Use of Metal Catalysts Bearing Schiff Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters
Abstract
:1. Introduction
2. Discussion
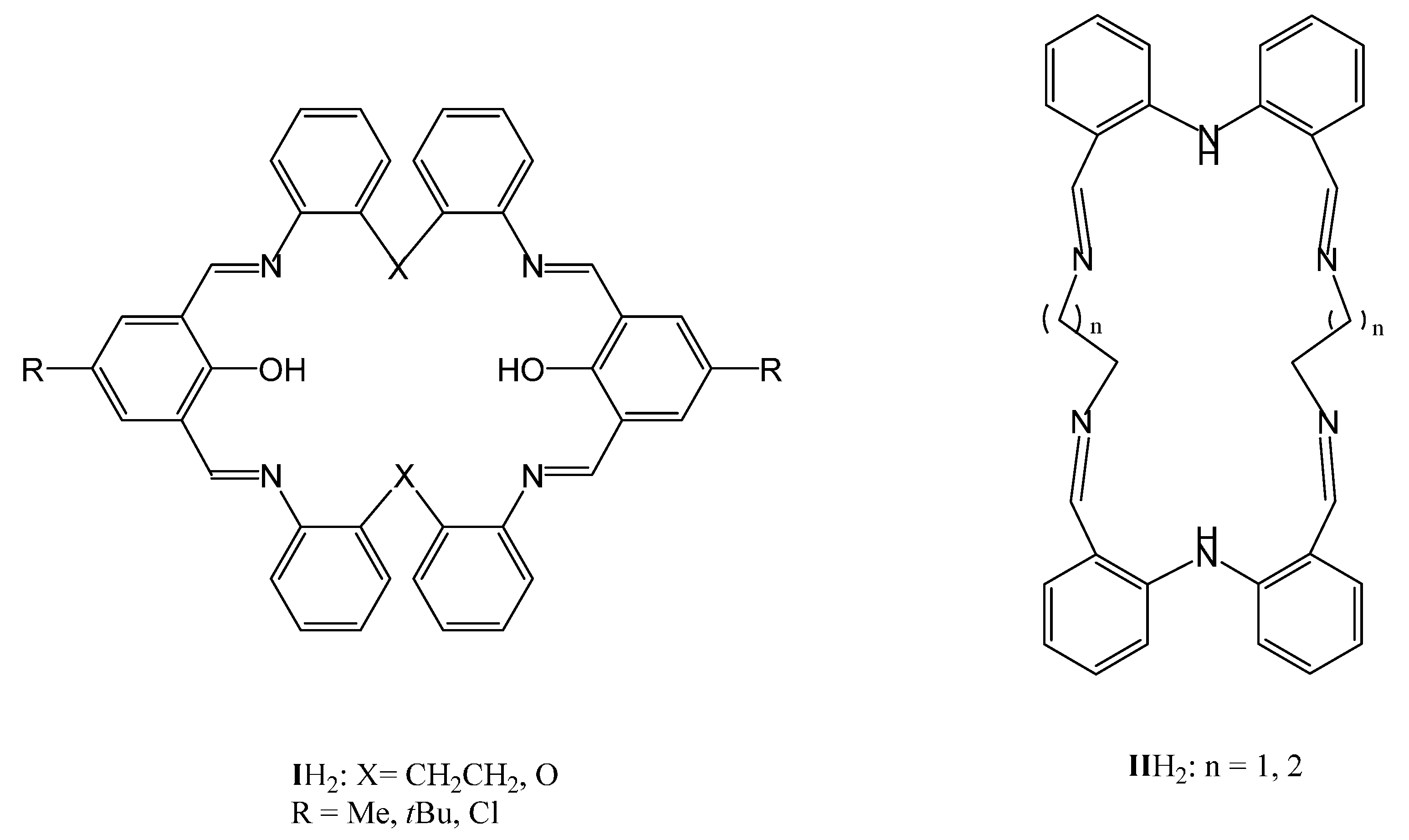
2.1. The Use of IH2 (X = CH2CH2)
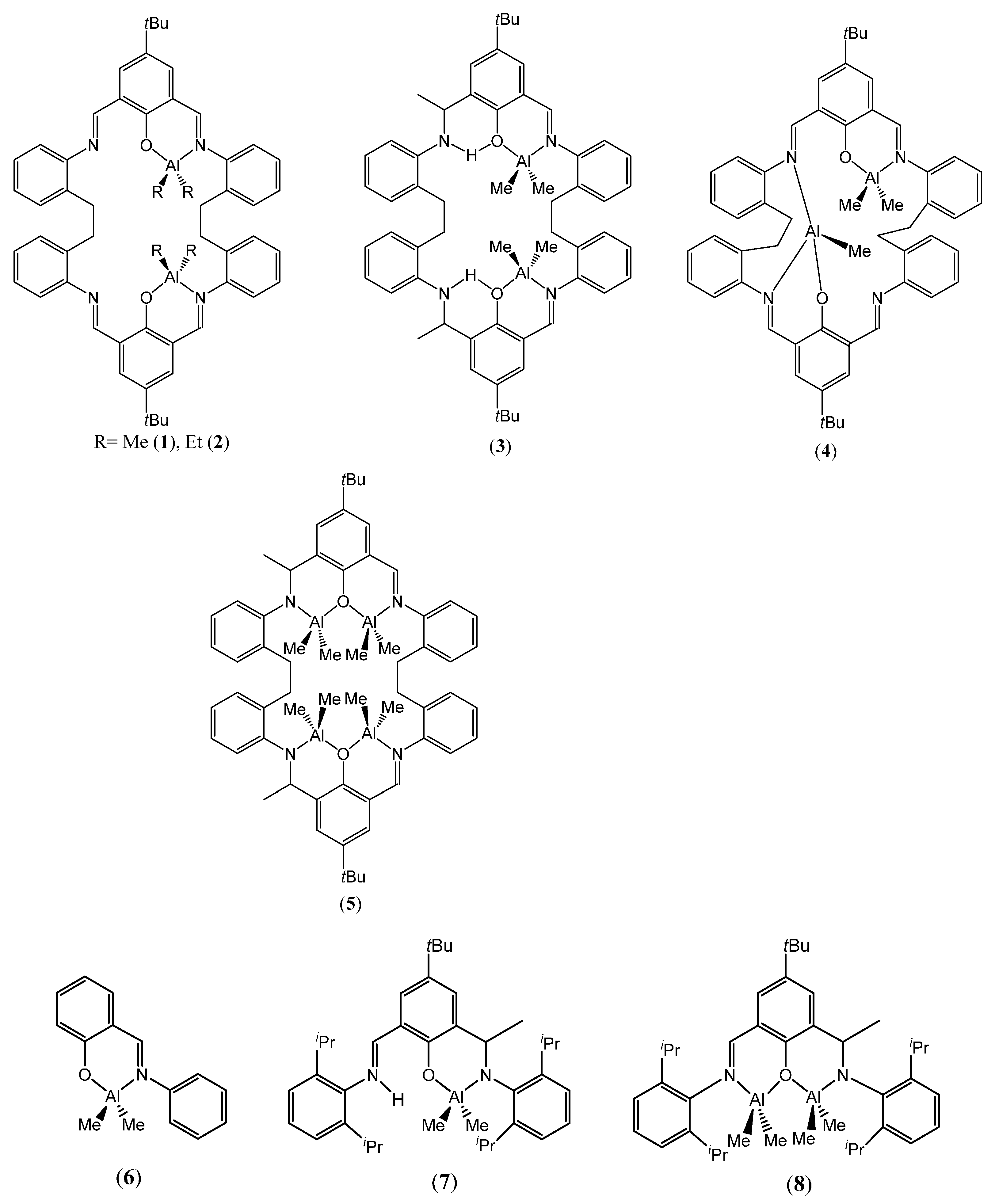
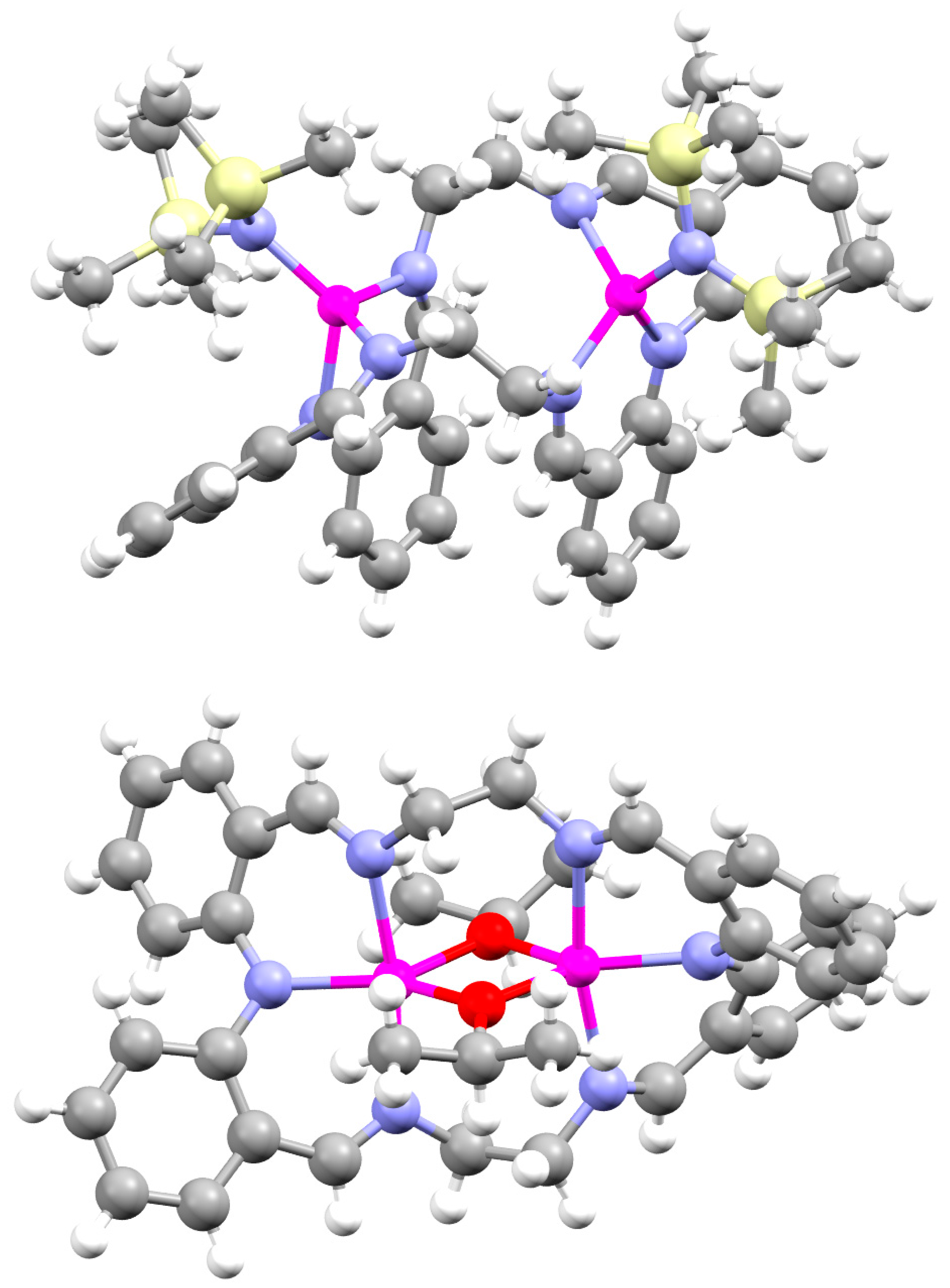
2.1.1. Organoaluminium Complexes
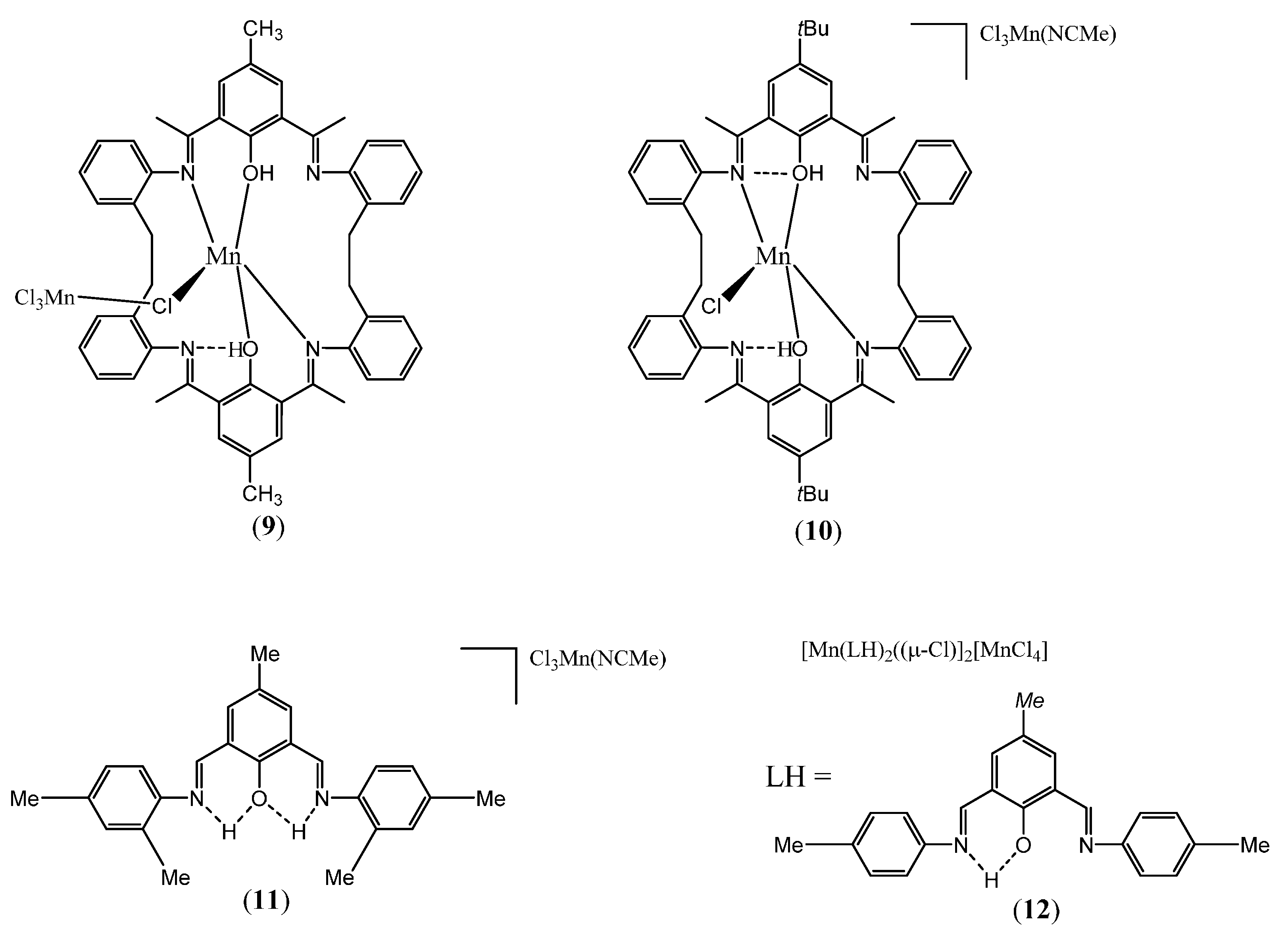
2.1.2. Manganese Complexes
2.2. Use of IH2 (X = O)
2.2.1. Organoaluminium Complexes
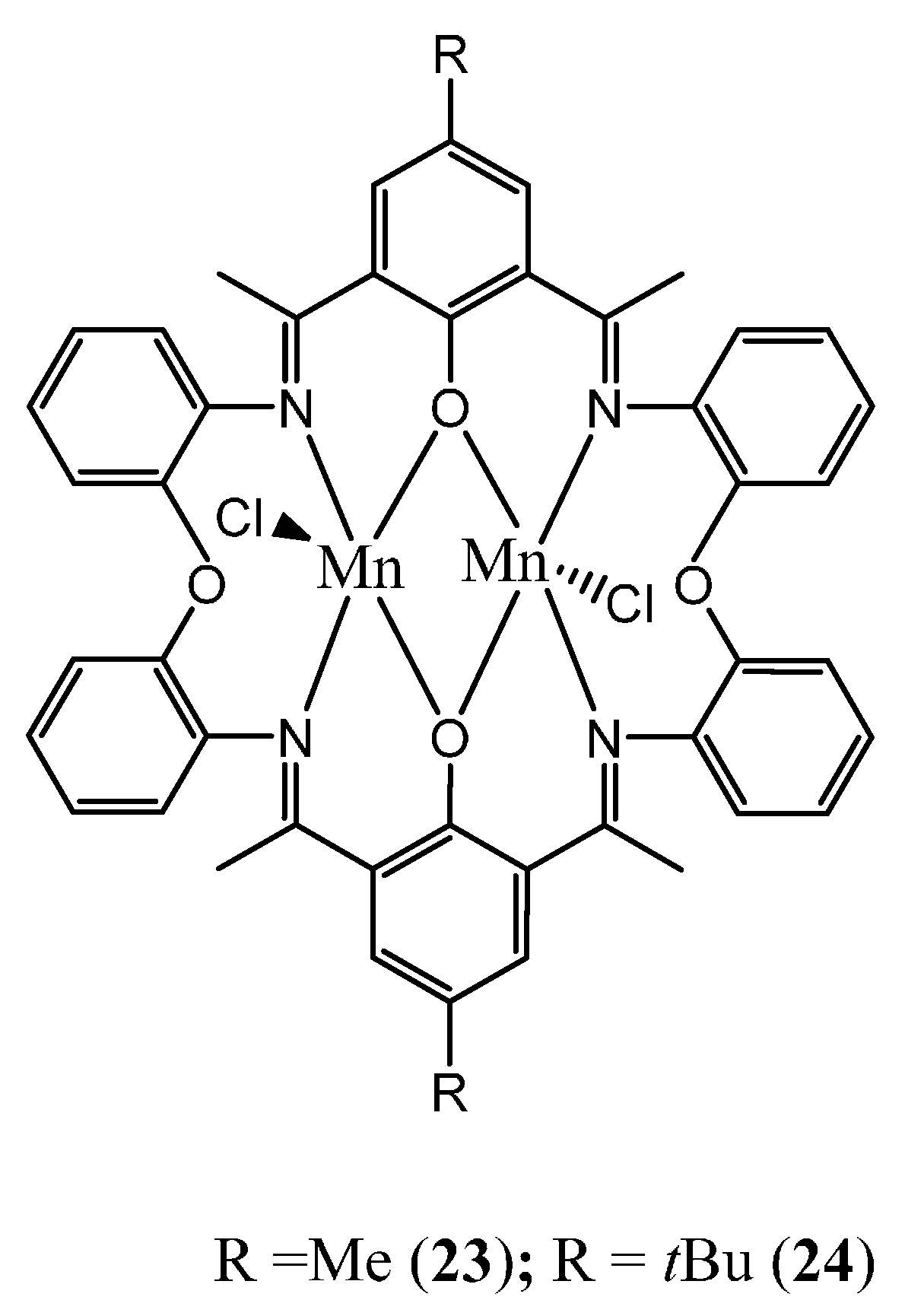
2.2.2. Manganese Complexes
2.3. Use of IIH2
3. Outlook
Conflicts of Interest
References
- Raquez, J.-M.; Mincheva, R.; Coulembier, O.; Dubois, P. Ring-opening Polymerization of Cyclic Esters: Industrial Synthesis, Properties, Applications, and Perspectives. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 761–777. [Google Scholar]
- Delferro, M.; Marks, T.J. Multinuclear Olefin Polymerization Catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Chen, H.-Y.; Huang, Y.-T.; Lu, W.-Y.; Chang, Y.-L.; Chiang, M.Y.; Lai, Y.-C.; Chen, H.-Y. Improvement in Titanium Complexes Bearing Schiff Base Ligands in the Ring-Opening Polymerization of l-Lactide: A Dinuclear System with Hydrazine-Bridging Schiff Base Ligands. Inorg. Chem. 2016, 55, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Clowes, L.; Redshaw, C.; Hughes, D.L. Vanadium-based pro-catalysts bearing depleted 1,3-calix[4]arenes for ethylene or ε-caprolactone polymerization. Inorg. Chem. 2011, 50, 7838–7845. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.J.; Lancaster, S.J.; Wright, J.A.; Elsegood, M.R.J.; Redshaw, C. Zinc calixarene complexes for the Ring Opening Polymerization of Cyclic Esters. Dalton Trans. 2014, 43, 18001–18809. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, K.-Q.; Feng, C.; Elsegood, M.R.J.; Prior, T.J.; Sun, X.; Redshaw, C. Ethyleneglycol Tungsten Complexes of calix[6 and 8]arenes: Synthesis, Characterization and ROP of ε-caprolactone. Dalton Trans. 2014, 43, 13612–13619. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.D.; Gagnon, K.J.; Teat, S.J.; McIntosh, R.D. Flexible macrocycles as versatile supports for catalytically active metal clusters. Chem. Commun. 2016, 52, 9071–9073. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C. Metallocalixarene catalysts: α-olefin polymerisation and ROP of cyclic esters. Dalton Trans. 2016, 45, 9018–9030. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, H.; Mao, X.; Pan, X.; Wu, J. Structures of potassium calix[4]arene crown ether inclusion complexes and application in polymerization of rac-lactide. Dalton Trans. 2016, 45, 9636–9645. [Google Scholar] [CrossRef] [PubMed]
