Activated Carbon Supported Mo-Ti-N Binary Transition Metal Nitride as Catalyst for Acetylene Hydrochlorination
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Catalysts
2.3. Characterization Techniques
2.4. Experimental Measurements
2.5. Analytical Methods and Criteria
3. Results and Discussion
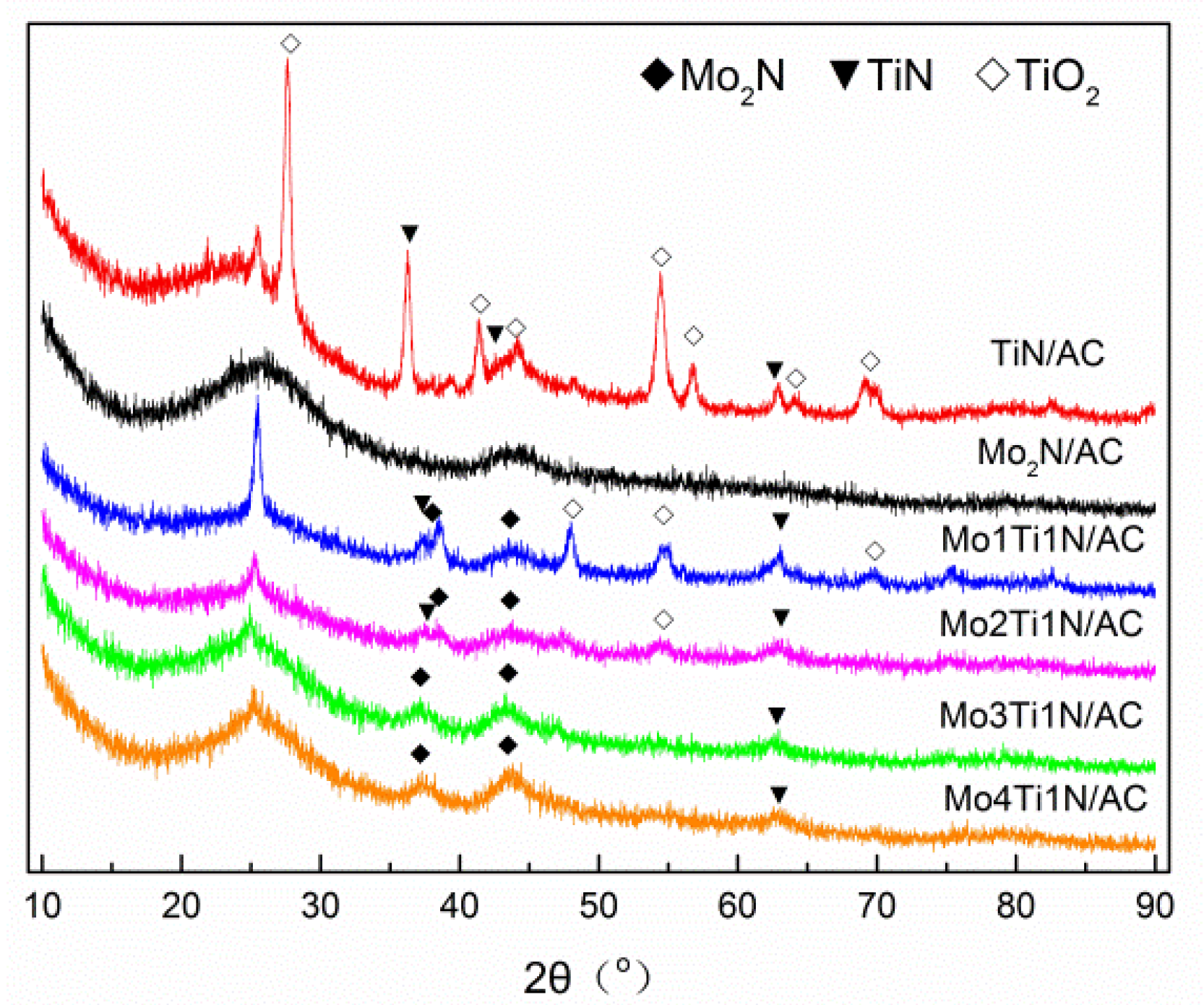
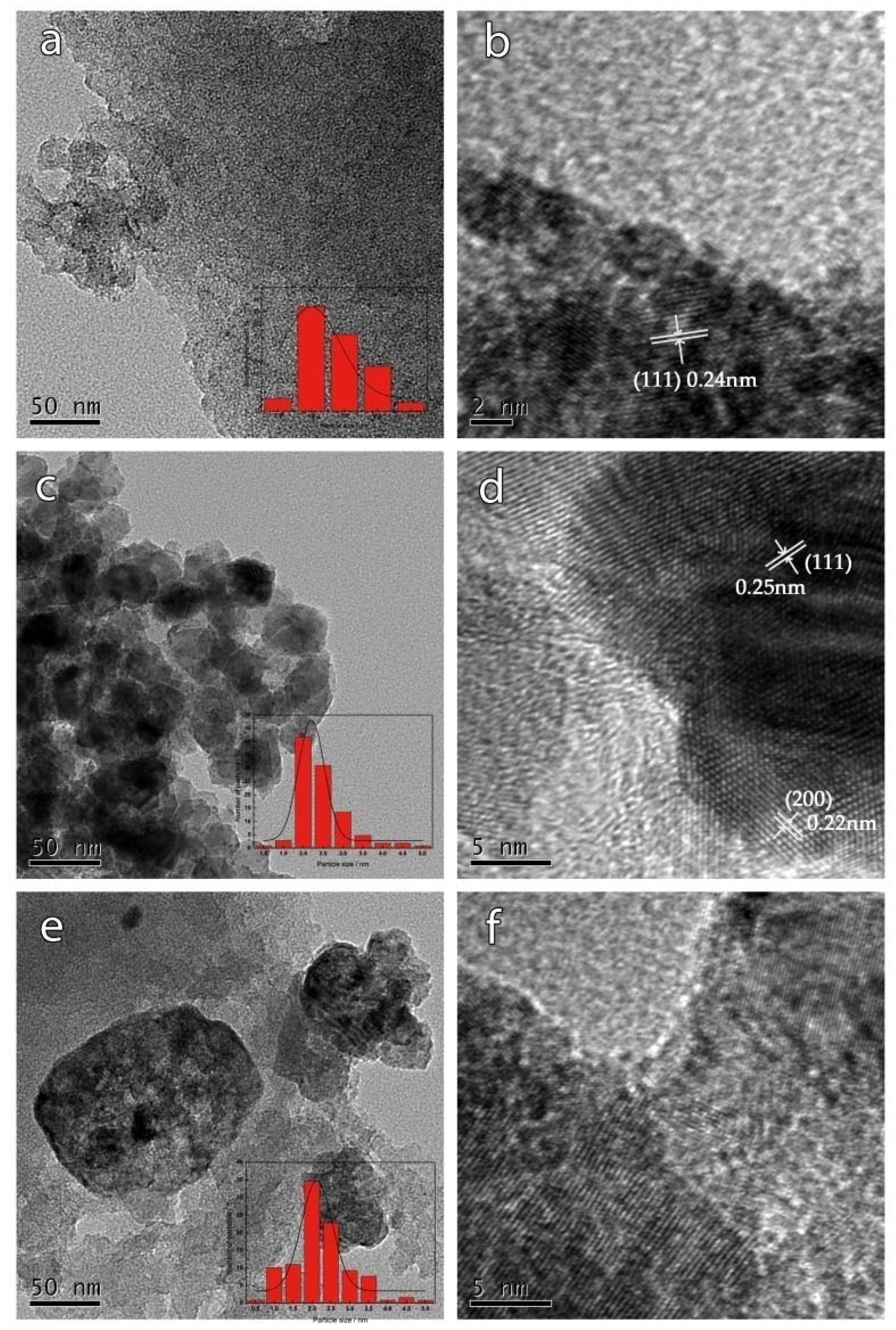
3.1. Morphology of Samples
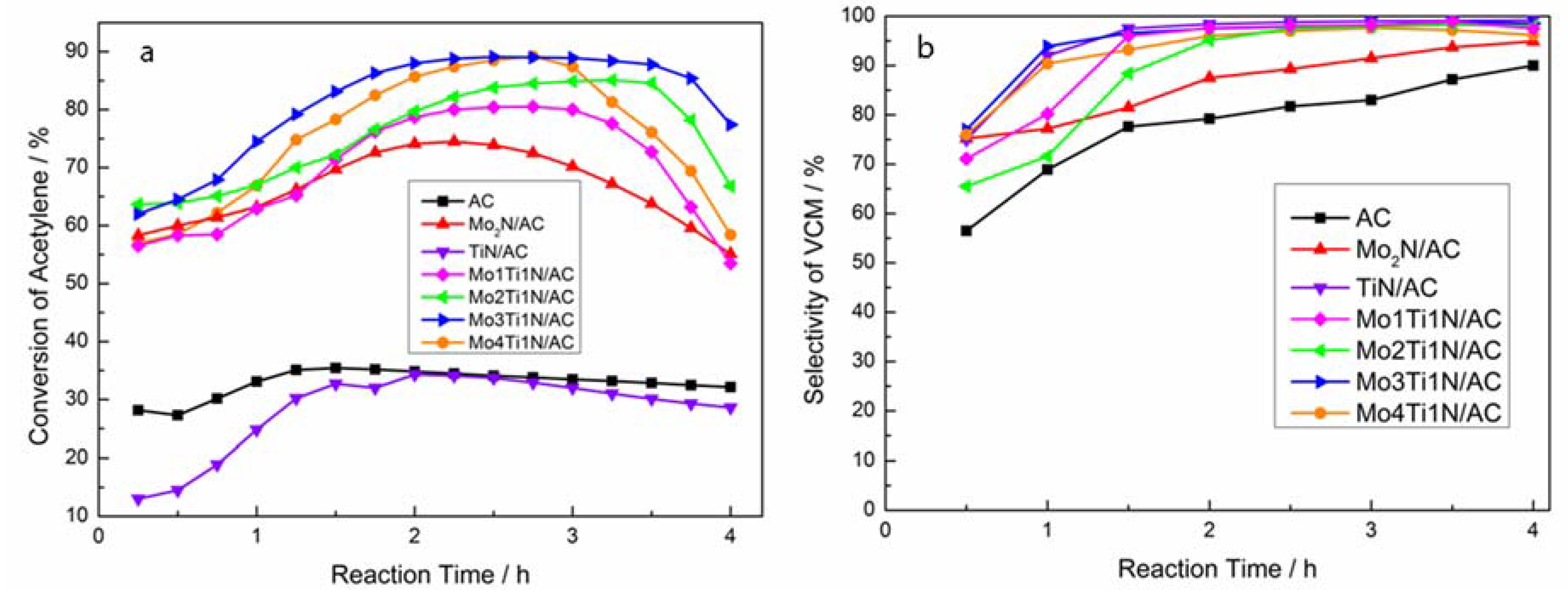
3.2. Catalytic Performance of Catalysts in Acetylene Hydrochlorination Reaction
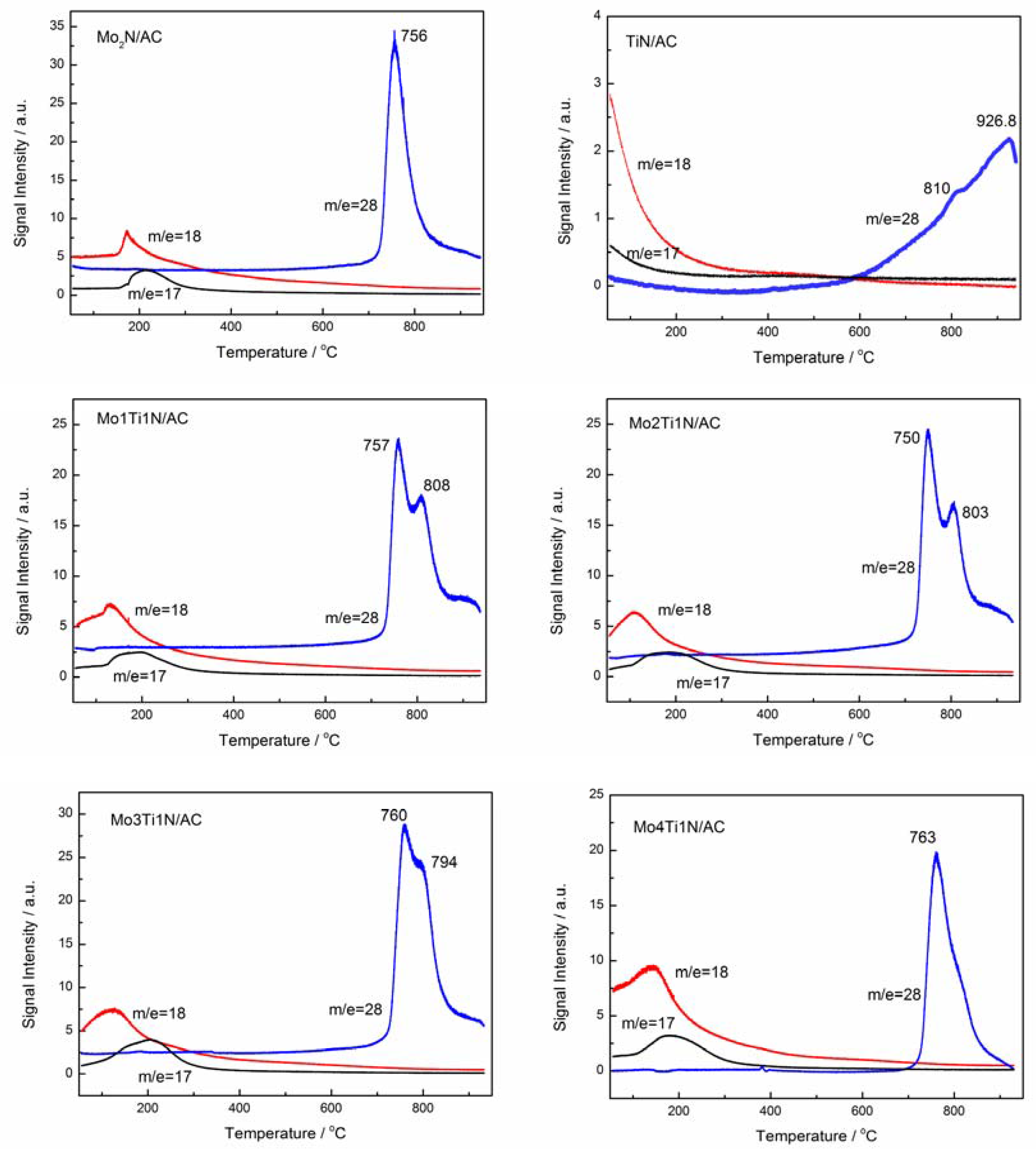
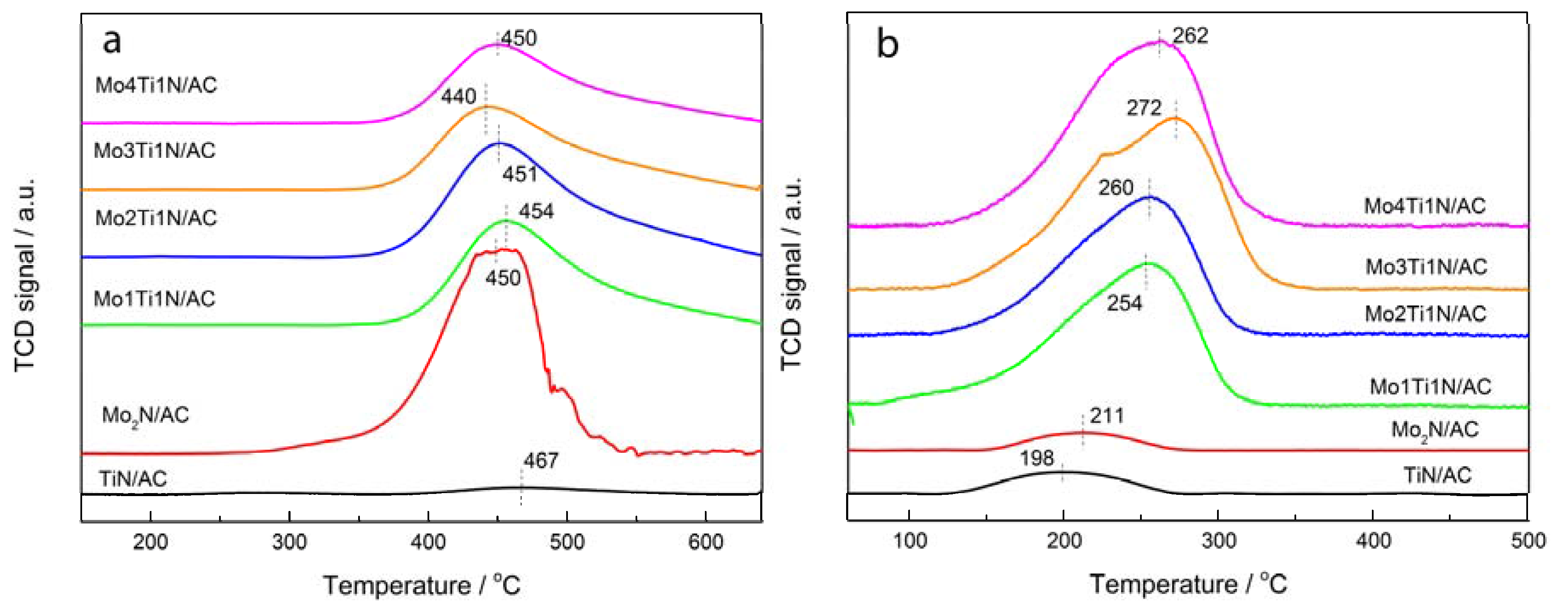
3.3. Characteristics of Fresh and Used Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, M.; Wang, Q.; Chen, K.; Wang, Y.; Huang, C.; Dai, H.; Yu, F.; Kang, L.; Dai, B. Development of a heterogeneous non-mercury catalyst for acetylene hydrochlorination. ACS Catal. 2015, 5, 5306–5316. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, N.; Li, W.; Dai, B. Progress on cleaner production of vinyl chloride monomers over non-mercury catalysts. Front. Chem. Sci. Eng. 2011, 5, 514–520. [Google Scholar] [CrossRef]
- Dai, B.; Wang, Q.; Yu, F.; Zhu, M. Effect of au nano-particle aggregation on the deactivation of the AUCL3/ac catalyst for acetylene hydrochlorination. Sci. Rep. 2014, 5, 10553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, B.; Wang, X.; Xu, L.; Zhu, M. Hydrochlorination of acetylene to vinyl chloride monomer over bimetallic au–la/sac catalysts. J. Ind. Eng. Chem. 2012, 18, 49–54. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Li, W.; Han, Y.; Gu, J.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au–Co(iii)/sac catalysts for vinyl chloride monomer production. Green Chem. 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Conte, M.; Carley, A.F.; Attard, G.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Hydrochlorination of acetylene using supported bimetallic au-based catalysts. J. Catal. 2008, 257, 190–198. [Google Scholar] [CrossRef]
- Nkosi, B.; Adams, M.D.; Coville, N.J.; Hutchings, G.J. Hydrochlorination of acetylene using carbon-supported gold catalysts: A study of catalyst reactivation. J. Catal. 1991, 128, 378–386. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, W.; Guo, C.; Li, W. Acetylene hydrochlorination over bimetallic ru-based catalysts. RSC Adv. 2013, 3, 21062–21068. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Zhang, H.; Pu, Y.; Sun, M.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over ru catalysts enhanced by carbon nanotubes. RSC Adv. 2015, 5, 9002–9008. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.; Wang, X.; Zhang, H.; Yu, L.; Dong, Y.; Li, W. Bimetallic Au–Ni/CsS catalysts for acetylene hydrochlorination. Catal. Sci. Technol. 2014, 4, 4426–4432. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, J.; Cheng, X.; Wang, L.; Yang, H.; Shen, B. An au–cu bimetal catalyst for acetylene hydrochlorination with renewable γ-Al2O3 as the support. RSC Adv. 2015, 5, 16727–16734. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.; Di, X.; Xu, J.; Xu, J.; Feng, F.; Jun, N.; Li, X. Nitrogen-modified activated carbon supported bimetallic gold–cesium (i) as highly active and stable catalyst for the hydrochlorination of acetylene. RSC Adv. 2015, 5, 6925–6931. [Google Scholar] [CrossRef]
- Wang, B.; Yu, L.; Zhang, J.; Pu, Y.; Zhang, H.; Li, W. Phosphorus-doped carbon supports enhance gold-based catalysts for acetylene hydrochlorination. RSC Adv. 2014, 4, 15877–15885. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Zhang, H.; Chen, K.; Wang, Q.; Li, X. Melamine modification of spherical activated carbon and its effects on acetylene hydrochlorination. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 1147–1151. [Google Scholar]
- Li, X.; Zhu, M.; Dai, B. AuCl3 on polypyrrole-modified carbon nanotubes as acetylene hydrochlorination catalysts. Appl. Catal. B Environ. 2013, 142, 234–240. [Google Scholar] [CrossRef]
- Dai, B.; Chen, K.; Wang, Y.; Kang, L.; Zhu, M. Boron and nitrogen doping in graphene for the catalysis of acetylene hydrochlorination. ACS Catal. 2015, 5, 2541–2547. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; Ren, P.; Wu, X.; Sun, L.; Jiao, F.; Bao, X. Silicon carbide-derived carbon nanocomposite as a substitute for mercury in the catalytic hydrochlorination of acetylene. Nat. Commun. 