Potential of Pervaporation and Vapor Separation with Water Selective Membranes for an Optimized Production of Biofuels—A Review
Abstract
:1. Introduction
2. Pervaporation and Vapor Permeation Principles
2.1. Pervaporation (PV)
2.2. Vapor Permeation (VP)
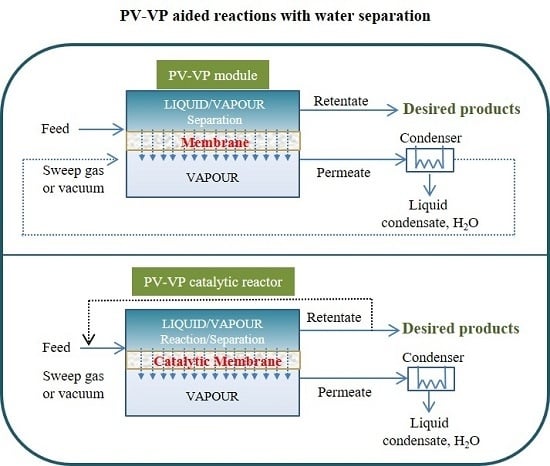
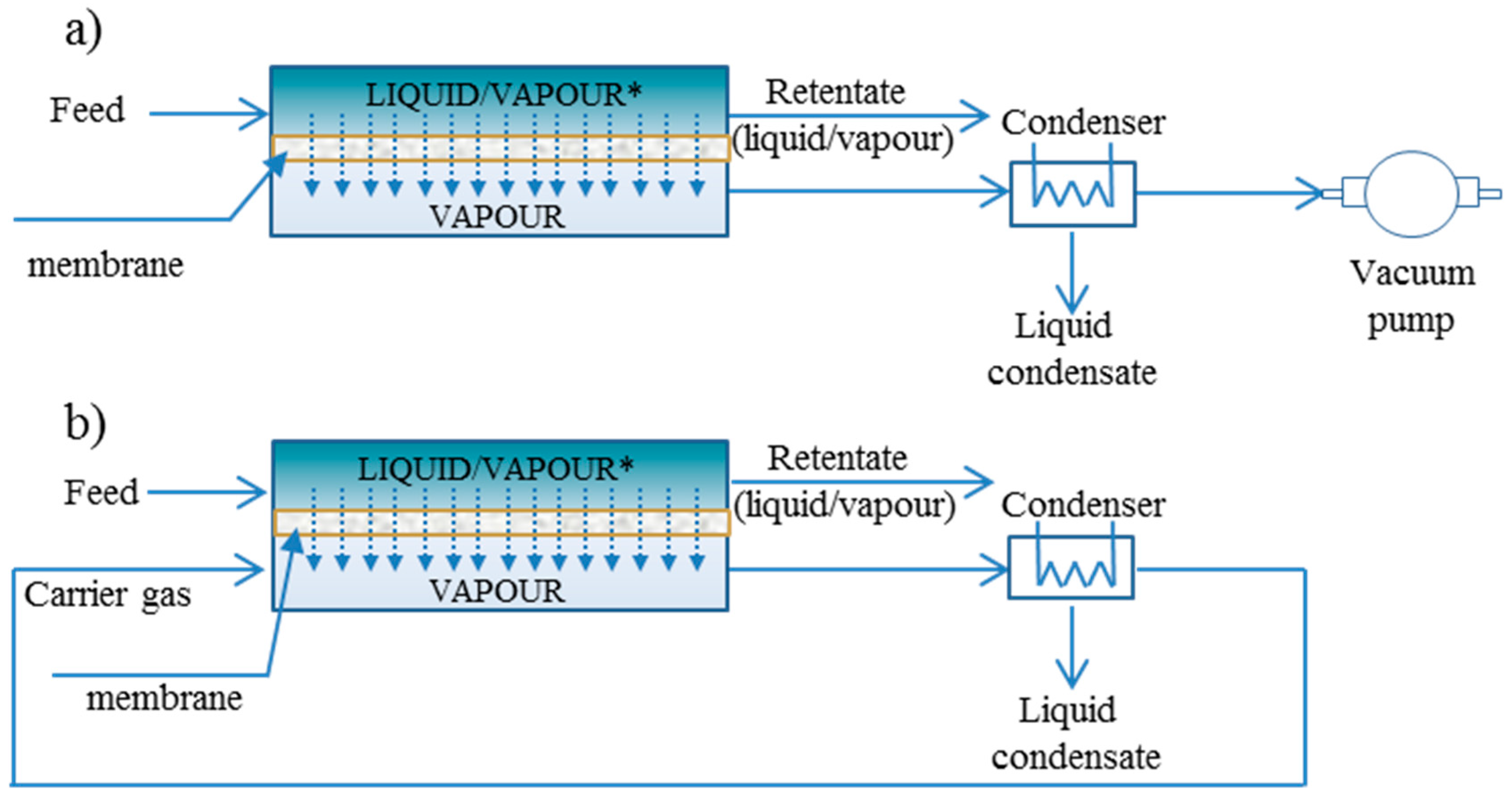
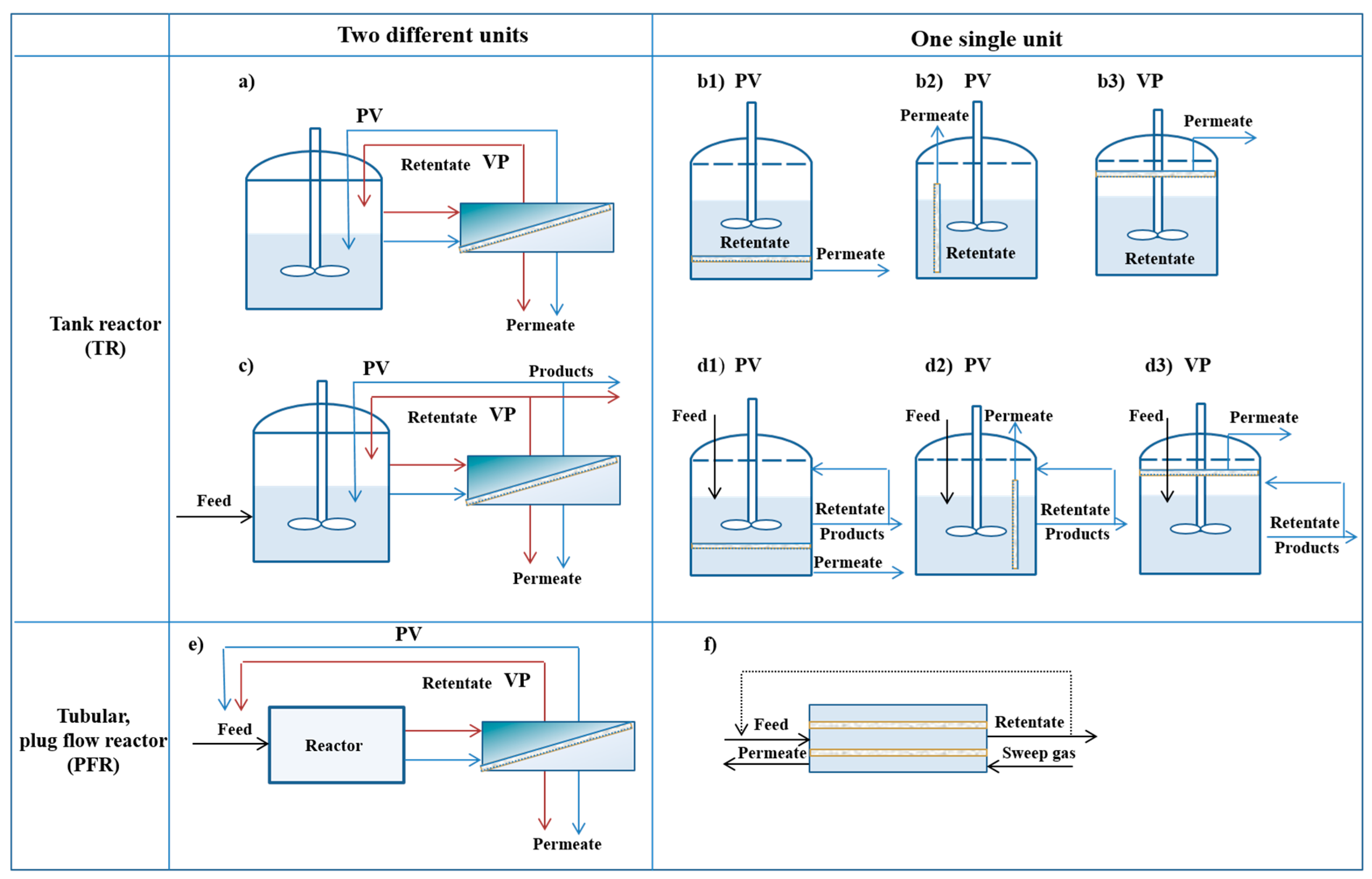
3. Membrane Reactors and Process Configurations
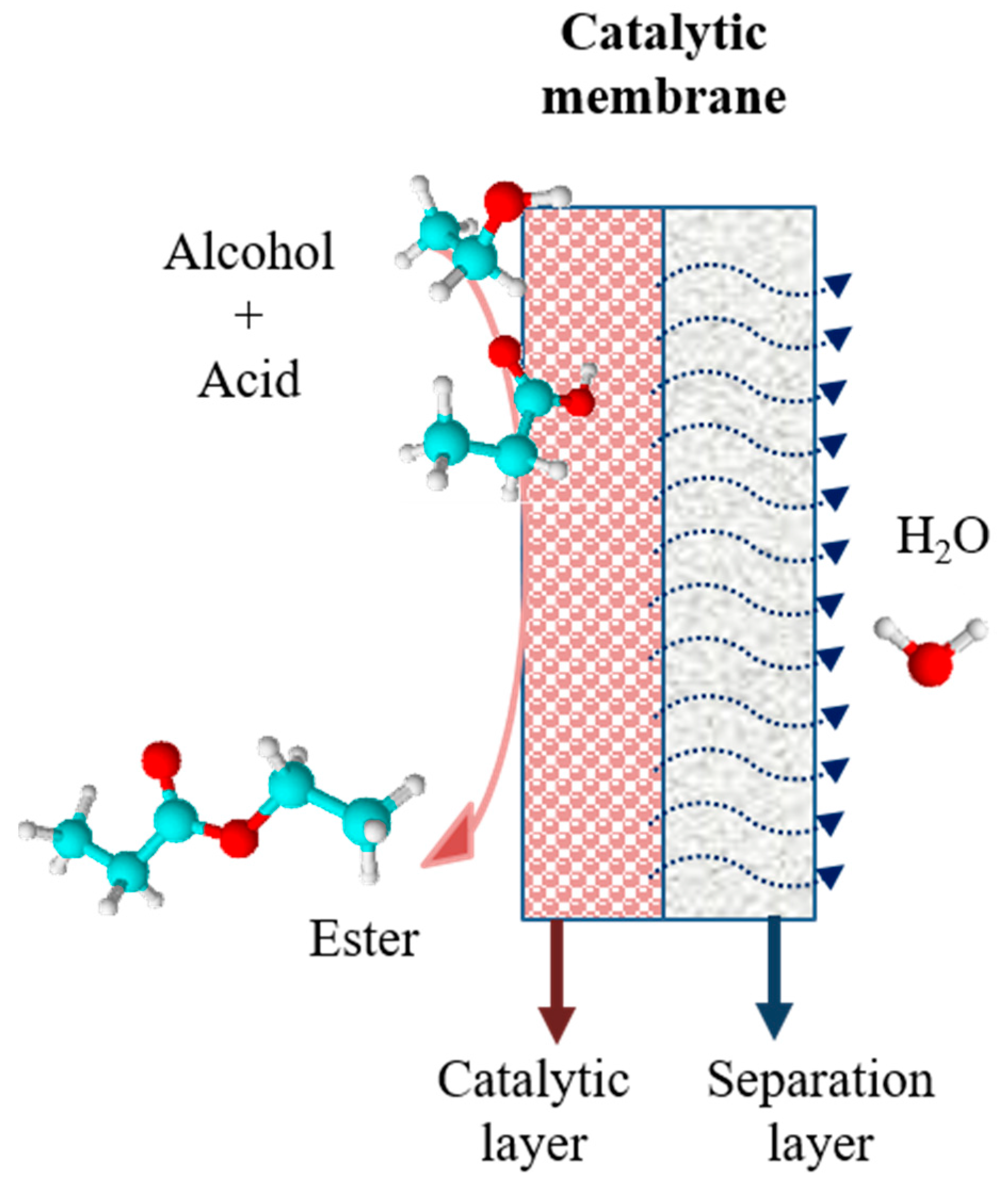
4. Membranes
5. PV and VP Applications
5.1. Model Reactions in PV Assisted Processes
5.2. Model Reactions in VP Assisted Processes
6. Biofuels and Fuel Additives Production Aided by PV or VP Processes
6.1. Biodiesel Production

6.2. Biolubricants Production
6.3. Fuel Additives
7. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| BUT | Butanol |
| CHA | Chabazite |
| CMR | Catalytic Membrane Reactor |
| CSTR | Continuous Stirred Tank Reactor |
| CST+PV | Continuous Stirred Tank reactor with external Pervaporation unit |
| CST+VP | Continuous Stirred Tank reactor with external Vapor Permeation unit |
| CSTPV | Continuous Stirred Tank reactor with internal Pervaporation unit |
| CSTVP | Continuous Stirred Tank reactor with internal Vapor Permeation unit |
| DAG | di-acylglycerol |
| DBE | 1,1-dibutoxyethane |
| DBGEs | di-butylglycerol ethers |
| DTGEs | di-tert-butylglycerol ethers |
| ETBE | Ethyl tert-butyl ether |
| FAAEs | Fatty Acid Alkyl Esters |
| FAMEs | Fatty Acid Methyl Esters |
| FFAs | Free Fatty Acids |
| GLY | Glycerol |
| m0 | Initial Molar Ratio |
| MAG | mono-acylglycerol |
| MBGEs | mono-butylglycerol ethers |
| MER | Merlionite |
| MMM | Mixed Matrix Membrane |
| MR | Membrane Reactor |
| MTBE | Methyl tert-butyl ether |
| MTGEs | mono-tert-butylglycerol ethers |
| P | Permeability constant [m/h] |
| PW | Water Permeability constant |
| PAN | Polyacrylonitrile |
| PDMS | Polydimethylsiloxane |
| PEI | Poly-etherimide |
| PermSMBR | coupling of permselective membrane with SMBR |
| PES | Polyethersulfone |
| PFR | lug Flow Reactor |
| PFR+PV | Plug Flow Reactor with external Pervaporation unit |
| PFR+VP | Plug Flow Reactor with external Vapor Permeation unit |
| PFRPV | Plug Flow Reactor with internal Pervaporation unit |
| PHI | Phillipsite |
| POPMI | poly(4,4′)-oxydiphenylene pyromellitimide |
| PV | Pervaporation |
| PVA | Polyvinyl Alcohol |
| PVMR | Pervaporation Membrane Reactor |
| S | Effective membrane area [m2] |
| SBPV | Semi Batch Reactor with internal PV unit |
| SB+PV | Semi Batch Reactor with external PV unit |
| SMBR | Simulated Moving Bed Reactor |
| SOD | Sodalite |
| TAG | tri-acylglycerol |
| TAME | tert-amyl methyl ether |
| TBA | tert-butyl alcohol |
| TBGE | tri-butylglycerolether |
| TGs | Triacylglycerides |
| TR | Tank Reactor |
| TR+PV | Tank Reactor + external PV separation unit |
| TR+VP | Tank Reactor + external Vapor Permeation separation unit |
| TRPV | Tank Reactor with internal PV separation unit |
| TRVP | Tank Reactor with Vapor Permeation separation unit |
| TTGE | tri-tert-butylglycerol ether |
| V | Volume of reaction mixture [m3] |
| VP | Vapor Permeation |
| ZCMR | Zeolite Catalytic Membrane Reactor |
| ZIF-8 | Zeolite Imidazole Framework |
| ZMR | Zeolite Membrane Reactor |
References
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y.M. Liquid Separation by Membrane Pervaporation: A Review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Kiss, A.A. Process Intensification Technologies for Biodiesel Production. Reactive Separation Processes; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Kiss, A.A.; Bildea, C.S. A review of biodiesel production by integrated reactive separation technologies. J. Chem. Technol. Biotechnol. 2012, 87, 861–879. [Google Scholar] [CrossRef]
- Gubicza, L.; Kabiri-Badr, A.; Keoves, E.; Belafi-Bako, K. Large-scale enzymatic production of natural flavour esters in organic solvent with continuous water removal. J. Biotechnol. 2000, 84, 193–196. [Google Scholar] [CrossRef]
- Jeong, J.C.; Lee, S.B. Enzymatic esterification reaction in organic media with continuous water stripping: Effect of water content on reactor performance and enzyme agglomeration. Biotechnol. Tech. 1997, 11, 853–858. [Google Scholar] [CrossRef]
- Kvittingen, L.; Sjursnes, B.; Anthonsen, T.; Halling, P. Use of salt hydrates to buffer optimal water level during lipase catalysed in synthesis in organic media: A practical procedure for organic chemists. Tetrahedron 1992, 48, 2793–2802. [Google Scholar] [CrossRef]
- Wehtje, E.; Kaur, J.; Adlercreutz, P.; Chand, S.; Mattiasson, B. Water activity control in enzymatic esterification processes. Enzyme Microb. Technol. 1997, 21, 502–510. [Google Scholar] [CrossRef]
