Carbon-Modified Mesoporous Anatase/TiO2(B) Whisker for Enhanced Activity in Direct Synthesis of Hydrogen Peroxide by Palladium
Abstract
:1. Introduction
2. Results and Discussion
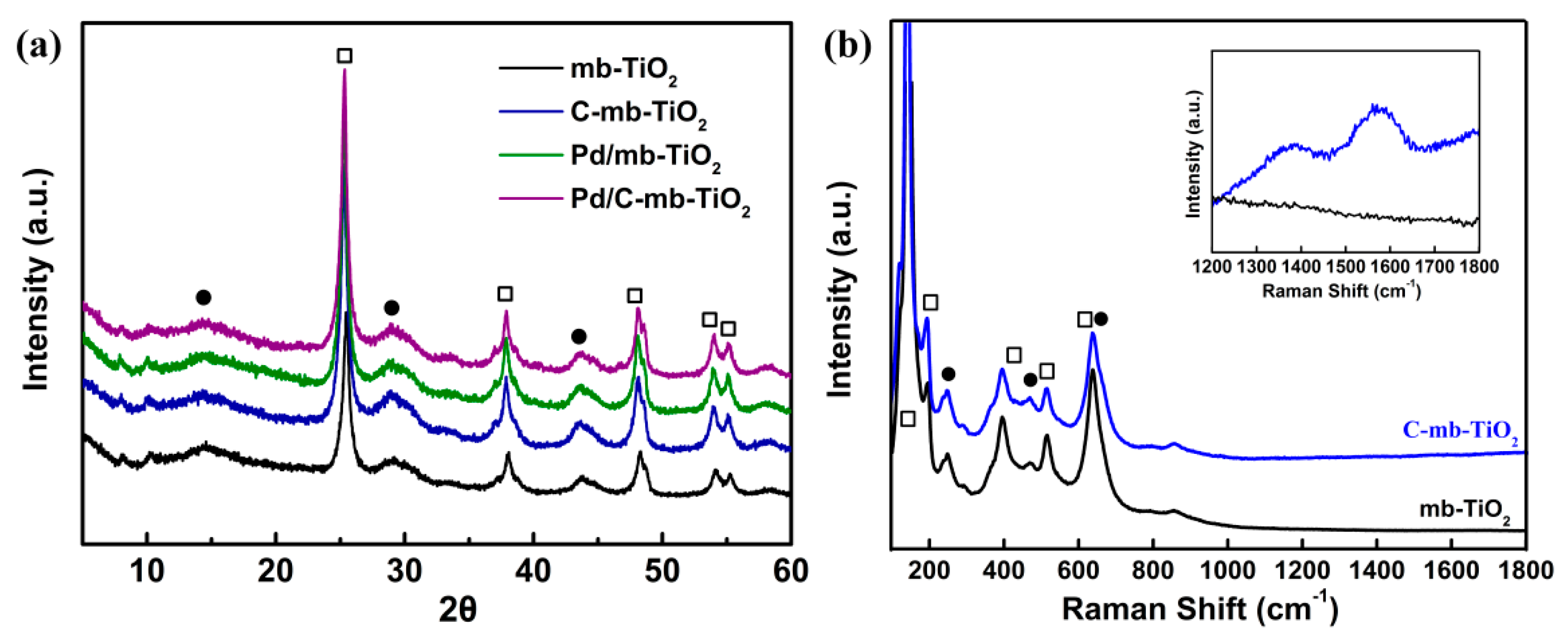
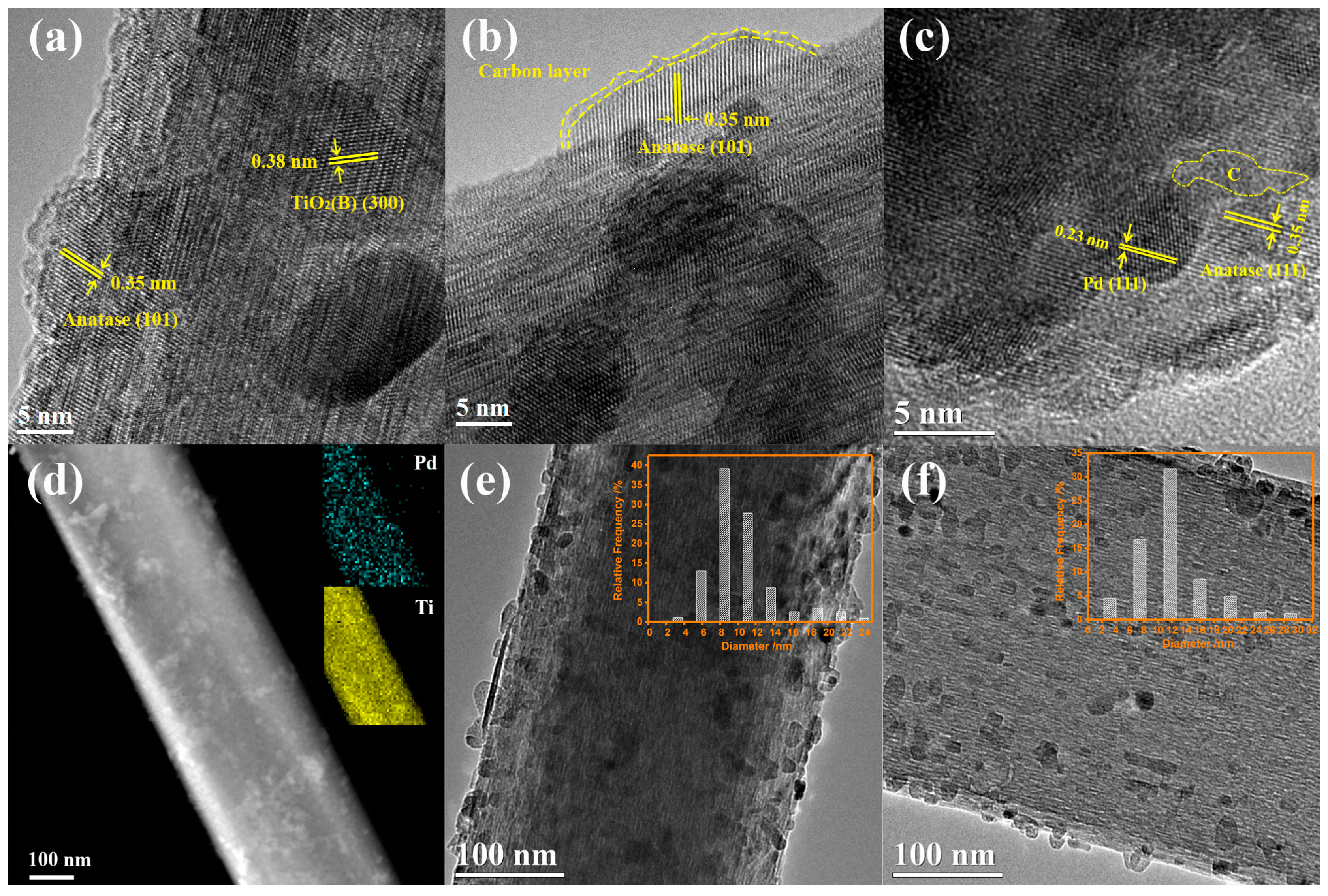
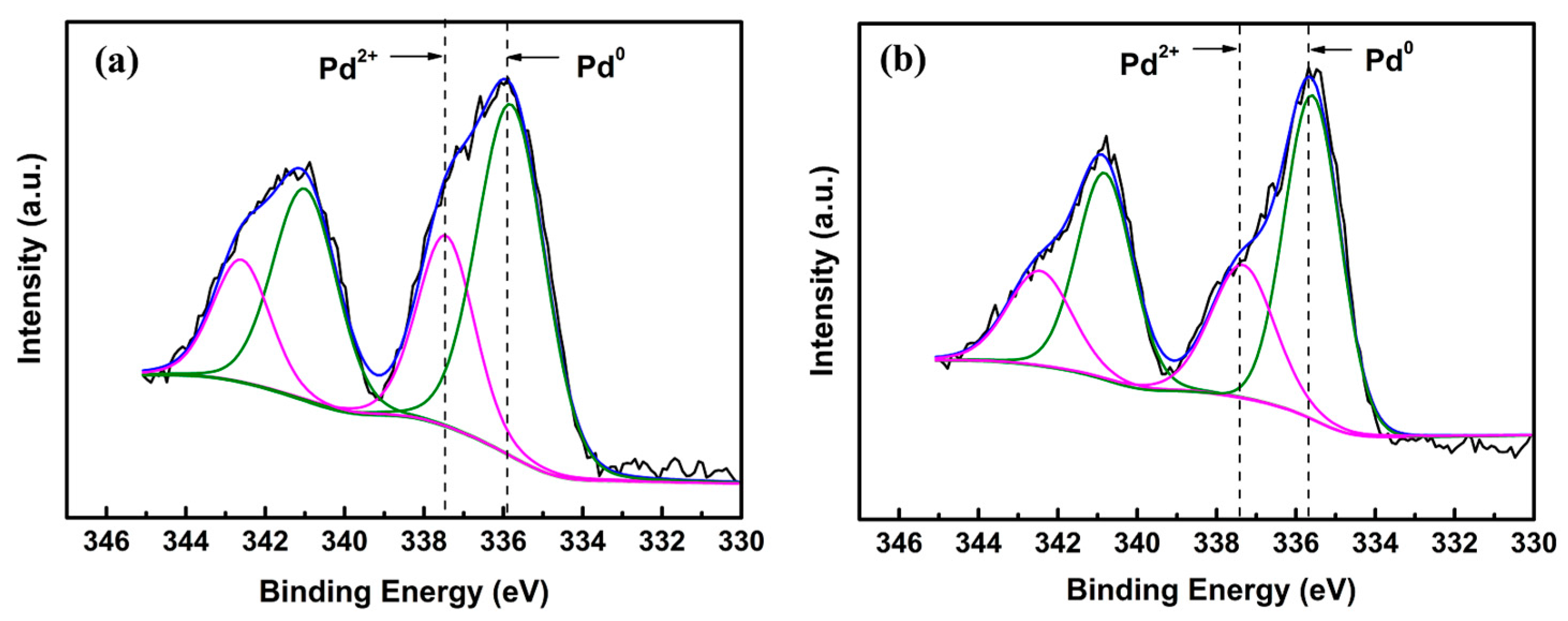
2.1. Structural Characterization of the Catalysts
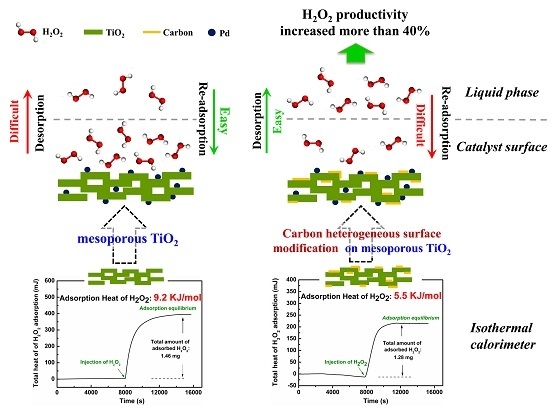
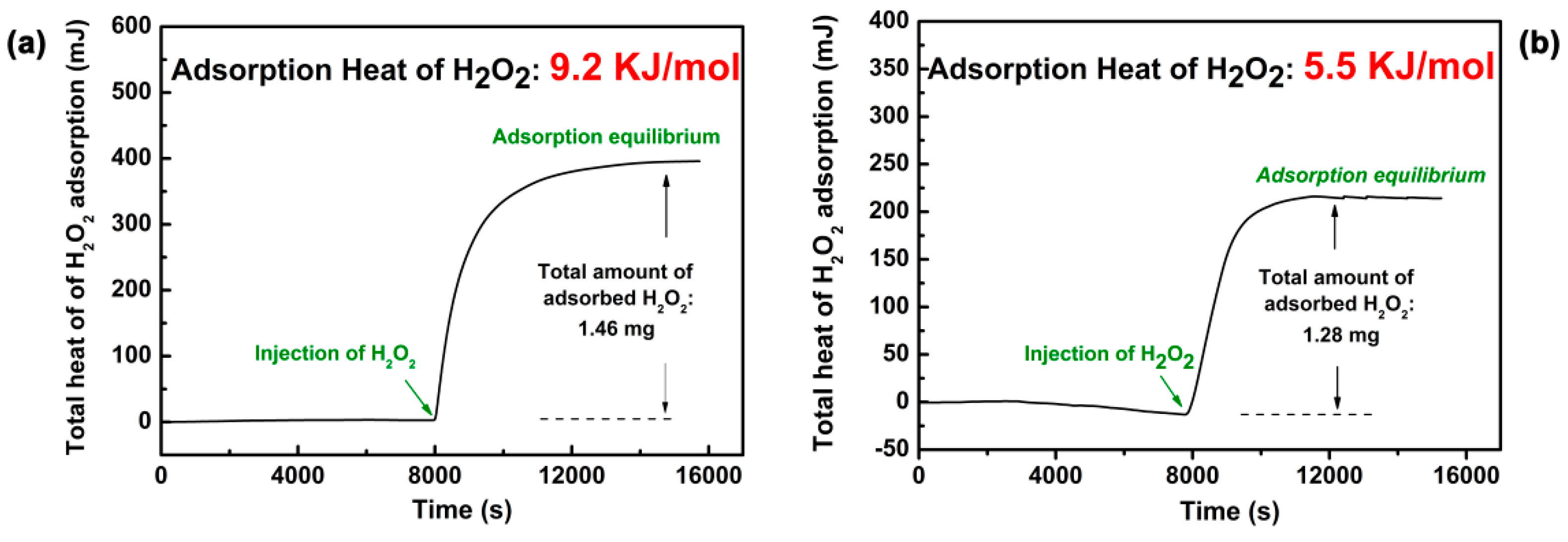
2.2. The Adsorption Properties of H2O2 on the Supports
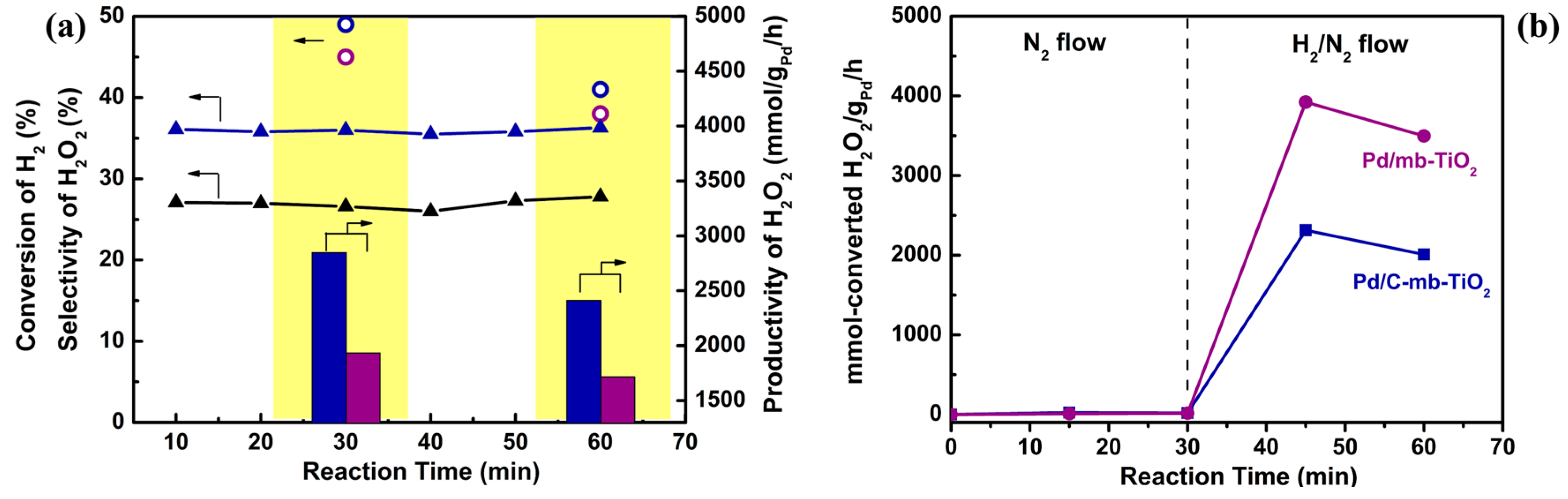
2.3. The Catalytic Activity of the Catalysts
3. Materials and Methods
3.1. Synthesis of Carbon-Modified TiO2 Whisker
3.2. Catalyst Characterization
3.3. Catalytic Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Landon, P.; Collier, P.J.; Papworth, A.J.; Kiely, C.J.; Hutchings, G.J. Direct formation of hydrogen peroxide from H2/O2 using a gold catalyst. Chem. Commun. 2002, 18, 2058–2059. [Google Scholar] [CrossRef]
