Unconventional Approaches Involving Cyclodextrin-Based, Self-Assembly-Driven Processes for the Conversion of Organic Substrates in Aqueous Biphasic Catalysis
Abstract
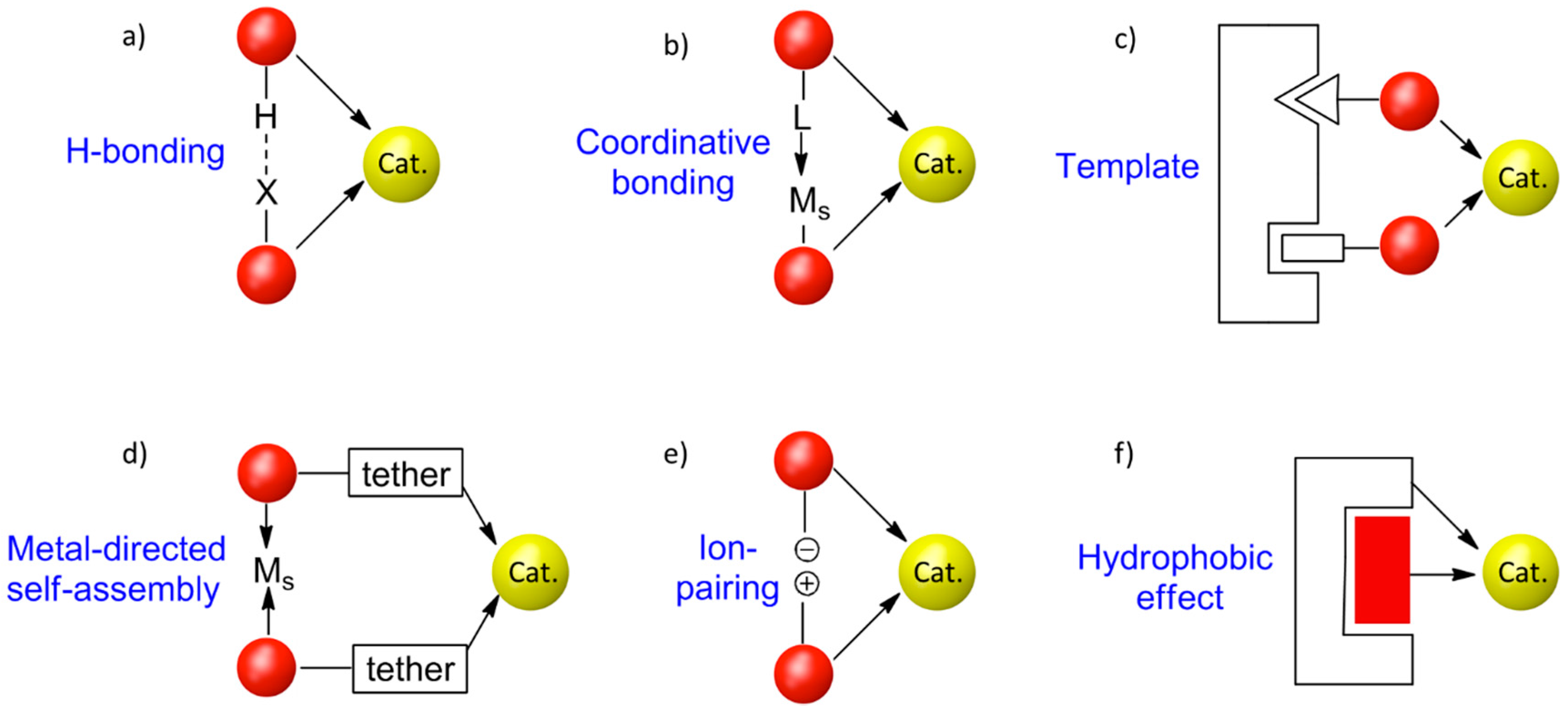
:1. Introduction
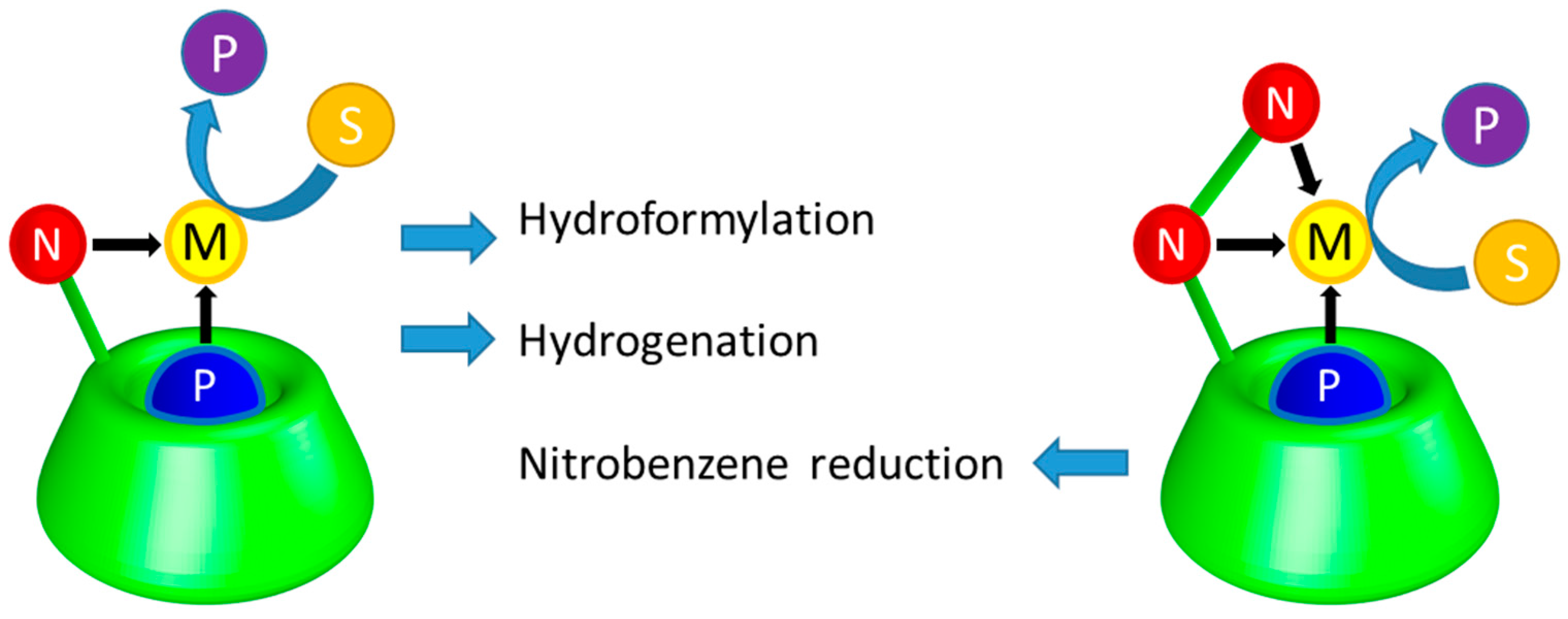
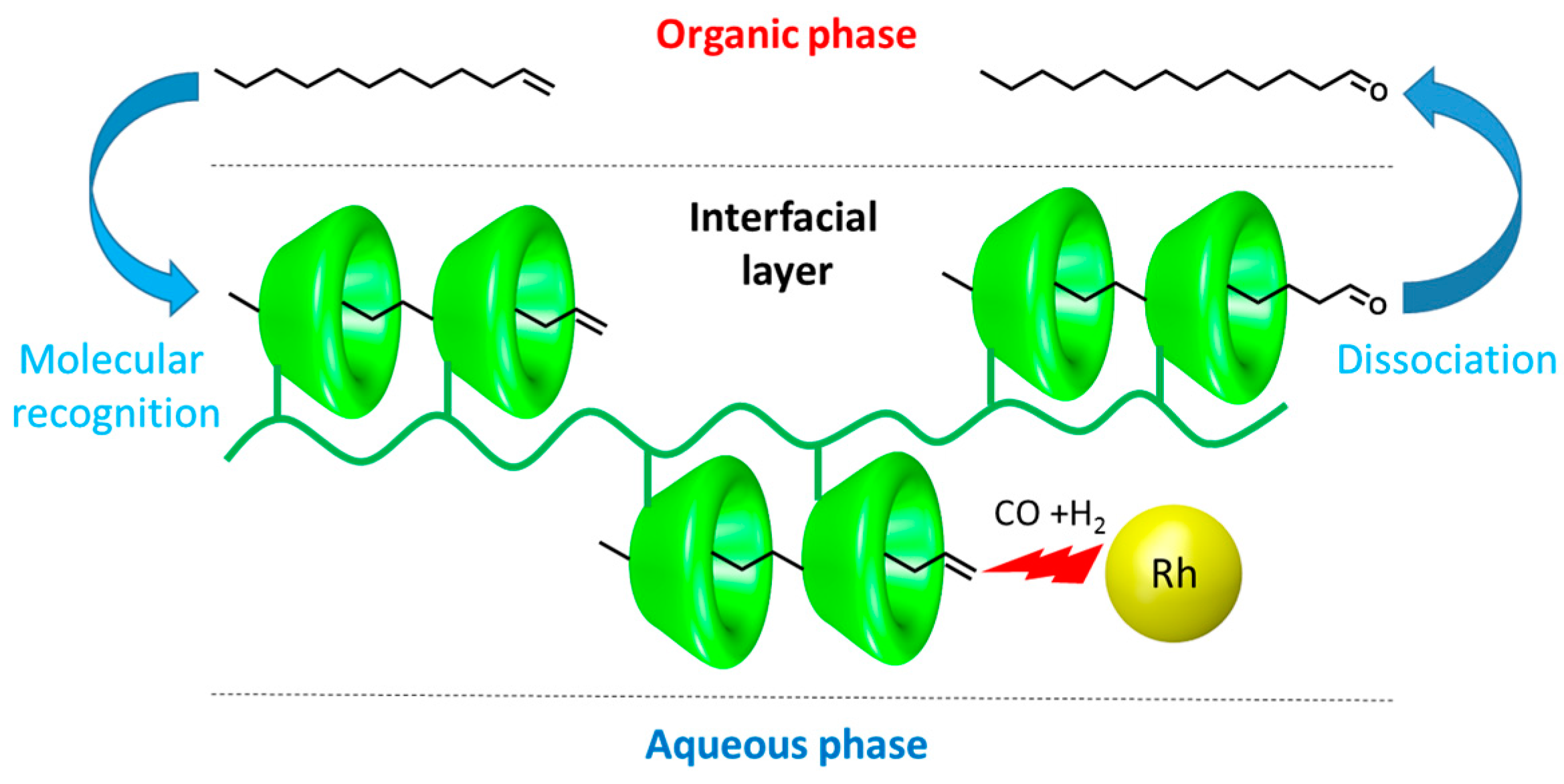
2. CD-Based Organometallic Catalysts Self-Assembled via Hydrophobic Effects
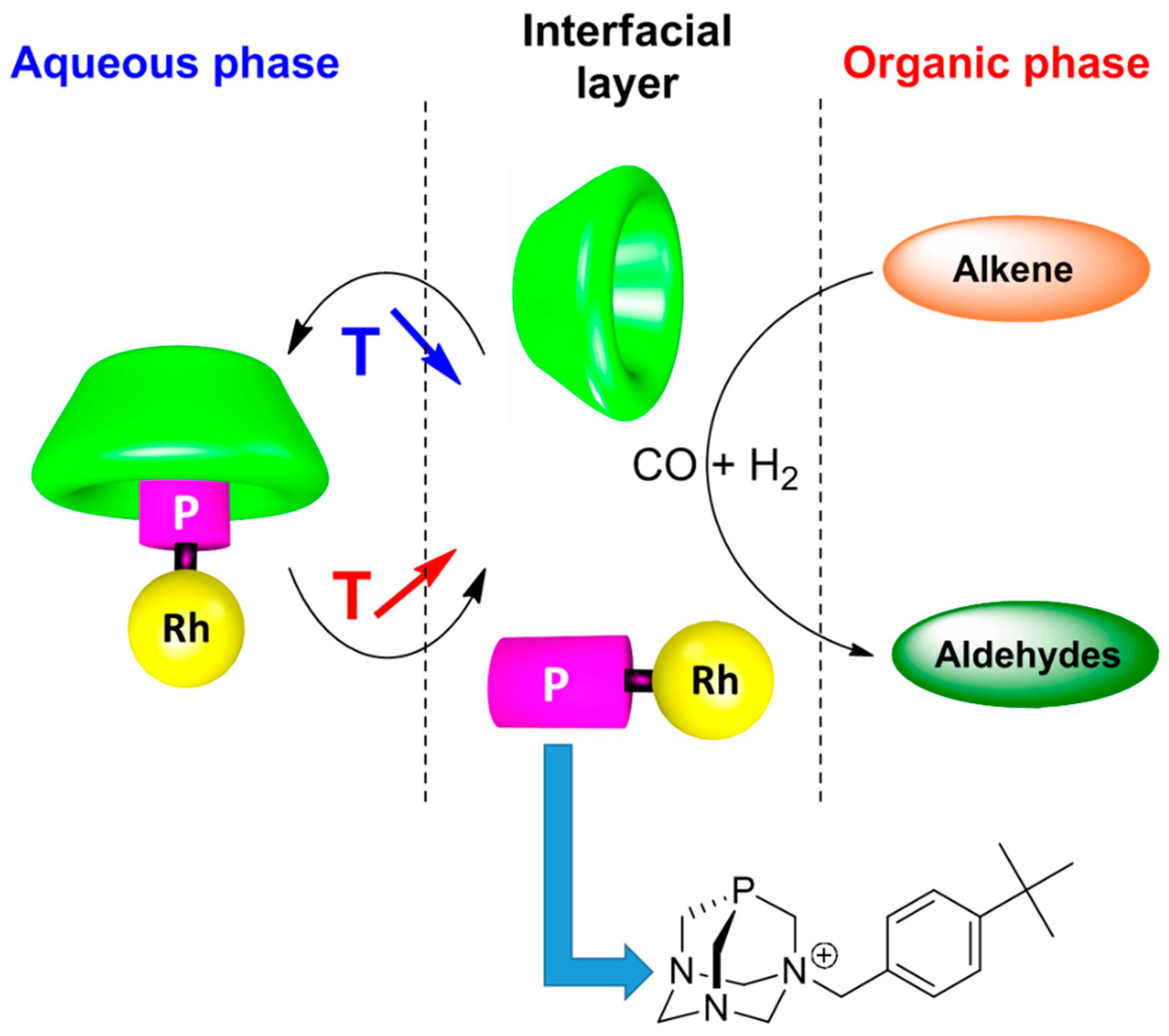
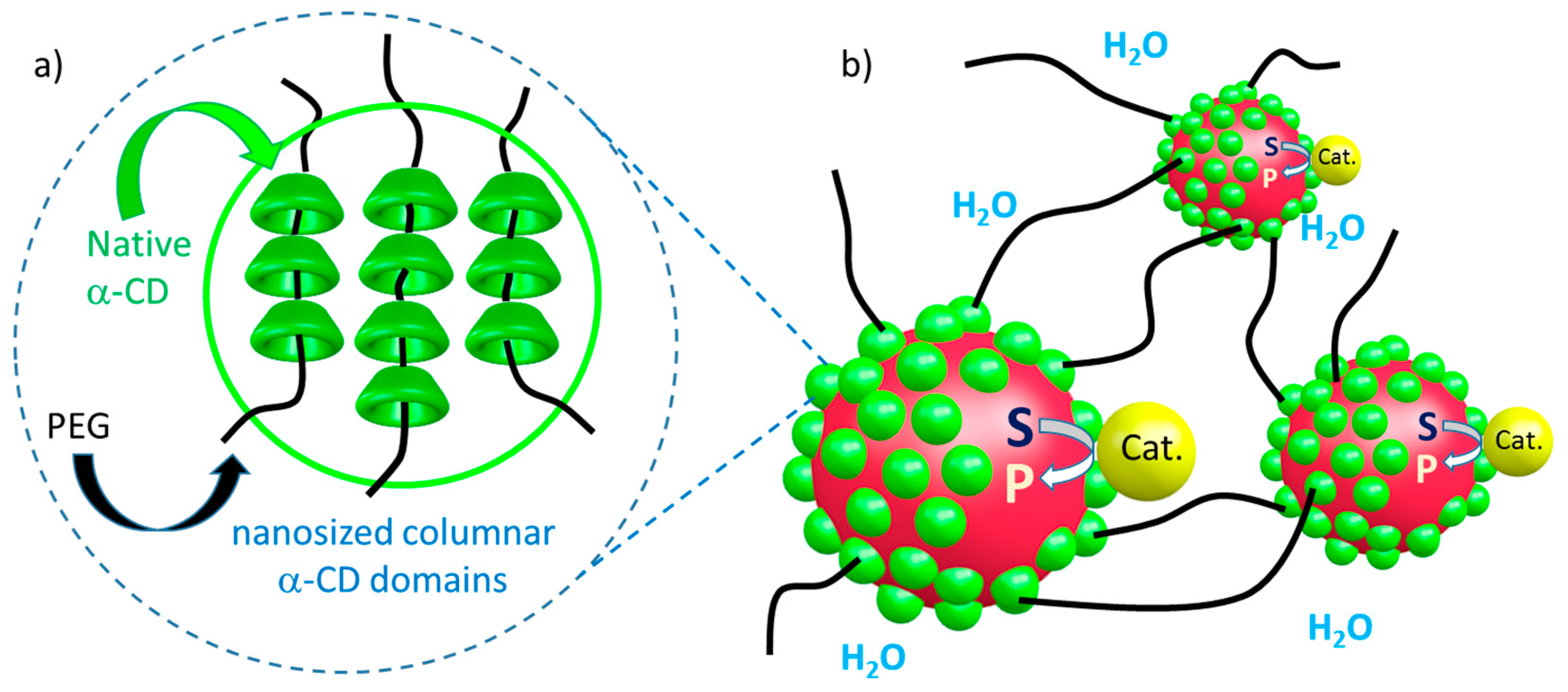
3. Control of Micelle Dynamics
4. Self-Emulsifying Catalytic System
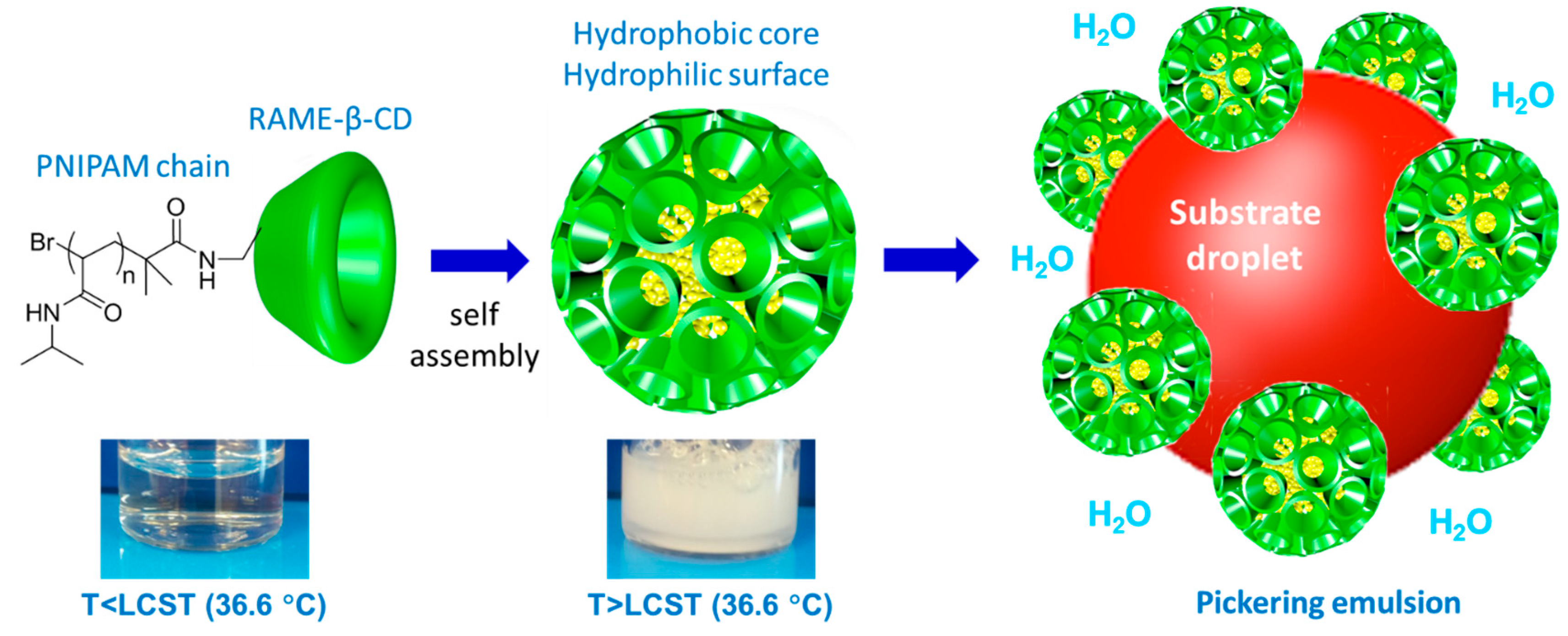
4.1. CD-Based Pickering-Like Emulsions
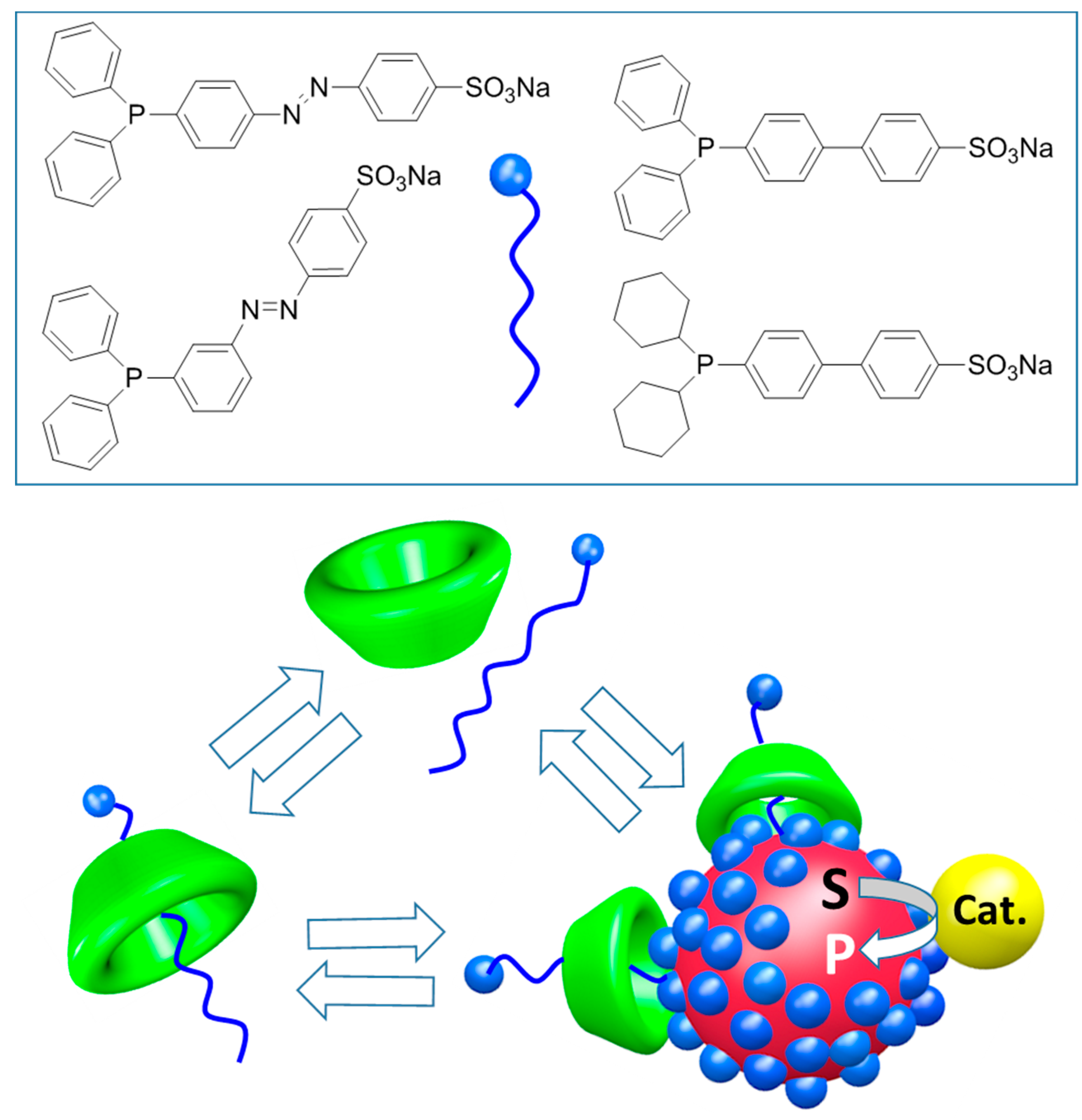
4.2. Multivalency with CD Dimers and CD Polymers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raynal, M.; Ballester, P.; Vidal-Ferrana, A.; van Leeuwen, P.W. Supramolecular catalysis. Part 1: Non-covalent interactions as a tool for building and modifying homogeneous catalysts. Chem. Soc. Rev. 2014, 43, 1660–1733. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Vidal-Ferrana, A. Supramolecular Catalysis; van Leeuwen, P.W., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–27. [Google Scholar]
- Catti, L.; Zhang, Q.; Tiefenbacher, K. Advantages of catalysis in self-assembled molecular capsules. Chem. Eur. J. 2016, 22, 9060–9066. [Google Scholar] [CrossRef] [PubMed]