- Kujawski, W. Application of Pervaporation and Vapor Permeation in Environmental Protection. Pol. J. Environ. Stud. 2000, 9, 13–26. [Google Scholar]
- Trusek-Holownia, A.; Noworyta, A. An integrated process: Ester synthesis in an enzymatic membrane reactor and water sorption. J. Biotechnol. 2007, 130, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yoshikawa, R.; Ying, C.; Kita, H.; Okamoto, K.I. Application of zeolite T membrane to vapor-permeation-aided esterification of lactic acid with ethanol. Chem. Eng. Sci. 2002, 57, 1577–1584. [Google Scholar] [CrossRef]
- Espro, C.; Bonura, G.; Arena, F.; Frusteri, F; Parmaliana, A.; Sini, F.; Solinas, V. Factors affecting the efficiency of Nafion-based catalytic membranes in the selective oxidation of light paraffins mediated by the Fenton system. Catal. Today 2004, 91–92, 215–218. [Google Scholar] [CrossRef]
- Silva, V.M.T.M.; Pereira, C.S.M.; Rodrigues, A.E. PermSMBR—A New Hybrid Technology: Application on Green Solvent and Biofuel production. AIChE J. 2011, 57, 1841–1851. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Green Fuel Production Using the PermSMBR Technology. Ind. Eng. Chem. Res. 2012, 51, 8928–8938. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Coupled PermSMBR—Process design and development for 1,1-dibutoxyethane production. Chem. Eng. Res. Des. 2014, 92, 2017–2026. [Google Scholar] [CrossRef]
- Graça, N.S.; Rodrigues, A.E. Application of membrane technology for the enhancement of 1,1-Diethoxybutane synthesis. Chem. Eng. Process. Process Intensif. 2017, 117, 45–57. [Google Scholar] [CrossRef]
- Faria, R.P.V.; Pereira, C.S.M.; Silva, V.M.T.M.; Loureiro, J.M.; Rodrigues, A.E. Sorption Enhanced Reactive Process for the Synthesis of Glycerol Ethyl Acetal. Chem. Eng. J. 2014, 258, 229–239. [Google Scholar] [CrossRef]
- Boutikos, P.; Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Performance Evaluation of Silica Membrane for Water—n-butanol Binary Mixture. Sep. Purif. Technol. 2014, 127, 18–28. [Google Scholar] [CrossRef]
- Faria, R.P.V.; Pereira, C.S.M.; Silva, V.M.T.M.; Loureiro, J.M.; Rodrigues, A.E. Glycerol valorisation as biofuels: Selection of a suitable solvent for an innovative process for the synthesis of GEA. Chem. Eng. J. 2013, 233, 159–167. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Rodrigues, A.E. Process Intensification: New technologies (SMBR and PermSMBR) for the synthesis of Acetals. Catal. Today 2013, 218–219, 148–152. [Google Scholar] [CrossRef]
- Drioli, E.; Fontananova, E. Membrane Materials for Addressing Energy and Environmental Challenges. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 395–420. [Google Scholar] [CrossRef] [PubMed]
- Unlu, D.; Hilmioglu, N.D. Pervaporation catalytic membrane reactor study for the production of ethyl acetate using Zr(SO4)2*4H2O coated chitosan membrane. J. Chem. Technol. Biotechnol. 2016, 91, 122–130. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Dutta, S.; Wu, K.C.-W. Integrated, Cascading Enzyme-/Chemocatalytic Cellulose Conversion using Catalysts based on Mesoporous Silica Nanoparticles. ChemSusChem 2014, 7, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chen, C.-T.; Chiu, Y.-T.; Wu, K.C.-W. An Effective Cellulose-to-Glucose-to-Fructose Conversion Sequence by Using Enzyme Immobilized Fe3O4-Loaded Mesoporous Silica Nanoparticles as Recyclable Biocatalysts. ChemCatChem 2013, 5, 2153–2157. [Google Scholar] [CrossRef]
- Alam, I.M.; De, S.; Singh, B.; Saha, B. Titanium hydrogenphosphate: An efficient dual acidic catalyst for 5-hydroxymethylfurfural (HMF) production. Appl. Catal. A 2014, 486, 42–48. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Ra, C.H.; Hong, Y.-K.; Kim, J.K.; Kong, I.-S.; Kim, S.-K.; Park, D.-H. Conversion of red-algae Gracilaria verrucosa to sugars, levulinic acid and 5-hydroxymethylfurfural. Bioprocess Biosyst. Eng. 2015, 38, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.-J.; Suzuki, N.; Yamauchi, Y.; Wu, K.C.-W. Cellulose-to-HMF conversion using crystalline mesoporous titania and zirconia nanocatalysts in ionic liquid systems. RSC Adv. 2013, 3, 2028–2034. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Lee, Y.-Y.; Peng, W.-H.; Wu, K.C.-W. Cellulosic conversion in ionic liquids (ILs): Effects of H2O/cellulose molar ratios, temperatures, times, and different ILs on the production of monosaccharides and 5-hydroxymethylfurfural (HMF). Catal. Today 2011, 174, 65–69. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Llorens, J.; Cunill, F. Technical and economical feasibility of zeolite NaA membrane-based reactors in liquid-phase etherification reactions. Chem. Eng. Process. Process Intensif. 2009, 48, 1072–1079. [Google Scholar] [CrossRef]