- Samanta, C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- Edwards, J.K.; Hutchings, G.J. Palladium and gold-palladium catalysts for the direct synthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 2008, 47, 9192–9198. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.; Murillo, R.; Agouram, S.; Dejoz, A.; Lazaro, M.J.; Torrente-Murciano, L.; Solsona, B. Highly dispersed encapsulated Au-Pd nanoparticles on ordered mesoporous carbons for the direct synthesis of H2O2 from molecular oxygen and hydrogen. Chem. Commun. 2012, 48, 5316–5318. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.K.; Solsona, B.; Ntainjua, N.E.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Switching off hydrogen peroxide hydrogenation in the direct synthesis process. Science 2009, 323, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, L.; Li, G.; Guo, H. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: Noble-metal catalytic method, fuel-cell method and plasma method. Catal. Sci. Technol. 2016, 6, 1593–1610. [Google Scholar] [CrossRef]
- Hu, B.; Deng, W.; Li, R.; Zhang, Q.; Wang, Y.; Delplanque-Janssens, F.; Paul, D.; Desmedt, F.; Miquel, P. Carbon-supported palladium catalysts for the direct synthesis of hydrogen peroxide from hydrogen and oxygen. J. Catal. 2014, 319, 15–26. [Google Scholar] [CrossRef]
