Rapid Jatropha-Castor Biodiesel Production with Microwave Heating and a Heterogeneous Base Catalyst Nano-Ca(OH)2/Fe3O4
Abstract
:1. Introduction
2. Results and Discussion
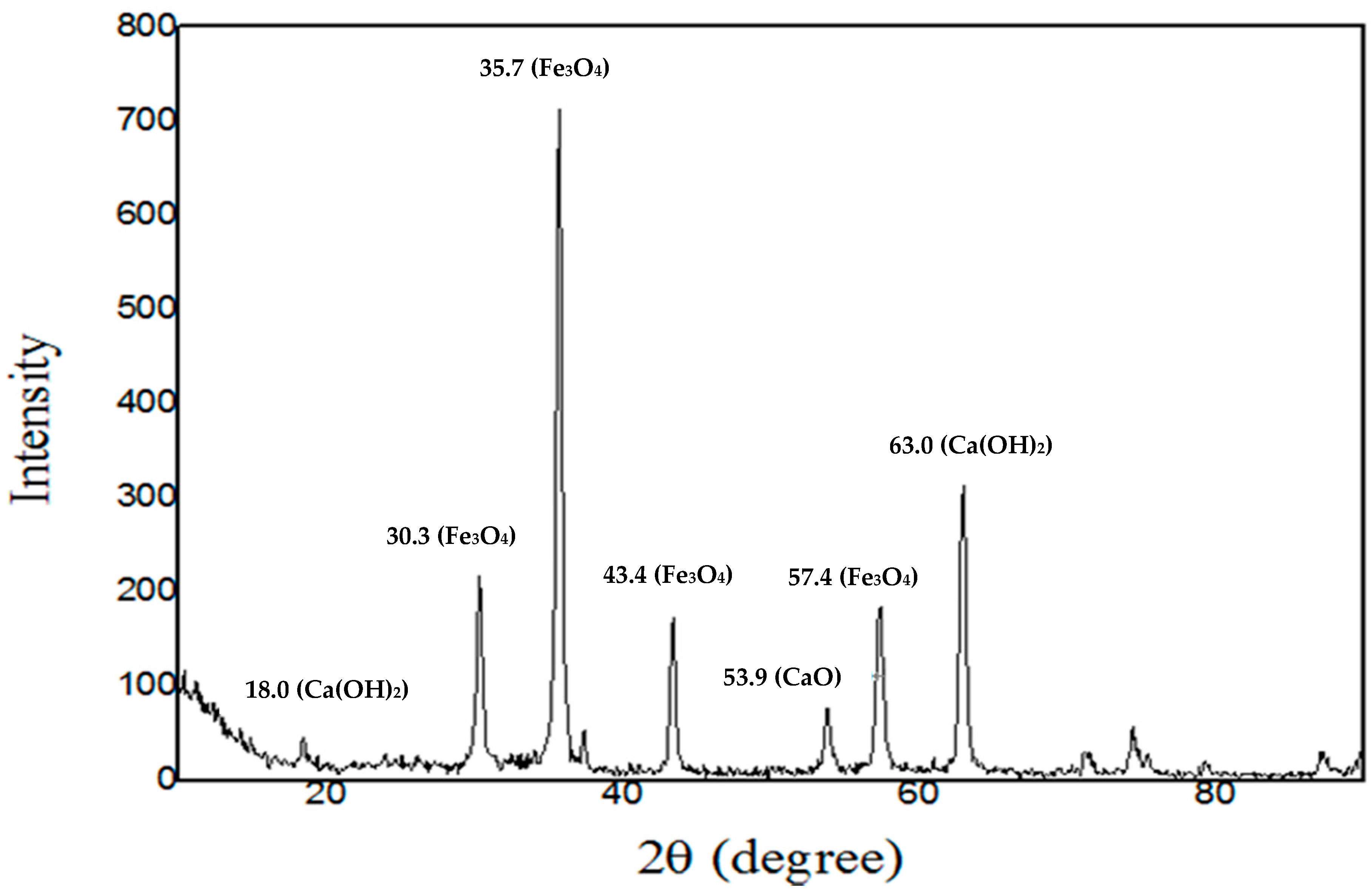
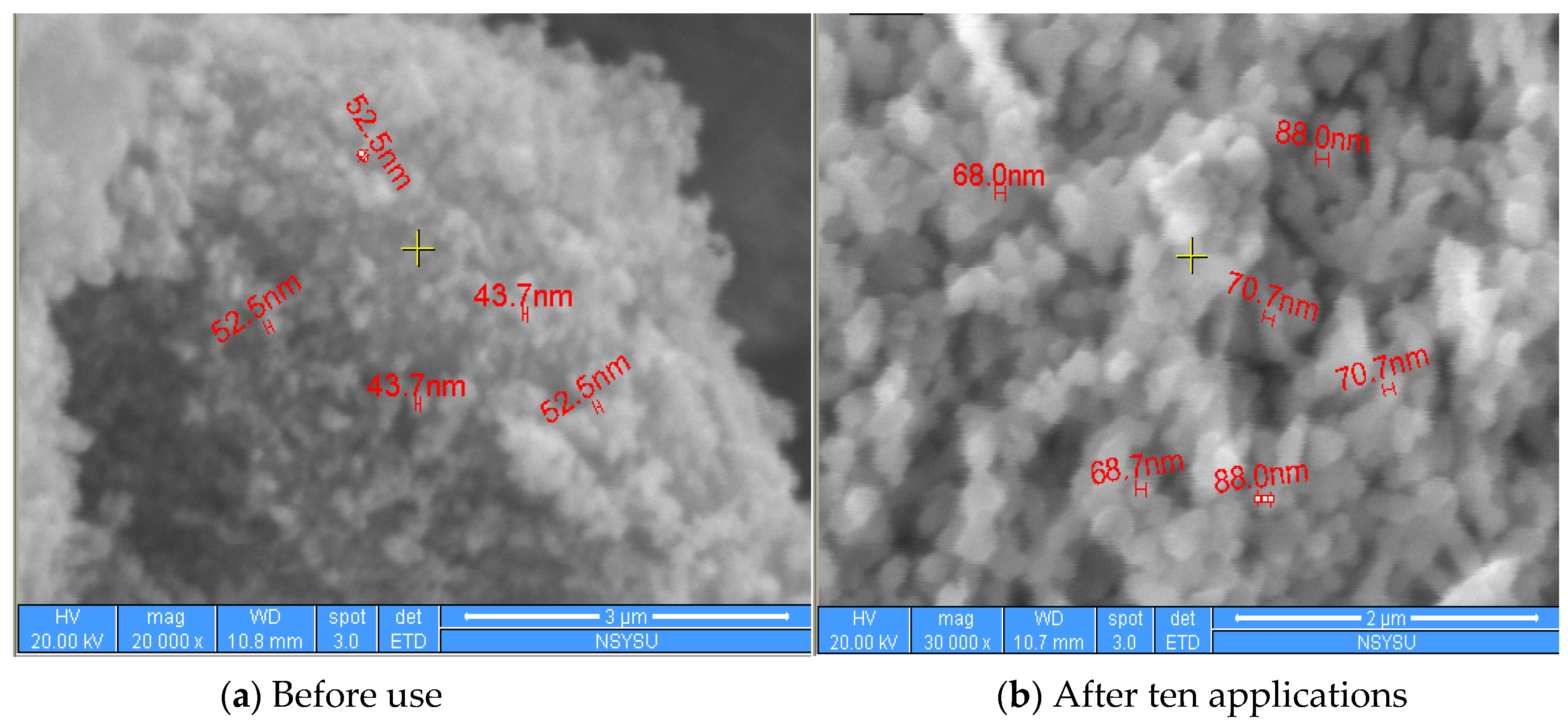
2.1. Characteristics of the Catalyst
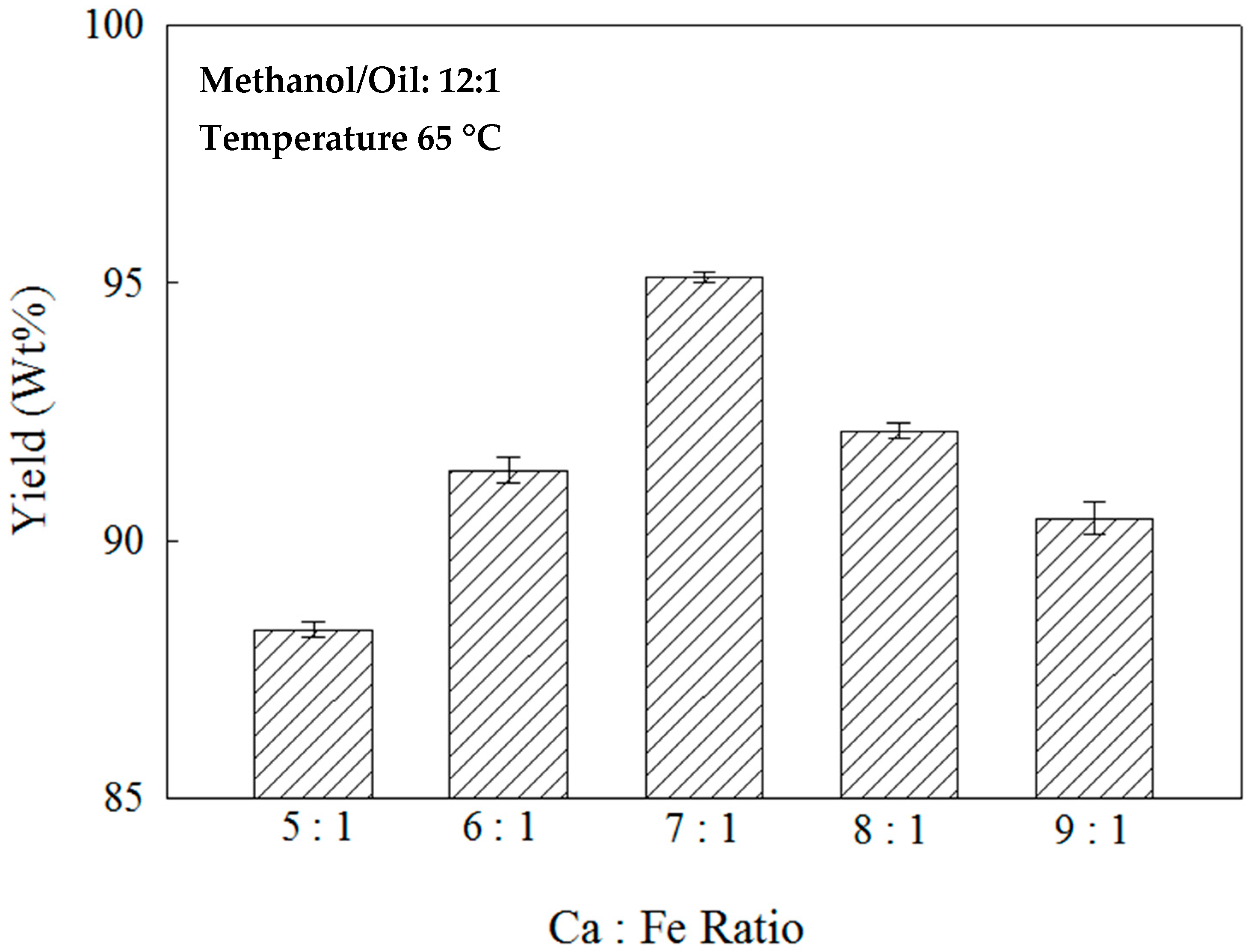
2.2. Effects of the Ca:Fe Ratio on the Nano-Ca(OH)2/Fe3O4 Catalyst and Methanol/Oil Ratio
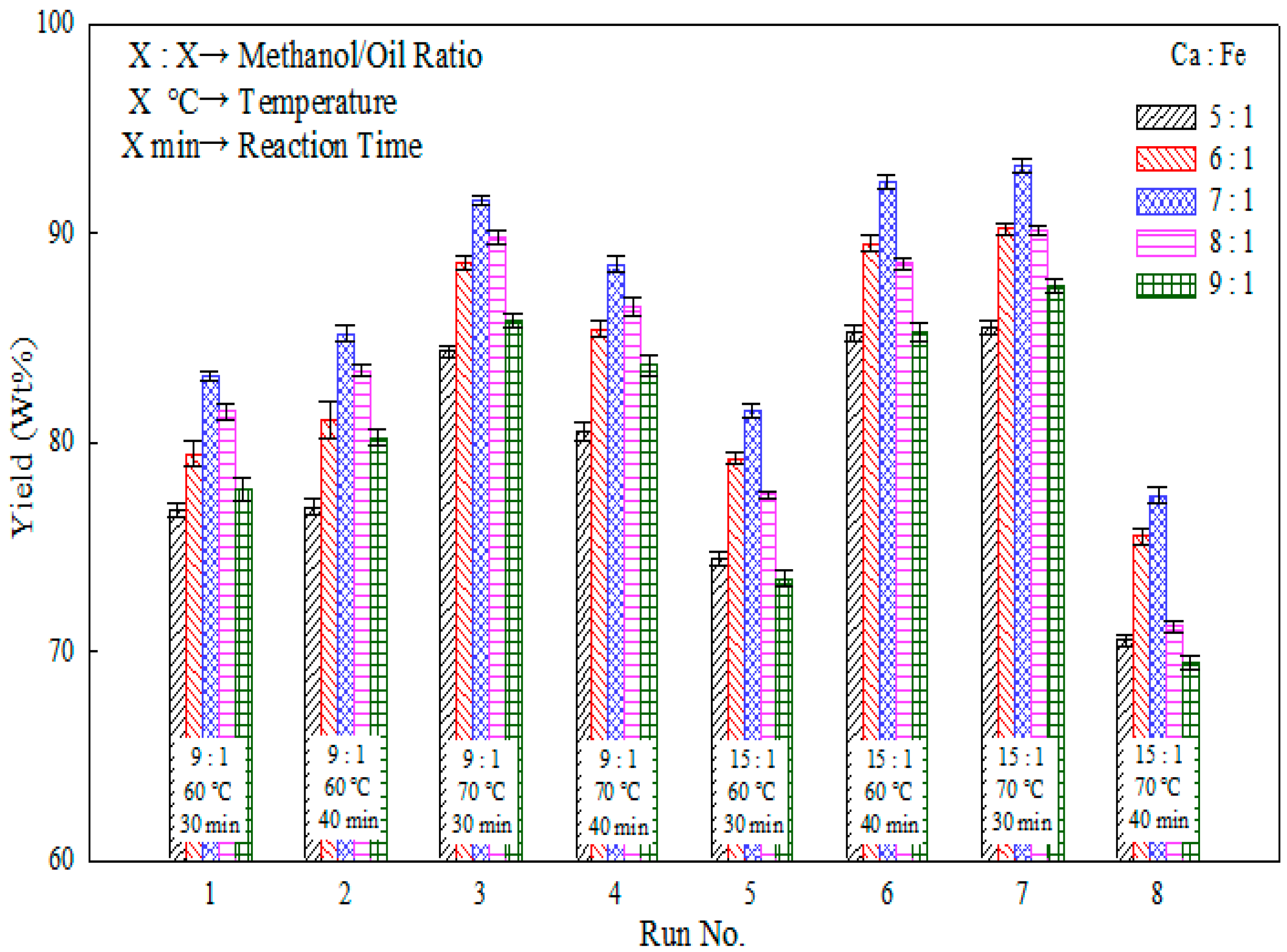
2.3. Effects of Temperature and Reaction Time
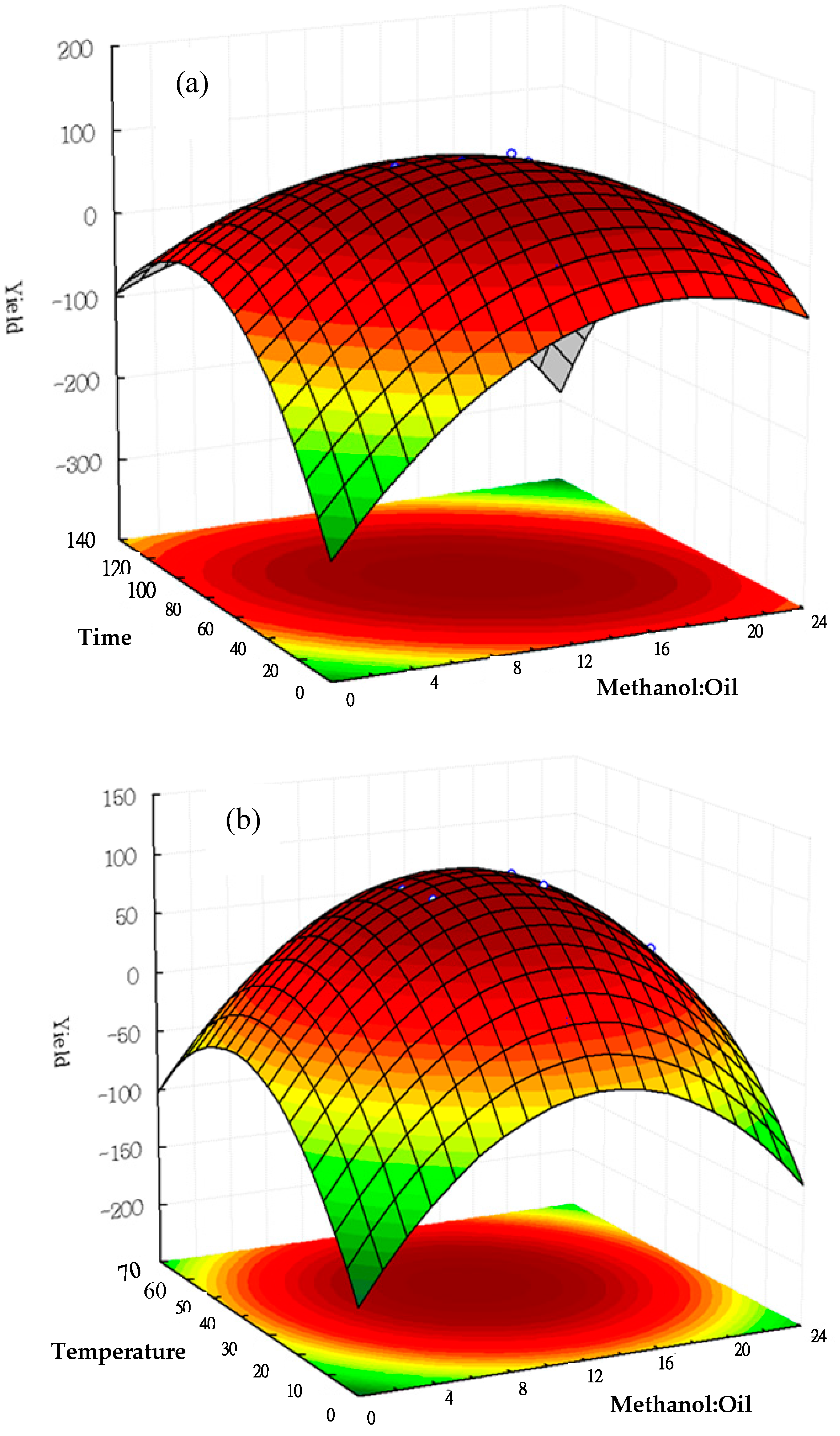
2.4. Discussion of Response Surface Methodology on Biodiesel Production
