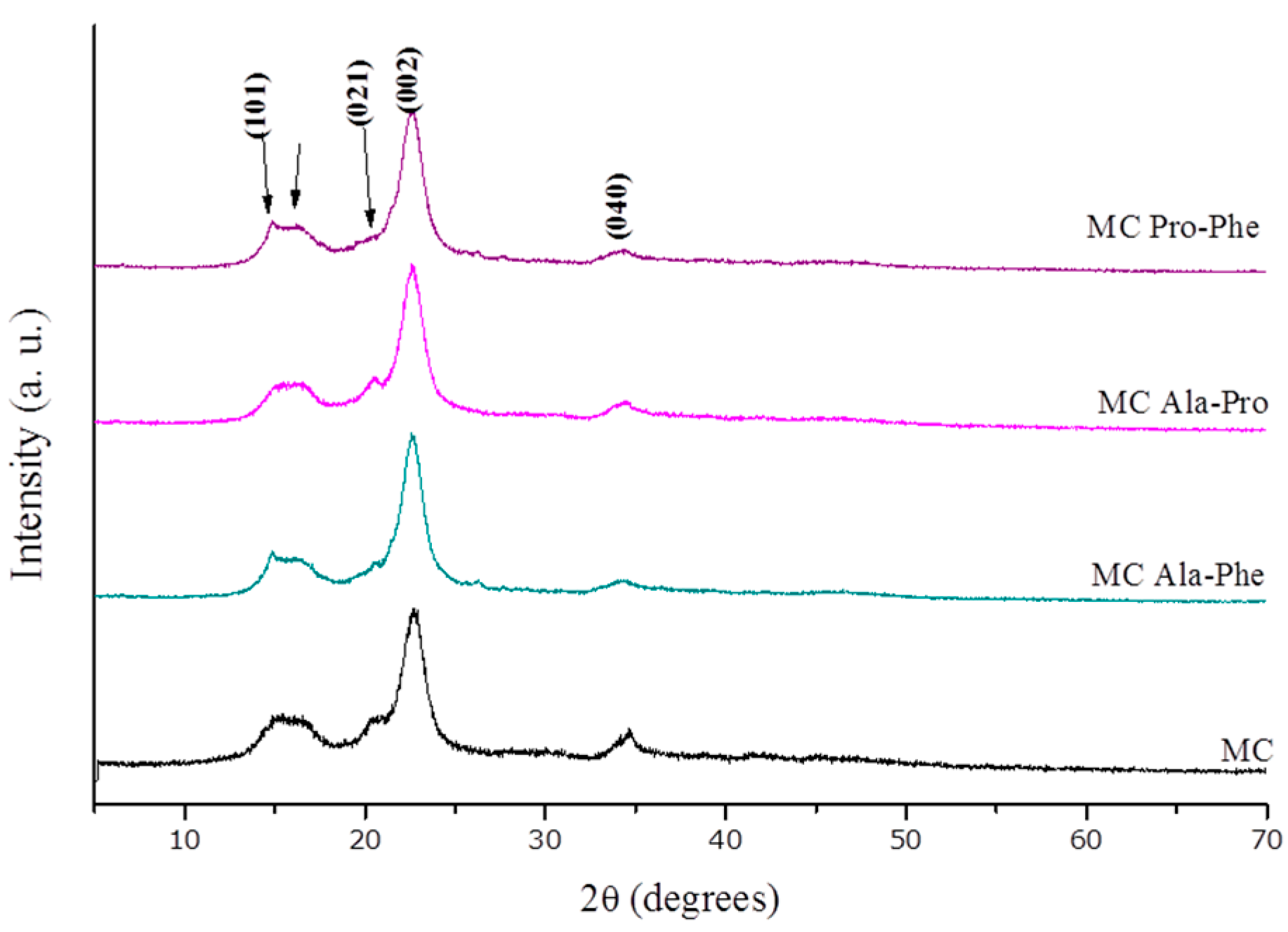
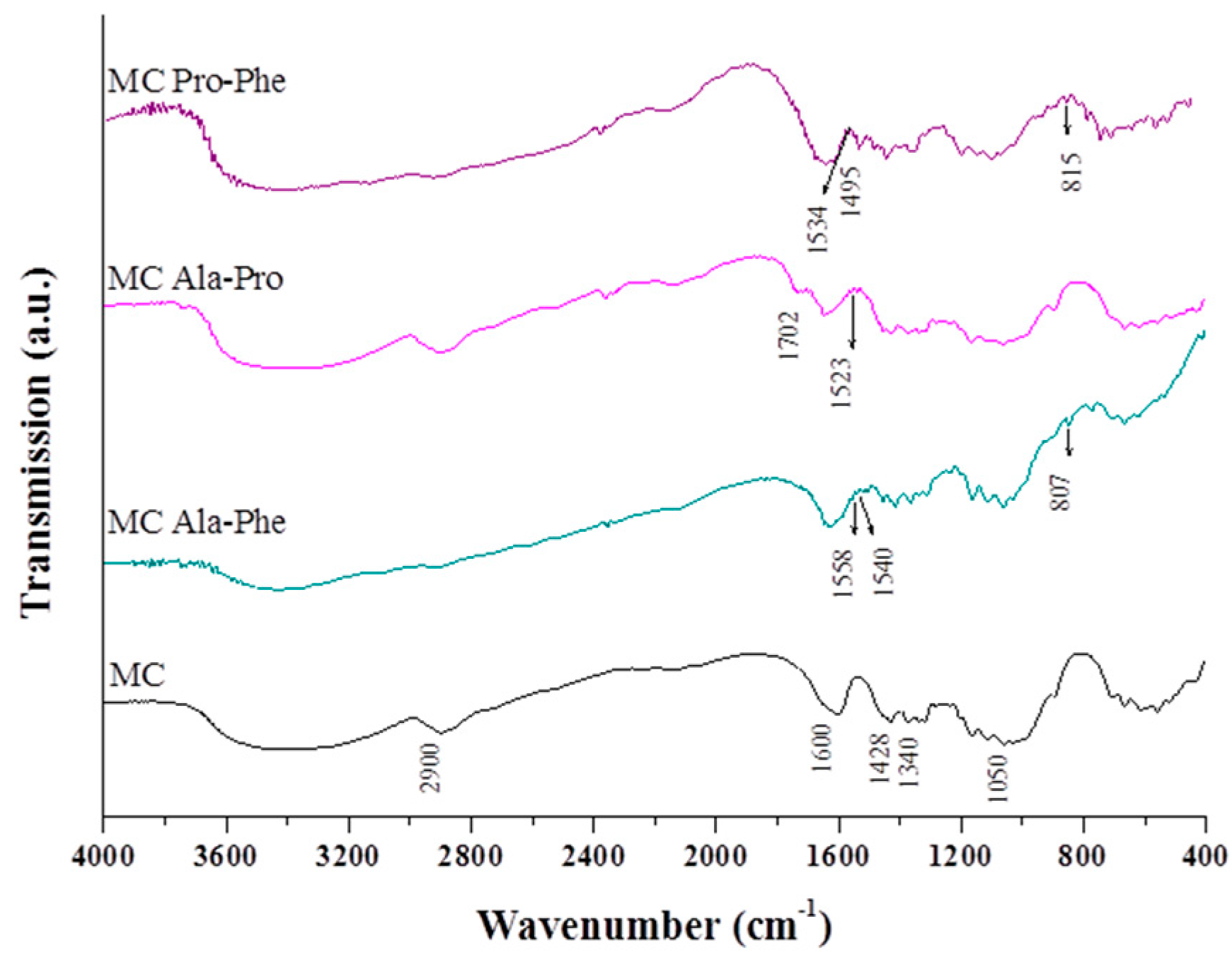
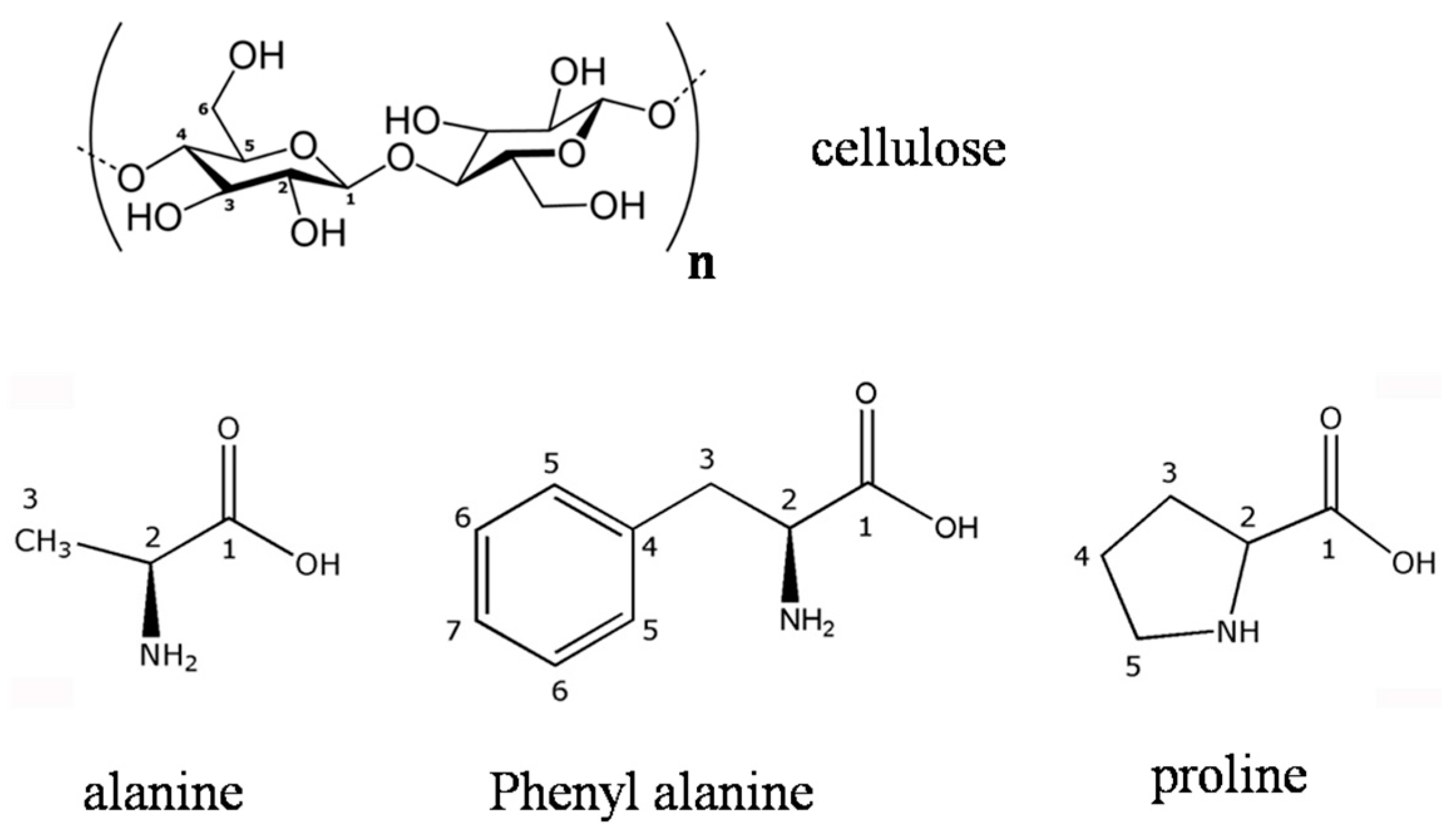
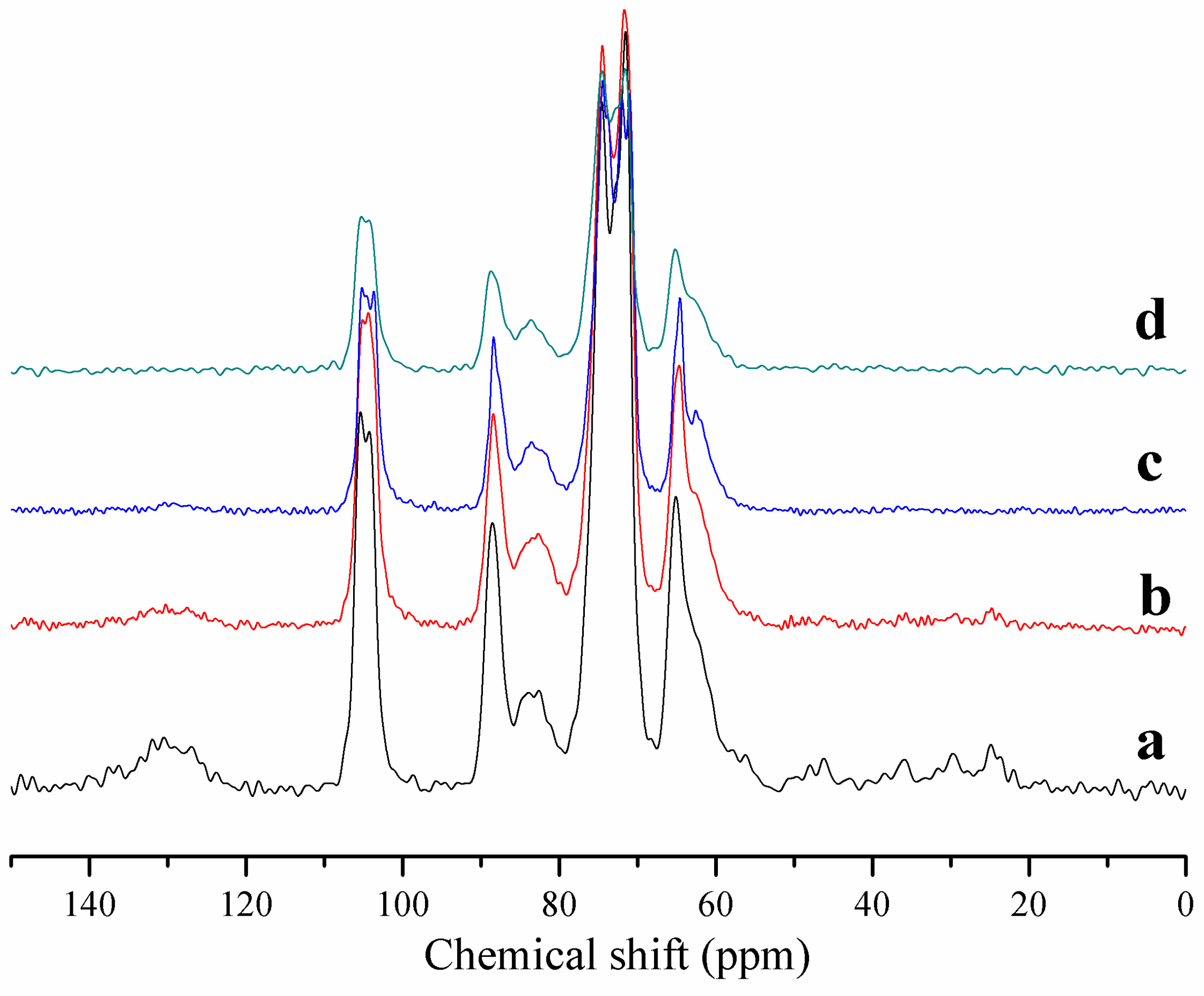
2.1. Modification of the Cellulose Surface
- (1)
- The broad band between 3200 and 3400 cm−1 was assigned to the ν vibration mode of O–H bonds.
- (2)
- The band due to the stretching C–H mode was observed close to 2900 cm−1.
- (3)
- The band at 1600 cm−1 was assigned to the deformation δ vibration coming from H–O–H (adsorbed onto cellulose surface).
- (4)
- The band between 1426 and 1430 cm−1 was due to flexion δ vibration of CH2 groups.
- (5)
- The band resulting from the stretching vibration of C–O and C–C bonds was observed close to 1000 cm−1.
