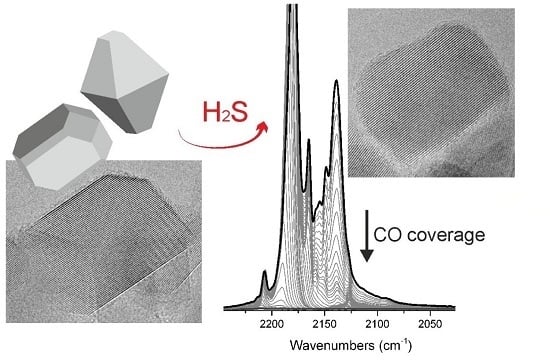
Sulfur-Doped TiO2: Structure and Surface Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology by XRD, Raman, and HRTEM Analyses
2.1.1. XRD Analysis
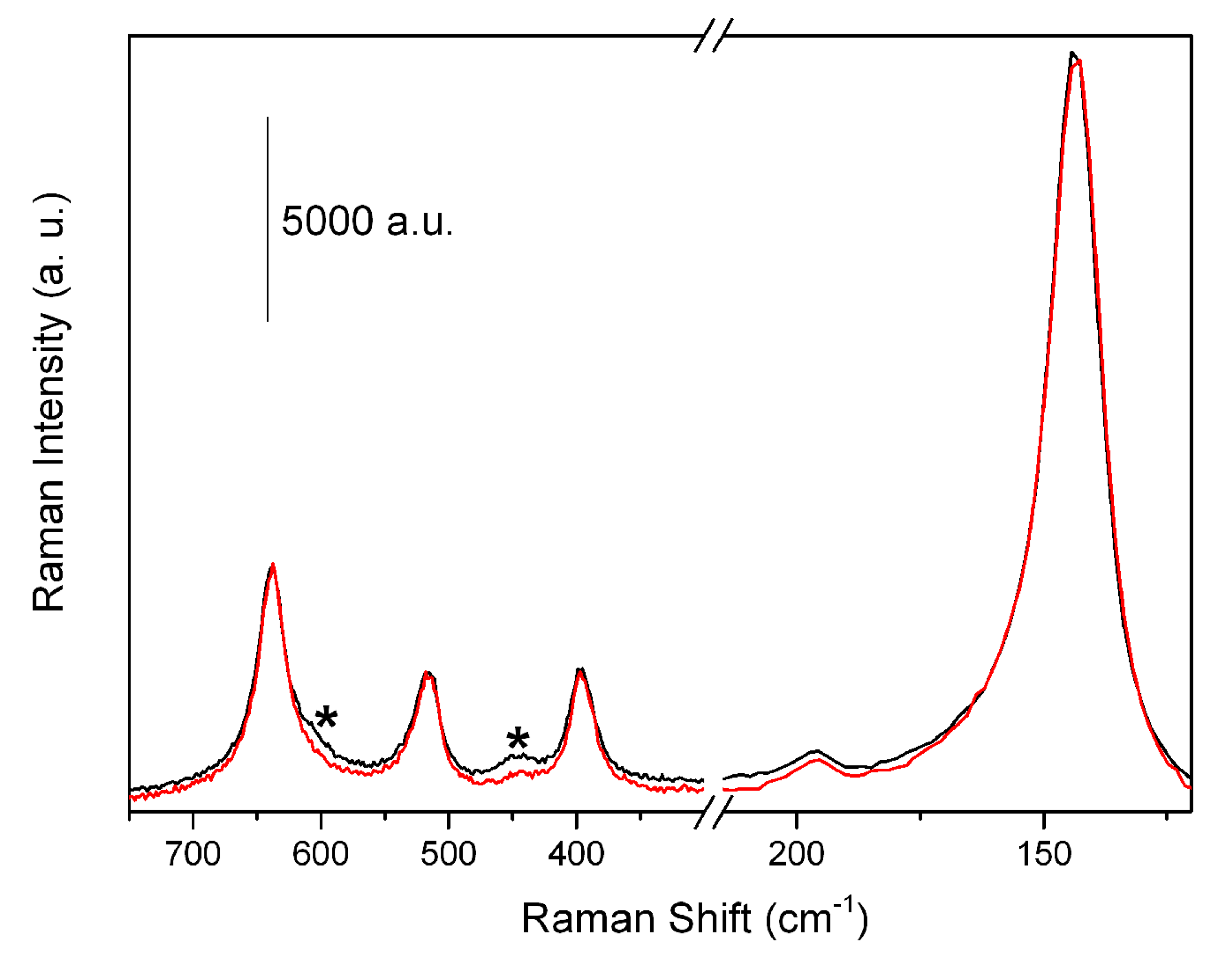
2.1.2. Raman Spectroscopy
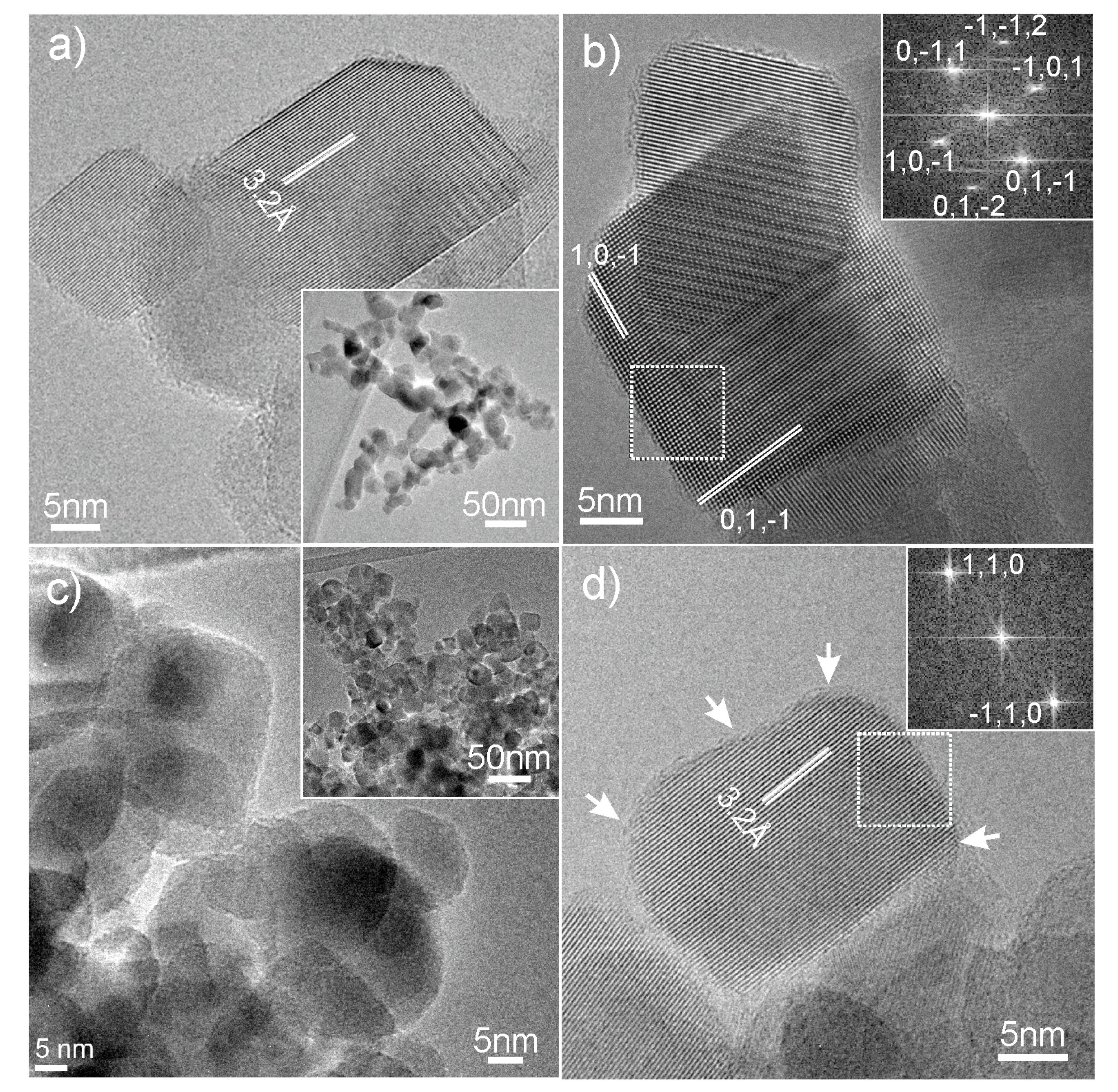
2.1.3. Surface Area and HRTEM Analysis
2.2. Surface Properties by FTIR and UV-Vis Spectroscopies
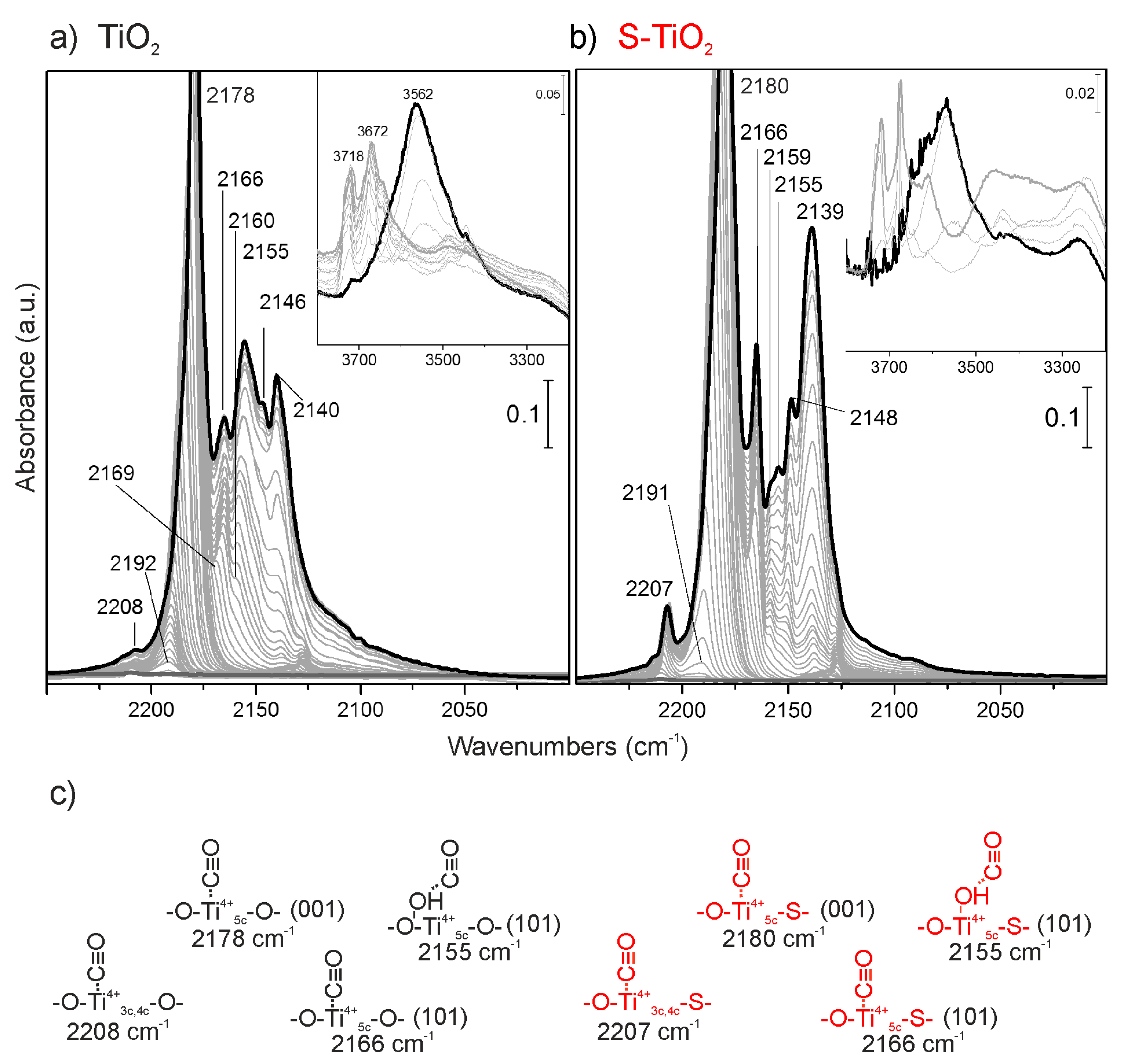
2.2.1. FTIR Spectroscopy
2.2.2. UV-Vis Spectroscopy
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Y.; Schmidt-Stein, F.; Baue, S.; Schmuki, P. Amphiphilic TiO2 nanotube arrays: An actively controllable drug delivery system. J. Am. Chem. Soc. 2009, 131, 4230–4232. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysioub, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Indoor air purification by photocatalyst TiO2 immobilized on an activated carbon filter installed in an air cleaner. Chem. Eng. Sci. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Cesano, F.; Pellerej, D.; Scarano, D.; Ricchiardi, G.; Zecchina, A. Radially organized pillars of TiO2 nanoparticles: Synthesis, characterization and photocatalytic tests. J. Photochem. Photob. A Chem. 2012, 242, 51–58. [Google Scholar] [CrossRef]
- Chatterjee, D.; Mahata, A. Visible light induced photo-degradation of organic pollutants on dye adsorbed TiO2 surface. J. Photochem. Photobiol. A 2002, 153, 199–204. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Carbon domains on MoS2/TiO2 system via catalytic acetylene oligomerization: Synthesis, structure and surface properties. Front. Chem. 2017. submitted. [Google Scholar]