- Leenders, S.H.; Gramage-Doria, R.; de Bruin, B.; Reek, J.N. Transition metal catalysis in confined spaces. Chem. Soc. Rev. 2015, 44, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Bocokić, V.; Kalkan, A.; Lutz, M.; Spek, A.L.; Gryko, D.T.; Reek, J.N. Capsule-controlled selectivity of a rhodium hydroformylation catalyst. Nat. Commun. 2013, 4, 2670. [Google Scholar] [CrossRef] [PubMed]
- Jouffroy, M.; Armspach, D.; Matt, D. Cyclodextrin and phosphorus(III): A versatile combination for coordination chemistry and catalysis. Dalton Trans. 2015, 44, 12942–12969. [Google Scholar] [CrossRef] [PubMed]
- Zaborova, E.; Deschamp, J.; Guieu, S.; Blériot, Y.; Poli, G.; Ménand, M.; Madec, D.; Prestat, G.; Sollogoub, M. Cavitand supported tetraphosphine: Cyclodextrin offers a useful platform for Suzuki-Miyaura cross-coupling. Chem. Commun. 2011, 47, 9206–9208. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, F.; Breit, B. Self-assembled bidentate ligands for Ru-catalyzed anti-Markovnikov hydration of terminal alkynes. Angew. Chem. Int. Ed. 2006, 45, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Kuil, M.; Goudriaan, P.E.; Tooke, D.M.; Spek, A.L.; van Leeuwen, P.W.; Reek, J.N. Rigid bis-zinc(II) salphen building blocks for the formation of template-assisted bidentate ligands and their application in catalysis. Dalton Trans. 