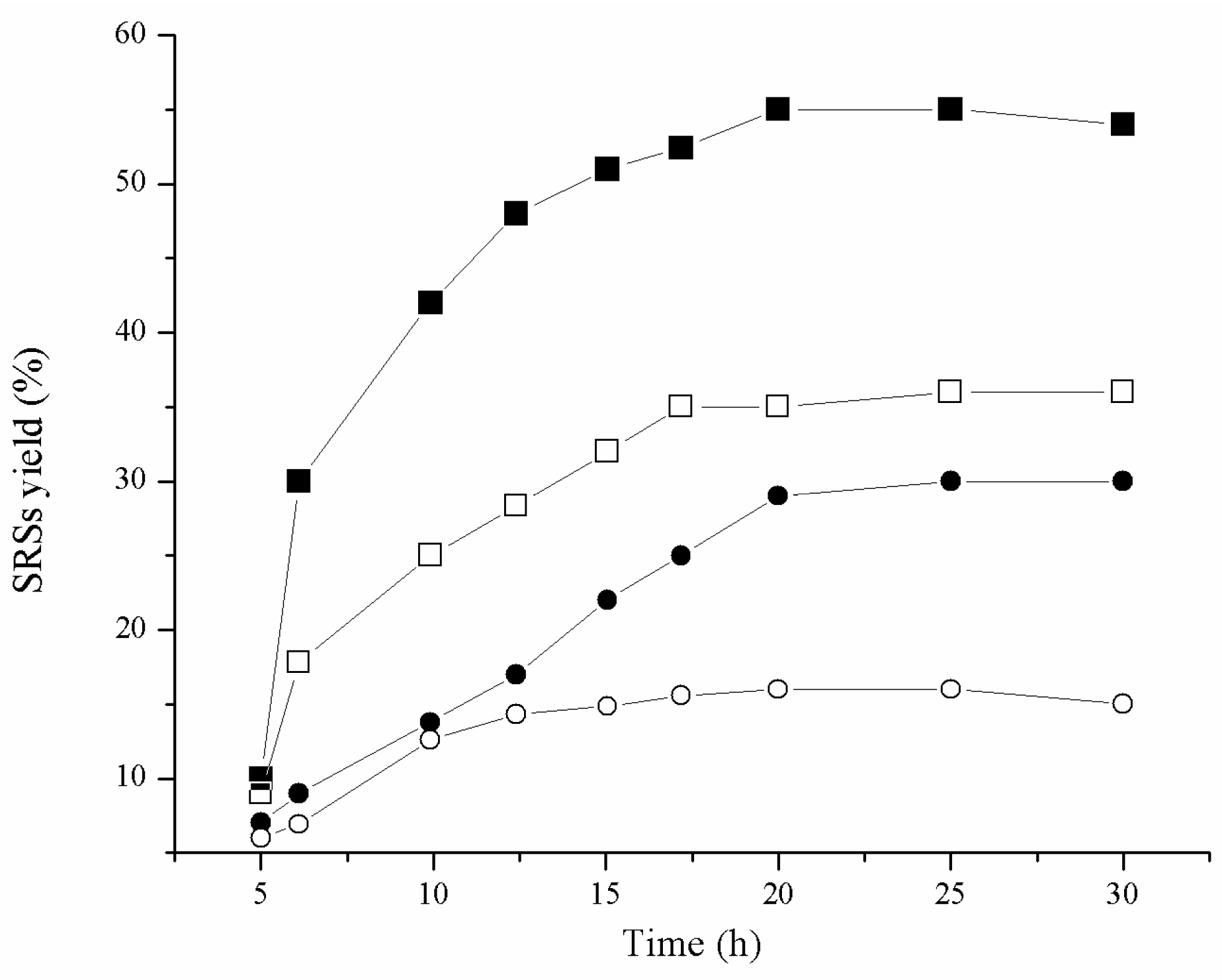
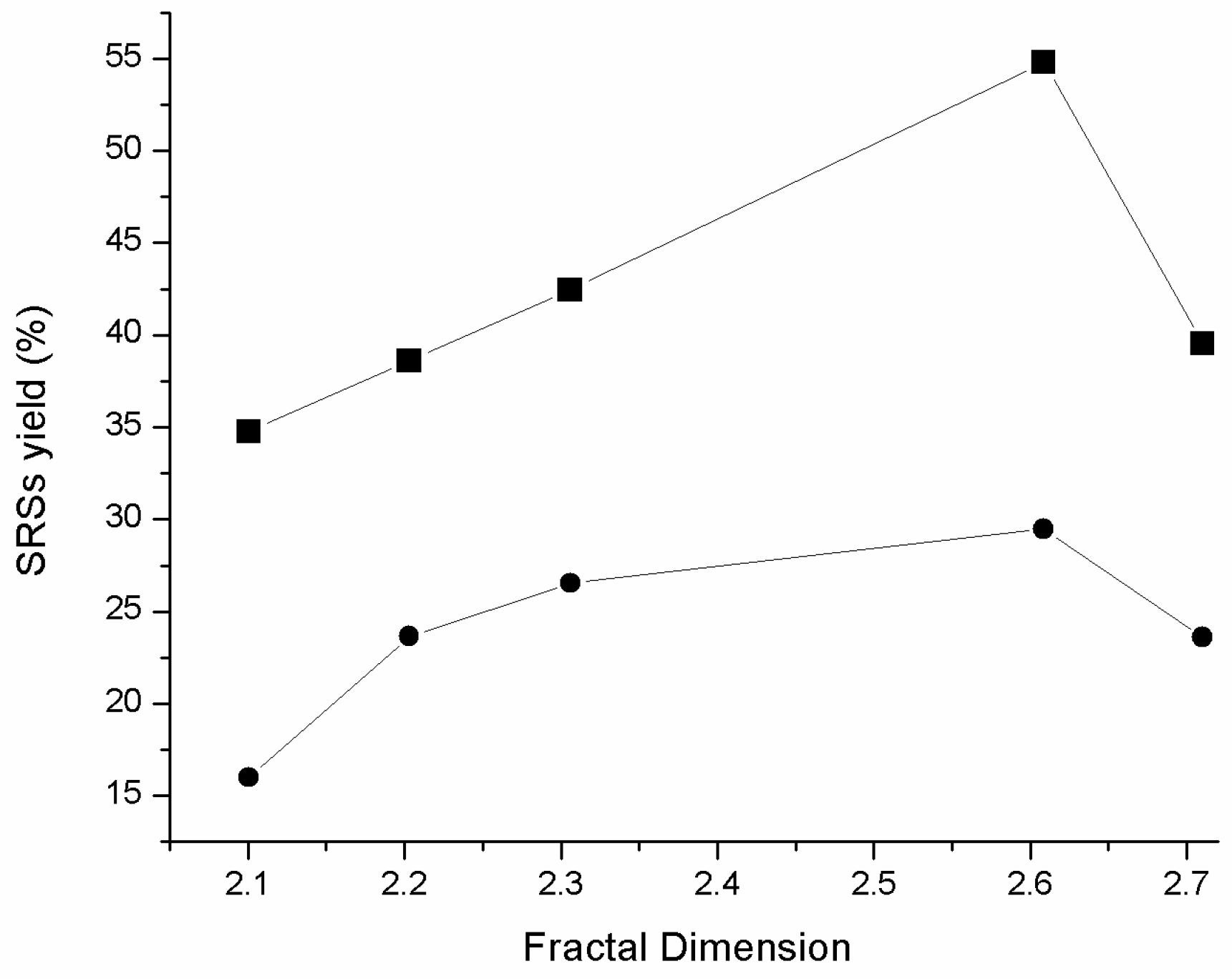
2.2. Catalytic Hydrolysis of Amino Acid-Modified Cellulose Materials
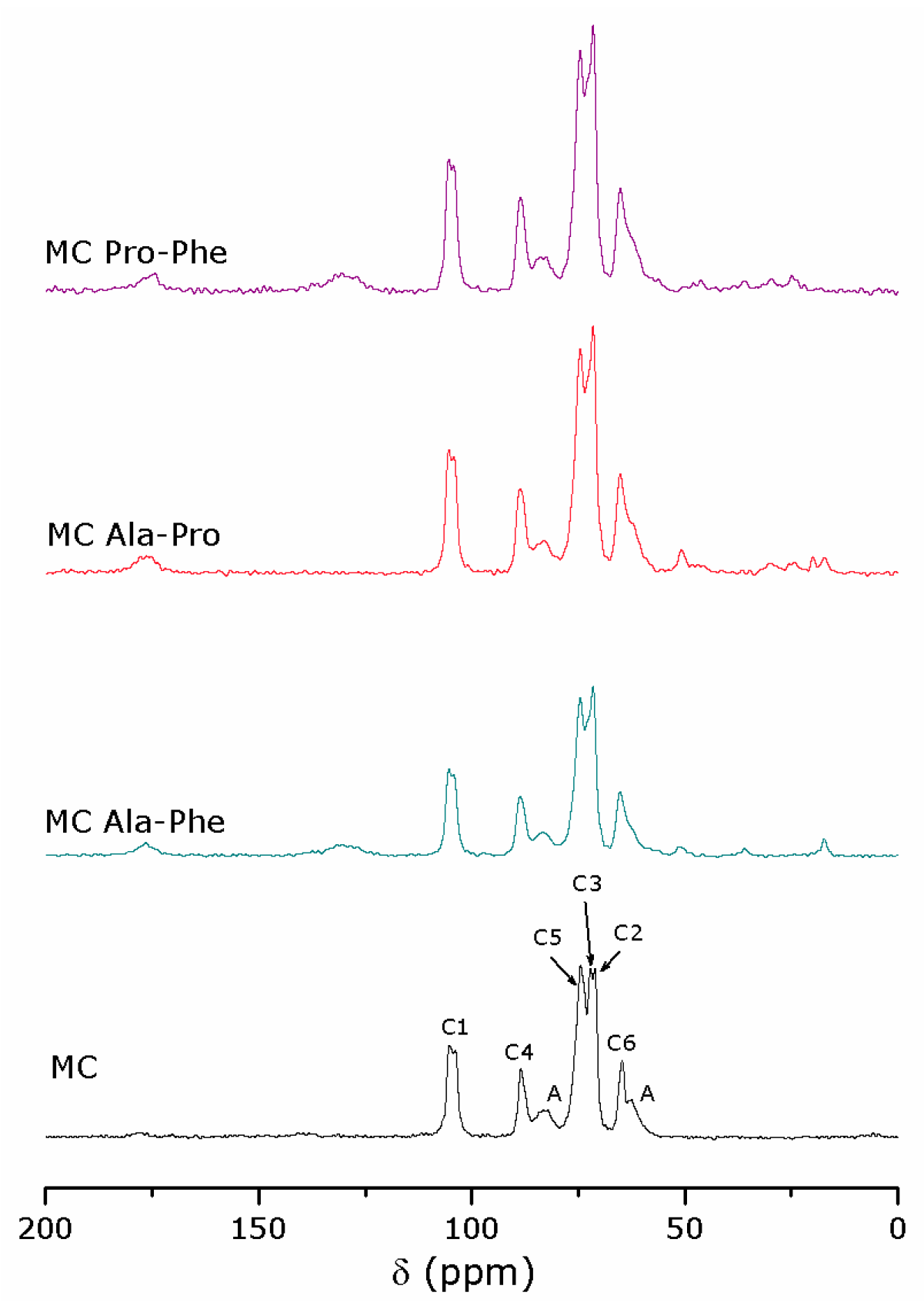
2.3. The Surface of Amino Acid-Modified Cellulose under Catalysis Conditions
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamm, B. Production of Platform Chemicals and SynGas from biomass. Angew. Chem. 2007, 119, 5146–5149. [Google Scholar] [CrossRef]
- Fan, L.T.; Gharpuray, M.Y.; Lee, H. Cellulose Hydrolysis; Springer: Berlin, Germany, 1987. [Google Scholar]
- Krassig, H.A. Cellulose-Structure; Accessibility and Reactivity; Gordon and Breach Science Publisher: Yverdon, Switzerland, 1993. [Google Scholar]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Takagaki, A.; Tagusagawa, C.; Domen, K. Glucose production from saccharides using layered transition metal oxide and exfoliated nanosheets as a water-tolerant solid acid catalyst. Chem. Commun. 2008, 5363–5365. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Tagusagawa, C.; Takagaki, A.; Iguchi, A.; Takanabe, K.; Kondo, J.N.; Ebitani, K.; Hayashi, S.; Tatsumi, T.; Domen, K. Highly active mesoporous Nb-W oxide solid acid catalyst. Angew. Chem. Int. Ed. 2010, 49, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Mladenov, R.; Wulfhorst, H.; Jager, G.; Spiess, A.C. Point by point analysis: How ionic liquid affects the enzymatic hydrolysis of native and modified cellulose. Green Chem. 2010, 12, 1959–1966. [Google Scholar] [CrossRef]
- Mok, W.S.; Antal, M.J., Jr.; Varhegyi, G. Productive and parasitic pathways in dilute acid-catalyzed hydrolysis of cellulose. Ind. Eng. Chem. Res. 1992, 31, 94–100. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Licursi, D.; Nassi, N. Levulinic acid production from waste biomass. BioResources 2012, 7, 1824–1835. [Google Scholar]
- Chen, H.; Yu, B.; Jin, S. Production of levulinic acid from steam exploded rice straw via solid superacid. Bioresour. Technol. 2011, 102, 3568–3570. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Hongzhang, C. Effect of the ash on enzymatic hydrolysis of steam-exploded rice straw. Bioresour. Technol. 2010, 101, 9114–9119. [Google Scholar] [CrossRef] [PubMed]
