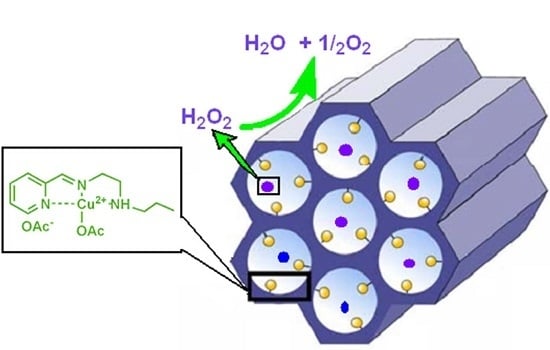
Highly ordered Nanomaterial Functionalized Copper Schiff Base Framework: Synthesis, Characterization, and Hydrogen Peroxide Decomposition Performance
Abstract
:1. Introduction
2. Results and Discussion
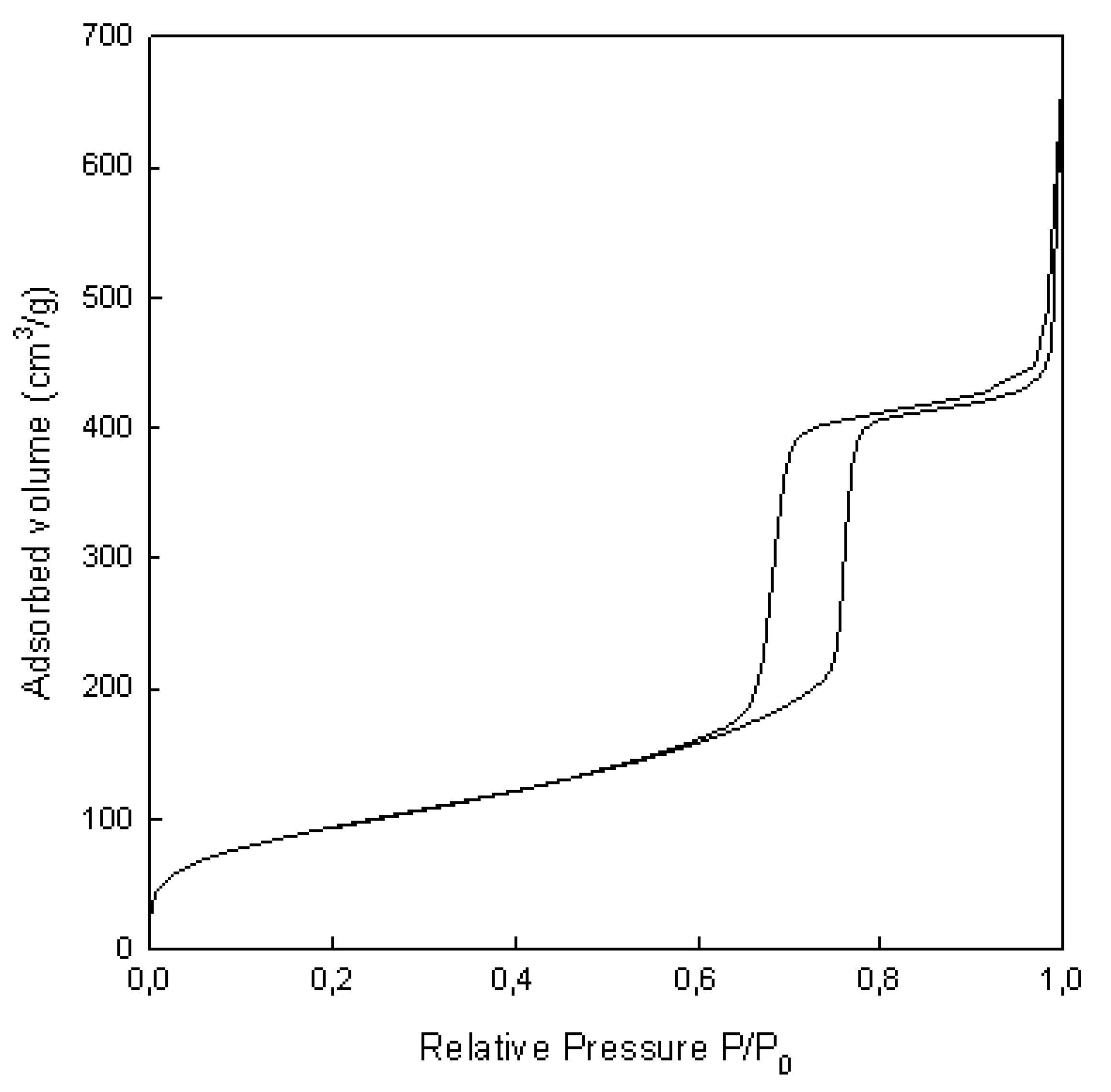
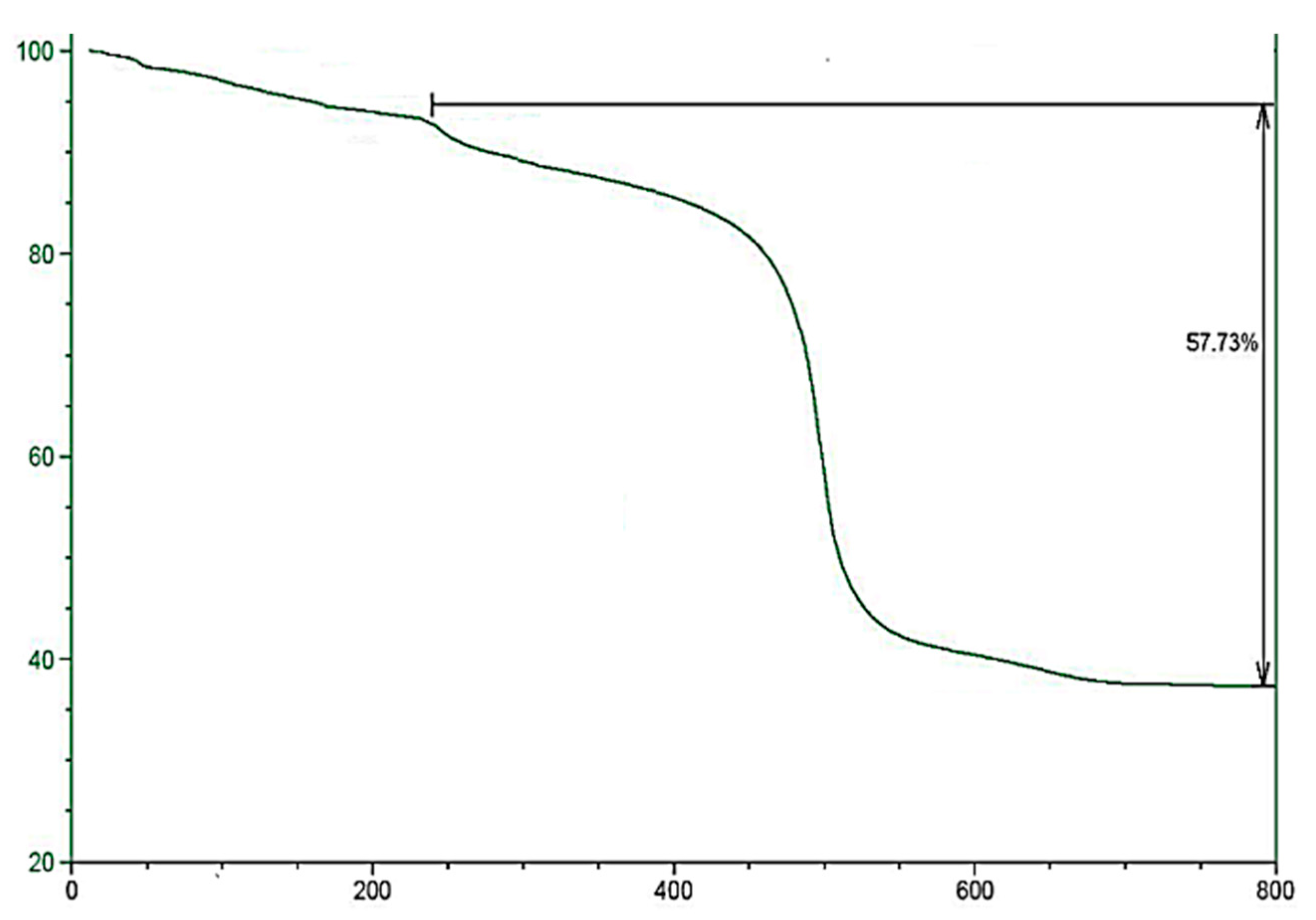
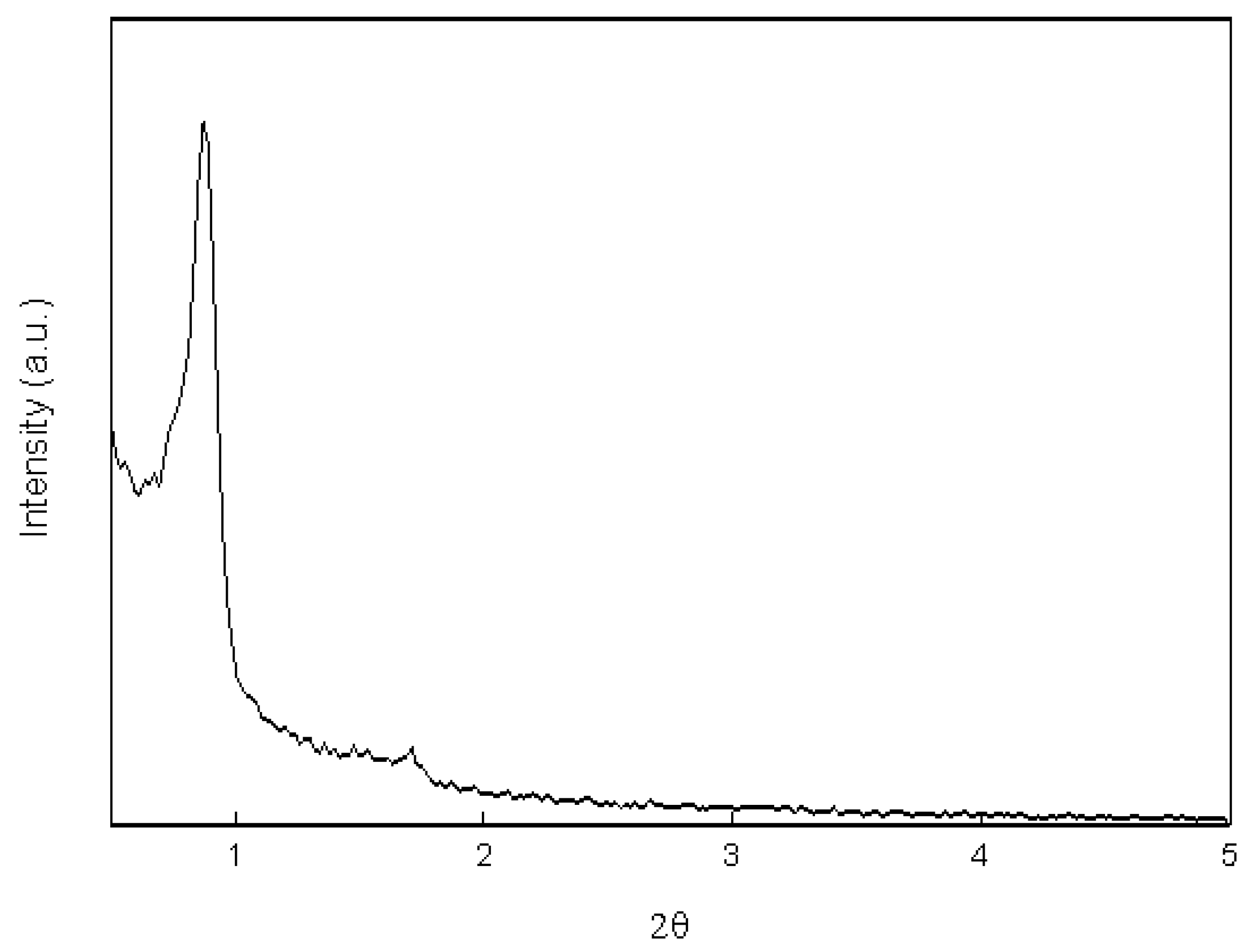
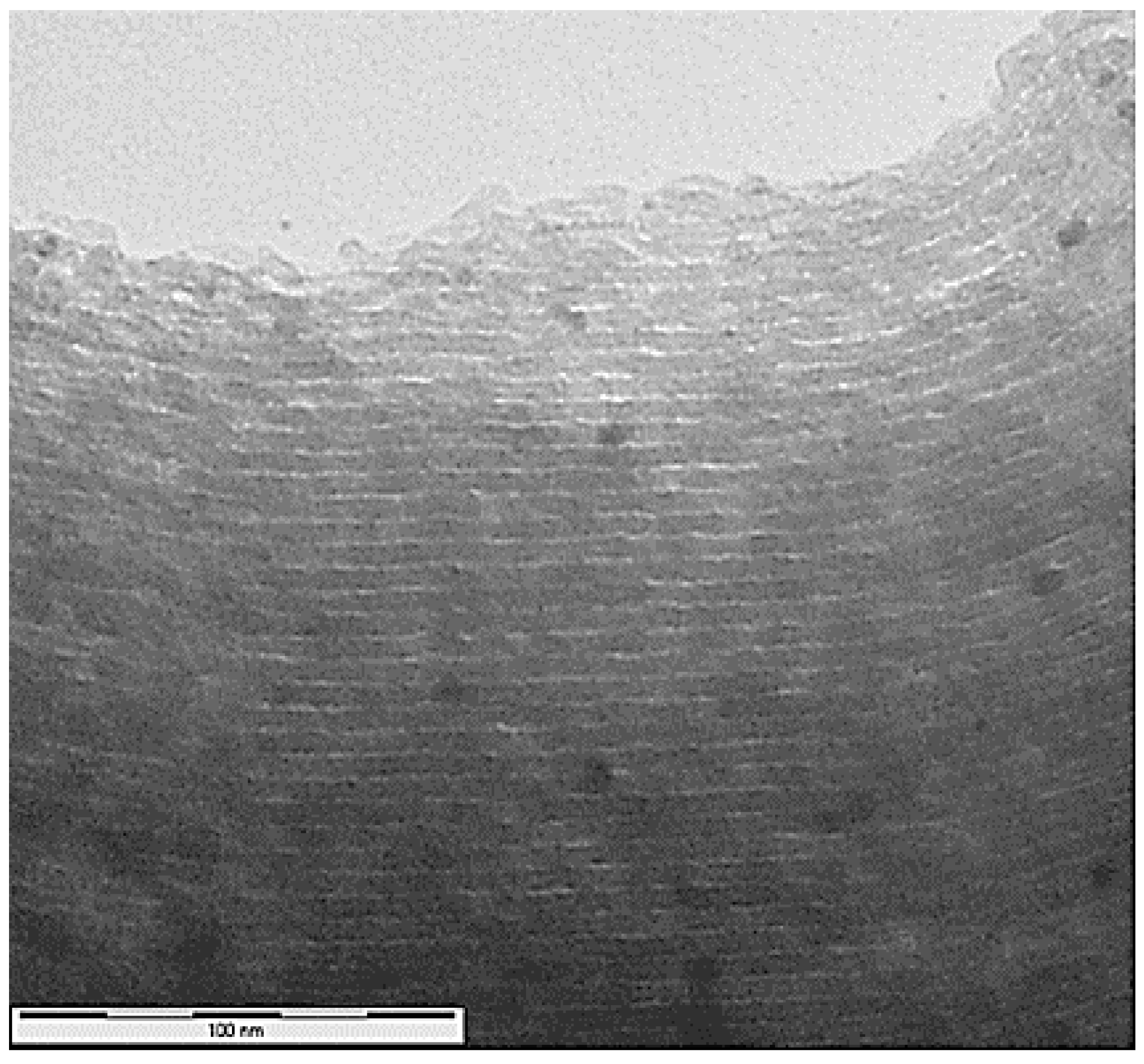
2.1. Morphology and Characterization of Immobilized Materials
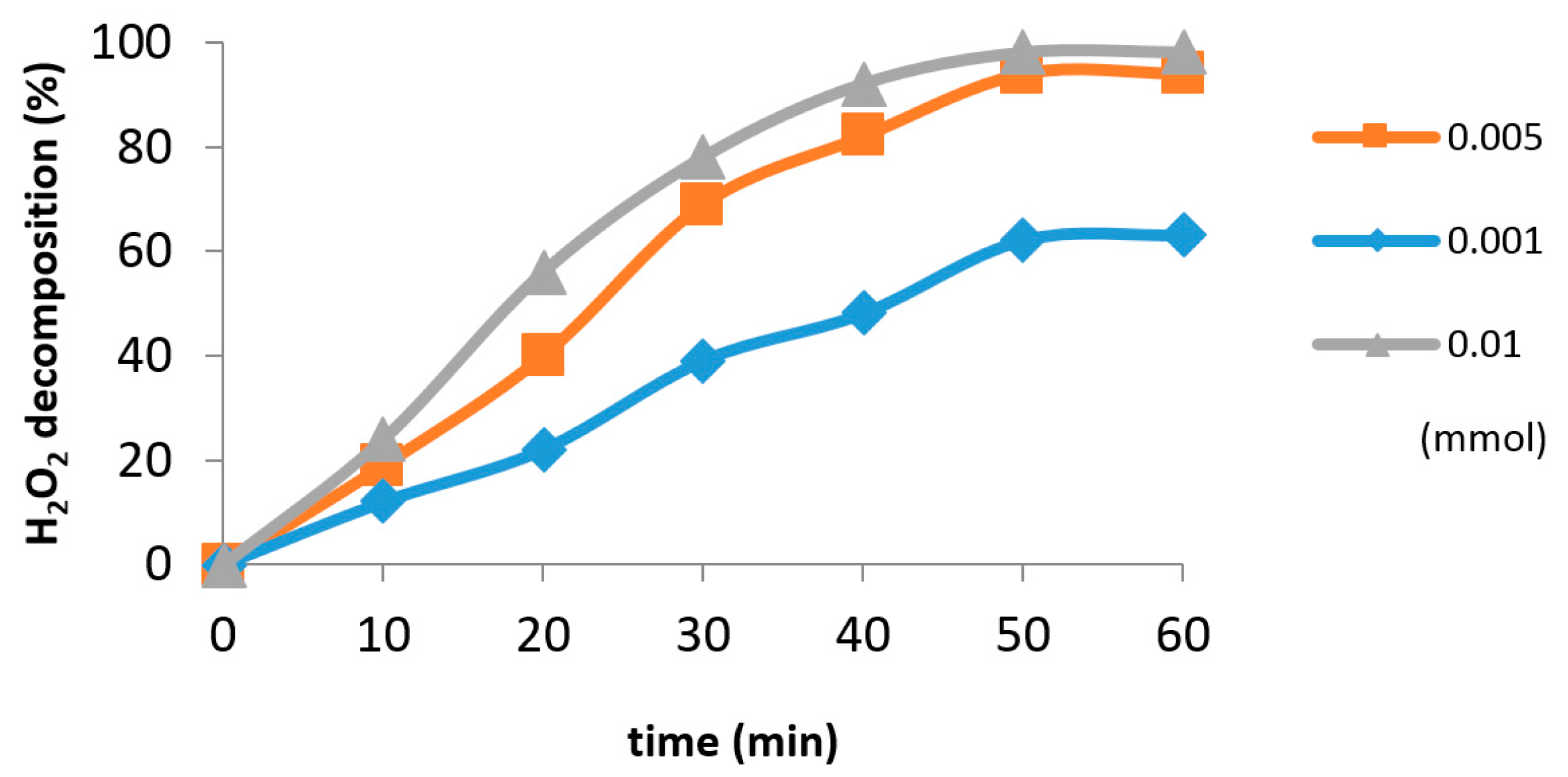
2.2. Catalytic Tests
2.3. Catalyst Reusability
3. Experimental Section
3.1. Materials
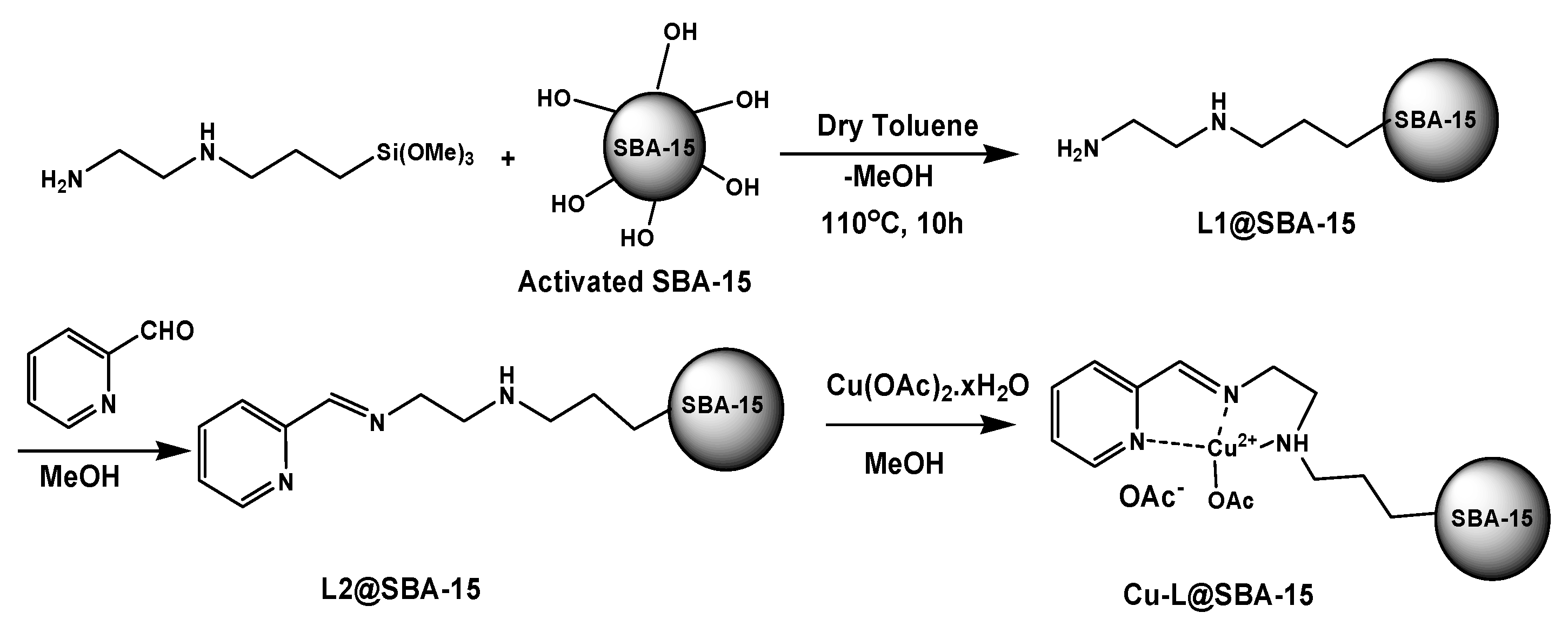
3.2. Preparation of L1@SBA-15
3.3. Preparation of L2@SBA-15
3.4. Preparation of Cu-L@SBA-15
3.5. General Procedure for Hydrogen Peroxide Decomposition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, J.H. Chemistry of Minimisation; Blackie Academic: London, UK, 1995; pp. 17–64. [Google Scholar]
- Clark, J.H. Handbook of Green Chemistry and Technology; Blackwell Science Ltd.: Oxford, UK, 2002; pp. 10–60. [Google Scholar]
- Kirschning, A. Immobilized Catalysts: Solid Phases, Immobilization and Applications; Springer: Berlin, Germany, 2004; Volume 242, pp. 241–271. [Google Scholar]
- Sherrington, D.C.; Kybett, A.P. Supported Catalysts and Their Applications; Royal Society of Chemistry: Cambridge, UK, 2001; pp. 48–54. [Google Scholar]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- DeVos, D.E.; Dams, M.; Sels, B.F.; Jacobs, P.A. Ordered