Newly Designed Ternary Metallic PtPdBi Hollow Catalyst with High Performance for Methanol and Ethanol Oxidation
Abstract
:1. Introduction
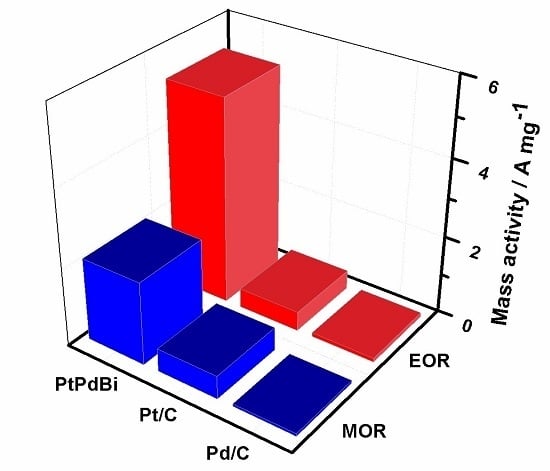
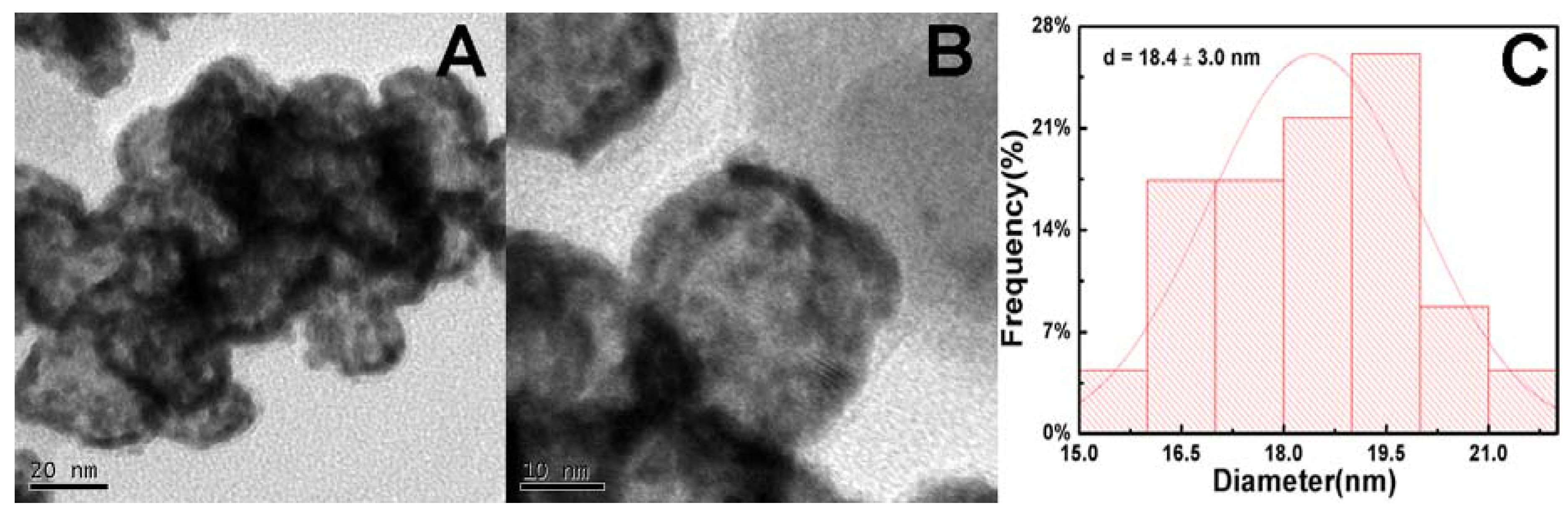
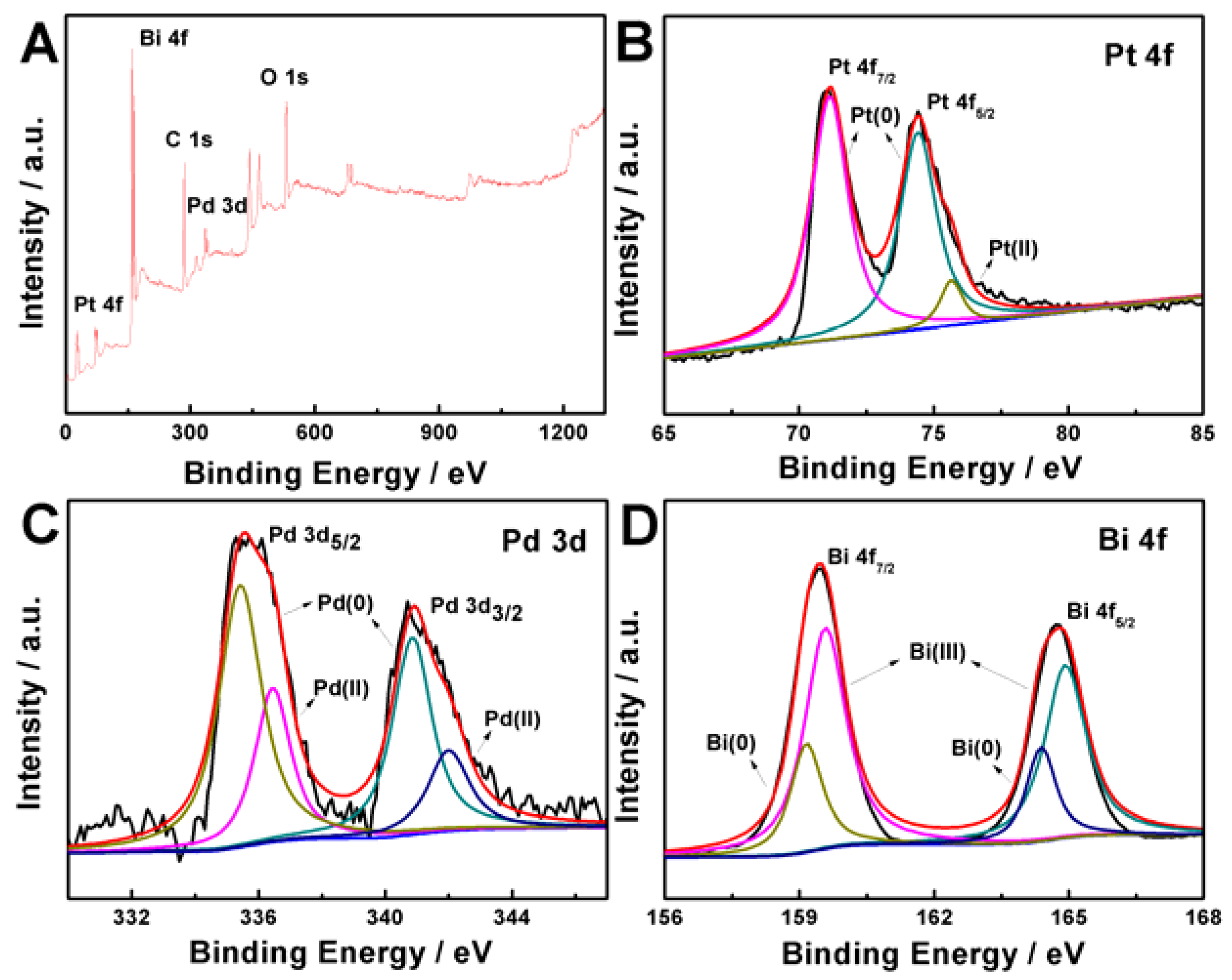
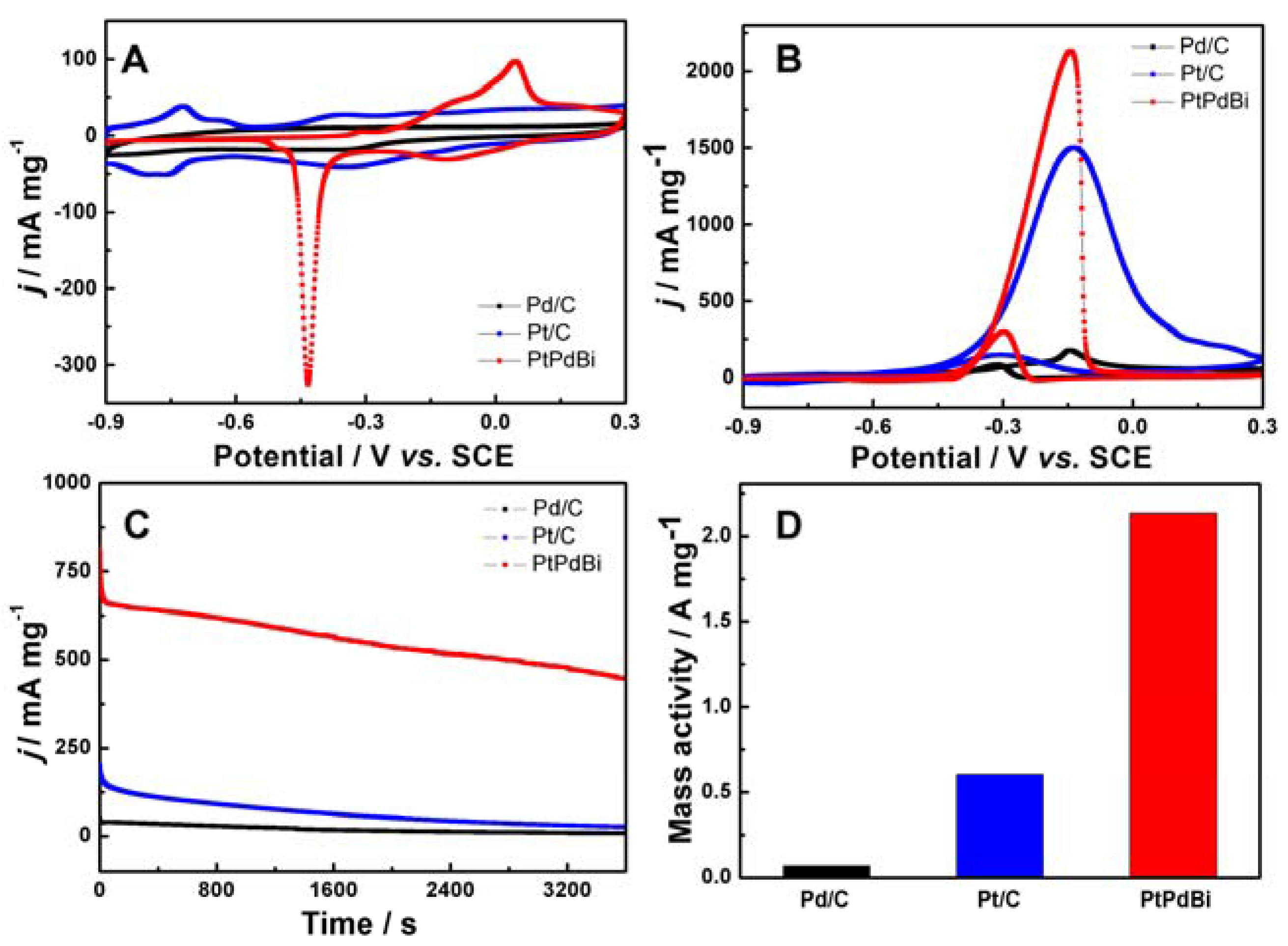
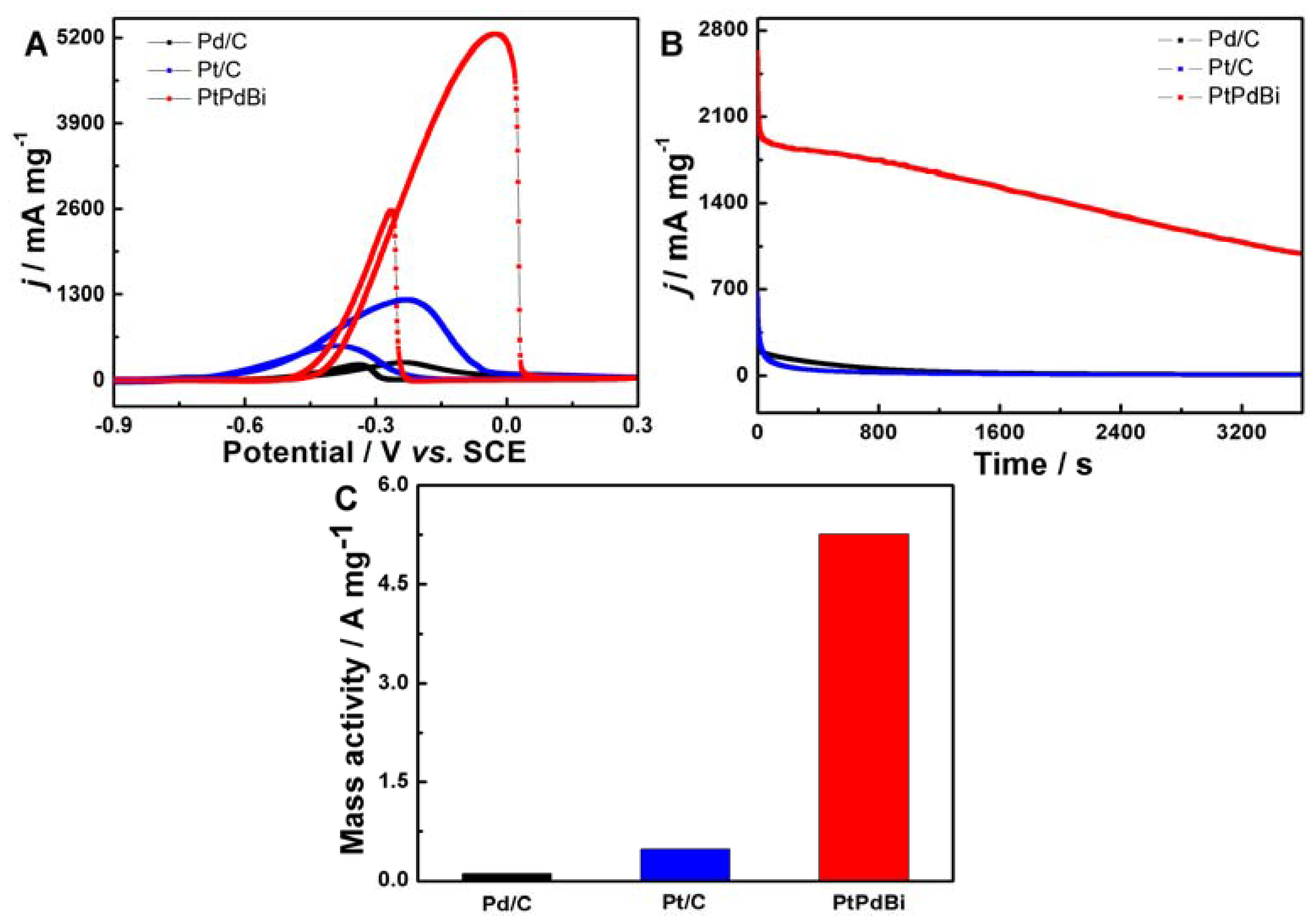
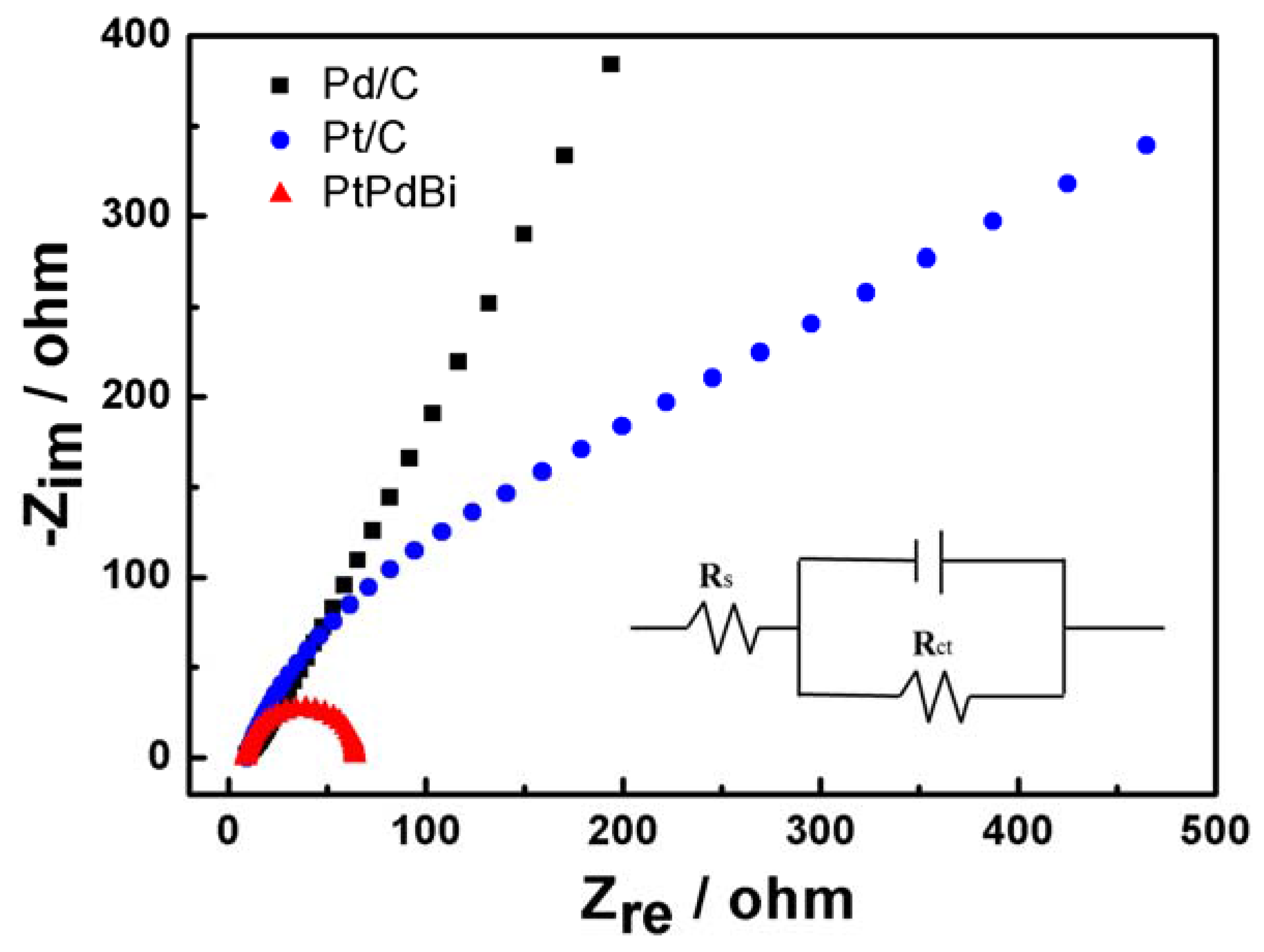
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of PtPdBi NPs
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, N.; Guo, S.; Zhu, X.; Guo, J.; Huang, X. Hierarchical Pt/PtxPb core/shell nanowires as efficient catalysts for electrooxidation of liquid fuels. Chem. Mater. 2016, 28, 4447–4452. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-M.; Gao, X.-Q.; Li, S.-N.; Chen, Y.; Lee, J.-M. Thermal decomposition synthesis of functionalized PdPt alloy nanodendrites with high selectivity for oxygen reduction reaction. NPG Asia Mater. 2015, 7, e219. [Google Scholar] [CrossRef]
- Hong, J.W.; Kang, S.W.; Choi, B.S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled synthesis of Pd-Pt alloy hollow nanostructures with enhanced catalytic activities for oxygen reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yue, R.; Wang, H.; Zou, C.; Du, J.; Jiang, F.; Du, Y.; Yang, P.; Wang, C. Dendritic Ag@Pt core-shell catalyst modified with reduced graphene oxide and titanium dioxide: Fabrication, characterization, and its photo-electrocatalytic performance. Int. J. Hydrogen Energy 2014, 39, 5764–5771. [Google Scholar] [CrossRef]