2014, 5, 3688–3694. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Zhao, W.; Zhu, M. The preparation of Cu-g-C3N4/AC catalyst for acetylene hydrochlorination. Catalysts 2016, 6, 193. [Google Scholar]
- Toth, L. Transition Metal Carbides and Nitrides; Elsevier: New York, NY, USA, 1971. [Google Scholar]
- Alhajri, N.S.; Anjum, D.H.; Hedhili, M.N.; Takanabe, K. Generation and characteristics of iv–vi transition metal nitride and carbide nanoparticles using a reactive mesoporous carbon nitride. ChemistrySelect 2016, 1, 290–296. [Google Scholar] [CrossRef]
- Ham, D.J.; Lee, J.S. Transition metal carbides and nitrides as electrode materials for low temperature fuel cells. Energies 2009, 2, 873–899. [Google Scholar] [CrossRef]
- Chen, W.-F.; Muckerman, J.T.; Fujita, E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Xiao, Y.; Fu, Z.; Zhan, G.; Wu, S.; Xiao, C.; Hu, G.; Wei, Z. Hollow and porous titanium nitride nanotubes as high-performance catalyst supports for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 13966–13975. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, Z.; Zhan, G.; Pan, Z.; Xiao, C.; Wu, S.; Chen, C.; Hu, G.; Wei, Z. Increasing pt methanol oxidation reaction activity and durability with a titanium molybdenum nitride catalyst support. J. Power Sources 2015, 273, 33–40. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Song, R.; Yang, S.; Song, H. Hybrid 2D–0D Graphene–VR Quantum Dots for Superior Lithium and Sodium Storage. Adv. Energy Mater. 2015, 6, 1502067. [Google Scholar] [CrossRef]
- Tian, X.; Luo, J.; Nan, H.; Fu, Z.; Zeng, J.; Liao, S. Binary transition metal nitrides with enhanced activity and durability for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 16801–16809. [Google Scholar] [CrossRef]
- Cao, B.; Veith, G.M.; Neuefeind, J.C.; Adzic, R.R.; Khalifah, P.G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 51, 19186–19192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, S.; Pan, X.; Wang, J.; Bao, X. Fen nanoparticles confined in carbon nanotubes for co hydrogenation. Energy Environ. Sci. 2011, 4, 4500–4503. [Google Scholar] [CrossRef]
- Podila, S.; Zaman, S.F.; Driss, H.; Alhamed, Y.A.; Al-Zahrani, A.A.; Petrova, L.A. Hydrogen production by ammonia decomposition using high surface area Mo2N and Co3Mo3N catalysts. Catal. Sci. Technol. 2013, 6, 1496–1506. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, S.; Ma, Y.; Tang, Y.; Giordano, C.; Antonietti, M. SiO2-surface-assisted controllable synthesis of taon and Ta3N5 nanoparticles for alkene epoxidation. Angew. Chem. 2012, 124, 985–989. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures; Wiley: New York, NY, USA, 1954; Volume 2. [Google Scholar]