- Basile, A.; Figoli, A.; Khayet, M. Pervaporation, Vapour Permeation and Membrane Distillation: Principles and Applications; Woodhead Publishing in Elsevier: Kidlington, UK, 2015. [Google Scholar]
- Marszałek, J.; Kamiński, W. Environmental impact of bioethanol production. Proc. ECOpole 2008, 2, 65–70. [Google Scholar]
- Sander, U.; Soukup, P.B. Design and operation of pervaporation plant for ethanol dehydration. J. Membr. Sci. 1988, 36, 463–475. [Google Scholar] [CrossRef]
- Sander, U.; Soukup, P.B. Practical experience with pervaporation systems for liquid and vapour separation. J. Membr. Sci. 1991, 62, 67–89. [Google Scholar]
- Ohlemann, B. Chemicals: Pervaporation and vapour permeation processes meet specialist needs. Filtr. Sep. 2012, 49, 18–22. [Google Scholar] [CrossRef]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Benedict, D.J.; Parulekar, S.J.; Tsai, S.-P. Esterification of Lactic Acid and Ethanol with/without Pervaporation. Ind. Eng. Chem. Res. 2003, 42, 2291. [Google Scholar] [CrossRef]
- Brinkmann, T.; Dijkstra, M.; Ebert, K.; Ohlrogge, K. Improved simulation of a vapour permeation module. J. Chem. Technol. Biotechnol. 2003, 78, 332–337. [Google Scholar] [CrossRef]
- Bolto, B.; Hoang, M.; Xie, Z. A review of water recovery by vapour permeation through membranes. Water Res. 2012, 46, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.-Y.; Lee, K.-R.; Fan, S.-C.; Liaw, D.-J.; Huang, J.; Lai, J.-Y. Development of aromatic polyamide membranes for pervaporation and vapor permeation. J. Membr. Sci. 2000, 164, 241–249. [Google Scholar] [CrossRef]
- Sander, U.; Janssen, H. Industrial application of vapour permeation. J. Membr. Sci. 1991, 61, 113–129. [Google Scholar] [CrossRef]
- Fan, S.C.; Li, C.L.; Wang, Y.C.; Lee, K.R.; Liaw, D.J.; Lai, J.Y. Application of aromatic polyamide membranes for pervaporation and vapor permeation. Desalination 2002, 148, 43–48. [Google Scholar] [CrossRef]
- Armor, J.N. Membrane catalysis: Where is it now, what needs to be done? Catal. Today 1995, 25, 199–207. [Google Scholar] [CrossRef]
- Giorno, L.; Drioli, E. Biocatalytic membrane reactors: Applications and perspectives. Trends Biotechnol. 2000, 18, 339–349. [Google Scholar] [CrossRef]
- Pabby, A.K.; Rizvi, S.S.H.; Requena, A.M.S. (Eds.) Handbook of Membrane Separations: Chemical, Pharmaceutical, and Biotechnological Applications, 2nd ed.; CRC Press, Taylor & Francis Group: Raton, FL, USA, 2015. [Google Scholar]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A.; Hai, F.I. Introduction—A Review of Membrane Reactors. In Membranes for Membrane Reactors; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 1–61. [Google Scholar]
- Parulekar, S.J. Analysis of Pervaporation-Aided Esterification of Organic Acids. Ind. Eng. Chem. Res. 2007, 46, 8490–8504. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Dragomirova, R.; Wohlrab, S. Zeolite membrane in Catalysis—From separate units to particle coatings. Catalysts 2015, 5, 2161–2222. [Google Scholar] [CrossRef]
- Lipnizki, F.; Field, R.W.; Ten, P.-K. Pervaporation-based hybrid process: A review of process design, applications and economics. J. Membr. Sci. 1999, 153, 183–210. [Google Scholar] [CrossRef]
- Armor, J.N. Applications of catalytic inorganic membrane reactors to refinery products. J. Membr. Sci. 1998, 147, 217–233. [Google Scholar] [CrossRef]
- Takht Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Diban, N.; Aguayo, A.T.; Bilbao, J.; Urtiaga, A.; Ortiz, I. Membrane reactors for in situ water removal: A review of applications. Ind. Eng. Chem. Res. 2013, 52, 10342–10354. [Google Scholar] [CrossRef]
- Lim, Y.S.; Park, B.; Hung, F.; Sahimi, M.; Tsotsis, T.T. Design issues of pervaporation membrane reactors for esterification. Chem. Eng. Sci. 2002, 57, 4933–4946. [Google Scholar] [CrossRef]
- Dams, A.; Krug, J. Pervaporation aided esterification alternatives in plant extension for an existing chemical process. In Proceedings of the Fifth International Conference on Pervaporation Process in the Chemical Industries, Heidelberg, Germany, 11–15 March 1991; Bakish, R., Ed.; Bakish Material Corporation: Englewood, NJ, USA, 1991; pp. 338–348. [Google Scholar]
- Peters, T.A. Catalytic Pervaporation Membranes for Close Integration of Reaction and Separation; Eindhoven University of Technology: Eindhoven, The Netherlands, 2006. [Google Scholar]
- Nguyen, Q.T.; M’Bareck, C.O.; David, M.O.; Métayer, M.; Alexandre, S. Ion-exchange membranes made of semi-interpenetrating polymer networks, used for pervaporation-assisted esterification and ion transport. Mater. Res. Innov. 2003, 7, 212–219. [Google Scholar] [CrossRef]