- Lim, E.J.; Kim, Y.; Choi, S.M.; Lee, S.; Noh, Y.; Kim, W.B. Binary PdM catalysts (M = Ru, Sn, or Ir) over a reduced graphene oxide support for electro-oxidation of primary alcohols (methanol, ethanol, 1-propanol) under alkaline conditions. J. Mater. Chem. A 2015, 3, 5491–5500. [Google Scholar] [CrossRef]
- Mao, J.; Liu, Y.; Chen, Z.; Wang, D.; Li, Y. Bimetallic Pd-Cu nanocrystals and their tunable catalytic properties. Chem. Commun. 2014, 50, 4588–4591. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Zhang, Y.; Gong, M.-X.; Tang, Y.-W.; Lu, T.-H.; Chen, Y.; Lee, J.-M. Facile synthesis of corallite-like Pt–Pd alloy nanostructures and their enhanced catalytic activity and stability for ethanol oxidation. J. Mater. Chem. A 2014, 2, 13840. [Google Scholar] [CrossRef]
- Li, S.; Lai, J.; Luque, R.; Xu, G. Designed multimetallic Pd nanosponges with enhanced electrocatalytic activity for ethylene glycol and glycerol oxidation. Energy Environ. Sci. 2016, 9, 3097–3102. [Google Scholar] [CrossRef]
- Mao, J.; Cao, T.; Chen, Y.; Wu, Y.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Seed-mediated synthesis of hexameric octahedral PtPdCu nanocrystals with high electrocatalytic performance. Chem. Commun. 2015, 51, 15406–15409. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Ping, Y.; Yan, J.