mesoporous and microporous molecular sieves functionalized with transition metal complexes as catalysts for selective organic transformations. Chem. Rev. 2002, 102, 3615–3640. [Google Scholar] [CrossRef]
- Taguchi, A.; Schìth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Shea, K.J.; Loy, D.A.; Webster, O. Arylsilsesquioxane gels and related materials. New hybrids of organic and inorganic networks. J. Am. Chem. Soc. 1992, 114, 6700–6710. [Google Scholar] [CrossRef]
- Loy, D.A.; Shea, K.J. Bridged polysilsesquioxanes. Highly porous hybrid organic-inorganic materials. Chem. Rev. 1995, 95, 1431–1442. [Google Scholar] [CrossRef]
- Hoffmann, F.; Corne-lius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-B.; Liu, X.-Q.; Zhou, H.-C. Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 2015, 44, 5092–5147. [Google Scholar] [CrossRef] [PubMed]
- Pater, J.P.G.; Jacobs, P.A.; Martens, J.A. Oligomerization of hex-1-ene over acidic aluminosilicate zeolites, MCM-41, and silica-alumina co-gel catalysts: A comparative study. J. Catal. 1999, 184, 262–267. [Google Scholar] [CrossRef]
- Liang, Y.; Anwander, R. Nanostructured catalysts via metal amide-promoted smart grafting. Dalton Trans. 2013, 42, 12521–12545. [Google Scholar] [CrossRef] [PubMed]
