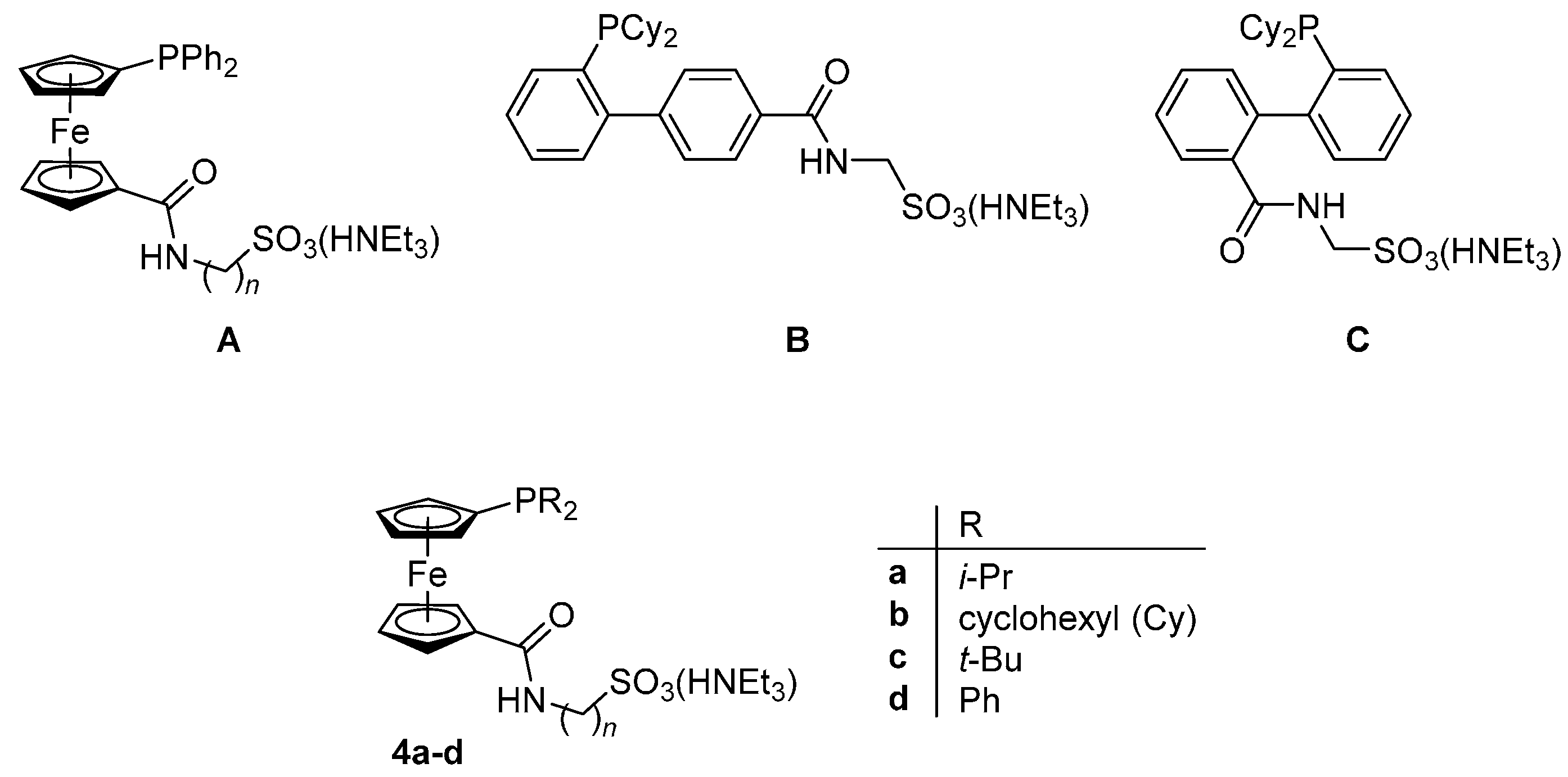
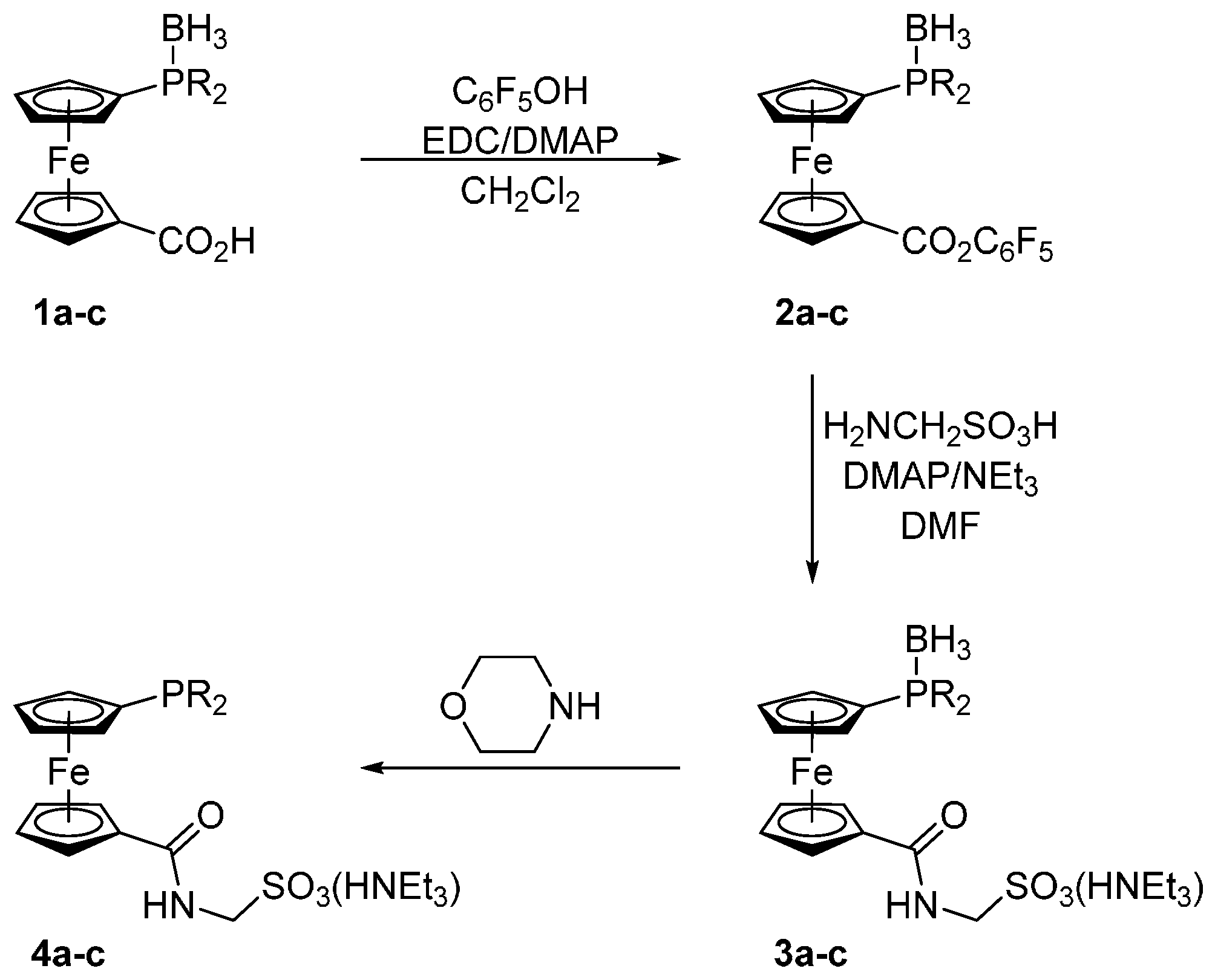
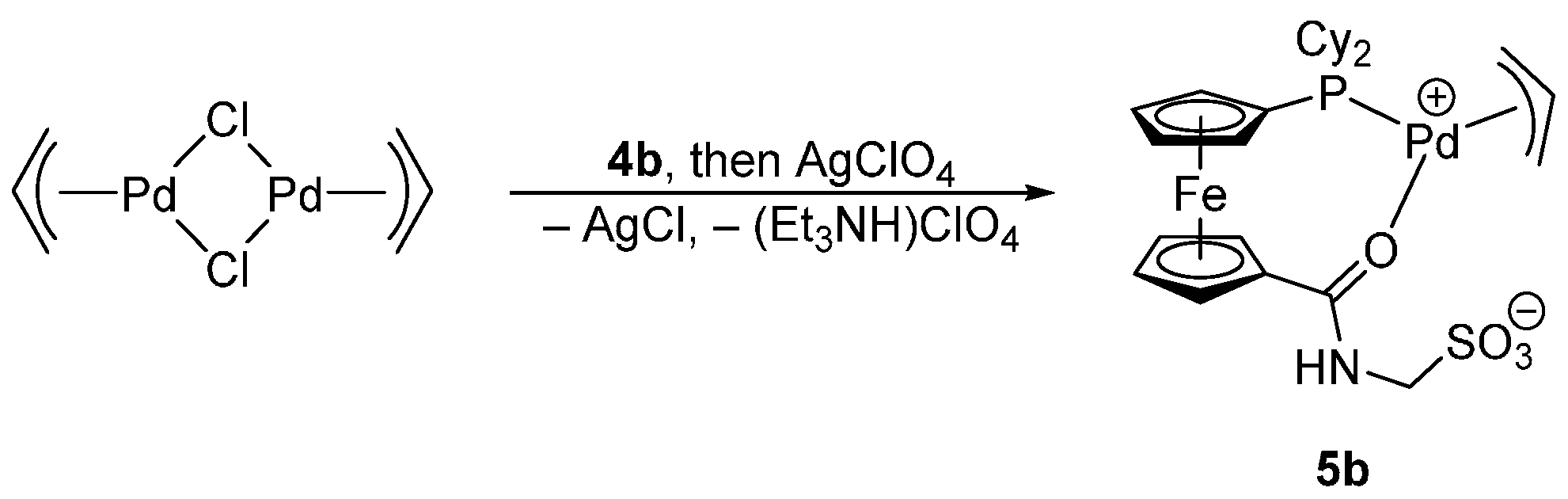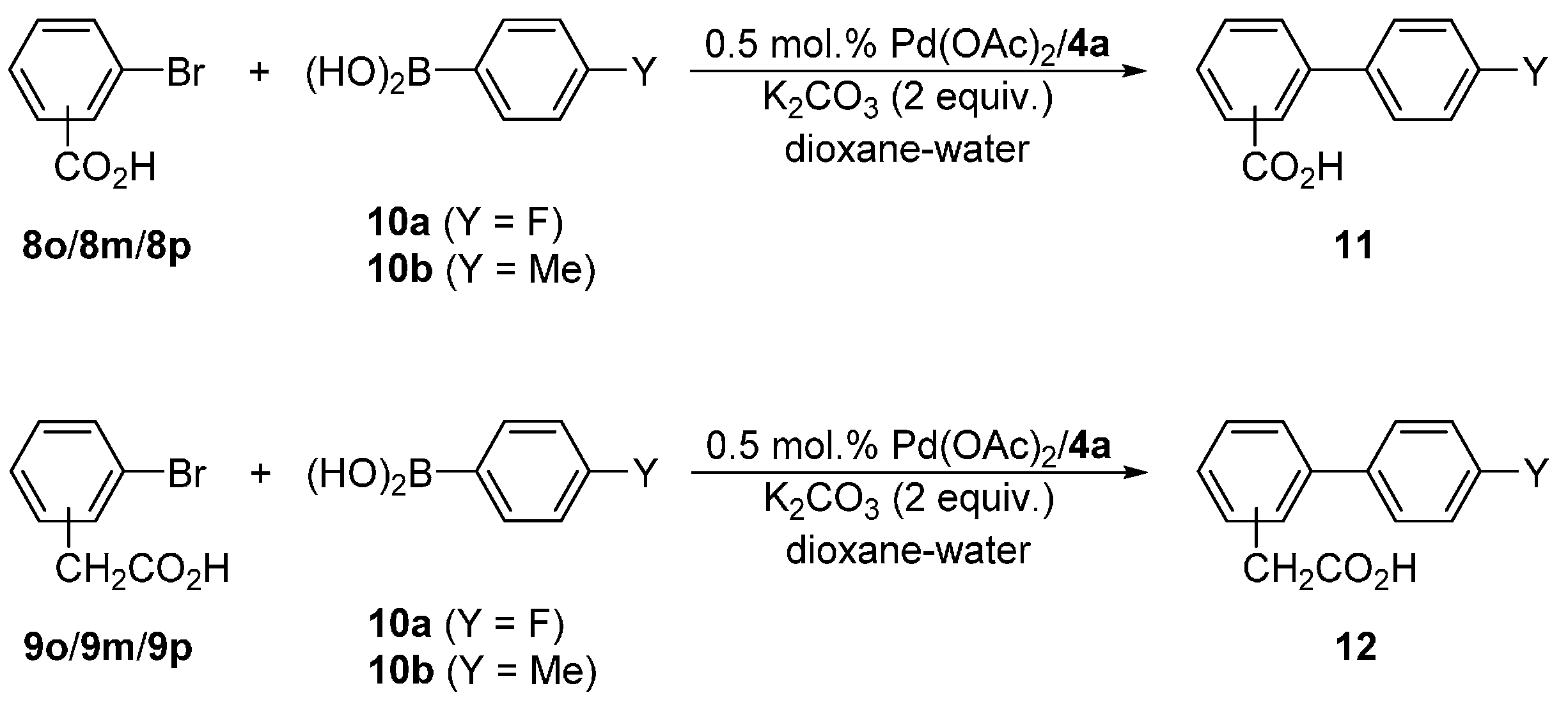3.2. General Procedure for the Synthesis of Esters H3B·R2PfcCO2C6F5 (2a–c)
The respective acids H3B·R2PfcCO2H (1a–c, 5.0 mmol), 1-ethyl-3-[3-(dimethylamino)propyl]-carbodiimide hydrochloride (1.15 g, 6.0 mmol), pentafluorophenol (1.10 g, 6.0 mmol) and 4-(dimethylamino)pyridine (122 mg, 1.0 mmol) were dissolved in dichloromethane (50 mL). After stirring overnight, brine (50 mL) was added to the reaction mixture and stirring was continued for another 10 min. Then, the organic layer was separated and washed with brine (50 mL). The combined aqueous phases were back-extracted with dichloromethane (20 mL). The combined organic layers were dried over anhydrous MgSO4 and evaporated to dryness. The crude product was purified by flash column chromatography over silica gel as described below.
3.2.1. Preparation of iPr2PfcCO2C6F5·BH3 (2a)
Ester 2a was synthesized from acid 1a (1.80 g, 5.0 mmol) following the general procedure and isolated as an orange solid. Ethyl acetate-hexane (1:8) was used during the chromatography. Only the first band from the product was collected. Yield: 2.46 g (94%).
