Cerium Coordination Polymer Based Composite Mimicking Peroxidase for Detection of Nitroaniline
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Pt Deposited Ce Coordination Polymer
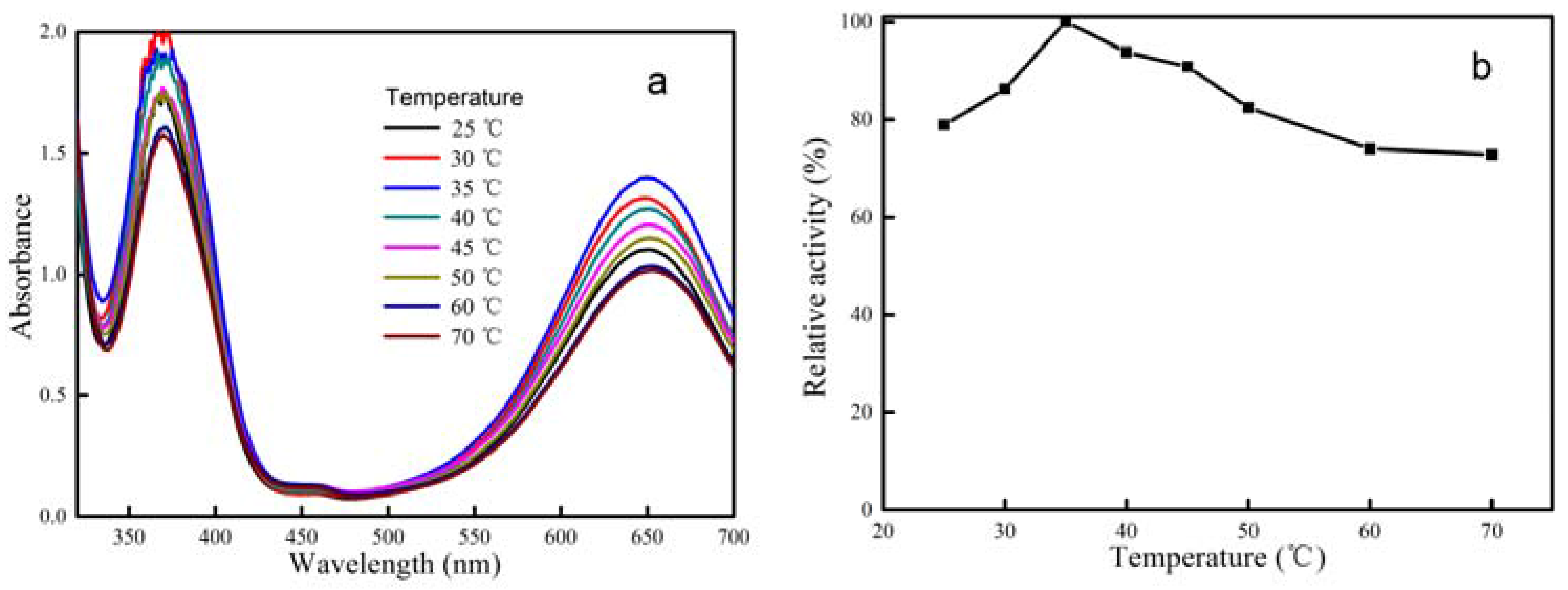
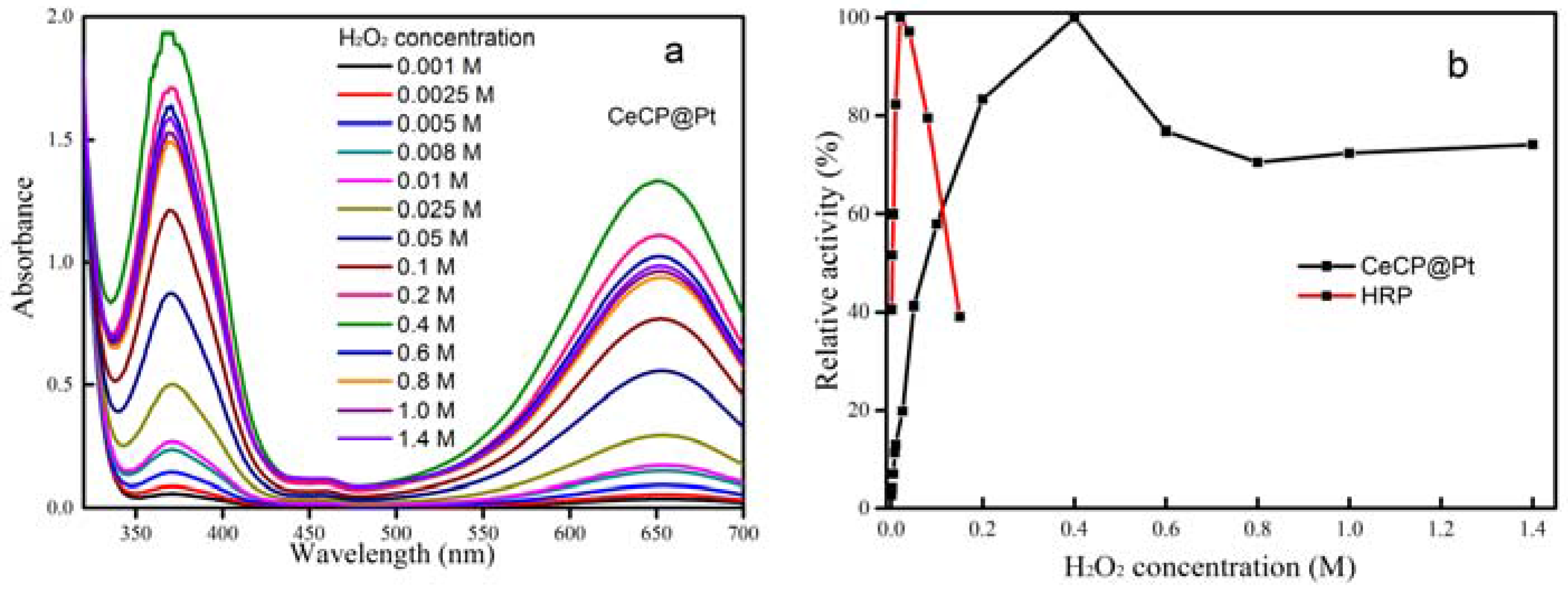
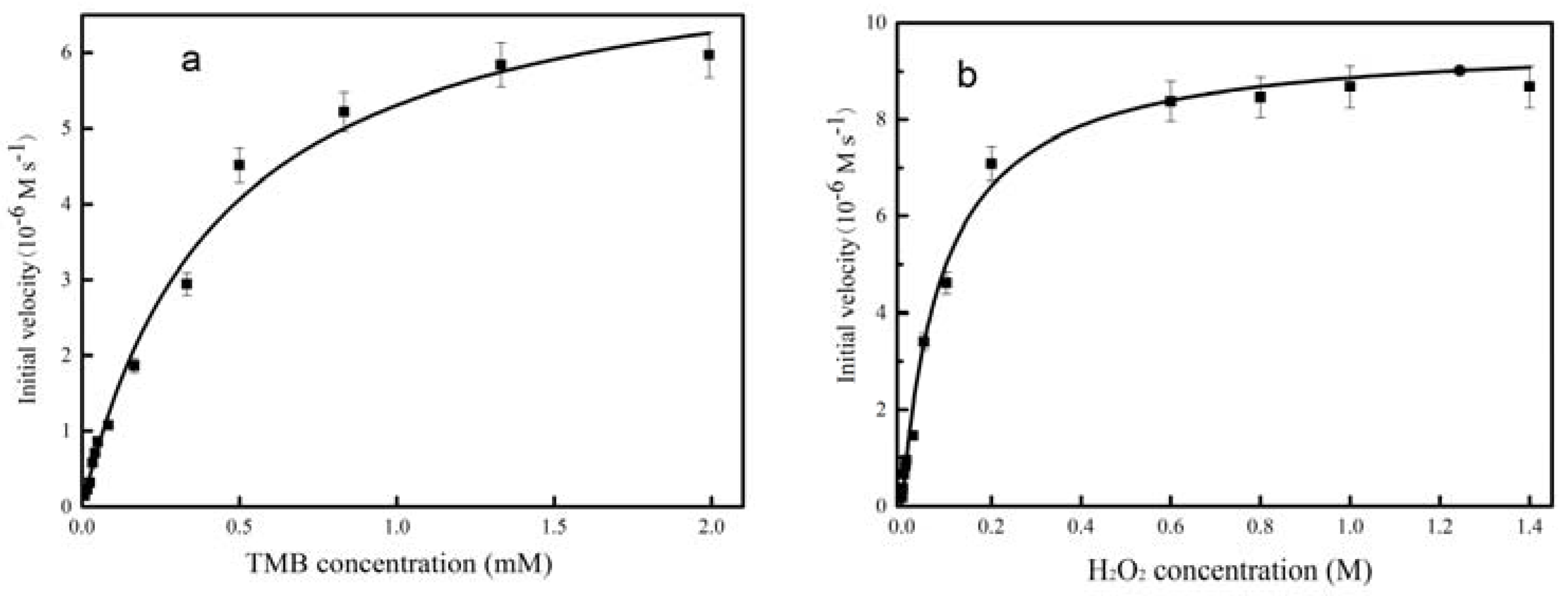
2.2. Peroxidase Activity of CeCP@Pt
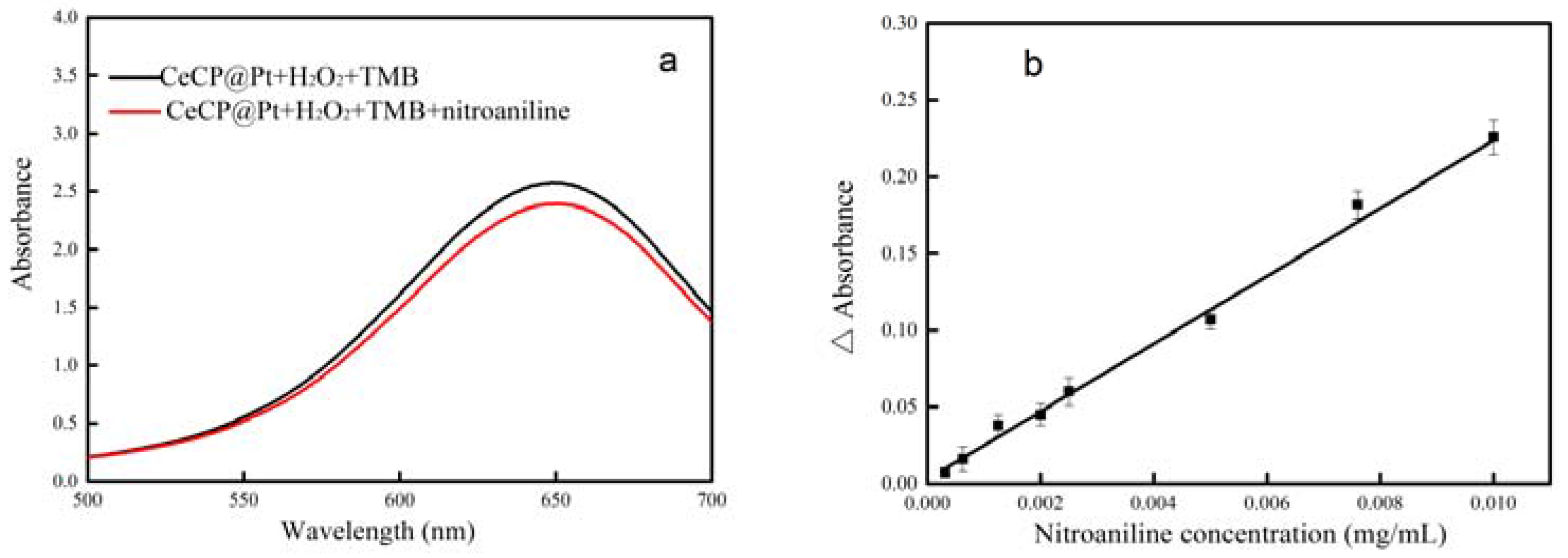
2.3. Detection of Nitroaniline by CeCP@Pt
3. Experimental Section
3.1. Materials
3.2. Preparation of CeCP@Pt
3.3. Measurement of Peroxidase Activity of CeCP@Pt
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, M.C.; Lee, D.; Jeong, S.H.; Lee, S.Y.; Kang, E. Nanodiamond–Gold Nanocomposites with the Peroxidase-Like Oxidative Catalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 34317–34326. [Google Scholar] [CrossRef] [PubMed]
- Woolridge, E.M. Mixed Enzyme Systems for Delignification of Lignocellulosic Biomass. Catalysts 2014, 4, 1–35. [Google Scholar] [CrossRef]
- Wang, S.; Cazelles, R.; Liao, W.C.; Vázquez-González, M.; Zoabi, A.; Abu-Reziq, R.; Willner, I. Mimicking Horseradish Peroxidase and NADH Peroxidase by Heterogeneous Cu2+-Modified Graphene