2007, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, L.; Lynikaite, B.; Cvengro, J.; Marchini, M.; Piarulli, U.; Gennari, C. Combinations of acidic and basic monodentate binaphtholic phosphites as supramolecular bidentate ligands for enantioselective Rh-catalyzed hydrogenations. Eur. J. Org. Chem. 2009, 2539–2547. [Google Scholar] [CrossRef]
- Moteki, S.A.; Takacs, J.M. Exploiting self-assembly for ligand-scaffold optimization: Substrate-tailored ligands for efficient catalytic asymmetric hydroboration. Angew. Chem. Int. Ed. 2008, 47, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Ohmatsu, K.; Ito, M.; Kunieda, T.; Ooi, T. Ion-paired chiral ligands for asymmetric palladium catalysis. Nat. Chem. 2012, 4, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, F.; Nau, W.M.; Schneider, H.-J. The hydrophobic effect revisited—Studies with supramolecular complexes imply high-energy water as a noncovalent driving force. Angew. Chem. Int. Ed. 2014, 53, 11158–11171. [Google Scholar] [CrossRef] [PubMed]
- Kataev, E.A.; Müller, C. Recent advances in molecular recognition in water: Artificial receptors and supramolecular catalysis. Tetrahedron 2014, 70, 137–167. [Google Scholar] [CrossRef]
