Selective Hydrogenolysis of Glycerol and Crude Glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 Promoted Cu/Al2O3 Catalysts
Abstract
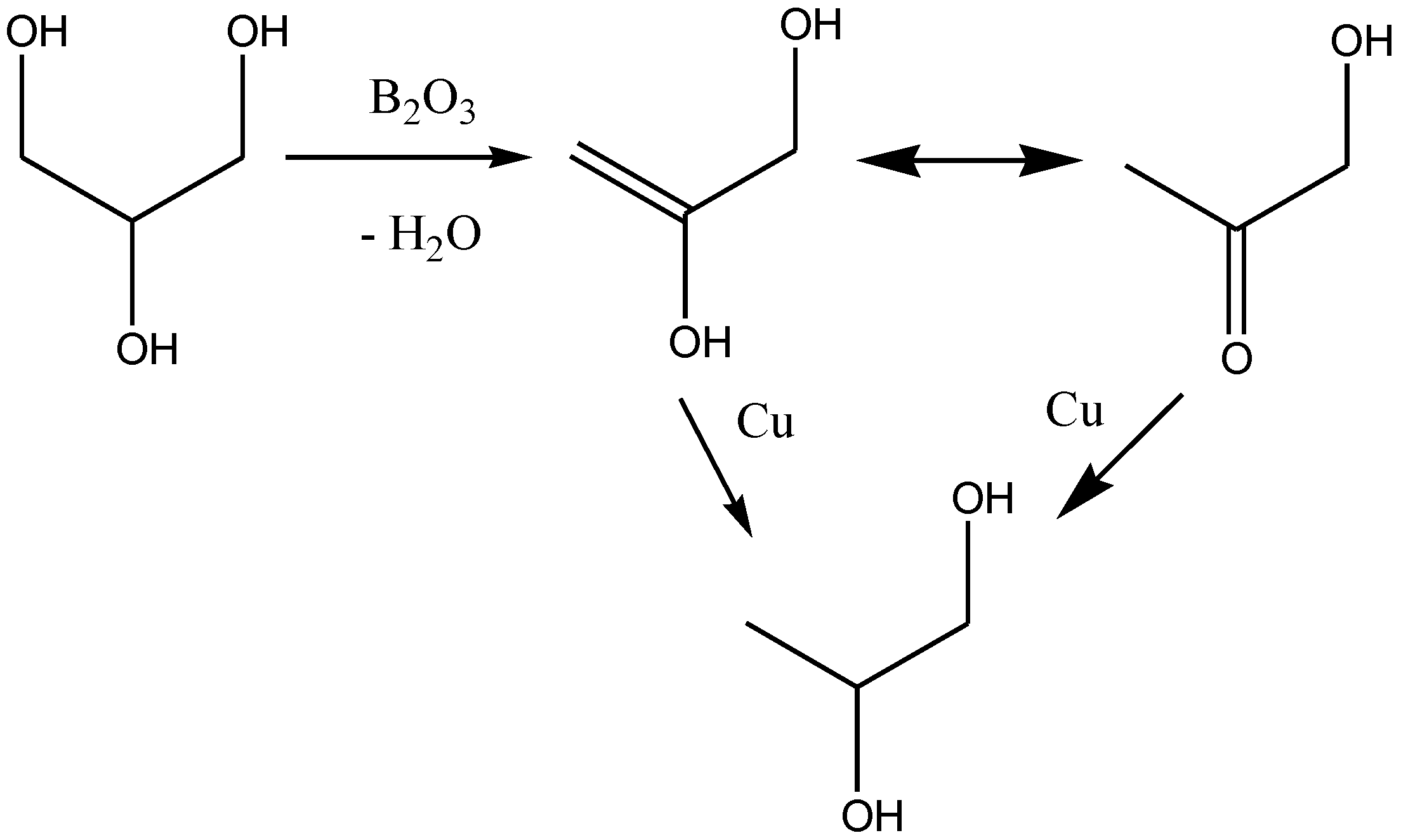
:1. Introduction
2. Results and Discussion
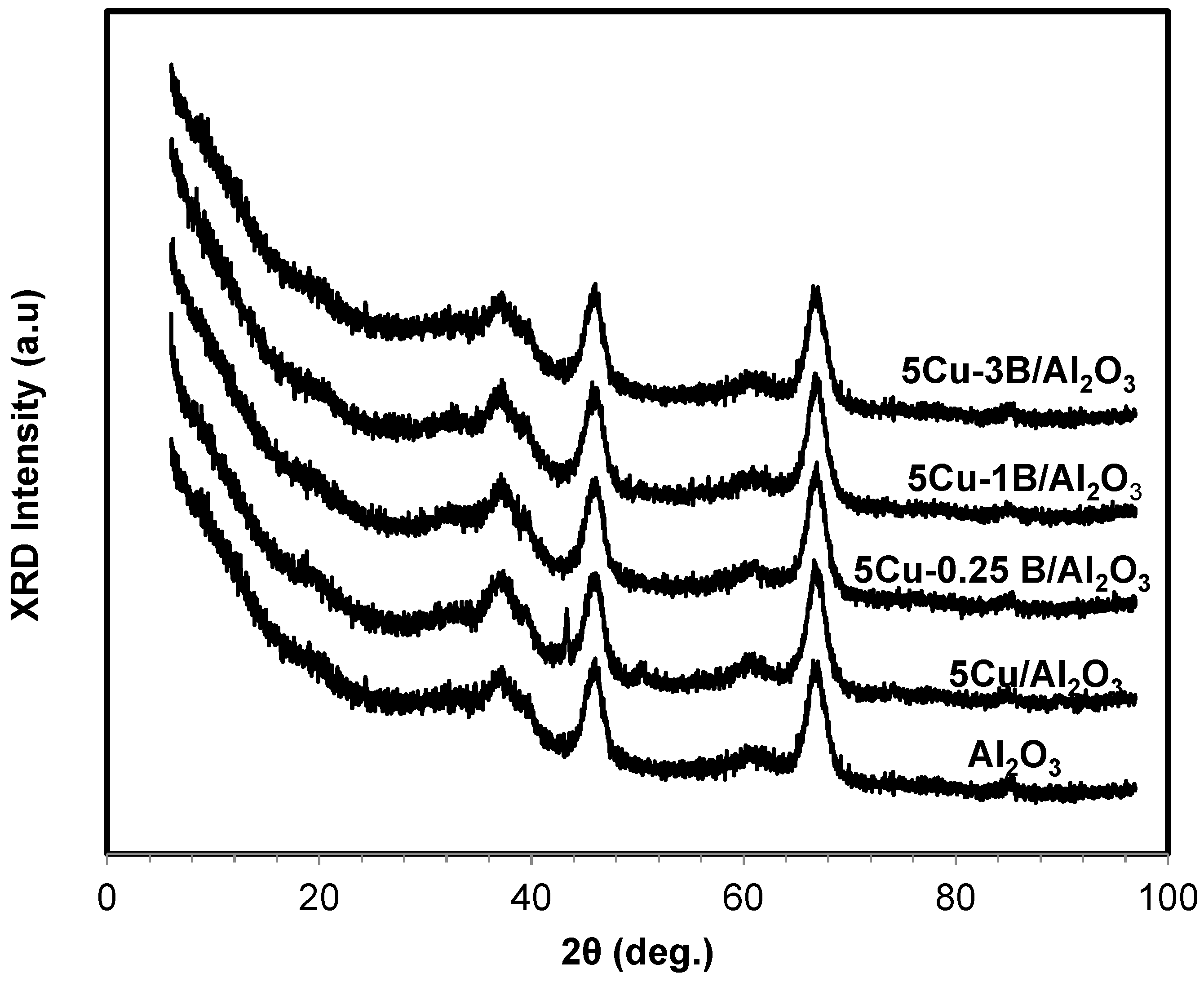
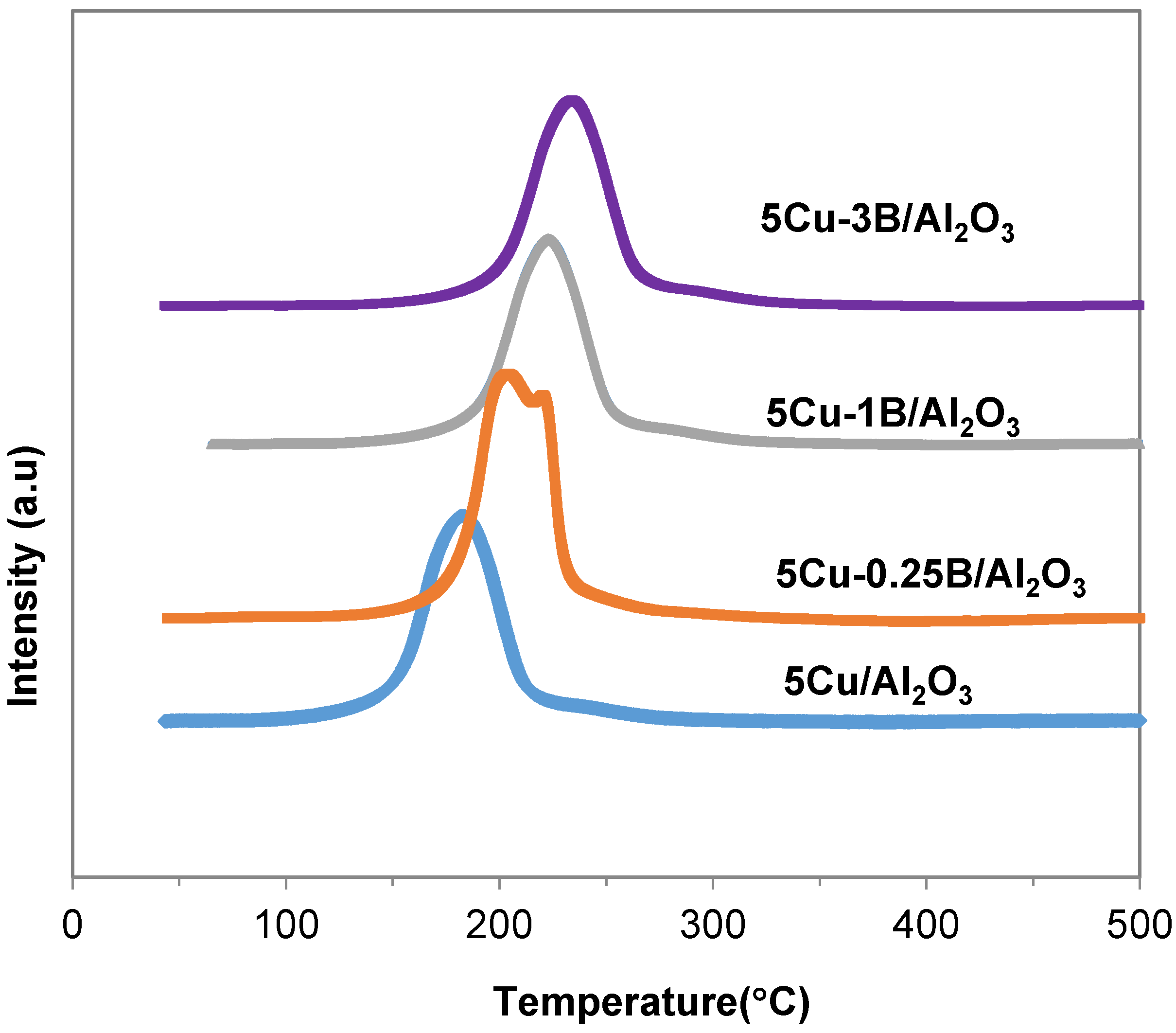
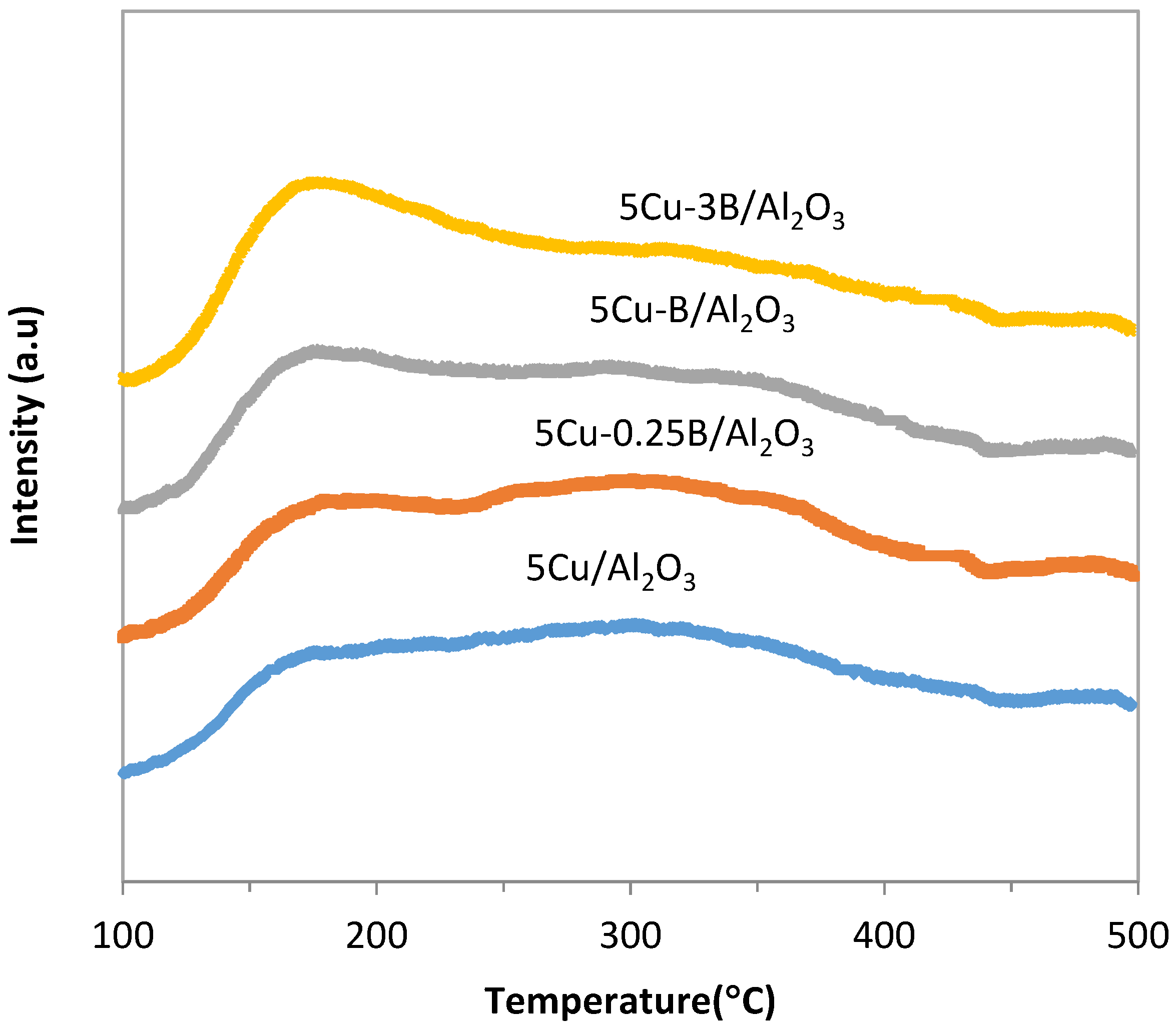
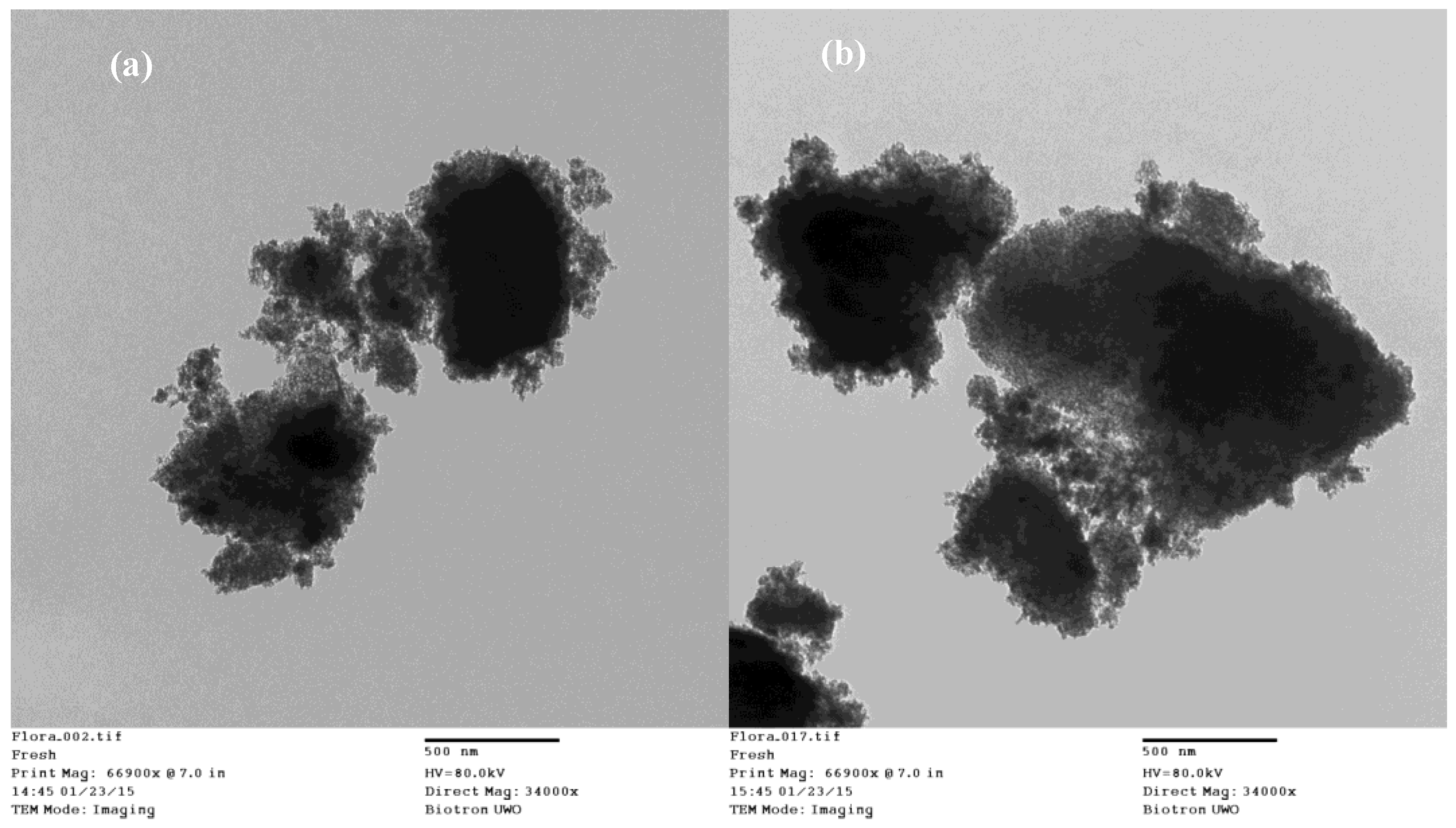
2.1. Catalyst Characterization
2.2. Influence of Process Parameters
2.2.1. Influence of Cu Loading
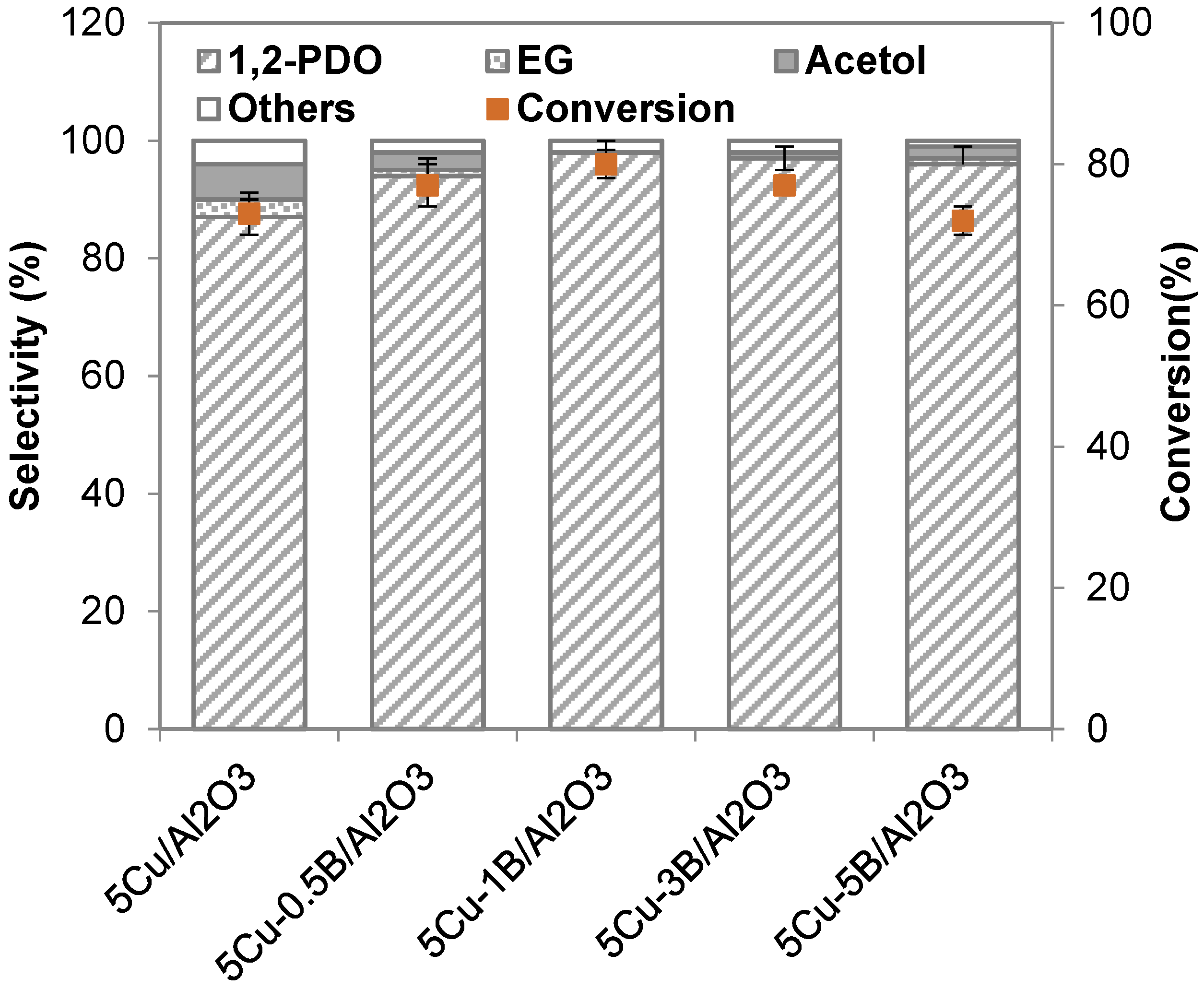
2.2.2. Influence of B Incorporation
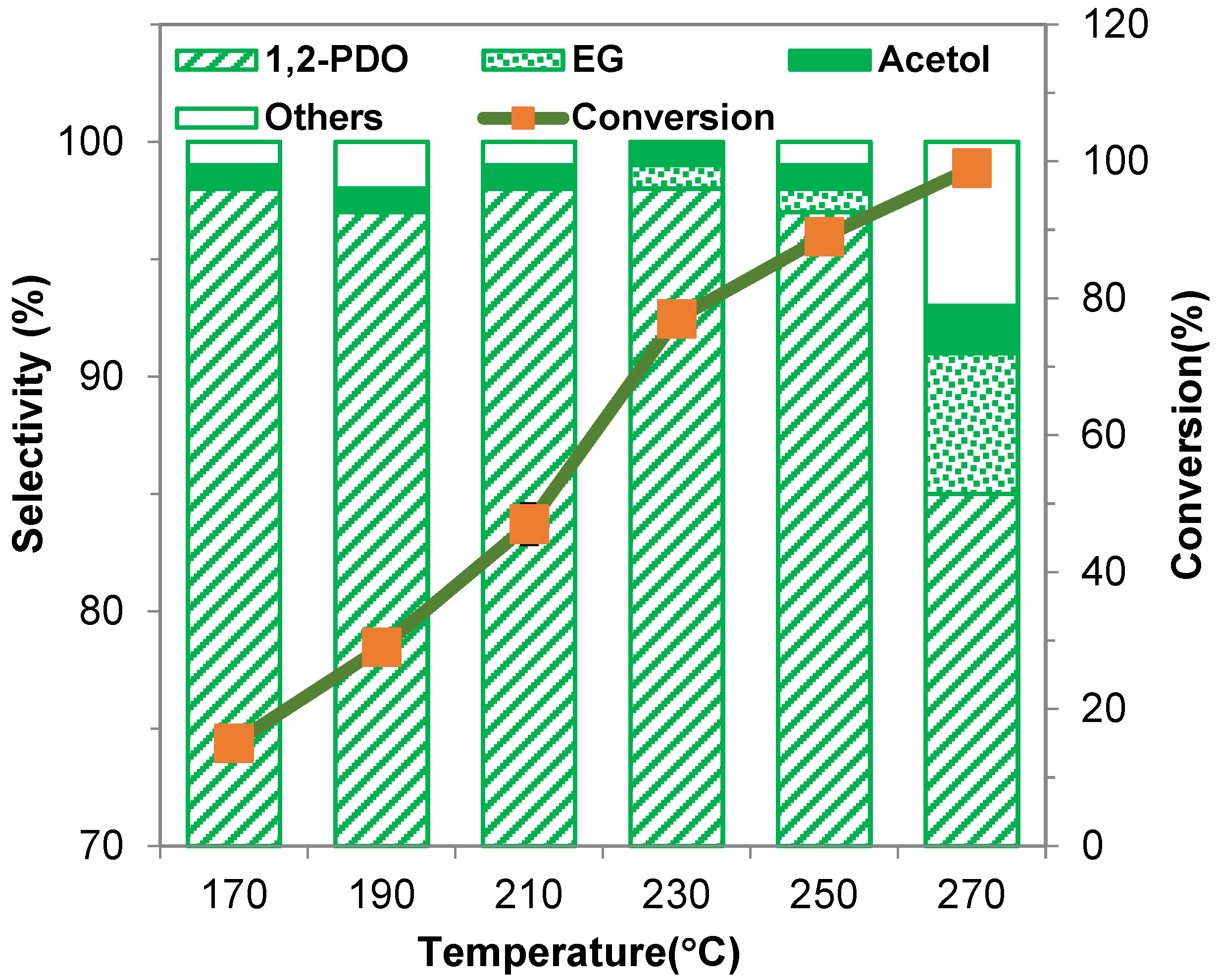
2.2.3. Influence of Temperature
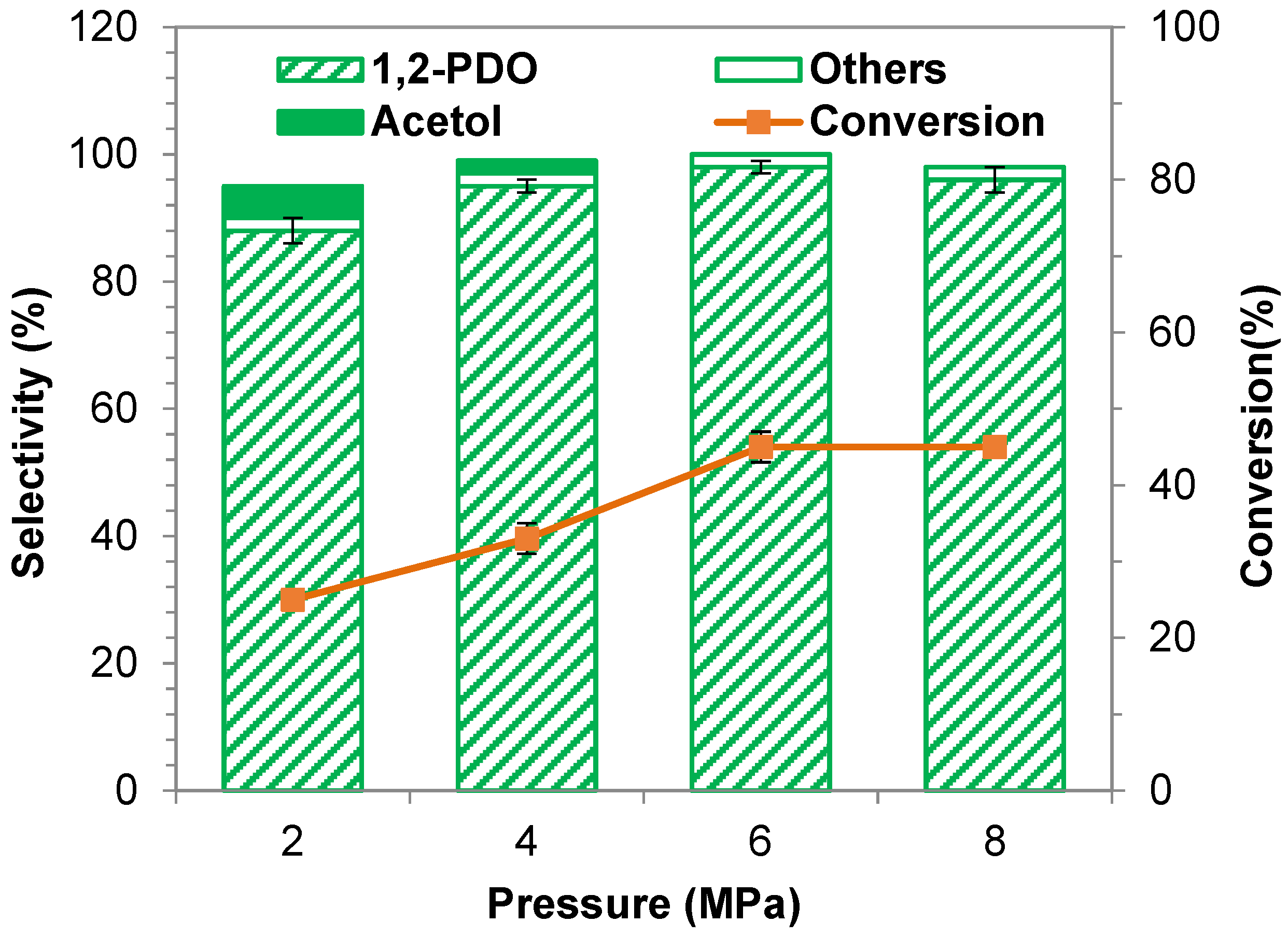
2.2.4. Influence of Hydrogen Pressure
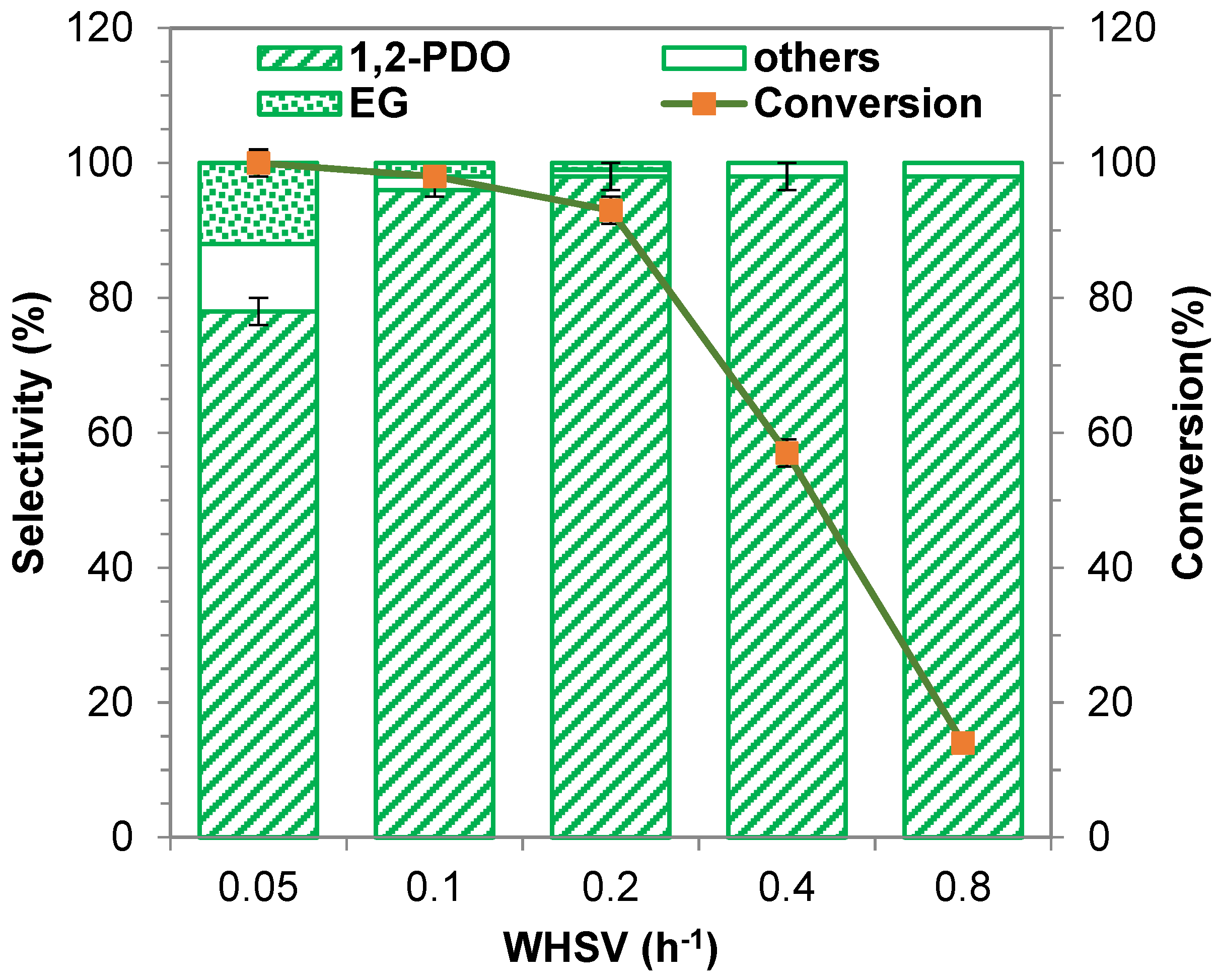
2.2.5. Influence of Weight Hourly Space Velocity (WHSV)
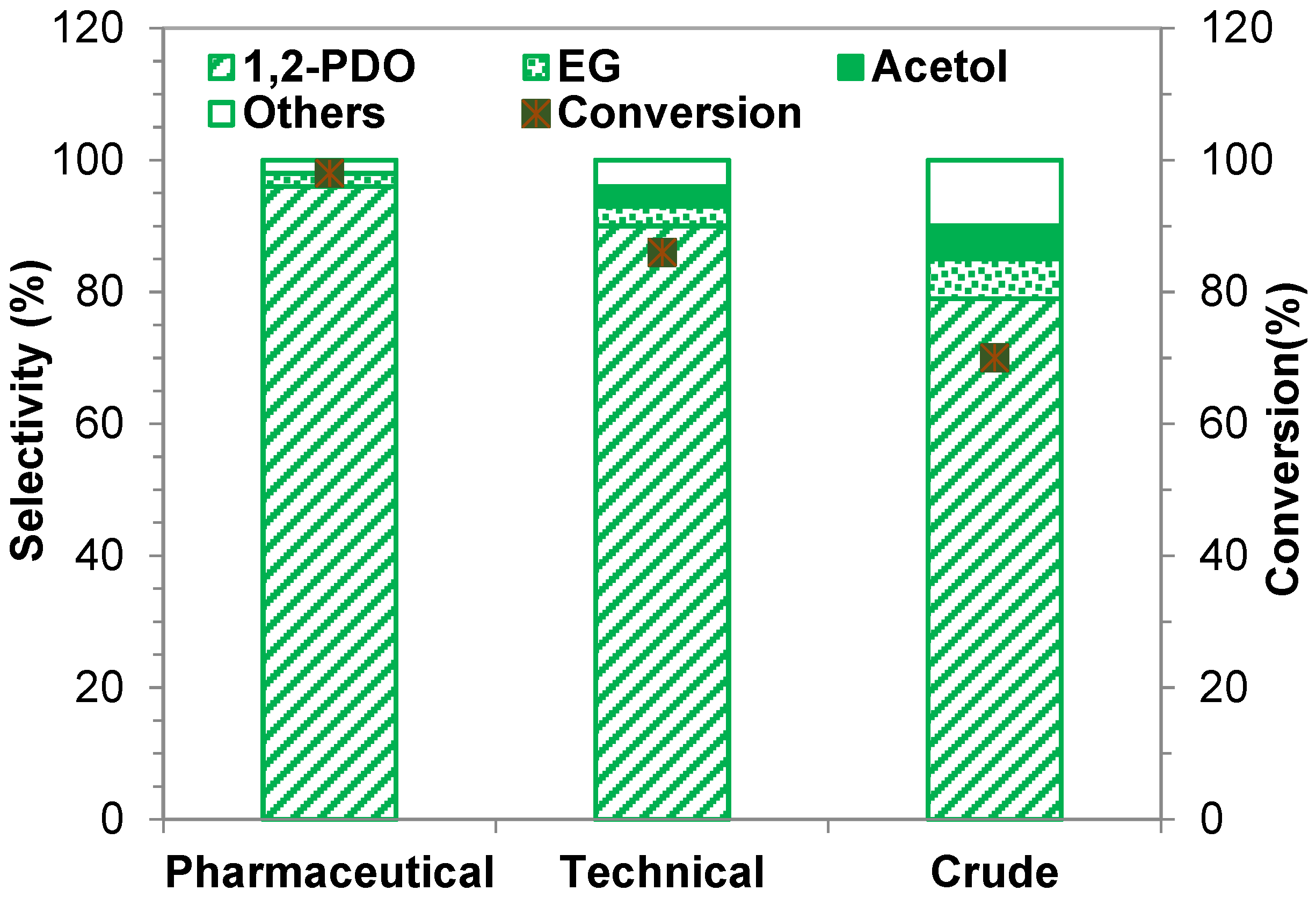
2.2.6. Influence of Glycerol Feedstock Purity
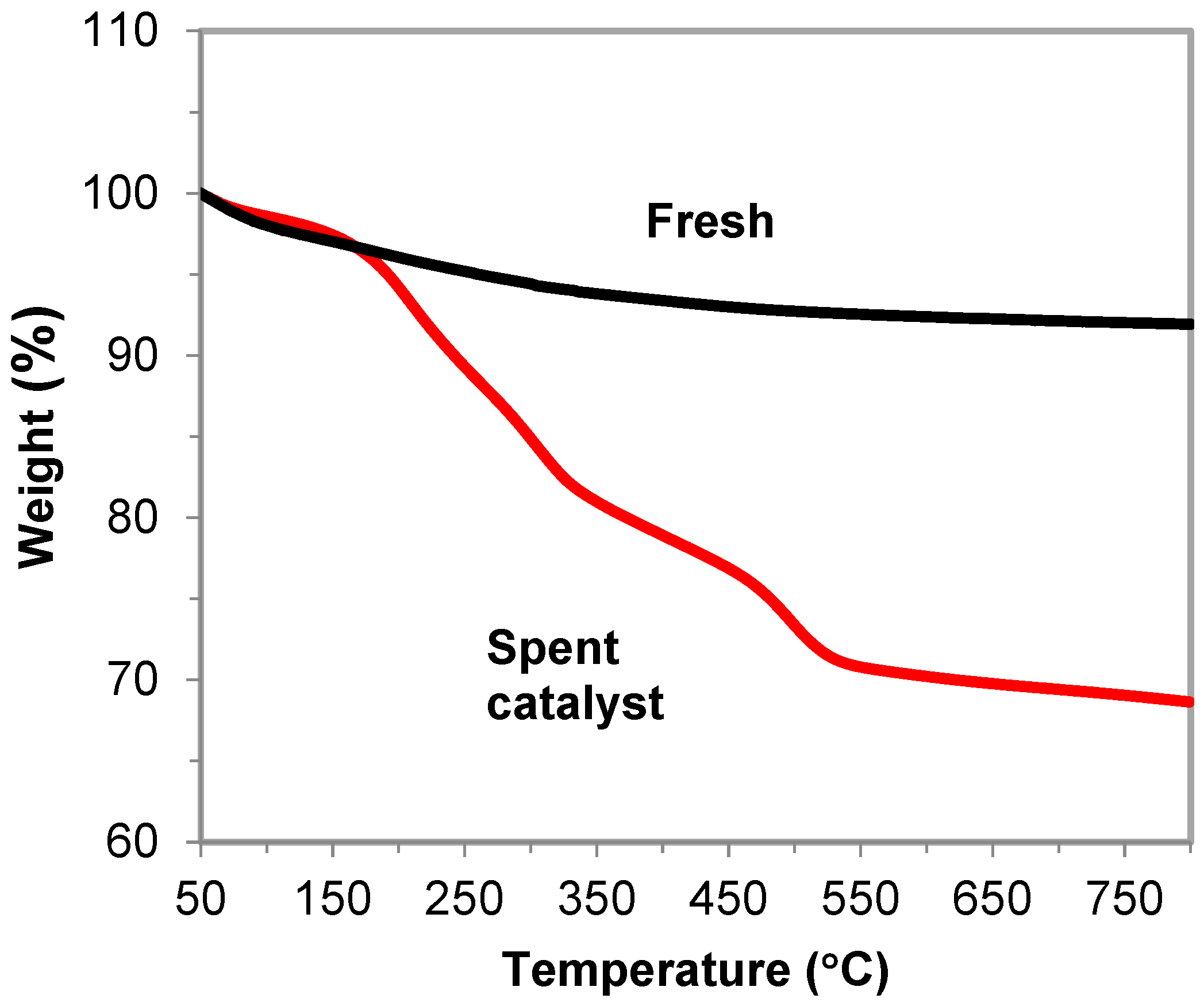
2.3. Long-Term Stability and Catalyst Deactivation
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Tests
3.5. Product Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maris, E.; Ketchie, W.; Murayama, M.; Davis, R. Glycerol hydrogenolysis on carbon-supported PtRu and AuRu bimetallic catalysts. J. Catal. 2007, 251, 281–294. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, L.; Xie, W.; Chen, P.; Hou, Z.; Zheng, X. Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts. Bioresour. Technol. 2010, 101, 7099–7103. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C. A new continuous-flow process for catalytic conversion of glycerol to oxygenated fuel additive: Catalyst screening. Appl. Energy 2014, 123, 75–81. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Ma, L.; He, D.; Li, Z. Promoting effect of rhenium on catalytic performance of Ru catalysts in hydrogenolysis of glycerol to propanediol. Catal. Commun. 2008, 9, 2489–2495. [Google Scholar] [CrossRef]
- Feng, J.; Fu, H.; Wang, J.; Li, R.; Chen, H.; Li, X. Hydrogenolysis of glycerol to glycols over ruthenium catalysts: Effect of support and catalyst reduction temperature. Catal. Commun. 2008, 9, 1458–1464. [Google Scholar] [CrossRef]
- Sharma, R.V.; Kumar, P.; Dalai, A.K. Selective hydrogenolysis of glycerol to propylene glycol by using Cu:Zn:Cr:Zr mixed metal oxides catalyst. Appl. Catal. A Gen. 2014, 477, 147–156. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Wang, J.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B Environ. 2011, 101, 431–440. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol on bimetallic Pd-Cu/solid-base catalysts prepared via layered double hydroxides precursors. Appl. Catal. A Gen. 2011, 403, 173–182. [Google Scholar] [CrossRef]
- Atia, H.; Armbruster, U.; Martin, A. Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 2008, 258, 71–82. [Google Scholar] [CrossRef]
- Auttanat, T.; Jongpatiwut, S.; Rirksomboon, T. Dehydroxylation of glycerol to propylene glycol over Cu-ZnO/Al2O3 Catalyst: Effect of feed purity. World Acad. Sci. Technol. 2012, 6, 400–403. [Google Scholar]