- Tian, X.; Luom, J.; Nan, H.; Zou, H.; Chen, R.; Shu, T.; Li, X.; Li, Y.; Song, H.; Liao, S.; et al. Transition metal nitride coated with atomic layers of pt as a low-cost, highly stable electrocatalyst for the oxygen reduction reaction. J. Am. Chem. Soc. 2016, 138, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, A.; Yang, X.; Au, C. On the catalytic nature of vn, Mo2N, and W2N nitrides for NO reduction with hydrogen. Appl. Catal. A Gen. 2004, 276, 223–230. [Google Scholar] [CrossRef]
- Chen, K.; Kang, L.; Zhu, M.; Dai, B. Mesoporous carbon with controllable pore sizes as a support of the AuCl3 catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2014, 5, 1035–1040. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Ni, J.; Zhang, T.; Xu, X.; Li, X. Activated-carbon-supported gold–cesium(i) as highly effective catalysts for hydrochlorination of acetylene to vinyl chloride. ChemPlusChem 2015, 80, 196–201. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.; Di, X.; Xu, J.; Gu, S.; Zhang, Q.; Ni, J.; Li, X. Activated carbon supported ternary gold-cesium(i)-indium(iii) catalyst for the hydrochlorination of acetylene. Catal. Sci. Technol. 2015, 5, 4973–4984. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Li, W.; Han, Y. Deactivation mechanism of AuCl3 catalyst in acetylene hydrochlorination reaction: A dft study. Rsc Adv. 2012, 2, 4814–4821. [Google Scholar] [CrossRef]