-M.; Wang, H.-L.; Wu, M.; Jiang, Q. Facile synthesis of AgAuPd/graphene with high performance for hydrogen generation from formic acid. J. Mater. Chem. A 2015, 3, 14535–14538. [Google Scholar] [CrossRef]
- Saleem, F.; Ni, B.; Yong, Y.; Gu, L.; Wang, X. Ultra-small tetrametallic Pt-Pd-Rh-Ag nanoframes with tunable behavior for direct formic acid/methanol oxidation. Small 2016, 12, 5261–5268. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Y.; Wu, H.; Chen, W. Nano-PtPd cubes on graphene exhibit enhanced activity and durability in methanol electrooxidation after CO stripping-cleaning. J. Phys. Chem. C 2013, 117, 2926–2938. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.Y.; Die, L.; Zhang, X.H.; Fan, Z.; Chen, J.H. Carbon nanotubes supported PtPd hollow nanospheres for formic acid electrooxidation. J. Power Sources 2009, 186, 62–66. [Google Scholar] [CrossRef]
- Xu, H.; Yan, B.; Zhang, K.; Wang, C.; Zhong, J.; Li, S.; Yang, P.; Du, Y. Facile synthesis of Pd-Ru-P ternary nanoparticle networks with enhanced electrocatalytic performance for methanol oxidation. Int. J. Hydrogen Energy 2017, 42, 11229–11238. [Google Scholar] [CrossRef]
- Gu, Z.; Bin, D.; Feng, Y.; Zhang, K.; Wang, J.; Yan, B.; Li, S.; Xiong, Z.; Wang, C.; Shiraishi, Y.; et al. Seed-mediated synthesis of cross-linked Pt-NiO nanochains for methanol oxidation. Appl. Surf. Sci. 2017, 411, 379–385. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, J.; Hou, Y. Synthesis and electrocatalytic properties of PtBi nanoplatelets and PdBi nanowires. Nanoscale 2014, 6, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.O.; Tusi, M.M.; de Oliveira Polanco, N.S.; da Silva, S.G.; Coelho dos Santos, M.; Spinacé, E.V. PdBi/C electrocatalysts for ethanol electro-oxidation in alkaline medium. Int. J. Hydrogen Energy 2011, 36, 10522–10526. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Holden, R.; Zhang, L.; Zhang, J.; Botton, G.A.; Couillard, M.; Nazar, L.F. Nanocrystalline intermetallics on mesoporous carbon for direct formic acid fuel cell anodes. Nat. Chem. 2010, 2, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sato, T.; Yamauchi, Y. Electrochemical synthesis of one-dimensional mesoporous Pt nanorods using the assembly of surfactant micelles in confined space. Angew. Chem. 2013, 52, 8050–8053. [Google Scholar] [CrossRef] [PubMed]
- Bin, D.; Yang, B.; Zhang, K.; Wang, C.; Wang, J.; Zhong, J.; Feng, Y.; Guo, J.; Du, Y. Design of PdAg hollow nanoflowers through galvanic replacement and their application for ethanol electrooxidation. Chem. Eur. J. 2016, 22, 16642–16647. [Google Scholar] [CrossRef] [PubMed]