1H NMR (CDCl3): δ 0.15–1.10 (br m, 3 H, BH3), 1.16 (dd, 3JHH = 7.1 Hz, 3JPH = 2.7 Hz, 6 H, CHMe2), 1.19 (dd, 3JHH = 7.1 Hz, 3JPH = 3.5 Hz, 6 H, CHMe2), 2.16 (d of sept, 2JPH = 10.1 Hz, 3JHH = 7.1 Hz, 2 H, CHMe2), 4.52 (vq, J′ = 1.8 Hz, 2 H, fc), 4.61 (d of vt, J’ ≈ 0.9, 1.9 Hz, 2 H, fc), 4.80 (vt, J′ = 2.0 Hz, 2 H, fc), 5.06 (vt, J′ = 2.0 Hz, 2 H, fc). 13C{1H} NMR (CDCl3): δ 17.16 (s, 2 C, CHMe2), 17.47 (d, 2JPC = 2 Hz, 2 C, CHMe2), 22.58 (d, 1JPC = 35 Hz, 2 C, CHMe2), 68.05 (s, Cipso-CO of fc), 70.65 (d, 1JPC = 53 Hz, Cipso-P of fc), 72.32 (s, 2 C, CH of fc), 73.44 (d, JPC = 7 Hz, 2 C, CH of fc), 73.59 (d, JPC = 6 Hz, 2 C, CH of fc), 75.43 (s, 2 C, CH of fc), 125.16 (s, Cipso of C6F5), 137.96 (dm, 1JFC = 253 Hz, Cmeta of C6F5), 139.45 (dm, 1JFC = 253 Hz, Cpara of C6F5), 141.48 (dm, 1JFC = 250 Hz, Cortho of C6F5), 167.45 (s, C=O). 19F{1H} NMR (CDCl3): δ = −162.60 (m, Fmeta of C6F5), −158.34 (t, 3JFF = 22 Hz, Fpara of C6F5), −153.08 (m, Fortho of C6F5) ppm. 31P{1H} NMR (CDCl3): δ 31.9 (br d). IR (Nujol): 2373 m, 2342 m, 1768 s, 1522 s, 1305 w, 1261 (s), 1203 w, 1169 w, 1142 w, 1072 s, 1058 m, 1023 m, 1011 m, 974 m, 885 m, 870 w, 852 w, 833 w, 753 w, 639 w, 611 w, 531 w, 505 w cm−1. MS (ESI+): m/z 549.1 ([M + Na]+), 565.1 ([M + K]+). Anal. Calc. for C23H25BF5FeO2P (526.06): C 52.51, H 4.79%. Found: C 52.50, H 4.69%.
3.2.2. Preparation of Cy2PfcCO2C6F5·BH3 (2b)
Ester 2b was obtained from acid 1b (2.20 g, 5.0 mmol) as described above and isolated as an orange solid. Ethyl acetate-hexane (1:10) was used during the chromatography. Only the first band from the product was collected. Yield: 2.54 g (84%).