Oxide Nanoparticles. Nano Lett. 2017, 17, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, J.; Lu, N.; Kim, M.J.; Ghale, K.; Xu, Y.; McKenzie, E.; Liu, J.; Ye, H. Pd–Ir Core–Shell Nanocubes: A Type of Highly Efficient and Versatile Peroxidase Mimic. ACS Nano 2015, 9, 9994–10004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J. Highly Dispersed CeO2 on TiO2 Nanotube: A Synergistic Nanocomposite with Superior Peroxidase-Like Activity. ACS Appl. Mater. Interfaces 2015, 7, 6451–6461. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, X.; Hu, J. Pt–DNA Complexes as Peroxidase Mimetics and Their Applications in Colorimetric Detection of H2O2 and Glucose. Anal. Methods 2012, 4, 2183–2187. [Google Scholar] [CrossRef]
- Tseng, C.W.; Chang, H.Y.; Chang, J.Y.; Huang, C.C. Detection of Mercury Ions Based on Mercury-Induced Switching of Enzyme-Like Activity of Platinum/Gold Nanoparticles. Nanoscale 2012, 4, 6823–6830. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Si, Y.; Zhang, N.; Sun, Z.; Wu, H.; Lin, Y. Platinum Nanocatalysts Loaded on Graphene Oxide Dispersed Carbon Nanotubes with Greatly Enhanced Peroxidase-Like Catalysis and Electrocatalysis Activities. Nanoscale 2014, 6, 8107–8116. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Q.; Deng, H.H.; Wu, G.W.; Peng, H.P.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. Platinum Nanoparticles/Graphene-Oxide Hybrid with Excellent Peroxidase-Like Activity and its Application for Cysteine Detection. Analyst 2015, 140, 5251–5256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lv, L.; Zhang, G.; Zhou, X.; Shen, A.; Hu, J. Core–Shell Fructus Broussonetia-Like Au@Ag@Pt Nanoparticles as Highly Efficient Peroxidase Mimetics for Supersensitive Resonance-Enhanced Raman Sensing. Anal. Methods 2016, 8, 2097–2105. [Google Scholar] [CrossRef]
- Ju, Y.; Kim, J. Dendrimer-Encapsulated Pt Nanoparticles with Peroxidase-Mimetic Activity as Biocatalytic Labels for Sensitive Colorimetric Analyses. Chem. Commun. 2015, 51, 13752–13755. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, J.; Jiang, P.; Wang, G.; Wu, X.; Zhao, H.; Zhang, C. Superior Peroxidase Mimetic Activity of Carbon Dots–Pt Nanocomposites Relies on Synergistic Effects. New J. Chem. 2015, 39, 4141–4146. [Google Scholar] [CrossRef]
- Chau, L.Y.; He, Q.; Qin, A.; Yipand, S.P.; Lee, T.M.H. Platinum Nanoparticles on Reduced Graphene Oxide as Peroxidase Mimetics for the Colorimetric Detection of Specific DNA Sequence. J. Mater. Chem. B 2016, 4, 4076–4083. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Song, Y. Antibody-Free Detection of Protein Phosphorylation Using Intrinsic Peroxidase-Like Activity of Latinum/Carbon Dot Hybrid Nanoparticles. Chem. Commun. 2016, 52, 7994–7997. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Jiang, Y.; Chi, M.; Nie, G.; Lu, X.; Wang, C. Palladium Nanoparticles Modified Electrospun CoFe2O4 Nanotubes with Enhanced Peroxidase-Like Activity for Colorimetric Detection of Hydrogen Peroxide. RSC Adv. 2016, 6, 33636–33642. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.L.; Ricchetti, L.B.; Pittman, C.U.; Anderson, R.; Mlsna, T.E. Rapid Removal of Salicylic Acid, 4-Nitroaniline, Benzoic Acid and Phthalic Acid from Wastewater Using Magnetized Fast Pyrolysis Biochar from Waste Douglas Fir. Chem. Eng. J. 2017, 319, 75–88. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, L.; Lee, H.K. Analysis of Aromatic Amines in Water Samples by Liquid-Liquid-Liquid Microextraction with HollowFibers and High-Performance Liquid Chromatography. J. Chromatogr. A 2002, 963, 239–248. [Google Scholar] [CrossRef]