- Barbelli, M.L.; Santori, G.F.; Nichio, N.N. Aqueous phase hydrogenolysis of glycerol to bio-propylene glycol over Pt-Sn catalysts. Bioresour. Technol. 2012, 111, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Dasari, M.; Kiatsimkul, P.-P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Deng, C.; Duan, X.; Zhou, J.; Chen, D.; Zhou, X.; Yuan, W. Size effects of Pt-Re bimetallic catalysts for glycerol hydrogenolysis. Catal. Today 2014, 234, 208–214. [Google Scholar] [CrossRef]
- D’Hondt, E.; Van de Vyver, S.; Sels, B.F.; Jacobs, P. Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen. Chem. Commun. 2008, 6011–6012. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Park, J.R.; Park, D.S.; Kwak, B.K.; Yi, J. Promoter effect of Pd in CuCr2O4 catalysts on the hydrogenolysis of glycerol to 1,2-propanediol. Green Chem. 2012, 14, 2638–2646. [Google Scholar] [CrossRef]
- Balaraju, M.; Rekha, V.; Prasad, P.S.S.; Devi, B.L.A.P.; Prasad, R.B.N.; Lingaiah, N. Influence of solid acids as co-catalysts on glycerol hydrogenolysis to propylene glycol over Ru/C catalysts. Appl. Catal. A Gen. 2009, 354, 82–87. [Google Scholar] [CrossRef]
- Zhou, C.-H.C.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, E.S.; Eggenhuisen, T.M.; Munnik, P.; de Jongh, P.E.; de Jong, K.P.; Lemonidou, A.A. Synthesis and performance of highly dispersed Cu/SiO2 catalysts for the hydrogenolysis of glycerol. Appl. Catal. B Environ. 2014, 145, 108–119. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; El Doukkali, M.; Güemez, M.B. Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source. J. Catal. 2011, 282, 237–247. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, X.; Xiu, J.; Wang, Y.; Williams, C.T.; Liang, C. Synergetic effect between Cu0 and Cu+ in the Cu-Cr catalysts for hydrogenolysis of glycerol. Catal. Today 2014, 234, 200–207. [Google Scholar] [CrossRef]