- Keng, P.Y.; Kim, B.Y.; Shim, I.B.; Sahoo, R.; Veneman, P.E.; Armstrong, N.R.; Yoo, H.; Pemberton, J.E.; Bull, M.M.; Griebel, J.J.; et al. Colloidal polymerization of polymer-coated ferromagnetic nanoparticles into cobalt oxide nanowires. ACS Nano 2009, 3, 3143–3157. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Han, S.; Sanedrin, R.J.; Galvez, C.; Ho, D.G.; Hernandez, B.; Zhou, F.; Selke, M. Formation of cobalt oxide nanotubes: Effect of intermolecular hydrogen bonding between Co(III) complex precursors incorporated onto colloidal templates. Nano Lett. 2002, 2, 289–293. [Google Scholar] [CrossRef]
- Yoo, E.; Okata, T.; Akita, T.; Kohyama, M.; Makamura, J.; Honma, I. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Daimon, H.; Lee, Y.; Kim, J.; Sun, S. Synthesis of monodisperse Pt nanocubes and their enhanced catalysis for oxygen reduction. J. Am. Chem. Soc. 2007, 129, 6974–6975. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, Y.; He, H.; Wang, J.; Zhou, W.; Abruna, H.D. Template-free synthesis of hollow structured Co3O4 nanoparticles as high-performance anodes for lithium-ion batteries. ACS Nano 2015, 9, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.; Tuysuz, H.; Duyckaerts, N.; Knossalla, J.; Wang, G.H.; Schuth, F. Hollow nano- and microstructures as catalysts. Chem. Rev. 2016, 116, 14056–14119. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, C.; Chen, L.; Chen, S. Facile dicyandiamide-mediated fabrication of well-defined CuO hollow microspheres and their catalytic application. Mater. Chem. Phys. 2010, 120, 296–301. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Rodrigues, T.S.; Taguchi, L.S.K.; Fajardo, H.V.; Balzer, R.; Probst, L.F.D.; Camargo, P.H.C. Pd-based nanoflowers catalysts: Controlling size, composition, and structures for the 4-nitrophenol reduction and BTX oxidation reactions. J. Mater. Sci. 2015, 51, 603–614. [Google Scholar] [CrossRef]
- Huang, L.; Han, Y.; Dong, S. Highly-branched mesoporous Au-Pd-Pt trimetallic nanoflowers blooming on reduced graphene oxide as an oxygen reduction electrocatalyst. Chem. Commun. 2016, 52, 8659–8662. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Wang, H.; Malgras, V.; Alothman, Z.A.; Yamauchi, Y.; Wang, L. Trimetallic PtPdRu dendritic nanocages with three-dimensional electrocatalytic surfaces. J. Phys. Chem. C 2015, 119, 19947–19953. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Ding, K.; Wu, P.; Abbas, S.C.; Ghausi, M.A.; Zhang, T.; Wang, Y. Newly designed PdRuBi/N-Graphene catalysts with synergistic effects for enhanced ethylene glycol electro-oxidation. Electrochim. Acta 2016, 191, 940–945. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Y.-T.; Zhao, B.; Wang, T.; Zhang, K.; Yuen, M.M.F.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Urchin-like Pd@CuO–Pd yolk–shell nanostructures: Synthesis, characterization and electrocatalysis. J. Mater. Chem. A 2015, 3, 13653–13661. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, K.; Yan, B.; Wang, J.; Wang, C.; Li, S.; Gu, Z.; Du, Y.; Yang, P. Ultra-uniform PdBi nanodots with high activity towards formic acid oxidation. J. Power Sources 2017, 356, 27–35. [Google Scholar] [CrossRef]