- Fu, L.; Chuang, K.T.; Fiedorow, R. Selective oxidation of hydrogen to hydrogen peroxide. Stud. Surf. Sci. Catal. 1992, 72, 33–41. [Google Scholar]
- Ouyang, L.; Da, G.; Tian, P.; Chen, T.; Liang, G.; Xu, J.; Han, Y. Insight into active sites of Pd–Au/TiO2 catalysts in hydrogen peroxide synthesis directly from H2 and O2. J. Catal. 2014, 311, 129–136. [Google Scholar] [CrossRef]
- Ouyang, L.; Tian, P.; Da, G.; Xu, X.; Ao, C.; Chen, T.; Si, R.; Xu, J.; Han, Y. The origin of active sites for direct synthesis of H2O2 on Pd/TiO2 catalysts: Interfaces of Pd and PdO domains. J. Catal. 2015, 321, 70–80. [Google Scholar] [CrossRef]
- Edwards, J.K.; Ntainjua, E.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Direct synthesis of H2O2 from H2 and O2 over gold, palladium, and gold-palladium catalysts supported on acid-pretreated TiO2. Angew. Chem. Int. Ed. 2009, 48, 8512–8515. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Chen, S.; Cao, W.; Zhang, S.; Li, L.; Ji, T.; Zhu, J.; Li, J.; Lu, X. The effect of H2O2 desorption on achieving improved selectivity for direct synthesis of H2O2 over TiO2(B)/anatase supported Pd catalyst. Catal. Commun. 