- Hick, S.M.; Griebel, C.; Restrepo, D.T.; Truitt, J.H.; Buker, E.J.; Bylda, C.; Blair, R.G. Mechanocatalysis for biomass-derived chemicals and fuels. Green Chem. 2010, 12, 468–474. [Google Scholar] [CrossRef]
- Hernández, M.; Lima, E.; Guzmán, A.; Vera, M.; Novelo, O.; Lara, V. A small change in the surface polarity of cellulose causes a significant improvement in its conversion to glucose and subsequent catalytic oxidation. Appl. Catal. B Environ. 2014, 144, 528–537. [Google Scholar] [CrossRef]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Trejo-O´Reily, J.A.; Cavaille, J.Y.; Gandini, A. The surface chemical modification of cellulosic fibers in view of their use in composite materials. Cellulose 1997, 4, 305–320. [Google Scholar] [CrossRef]
- Chan, W.C.; Higton, A.; Davies, J.S. Amino Acids. In Amino Acids, Peptides and Proteins; Davies, J.S., Ed.; RSC Publishing: Cambridge, UK, 2006; Volume 35, pp. 1–73. [Google Scholar]
- Park, S.; Baker, J.O.; Himmel, M.; Parrilla, P.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Johnson Ford, E.; Sharathkumar, M.; Shelby, T.; Rawlins, J. X-ray Diffraction of Cotton Treated with Neutralized Vegetable Oil-based Macromolecular Crosslinkers. J. Eng. Fibers Fabrics 2010, 5, 10–20. [Google Scholar]
- Oh, S.; Yoo, D.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Hinterstoisser, B.; Salmén, L. Application of dynamic 2D FTIR to cellulose. Vib. Spectrosc. 2000, 22, 111–118. [Google Scholar] [CrossRef]
- Wickholm, K.; Larsson, P.T.; Iversen, T. Assignment of non-crystalline forms in cellulose I by CP/MAS 13C NMR spectroscopy. Carbohydr. Res. 1998, 312, 123–129. [Google Scholar] [CrossRef]
- Pu, Y.; Ziemer, C.; Ragauskas, A.J. CP/MAS 13C NMR analysis of cellulase treated bleached softwood kraft pulp. Carbohydr. Res. 2006, 341, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Hotař, V. Fractal geometry for industrial data evaluation. Computers and Mathematics with Applications. Comput. Math. Appl. 2013, 66, 113–121. [Google Scholar] [CrossRef]
