Using Laccases in the Nanoflower to Synthesize Viniferin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coupling Efficiency and Loading Efficiency
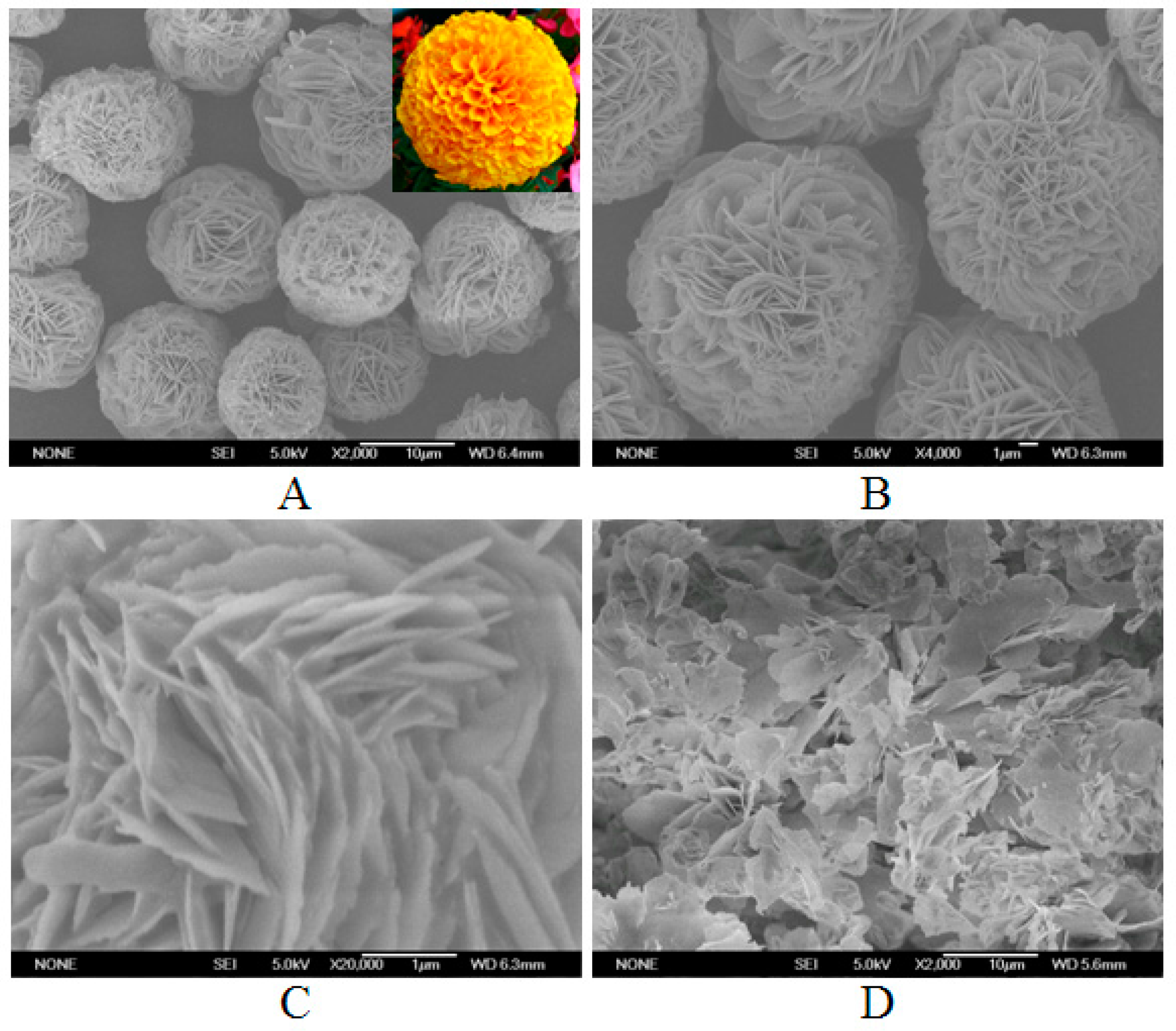
2.2. SEM
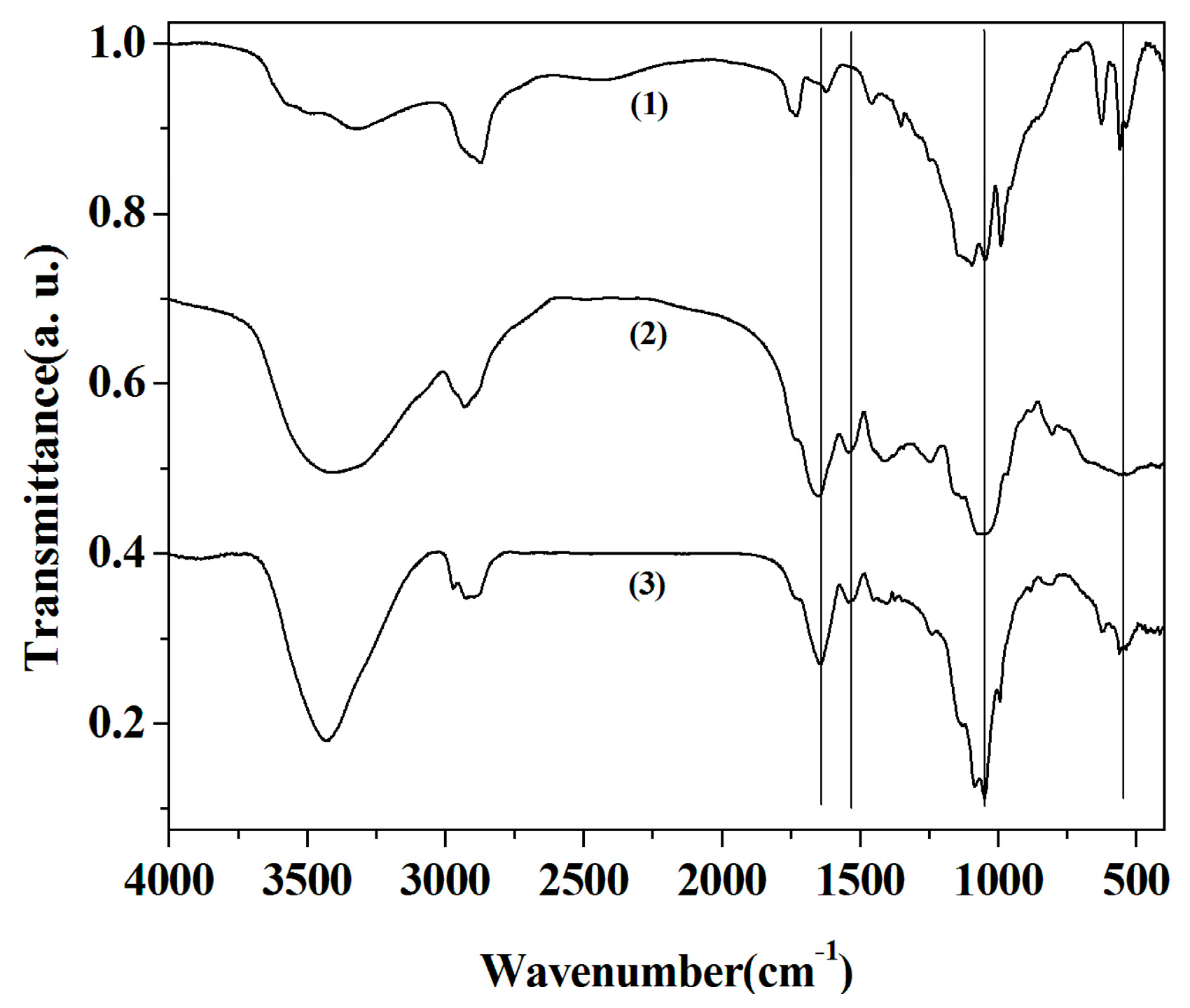
2.3. FTIR
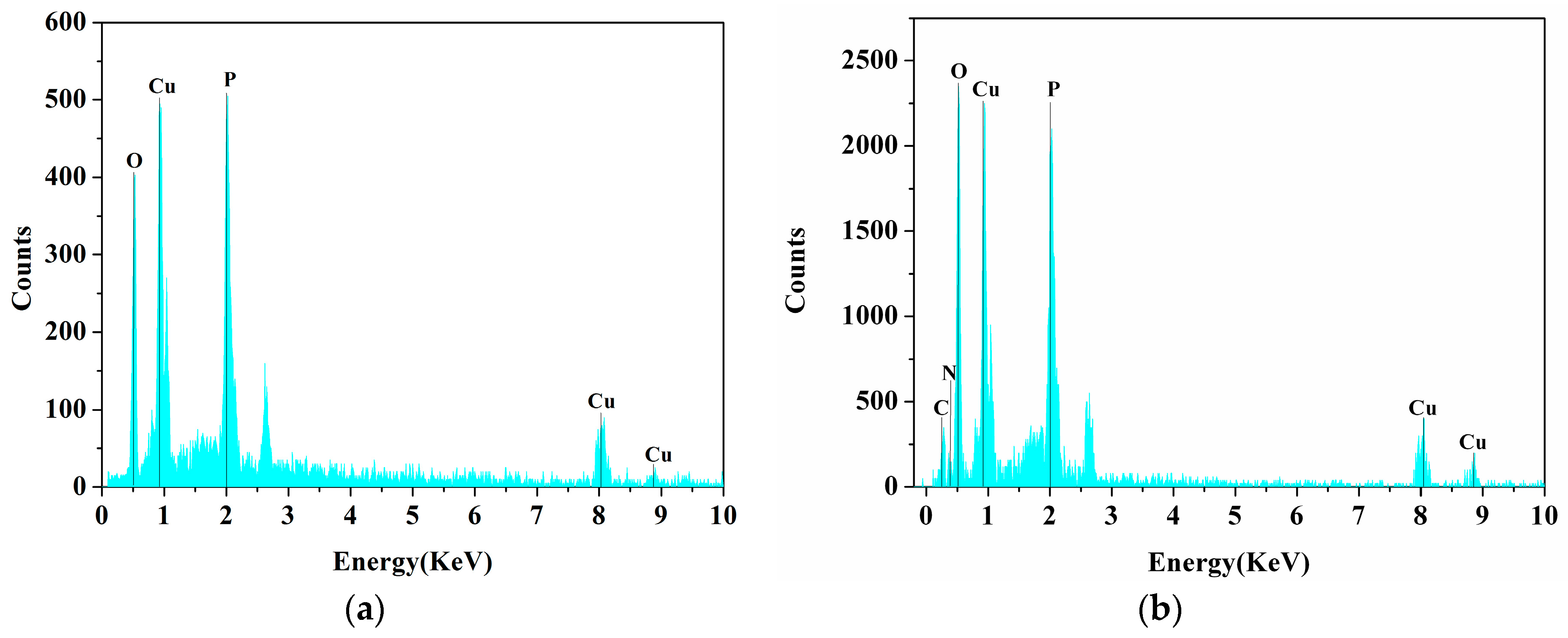
2.4. EDS Spectrum
2.5. Optimizing the Reaction Conditions for Synthesis of Viniferin
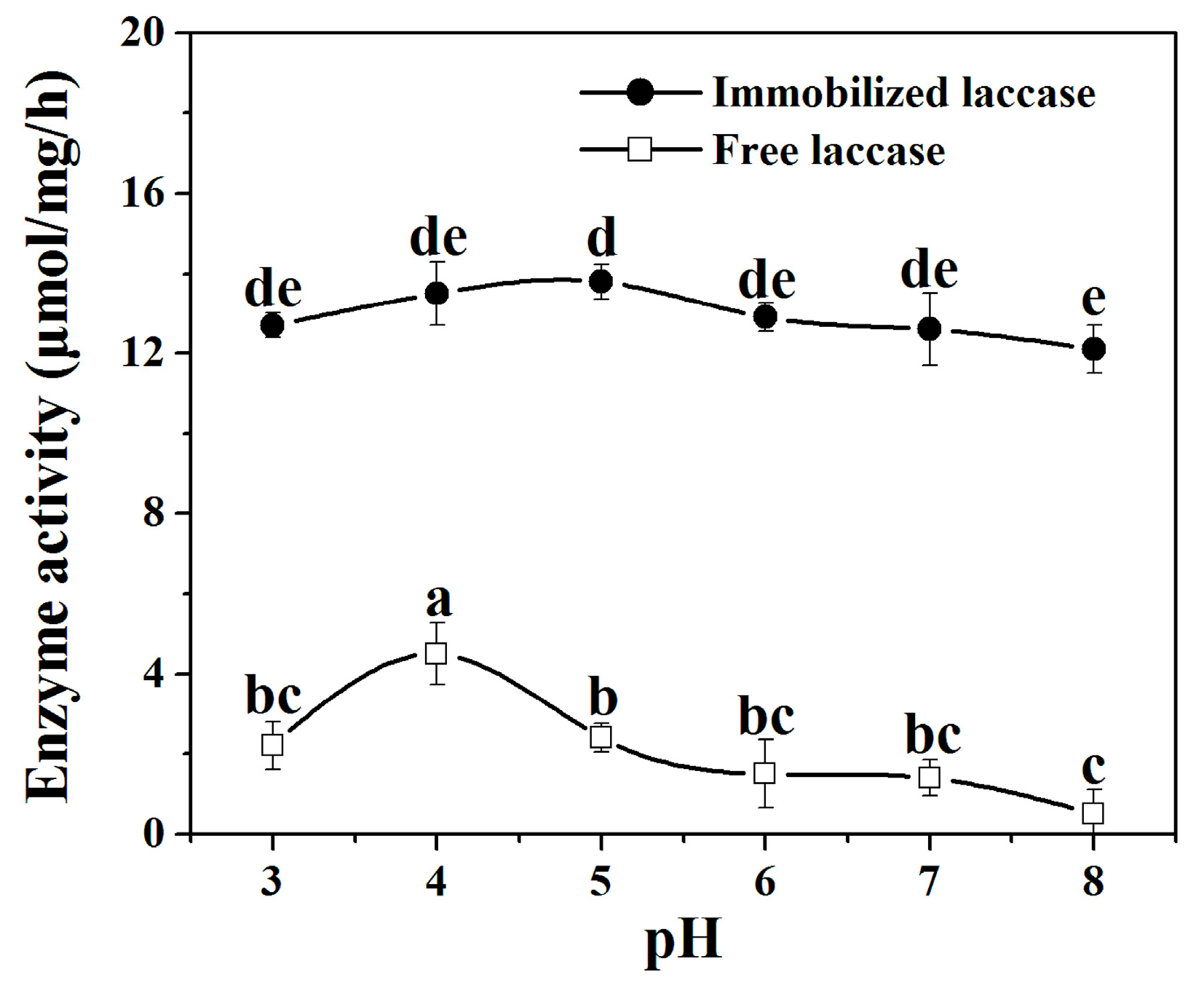
2.5.1. pH
2.5.2. Temperature
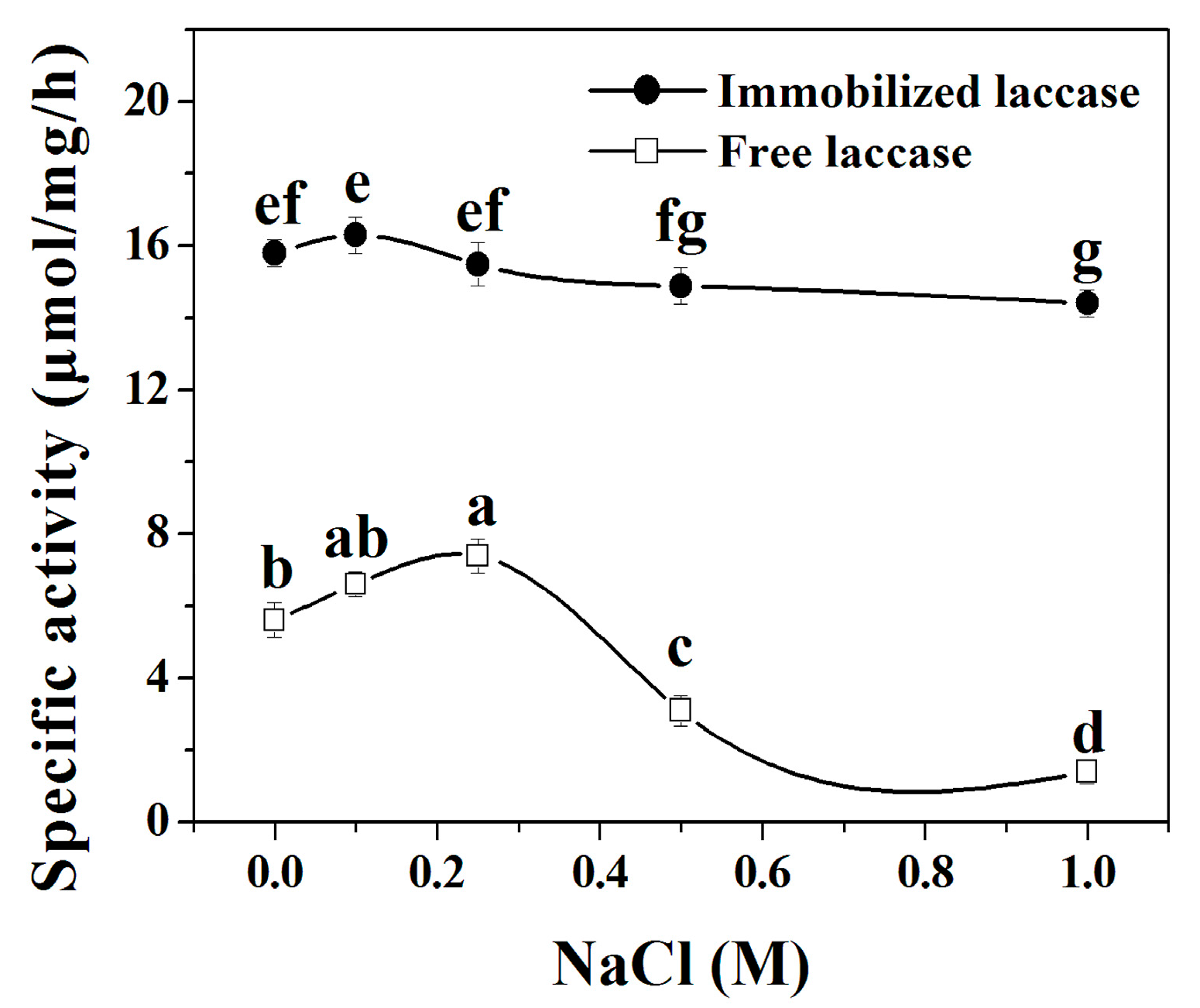
2.5.3. Ionic Strength
2.6. Comparison of Specific Activity
2.7. Reusability
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Laccase-Incorporated Nanoflower
3.3. Characterization of Laccase-Incorporated Nanoflower
3.4. Screening the Reaction Conditions
3.5. Synthesis of Viniferin under Optimum Conditions
3.6. Reusability
3.7. HPLC Analytical Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zghonda, N.; Yoshida, S.; Araki, M.; Kusunoki, M.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Greater effectiveness of ε-viniferin in red wine than its monomer resveratrol for inhibiting vascular smooth muscle cell proliferation and migration. Biosci. Biotechnol. Biochem. 2011, 75, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Zghonda, N.; Yoshida, S.; Ezaki, S.; Otake, Y.; Murakami, C.; Mliki, A.; Ghorbel, A.; Miyazaki, H. Ε-viniferin is more effective than its monomer resveratrol in improving the functions of vascular endothelial cells and the heart. Biosci. Biotechnol. Biochem. 