- Morozan, A.; Goellner, V.; Zitolo, A.; Fonda, E.; Donnadieu, B.; Jonesa, D.; Jaouen, F. Synergy between molybdenum nitride and gold leading to platinum-like activity for hydrogen evolution. Phys. Chem. Chem. Phys. 2015, 17, 4047–4053. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Fu, Z.; Sun, D.; Pan, Z.; Xiao, C.; Wu, S.; Chen, C.; Hu, G.; Wei, Z. Platinum nanoparticles decorated robust binary transition metal nitrideecarbon nanotubes hybrid as an efficient electrocatalyst for the methanol oxidation reaction. J. Power Sources 2016, 326, 84–92. [Google Scholar] [CrossRef]
| Samples | Mole Ratio | D (nm) | SBET (m2 g−1) | Vtot (cm3 g−1) | |||
|---|---|---|---|---|---|---|---|
| Fresh | Used | Fresh | Used | Fresh | Used | ||
| AC | - | 2.2 | 2.1 | 948 | 915 | 0.52 | 0.48 |
| Mo2N/AC | - | 2.1 | 1.9 | 811 | 40 | 0.43 | 0.19 |
| TiN/AC | - | 2.2 | 2.2 | 889 | 881 | 0.49 | 0.47 |
| Mo1Ti1N/AC | Mo:Ti = 1:1 | 2.2 | 2.6 | 649 | 30 | 0.36 | 0.19 |
| Mo2Ti1N/AC | Mo:Ti = 2:1 | 2.1 | 2.8 | 680 | 33 | 0.36 | 0.23 |
| Mo3Ti1N/AC | Mo:Ti = 3:1 | 2.2 | 2.7 | 772 | 32 | 0.42 | 0.20 |
| Mo4Ti1N/AC | Mo:Ti = 4:1 | 2.2 | 2.6 | 785 | 27 | 0.43 | 0.18 |
| Samples | Peak Area of Desorption C2H2 | Peak Area of Desorption HCl |
|---|---|---|
| Mo2N/AC | 12.6 | 0.06 |
| TiN/AC | 0.57 | 0.10 |
| Mo1Ti1N/AC | 7.32 | 0.76 |
| Mo2Ti1N/AC | 6.95 | 0.90 |
| Mo3Ti1N/AC | 6.55 | 0.96 |
| Mo4Ti1N/AC | 6.08 | 0.99 |