- Cravanzola, S.; Muscuso, L.; Cesano, F.; Agostini, G.; Damin, A.; Scarano, D.; Zecchina, A. MoS2 nanoparticles decorating titanate-nanotube surfaces: Combined microscopy, spectroscopy, and catalytic studies. Langmuir 2015, 31, 5469–5478. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Cesano, F.; Bertarione, S.; Bonino, F.; Bordiga, S.; Scarano, D.; Zecchina, A. Tailoring the activity of Ti-based photocatalysts by playing with surface morphology and silver doping. J. Photochem. Photob. A Chem. 2008, 196, 165–173. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wang, Z.; Cai, W.; Lu, F.; Zhang, J.; Yang, Y.; Ma, N.; Liu, Y. Visible-light-activated nanoparticle photocatalyst of iodine-doped titanium dioxide. Chem. Mater. 2005, 17, 1548–1552. [Google Scholar] [CrossRef]
- Dozzi, M.V.; D’Andrea, C.; Ohtani, B.; Valentini, G.; Selli, E. Fluorine-doped TiO2 materials: Photocatalytic activity vs. time-resolved photoluminescence. J. Phys. Chem. C 2013, 117, 25586–25595. [Google Scholar] [CrossRef]
- Cravanzola, S.; Jain, S.M.; Cesano, F.; Damin, A.; Scarano, D. Development of a multifunctional TiO2/MWCNT hybrid composite grafted on a stainless steel grating. RSC Adv. 2015, 5, 103255–103264. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansaric, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Damin, A.; Agostini, G.; Usseglio, S.; Vitillo, J.G.; Lamberti, C.; Spoto, G.; Scarano, D.; Zecchina, A. Oriented TiO2 nanostructured pillar arrays: Synthesis and characterization. Adv. Mater. 2008, 20, 3342–3348. [Google Scholar] [CrossRef]
- Ramandi, S.; Entezari, M.H.; Ghows, N. Sono-synthesis of solar light responsive S–N–C–tri doped TiO2 photo-catalyst under optimized conditions for degradation and mineralization of diclofenac. Ultrason. Sonochem. 2017, 38, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Brindha, A.; Sivakumar, T. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol. A 2017, 340, 146–156. [Google Scholar] [CrossRef]