- Dubuc, B.; Tricot, C.; Zucker, S.W. Evaluating the fractal dimension of profiles. Phys. Rev. A Gen. Phys. 1989, 39, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fan, C.; Cheng, M.; Wang, X. Hydrolysis of cellulose over CsxH3-xPW12O40 (X = 1–3) Heteropoly acid catalysts. Chem. Eng. Technol. 2011, 34, 482–486. [Google Scholar] [CrossRef]
- Shimizu, K.; Furukawa, H.; Kobayashi, N.; Itaya, Y.; Satsuma, A. Effects of Brønsted and Lewis acidities on activity and selectivity of heteropolyacid-based catalysts for hydrolysis of cellobiose and cellulose. Green Chem. 2009, 11, 1627–1632. [Google Scholar] [CrossRef]
- Camacho, F.; Gonzalez-Tello, P.; Jurado, E.; Robles, A. Microcrystalline-Cellulose Hydrolysis with Concentrated Sulphuric Acid. J. Chem. Tech. Biotechnol. 1996, 67, 350. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Z.; Yin, D.; Xu, Q.; Liu, F.; Lu, C.; Mao, L. Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem. 2010, 12, 696–700. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Solid acid and microwave-assisted hydrolysis of cellulose in ionic liquid. Carbohydr. Res. 2009, 344, 2069–2072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Benoit, M.; De Oliveira Vigier, K.; Barrault, J.; Jégou, G.; Philippe, M.; Jérôme, F. Pretreatment of microcrystalline cellulose by ultrasounds: Effect of particle size in the heterogeneously-catalyzed hydrolysis of cellulose to glucose. Green Chem. 2013, 15, 963–969. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, F.; Zeng, H.-Y.; Guo, F. Production of glucose by hydrolysis of cellulose at 423 K in the presence of activated hydrotalcite nanoparticles. Bioresour. Technol. 2011, 102, 8017–8021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kwak, J.H.; Wang, Y.; Franz, J.A.; White, J.M.; Holladay, J.E. Effects of crystallinity on dilute acid hydrolysis of cellulose by cellulose ball-milling study. Energy Fuel 2006, 20, 807–811. [Google Scholar] [CrossRef]
- SDP v4.1 Copyright© 2004. XPS International, LLC; Compiled 17 January 2004.