1H NMR (CDCl3): δ 0.15–1.05 (br m, 3 H, BH3), 1.10–1.40 (m, 10 H, Cy), 1.65–1.73 (m, 2 H, Cy), 1.75–2.01 (m, 10 H, Cy), 4.49 (vq, J′ = 1.8 Hz, 2 H, fc), 4.60 (d of vt, J′ ≈ 0.9, 1.8 Hz, 2 H, fc), 4.78 (vt, J′ = 2.0 Hz, 2 H, fc), 5.04 (vt, J′ = 2.0 Hz, 2 H, fc). 13C{1H} NMR (CDCl3): δ 25.89 (d, JPC = 1 Hz, 2 C, CH2 of Cy), 26.77 (d, JPC = 2 Hz, 2 C, CH2 of Cy), 26.88 (d, JPC = 1 Hz, 2 C, CH2 of Cy), 26.95 (s, 2 C, CH2 of Cy), 27.27 (d, JPC = 2 Hz, 2 C, CH2 of Cy), 32.33 (d, 1JPC = 34 Hz, 2 C, CH of Cy), 68.03 (s, Cipso-CO of fc), 71.31 (d, 1JPC = 53 Hz, Cipso-P of fc), 72.34 (s, 2 C, CH of fc), 73.42 (d, JPC = 6 Hz, 2 C, CH of fc), 73.58 (d, JPC = 7 Hz, 2 C, CH of fc), 75.39 (s, 2 C, CH of fc), 125.18 (m, Cipso of C6F5), 137.95 (dm, 1JFC = 252 Hz, Cmeta of C6F5), 139.44 (dm, 1JFC = 253 Hz, Cpara of C6F5), 141.38 (dm, 1JFC = 251 Hz, Cortho of C6F5), 167.46 (s, C=O). 19F{1H} NMR (CDCl3): δ = −162.62 (m, Fmeta of C6F5), −158.33 (t, 3JFF = 22 Hz, Fpara of C6F5), −153.10 (m, Fortho of C6F5) ppm. 31P{1H} NMR (CDCl3): δ 24.3 (br d). IR (Nujol): 2373 m, 2342 m, 1768 s, 1522 s, 1305 w, 1261 m, 1203 w, 1169 w, 1142 w, 1072 s, 1058 m, 1023 m, 1011 s, 974 m, 885 m, 870 w, 852 w, 833 w, 753 w, 639 w, 611 w, 531 w, 505 w cm−1. MS (ESI+): m/z 629.2 ([M + Na]+), 645.1 ([M + K]+). Anal. Calc. for C29H33BF5FeO2P (606.18): C 57.50, H 5.50%. Found: C 57.46, H 5.49%.
3.2.3. Preparation of H3B·tBu2PfcCO2C6F5 (2c)
Ester 2c was synthesized from 1c (1.94 g, 5.0 mmol) by using the general procedure and isolated as an orange solid. Ethyl acetate-hexane (1:8) was used during the column chromatography. Only the first band from the product was collected. Yield: 2.06 g (93%).
1H NMR (CDCl3): δ 0.20–1.20 (br m, 3 H, BH3), 1.30 (d, 3JPH = 12.9 Hz, 18 H, CMe3), 4.62–4.65 (m, 4 H, fc), 4.80 (vt, J′ = 2.0 Hz, 2 H, fc), 5.03 (vt, J′ = 2.0 Hz, 2 H, fc). 13C{1H} NMR (CDCl3): δ 28.64 (d, 2JPC = 2 Hz, 6 C, CMe3), 33.42 (d, 1JPC = 27 Hz, 2 C, CMe3), 67.95 (s, Cipso-CO of fc), 72.46 (s, 2 C, CH of fc), 72.76 (d, 1JPC = 48 Hz, Cipso-P of fc), 73.51 (d, JPC = 6 Hz, 2 C, CH of fc), 74.91 (d, JPC = 6 Hz, 2 C, CH of fc), 75.92 (s, 2 C, CH of fc), 125.18 (m, Cipso of C6F5), 137.92 (dm, 1JFC = 252 Hz, Cmeta of C6F5), 139.44 (dm, 1JFC = 253 Hz, Cpara of C6F5), 143.37 (dm, 1JFC = 251 Hz, Cortho of C6F5), 167.45 (s, C=O). 19F{1H} NMR (CDCl3): δ = −162.60 (m, Fmeta of C6F5), −158.35 (t, 3JFF = 22 Hz, Fpara of C6F5), −153.01 (m, Fortho of C6F5) ppm. 31P{1H} NMR (CDCl3): δ 45.5 (br d). IR (Nujol): 2391 m, 2363 m, 2344 m, 1752 s, 1521 s, 1304 w, 1267 s, 1162 w, 1144 w, 1080 s, 1057 w, 1026 m, 1006 w, 995 m, 980 w, 910 w, 895 m, 874 w, 852 w, 833 m, 815 w, 755 w, 647 w, 626 w, 573 w, 531 w, 514 w cm−1. MS (ESI+): m/z 577.2 ([M + Na]+), 593.1 ([M + K]+). Anal. Calc. for C25H29BF5FeO2P (554.11): C 54.19, H 5.28%. Found: C 54.18, H 5.22%.
3.3. General Procedure for the Synthesis of Amides R2PfcCONHCH2SO3(HNEt3)·BH3 (3a–c)
The respective ester 1 (3.0 mmol), aminomethanesulfonic acid (0.51 g, 4.5 mmol) and 4-(dimethylamino)pyridine (122 mg, 1.0 mmol) were dissolved in a mixture of N,N-dimethylformamide (10 mL) and triethylamine (2 mL). After stirring overnight, the solvents were evaporated under vacuum and the residual DMF was removed by trituration with diethyl ether (50 mL). The crude product was purified by flash column chromatography over silica gel. The column was packed in CH2Cl2–MeOH–Et3N (100:5:5) and the product was eluted with CH2Cl2–MeOH (100:5). The first orange band was collected and evaporated. The solid residue was triturated with diethyl ether (3 × 20 mL) to remove residual triethylamine and, finally, dried under vacuum.
3.3.1. Preparation of iPr2PfcCONHCH2SO3(HNEt3)·BH3 (3a)
Amide 3a was synthesized from ester 2a (1.58 g, 3.0 mmol) following the general procedure described above and isolated as an orange crystalline solid after crystallization from hot ethyl acetate. Yield: 1.32 g (79%).
1H NMR (CD2Cl2): δ 0.10–1.10 (br m, 3 H, BH3), 1.11 (dd, 3JHH = 7.1 Hz, 3JPH = 1.4 Hz, 6 H, CHMe2), 1.15 (d, 3JHH = 7.1 Hz, 6 H, CHMe2), 1.31 (t, 3JHH = 7.3 Hz, 9 H, CH3 of Et3NH+), 2.13 (d of sept, 2JPH = 10.1 Hz, 3JHH = 7.1 Hz, 2 H, CHMe2), 3.10 (q, 3JHH = 7.3 Hz, 6 H, CH2 of Et3NH+), 4.40 (vq, J′ = 1.7 Hz, 2 H, fc), 4.43 (d, 3JHH = 6.6 Hz, 2 H, CH2N), 4.50 (vt, J′ = 2.0 Hz, 2 H, fc), 4.65 (d of vt, J′ ≈ 0.9, 1.9 Hz, 2 H, fc), 4.83 (vt, J′ = 2.0 Hz, 2 H, fc), 6.92 (t, 3JHH = 6.5 Hz, 1 H, NH). 13C{1H} NMR (CD2Cl2): δ 8.85 (s, 3 C, CH3 of Et3NH+), 17.30 (s, 2 C, CHMe2), 17.62 (d, 2JPC = 2 Hz, 2 C, CHMe2), 22.82 (d, 1JPC = 35 Hz, 2 C, CHMe2), 46.46 (s, 3 C, CH2 of Et3NH+), 56.05 (s, CH2N), 69.36 (d, 1JPC = 55 Hz, Cipso-P of fc), 70.09 (s, 2 C, CH of fc), 73.35 (s, 2 C, CH of fc), 73.59 (s, 2 C, CH of fc), 73.66 (d, JPC = 1 Hz, 2 C, CH of fc), 76.96 (s, Cipso-CO of fc), 169.54 (s, C=O). 31P{1H} NMR (CD2Cl2): δ 31.6 (br d). IR (Nujol): 3312 m, 2376 w, 2360 m, 2332 m, 1654 s, 1533 s, 1499 m, 1366 m, 1313 w, 1285 w, 1245 m, 1216 m, 1165 s, 1070 m, 1035 m, 1012 m, 980 m, 899 w, 888 w, 868 w, 852 w, 840 m, 831 m, 812 w, 797 w, 754 m, 692 w, 667 w, 632 w, 615 m, 585 w, 535 w, 515 m cm−1. MS (ESI+): m/z 555.2 ([M + H]+), 656.4 ([M + HNEt3]+); MS (ESI−): m/z 451.9 ([M − HNEt3]+). Anal. Calc. for C24H44BFeN2O4PS (554.30): C 52.00, H 8.00, N 5.06%. Found: C 51.90, H 8.03, N 4.93%.