2012, 76, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Billard, C.; Izard, J.-C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.-P. Comparative antiproliferative and apoptotic effects of resveratrol, ϵ-viniferin and vine-shots derived polyphenols (Vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Quiney, C.; Dauzonne, D.; Kern, C.; Fourneron, J.-D.; Izard, J.-C.; Mohammad, R.M.; Kolb, J.-P.; Billard, C. Flavones and polyphenols inhibit the NO pathway during apoptosis of leukemia B-cells. Leuk. Res. 2004, 28, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Namao, K.; Kamijou, A.; Matsuoka, S.; Nakano, M.; Terao, K.; Ohizumi, Y. Powerful hepatoprotective and hepatotoxic plant oligostilbenes, isolated from the oriental medicinal plant Vitis coignetiae (Vitaceae). Experientia 1995, 51, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Piver, B.; Berthou, F.; Dreano, Y.; Lucas, D. Differential inhibition of human cytochrome P450 enzymes by ε-viniferin, the dimer of resveratrol: Comparison with resveratrol and polyphenols from alcoholized beverages. Life Sci. 2003, 73, 1199–1213. [Google Scholar] [CrossRef]
- Takaya, Y.; Terashima, K.; Ito, J.; He, Y.-H.; Tateoka, M.; Yamaguchi, N.; Niwa, M. Biomimic transformation of resveratrol. Tetrahedron 2005, 61, 10285–10290. [Google Scholar] [CrossRef]