- Cesano, F.; Agostini, G.; Scarano, D. Nanocrystalline TiO2 micropillar arrays grafted on conductive glass supports: Microscopic and spectroscopic studies. Thin Solid Films 2015, 590, 200–206. [Google Scholar] [CrossRef]
- Uddin, M.J.; Daramola, D.E.; Velasquez, E.; Dickens, T.J.; Yan, J.; Hammel, E.; Cesano, F.; Okoli, O.I. A high efficiency 3D photovoltaic microwire with carbon nanotubes (CNT)-quantum dot (QD) hybrid interface. PSS RRL 2014, 8, 898–903. [Google Scholar] [CrossRef]
- Hui, F.; Bu, C. DFT description on electronic structure and optical absorption properties of anionic S-doped anatase TiO2. J. Phys. Chem. B 2006, 110, 17866–17871. [Google Scholar]
- Smith, M.F.; Setwong, K.; Tongpool, R.; Onkaw, D.; Naphattalung, S.; Limpijumnong, S.; Rujirawat, S. Identification of bulk and surface sulfur impurities in TiO2 by synchrotron X-ray absorption near edge structure. Appl. Phys. Lett. 2007, 91, 142107. [Google Scholar] [CrossRef]
- Liu, G.; Sun, C.; Smith, S.C.; Wang, L.; Lu, G.Q.; Cheng, H.-M. Sulfur doped anatase TiO2 single crystals with a high percentage of {0 0 1} facets. J. Colloid Interface Sci. 2010, 349, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-H.; Kim, T.K.; Boo, J.-H. Physical property and photo-catalytic activity of sulfur doped TiO2 catalysts responding to visible light. Catal. Today 2012, 185, 259–262. [Google Scholar] [CrossRef]
- Yang, G.; Yan, Z.; Xiao, T. Low-temperature solvothermal synthesis of visible-light-responsive S-doped TiO2 nanocrystal. Appl. Surf. Sci. 2012, 258, 4016–4022. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Livraghi, S.; Giamello, E.; Selli, E. Photocatalytic activity of S- and F-doped TiO2 in formic acid mineralization. Photochem. Photobiol. Sci. 2011, 10, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Y.; Li, W.; Jin, R.; Tang, S.; Hu, W. Adsorption and interaction of H2S/SO2 on TiO2. Catal. Today 1999, 50, 39–47. [Google Scholar]