3.3.2. Preparation of Cy2PfcCONHCH2SO3(HNEt3)·BH3 (3b)
Amide 3b was synthesized from 2b (1.82 g, 3.0 mmol) according to the general procedure and was isolated as an orange crystalline solid after crystallization from hot ethyl acetate. Yield: 1.67 g (88%).
1H NMR (CD2Cl2): δ 0.04–1.04 (br m, 3 H, BH3), 1.08–1.38 (m, 10 H, Cy), 1.31 (t, 3JHH = 7.3 Hz, 9 H, CH3 of Et3NH+), 1.64–1.71 (m, 2 H, Cy), 1.74–1.99 (m, 10 H, Cy), 3.11 (q, 3JHH = 7.3 Hz, 6 H, CH2 of Et3NH+), 4.37 (vq, J′ = 1.8 Hz, 2 H, fc), 4.42 (d, 3JHH = 6.6 Hz, 2 H, CH2N), 4.47 (vt, J′ = 2.0 Hz, 2 H, fc), 4.64 (d of vt, J′ ≈ 0.9, 1.8 Hz, 2 H, fc), 4.80 (vt, J′ = 1.9 Hz, 2 H, fc), 6.85 (t, 3JHH = 6.6 Hz, 1 H, NH). 13C{1H} NMR (CD2Cl2): δ 8.87 (s, 3 C, CH3 of Et3NH+), 26.39 (d, JPC = 2 Hz, 2 C, CH2 of Cy), 27.15–27.30 (m, 6 C, CH2 of Cy), 27.57 (d, JPC = 2 Hz, 2 C, CH2 of Cy), 32.49 (d, 1JPC = 35 Hz, 2 C, CH of Cy), 46.50 (s, 3 C, CH2 of Et3NH+), 56.02 (s, CH2N), 69.99 (d, 1JPC = 55 Hz, Cipso-P of fc), 70.04 (s, 2 C, CH of fc), 73.35 (s, 2 C, CH of fc), 73.48 (d, JPC = 7 Hz, 2 C, CH of fc), 73.70 (d, JPC = 7 Hz, 2 C, CH of fc), 76.98 (s, Cipso-CO of fc), 169.44 (s, C=O). 31P{1H} NMR (CD2Cl2): δ 24.1 (br d). IR (Nujol): 3531 m, 3456 m, 3337 m, 2378 m, 2334 m, 1648 s, 1523 m, 1296 w, 1253 w, 1211 m, 1168 s, 1053 m, 890 w, 853 w, 826 w, 765 w, 635 w, 616 w, 528 w, 506 w cm−1. MS (ESI+): m/z 635.2 ([M + H]+), 736.5 ([M + HNEt3]+); MS (ESI−): m/z 532.0 ([M − HNEt3]+). Anal. Calc. for C30H52BFeN2O4PS (634.43): C 56.79, H 8.26, N 4.42%. Found: C 56.64, H 8.31, N 4.29%.
3.3.3. Preparation of tBu2PfcCONHCH2SO3(HNEt3)·BH3 (3c)
Amide 3c was synthesized from 2c (1.66 g, 3.0 mmol) according to the general procedure and isolated as an orange solid. The compound decomposes upon attempted crystallization. Yield: 1.62 g (92%).
1H NMR (CD2Cl2): δ 0.15–1.15 (br m, 3 H, BH3), 1.27 (d, 3JPH = 12.8 Hz, 18 H, CMe3), 1.31 (t, 3JHH = 7.3 Hz, 9 H, CH3 of Et3NH+), 3.10 (q, 3JHH = 7.3 Hz, 6 H, CH2 of Et3NH+), 4.43 (d, 3JHH = 6.6 Hz, 2 H, CH2N), 4.48 (vt, J′ = 2.0 Hz, 2 H, fc), 4.50 (vq, J′ = 1.7 Hz, 2 H, fc), 4.69 (d of vt, J′ ≈ 0.9, 1.9 Hz, 2 H, fc), 4.80 (vt, J′ = 2.0 Hz, 2 H, fc), 6.91 (t, 3JHH = 6.5 Hz, 1 H, NH). 13C{1H} NMR (CD2Cl2): δ 8.86 (s, 3 C, CH3 of Et3NH+), 28.84 (d, 2JPC = 2 Hz, 6 C, CMe3), 33.60 (d, 1JPC = 28 Hz, 2 C, CMe3), 46.44 (s, 3 C, CH2 of Et3NH+), 56.03 (s, CH2N), 70.23 (s, 2 C, CH of fc), 71.66 (d, 1JPC = 49 Hz, Cipso-P of fc), 73.49 (d, JPC = 6 Hz, 2 C, CH of fc), 73.85 (s, 2 C, CH of fc), 75.16 (d, JPC = 7 Hz, 2 C, CH of fc), 76.93 (s, Cipso-CO of fc), 169.42 (s, C=O). 31P{1H} NMR (CD2Cl2): δ 45.1 (br m). IR (Nujol): 3296 m, 2382 m, 2363 m, 2343 m, 1656 s, 1517 m, 1498 m, 1316 w, 1299 w, 1251 w, 1211 m, 1161 s, 1068 w, 1039 s, 1014 m, 983 m, 895 w, 858 w, 838 w, 814 w, 751 w, 647 w, 613 w, 598 w, 516 w cm−1. MS (ESI+): m/z 684.4 ([M + HNEt3]+), 605.3 ([M + Na]+); MS (ESI−): m/z 479.9 ([M − HNEt3]+). Anal. Calc. for C26H48BFeN2O4PS·0.1CH2Cl2 (590.84): C 53.05, H 8.22, N 4.74%. Found: C 52.98, H 7.88, N 4.49%.
3.4. General Procedure for Deprotection of Borane Aducts 3
A Schlenk flask was charged with a borane adduct 3 (1.5 mmol) and freshly distilled morpholine (10 mL). The reaction mixture was thoroughly degassed by five freeze–pump–thaw cycles and then heated at 65 °C for 16 h. Next, the morpholine was removed under vacuum and the crude product was purified by flash column chromatography over silica gel. The chromatographic column was packed in CH2Cl2–MeOH–Et3N (100:5:5) and the product was eluted with CH2Cl2–MeOH (100:5). The first orange band was collected and evaporated. The residue was triturated with diethyl ether (3 × 20 mL) to remove residual triethylamine.
3.4.1. Preparation of iPr2PfcCONHCH2SO3(HNEt3) (4a)
Amide 4a was prepared from 3a (0.831 g, 1.5 mmol) as outlined above and isolated as an orange crystalline solid after crystallization from hot ethyl acetate. Yield: 0.657 g (81%).
1H NMR (CD2Cl2): δ 1.05 (dd, 3JHH = 7.0 Hz, 3JPH = 2.1 Hz, 6 H, CHMe2), 1.08 (dd, 3JHH = 7.0 Hz, 3JPH = 4.2 Hz, 6 H, CHMe2), 1.32 (t, 3JHH = 7.3 Hz, 9 H, CH3 of Et3NH+), 1.91 (d of sept, 2JPH = 2.0 Hz, 3JHH = 7.3 Hz, 2 H, CHMe2), 3.12 (q, 3JHH = 7.3 Hz, 6 H, CH2 of Et3NH+), 4.25 (vq, J′ = 1.6 Hz, 2 H, fc), 4.31 (vt, J′ = 1.9 Hz, 2 H, fc), 4.41 (d, 3JHH = 6.5 Hz, 2 H, CH2N), 4.47 (vt, J′ = 1.8 Hz, 2 H, fc), 4.68 (vt, J′ = 1.9 Hz, 2 H, fc), 6.77 (t, 3JHH = 5.8 Hz, 1 H, NH). 13C{1H} NMR (CD2Cl2): δ 8.90 (s, 3 C, CH3 of Et3NH+), 20.03 (d, 2JPC = 11 Hz, 2 C, CHMe2), 20.25 (d, 2JPC = 15 Hz, 2 C, CHMe2), 23.76 (d, 1JPC = 12 Hz, 2 C, CHMe2), 46.61 (s, 2 C, CH2 of Et3NH+), 56.08 (s, CH2N), 69.66 (s, 2 C, CH of fc), 72.21 (d, JPC = 2 Hz, 2 C, CH of fc), 72.75 (s, 2 C, CH of fc), 73.13 (d, JPC = 10 Hz, 2 C, CH of fc), 76.28 (s, Cipso-CO of fc), 76.28 (d, 1JPC = 10 Hz, Cipso-P of fc), 170.09 (s, C=O). 31P{1H} NMR (CD2Cl2): δ − 0.1 (s). IR (Nujol): 3509 s, 3448 s, 3285 s, 1659 s, 1637 s, 1540 s, 1342 w, 1315 m, 1286 m, 1243 m, 1209 m, 1157 s, 1073 w, 1038 s, 964 w, 893 w, 834 m, 769 w, 658 w, 635 w, 616 m, 595 w, 541 w, 525 w, 517 w cm−1. MS (ESI+): m/z 440.0 ([M + H − NEt3]+), 541.2 ([M + H]+); MS (ESI−): m/z 437.9 ([M − HNEt3]+). Anal. Calc. for C24H41BFeN2O4PS (540.47): C 53.33, H 7.65, N 5.18%. Found: C 53.07, H 7.69, 5.24%.