| Samples | Binding Energy/eV (Area/%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mo 3d | Ti 2p | N 1s | |||||||
| Moδ+ (2 < δ < 4) | TiO2 | TiN | Ti-O-N | Pyridinic-N | Mo-N | Ti-N | Pyrrolic-N | ||
| Mo2N/AC | 229.9 (61.1) | 233.1 (38.8) | - | - | - | 397.9 (27.4) | 398.5 (43.2) | 399.3 (29.4) | |
| TiN/AC | - | - | 464.3 (29.0) | 460.5 (11.5) | 458.5 (59.5) | - | - | - | - |
| Mo3Ti1N/AC | 229.9 (54.5) | 233.1 (45.4) | - | - | - | - | 398.6 (76.4) | 397.1 (23.6) | - |
| Catalyst | Amount of Carbon Deposition (%) |
|---|---|
| Mo2N/AC | 6.63 |
| TiN/AC | 1.88 |
| Mo1Ti1N/AC | 10.9 |
| Mo2Ti1N/AC | 9.3 |
| Mo3Ti1N/AC | 9.2 |
| Mo4Ti1N/AC | 11.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Zhu, M.; Zhang, H.; Yu, F.; Wang, C.; Dai, B. Activated Carbon Supported Mo-Ti-N Binary Transition Metal Nitride as Catalyst for Acetylene Hydrochlorination. Catalysts 2017, 7, 200. https://doi.org/10.3390/catal7070200
Dai H, Zhu M, Zhang H, Yu F, Wang C, Dai B. Activated Carbon Supported Mo-Ti-N Binary Transition Metal Nitride as Catalyst for Acetylene Hydrochlorination. Catalysts. 2017; 7(7):200. https://doi.org/10.3390/catal7070200
Chicago/Turabian StyleDai, Hui, Mingyuan Zhu, Haiyang Zhang, Feng Yu, Chao Wang, and Bin Dai. 2017. "Activated Carbon Supported Mo-Ti-N Binary Transition Metal Nitride as Catalyst for Acetylene Hydrochlorination" Catalysts 7, no. 7: 200. https://doi.org/10.3390/catal7070200