- Van Der Voort, P.; Esquivel, D.; De Canck, E.; Goethals, F.; Van Driessche, I.; Romero-Salguero, F.J. Periodic mesoporous organosilicas: from simple to complex bridges; a comprehensive overview of functions, morphologies and applications. Chem. Soc. Rev. 2013, 42, 3913–3955. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Zhang, L.; Li, C. Functionalized periodic mesoporous organosilicas for catalysis. J. Mater. Chem. 2009, 19, 1945–1955. [Google Scholar] [CrossRef]
- Meng, X.; Nawaz, F.; Xiao, F.-S. Templating route for synthesizing mesoporous zeolites with improved catalytic properties. Nano Today 2009, 4, 292–301. [Google Scholar] [CrossRef]
- Hata, H.; Saeki, S.; Kimura, T.; Sugahara, Y.; Kuroda, K. Adsorption of taxol into ordered mesoporous silicas with various pore diameters. Chem. Mater. 1999, 11, 1110–1119. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Yang, P.D.; Huo, Q.S.; Chmelka, B.F.; Stucky, G.D. Topological construction of mesoporous materials. Curr. Opin. Solid State Mater. Sci. 1998, 3, 111–121. [Google Scholar] [CrossRef]
- Waki, M.; Maegawa, Y.; Hara, K.; Goto, Y.; Yamada, Y.; Mizoshita, N.; Tani, T.; Chun, W.-J.; Muratsugu, S.; Tada, M.; et al. A solid chelating ligand: periodic mesoporous organosilica containing 2, 2′-bipyridine within the pore walls. J. Am. Chem. Soc. 2014, 136, 4003–4011. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Morey, M.S.; Davidson, A.; Stucky, G.D. Silica-based, cubic mesostructures: Synthesis, characterization and relevance for catalysis. J. Porous Mater. 1998, 5, 195–204. [Google Scholar] [CrossRef]