3.4.2. Preparation of Cy2PfcCONHCH2SO3(HNEt3) (4b)
Amide 4b was synthesized from 3b (0.952 g, 1.5 mmol) by following the general procedure and isolated as an orange crystalline solid after crystallization from hot ethyl acetate. Yield: 0.78 g (84%).
1H NMR (CD2Cl2): δ 0.96–1.37 (m, 10 H, Cy), 1.32 (t, 3JHH = 7.3 Hz, 9 H, CH3 of Et3NH+), 1.61–1.82 (m, 10 H, Cy), 1.86–1.95 (m, 2 H, Cy), 3.12 (q, 3JHH = 7.3 Hz, 6 H, CH2 of Et3NH+), 4.22 (vq, J′ = 1.6 Hz, 2 H, fc), 4.29 (vt, J′ = 1.9 Hz, 2 H, fc), 4.42 (d, 3JHH = 6.5 Hz, 2 H, CH2N), 4.46 (vt, J′ = 1.8 Hz, 2 H, fc), 4.67 (vt, J′ = 1.9 Hz, 2 H, fc), 6.86 (t, 3JHH = 6.3 Hz, 1 H, NH). 13C{1H} NMR (CD2Cl2): δ 8.91 (s, 3 C, CH3 of Et3NH+), 26.87 (d, JPC = 1 Hz, 2 C, CH2 of Cy), 27.65 (d, JPC = 9 Hz, 2 C, CH2 of Cy), 27.78 (d, JPC = 11 Hz, 2 C, CH2 of Cy), 30.48 (d, JPC = 2 Hz, 2 C, CH2 of Cy), 30.60 (s, 2 C, CH2 of Cy), 33.73 (d, JPC = 12 Hz, 2 C, CH of Cy), 46.63 (s, 3 C, CH2 of Et3NH+), 56.09 (s, CH2N), 69.67 (s, 2 C, CH of fc), 72.16 (d, JPC = 3 Hz, 2 C, CH of fc), 72.76 (d, JPC = 1 Hz, 2 C, CH of fc), 73.32 (d, JPC = 10 Hz, 2 C, CH of fc), 76.23 (s, Cipso-CO of fc), 170.21 (s, C=O). One ferrocene resonance (Cipso-P) was not identified, presumably due to overlaps. 31P{1H} NMR (CD2Cl2): δ − 8.3 (s). IR (Nujol): 3309 s, 1653 s, 1533 s, 1314 m, 1284 m, 1247 w, 1217 m, 1169 s, 1077 w, 1044 s, 966 w, 891 w, 847 w, 831 w, 771 w, 613 m, 547 w, 533 w, 516 w cm−1. MS (ESI+): m/z 621.3 ([M + H]+); MS (ESI−): m/z 518.0 ([M − HNEt3]+). Anal. Calc. for C30H49BFeN2O4PS (620.59): C 58.06, H 7.96, N 4.52%. Found: C 57.81, H 7.82, N 4.50%.
3.4.3. Preparation of tBu2PfcCONHCH2SO3(HNEt3) (4c)
Amide 4c was synthesized from 3c (0.874 g, 1.5 mmol) as described above and isolated as an orange solid. Yield: 0.605 g (71%).
1H NMR (CD2Cl2): δ 1.18 (d, 3JPH = 11.2 Hz, 18 H, CMe3), 1.32 (t, 3JHH = 6.0 Hz, 9 H, CH3 of Et3NH+), 3.12 (q, 3JHH = 6.4 Hz, 6 H, CH2 of Et3NH+), 4.32–4.45 (m, 4 H, fc), 4.42 (d, 3JHH = 6.5 Hz, 2 H, CH2N), 4.54 (vt, J′ = 1.8 Hz, 2 H, fc), 4.71 (vt, J′ = 1.9 Hz, 2 H, fc), 6.76 (t, 3JHH = 5.4 Hz, 1 H, NH). 13C{1H} NMR (CD2Cl2): δ 8.92 (s, 3 C, CH3 of Et3NH+), 30.92 (d, 2JPC = 13 Hz, 6 C, CMe3), 33.00 (d, 1JPC = 20 Hz, 2 C, CMe3), 46.58 (s, 3 C, CH2 of Et3NH+), 56.10 (s, CH2N), 69.72 (s, 2 C, CH of fc), 72.17 (d, JPC = 3 Hz, 2 C, CH of fc), 73.34 (s, 2 C, CH of fc), 74.65 (d, JPC = 12 Hz, 2 C, CH of fc), 76.11 (s, Cipso-CO of fc), 170.00 (s, C=O). One ferrocene resonance (Cipso-P) was not identified, presumably due to overlaps. 31P{1H} NMR (CD2Cl2): δ 27.3 (s). IR (Nujol): 3448 s, 3324 s, 1644 s, 1541 s, 1317 m, 1292 m, 1186 s, 1072 w, 1039 s, 936 w, 896 w, 837 m, 812 m, 756 w, 734 w, 617 m, 522 m cm−1. MS (ESI+): m/z 468.1 ([M + H − NEt3]+), 569.2 ([M + H]+); MS (ESI−): m/z 465.9 ([M − HNEt3]+). Anal. Calc. for C26H45FeN2O4PS·0.3CH2Cl2 (594.00): C 53.18, H 7.74, N 4.72%. Found: C 53.16, H 7.75, N 4.53%.
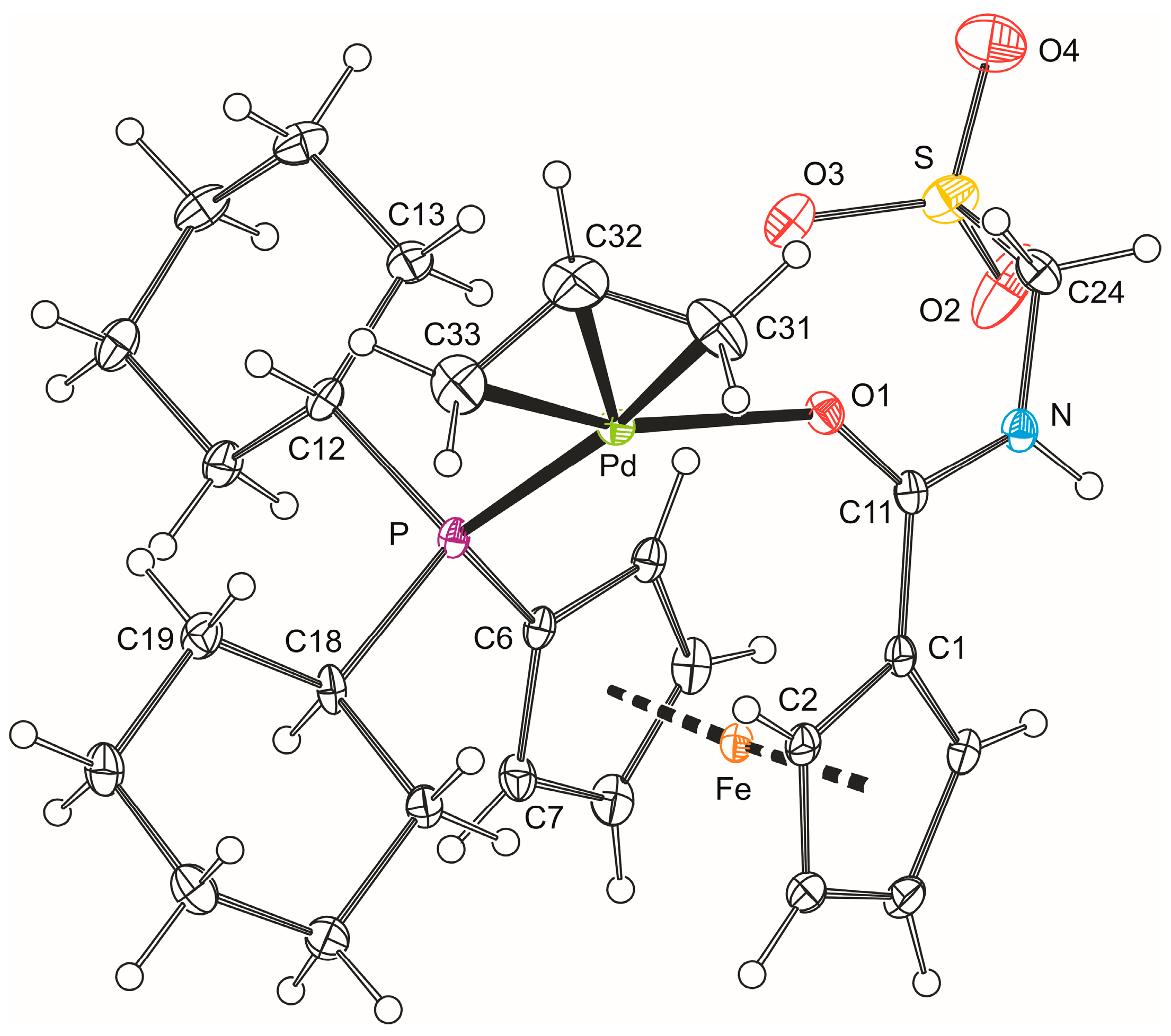
3.4.4. Preparation of [Pd(Cy2PfcCONHCH2SO3-κ2O,P)(η3-C3H5)] (5b)
Solid [Pd(μ-Cl)(η3-C3H5)]2 (18.3 mg, 0.05 mmol) was added to a solution of 4b (62.1 mg, 0.1 mmol) in dichloromethane (5 mL). After stirring for 30 min, a solution of AgClO4 (21.0 mg, 0.1 mmol) in benzene (1 mL) was added causing immediate precipitation of AgCl. The resulting mixture was stirred for 1 h and filtered through a syringe filter (PTFE, 0.45 μm pore size). The filtrate was evaporated under vacuum to afford a crude product, which was purified by two subsequent crystallizations by liquid-phase diffusion of diethyl ether into a chloroform solution of the complex to fully remove triethylammonium perchlorate. Yield after two crystallizations: 41 mg (62%).