- Machut, C.; Patrigeon, J.; Tilloy, S.; Bricout, H.; Hapiot, F.; Monflier, E. Self-assembled supramolecular bidentate ligands for aqueous organometallic catalysis. Angew. Chem. Int. Ed. 2007, 46, 3040–3042. [Google Scholar] [CrossRef] [PubMed]
- Patrigeon, J.; Hapiot, F.; Canipelle, M.; Menuel, S.; Monflier, E. Cyclodextrin-based supramolecular P,N bidentate ligands and their platinum and rhodium complexes. Organometallics 2010, 29, 6668–6674. [Google Scholar] [CrossRef]
- Hapiot, F.; Bricout, H.; Tilloy, S.; Monflier, E. Functionalized cyclodextrins as first and second coordination sphere ligands for aqueous organometallic catalysis. Eur. J. Inorg. Chem. 2012, 1571–1578. [Google Scholar] [CrossRef]
- Potier, J.; Guerriero, A.; Menuel, S.; Monflier, E.; Peruzzini, M.; Hapiot, F.; Gonsalvi, L. Cyclodextrins as first and second sphere ligands for Rh(I) complexes of lower-rim PTA derivatives for use as catalysts in aqueous phase hydrogenation. Catal. Commun. 2015, 63, 74–78. [Google Scholar] [CrossRef]
- Menuel, S.; Bertaut, E.; Monflier, E.; Hapiot, F. Cyclodextrin-based PNN supramolecular assemblies: A new class of pincer-type ligands for aqueous organometallic catalysis. Dalton Trans. 2015, 44, 13504–13512. [Google Scholar] [CrossRef] [PubMed]