- Tong, C.; Guo, Y.; Liu, W. Simultaneous Determination of Five Nitroaniline and Dinitroaniline Isomers in Wastewaters by Solid-Phase Extraction and High-Performance Liquid Chromatography with Ultraviolet Detection. Chemosphere 2010, 81, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, F.; Lin, X.; Wang, C.; Fu, Y.; Wang, X.; Zhao, Y.; Li, G. Selective Fluorescence Sensors for Detection of Nitroaniline and Metal Ions Based on Ligand-Based Luminescent Metal-Organic Frameworks. J. Solid State Chem. 2015, 232, 96–101. [Google Scholar] [CrossRef]
- Ezugwu, C.I.; Mousavi, B.; Asraf, M.A.; Luo, Z.; Verpoort, F. Post-Synthetic Modified MOF for Sonogashira Cross-Coupling and Knoevenagel Condensation Reactions. Chem. Eng. J. 2016, 300, 177–184. [Google Scholar] [CrossRef]
- Chen, J.; Yu, C.; Zhao, Y.; Niu, Y.; Zhang, L.; Yu, Y.; Wu, J.; He, J. A Novel Non-Invasive Detection Method for the FGFR3 Gene Mutation in Maternal Plasma for a Fetal Achondroplasia Diagnosis Based on Signal Amplification by Hemin-MOFs/PtNPs. Biosens. Bioelectron. 