- Panyad, S.; Jongpatiwut, S.; Sreethawong, T.; Rirksomboon, T.; Osuwan, S. Catalytic dehydroxylation of glycerol to propylene glycol over Cu–ZnO/Al2O3 catalysts: Effects of catalyst preparation and deactivation. Catal. Today 2011, 174, 59–64. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Miyazawa, T.; Kusunoki, Y.; Kunimori, K.; Tomishige, K. Glycerol conversion in the aqueous solution under hydrogen over Ru/C + an ion-exchange resin and its reaction mechanism. J. Catal. 2006, 240, 213–221. [Google Scholar] [CrossRef]
- Schmidt, S.R.; Tanielyan, S.K.; Marin, N.; Alvez, G.; Augustine, R.L. Selective conversion of glycerol to propylene glycol over fixed bed Raney® Cu catalysts. Top. Catal. 2010, 53, 1214–1216. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Mizugaki, T.; Arundhathi, R.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Selective hydrogenolysis of glycerol to 1,3-propanediol catalyzed by Pt nanoparticles–AlOx/WO3. Chem. Lett. 2013, 42, 729–731. [Google Scholar] [CrossRef]
- Furikado, I.; Miyazawa, T.; Koso, S.; Shimao, A.; Kunimori, K.; Tomishige, K. Catalytic performance of Rh/SiO2 in glycerol reaction under hydrogen. Green Chem. 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, H.; Cui, F.; Zuo, J.; Chen, J.; Xia, C. Effects of the precipitation agents and rare earth additives on the structure and catalytic performance in glycerol hydrogenolysis of Cu/SiO2 catalysts prepared by precipitation-gel method. Catal. Today 2014, 234, 223–232. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Xue, J.; Zuo, J.; Chen, J.; Xia, C. Cu/SiO2 catalysts prepared by hom- and heterogeneous deposition–precipitation methods: Texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Today 2012, 183, 42–51. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H. Selective hydrogenolysis of glycerol to propylene glycol on Cu-ZnO catalysts. Catal. Lett. 2007, 117, 62–67. [Google Scholar] [CrossRef]
- Bienholz, A.; Schwab, F.; Claus, P. Hydrogenolysis of glycerol over a highly active CuO/ZnO catalyst prepared by an oxalate gel method: Influence of solvent and reaction temperature on catalyst deactivation. Green Chem. 2010, 12, 290–295. [Google Scholar] [CrossRef]
- Hao, S.-L.; Peng, W.-C.; Zhao, N.; Xiao, F.-K.; Wei, W.; Sun, Y.-H. Hydrogenolysis of glycerol to 1,2-propanediol catalyzed by Cu-H4SiW12O40/Al2O3 in liquid phase. J. Chem. Technol. Biotechnol. 2010, 85, 1499–1503. [Google Scholar] [CrossRef]
- Vila, F.; López Granados, M.; Ojeda, M.; Fierro, J.L.G.; Mariscal, R. Glycerol hydrogenolysis to 1,2-propanediol with Cu/γ-Al2O3: Effect of the activation process. Catal. Today 2012, 187, 122–128. [Google Scholar] [CrossRef]
- Niu, L.; Wei, R.; Yang, H.; Li, X.; Jiang, F.; Xiao, G. Hydrogenolysis of glycerol to propanediols over Cu-MgO/USY catalyst. Chin. J. Catal. 2013, 34, 2230–2235. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Catalytic production of 1,2-propanediol from glycerol in bio-ethanol solvent. Bioresour. Technol. 2012, 104, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xia, Z.; Li, J.; Lai, W.; Yi, X.; Chen, B.; Fang, W.; Wan, H. Promoting effect of boron with high loading on Ni-based catalyst for hydrogenation of thiophene-containing ethylbenzene. Catal. Commun. 2012, 21, 18–21. [Google Scholar] [CrossRef]