2.5. Catalyst Stability
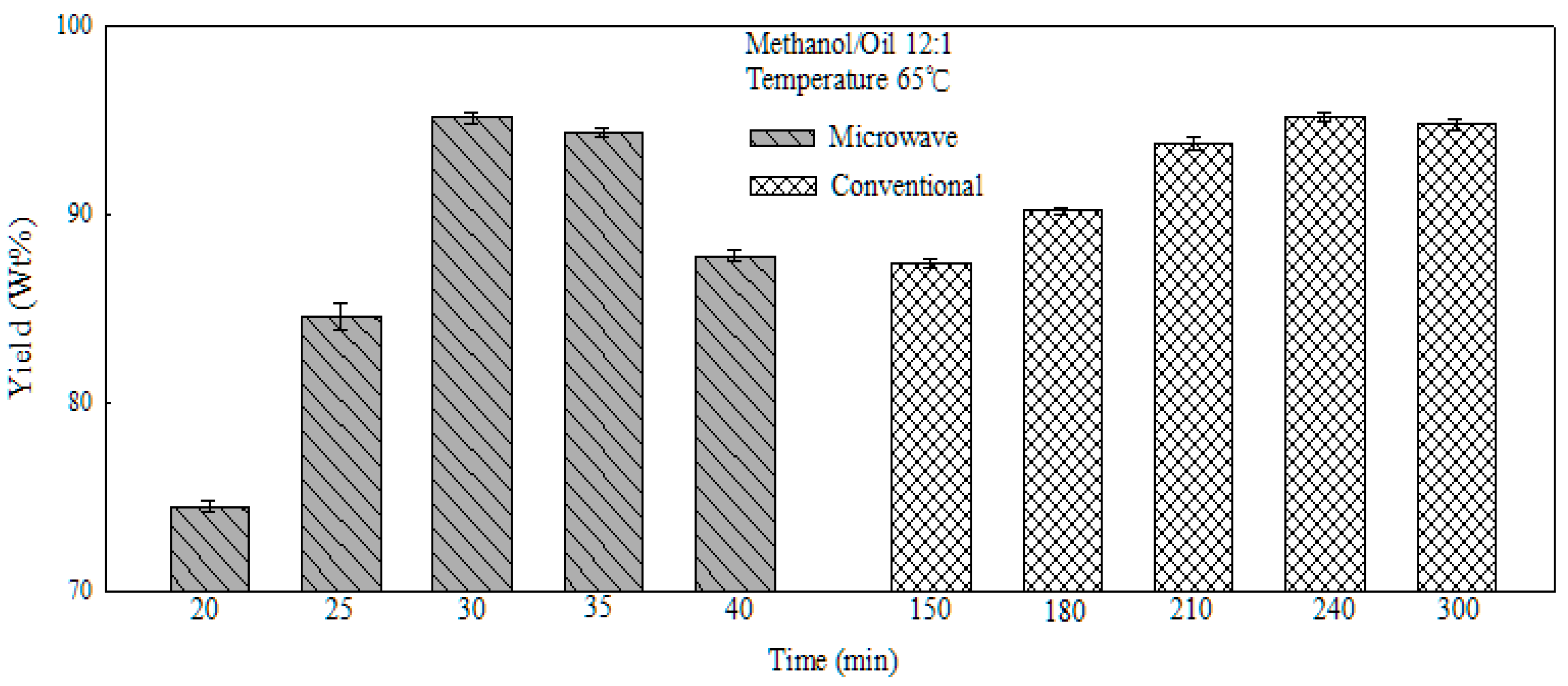
2.6. Comparison of Traditional and Microwave Heating
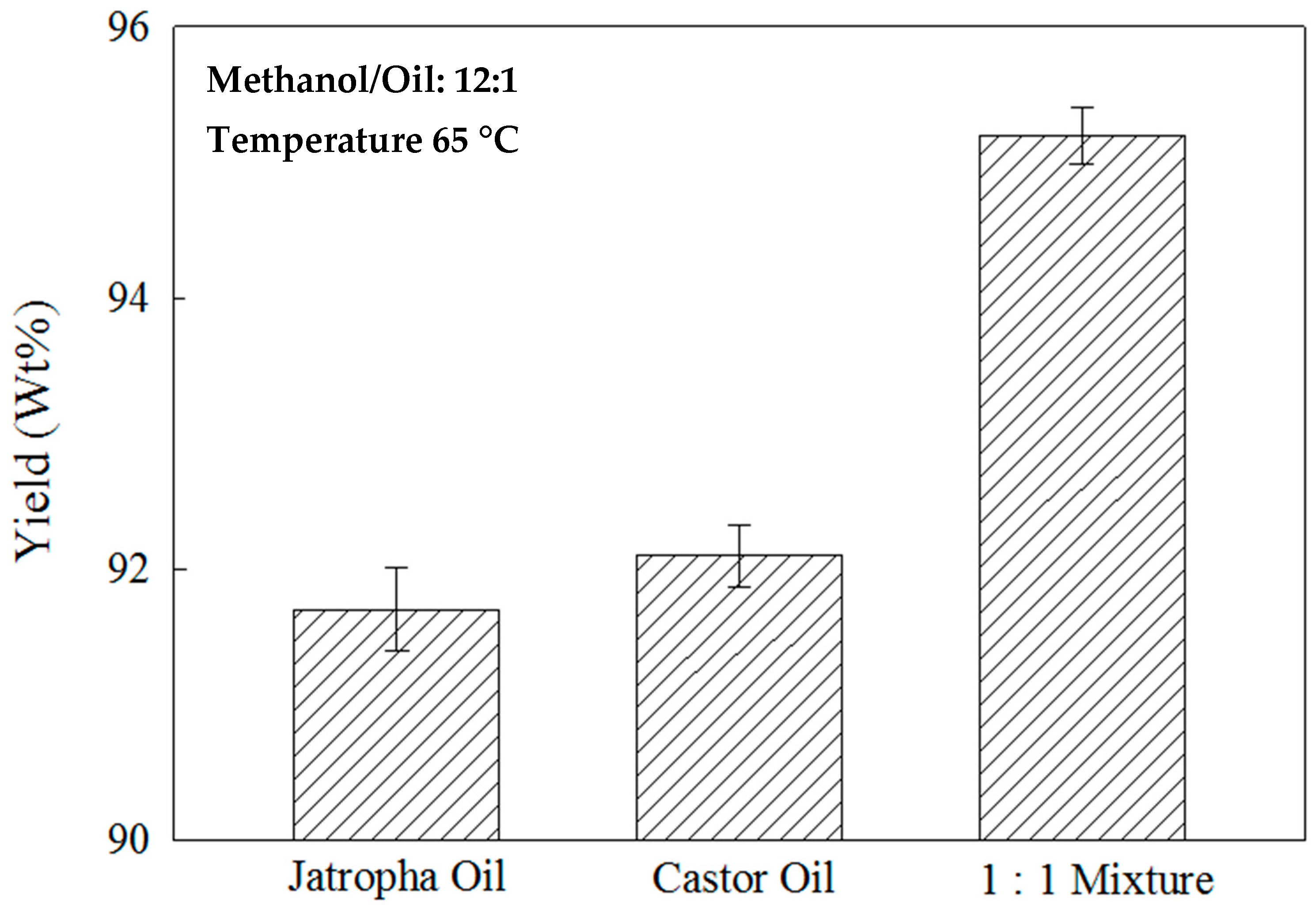
2.7. Effects of the Feedstock Oil
3. Methodology
3.1. Materials for Experimentation
3.2. Synthesis and Recycling of the Nano-Ca(OH)2/Fe3O4 Catalyst
3.3. Characterization of the Catalyst
3.4. Transesterification for 1:1 Mixed Jatropha-Castor Oil Biodiesel with Methanol
3.5. Product Analysis
3.6. Design of the Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| CaCl2 | Calcium chloride |
| Ca(OH)2 | Calcium Hydroxide |