- Morigami, Y. The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane. Sep. Purif. Technol. 2001, 25, 251–260. [Google Scholar] [CrossRef]
- Kondo, M.; Komori, M.; Kita, H.; Okamoto, K.-I. Tubular-type pervaporation module with zeolite NaA membrane. J. Membr. Sci. 1997, 133, 133–141. [Google Scholar] [CrossRef]
- Cui, Y.; Kita, H.; Okamoto, K.-I. Zeolite T membrane: Preparation, characterization, pervaporation of water/organic liquid mixtures and acid stability. J. Membr. Sci. 2004, 236, 17–27. [Google Scholar] [CrossRef]
- Li, G.; Kikuchi, E.; Matsukata, M. Separation of water-acetic acid mixtures by pervaporation using a thin mordenite membrane. Sep. Purif. Technol. 2003, 32, 199–206. [Google Scholar] [CrossRef]
- Lovallo, M.C.; Tsapatsis, M.; Okubo, T. Preparation of an Asymmetric Zeolite L Film. Chem. Mater. 1996, 8, 1579–1583. [Google Scholar] [CrossRef]
- Pina, M.P.; Arruebo, M.; Felipe, M.; Fleta, F.; Bernal, M.P.; Coronas, J.; Menéndez, M.; Santamaría, J. A semi-continuous method for the synthesis of NaA zeolite membranes on tubular supports. J. Membr. Sci. 2004, 244, 141–150. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Chen, J.-T.; Chang, C.-H.; Liao, K.-S.; Tung, K.-L.; Price, W.E.; Yamauchi, Y.; Wu, K.C.-W. A Drying-Free, Water-Based Process for Fabricating Mixed-Matrix Membranes with Outstanding Pervaporation Performance. Angew. Chem. Int. Ed. 2016, 55, 12793–12796. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bornscheuer, U.T.; Schmid, R.D. Efficient water removal in lipase-catalyzed esterifications using a low-boiling-point azeotrope. Biotechnol. Bioeng. 2002, 78, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [PubMed]
- Krupiczka, R.; Koszorz, Z. Activity-based model of the hybrid process of an esterification reaction coupled with pervaporation. Sep. Purif. Technol. 1999, 16, 55–59. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Salt, Y.; Keleşer, S.; Dinçer, S. The esterification of acetic acid with ethanol in a pervaporation membrane reactor. Desalination 2009, 245, 662–669. [Google Scholar] [CrossRef]
- Sanz, M.T.; Gmehling, J. Esterification of acetic acid with isopropanol coupled with pervaporation Part I: Kinetics and pervaporation studies. Chem. Eng. J. 2006, 123, 1–8. [Google Scholar] [CrossRef]
- Domingues, L.; Recasens, F.; Larrayoz, A. Studies of a pervaporation reactor: Kinetics and equilibrium shift in benzyl alcohol acetylation. Chem. Eng. Sci. 1999, 54, 1461–1465. [Google Scholar] [CrossRef]
- Sekulic, J. Mesoporous and Microporous Titania Membranes. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2004. [Google Scholar]
- Inoue, T.; Nagase, T.; Hasegawa, Y.; Kiyozumi, Y.; Sato, K.; Nishioka, M.; Hamakawa, S.; Mizukami, F. Stoichiometric Ester Condensation Reaction Processes by Pervaporative Water Removal via Acid-Tolerant Zeolite Membranes. Ind. Eng. Chem. Res. 2007, 46, 3743–3750. [Google Scholar] [CrossRef]
- De la Iglesia, Ó.; Mallada, R.; Menéndez, M.; Coronas, J. Continuous zeolite membrane reactor for esterification of ethanol and acetic acid. Chem. Eng. J. 2007, 131, 35–39. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshikawa, R.; Ying, C.; Kita, H.; Okamoto, K.I. Application of zeolite membranes to esterification reactions. Catal. Today 2001, 67, 121–125. [Google Scholar] [CrossRef]
- Zhang, W.; Na, S.; Li, W.; Xing, W. Kinetic modelling of pervaporation aided esterification of propionic acid and ethanol using T-type zeolite membrane. Ind. Eng. Chem. Res. 2015, 54, 4940–4946. [Google Scholar] [CrossRef]
- Zhu, Y.; Minet, R.G.; Tsotsis, T.T. A continuous pervaporation membrane reactor for the study of esterification reactions using a composite polymeric/ceramic membrane. Chem. Eng. Sci. 1996, 51, 4103–4113. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y.M. Studies of a membrane reactor: Esterification facilitated by pervaporation. Chem. Eng. Sci. 1996, 51, 4673–4679. [Google Scholar] [CrossRef]
- Li, X.; Wang, L. Kinetic model for an esterification process coupled by pervaporation. J. Membr. Sci. 2001, 186, 19–24. [Google Scholar]
- Liu, Q.; Zhang, Z.; Chen, H. Study on the coupling of esterification with pervaporation. J. Membr. Sci. 2001, 182, 173–181. [Google Scholar] [CrossRef]
- Liu, Q.L.; Chen, H.F. Modeling of esterification of acetic acid with n-butanol in the presence of Zr(SO4)2 4H2O coupled pervaporation. J. Membr. Sci. 2002, 196, 171–178. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijin, F. Application of sodalite membrane reactor in esterification-coupling reaction and separation. Catal. Today 2010, 156, 132–139. [Google Scholar] [CrossRef]
- David, M.O.; Gref, R.; Nguyen, T.Q.; Neek, J. Pervaporation esterification coupling. Part I. Basic kinetic model. Trans. IChemE 1991, 69, 335–340. [Google Scholar]