- Yao, C.S.; Lin, M.; Wang, Y.H. Synthesis of the active stilbenoids by photooxidation reaction of trans-ϵ-viniferin. Chin. J. Chem. 2004, 22, 1350–1355. [Google Scholar] [CrossRef]
- Pezet, R. Purification and characterization of a 32-kDa laccase-like stilbene oxidase produced by Botrytis cinerea pers.: Fr. FEMS Microbiol. Lett. 1998, 167, 203–208. [Google Scholar] [CrossRef]
- Nicotra, S.; Cramarossa, M.R.; Mucci, A.; Pagnoni, U.M.; Riva, S.; Forti, L. Biotransformation of resveratrol: Synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens. Tetrahedron 2004, 60, 595–600. [Google Scholar] [CrossRef]
- Zhang, H.; Xun, E.; Wang, J.; Chen, G.; Cheng, T.; Wang, Z.; Ji, T.; Wang, L. Immobilization of laccase for oxidative coupling of trans-resveratrol and its derivatives. Int. J. Mol. Sci. 2012, 13, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Klapiszewski, L.; Jedrzak, A.; Nowicki, M.; Moszynski, D.; Jesionowski, T. Lipase B from Candida antarctica immobilized on a silica-lignin matrix as a stable and reusable biocatalytic system. Catalysts 2016, 7, 14. [Google Scholar] [CrossRef]
- Rodriguez-Colinas, B.; Fernandez-Arrojo, L.; Santos-Moriano, P.; Ballesteros, A.O.; Plou, F.J. Continuous packed bed reactor with immobilized β-galactosidase for production of galactooligosaccharides (GOS). Catalysts 2016, 6, 189. [Google Scholar] [CrossRef]