| XRD | X-ray diffraction analysis |
| ESEM | Environmental scanning electron microscope |
| EDS | Energy Dispersive Spectroscopy |
References
- Haas, M.J.; McAloon, A.J.; Yee, W.C.; Foglia, T.A. A process model to estimate biodiesel production costs. Bioresour. Technol. 2006, 97, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G.; Van Gerpen, J.H.; Krahl, J. The Biodiesel Handbook; AOCS Press: Champaign, IL, USA, 2005. [Google Scholar]
- Lin, Y.C.; Lee, W.J.; Hou, H.C. PAH emissions and energy efficiency of palm-biodiesel blends fueled on diesel generator. Atmos. Environ. 2006, 40, 3930–3940. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lee, W.J.; Wu, T.S.; Wang, C.T. Comparison of PAH and regulated harmful matter emissions from biodiesel blends and paraffinic fuel blends on engine accumulated mileage test. Fuel 2006, 85, 2516–2523. [Google Scholar] [CrossRef]
- Yuan, C.S.; Lin, H.Y.; Lee, W.J.; Lin, Y.C.; Wu, T.S.; Chen, K.F. A new alternative fuel for reduction of polycyclic aromatic hydrocarbon and particulate matter emissions from diesel engines. J. Air Waste Manag. Assoc. 2007, 57, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Tsai, C.H.; Yang, C.R.; Wu, C.J.; Wu, T.Y.; Chang-Chien, G.P. Effects on aerosol size distribution of polycyclic aromatic hydrocarbons from the heavy-duty diesel generator fueled with feedstock palm-biodiesel blends. Atmos. Environ. 2008, 42, 6679–6688. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, S.H.; Chen, Y.M.; Wu, T.Y. A new alternative paraffinic–palmbiodiesel fuel for reducing polychlorinated dibenzo-p-dioxin/dibenzofuran emissions from heavy-duty diesel engines. J. Hazard. Mater. 2011, 185, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, M.; Mondragón, F.; Agudelo, J.R.; Santamaría, A. Influence of palm oil biodiesel on the chemical and morphological characteristics of particulate matter emitted by a diesel engine. Atmos. Environ. 2012, 62, 220–227. [Google Scholar] [CrossRef]
- Sooknoi, T.; Danuthai, T.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Deoxygenation of methylesters over CsNaX. J. Catal. 2008, 258, 199–209. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. A Comprehensive Analysis of Biodiesel Impacts on Exhaust Emissions Draft Technical Report; EPA420-P-02-001; United States Environmental Protection Agency: Washington, DC, USA, 2002.