- Gregoliński, J.; Lisowski, J.; Lis, T. New 2 + 2, 3 + 3 and 4 + 4 macrocycles derived from 1,2-diaminocyclohexane and 2,6-diformylpyridine. Org. Biomol. Chem. 2005, 3, 3161–3166. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.K.-H.; MacLachlan, M.J. [6 + 6] Schiff-base macrocycles with 12 imines: Giant analogues of cyclohexane. Chem. Commun. 2006, 23, 2480–2482. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, A.; Redshaw, C.; Hughes, D.L. Multi-Nuclear Alkylaluminium Macrocyclic Schiff Base Complexes: Influence of Pro-catalyst Structure on the Ring Opening Polymerisation of ε-Caprolactone. Chem. Commun. 2008, 39, 4717–4719. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, K.-Q.; Prior, T.J.; Hughes, D.L.; Arbaoui, A.; Bian, T.; Chao, Y.; Elsegood, M.R.J.; Redshaw, C. Structure and emission studies of Schiff-base [2 + 2] macrocycles derived from 2,2′-oxydianiline and the ROP capability of their organoaluminium complexes. Dalton Trans. 2016, 45, 11990–12005. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, K.-Q.; Wang, B.-Q.; Redshaw, C.; Elsegood, M.R.J.; Zhao, J.