- Johnson-White, B.; Zeinali, M.; Shaffer, K.M.; Patterson, C.H.; Charles, P.T.; Markowitz, M.A. Detection of organics using porphyrin embedded nanoporous organosilicas. Biosens. Bioelectron. 2007, 22, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Jo, C.; Lee, J. A mini review of designed mesoporous materials for energy-storage applications: from electric double-layer capacitors to hybrid supercapacitors. Nanoscale 2016, 8, 7827–7833. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesoporous materials as gas sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Park, S.S.; Fuping, D.; Hong, S.H.; Selvaraj, M.; Ha, C.S. Step-up synthesis of amidoxime-functionalised periodic mesoporous organosilicas with an amphoteric ligand in the framework for drug delivery. J. Mater. Chem. 2012, 22, 9100–9108. [Google Scholar] [CrossRef]
- Du, X.; Li, X.; Xiong, L.; Zhang, X.; Kleitz, F.; Qiao, S.Z. Mesoporous silica nanoparticles with organo-bridged silsesquioxane framework as innovative platforms for bioimaging and therapeutic agent delivery. Biomaterials 2016, 91, 90–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Luque, R.; Balu, A.M.; Campelo, J.M.; Gracia, M.D.; Losada, E.; Pineda, A.; Romero, A.A.; Serrano-Ruiz, J.C. Catalytic applications of mesoporous silica-based materials. Catalysis 2012, 24, 253–280. [Google Scholar]