- Ma, L.; He, D. Influence of catalyst pretreatment on catalytic properties and performances of Ru–Re/SiO2 in glycerol hydrogenolysis to propanediols. Catal. Today 2010, 149, 148–156. [Google Scholar] [CrossRef]
- Perosa, A.; Tundo, P. Selective Hydrogenolysis of Glycerol with Raney Nickel. Ind. Eng. Chem. Res. 2005, 44, 8535–8537. [Google Scholar] [CrossRef]
- Gandarias, I.; Fernández, S.G.; El Doukkali, M.; Requies, J.; Arias, P.L. Physicochemical Study of Glycerol Hydrogenolysis Over a Ni–Cu/Al2O3 Catalyst Using Formic Acid as the Hydrogen Source. Top. Catal. 2013, 56, 995–1007. [Google Scholar] [CrossRef]
- Lewandowski, M.; Sarbak, Z. The effect of boron addition on hydrodesulfurization and hydrodenitrogenation activity of NiMo/Al2O3 catalysts. Fuel 2000, 79, 487–495. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, L.; Guo, X.; Mao, J.; Zhang, S. Selective hydrogenolysis of glycerol to propanediols on supported Cu-containing bimetallic catalysts. Green Chem. 2010, 12, 1835–1843. [Google Scholar] [CrossRef]
- Guerreiro, E.D.; Gorriz, O.F.; Rivarola, J.B.; Arrúa, L.A. Characterization of Cu/SiO2 catalysts prepared by ion exchange for methanol dehydrogenation. Appl. Catal. A Gen. 1997, 165, 259–271. [Google Scholar] [CrossRef]
- Tan, K.F.; Chang, J.; Borgna, A.; Saeys, M. Effect of boron promotion on the stability of cobalt Fischer–Tropsch catalysts. J. Catal. 2011, 280, 50–59. [Google Scholar]