-L.; Yamato, T. Manganese coordination chemistry of bis(imino)phenoxide derived [2 + 2] Schiff-base macrocyclic ligands. Dalton Trans. 2016, 45, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Bennington, M.S.; White, A.J.P.; Williams, C.K.; Brooker, S. Macrocyclic Dizinc(II) Alkyl and Alkoxide Complexes: Reversible CO2 Uptake and Polymerization Catalysis Testing. Inorg. Chem. 2015, 54, 11842–11851. [Google Scholar] [CrossRef] [PubMed]
- Thevenon, A.; Romain, C.; Bennington, M.S.; White, A.J.P.; Davidson, H.J.; Brooker, S.; Williams, C.K. Dizinc Lactide Polymerization Catalysts: Hyperactivity by Control of Ligand Conformation and Metallic Cooperativity. Angew. Chem. Int. Ed. 2016, 55, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, S.; Zhou, S. Aluminium alkyl complexes: Synthesis, structure, and application in ROP of cyclic esters. Dalton Trans. 2016, 45, 4471–4485. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, T.; Pappalardo, D. Aluminium Alkyl Complexes Bearing Salicylaldiminato Ligands: Versatile Initiators in the Ring-Opening Polymerization of Cyclic Esters. Catalysts 2017, 7, 64–81. [Google Scholar] [CrossRef]