1H NMR (CDCl3): δ 1.11–1.44 (m, 11 H, Cy), 1.67–2.09 (m, 11 H, Cy), 4.41 (br m, 2 H, fc), 4.53 (br m, 2 H, fc), 4.60 (br d, 3JHH = 5.4 Hz, 2 H, CH2N), 4.76 (br m, 2 H, fc), 4.87 (br m, 2 H, fc), 5.67 (quint, 3JHH = 9.9 Hz, 1 H, CH of C3H5), 7.14 (br s, 1 H, NH). 31P{1H} NMR (CDCl3): δ 27.7 (s). IR (Nujol): 3240 s, 1588 s, 1566 s, 1329 w, 1297 m, 1258 m, 1249 w, 1226 w, 1204 s, 1175 s, 1091 s, 1039 s, 1007 m, 976 w, 959 w, 937 w, 921 w, 846 m, 836 w, 771 w, 743 m, 619 m, 604 w, 594 w, 548 w, 529 w, 500 w. MS (ESI+): m/z 688.1 ([M + Na]+). Anal. Calc. for C27H38FeNO4PPdS (665.86): C 48.70, H 5.75, N 2.10%. Found: C 48.52, H 5.68, N 2.04%.
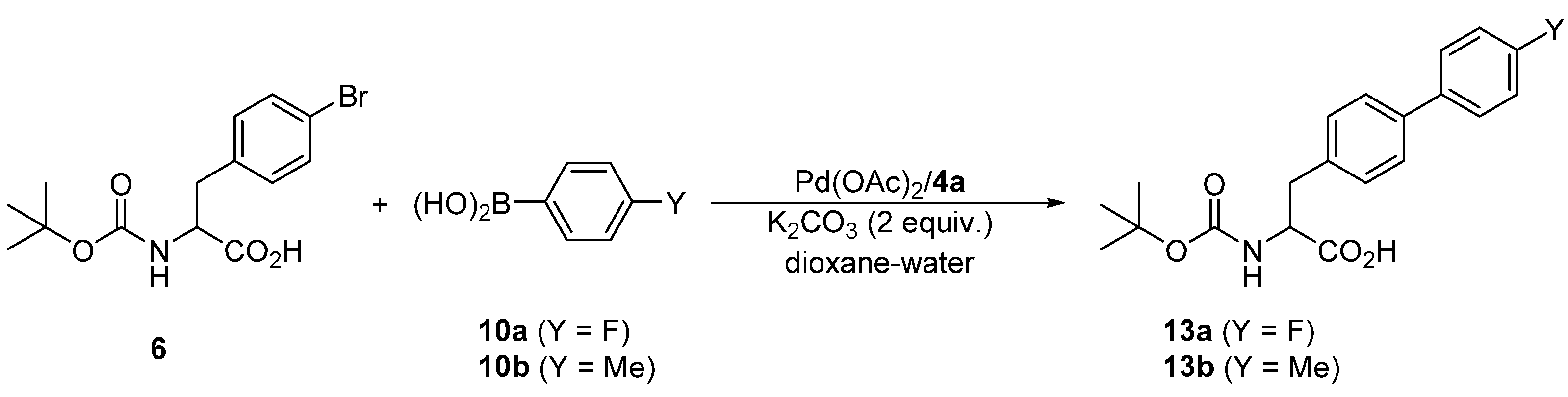
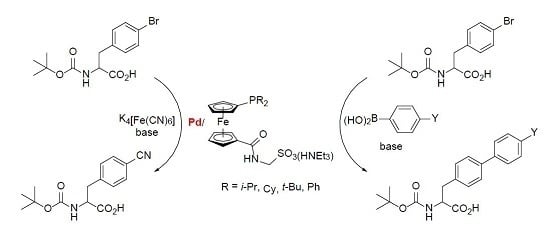
3.6. Catalytic Tests in Pd-Catalyzed Suzuki–Miyaura Cross-Coupling
The procedure was modified from ref. [
28]. Thus, palladium(II) acetate (1.0 or 0.5 mol. %) and the respective ligand
4 (1 or 2 equiv. with respect to Pd) were placed into a Schlenk tube and dissolved in dichloromethane (2 mL) under argon. The mixture was stirred for 5 min and then evaporated under vacuum. Next, aryl halide (1.00 mmol), boronic acid (1.15 mmol) and K
2CO
3 (2.00 mmol) were added to the Schlenk tube and the reaction vessel was filled with argon and sealed with a rubber septum. Degassed water (2 mL) and dioxane (2 mL) were introduced and the reaction flask was placed into a preheated oil bath (40 °C). After stirring for 6 h, the reaction was terminated by cooling on ice and the simultaneous addition of 3 M aqueous HCl (3 mL), water (7 mL) and ethyl acetate (10 mL). The aqueous layer was separated and extracted with ethyl acetate (3 × 15 mL). The organic layers were combined, washed with brine (30 mL), dried over MgSO
4 and evaporated under reduced pressure. Conversion was determined by
1H NMR spectroscopy.
If appropriate, the coupling products were isolated as follows. K2CO3 (ca. 300 mg) was added to the crude product and the mixture was dissolved in water (10 mL). Brine (10 mL) and NaCl (ca. 300 mg) were added to the solution and the resulting precipitate was filtered. The collected precipitate was dissolved in boiling water (100 mL) and filtered immediately by suction to remove the by-product resulting from self-coupling of aryl boronic acids. The filtrate was cooled to laboratory temperature and acidified with dilute hydrochloric acid until pH 3–4 was reached whereupon a white precipitate formed. The separated product was filtered off, washed with distilled water (20 mL) and then taken up with ethyl acetate (30 mL). The solution was dried over MgSO4 and evaporated under reduced pressure to afford pure coupling product, which was analyzed by 1H NMR spectroscopy. Characterization data of the coupling products were as follows.
2-(4-Fluorophenyl)benzoic acid (
11oa).
1H NMR (CDCl
3): δ 7.13–7.01 (m, 2 H), 7.31–7.25 (m, 2 H), 7.33 (dd,
J = 7.8, 1.1 Hz, 1H), 7.43 (td,
J = 7.6, 1.4 Hz, 1H), 7.57 (td,
J = 7.6, 1.4 Hz), 7.96 (dd,
J = 7.8, 1.1 Hz, 1H), (all aromatics). The collected data correspond to those in the literature; see ref. [
29].
3-(4-Fluorophenyl)benzoic acid (
11ma).
1H NMR (dmso-
d6): δ 7.28–8.17 (m, 8 H, aromatics), 13.16 (br s, 1 H, CO
2H). The NMR data are in line with those in the literature (see ref. [
27]).
4-(4-Fluorophenyl)benzoic acid (
11pa).
1H NMR (dmso-
d6): δ 7.30–7.37 (m, 2 H, aromatics), 7.75–7.83 (m, 4 H, aromatics), 7.98–8.04 (m, 2 H, aromatics), 13.02 (br s, 1 H, CO
2H). The data are in accordance with those in the literature; see ref. [
27].
2-(4-Tolyl)benzoic acid (
11ob).
1H NMR (CDCl
3): δ 2.38 (s, 3 H, CH
3), 7.26–7.14 (m, 4 H, aromatics), 7.35 (dd,
J = 7.7, 0.9 Hz, 1 H, aromatics), 7.39 (td,
J = 7.6, 1.4 Hz, 1 H, aromatics), 7.53 (td,
J = 7.6, 1.4 Hz, 1 H, aromatics), 7.92 (dd,
J = 7.8, 1.1 Hz, 1 H, aromatics). The analytical data are in agreement with those in the literature, see ref. [
28].
3-(4-Tolyl)benzoic acid (
11mb).
1H NMR (dmso-
d6): δ 2.35 (s, 3 H, CH
3), 7.27–8.19 (m, 8 H, aromatics). The NMR data are in agreement with those in the literature; see ref. [
27].
4-(4-Tolyl)benzoic acid (
11pb).
1H NMR (CDCl
3): δ 2.42 (s, 3 H, CH
3) 7.27–7.32 (m, 2 H, aromatics), 7.52–7.57 (m, 2 H, aromatics), 7.66–7.71 (m, 2 H, aromatics), 8.13–8.18 (m, 2 H, aromatics). These data correspond with those in the literature; see ref. [
27].