- Blaszkiewicz, C.; Bricout, H.; Léonard, E.; Len, C.; Landy, D.; Cézard, C.; Djedaïni-Pilard, F.; Monflier, E.; Tilloy, S.A. Cyclodextrin dimer as a supramolecular reaction platform for aqueous organometallic catalysis. Chem. Commun. 2013, 49, 6989–6991. [Google Scholar] [CrossRef] [PubMed]
- Six, N.; Guerriero, A.; Landy, D.; Peruzzini, M.; Gonsalvi, L.; Hapiot, F.; Monflier, E. Supramolecularly controlled surface activity of an amphiphilic ligand. Application to aqueous biphasic hydroformylation of higher olefins. Catal. Sci. Technol. 2011, 1, 1347–1353. [Google Scholar] [CrossRef]
- Leclercq, L.; Schmitzer, A.R. Assembly of tunable supramolecular organometallic catalysts with cyclodextrins. Organometallics 2010, 29, 3442–3449. [Google Scholar] [CrossRef]
- Qi, M.; Tan, P.Z.; Xue, F.; Malhi, H.S.; Zhang, Z.-X.; Young, D.J.; Hor, T.S.A. A supramolecular recyclable catalyst for aqueous Suzuki–Miyaura coupling. RSC Adv. 2015, 5, 3590–3596. [Google Scholar] [CrossRef]
- Yin, Y.; Jiao, S.; Zhang, R.; Wang, X.; Zhang, L.; Yang, L. Construction of a soluble supramolecular glutathione peroxidase mimic based on host-guest interaction. Curr. Organocatal. 2015, 2, 64–70. [Google Scholar] [CrossRef]