- Florjanczyk, Z.; Plichta, A.; Sobczak, M. Ring opening polymerization initiated by methylaluminoxane/AlMe3 complexes. Polymer 2006, 47, 1081–1090. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, K.-Q.; Elsegood, M.R.J.; Prior, T.J.; Sun, X.; Mo, S.; Redshaw, C. Organoaluminium complexes of o-,m-,p-anisidines: Synthesis, structural studies and ROP of ε-caprolactone. Catal. Sci. Technol. 2014, 4, 3025–3031. [Google Scholar] [CrossRef]
- Antelmann, B.; Chisholm, M.H.; Iyer, S.S.; Huffman, J.C.; Navarro-Llobet, D.; Pagel, M.; Simonsick, W.J.; Zhong, W. Molecular Design of Single Site Catalyst Precursors for the Ring-Opening Polymerization of Cyclic Ethers and Esters.2. Can Ring-Opening Polymerization of Propylene Oxide Occur by a Cis-Migratory Mechanism? Macromolecules 2001, 34, 3159–3175. [Google Scholar] [CrossRef]
- Braune, W.; Okuda, J. An Efficient Method for Controlled Propylene Oxide Polymerization: The Significance of Bimetallic Activation in Aluminium Lewis Acids. Angew. Chem. Int. Ed. 2003, 42, 64–68. [Google Scholar] [CrossRef]
- Al-Khafaji, Y.; Sun, X.; Prior, T.J.; Elsegood, M.R.J.; Redshaw, C. Tetraphenolate niobium and tantalum complexes for the ring opening polymerization of ɛ-caprolactone. Dalton Trans. 2015, 44, 12349–12356. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, Y.; Prior, T.J.; Elsegood, M.R.J.; Redshaw, C. Molybdenum(VI) imido complexes derived from chelating phenols: Synthesis, characterization and ɛ-caprolactone ROP capability. Catalysts 2015, 5, 1928–1947. [Google Scholar] [CrossRef]