2017, 89, 69–72. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Lu, X.; Wei, M.; Zhuang, W.; Yang, Z.; Feng, X. Carbon heterogeneous surface modification on a mesoporous TiO2-supported catalyst and its enhanced hydrodesulfurization performance. Chem. Commun. 2012, 48, 11525–11527. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Zhou, Y.; Bai, Y.; Feng, X.; Yang, Z.; Lu, L.; Lu, X.; Chan, K. Enhanced photocatalytic activity in anatase/TiO2(B) core-shell nanofiber. J. Phys. Chem. C 2008, 112, 20539–20545. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Liu, C.; Yang, Z.; Feng, X.; Lu, X.; van der Laak, N.K.; Chan, K. Highly thermal stable and highly crystalline anatase TiO2 for photocatalysis. Environ. Sci. Technol. 2009, 43, 5423–5428. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, W.; Liu, C.; Yang, Z.; Feng, X.; Lu, X.; Chan, K. Stability of Pt nanoparticles and enhanced photocatalytic performance in mesoporous Pt-(anatase/TiO2(B)) nanoarchitecture. J. Mater. Chem. 2009, 19, 7055–7061. [Google Scholar] [CrossRef]
- Li, L.; Shi, K.; Tu, R.; Qian, Q.; Li, D.; Yang, Z.; Lu, X. Black TiO2(B)/anatase bicrystalline TiO2−x nanofibers with enhanced photocatalytic performance. Chin. J. Catal. 2015, 36, 1943–1948. [Google Scholar] [CrossRef]
- Ji, T.; Li, L.; Wang, M.; Yang, Z.; Lu, X. Carbon-protected Au nanoparticles supported on mesoporous TiO2 for catalytic reduction of p-nitrophenol. RSC Adv. 2014, 4, 29591–29594. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, D.; Zhang, H.; Cao, Y.; Li, G.; Yang, H.; Gao, X. Electrochemical sodium storage of TiO2(B) nanotubes for sodium ion batteries. RSC Adv. 2013, 3, 12593–12597. [Google Scholar] [CrossRef]
- Chao, K.; Cheng, W.; Yu, T.; Lu, S. Large enhancements in hydrogen production of TiO2 through a simple carbon decoration. Carbon 2013, 62, 69–75. [Google Scholar] [CrossRef]
- He, Y.; Feng, J.; Du, Y.; Li, D. Controllable synthesis and acetylene hydrogenation performance of supported Pd nanowire and cuboctahedron catalysts. ACS Catal. 2012, 2, 1703–1710. [Google Scholar] [CrossRef]