- Łukaszewski, M. Electrochemical methods of real surface area determination of noble metal electrodes—An overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Ren, F.; Zhai, C.; Zhu, M.; Wang, C.; Wang, H.; Bin, D.; Guo, J.; Yang, P.; Du, Y. Facile synthesis of PtAu nanoparticles supported on polydopamine reduced and modified graphene oxide as a highly active catalyst for methanol oxidation. Electrochim. Acta 2015, 153, 175–183. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Li, S.; Xu, H.; Zhang, K.; Yan, B.; Du, Y. Newly Designed Ternary Metallic PtPdBi Hollow Catalyst with High Performance for Methanol and Ethanol Oxidation. Catalysts 2017, 7, 208. https://doi.org/10.3390/catal7070208
Xiong Z, Li S, Xu H, Zhang K, Yan B, Du Y. Newly Designed Ternary Metallic PtPdBi Hollow Catalyst with High Performance for Methanol and Ethanol Oxidation. Catalysts. 2017; 7(7):208. https://doi.org/10.3390/catal7070208
Chicago/Turabian StyleXiong, Zhiping, Shumin Li, Hui Xu, Ke Zhang, Bo Yan, and Yukou Du. 2017. "Newly Designed Ternary Metallic PtPdBi Hollow Catalyst with High Performance for Methanol and Ethanol Oxidation" Catalysts 7, no. 7: 208. https://doi.org/10.3390/catal7070208