- Ge, F.; Dan, Y.; Al-Khafaji, Y.; Prior, T.J.; Jiang, L.; Elsegood, M.R.J.; Redshaw, C. Vanadyl phenolate complexes for ring opening homo- and co-polymerisation of ε-caprolactone, l-lactide and rac-lactide. RSC Adv. 2016, 6, 4792–4802. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; White, A.J.P.; Williams, D.J. Synthesis and structural characterisation of aluminium imino-amide and pyridyl-amide complexes: Bulky monoanionic N,N chelate ligands via methyl group transfer. J. Organomet. Chem. 1998, 550, 453–456. [Google Scholar] [CrossRef]
- Gibson, V.C.; Nienhuis, D.; Redshaw, C.; White, A.J.P.; Williams, D.J. Bis(imino)phenoxide complexes of aluminium. Dalton Trans. 2004, 11, 1761–1765. [Google Scholar] [CrossRef] [PubMed]
- Gibson, V.C.; Redshaw, C.; Solan, G.A.; White, A.J.P.; Williams, D.J. Aluminum Alkyl-Mediated Route to Novel N,N,O-Chelates for Five-Coordinate Iron(II) Chloride Complexes: Synthesis, Structures, and Ethylene Polymerization Studies. Organometallics 2007, 26, 5119–5123. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C.; Hughes, D.L. One-pot conversion of tetraiminodiphenols to diiminodiaminodiphenols via methyl transfer at aluminum. Supramol. Chem. 2009, 21, 35. [Google Scholar] [CrossRef]