- La Sorella, G.; Strukul, G.; Scarso, A. Recent advances in catalysis in micellar media. Green Chem. 2015, 17, 644–683. [Google Scholar] [CrossRef]
- Leclercq, L.; Lacour, M.; Sanon, S.H.; Schmitzer, A.R. Thermoregulated microemulsions by cyclodextrin sequestration: A new approach to efficient catalyst recovery. Chem. Eur. J. 2009, 15, 6327–6331. [Google Scholar] [CrossRef] [PubMed]
- Kairouz, V.; Schmitzer, A.R. Imidazolium-functionalized β-cyclodextrin as a highly recyclable multifunctional ligand in water. Green Chem. 2014, 16, 3117–3124. [Google Scholar] [CrossRef]
- Ferreira, M.; Bricout, H.; Azaroual, N.; Landy, D.; Tilloy, S.; Hapiot, F.; Monflier, E. Cyclodextrin/amphiphilic phosphane mixed systems and their applications in aqueous organometallic catalysis. Adv. Synth. Catal. 2012, 354, 1337–1346. [Google Scholar] [CrossRef]
- Bricout, H.; Léonard, E.; Len, C.; Landy, D.; Hapiot, F.; Monflier, E. Impact of cyclodextrins on the behavior of amphiphilic ligands in aqueous organometallic catalysis. Beilstein J. Org. Chem. 2012, 8, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Potier, J.; Menuel, S.; Lyskawa, J.; Fournier, D.; Stoffelbach, F.; Monflier, E.; Woisel, P.; Hapiot, F. Thermoresponsive self-assembled cyclodextrin-end-decorated PNIPAM for aqueous catalysis. Chem. Commun. 2015, 51, 2328–2330. [Google Scholar] [CrossRef] [PubMed]