- Karimi, B.; Mansouri, F.; Khorasani, M. Recent progress in design and application of functional ordered/periodic mesoporous silicas (OMSS) and organosilicas (PMOS) as catalyst support in carbon-carbon coupling reactions. Curr. Org. Chem. 2016, 20, 349–380. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Airoldi, C. Different neutral surfactant template extraction routes for synthetic hexagonal mesoporous silicas. J. Mater. Chem. 2002, 12, 3823–3826. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Melero, J.A.; van Grieken, R.; Morales, G. Advances in the synthesis and catalytic applications of organosulfonic-functionalized mesostructured materials. Chem. Rev. 2006, 106, 3790–3812. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kruk, M. Versatile surfactant/swelling-agent template for synthesis of large-pore ordered mesoporous silicas and related hollow nanoparticles. Chem. Mater. 2015, 27, 679–689. [Google Scholar] [CrossRef]
- Han, L.; Che, S. Anionic surfactant templated mesoporous silicas (AMSs). Chem. Soc. Rev. 2013, 42, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.; Silva, A.M.; Pinho, M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Graphene-based materials for the catalytic wet peroxide oxidation of highly concentrated 4-nitrophenol solutions. Catal. Today 2015, 249, 204–212. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Rathi, A.K.; Gawande, M.B.; Hola, K.; Goswami, A.; Kalytchuk, S.; Karakassides, M.A.; Kouloumpis, A.; Gournis, D.; Deligiannakis, Y.; et al. Fe(III)-functionalized carbon dots—Highly efficient photoluminescence redox catalyst for hydrogenations of olefins and decomposition of hydrogen peroxide. Appl. Mater. Today 2017, 7, 179–184. [Google Scholar] [CrossRef]