2-(4-Fluorophenyl)phenylacetic acid (12oa). 1H NMR (dmso-d6): δ 3.50 (s, 2 H, CH2), 7.21–7.38 (m, 8 H, aromatics), 12.27 (br s, 1 H, CO2H). 13C{1H} NMR (dmso-d6): δ 38.49 (s, CH2), 115.06 (d, 2JCF = 21 Hz, 2 C, aromatic CH), 126.91 (s, CH), 127.45 (s, CH) 129.76 (s, aromatic CH), 130.79 (s, CH), 130.83 (d, 3JCF = 7.9 Hz, 2 C, aromatic CH), 132.53 (s, aromatic C), 137.06 (d, 4JCF = 3 Hz, aromatic C), 140.76 (s, aromatic C), 161.42 (d, 1JCF = 244 Hz, aromatic C), 172.67 (s, CO2H). HRMS (ESI−) calc. for C14H10FO2 ([M − H]−): 229.0665, found 229.0670.
3-(4-Fluorophenyl)phenylacetic acid (12ma). 1H NMR (dmso-d6): δ 3.65 (s, 2 H, CH2), 2.24–2.33 (m, 3 H, aromatics), 7.37–7.43 (m, 1 H, aromatics), 7.50–7.55 (m, 2 H, aromatics), 7.65–7.71 (m, 2 H, aromatics), 12.35 (br s, 1 H, CO2H). 13C{1H} NMR (dmso-d6): δ 40.61 (s, CH2), 115.68 (d, 2JCF = 21 Hz, 2 C aromatic CH), 124.88 (s, aromatic CH), 127.79 (s, aromatic CH), 128.56 (s, aromatic CH), 128.60 (d, 3JCF = 7.9 Hz, 2 C aromatic CH) 128.83 (s, aromatic CH) 135.72 (s, aromatic C), 136.50 (d, 4JCF = 3 Hz, aromatic C), 139.04 (s, aromatic C), 161.82 (d, 1JCF = 244 Hz, aromatic C), 172.62 (s, CO2H). HRMS (ESI−) calc. for C14H10FO2 ([M − H]−): 229.0665, found 229.0669.
4-(4-Fluorophenyl)phenylacetic acid (
12pa).
1H NMR (dmso-
d6): δ 3.61 (s, 2 H, CH
2), 7.24–7.74 (m, 8 H, aromatics), 12.38 (br s, 1 H, CO
2H). The data are in accordance with those in the literature; see ref. [
30].
2-(4-Tolyl)phenylacetic acid (
12ob).
1H NMR (CDCl
3): δ 2.39 (s, 3 H, CH
3), 3.59 (s, 2 H, CH
2), 7.25–7.55 (m, 8 H, aromatics). The NMR parameters are in agreement with those in the literature; see ref. [
31].
3-(4-Tolyl)phenylacetic acid (12mb). 1H NMR (dmso-d6): δ 2.34 (s, 3 H, CH3), 3.64 (s, 2 H, CH2), 7.20–7.56 (m, 8 H, aromatics), 12.35 (br s, 1 H, CO2H). 13C{1H} NMR (dmso-d6): δ 20.67 (s, CH3), 40.66 (s, CH2), 124.68 (s, aromatic CH), 126.45 (s, 2 C, aromatic CH), 127.56 (s, aromatic CH), 128.17(s, aromatic CH), 128.78(s, aromatic CH), 129.51 (s, 2 C, aromatic CH), 135.62 (s, aromatic C), 136.72 (s, aromatic C), 137.15(s, aromatic C), 140.013 (s, aromatic C), 172.69 (s, CO2H). HRMS (ESI−) calc. for C15H13O2 ([M − H]−): 225.0916, found 225.0957.
4-(4-Tolyl)phenylacetic acid (
12pb).
1H NMR (CDCl
3): δ 2.39 (s, 3 H, CH
3), 3.59 (s, 2 H, CH
2), 7.25–7.55 (m, 8 H, aromatics). The data correspond with those in the literature (ref. [
27]).
Rac-2-{[(
tert-butyloxy)carbonyl]amino}-3-(4′-fluorobiphenyl-4-yl)propionic acid (
13a).
1H NMR (dmso-
d6): δ 1.32 (s, 9 H, CMe
3) 2.83–2.89 (m, 1 H, CH
2), 3.03–3.08 (m, 1 H, CH
2), 4.01–4.15 (m, 1 H, CH), 7.16–7.09 (m, 1 H), 7.13 (d,
3JHH = 8.3 Hz, 1 H, NH), 7.24–7.30 (m, 2 H, aromatics), 7.33 (d,
J = 8.1 Hz, 2 H, aromatics), 7.56 (d,
J = 8.2 Hz, 2 H, aromatics), 12.65 (br s, 1 H, CO
2H). The data are in accordance with those reported in ref. [
32].
Rac-2-{[(
tert-butyloxy)carbonyl]amino}-3-(4′-methylbiphenyl-4-yl)propionic acid (
13b).
1H NMR (dmso-
d6): δ 1.32 (s, 9 H, CMe
3), 2.33 (s, 3 H, CH
3), 2.76–3.08 (m, 2 H, CH
2), 4.01‑4.16 (m, 1 H, CH), 7.12 (d,
3JHH = 8.3 Hz, 1 H, NH), 7.24–7.26 (m, 2 H, aromatics), 7.30–7.32 (m, 2 H, aromatics), 7.51–7.56 (m, 4 H, aromatics), 12.63 (br s, 1 H, CO
2H). The analytical data are in agreement with those in the literature (see ref. [
8]).
3.7. X-ray Crystallography
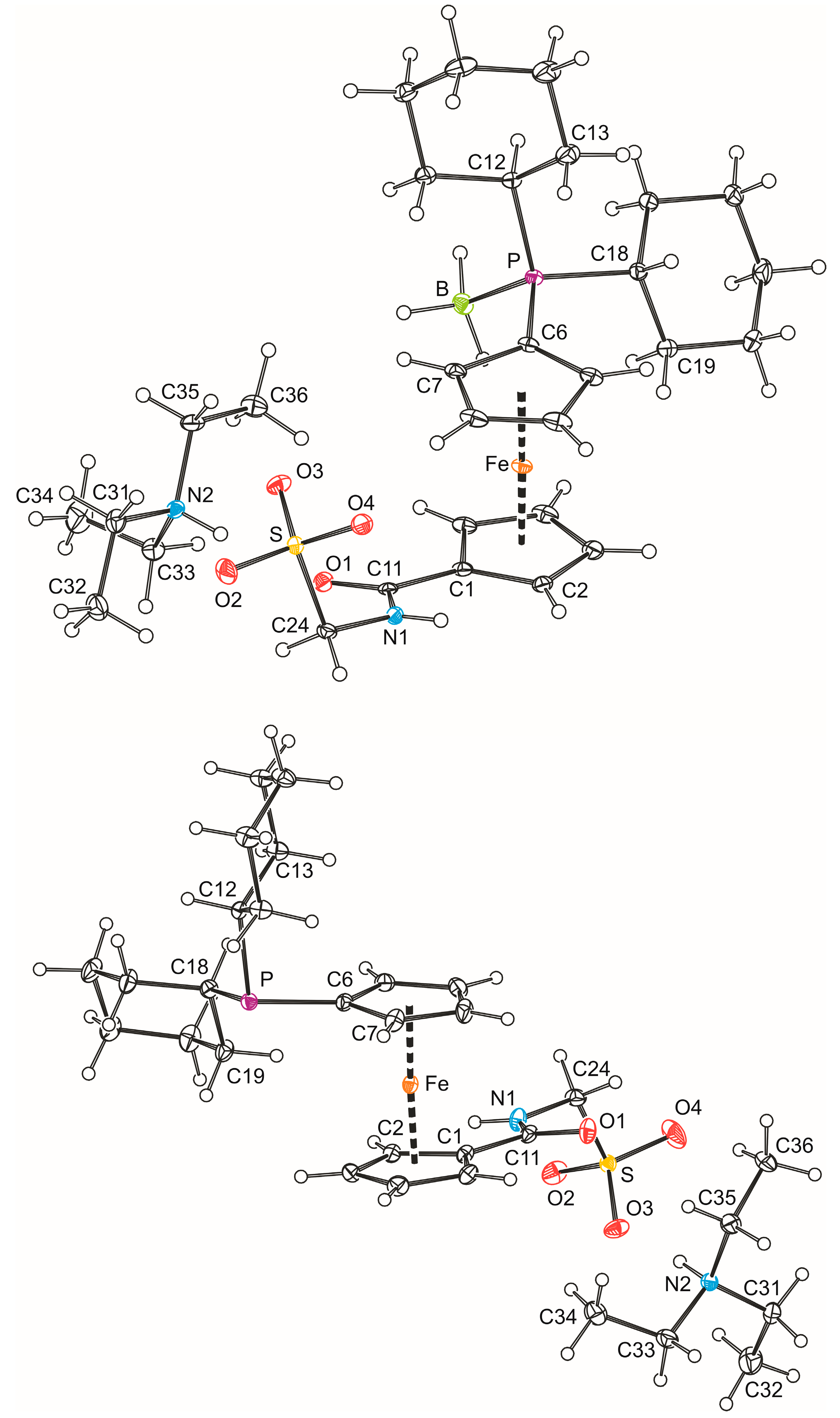
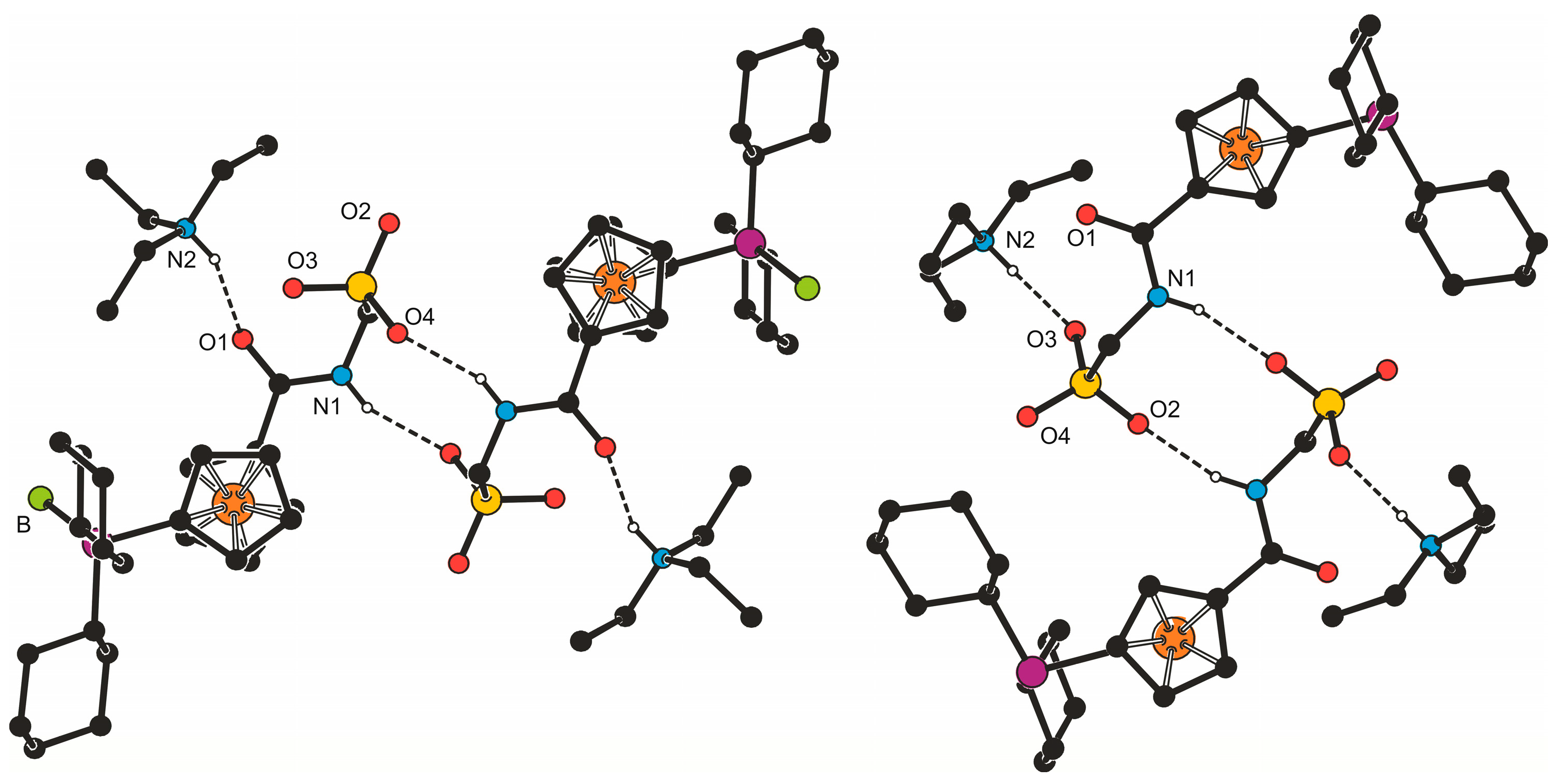
The crystals of 3b·CH2Cl2 (orange prism, 0.17 × 0.20 × 0.25 mm3) and 4b (orange plate, 0.07 × 0.17 × 0.23 mm3) were grown from dichloromethane–hexane and ethyl acetate–heptane, respectively. Full-set diffraction data for both compounds (±h ± k ± l, θmax = 27.5°) were collected on a Bruker D8 VENTURE Kappa Duo PHOTON100 diffractometer equipped with IµS micro-focus sealed tube (MoKα radiation, λ = 0.71073 Å) and a Cryostream cooling device (Oxford Cryosystems) at 150(2) K.