- Glatter, O. Convolution square root of band-limited symmetrical functions and its application to small-angle X-ray scattering. J. Appl. Crystallogr. 1981, 14, 101–108. [Google Scholar] [CrossRef]
- Glatter, O.; Hainish, B. Improvements in real-space deconvolution of small angle scattering data. J. Appl. Crystallogr. 1984, 17, 435–441. [Google Scholar] [CrossRef]
- Glatter, O. Scattering studies on colloids of biological interest (amphiphilic systems). Prog. Colloid Polym. Sci. 1991, 84, 46–54. [Google Scholar]
- Harrison, A. Fractals in Chemistry; Oxford University Press Inc.: New York, NY, USA, 1995. [Google Scholar]
- Ibarra, I.A.; Loera, S.; Laguna, H.; Lima, E.; Lara, V. Irreversible adsorption of an Aztec dye on fractal surfaces. Chem. Mater. 2005, 17, 5763–5769. [Google Scholar] [CrossRef]
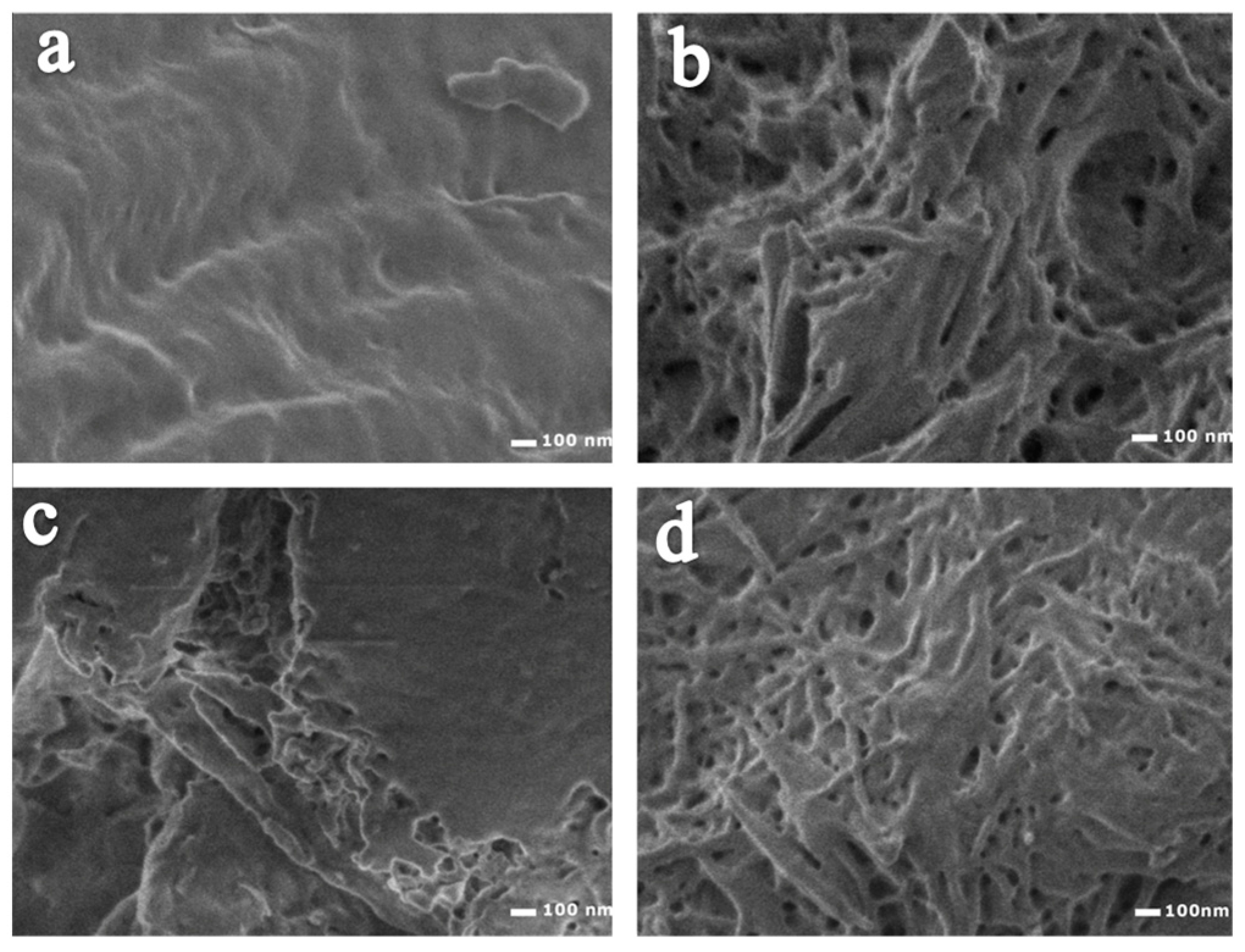
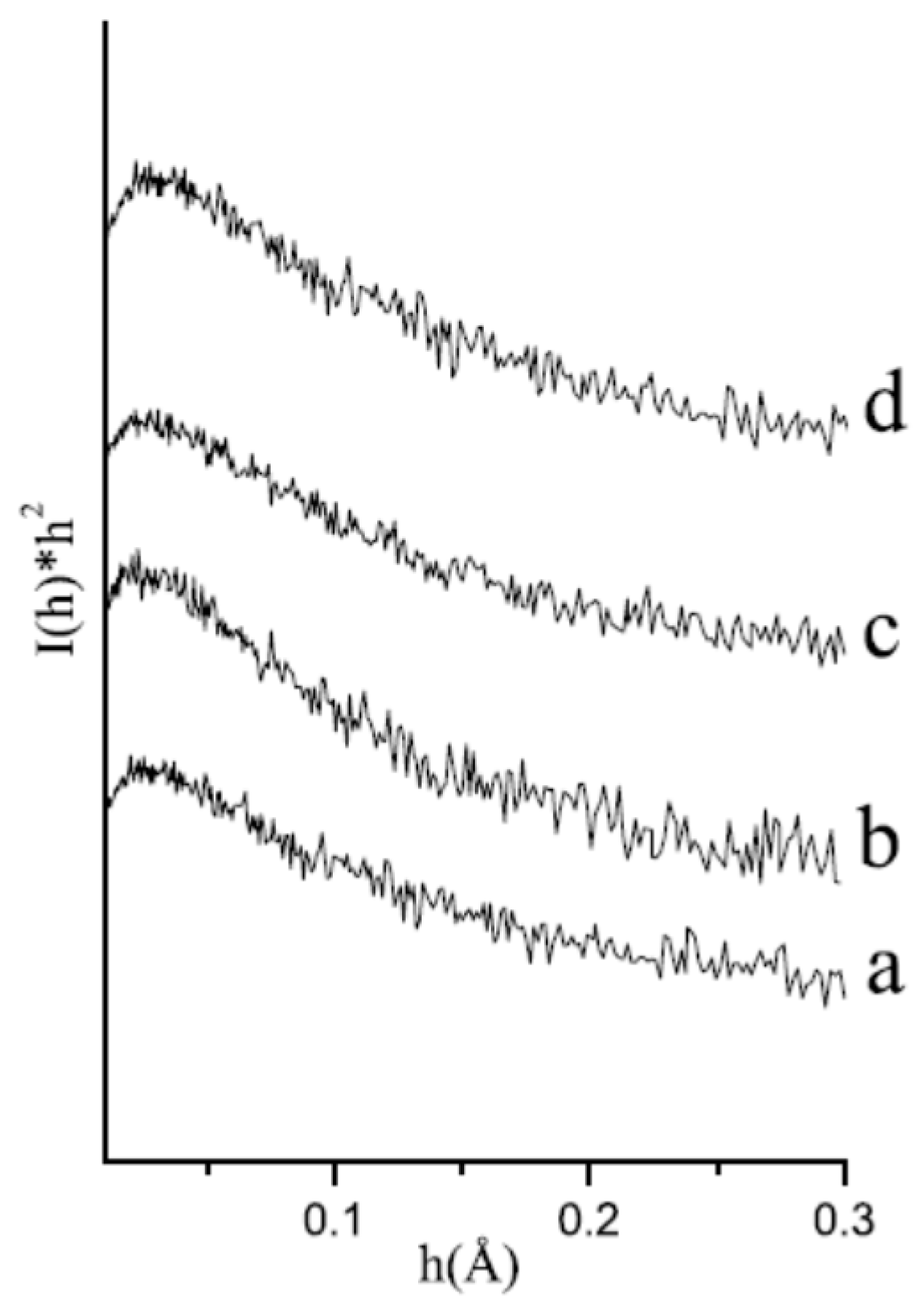
| Code Sample | Amino Acids | Amount (wt %) of Chemical Elements | Amount (wt %) of Amino Acids | ||
|---|---|---|---|---|---|
| C | O | N | Data | ||
| MC | - | 47.40 | 52.60 | - | - |
| MC Ala-Pro | Alanine and proline | 47.64 | 51.80 | 0.56 | 3.8 |
| MC Ala-Phe | Alanine and phenylalanine | 48.33 | 51.17 | 0.50 | 4.2 |
| MC Pro-Phe | Proline and phenylalanine | 48.01 | 51.58 | 0.41 | 3.9 |
| Sample | Fractal Dimension | Particle Size (µm) |
|---|---|---|
| MC | 2.1 | 49 |
| MC Ala-Pro | 2.2 | 68 |
| MC Ala-Phe | 2.3 | 72 |