- Tamami, B.; Ghasemi, S. Catalytic activity of Schiff-base transition metal complexes supported on crosslinked polyacrylamides for hydrogen peroxide decomposition. J. Organomet. Chem. 2015, 794, 311–317. [Google Scholar] [CrossRef]
- Demetgül, C. Synthesis of the ketimine of chitosan and 4, 6-diacetylresorcinol, and study of the catalase-like activity of its copper chelate. Carbohydr. Polym. 2012, 89, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Karimi, N.; Saidi, M.R.; Primo, A.; Varma, R.S.; Luque, R. Unprecedented selective oxidation of styrene derivatives using a supported iron oxide nanocatalyst in aqueous medium. Adv. Synth. Catal. 2012, 354, 1707–1711. [Google Scholar] [CrossRef]
- Rajabi, F.; Nourian, S.; Ghiassian, S.; Balu, A.M.; Saidi, M.R.; Serrano-Ruiz, J.C.; Luque, R. Heterogeneously catalysed Strecker-type reactions using supported Co (II) catalysts: Microwave vs. conventional heating. Green Chem. 2011, 13, 3282–3289. [Google Scholar] [CrossRef]
- Rajabi, F.; Schaffner, D.; Follmann, S.; Wilhelm, C.; Ernst, S.; Thiel, W.R. Electrostatic grafting of a palladium n-heterocyclic carbene catalyst on a periodic mesoporous organosilica and its application in the Suzuki-miyaura reaction. ChemCatChem 2015, 7, 3513–3518. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Grosjean, A.; Soroka, I.; Jonsson, M. kinetics and mechanism of the reaction between H2O2 and tungsten powder in water. J. Phys. Chem. C 2015, 119, 22560–22569. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J. Molecular assembly of Schiff base interactions: Construction and application. Chem. Rev. 2015, 115, 1597–1621. [Google Scholar] [CrossRef] [PubMed]
- Casellato, U.; Vigato, P.A. Transition metal complexes with binucleating ligands. Coord. Chem. Rev. 1977, 23, 31–50. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K.; Lin, C.C. Polymer-supported Schiff base complexes in oxidation reactions. Coord. Chem. Rev. 2009, 253, 1926–1946. [Google Scholar] [CrossRef]
- Cozzi, P.G. Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Liu, X.; Novoa, N.; Manzur, C.; Carrillo, D.; Hamon, J.-R. New organometallic Schiff-base copper complexes as efficient “click” reaction precatalysts. New J. Chem. 2016, 40, 3308–3313. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Y.; Li, Q.; Ma, T.; Xu, J.; Zhu, T.; Xie, J.; Zhu, W.; Cao, Z.; Dong, K.; et al. Ternary dinuclear copper (II) complexes of a reduced schiff Base ligand with diimine coligands: DNA binding, cytotoxic cell apoptosis, and apoptotic mechanism. Chem. Biol. Drug Des. 2016, 87, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd (II) and Cu (II) complexes of polydentate Schiff base ligands: synthesis, characterization, properties and biological activity. Inorg. Chimica Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Chaviara, A.T.; Cox, P.J.; Repana, K.H.; Pantazaki, A.A.; Papazisis, K.T.; Kortsaris, A.H.; Kyriakidis, D.A.; Nikolov, G.S.; Bolos, C.A. The