- Armitage, A.P.; Boyron, O.; Champouret, Y.D.M.; Patel, M.; Singh, K.; Solan, G.A. Dimethyl-Aluminium Complexes Bearing naphthyl-Substituted Pyridine-Alkylamides as Pro-Initiators for the Efficient ROP of ε-Caprolactone. Catalysts 2015, 5, 1425–1444. [Google Scholar] [CrossRef]
- Redshaw, C.; Rowan, M.A.; Warford, L.; Homden, D.M.; Arbaoui, A.; Elsegood, M.R.J.; Dale, S.H.; Yamato, T.; Pérez Casas, C.; Matsui, S.; et al. Oxo- and Imidovanadium Complexes incorporating Methylene- and Dimethyleneoxa-Bridged Calix[3]- and -[4]arenes: Synthesis, Structures and Ethylene Polymerisation Catalysis. Chem. Eur. J. 2007, 13, 1090–1107. [Google Scholar] [CrossRef] [PubMed]
- Castro-Osma, J.A.; Alonso-Moreno, C.; Márquez-Segovia, I.; Otero, A.; Lara-Sánchez, A.; Fernández-Baeza, J.; Rodriguez, A.M.; Sánchez-Barba, L.F.; Garcia-Martinez, J.C. Synthesis, structural characterization and catalytic evaluation of the ring opening polymerization of discrete five-coordinate alkyl aluminium complexes. Dalton Trans. 2013, 42, 9325–9337. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.J.; Lancaster, S.J.; Redshaw, C. Highly Selective and Immortal Magnesium Calixarene Complexes for Ring Opening Polymerization of rac-Lactide. ChemCatChem 2014, 6, 1892–1898. [Google Scholar] [CrossRef]
- Cameron, S.A.; Brooker, S. Metal-Free and Dicopper(II) Complexes of Schiff Base [2 + 2] Macrocycles Derived from 2,2′-Iminobisbenzaldehyde: Syntheses, Structures, and Electrochemistry. Inorg. Chem. 2011, 50, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chang, F.-F.; Zhao, P.-C.; Feng, G.-F.; Zhang, K.; Huang, W. Construction of Chiral [4 + 4] and [2 + 2] Schiff-Base Macrocyclic Zinc(II) Complexes Influenced by Counterions and Pendant Arms. Inorg. Chem. 2016, 55, 8260–8262. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redshaw, C. Use of Metal Catalysts Bearing Schiff Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2017, 7, 165. https://doi.org/10.3390/catal7050165
Redshaw C. Use of Metal Catalysts Bearing Schiff Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts. 2017; 7(5):165. https://doi.org/10.3390/catal7050165
Chicago/Turabian StyleRedshaw, Carl. 2017. "Use of Metal Catalysts Bearing Schiff Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters" Catalysts 7, no. 5: 165. https://doi.org/10.3390/catal7050165