- Potier, J.; Menuel, S.; Chambrier, M.-H.; Burylo, L.; Blach, J.-F.; Woisel, P.; Monflier, E.; Hapiot, F. Pickering emulsions based on supramolecular hydrogels: Application to higher olefins’ hydroformylation. ACS Catal. 2013, 3, 1618–1621. [Google Scholar] [CrossRef]
- Potier, J.; Menuel, S.; Monflier, E.; Hapiot, F. Synergetic effect of randomly methylated β-cyclodextrin and a supramolecular hydrogel in Rh-catalyzed hydroformylation of higher olefins. ACS Catal. 2014, 4, 2342–2346. [Google Scholar] [CrossRef]
- Barnard, A.; Smith, D.K. Self-assembled multivalency: Dynamic ligand arrays for high-affinity binding. Angew. Chem. Int. Ed. 2012, 51, 6572–6581. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.; Huskens, J.; Reinhoudt, D.N. Multivalency in supramolecular chemistry and nanofabrication. Org. Biomol. Chem. 2004, 7, 3409–3424. [Google Scholar] [CrossRef] [PubMed]
- Six, N.; Menuel, S.; Bricout, H.; Hapiot, F.; Monflier, E. Ditopic cyclodextrin-based receptors: New perspectives in aqueous organometallic catalysis. Adv. Synth. Catal. 2010, 352, 1467–1475. [Google Scholar] [CrossRef]