2017, 91, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, J.; Ni, Y.; Men, W.; Tagami, K.; Uchida, S. High-Performance Method for Determination of Pu Isotopes in Soil and Sediment Samples by Sector Field-Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2017, 89, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dun, K.; Song, X.; Gao, Q.; Wu, H.; Ma, J.; Ji, P.; Feng, W. Specific Immobilization of d-amino Acid Oxidase Mimicking Multi-Enzyme Catalysis. Green Chem. 2015, 17, 4465–4472. [Google Scholar] [CrossRef]
- Su, L.; Feng, J.; Zhou, X. Colorimetric Detection of Urine Glucose Based ZnFe2O4 Magnetic Nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, M.; Li, H. Irregular-Shaped Platinum Nanoparticles as Peroxidase Mimics for Highly Efficient Colorimetric Immunoassay. Anal. Chim. Acta 2013, 776, 79–86. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Y.; Yuan, J. Au@Pt Nanostructures as Oxidase and Peroxidase Mimetics for Use in Immunoassays. Biomaterials 2011, 32, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Siyo, B.; Schneider, M.; Pohl, M.M. Synthesis, Characterization, and Application of PVP-Pd NP in the Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF). Catal. Lett. 2014, 144, 498–506. [Google Scholar] [CrossRef]
- Deshmukh, A.; Bandyopadhyay, S.; James, A.; Patra, A. Trace level detection of nitroanilines using a solution processable fluorescent porous organic polymer. J. Mater. Chem. C 2016, 4, 4427–4433. [Google Scholar] [CrossRef]
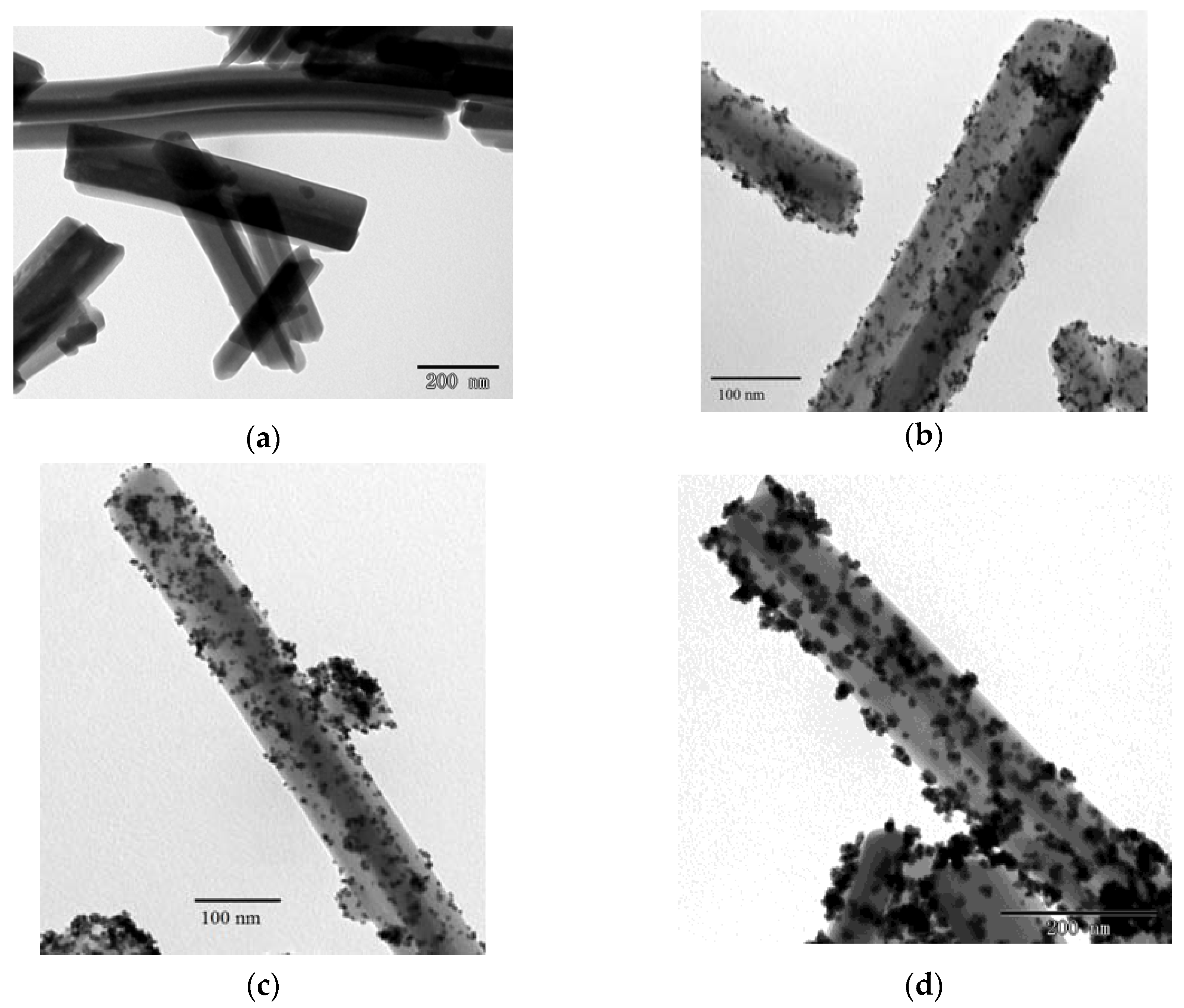
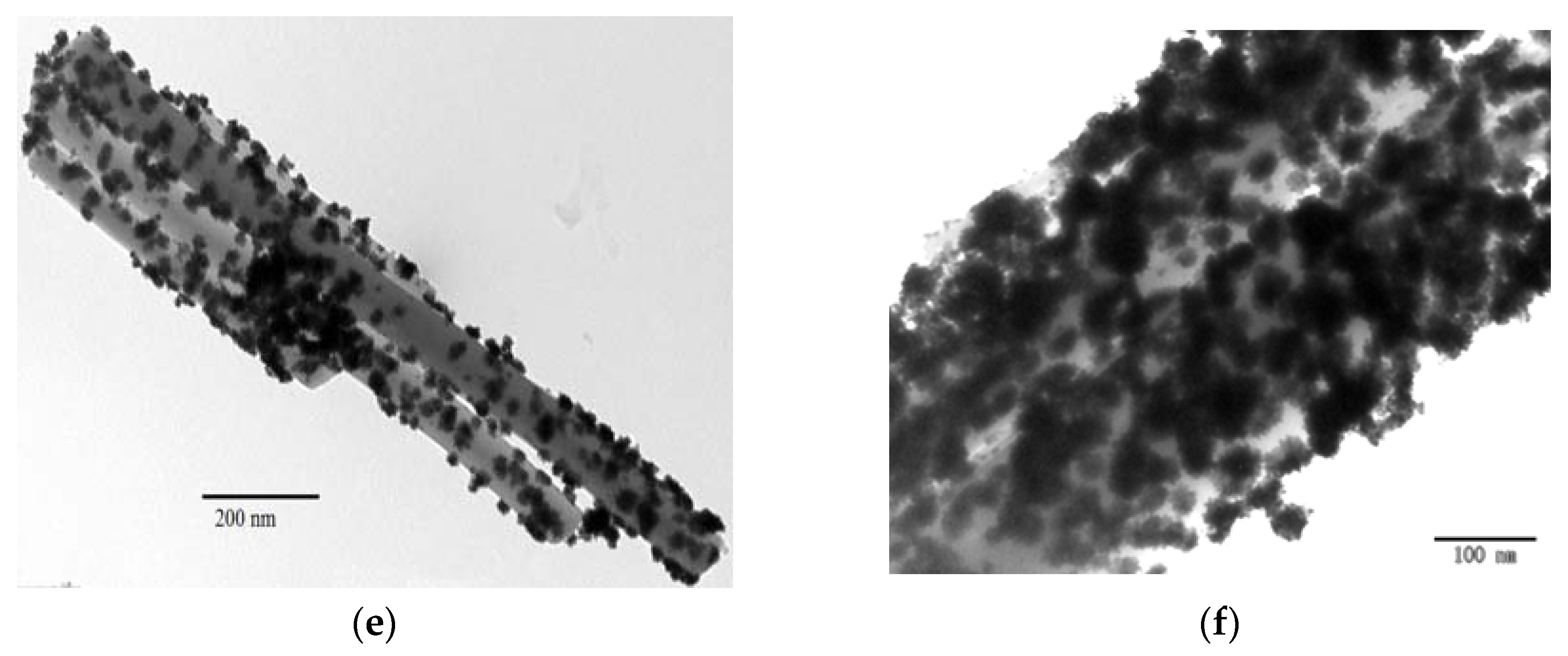
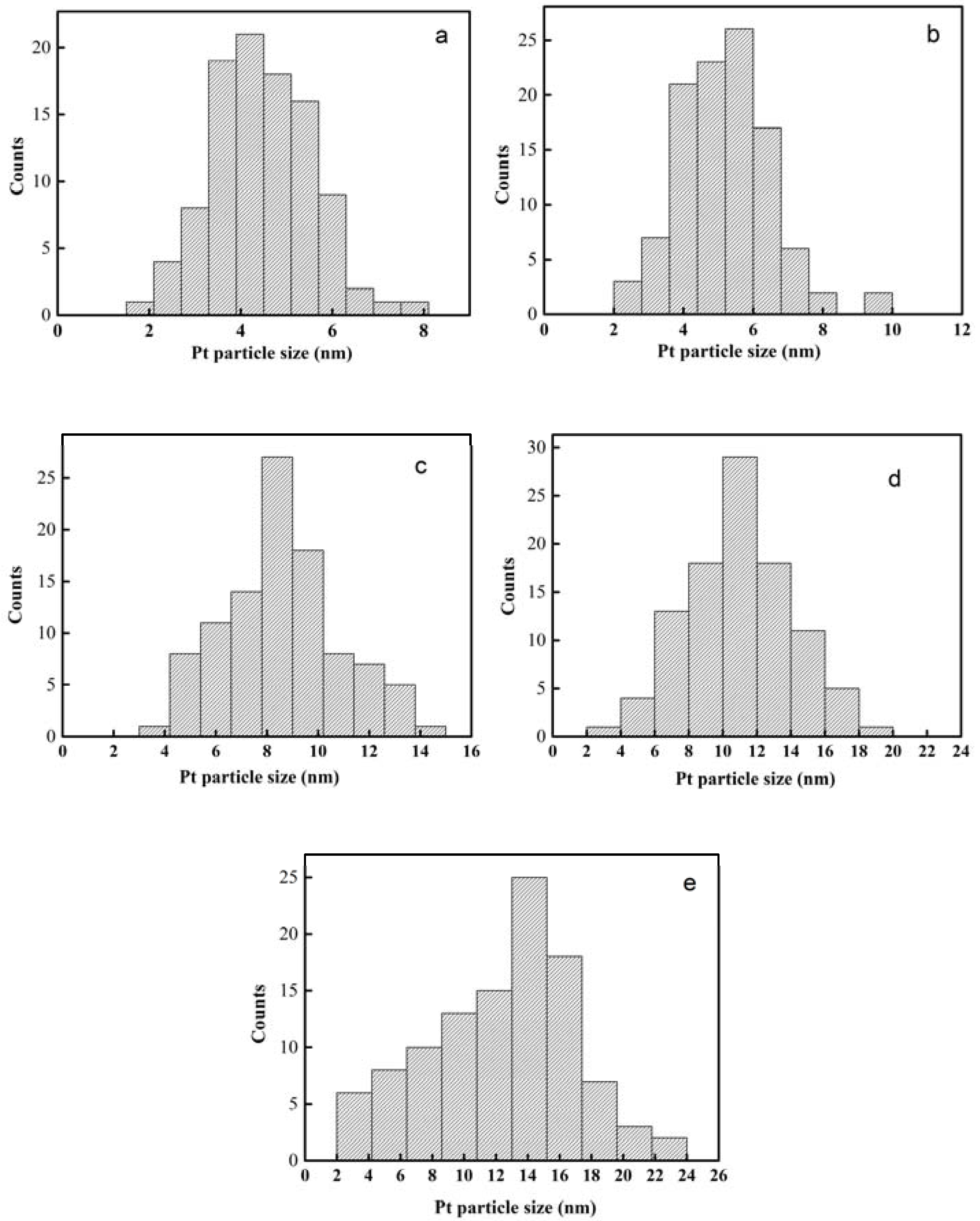
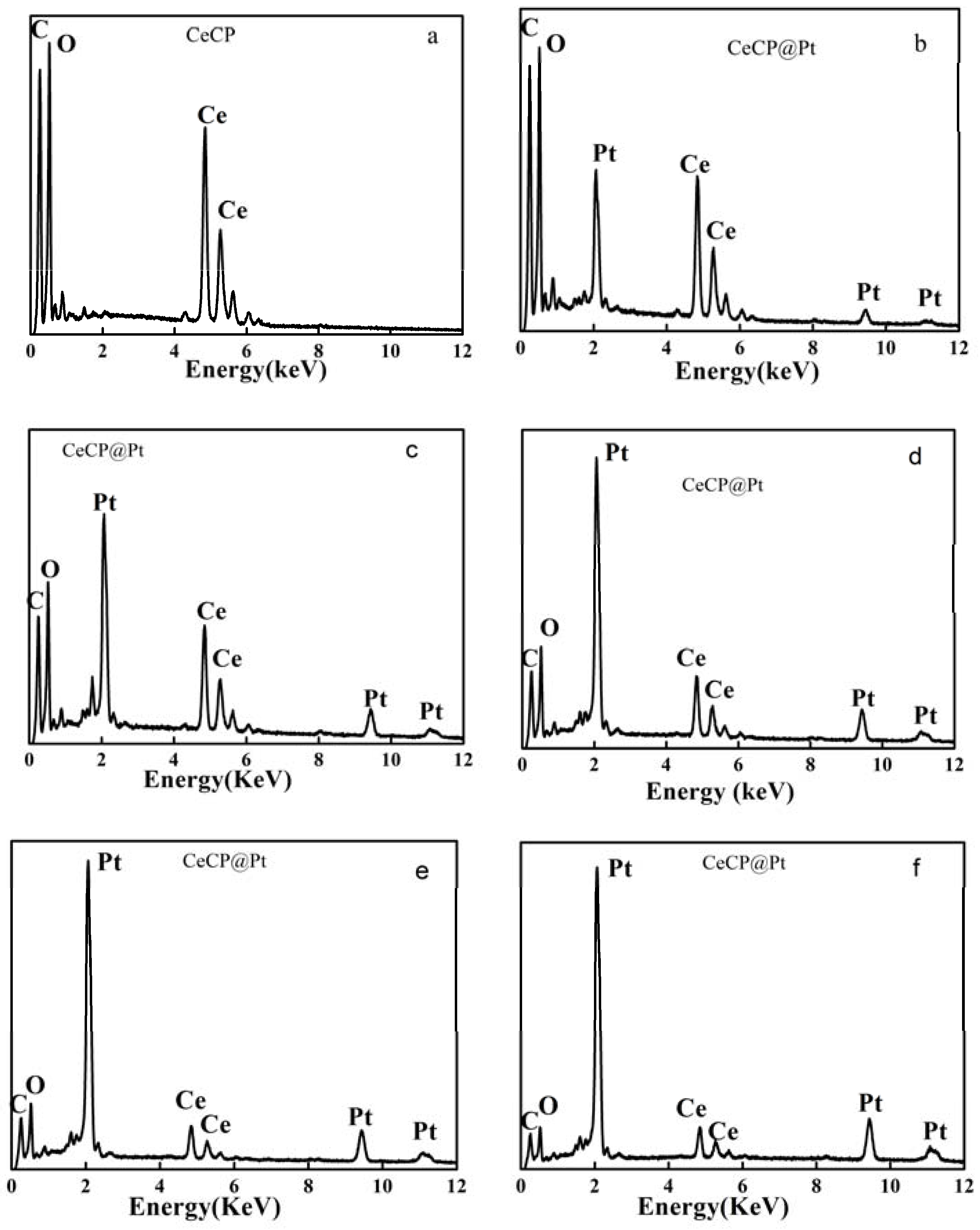
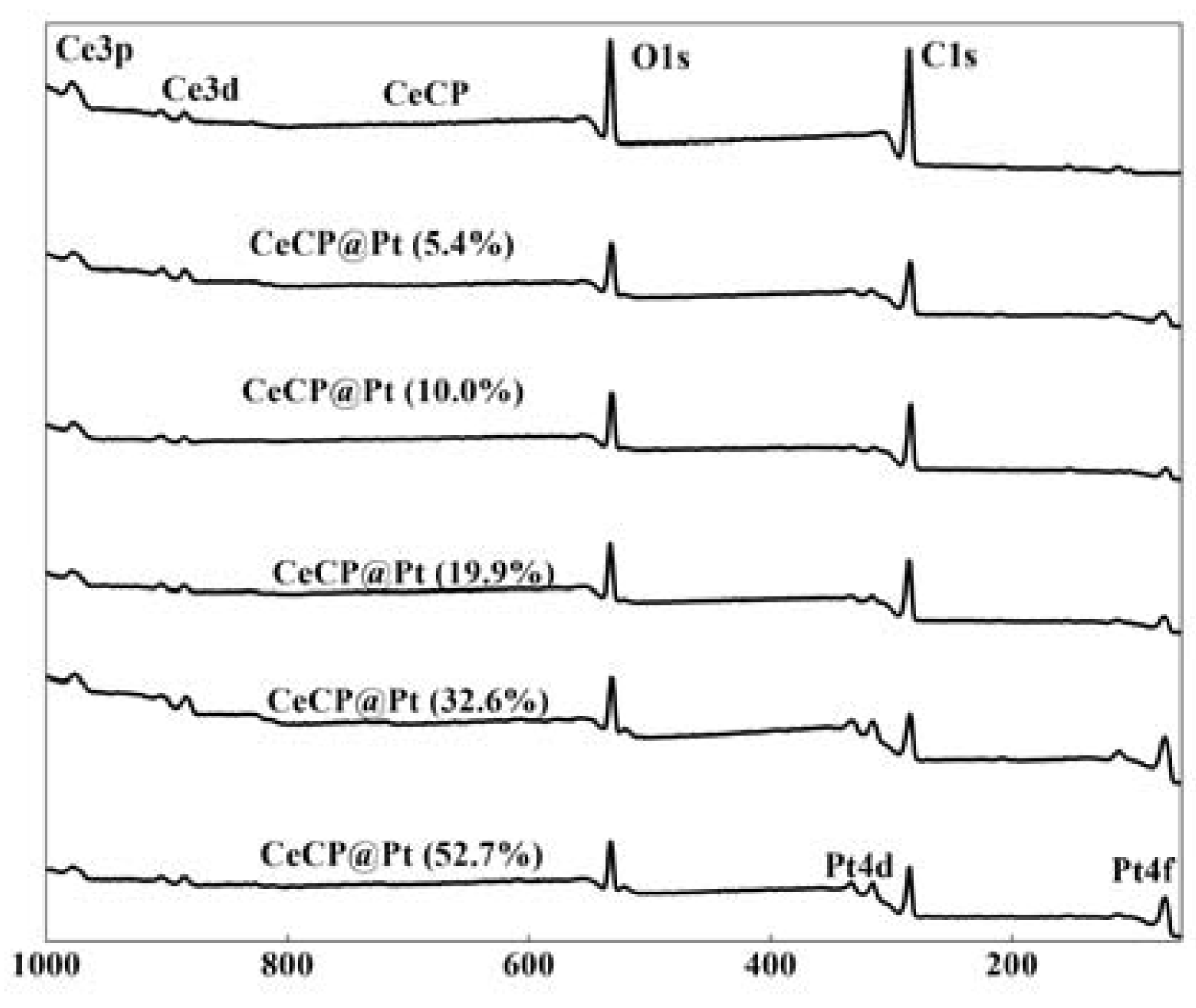

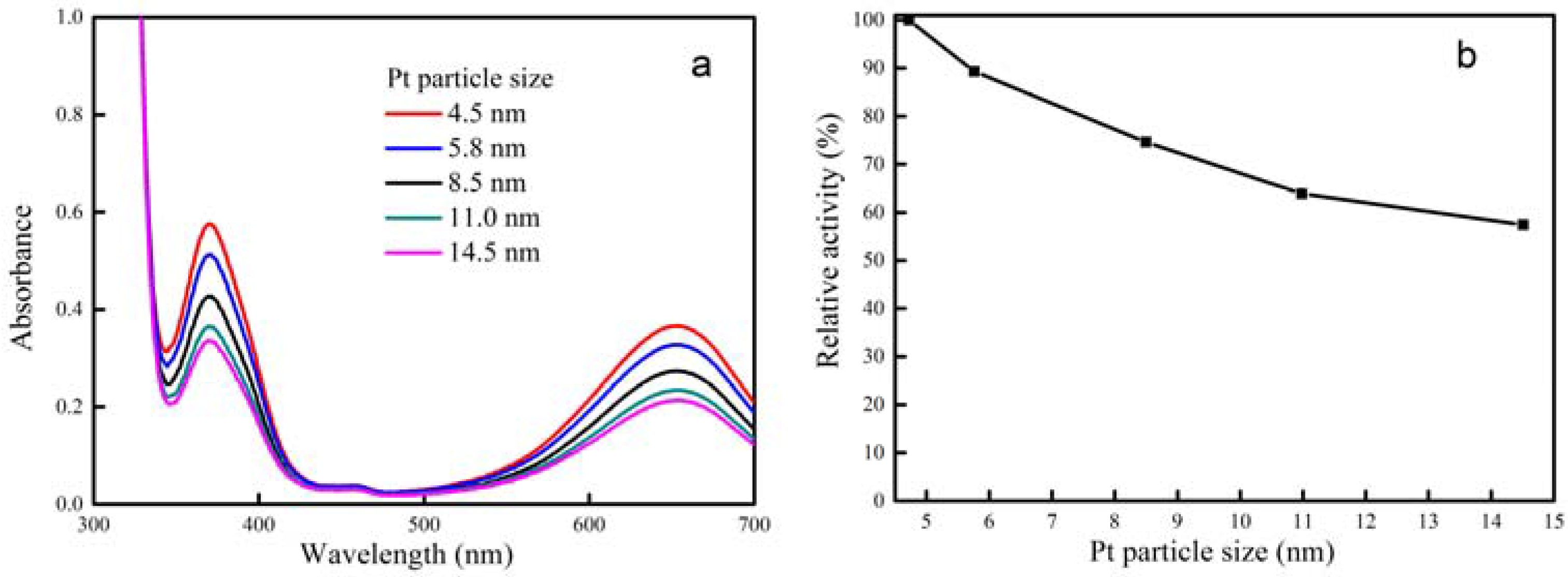

| Percentage of Pt Loaded (%) | Average Size of Pt Particle (nm) |
|---|---|
| 5.4 | 4.5 |
| 10 | 5.8 |
| 19.9 | 8.5 |
| 32.6 | 11.0 |
| 52.7 | 14.5 |
| Catalyst | Substrate | Km [mM] | Vmax [Ms−1] | Kcat [s−1] | Refrences |
|---|---|---|---|---|---|
| Au@Pd particles | TMB | 0.17 | 2.00 × 10−6 | 2.1 × 104 | [6] |
| H2O2 | 1100 | 4.40 × 10−6 | 4.6 × 104 | [6] | |
| HRP | TMB | 0.434 | 10 × 10−8 | 0.4 × 104 | [25] |
| H2O2 | 3.70 | 8.71 × 10−8 | 0.348 × 104 | [25] | |
| Fe3O4 particles | TMB | 0.098 | 3.4 × 10−8 | 3.0 × 104 | [25] |