- Araujo, V.K.W.S.; Hamacher, S.; Scavarda, L.F. Economic assessment of biodiesel production from waste frying oils. Bioresour. Technol. 2010, 101, 4415–4422. [Google Scholar] [CrossRef] [PubMed]
- Dmytryshyn, S.; Dalai, A.; Chaudhari, S.; Mishra, H.; Reaney, M. Synthesis and characterization of vegetable oil derived esters: Evaluation for their diesel additive properties. Bioresour. Technol. 2004, 92, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Vicente, G.; Martı́nez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, L.; Zhang, Y.; Li, S.; Tian, S.; Wang, B. Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification. Fuel Process. Technol. 2012, 95, 84–89. [Google Scholar] [CrossRef]
- Bak, Y.C.; Choi, J.H.; Kim, S.B.; Kang, D.W. Production of bio-diesel fuels by transesterification of rice bran oil. Korean J. Chem. Eng. 1996, 13, 242–245. [Google Scholar] [CrossRef]
- Freedman, B.; Pryde, E.; Mounts, T. Variables affecting the yields of fatty esters from transesterified vegetable oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Apostolakou, A.; Kookos, I.; Marazioti, C.; Angelopoulos, K. Techno-economic analysis of a biodiesel production process from vegetable oils. Fuel Process. Technol. 2009, 90, 1023–1031. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Brito, A.; Borges, M.; Otero, N. Zeolite Y as a heterogeneous catalyst in biodiesel fuel production from used vegetable oil. Energy Fuels 2007, 21, 3280–3283. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Kawashima, A.; Matsubara, K.; Honda, K. Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresour. Technol. 2009, 100, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Kusakabe, K. Transesterification of sunflower oil in a countercurrent trickle-bed reactor packed with a CaO catalyst. Chem. Eng. Process. Process Intensif. 2011, 50, 650–654. [Google Scholar] [CrossRef]
- Umdu, E.S.; Tuncer, M.; Seker, E. Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour. Technol. 2009, 100, 2828–2831. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Yu, P.; Wang, S.; Luo, Y. Application of sodium aluminate as a heterogeneous base catalyst for biodiesel production from soybean oil. Energy Fuels 2009, 23, 1089–1092. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S.; He, H. Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol. Fuel 2008, 87, 1076–1082. [Google Scholar] [CrossRef]
- Di Serio, M.; Cozzolino, M.; Giordano, M.; Tesser, R.; Patrono, P.; Santacesaria, E. From Homogeneous to Heterogeneous Catalysts in Biodiesel Production. Ind. Eng. Chem. Res. 2007, 46, 6379–6384. [Google Scholar]
- Ying, M.; Chen, G. Study on the production of biodiesel by magnetic cell biocatalyst based on lipase-producing Bacillus subtilis. Appl. Biochem. Biotechnol. 2007, 137, 793–803. [Google Scholar] [PubMed]
- Xie, W.; Ma, N. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO-Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Liu, C.; Lv, P.; Yuan, Z.; Yan, F.; Luo, W. The nanometer magnetic solid base catalyst for production of biodiesel. Renew. Energy 2010, 35, 1531–1536. [Google Scholar] [CrossRef]
- Dai, Y.M.; Wu, J.S.; Chen, C.C.; Chen, K.C. Evaluating the optimum operating parameters on transesterification reaction for biodiesel production over a LiAlO2 catalyst. Chem. Eng. J. 2015, 280, 370–376. [Google Scholar] [CrossRef]
- Dai, Y.M.; Kao, I.H.; Chen, C.C. Evaluating the optimum operating parameters of biodiesel production process from soybean oil using the Li2TiO3 catalyst. J. Taiwan Inst. Chem. Eng. 2017, 70, 260–266. [Google Scholar] [CrossRef]
- Azcan, N.; Danisman, A. Alkali catalyzed transesterification of cottonseed oil by microwave irradiation. Fuel 2007, 86, 2639–2644. [Google Scholar] [CrossRef]