- Caro, J. Volume 3.01 Basic aspects of membrane reactors. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 1–24. [Google Scholar]
- Saracco, G.; Specchia, V. Catalytic inorganic membrane reactor: Present experience and future opportunities. Catal. Rev. 1994, 36, 305–384. [Google Scholar] [CrossRef]
- Téllez, C.; Menéndez, M. Zeolite membrane reactors. In Membranes for Membrane Reactors; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 243–273. [Google Scholar]
- Gallucci, F.; Fernandez, E.; Corengia, P.; Van sint Annaland, M. Recent advances on membranes and membranes reactor for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Thomas, S.; Hamel, C.; Seidel-Morgenstern, A. Basic Problems of chemical reaction enginnering and potential of membrane reactors. In Membrane Reactors; Wiley-VCH Verlag GmbH & Co. KGaA: Winheim, Germany, 2010; pp. 1–27. [Google Scholar]
- Bernal, M.P.; Coronas, J.; Menendez, M.; Santamaria, J. Coupling of reaction and separation at the microscopic level: Esterification processes in a H-ZSM5 mebrane reactor. Chem. Eng. Sci. 2002, 57, 1557–1562. [Google Scholar] [CrossRef]
- Peters, T.A.; Benes, N.R.; Keurentjes, J.T.F. Zeolite-coated ceramic pervaporation membranes; pervaporation-esterification coupling and reactor evaluation. Ind. Eng. Chem. Res. 2005, 44, 9490–9496. [Google Scholar] [CrossRef]
- De la Iglesia, O.; Irusta, S.; Mallada, R.; Menéndez, M.; Coronas, J.; Santamarìa, J. Preparation and characterization of two-layered mordenite ZSM5 bifunctional membranes. Microporous Mesoporous Mater. 2006, 93, 318–324. [Google Scholar] [CrossRef]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Optimization of Geometric Properties of a Monolithic Catalyst for the Selective Hydrogenation of Phenylacetylene. Ind. Eng. Chem. Res. 2001, 40, 2801–2809. [Google Scholar] [CrossRef]
- Bagnall, L.; Cavell, K.; Hodges, A.M.; Mau, A.W.; Seen, A.J. The use of catalytically active pervaporation membranes in esterification reactions to simultaneously increase product yield and permselectivity flux. J. Membr. Sci. 1993, 85, 291–299. [Google Scholar] [CrossRef]
- David, M.O.; Nguyen, T.Q.; Neel, J. Pervaporation membranes endowed with catalytic properties, based on polymer blends. J. Membr. Sci. 1992, 73, 129–141. [Google Scholar] [CrossRef]
- Zhang, W.; Qing, W.; Chen, N.; Ren, Z.; Chen, J.; Sun, W. Enhancement of esterification conversion using novel composite catalytically active pervaporation membranes. J. Membr. Sci. 2013, 451, 285–292. [Google Scholar] [CrossRef]
- Ziobrowski, Z.; Kiss, K.; Rotkegel, A.; Nemest Othy, N.; Krupiczka, R.; Gubicza, L. Pervaporation aided enzymatic production of glycerol monostearate in organic solvents. Desalination 2009, 241, 212–217. [Google Scholar] [CrossRef]
- Kita, H.; Tanaka, K.; Okamoto, K.; Yamamoto, M. The Esterification of Oleic Acid with Ethanol Accompanied by Membrane Separation. Chem. Lett. 1987, 16, 2053–2056. [Google Scholar] [CrossRef]
- Yamamoto, M.; Munehisa, N.; Kaibara, M.; Horii, K.; Tanaka, K.; Kita, H.; Okamoto, K. Vapor-Permeation-Aided Esterification of Oleic Acid. Application of pressurized vapor circulation system and Zeolite membrane. Membrane 1995, 20, 143–148. [Google Scholar] [CrossRef]
- Okamoto, K.I.; Kita, H. Zeolite NaA membrane: Preparation, single—Gas permeation, and pervaporation and vapor permeation of water/organic liquid mixtures. Ind. Eng. Chem. Res. 2001, 40, 163–175. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nagase, T.; Kiyozumi, Y.; Hanaoka, T.; Mizukami, F. Influence of acid on the permeation properties of NaA-type zeolite membranes. J. Membr. Sci. 2010, 349, 189–194. [Google Scholar] [CrossRef]
- Ameri, E.; Moheb, A.; Roodpeyma, S. Vapor-permeation-aided esterification of isopropanol/propionic acid using NaA and PERVAP® 2201 membranes. Chem. Eng. J. 2010, 162, 355. [Google Scholar] [CrossRef]
- Jafar, J.J.; Budd, P.M.; Hugher, R. Enhancement of esterification reaction yield using zeolite a vapour permeation membrane. J. Membr. Sci. 2002, 199, 117–123. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Mizukami, F.; Kowata, Y.; Hanaoka, T. Application of a CHA-type zeolite membrane to the esterification of adipic acid with isopropyl alcohol using sulfuric acid catalyst. J. Membr. Sci. 2012, 415, 368–374. [Google Scholar] [CrossRef]