Selected crystallographic data for 3b·CH2Cl2: C31H54BCl2FeN2O4PS·CH2Cl2 (M = 719.35 g mol−1), triclinic, space group P − 1 (no. 2); a = 8.3583(3) Å, b = 11.0812(4) Å, c = 20.6164(8) Å, α = 99.276(1)°, β = 100.551(1)°, γ = 102.947(1)°; V = 1788.3(1) Å3, Z = 2, Dcalc = 1.336 g mL−1, F(000) = 764, μ(MoKα) = 0.711 mm−1. A total of 37,660 diffractions were collected, of which were 8211 unique (Rint = 2.93%) and 7117 observed according to the Io > 2σ(Io) criterion.
Selected crystallographic data for 4b: C30H49FeN2O4PS (M = 620.59 g mol−1), monoclinic, space group P21/c (no. 14); a = 17.3904(6) Å, b = 9.1114(3) Å, c = 19.7014(7) Å, β = 97.552(1)°; V = 3094.6(2) Å3, Z = 4, Dcalc = 1.332 g mL−1, F(000) = 1328, μ(MoKα) = 0.643 mm−1. A total of 82,146 diffractions were collected, of which were 7125 unique (Rint = 4.12%) and 3275 observed according to the Io > 2σ(Io) criterion.
The structures of
3b·CH
2Cl
2 and
4b were solved by direct methods (XT2014 [
33]) and refined by unrestricted least-squares against
F2 (SHELXL-97 [
34] or SHELXL-2014 [
35]). All non-hydrogen atoms were refined with anisotropic displacement parameters. The amide hydrogens H1N were identified on an electron density map and refined as riding atom with
Uiso(H1N) set to 1.2
Ueq(N). Hydrogen atoms in the CH
n groups were included in their theoretical positions using the standard HFIX instructions in SHELXL-2014 and refined as riding atoms. A recent version of the PLATON program [
36] was used to perform all geometric calculations and prepare the structural diagrams.
In the case of 3b·CH2Cl2, the refinement converged (Δ/σ ≤ 0.002, 391 parameters) to R = 2.96% and wR = 7.02% for the observed diffractions and to R = 3.84% and wR = 7.54% for all diffractions. Extremes on the final difference electron density map were: Δρmax = 0.72, Δρmin = −0.79 e Å−3. CCDC deposition number: 1545049.
For 4b, the refinement converged (Δ/σ ≤ 0.002, 355 parameters) to R = 2.68% and wR = 6.53% for the observed diffractions and to R = 3.31% and wR = 6.83% for all diffractions. Extremes on the final difference electron density map were: Δρmax = 0.36, Δρmin = −0.36 e Å−3. CCDC deposition number: 1545048.
An orange, bar-like crystal of 5b·CH2Cl2 with approximate dimensions of 0.14 × 0.25 × 0.36 mm3 was obtained by recrystallization of the complex from dichloromethane–diethyl ether. Full-diffraction data (±h ± k ± l, θmax = 27.56°) were collected at 150(2) K with a Nonius Kappa CCD diffractometer equipped with a Bruker APEX-II image plate detector and a Cryostream cooling device (Oxford Cryosystems) using MoKα radiation (λ = 0.71073 Å). A total of 26,856 diffractions were collected, of which 7049 were independent (Rint = 2.98%) and 5970 observed according to the Io > 2σ(Io) criterion. Selected crystallographic data: C27H38FeNO4PPdS·CH2Cl2 (M = 750.79 g mol−1), monoclinic, space group P2/c (no. 13); a = 17.257(1) Å, b = 10.4304(6) Å, c = 17.2618(8) Å, β = 100.882(2)°; V = 3051.2(3) Å3, Z = 4, Dcalc = 1.634 g mL−1, F(000) = 1535, μ(MoKα) = 1.395 mm−1.
The structure was solved and refined as described above for 3b·CH2Cl2 and 4b. The refinement converged (Δ/σ ≤ 0.002, 352 parameters) to R = 3.30% and wR = 7.74% for the observed diffractions and to R = 4.26% and wR = 8.22% for all diffractions. The final difference electron density map showed peaks of no chemical significance (Δρmax = 1.51, Δρmin = −0.75 e Å−3). CCDC deposition number: 1544852.