- Yin, A.; Qu, J.; Guo, X.; Dai, W.-L.; Fan, K. The influence of B-doping on the catalytic performance of Cu/HMS catalyst for the hydrogenation of dimethyloxalate. Appl. Catal. A Gen. 2011, 400, 39–47. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, H.; Zhao, Y.; Wang, B.; Geng, Y.; Lv, J.; Wang, S.; Gong, J.; Ma, X. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant. J. Catal. 2013, 297, 142–150. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Gandarias, I.; Arias, P.L. Glycerol hydrogenolysis into propanediols using in situ generated hydrogen—A critical review. Eur. J. Lipid Sci. Technol. 2013, 115, 9–27. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A. Glycerol transformation to value added C3 diols: Reaction mechanism, kinetic, and engineering aspects. Wiley Interdiscip. Rev. Energy Environ. 2014, 4, 486–520. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Guo, X.; Mao, J.; Zhang, S. Ag/Al2O3 for glycerol hydrogenolysis to 1,2-propanediol: Activity, selectivity and deactivation. Green Chem. 2012, 14, 156–163. [Google Scholar] [CrossRef]

| Catalyst | BET Surface Area (m2/g) | Total Pore Volume (cc/g) | Pore Diameter (Å) | Amount of Cu (wt%) 1 | Amount of B (wt%) 1 | Amount of Al (wt%) 1 |
|---|---|---|---|---|---|---|
| Al2O3 | 231 | 0.54 | 103 | |||
| 5 Cu/Al2O3 | 152 | 0.47 | 100 | |||
| 5 Cu-0.25B/Al2O3 | 167 | 0.49 | 96 | |||
| 5 Cu-1B/Al2O3 | 131 | 0.47 | 99 | 4.8 | 0.9 | 92 |
| 5 Cu-3B/Al2O3 | 109 | 0.32 | 87 | |||
| 5 Cu-1B/Al2O3 (Spent) | 91 | 0.25 | 83 | 4.5 | 0.2 | 30 |
| Catalyst | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| 1,2-PDO | EG | Acetol | Others 1 | ||