- Unlu, D.; Hilmioglu, N.D. Bioadditive Synthesis from Glycerol by Esterification Using Catalytic Chitosan Membrane. In Progress in Clean Energy, Volume I: Analysis and Modeling; Dincer, I., Colpan, C.O., Kizilkan, O., Ezan, M.A., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Cannilla, C.; Bonura, G.; Arena, F.; Rombi, E.; Frusteri, F. How surface and textural properties affect the behaviour of Mn-based catalysts during transesterification reaction to produce biodiesel. Catal. Today 2012, 195, 32–43. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Farobie, O.; Matsumura, Y. A comparative study of biodiesel production using methanol, ethanol, and tert-butyl methyl ether (MTBE) under supercritical conditions. Bioresour. Technol. 2015, 191, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. Membrane biodiesel production and refining technology: A critical review. Renew. Sustain. Energy Rev. 2011, 15, 5051–5062. [Google Scholar] [CrossRef]
- Shuit, S.H.; Ong, Y.T.; Lee, K.T.; Subhash, B.; Tan, S.H. Membrane technology as a promising alternative in biodiesel production: A review. Biotechnol. Adv. 2012, 30, 1364–1380. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Dubé, M.A.; Tremblay, A.Y. High-purity fatty acid methyl ester production from canola, soybean, palm, and yellow grease lipids by means of a membrane reactor. Biomass Bioenergy 2008, 32, 1028–1036. [Google Scholar] [CrossRef]
- Waldburger, R.M.; Widmer, F. Membrane reactors in chemical production processes and the application to the pervaporation-assisted esterification. Chem. Eng. Technol. 1996, 19, 117–126. [Google Scholar] [CrossRef]
- Assabumrungrat, S.; Phongpatthanapanich, J.; Praserthdam, P.; Tagawa, T.; Goto, S. Theoretical study on the synthesis of methyl acetate from methanol and acetic acid in pervaporation membrane reactor: Effect of continuous flow modes. Chem. Eng. J. 2003, 95, 57–65. [Google Scholar] [CrossRef]
- Okamoto, K.; Yamamoto, M.; Otoshi, Y.; Semoto, T.; Yano, M.; Tanaka, K.; Kira, H. Pervaporation-aided esterification of oleic acid. J. Chem. Eng. Jpn. 1993, 26, 475–481. [Google Scholar] [CrossRef]
- Sarkar, B.; Sridhar, S.; Saravanan, K.; Kale, V. Preparation of fatty acid methyl ester through temperature gradient driven pervaporation process. Chem. Eng. J. 2010, 162, 609–615. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Salim, V.M.M.; Borges, C.P. Ethyl oleate production by means of pervaporation-assisted esterification using heterogeneous catalysisi. Braz. J. Chem. Eng. 2010, 27, 609–617. [Google Scholar] [CrossRef]
- Okamoto, K.I.; Yamamoto, M.; Noda, S.; Semoto, T.; Otoshi, Y.; Tanaka, K.; Kita, H. Vapor-permeation-aided esterification of oleic acid. Ind. Eng. Chem. Res. 1994, 33, 849–853. [Google Scholar] [CrossRef]
- Rewagad, R.R.; Kiss, A.A. Modeling and simulation of a pervaporation process for fatty ester synthesis. Chem. Eng. Commun. 2012, 199, 1357–1374. [Google Scholar] [CrossRef]
- Dörmő, N.; Bélafi-Bakó, K.; Bartha, L.; Ehrenstein, U.; Gubicza, L. Manufacture of an environmental-safe biolubricant from fusel oil by enzymatic esterification in solvent-free system. Biochem. Eng. J. 2004, 21, 229–234. [Google Scholar] [CrossRef]
- Koszorz, Z.; Nemestothy, N.; Ziobrowski, Z.; Belafi-Bako, K.; Krupiczka, R. Influence of pervaporation process parameters on enzymatic catalyst deactivation. Desalination 2004, 162, 307–313. [Google Scholar] [CrossRef]
- Chamberlain, R.; Borges, C.P.; Habert, A.C.; Nobrega, R. Fractionation of fusel oil coupling pervaporation and distillation. In Proceedings of the Seventh International Conference on Pervaporation Processes in the Chemical Industry, Reno, Nevada, 26 February–1 March 1995; Bakish, R., Ed.; Bakish Material Corporation: Englewood, NJ, USA, 1995; pp. 271–284. [Google Scholar]
- Beatrice, C.; Di Blasio, G.; Lazzaro, M.; Cannilla, C.; Bonura, G.; Frusteri, F.; Asdrubali, F.; Baldinelli, G.; Presciutti, A.; Fantozzi, F.; et al. Technologies for energetic exploitation of biodiesel chain derived glycerol: Oxy-fuels production by catalytic conversion. Appl. Energy 2013, 102, 63–71. [Google Scholar] [CrossRef]
- Salomón, M.; Coronas, J.; Menéndez, M.; Santamarı́a, J. Synthesis of MTBE in zeolite membrane reactors. Appl. Catal. A Gen. 2000, 200, 201–210. [Google Scholar] [CrossRef]
- Assabumrungrat, S.; Kiatkittipong, W.; Praserthdam, P.; Goto, S. Simulation of pervaporation membrane reactors for liquid phase synthesis of ethyl tert-butyl ether from tert-butyl alcohol and ethanol. Catal. Today 2003, 79–80, 249–257. [Google Scholar] [CrossRef]
- Yang, B.-L.; Goto, S. Pervaporation with reactive distillation for the production of ethyl tert-butyl ether. Sep. Sci. Technol. 1997, 32, 971–981. [Google Scholar] [CrossRef]
- Norkobilov, A.; Gorri, D.; Ortiz, I. Process flowsheet analysis of pervaporation-based hybrid processes in the production of ethyl tert-butyl ether. J. Chem. Technol. Biotechnol. 2017, 92, 1167–1177. [Google Scholar] [CrossRef]