- Suppalakpanya, K.; Ratanawilai, S.; Tongurai, C. Production of ethyl ester from crude palm oil by two-step reaction with a microwave system. Fuel 2010, 89, 2140–2144. [Google Scholar] [CrossRef]
- Suppalakpanya, K.; Ratanawilai, S.; Tongurai, C. Production of ethyl ester from esterified crude palm oil by microwave with dry washing by bleaching earth. Appl. Energy 2010, 87, 2356–2359. [Google Scholar] [CrossRef]
- Koberg, M.; Abu-Much, R.; Gedanken, A. Optimization of bio-diesel production from soybean and wastes of cooked oil: Combining dielectric microwave irradiation and a SrO catalyst. Bioresour. Technol. 2011, 102, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J.; Esteban, A. Kinetics of sunflower oil methanolysis. Ind. Eng. Chem. Res. 2005, 44, 5447–5454. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel production, properties, and feedstocks. In Vitro Cell. Dev. Biol. Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, K.; Singh, J.S.; Kumar, A.; Singh, B.; Singh, R.P. Jatropha curcas: A potential biofuel plant for sustainable environmental development. Renew. Sustain. Energy Rev. 2012, 16, 2870–2883. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, K.; Kumar, S.; Kaushik, N.; Roy, S. Genetic variability and divergence studies in seed traits and oil content of Jatropha (Jatropha curcas L.) accessions. Biomass Bioenergy 2007, 31, 497–502. [Google Scholar] [CrossRef]
- Scholz, V.; da Silva, J.N. Prospects and risks of the use of castor oil as a fuel. Biomass Bioenergy 2008, 32, 95–100. [Google Scholar] [CrossRef]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yang, P.M.; Chen, S.C.; Lin, J.F. Improving biodiesel yields from waste cooking oil using ionic liquids as catalysts with a microwave heating system. Fuel Process. Technol. 2013, 115, 57–62. [Google Scholar] [CrossRef]
- Dorado, M.; Ballesteros, E.; Arnal, J.; Gomez, J.; Lopez, F. Exhaust emissions from a Diesel engine fueled with transesterified waste olive oil. Fuel 2003, 82, 1311–1315. [Google Scholar] [CrossRef]


| Catalyst | Specific Surface Area (m3/g) | Grain Size (nm) |
|---|---|---|
| Fe3O4 | 24.8169 | 25.6254 |
| nano-Ca(OH)2/Fe3O4 | 34.7892 | 26.0871 |
| Acidity (mg KOH/g) | SV (mg KOH/g) | MW (g/mole) | Viscosity (mm2/s) | Density (kg/m3) | |
|---|---|---|---|---|---|
| Jatropha Oil | 2.10 | 195.12 | 861.01 | 38.10 | 921 |
| Castor Oil | 0.64 | 182.37 | 921.20 | 84.00 | 920 |
| Run No. | Methanol/Oil Ratio | Heating Temperature (°C) | Reaction Time (min) | Yield (wt %) |
|---|---|---|---|---|
| 1 | 9:1 | 60 | 30 | 83.4 |
| 2 | 9:1 | 60 | 40 | 85.3 |
| 3 | 9:1 | 70 | 30 | 91.6 |
| 4 | 9:1 | 70 | 40 | 88.9 |
| 5 | 15:1 | 60 | 30 | 81.2 |
| 6 | 15:1 | 60 | 40 | 92.4 |
| 7 | 15:1 | 70 | 30 | 93.5 |
| 8 | 15:1 | 70 | 40 | 77.8 |
| 9 | 2:1 | 65 | 35 | 0 |
| 10 | 22:1 | 65 | 35 | 24.1 |
| 11 | 12:1 | 10 | 35 | 0 |
| 12 | 12:1 | 120 | 35 | 8.2 |
| 13 | 12:1 | 65 | 6 | 0 |
| 14 | 12:1 | 65 | 65 | 43.8 |
| 15 | 12:1 | 65 | 35 | 94.8 |
| 16 | 12:1 | 65 | 35 | 95.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-L.; Lin, Y.-C.; Jhang, S.-R.; Cheng, W.L.; Chen, S.-C.; Mao, S.-Y. Rapid Jatropha-Castor Biodiesel Production with Microwave Heating and a Heterogeneous Base Catalyst Nano-Ca(OH)2/Fe3O4. Catalysts 2017, 7, 203. https://doi.org/10.3390/catal7070203
Chang K-L, Lin Y-C, Jhang S-R, Cheng WL, Chen S-C, Mao S-Y. Rapid Jatropha-Castor Biodiesel Production with Microwave Heating and a Heterogeneous Base Catalyst Nano-Ca(OH)2/Fe3O4. Catalysts. 2017; 7(7):203. https://doi.org/10.3390/catal7070203
Chicago/Turabian StyleChang, Ken-Lin, Yuan-Chung Lin, Syu-Ruei Jhang, Way Lee Cheng, Shang-Cyuan Chen, and Sung-Yuan Mao. 2017. "Rapid Jatropha-Castor Biodiesel Production with Microwave Heating and a Heterogeneous Base Catalyst Nano-Ca(OH)2/Fe3O4" Catalysts 7, no. 7: 203. https://doi.org/10.3390/catal7070203