| Al2O3 | 5 ± 1.2 | 38 ± 0.3 | - | 57 ± 3 2 | 5 ± 0.4 |
| 1Cu/Al2O3 | 26 ± 2.3 | 85 ± 3.0 | 2 ± 0.2 | 11 ± 0.1 | 2 ± 0.2 |
| 3Cu/Al2O3 | 45 ± 2.0 | 88 ± 2.0 | 1 ± 0.1 | 7 ± 0.6 | 4 ± 0.6 |
| 5Cu/Al2O3 | 71 ± 3.0 | 87 ± 1.0 | 3 ± 0.2 | 6 ± 0.2 | 2 ± 0.1 |
| 10Cu/Al2O3 | 70 ± 2.0 | 87 ± 2.0 | 1 ± 0.1 | 4 ± 0.5 | 3 ± 0.3 |
| 15Cu/Al2O3 | 68 ± 1.0 | 86 ± 3.0 | 2 ± 0.2 | 6 ± 0.4 | 3 ± 0.4 |
| Glycerol Grade | Purity (%) | Water (%) | Ash (%) | MONG 1 (%) |
|---|---|---|---|---|
| Pharmaceutical | 99.9 | 0.1 | <0.001 | N.D |
| Technical | 91.6 | 4.3 | 1.4 | 2.7 |
| Crude | 54.7 | 12.8 | 7.3 | 25.2 |
| Catalyst | Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|
| 1,2-PDO | EG | Acetol | 1-PrOH | 2-PrOH | EtOH | ||
| 5Cu-1B/Al2O3 | 98 ± 2 | 98 ± 2.0 | 0.6 ± 0.03 | 0.2 ± 0.04 | 0.6 ± 0.02 | 0.4 ± 0.04 | 0.2 ± 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanda, M.R.; Yuan, Z.; Shui, H.; Xu, C. Selective Hydrogenolysis of Glycerol and Crude Glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 Promoted Cu/Al2O3 Catalysts. Catalysts 2017, 7, 196. https://doi.org/10.3390/catal7070196
Nanda MR, Yuan Z, Shui H, Xu C. Selective Hydrogenolysis of Glycerol and Crude Glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 Promoted Cu/Al2O3 Catalysts. Catalysts. 2017; 7(7):196. https://doi.org/10.3390/catal7070196
Chicago/Turabian StyleNanda, Malaya R., Zhongshun Yuan, Hengfu Shui, and Chunbao (Charles) Xu. 2017. "Selective Hydrogenolysis of Glycerol and Crude Glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 Promoted Cu/Al2O3 Catalysts" Catalysts 7, no. 7: 196. https://doi.org/10.3390/catal7070196