- Chen, P.-C.; Qian, Y.-C.; Fang, F.; Zhu, X.-Y.; Huang, X.-J. Adsorption and activity of lipase on polyphosphazene-modified polypropylene membrane surface. Catalysts 2016, 6, 174. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M. Chitosan–collagen coated magnetic nanoparticles for lipase immobilization—New type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef]
- Sasai, Y.; Kanno, H.; Doi, N.; Yamauchi, Y.; Kuzuya, M.; Kondo, S.-I. Synthesis and characterization of highly stabilized polymer–trypsin conjugates with autolysis resistance. Catalysts 2016, 7, 4. [Google Scholar] [CrossRef]
- Belhacene, K.; Elagli, A.; Vivien, C.; Treizebré, A.; Dhulster, P.; Supiot, P.; Froidevaux, R. Investigation of the effect of plasma polymerized siloxane coating for enzyme immobilization and microfluidic device conception. Catalysts 2016, 6, 209. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.; Liu, D.; Feng, W. Enhancing the enzymatic activity of a heme-dependent peroxidase through genetic modification. Catalysts 2016, 6, 166. [Google Scholar] [CrossRef]
- Lei, C.; Soares, T.A.; Shin, Y.; Liu, J.; Ackerman, E.J. Enzyme specific activity in functionalized nanoporous supports. Nanotechnology 2008, 19, 125102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-X.; Dill, K.A. Stabilization of proteins in confined spaces. Biochemistry 2001, 40, 11289–11293. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19, 279–286. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, M.; Lu, M.; Li, Z. Encapsulation of β-galactosidase from Aspergillus oryzae based on “fish-in-net” approach with molecular imprinting technique. J. Mol. Catal. B Enzym. 2010, 63, 75–80. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein-inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Otari, S.V.; Kang, Y.C.; Lee, J.-K. Protein–inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017, 7, 3488–3494. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Li, F.; Yue, H.; He, C.; Xie, F.; Wang, Z. Enantioselective transesterification of (R,S)-2-pentanol catalyzed by a new flower-like nanobioreactor. RSC Adv. 2014, 4, 33998–34002. [Google Scholar] [CrossRef]
- Cho, I.S.; Kim, D.W.; Lee, S.; Kwak, C.H.; Bae, S.T.; Noh, J.H.; Yoon, S.H.; Jung, H.S.; Kim, D.W.; Hong, K.S. Synthesis of Cu2PO4OH hierarchical superstructures with photocatalytic activity in visible light. Adv. Funct. Mater. 2008, 18, 2154–2162. [Google Scholar] [CrossRef]
- Yang, W.-J.; Griffiths, P.R.; Byler, D.M.; Susi, H. Protein conformation by infrared spectroscopy: Resolution enhancement by fourier self-deconvolution. Appl. Spectrosc. 1985, 39, 282–287. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [PubMed]