- Wei, F.; Ni, L.; Cui, P. Preparation and characterization of N–S-codoped TiO2 photocatalyst and its photocatalytic activity. J. Hazard. Mater. 2008, 156, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Huo, Y.; Zhu, J. Supercritical preparation of a highly active S-doped TiO2 photocatalyst for methylene blue mineralization. Environ. Sci. Technol. 2007, 41, 4410–4414. [Google Scholar] [CrossRef] [PubMed]
- Travert, A.; Maugé, F. IR study of hydrotreating catalysts in working conditions: Comparison of the acidity present on the sulfided phase and on the alumina support. Stud. Surf. Sci. Catal. 1999, 127, 269–277. [Google Scholar]
- Barba, D.; Cammarota, F.; Vaiano, V.; Salzano, E.; Palma, V. Experimental and numerical analysis of the oxidative decomposition of H2S. Fuel 2017, 198, 68–75. [Google Scholar] [CrossRef]
- Jüngst, E.; Nehb, W. Hydrogen sulfide to sulfur (Claus process). In Handbook of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2008; pp. 2609–2623. [Google Scholar]
- Huang, W.-F.; Chen, H.-T.; Lin, M.C. Density functional theory study of the adsorption and reaction of H2S on TiO2 rutile (110) and anatase (101) surfaces. J. Phys. Chem. C 2009, 113, 20411–20420. [Google Scholar] [CrossRef]
- Arrouvel, C.; Toulhoat, H.; Breysse, M.; Raybaud, P. Effects of PH2O, PH2S, PH2 on the surface properties of anatase-TiO2 and g-Al2O3: A DFT study. J. Catal. 2004, 226, 260–272. [Google Scholar] [CrossRef]
- Fahmi, A.; Ahdjoudj, J.; Minot, C. A theoretical study of H2S and MeSH adsorption on TiO2. Surf. Sci. 1996, 352–354, 529–533. [Google Scholar] [CrossRef]