- Potier, J.; Menuel, S.; Fournier, D.; Fourmentin, S.; Woisel, P.; Hapiot, F.; Monflier, E. Cooperativity in aqueous organometallic catalysis: Contribution of cyclodextrin-substituted polymers. ACS Catal. 2012, 2, 1417. [Google Scholar] [CrossRef]
- Potier, J.; Menuel, S.; Mathiron, D.; Bonnet, V.; Hapiot, F.; Monflier, E. Cyclodextrin-grafted polymers functionalized with phosphanes: A new tool for aqueous organometallic catalysis. Beilstein J. Org. Chem. 2014, 10, 2642–2648. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hapiot, F.; Monflier, E. Unconventional Approaches Involving Cyclodextrin-Based, Self-Assembly-Driven Processes for the Conversion of Organic Substrates in Aqueous Biphasic Catalysis. Catalysts 2017, 7, 173. https://doi.org/10.3390/catal7060173
Hapiot F, Monflier E. Unconventional Approaches Involving Cyclodextrin-Based, Self-Assembly-Driven Processes for the Conversion of Organic Substrates in Aqueous Biphasic Catalysis. Catalysts. 2017; 7(6):173. https://doi.org/10.3390/catal7060173
Chicago/Turabian StyleHapiot, Frédéric, and Eric Monflier. 2017. "Unconventional Approaches Involving Cyclodextrin-Based, Self-Assembly-Driven Processes for the Conversion of Organic Substrates in Aqueous Biphasic Catalysis" Catalysts 7, no. 6: 173. https://doi.org/10.3390/catal7060173