- Laidler, K.J.; Bunting, P.S. The Chemical Kinetics of Enzyme Action, 2nd ed.; Clarendon Press Oxford: Oxford, UK, 1973; p. 63. [Google Scholar]
- Murphy, K.P. Stabilization of protein structure. In Protein Structure, Stability, and Folding; Springer: New York, NY, USA, 2001; pp. 1–16. [Google Scholar]
- Xu, L.; Ke, C.; Huang, Y.; Yan, Y. Immobilized Aspergillus niger lipase with SiO2 nanoparticles in sol-gel materials. Catalysts 2016, 6, 149. [Google Scholar] [CrossRef]
- Rivero, C.W.; Palomo, J.M. Covalent immobilization of Candida rugosa lipase at alkaline pH and their application in the regioselective deprotection of per-O-acetylated thymidine. Catalysts 2016, 6, 115. [Google Scholar] [CrossRef]
- Murugesan, K.; Kim, Y.-M.; Jeon, J.-R.; Chang, Y.-S. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J. Hazard. Mater. 2009, 168, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Peisach, J.; Levine, W.G. A comparison of the enzymic activities of pig ceruloplasmin and Rhus vernicifera laccase. J. Biol. Chem. 1965, 240, 2284–2289. [Google Scholar] [PubMed]
- Yabuki, S. How to lengthen the long-term stability of enzyme membranes: Trends and strategies. Catalysts 2017, 7, 36. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Rodrigues de Melo, R.; Palomo, J.M.; Maltempi de Souza, E.; Krieger, N.; Mateo, C. New tailor-made alkyl-aldehyde bifunctional supports for lipase immobilization. Catalysts 2016, 6, 191. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Enzyme | Specific Activity (µmol/mg/h) |