- Aguirre, I.; Güemez, M.B.; van Veen, H.M.; Motelica, A.; Vente, J.F.; Arias, P.L. Acetalization reaction of ethanol with butyraldehyde coupled with pervaporation. Semi-batch pervaporation studies and resistance of HybSi® membranes to catalyst impacts. J. Membr. Sci. 2011, 371, 179–188. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Glycerol Etherification with TBA: High Yield to Poly-Ethers Using a Membrane Assisted Batch Reactor. Environ. Sci. Technol. 2014, 48, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Kiatkittipong, W.; Intaracharoen, P.; Laosiripojana, N.; Chaisuk, C.; Praserthdam, P.; Assabumrungrat, S. Glycerol ethers synthesis from glycerol etherification with tert-butyl alcohol in reactive distillation. Comput. Chem. Eng. 2011, 35, 2034–2043. [Google Scholar] [CrossRef]
- Vlad, E.; Bildea, C.S.; Bozga, G. Design and Control of glycerol-tert-butyl alcohol etherification process. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; Di Blasi, O. Catalytic etherification of glycerol by tert-butyl alcohol to produce oxygenated additives for diesel fuel. Appl. Catal. A 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Ozbay, N.; Oktar, N.; Dogu, G.; Dogu, T. Effect of sorption enhancement and isobutene formation on etherification of glycerol with tert-butyl alcohol in a flow reactor. Ind. Eng. Chem. Res. 2012, 51, 8788–8795. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Mezzapica, A.; Frusteri, L.; Frusteri, F. An Original Catalytic Reactor Coupled with Tubular Membrane to Efficiently Produce Biofuels by Etherification of Glycerol with TBA. Proceeding of the 5th International Conference on Engineering for Waste and Biomass Valorisation (WasteEng2014), Rio de Janeiro, Brazil, 25–28 August 2014; pp. 1235–1247. [Google Scholar]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Batch reactor coupled with water permselective membrane: Study of glycerol etherification reaction with butanol. Chem. Eng. Process. 2015, 282, 187–193. [Google Scholar] [CrossRef]
- Gaudin, P.; Jacquot, R.; Marion, P.; Pouilloux, Y.; Jérôme, F. Acid-Catalyzed Etherification of Glycerol with Long-Alkyl-Chain Alcohols. ChemSusChem 2011, 4, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; De Oliveira Vigier, K.; Pera-Titus, M.; Pouilloux, Y.; Clacens, J.-M.; Decampo, F.; Jérôme, F.; Weckhuysen, B.M. Catalytic etherification of glycerol with short chain alkyl alcohols in the presence of Lewis acids. Green Chem. 2013, 15, 901–909. [Google Scholar] [CrossRef]

| Highlights of PV and VP Processes |
|---|
| Mixtures that at normal distillation form azeotropes and/or require a large number of theoretical stages can easily and economically be separated even without the use of entrained agents (chemicals). |
| High product purity is obtained and no environmental pollution occurs. |
| Multicomponent mixtures with small difference in boiling points can be dehydrated effectively and economically. |
| The feed mixtures could be in either liquid or vapor form. |
| Low energy consumption is required. |
| Small units can operate economically exploiting the modular design of the membrane system. |
| High degrees of feed mixture flexibility. |
| High final product qualities are obtained |
| Modularly, compact design and factory-preassembled systems are simple to operate and can be started up and shut down rapidly. |
| Pervaporation | Vapor Permeation |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Membrane | Nature of Membrane | Reaction Studied | Technology * | Reference |
|---|---|---|---|---|
| Zeolite T | Zeolite membrane | Esterification lactic acid + ethanol | TRVP | [11] |
| Pervatech BV | Microporous silica membrane | Esterification lactic acid + ethanol Acetalization acetaldehyde + ethanol (additive) | PermSMBR | [13] |
| Pervatech BV | Microporous silica membrane | Acetalization acetaldehyde + butanol (additive) | PermSMBR | [14,15] |
| Pervatech BV | Microporous silica membrane | Acetalization butyraldehyde + ethanol (additive) | PermSMBR | [16] |
| Pervatech BV | Microporous silica membrane | Dehydration n-butanol | SMBR+PV | [18] |
| NaA | Catalytic zeolite membrane | Etherification of n-pentanol (blending additive) | ExtractorCMR † ZCMR †† | [29] |
| GFT-1005 membrane | organic acid-compatible PVA-based membrane | Esterification lactic acid + ethanol | TR+PV | [36] |
| Mordenite membrane | Catalytic membrane | Dehydration of acetic acid | TRPV | [61] |
| Pervap 2200 | Hydrophilic polymeric membrane | Enzymatic esterification glucose + fatty acids | TRVP | [65] |
| PERVAP GFT-1005 | Hydrophilic polymeric membrane | Esterification acetic acid + ethanol | PVMR | [67] |
| PDMS | Hydrophobic cross-linked membrane | Esterification acetic acid + ethanol | TRPV | [68] |