| H2O2 | 150 | 9.8 × 10−8 | 8.6 × 104 | [25] | |
| Pt particles | TMB | 1.2 | 1.30 × 10−6 | 2.3 × 104 | [26] |
| H2O2 | 770 | 1.9 × 10−6 | 1.6 × 104 | [26] | |
| Pd-Ir cubes | TMB | 0.13 | 6.5 × 10−8 | 1.90 × 106 | [27] |
| H2O2 | 340 | 5.1 × 10−8 | 1.50 × 106 | [27] | |
| ZnFe2O4 | TMB | 0.85 | 1.33 × 10−7 | 4.36 × 1010 | [28] |
| H2O2 | 1.66 | 7.74 × 10−8 | 2.54 × 1010 | [28] | |
| CeCP@Pt | TMB | 0.4585 | 7.71 × 10−6 | 1.19 × 109 | This work |
| H2O2 | 92.7 | 9.69 × 10−6 | 0.947 × 109 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zheng, K.; Ji, P. Cerium Coordination Polymer Based Composite Mimicking Peroxidase for Detection of Nitroaniline. Catalysts 2017, 7, 206. https://doi.org/10.3390/catal7070206
Wang X, Zheng K, Ji P. Cerium Coordination Polymer Based Composite Mimicking Peroxidase for Detection of Nitroaniline. Catalysts. 2017; 7(7):206. https://doi.org/10.3390/catal7070206
Chicago/Turabian StyleWang, Xi, Kunkun Zheng, and Peijun Ji. 2017. "Cerium Coordination Polymer Based Composite Mimicking Peroxidase for Detection of Nitroaniline" Catalysts 7, no. 7: 206. https://doi.org/10.3390/catal7070206