- Datye, A.K.; Bravo, J.; Nelson, T.R.; Atanasova, P.; Lyubovsky, M.; Pfefferle, L. Catalyst microstructure and methane oxidation reactivity during the Pd↔PdO transformation on alumina supports. Appl. Catal. A Gen. 2000, 198, 179–196. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Z.; Chu, Y.; Gu, D.; Yin, G. Ultrahigh stable carbon riveted Pt/TiO2-C catalyst prepared by in situ carbonized glucose for proton exchange membrane fuel cell. Energy Environ. Sci. 2011, 4, 728–735. [Google Scholar] [CrossRef]
- Song, W.; Chen, Z.; Yang, C.; Yang, Z.; Tai, J.; Nan, Y.; Lu, H. Carbon-coated, methanol-tolerant platinum/graphene catalysts for oxygen reduction reaction with excellent long-term performance. J. Mater. Chem. A 2015, 3, 1049–1057. [Google Scholar] [CrossRef]
- Ao, C.; Tian, P.; Ouyang, L.; Da, G.; Xu, X.; Xu, J.; Han, Y. Dispersing Pd nanoparticles on N-doped TiO2: A highly selective catalyst for H2O2 synthesis. Catal. Sci. Technol. 2016, 6, 5060–5068. [Google Scholar] [CrossRef]
- Thetford, A.; Hutchings, G.J.; Taylor, S.H.; Willock, D.J. The decomposition of H2O2 over the components of Au/TiO2 catalysts. Proc. R. Soc. A 2011, 467, 1885–1899. [Google Scholar] [CrossRef]