- Markovits, A.; Ahdjoudj, J.; Minot, C. Theoretical study of the TiO2 and MgO surface acidity and the adsorption of acids and bases. Mol. Eng. 1997, 7, 245–261. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J. Adsorption and dissociation of H2S on nitrogen-doped TiO2 anatase nanoparticles: Insights from DFT computations. Surf. Interface 2017, 8, 15–27. [Google Scholar] [CrossRef]
- Ma, H.L.; Yang, J.Y.; Dai, Y.; Zhang, Y.B.; Lu, B.; Ma, G.H. Raman study of phase transformation of TiO2 rutile single crystal irradiated by infrared femtosecond laser. Appl. Surf. Sci. 2007, 253, 7497–7500. [Google Scholar] [CrossRef]
- Mino, L.; Spoto, G.; Bordiga, S.; Zecchina, A. Particles morphology and surface properties as investigated by HRTEM, FTIR, and periodic DFT calculations: From pyrogenic TiO2 (P25) to nanoanatase. J. Phys. Chem. C 2012, 116, 17008–17018. [Google Scholar] [CrossRef]
- Martra, G. Lewis acid and base sites at the surface of microcrystalline TiO2 anatase: Relationships between surface morphology and chemical behaviour. Appl. Catal. A 2000, 200, 275–285. [Google Scholar] [CrossRef]
- Spoto, G.; Morterra, C.; Marchese, L.; Orio, L.; Zecchina, A. The morphology of TiO2 microcrystals and their adsorptive properties towards CO: A HRTEM and FTIR study. Vacuum 1990, 41, 37–39. [Google Scholar] [CrossRef]
- Signorile, M.; Damin, A.; Budnyk, A.; Lamberti, C.; Puig-Molina, A.; Beato, P.; Bordiga, S. MoS2 supported on P25 titania: A model system for the activation of a HDS catalyst. J. Catal. 2015, 328, 225–235. [Google Scholar] [CrossRef]
- Cesano, F.; Bertarione, S.; Uddin, M.J.; Agostini, G.; Scarano, D.; Zecchina, A. Designing TiO2 based nanostructures by control of surface morphology of pure and silver loaded titanate nanotubes. J. Phys. Chem. C 2010, 114, 169–178. [Google Scholar] [CrossRef]
- Bordiga, S.; Scarano, D.; Spoto, G.; Zecchina, A.; Lamberti, C.; Otero Areán, C. Infrared study of carbon monoxide adsorption at 77 K on faujasites and ZSM-5 zeolites. Vib. Spectrosc. 1993, 5, 69–74. [Google Scholar] [CrossRef]