| MC Pro-Phe | 2.6 | 86 |
| MC Phe | 2.7 | 70 |
| Sample | Time (h) | Cellulose Conversion (%) | Glucose Selectivity (%) | ||
|---|---|---|---|---|---|
| H2SO4 | H3PW12O40 | H2SO4 | H3PW12O40 | ||
| MC | 5 | 3 | 7 | 21 | 26 |
| 10 | 5 | 15 | 33 | 39 | |
| 15 | 13 | 26 | 49 | 70 | |
| 20 | 15 | 34 | 87 | 89 | |
| 25 | 15 | 33 | 91 | 90 | |
| MC Pro-Phe | 5 | 5 | 6 | 6 | 28 |
| 10 | 10 | 15 | 35 | 43 | |
| 15 | 20 | 51 | 79 | 87 | |
| 20 | 29 | 54 | 86 | 91 | |
| 25 | 29 | 55 | 93 | 92 | |
| Sample | Reaction Time (h) | Fractal Dimension |
|---|---|---|
| MC | 1 | 2.2 |
| 5 | 2.2 | |
| 10 | 2.3 | |
| 12 | 2.3 | |
| MC Pro-Phe | 1 | 2.6 |
| 5 | 2.7 | |
| 10 | 2.9 | |
| 12 | 2.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, M.; Hernández, M.; Ibarra, I.A.; Guzmán, A.; Lara, V.; Lima, E. Cellulose with a High Fractal Dimension Is Easily Hydrolysable under Acid Catalysis. Catalysts 2017, 7, 162. https://doi.org/10.3390/catal7050162
Díaz M, Hernández M, Ibarra IA, Guzmán A, Lara V, Lima E. Cellulose with a High Fractal Dimension Is Easily Hydrolysable under Acid Catalysis. Catalysts. 2017; 7(5):162. https://doi.org/10.3390/catal7050162
Chicago/Turabian StyleDíaz, Mariana, Magali Hernández, Ilich A. Ibarra, Ariel Guzmán, Victor Lara, and Enrique Lima. 2017. "Cellulose with a High Fractal Dimension Is Easily Hydrolysable under Acid Catalysis" Catalysts 7, no. 5: 162. https://doi.org/10.3390/catal7050162