| PERVAP 2201 | Hydrophilic polymeric membrane | Esterification acetic acid + isopropanol | PVMR | [69] |
| PERVAP GFT-1005 | PVA polymeric membrane supported on polyacrylonitrile layered onto cellulose material | Esterification acetic acid + benzyl alcohol | TRPV | [70,71] |
| MER, PHI, CHA | Acid-tolerant hydrophilic zeolite membrane | Ester condensation between various kinds of carboxylic and alcohols at <50 °C | TRPV | [72] |
| Mordenite/α-Al2O3 Zeolite A/α-Al2O3 | Zeolite membrane | Esterification acetic acid + ethanol | PFRPV | [73] |
| Zeolite T | Zeolitic membrane | Esterification acetic acid+ ethanol | TRPV | [74,75] |
| Polyetherimid based membrane | Polymeric/ceramic membrane | Esterification acetic acid + ethanol | PFRPV | [76,77] |
| PVA/ceramic composite | Hydrophilic polymeric membrane | Esterification acetic acid + n-butanol | TRPV | [78,79] |
| SOD | Tubular hydroxyl sodalite membrane | Esterification acetic acid + 1-butanol | TR+PV | [80,81] |
| H-ZSM-5/α-Al2O3 | Zeolite catalytic membrane | Esterification acetic acid + ethanol | PFRPV ZMR ††† AZMR †††† | [82,83,84,85,86,87,88] |
| H-USY/TNO-TPD | Composite catalytic membrane: zeolite coating on top of ceramic hollow fiber silica membrane | Esterification acetic acid + butanol | CMR | [89] |
| H-ZSM-5/mordenite | Bi-functional zeolite membrane | Esterification acetic acid + ethanol | CMR | [90,91,92] |
| PVA/PES + catalytic PVA/PES | Catalytically active membrane Support layer: PES porous membrane; separation layer: PVA membrane; porous catalytic layer | Esterification acetic acid + n-butanol | TR-CMR | [93,94] |
| Sulzer Pervap 2001 | Hydrophilic polymeric membrane | Enzymatic esterification glycerol + stearic acid | TR+PV | [95] |
| Ultem | Polyimide membrane | Esterification oleic acid + ethanol (biodiesel) | TRVP | [96,97,98] |
| NaA | Tubular nanoporous zeolite membrane | Esterification of propionic acid + isopropanol | SB+VP | [99,100] |
| NaA/carbon-zirconia support | Tubular zeolite layer on porous α-Al2O3 tube | Esterification lactic acid + ethanol | TRVP TRPV | [101] |
| CHA | Polycrystalline zeolite membrane | Esterification of adipic acid + isopropyl alcohol | TRVP | [102] |
| Zr(SO4)3·4H2O/chitosan | Catalytic membrane | Esterification glycerol + acetic acid (additive) | TRPV(CMR) | [22,103,104] |
| PVA | Hydrophilic polymeric membrane | Esterification acetic acid + methanol | SBPV PFRPV CSTPV | [105,106,107,108,109,110,111,112] |
| PEI-POPMI | Aromatic polyimide hollow fiber | Esterification oleic acid + ethanol (biodiesel) | TRPV | [113] |
| PVA/PES substrate | Composite hydrophilic polymeric membrane | Esterification oleic acid + methanol (biodiesel) | TRPV | [114] |
| PERVAP 1000 | Crosslinked PVA layer coated onto a PAN substrate | Esterification oleic acid + ethanol (biodiesel) | TR+PV | [115] |
| PEI-POPMI | Aromatic polyimide hollow fiber | Esterification oleic acid + ethanol (biodiesel) | TRVP | [116,117] |
| PERVAP 2201 PERVAP 2202 CMC-VP-43 | Hydrophilic polymeric membrane | Enzymatic esterification fusel oil + oleic acid (biolubricant) | TR+PV | [118] |
| PERVAP-1005 | Hydrophilic polymeric membrane | Enzymatic esterification of oleic acid + i-amyl alcohol (bio-lubricant) | TR+PV | [119,120,121] |
| MOR/ZSM5/CHA NaA | Zeolite tubular membrane | Etherification methanol + tert-butyl alcohol (oxygenate additive) | ZCMR †††† | [122] |
| PVA | Hydrophilic polymeric membrane | Enzymatic etherification ethanol + tert-butyl alcohol (oxygenate additive) | SBPV PFRPV CSTPV | [123,124,125] |
| HybSi (ENC) | Inorganic/organic hybrid membrane | Esterification bioethanol + butanal (additive) | SBPV | [126] |
| HybSi | γ-Al2O3 tubular phase coated with organic polymer | Etherification glycerol + tert-butyl alcohol (additive) | TR+VP | [127,128,129,130,131,132] |
| HybSi | γ-Al2O3 tubular phase coated with organic polymer | Etherification glycerol + butanol (additive) | TR+VP | [133] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannilla, C.; Bonura, G.; Frusteri, F. Potential of Pervaporation and Vapor Separation with Water Selective Membranes for an Optimized Production of Biofuels—A Review. Catalysts 2017, 7, 187. https://doi.org/10.3390/catal7060187
Cannilla C, Bonura G, Frusteri F. Potential of Pervaporation and Vapor Separation with Water Selective Membranes for an Optimized Production of Biofuels—A Review. Catalysts. 2017; 7(6):187. https://doi.org/10.3390/catal7060187
Chicago/Turabian StyleCannilla, Catia, Giuseppe Bonura, and Francesco Frusteri. 2017. "Potential of Pervaporation and Vapor Separation with Water Selective Membranes for an Optimized Production of Biofuels—A Review" Catalysts 7, no. 6: 187. https://doi.org/10.3390/catal7060187