- Mino, L.; Ferrari, A.M.; Lacivita, V.; Spoto, G.; Bordiga, S.; Zecchina, A. CO adsorption on anatase nanocrystals: A combined experimental and periodic DFT study. J. Phys. Chem. C 2011, 115, 7694–7700. [Google Scholar] [CrossRef]
- Davydov, A.; Chuang, K.T.; Sanger, A.R. Mechanism of H2S oxidation by ferric oxide and hydroxide surfaces. J. Phys. Chem. B 1998, 102, 4745–4752. [Google Scholar] [CrossRef]
- Murphy, A.B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Zhou, W.; Liu, Z.; Xie, G.; Wang, Y.; Du, Y. High quality sulfur -doped titanium dioxide nanocatalysts with visible light photocatalytic activity from non-hydrolytic thermolysis synthesis. Inorg. Chem. Front. 2014, 1, 521–525. [Google Scholar] [CrossRef]
- Ho, W.; Yu, J.C.; Lee, S. Low-temperature hydrothermal synthesis of S-doped TiO2 with visible light photocatalytic activity. J. Solid State Chem. 2006, 179, 1171–1176. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The electronic origin of the visible-light absorption properties of C-, N- and S-doped TiO2 nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, D. Sulfur-doped hhighly ordered TiO2 nanotubular arrays with visible light response. J. Phys. Chem. C 2008, 112, 5405–5409. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-Doped TiO2: Structure and Surface Properties. Catalysts 2017, 7, 214. https://doi.org/10.3390/catal7070214
Cravanzola S, Cesano F, Gaziano F, Scarano D. Sulfur-Doped TiO2: Structure and Surface Properties. Catalysts. 2017; 7(7):214. https://doi.org/10.3390/catal7070214
Chicago/Turabian StyleCravanzola, Sara, Federico Cesano, Fulvio Gaziano, and Domenica Scarano. 2017. "Sulfur-Doped TiO2: Structure and Surface Properties" Catalysts 7, no. 7: 214. https://doi.org/10.3390/catal7070214