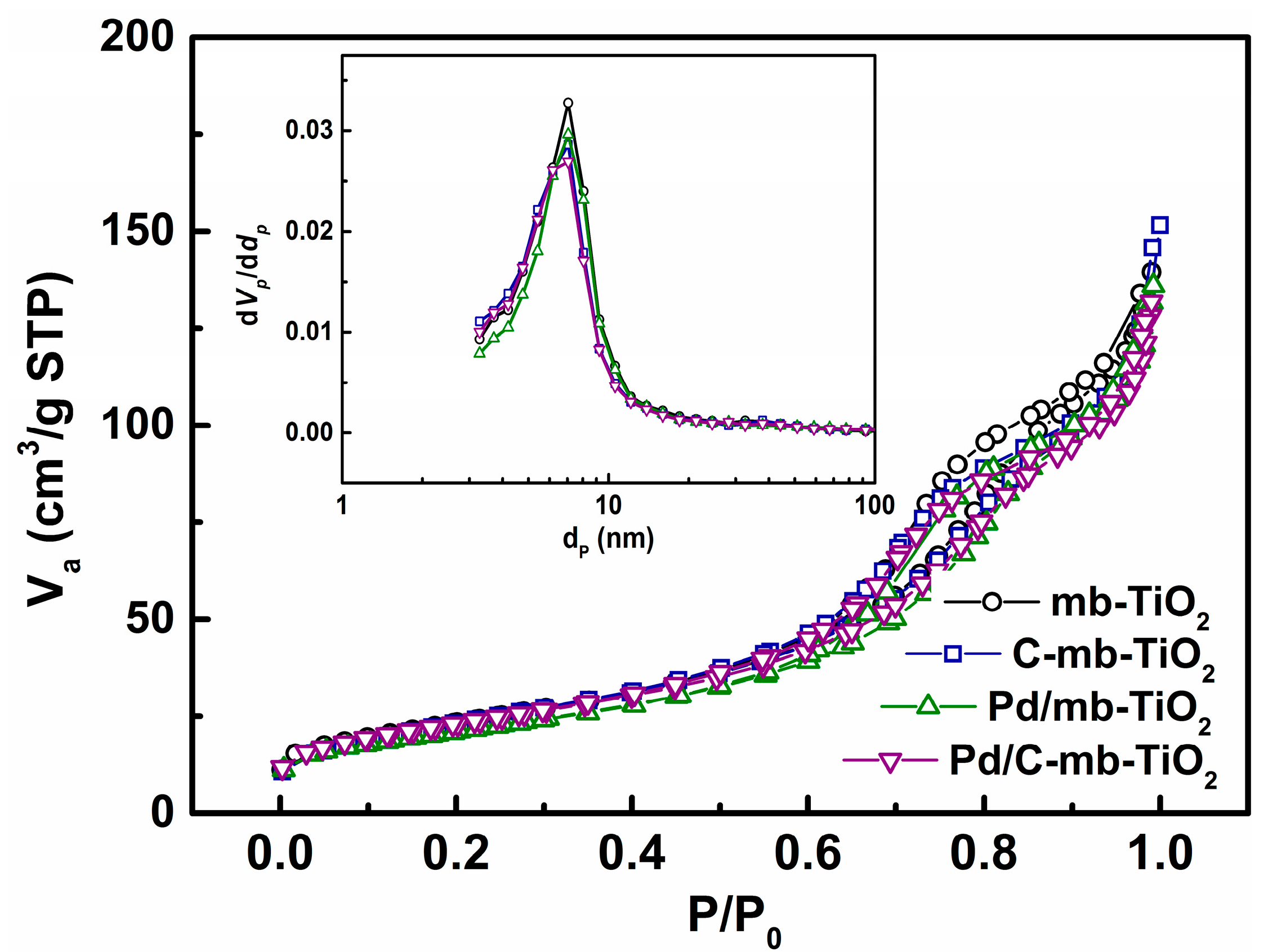
| Sample | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| mb-TiO2 | 82.2 | 0.22 | 10.5 |
| C-mb-TiO2 | 81.8 | 0.22 | 10.4 |
| Pd/mb-TiO2 | 78 | 0.20 | 10.2 |
| Pd/C-mb-TiO2 | 79 | 0.19 | 10.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, R.; Li, L.; Zhang, S.; Chen, S.; Li, J.; Lu, X. Carbon-Modified Mesoporous Anatase/TiO2(B) Whisker for Enhanced Activity in Direct Synthesis of Hydrogen Peroxide by Palladium. Catalysts 2017, 7, 175. https://doi.org/10.3390/catal7060175
Tu R, Li L, Zhang S, Chen S, Li J, Lu X. Carbon-Modified Mesoporous Anatase/TiO2(B) Whisker for Enhanced Activity in Direct Synthesis of Hydrogen Peroxide by Palladium. Catalysts. 2017; 7(6):175. https://doi.org/10.3390/catal7060175
Chicago/Turabian StyleTu, Rui, Licheng Li, Suoying Zhang, Shuying Chen, Jun Li, and Xiaohua Lu. 2017. "Carbon-Modified Mesoporous Anatase/TiO2(B) Whisker for Enhanced Activity in Direct Synthesis of Hydrogen Peroxide by Palladium" Catalysts 7, no. 6: 175. https://doi.org/10.3390/catal7060175