unexpected formation of biologically active Cu(II) Schiff mono-base complexes with 2-thiophene-carboxaldehyde and dipropylenetriamine: Crystal and molecular structure of CudptaSCl2. J. Inorg. Biochem. 2005, 99, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Spear, E.B. Catalytic decomposition of hydrogen peroxide under high pressures of oxygen. J. Am. Chem. Soc. 1908, 30, 195–209. [Google Scholar] [CrossRef]
- McKee, D.W. Catalytic decomposition of hydrogen peroxide by metals and alloys of the platinum group. J. Catal. 1969, 14, 355–364. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, F.; Pinilla-de Dios, M.; Luque, R. Highly ordered Nanomaterial Functionalized Copper Schiff Base Framework: Synthesis, Characterization, and Hydrogen Peroxide Decomposition Performance. Catalysts 2017, 7, 216. https://doi.org/10.3390/catal7070216
Rajabi F, Pinilla-de Dios M, Luque R. Highly ordered Nanomaterial Functionalized Copper Schiff Base Framework: Synthesis, Characterization, and Hydrogen Peroxide Decomposition Performance. Catalysts. 2017; 7(7):216. https://doi.org/10.3390/catal7070216
Chicago/Turabian StyleRajabi, Fatemeh, María Pinilla-de Dios, and Rafael Luque. 2017. "Highly ordered Nanomaterial Functionalized Copper Schiff Base Framework: Synthesis, Characterization, and Hydrogen Peroxide Decomposition Performance" Catalysts 7, no. 7: 216. https://doi.org/10.3390/catal7070216