|---|---|
| Free laccase 1 | 7.4 ± 0.5 |
| Free laccase in the presence of copper phosphate crystals 2 | 7.4 ± 0.3 |
| Free laccase in the presence of copper sulfate 3 | 8.9 ± 0.4 |
| Laccase in the nanoflowers 4 | 16.3 ± 0.5 |
| Copper phosphate crystals without laccase 5 | 0.0 |
| Recycles 1 | Relative Activity (%) |
|---|---|
| 1 | 100.0 ± 0.5a |
| 2 | 99.5 ± 0.7ab |
| 3 | 98.1 ± 0.2bc |
| 4 | 97.7 ± 0.4c |
| 5 | 96.9 ± 0.9cd |
| 6 | 96.1 ± 0.6de |
| 7 | 95.6 ± 0.3de |
| 8 | 94.8 ± 0.3ef |
| 9 | 93.9 ± 0.5fg |
| 10 | 93.2 ± 0.6g |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Li, H.; Zhu, X.; Li, S.; Wang, Z.; Wang, L.; Li, Z.; Chen, G. Using Laccases in the Nanoflower to Synthesize Viniferin. Catalysts 2017, 7, 188. https://doi.org/10.3390/catal7060188
Wu Z, Li H, Zhu X, Li S, Wang Z, Wang L, Li Z, Chen G. Using Laccases in the Nanoflower to Synthesize Viniferin. Catalysts. 2017; 7(6):188. https://doi.org/10.3390/catal7060188
Chicago/Turabian StyleWu, Zhuofu, Heng Li, XueJun Zhu, Shuai Li, Zhi Wang, Lei Wang, Zhengqiang Li, and Guang Chen. 2017. "Using Laccases in the Nanoflower to Synthesize Viniferin" Catalysts 7, no. 6: 188. https://doi.org/10.3390/catal7060188







